Summary
In animal models, time-restricted feeding (TRF) can prevent and reverse aspects of metabolic diseases. Time-restricted eating (TRE) in human pilot studies reduces the risks of metabolic diseases in otherwise healthy individuals. However, patients with diagnosed metabolic syndrome often undergo pharmacotherapy, and it has never been tested whether TRE can act synergistically with pharmacotherapy in animal models or humans. In a single-arm, paired-sample trial, 19 participants with metabolic syndrome and a baseline mean daily eating window of ≥ 14 hours, the majority of whom were on a statin and/or antihypertensive therapy, underwent 10-hour of TRE (all dietary intake within a consistent self-selected 10-hour window) for 12-weeks. We found this TRE intervention improves cardiometabolic health for patients with metabolic syndrome receiving standard medical care including high rates of statin and antihypertensive use. TRE is a potentially powerful lifestyle intervention that can be added to standard medical practice to treat metabolic syndrome.
Keywords: metabolic syndrome, time-restricted eating, TRE, TRF, circadian rhythm, obesity, impaired glucose tolerance, hypertension, dyslipidemia
Graphical Abstract
eTOC Blurb
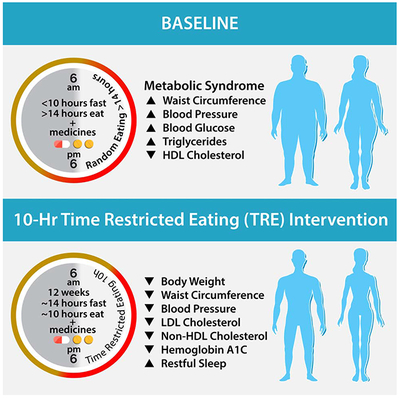
Wilkinson and Manoogian et al. studied the impact of time-restricted eating in metabolic syndrome by reducing participant’s daily eating window from ≥ 14 hours to a self-selected 10-hour window for 12-weeks. Time-restricted eating led to weight loss, healthier body composition, lower blood pressure, and decreased levels of cardiovascular disease-promoting lipids.
Introduction
Metabolic syndrome is defined by the presence of multiple, related risk factors for type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD) (Alberti et al., 2009; Sperling et al., 2015). It is highly prevalent, affecting ~30% of the U.S. population and confers a 5-fold increase in the risk of T2DM and doubles the risk for CVD over 5-10 years (Alberti et al., 2009; Ervin, 2009). Features of metabolic syndrome include abdominal obesity, elevated blood pressure, insulin resistance, a proinflammatory and prothrombotic state, and atherogenic dyslipidemia (high triglycerides, high apolipoprotein B, high low-density lipoprotein particle (LDL-p) number, and low high-density lipoprotein cholesterol (HDL-C)). The first line of therapy for metabolic syndrome is aggressive diet and lifestyle interventions: reducing caloric intake, adopting a healthy diet, and increasing physical activity (Sperling et al., 2015). However, these approaches are often insufficient to effectively manage the disease, the disease gradually worsens, and patients are commonly put on medications to treat their symptoms. Treating metabolic syndrome is of crucial importance in preventing progression to T2DM and in reducing morbidity and mortality from T2DM and CVD. Thus, there is a critical unmet need for lifestyle interventions in metabolic syndrome which are effective, easy for clinicians to teach to patients during routine care, and intuitive for patients to adopt and maintain, either to prevent or work as an “add-on” to pharmacological treatment.
Time-restricted eating (TRE) is an emerging dietary intervention that aims to maintain a consistent daily cycle of feeding and fasting to support robust circadian rhythms (Panda, 2016). Circadian regulation of the endocrine system, autonomic nervous system, and nutrient metabolism contribute to metabolic and physiological homeostasis (Asher and Sassone-Corsi, 2015; Panda, 2016). Both erratic eating patterns and eating over an extended period of time during the 24 h day can disrupt circadian rhythms. Chronic circadian disruption can increase the risk for components of metabolic syndrome including obesity, hypertension, insulin resistance, inflammation, and dyslipidemia (Lunn et al., 2017; Mohebbi et al., 2012; Puttonen et al., 2010; Yuan et al., 2018). Observational studies in humans have also shown a correlation between irregular eating times and increased risk for metabolic syndrome and other cardiometabolic diseases (Pot et al., 2016; Sierra-Johnson et al., 2008; Wennberg et al., 2016). Conversely, in animal models, maintaining a daily rhythm of feeding and fasting by TRF (time-restricted feeding refers to animal studies, TRE in human studies) sustains robust daily rhythms and prevents as well as reverses metabolic diseases (Mattson et al., 2014; Sulli et al., 2018; Zarrinpar et al., 2016). TRF is effective in reversing aspects of metabolic syndrome in mice in both sexes by reducing body weight, adiposity, glucose intolerance, plasma cholesterol, and plasma triglycerides (Chaix et al., 2014; Chung et al., 2016), and improving heart function in Drosophila (Gill et al., 2015).
There is potential for humans to adopt TRE for health benefits. Objective longitudinal monitoring of human eating habits over several days has found that over 50% of people eat within a window of >15 h (Gill and Panda, 2015; Gupta et al., 2017) and only ~10% of adults habitually maintain a ≥12 h window of fasting. Prior studies have shown that overweight or obese adults who habitually eat for ≥14 h can safely adopt an 8-10 h interval of TRE over several weeks and achieve weight loss (Gabel et al., 2018a; Gabel et al., 2018b; Gill and Panda, 2015; Hutchison et al., 2019). To date, TRE has primarily been studied in healthy humans (Antoni et al., 2018; LeCheminant et al., 2013; Moro et al., 2016; Tinsley et al., 2017), or in those who are overweight or obese but who are otherwise without metabolic disease (Gabel et al., 2018a; Gabel et al., 2018b; Gill and Panda, 2015; Hutchison et al., 2019). In these studies, TRE inadvertently reduced energy intake and led to a reduction in body weight. In men with prediabetes, 6 h TRE, without reducing caloric intake, reduced signs of insulin resistance (Sutton et al., 2018). In men who were overweight, a 9 h TRE decreased triglycerides, improved glycemic response to meals and reduced mean fasting glucose assessed by CGM (Hutchison et al., 2019). An extensive literature review on the effect of meal timing on cardiometabolic health has prompted the American Heart Association (AHA) to suggest that nightly fasting may reduce the risk for CVD (St-Onge et al., 2017).
Self-reported adverse effects of TRE are rare, but one crossover study (6 h TRE, 8 am-2 pm compared to 12 h, 8 am-8 pm, 5 weeks in each arm) included reports of vomiting (n=1), headaches, increased thirst, and diarrhea (n=2) (Sutton et al., 2018). Another study (8 h TRE, 10 am-6 pm, 12 weeks) reported a non-significant increase in mean percent occurrences of nausea, diarrhea, constipation, and dizziness (Gabel et al., 2018b). Resistance training combined with 8 h TRE lead to significant reductions in total testosterone and total triiodothyronine (T3) with TRE after 8 weeks (Moro et al., 2016); total testosterone remained within the reference range and T3 was just below the lower limit of normal. TRE of 13 h (6 am-7 pm) compared to ad lib, (a cross-over study, 2 weeks per arm) reported increased hunger before breakfast and a trend toward increased fatigue with TRE (driven in part by a reduction in fatigue during control) (LeCheminant et al., 2013). However, decreased hunger at bedtime and increased morning- and overall-energy was reported after 16 weeks of 10-12 h TRE (time of day decided by the participant). After a one-year follow-up, energy levels were still significantly increased, but hunger at bedtime was no longer significantly different, although still less than at baseline (Gill and Panda, 2015).
Although not considered a medically relevant adverse effect, a separate study which delayed breakfast and advanced dinner by 1.5 h each (resulting in an ~8.5 h eating interval) reported that TRE affected opportunities for people to engage in evening social eating and drinking activities (Antoni et al., 2018), which could conceivably have implications for long-term adherence. It is also important to note that in all TRE studies in which negative effects were reported, the timing of the TRE was pre-determined for the participant. Taking an individual’s schedule and personal preference into consideration and letting the participants choose their own TRE interval is likely an important factor for adherence, efficacy, and reducing adverse effects.
As part of routine medical care, patients with metabolic syndrome are counseled by healthcare providers to adopt increased physical activity and reduced caloric intake (Sperling et al., 2015). However, TRE as a behavioral intervention has never been studied in patients with metabolic syndrome. Components of metabolic syndrome (i.e. elevated blood pressure, abnormal lipids) are also frequently treated with pharmacotherapy. It is not known whether this high-risk group of patients with metabolic syndrome, who have already received standard medical care including diet/physical activity counseling and pharmacotherapy, will benefit from adopting TRE as an additional therapy.
Based on TRF studies in rodents with pre-existing diet-induced obesity (Chaix et al., 2014), we hypothesized that a 10 h TRE intervention in patients with metabolic syndrome (and a ≥ 14 h/day baseline eating interval) would result in a significant improvement in mean blood glucose, fasting insulin, triglycerides, and the inflammatory marker high sensitivity c-reactive protein (hs-CRP). We also anticipated weight loss and improvements in cardiometabolic health including a reduction in blood pressure and atherogenic lipid levels. We observed significant reductions (p<0.05) in body weight (mean±SD −3.3±3.20 kg (−3%)), waist circumference (−4.5±6.72 cm (−4%)), body mass index (−1.1±0.97 kg/m2, (−3%)), percent body fat (−1.0±0.91%, (−3%)), visceral fat rating (−0.6±0.77 (−3%)), systolic and diastolic blood pressure (−5.1±9.51 mmHg (−4%) and −6.5±7.94 mmHg (−8%), respectively), total cholesterol (−13.2±24.29 mg/dL (−7%)), low-density lipoprotein cholesterol (LDL-C) (−11.9±19.01 mg/dL (−11%)), and non-high-density lipoprotein cholesterol (non-HDL-C) −11.6±22.94 mg/dL (−9%)). Although we did not detect a significant improvement in average glucose, fasting insulin, triglycerides, and hs-CRP in the entire cohort, with the exception of hs-CRP these parameters modestly changed in the desirable direction. Participants with elevated fasting glucose (≥100 mg/dL) and/or HbA1c (≥5.7%) at baseline (n=12), had a significant reduction in HbA1c (−0.22±0.32% (3.7%), p = 0.04). Improvements were observed despite no change in physical activity and were independent of weight.
Results and Discussion.
Participants, adherence, and adverse effects
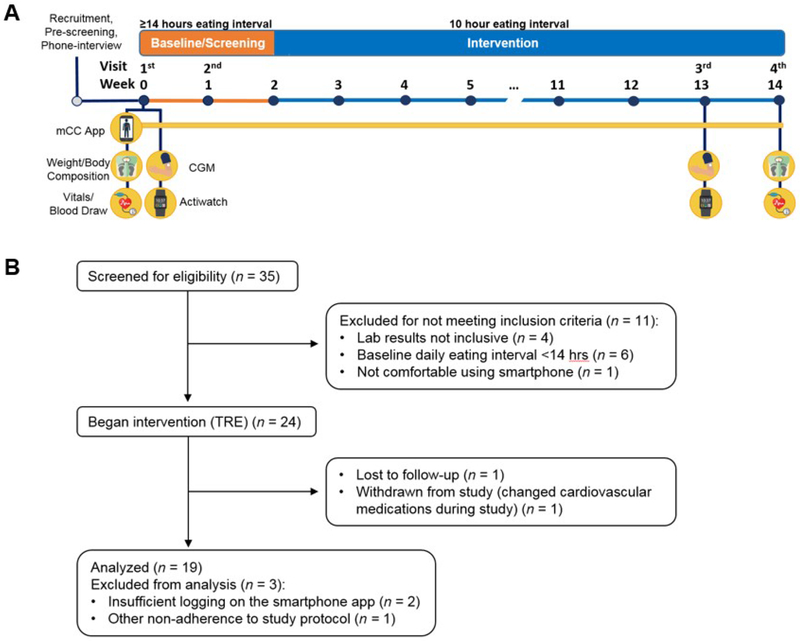
This study was conducted with approval from the Institutional Review Board at UC San Diego (UCSD) and The Salk Institute for Biological Studies. All participants provided written informed consent. Participants were recruited from UCSD clinics, were diagnosed with metabolic syndrome (using AHA/National Heart, Lung, and Blood Institute cut points for waist circumference) (Alberti et al., 2009) and a self-reported eating interval of ≥14 h per day. Our study design is summarized in Figure 1A.
Figure 1. Study Design and CONSORT flow diagram.
(A) Study design. Prior to baseline, participants were screened with a phone interview. At the first visit (day 1), questionnaires, vitals, and blood were collected. Participants also had the CGM applied, were given an actiwatch, and were trained how to use the myCircadianClock (mCC) app. Week 0-1: Participants wore the CGM and actiwatch, and logged all food and beverages on the mCC app. mCC data were used to screen participants for a ≥ 14 h eating interval. At visit 2 (~day 7) the CGM was removed and the actiwatch was returned. At the end of week 2, if participants qualified for the study, they were instructed to select a 10 h eating window and start their 12-week time-restricted eating intervention. The mCC app was used throughout intervention to log food and beverage intake and sleep. 7-10 days before the end of intervention, participants came for the 3rd visit and had another CGM applied and were provided an actiwatch. At visit 4, the CGM was removed, the actiwatch was returned, and all assays taken at baseline were repeated. CGM, continuous glucose monitor. mCC, myCircadianClock smartphone app. (B) CONSORT flow diagram describing the process of patient enrollment, intervention, and data analysis. TRE, time-restricted eating.
Thirty-five participants were enrolled. After screening to meet eligibility criteria, 25 began the TRE intervention, and 19 were included in the final analysis (Figure 1B); 13 men and 6 women, 63% non-Hispanic white, age 59±11.14 (mean±SD) years. All participants met 3 or more metabolic syndrome criteria at the time of enrollment (Supplemental Table 1). Sixteen participants (84%) were taking at least one medication, and 3 (16%) were taking no medications. Overall, statin and antihypertensive use were high, (79% and 63%, respectively; Supplemental Table 2). Most participants were obese, with a body weight of 97.84±19.73 kg and a BMI of 33.06±4.76 kg/m2. Twelve of the 19 participants had an elevated fasting glucose of ≥100 mg/dL (107.2±16.62 mg/dL) and/or elevated HbA1c of ≥ 5.7% (5.90±0.41%) at baseline (Supplemental Table 1). Time of day data for fasting lab draws was available for all 19 participants, while the time of day data for last dietary intake prior to overnight fasting for lab draws (and thus, fasting duration prior to lab draws) was available for n=8. Baseline fasting labs were drawn at 09:31±1:30 h (after a 13.75±0.76 h fast), and at the end of intervention fasting labs were drawn at 09:03±1:14 h (after a 13.38±0.84 h fast).
Participants used a validated app – myCircadianClock (mCC) – to log their caloric intake during the 2-week baseline and 12-week intervention periods. Mean percent adherence to logging (a minimum of two calorie-containing entries over a minimum of 5 h for a given day) was 94.30±7.25% during the 2-week baseline, and 85.61±12.39% during the 12-week intervention.
Unlike a 6 h TRE intervention which reported several adverse events including vomiting, headaches, increased thirst, and diarrhea (Sutton et al., 2018), participants in our 10 h TRE intervention did not report any of these adverse events. One participant reported muscle discomfort associated with implantation and use of the CGM, but no other adverse events occurred. Missing data and data excluded from our final analysis are described in STAR Methods.
Changes in eating pattern
As seen in previous longitudinal monitoring of daily eating patterns (Gill and Panda, 2015; Gupta et al., 2017), we also found the eating pattern of the participants at baseline varied from day to day (Supplemental Figure 1). Collating all calorie-ingestion events during the baseline period revealed the eating events were spread over a wide window of the 24 h day (Figure 2). To account for such day to day variation in the time of ingestion of calorie-containing food or beverages, we define the eating window as the time interval during which 95% of all calorie-containing ingestion events occur during baseline or intervention period. The 95% eating window at baseline was 15.13±1.13 h.
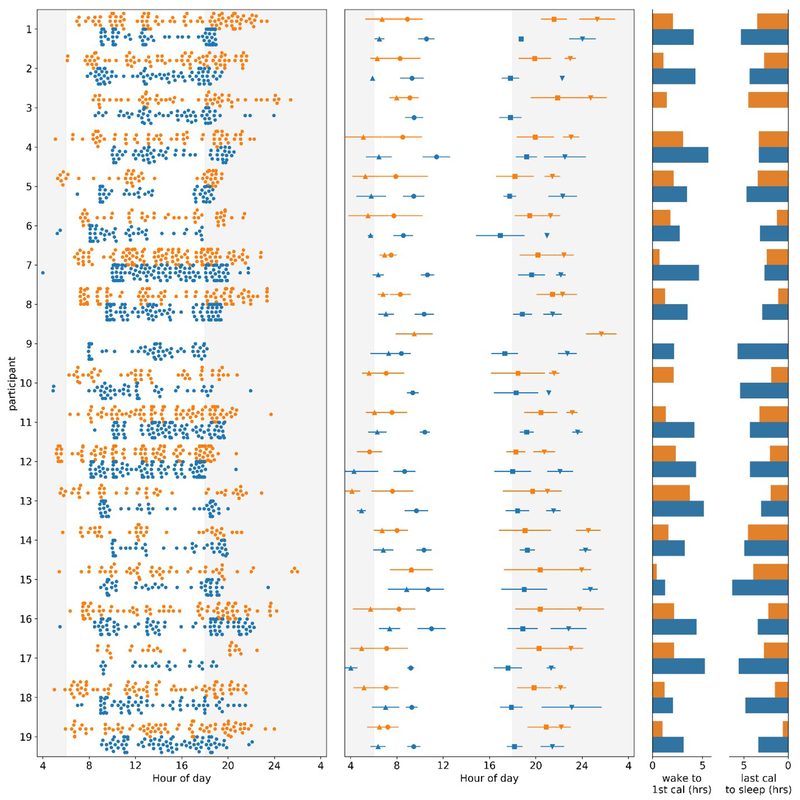
Figure 2. Variance in time of first and last calorie and sleep and wake times decreases in TRE intervention.
For all panels, baseline data is in orange, and intervention in blue. Y-axis: each orange/blue combination represents an individual participant. For the left and middle panels, X-axis: clock hour for eating event (4=4AM, 24=midnight). Left Panel: All food and beverage events from two weeks of baseline and the last two weeks of TRE intervention for each participant. Data from each two-week interval was randomly sampled to have the same number of entries at baseline and end of intervention. Middle Panel: Mean and standard deviation for first calorie (●), last calorie (■), wake (▲), and sleep onset (▼) at baseline and intervention. First and last calorie data was only used for days that logging adherence was met. Right Panel: Mean time (in h) between wake and first calorie (left) and last calorie and sleep onset (right) at baseline and intervention. Data were analyzed for each day that the watch was worn and logging adherence was achieved, and then averaged. Note: missing data for participant 9 (eating times at baseline) and participant 3 (sleep times at intervention).
We found three major trends in self-selected eating patterns with TRE which may help to explain the observed benefits in metabolic syndrome. These trends include: (1) maintaining a shortened daily eating window (~10 h) and nightly fasting (~14 h) was feasible for an extended period of time, (2) TRE was achieved through a combination of moderate delays and advances in meal timing rather than skipping meals, (3) regularity in timing of caloric intake was increased.
During the 12-week intervention, the eating window was significantly reduced by an average of 28.75% (4.35±1.32 h) to 10.78±1.18 h (p=8.847E-11; Figure 2, Table 1). The 95% eating window accounts for day to day changes in the eating pattern and is typically longer than the average daily eating interval (time interval between the first and the last calorie of a 24 h day), which, for the majority of the days was <11 h (Supplemental Figure 1). During the intervention, participants ate outside of their eating window by more than 1 h either before or after their self-designated eating window on 7.12±7.55% of days. Such occasional deviation from a designated eating window has been shown to have very little adverse impact on the metabolic benefits of TRF in mice (Chaix et al., 2014).
Table 1.
Changes in health metrics from baseline to end of 12-weeks of time-restricted eating.
| Baseline (mean (SD)) | TRE (mean (SD)) | Change TRE-Baseline (mean (SD)) | Percent change | p-value | |
|---|---|---|---|---|---|
| Weight, BMI, Body Fat, and Blood Pressure | |||||
| Daily Eating Interval* | 15.13 (1.13) | 10.78 (1.18) | −4.35 (1.32) | −29% | 8.847E-11 |
| Weight (kg) | 97.84 (19.73) | 94.54 (18.38) | −3.30 (3.20) | −3% | 0.00028 |
| BMI (kg/m2) | 33.06 (4.76) | 31.97 (4.44) | −1.09 (0.97) | −3% | 0.00011 |
| Percent Body Fat (%) | 36.62 (4.18) | 35.61 (4.02) | −1.01 (0.91) | −3% | 0.00013 |
| Waist Circumference (cm) | 109.14 (11.21) | 104.68 (14.79) | −4.46 (6.72) | −4% | 0.0097 |
| Visceral Fat Rating | 16.68 (5.97) | 16.11 (5.89) | −0.58 (0.77) | −3% | 0.004 |
| Systolic BP (mmHg)** | 127.88 (8.89) | 122.76 (13.35) | −5.12 (9.51) | −4% | 0.041 |
| Diastolic BP (mmHg)** | 78.47 (8.74) | 72.00 (10.75) | −6.47 (7.94) | −8% | 0.004 |
| Lipids and Inflammation | |||||
| Total Cholesterol (mg/dL) | 181.42 (35.80) | 168.26 (39.65) | −13.16 (24.29) | −7% | 0.030 |
| LDL-C (mg/dL)* | 104.33 (32.30) | 92.39 (37.82) | −11.94 (19.01) | −11% | 0.016 |
| non-HDL-C (mg/dL) | 134.42 (33.93) | 122.79 (37.85) | −11.63 (22.94) | −9% | 0.040 |
| LDL-p (nmol/L)** | 1441.41 (457.17) | 1334.65 (440.58) | −107.76 (249.62) | −7% | 0.094 |
| HDL-C (mg/dL) | 47.00 (12.49) | 45.47 (12.42) | −1.53 (3.19) | −3% | 0.051 |
| Triglycerides (mg/dL) | 161.16 (87.30) | 158.53 (94.24) | −2.63 (57.68) | −2% | 0.845 |
| hs-CRP (mg/L)** | 1.59 (1.31) | 1.77 (1.44) | 0.18 (1.19) | 11% | 0.537 |
| ALT (units/L) | 28.68 (10.74) | 25.79 (11.48) | −2.89 (7.15) | −10% | 0.095 |
| AST (units/L) | 26.79 (6.79) | 24.37 (7.37) | −2.42 (5.98) | −9% | 0.094 |
| Cardiometabolic Factors | |||||
| Blood Glucose (CGM) (mg/dL)* | 106.72 (14.77) | 101.77 (12.36) | −4.94 (16.32) | −5% | 0.194 |
| Fasting Blood Glucose (mg/dL) | 106.74 (18.01) | 101.00 (11.60) | −5.74 (13.54) | −5% | 0.081 |
| HbA1c (%) | 5.71 (0.45) | 5.57 (0.40) | −0.14 (0.29) | −2% | 0.058 |
| Fasting Insulin (uIU/mL) | 17.68 (13.36) | 14.05 (8.03) | −3.63 (8.01) | −21% | 0.064 |
| HOMA-IR | 5.10 (5.70) | 3.56 (2.28) | −1.54 (3.95) | −30% | 0.107 |
| Sleep and Activity | |||||
| Daily Activity Counts* | 225891.28 (69765.83) | 212610.25 (71847.35) | −13281.03 (29037.07) | −6% | 0.069 |
| mCC app: % days reported feeling rested from sleep*** | 69.88 (25.61) | 88.16 (21.89) | 16.28 (24.88) | 23% | 0.019 |
| PSQI Total Score | 6.16 (3.92) | 5.47 (3.67) | −0.68 (2.06) | −11% | 0.164 |
| Sleep Duration in Minutes* | 437.48 (66.67) | 449.93 (60.87) | 12.45 (49.58) | 3% | 0.302 |
| Percent Sleep* | 92.29 (2.15) | 92.12 (2.29) | −0.17 (1.99) | 0% | 0.728 |
| Thyroid Function and Blood Cells | |||||
| Thyroid Stimulating Hormone (TSH) (mIU/L) | 1.94 (0.753) | 2.18 (0.872) | 0.243 (0.599) | 13% | 0.094 |
| White Blood Cell Count (1000/mm3) | 5.83 (1.30) | 5.73 (1.24) | −0.095 (1.11) | −2% | 0.714 |
| Hemoglobin (g/dL) | 14.65 (1.78) | 14.58 (1.76) | −0.074 (0.737) | 0% | 0.668 |
| Hematocrit (%) | 43.51 (5.02) | 43.53 (4.96) | 0.021 (1.89) | 0% | 0.962 |
| Platelet Count (1000/mm3) | 242.26 (61.50) | 244.00 (62.13) | 1.74 (25.93) | 1% | 0.774 |
n=19 unless noted:
n=18
n=17
n=16
mCC, myCircadianClock smartphone app, BMI, body mass index, BP, blood pressure, HDL-C, high-density lipoprotein cholesterol, LDL-C, low-density lipoprotein cholesterol, LDL-p, low-density lipoprotein particle number, hs-CRP, high-sensitivity C-reactive protein, ALT, alanine aminotransferase, AST, aspartate aminotransferase, CGM, continuous glucose monitor, HbA1c, hemoglobin A1c, HOMA-IR, Homeostatic Model Assessment of Insulin Resistance, PSQI, Pittsburgh Sleep Quality Index. HOMA-IR is obtained using the following equation: (fasting glucose*fasting insulin)/405.
Longitudinal measurement of food intake and sleep enabled us to assess how participants self-adopted TRE. Participants chose to begin their 10 h eating window between 8 am-10 am and end the eating window between 6 pm-8 pm. they achieved this by delaying the time of the first calorie and advancing their last calorie. Based on their 95% eating window, participants delayed their first calorie by 2.09±1.20 h and advanced their last calorie by 2.08±1.32 h relative to baseline. For most participants three clusters of food intake roughly representing breakfast, lunch, and dinner were apparent during the intervention period (Figure 2). Furthermore, to determine the type of first meal, we assigned a breakfast score of 0, 0.5, or 1 (0=lunch item, 0.5=lunch or breakfast item, and 1=breakfast item) to the first two items logged before 11 am at baseline and the last two week of intervention. There was no significant difference (p=0.84) of the breakfast score between baseline (0.8102) and the last two weeks of intervention (0.8282; STAR Methods). There are not clear objective standards for what defines breakfast; whether it is the meal that breaks an overnight fast, or a meal that is consumed immediately after waking up. However, based on the observation that the participants continued to consume the same food items in their morning meals and there were three clear clusters of meals during the intervention period, we conclude that the participants did not skip breakfast but rather delayed it.
Based on days that participants wore the actiwatch (passive sleep measure) and met good logging criteria on the mCC app, participants significantly increased the time between wake and first calorie (+1.81±0.96 h, p=7.72E-7) and between last calorie and sleep onset (+1.53±1.18 h, p=6.48E-5) during the end of TRE compared to baseline. During TRE intervention the time between wake and first calorie was 3.64±1.22 h, and the time between the last calorie and sleep onset was 4.02±1.19 h (Figure 2).
A key aspect of eating patterns is the variability in first and last calorie. Studies have shown that irregular meal timing negatively impacts cardiometabolic health (Pot et al., 2016; Sierra-Johnson et al., 2008; Wennberg et al., 2016).
The regularity of the first and last calorie also increased during the TRE intervention. The variance of the time of first caloric intake was 2.77 h during baseline and significantly decreased by 40.96% to 1.63 h on TRE (p=0.001 Levene’s Test). The variance of the time of the last calorie was also significantly decreased by 44.23% from 4.12 h during baseline to 2.30 h during TRE (p=0.0001; Figure 2). Although the variance of time of first and last calorie was decreased, the variance in the time between wake and first calorie increased from 1.97 h during baseline to 2.79 h during TRE (p=0.006). This may be due to a more stable eating time that does not adjust to changes in wake time. There were no changes in the variance in time between last calorie and sleep onset between baseline (3.76 h) and TRE (3.71, p=0.981; Figure 2). These data were assessed on all days of good app logging (a minimum of 2 caloric entries at least 5 hours apart) throughout baseline and the last two weeks of the TRE intervention.
Impact of 10 h TRE on caloric intake, sleep, and physical activity
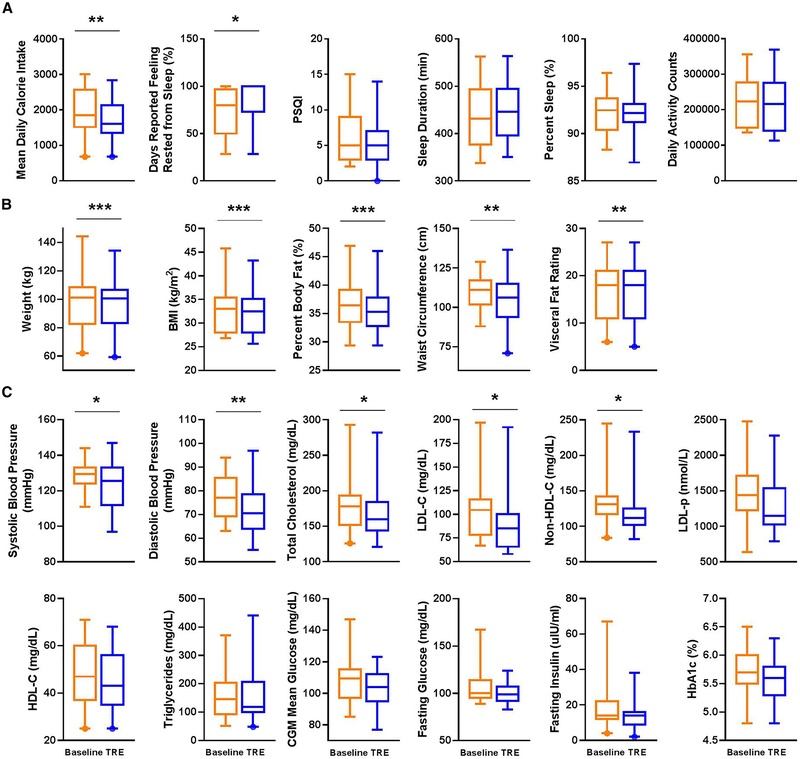
Caloric intake was estimated based on the photo and/or annotation entries on the mCC app. Despite no recommendations to change dietary quantity or quality, there was an 8.62±14.47% decrease in mean daily caloric intake during intervention (1792.00±578.08 calories) compared to baseline (1990.59±644.89 calories; p=0.007; Figure 3A, Table 1).
Figure 3. TRE intervention improves restfulness and body composition and significantly reduces weight, blood pressure, and atherogenic lipids.
(A) Mean daily caloric intake, sleep parameters, and activity counts at baseline (orange) and end of 12-weeks of time-restricted eating (TRE) intervention (blue). (B) Weight and body composition end-points, (C) blood pressure, lipid, and glucose end-points. Box plot with whiskers displaying 5-95% interval and outliers. *p<0.05, **p<0.01, ***p<0.001. PSQI, Pittsburgh Sleep Quality Index. BMI, body mass index, HDL-C, high-density lipoprotein cholesterol, LDL-C, low-density lipoprotein cholesterol, LDL-p, low-density lipoprotein particle number, CGM, continuous glucose monitor, HbA1c, hemoglobin A1c.
None of the participants were shift-workers and all had a relatively consistent sleep pattern. At baseline, participants slept for 437.48±66.67 min, which is in line with the recommended sleep duration (Hirshkowitz et al., 2015). TRE led to a modest, but not significant, increase in sleep duration to 449.93±60.87 min for the entire cohort (+12.45±49.58 minutes of sleep, p=0.302). We also did not find significant changes in an objective measure of sleep efficiency (percent time asleep during sleep interval −0.17±1.99%, p=0.728). However, this is likely due to large differences between participants at baseline. Overall, 16 of 19 participants demonstrated increased duration of sleep and/or increased sleep efficiency at the end of the intervention (percent sleep, Figure 3A, Table 1).
Interestingly, the variance in wake time decreased by 35.53% from 3.42 h at baseline to 2.21 h during intervention (p=0.035). Likewise, there was a trend toward a decrease in the variance of sleep onset from 3.05 h during baseline to 2.05 h during intervention (p=0.142; Figure 2).
To assess the subjective report of sleep satisfaction we used PSQI, which asks participants to rate many factors of sleep over the past month. Additionally, through the mCC app, we asked participants to rate their sleep immediately after waking up. Such repeated self-assessment of sleep quality immediately after waking up on multiple days is likely to report an accurate assessment of subjective sleep quality. Remarkably, TRE led to a significant improvement in morning restfulness based on daily morning momentary assessment of sleep quality in the mCC app. Specifically, the participants reported restful sleep only 69.88±25.61% of days at baseline, which increased significantly by as much as 23% to 88.16±21.89% (p=0.019; Figure 3A, Table 1). There was a small but non-significant, trend toward improved sleep (decreased score) as reported on the PSQI (−0.68±2.06, p=0.164). Overall, modest changes in the indirect measure of sleep from actigraphy coupled with significant changes in subjective rating of sleep immediately after waking up indicates TRE impacts sleep. However, more direct analysis of the effects of TRE on sleep stages and arousal threshold should be performed in future studies.
There were no significant changes in activity (measured by actigraphy) at the end of TRE compared to baseline, but there was a trend toward a reduction in physical activity (mean change in daily activity counts by Philips Actiwatch −13281.03±29037.07, p=0.069; Figure 3A, Table 1).
TRE reduced body weight and improved body composition
With 10 h TRE for 12-weeks, we observed significant reductions in body weight from baseline (−3.30±3.20 kg (−3%), p=0.00028). The average rate of body weight change was ~275g/week, which is considered within the safe range for weight loss (Weinsier et al., 1995). The body weight change also translated to a significant reduction in body mass index (−1.09±0.97 kg/m2 (−3%), p=0.00011). The decrease in body weight accompanied desirable reductions in percent body fat (−1.01±0.91%(−3%), p=0.00013). Some of the body fat loss was loss in abdominal fat because we observed a significant reduction in visceral fat rating (−0.58±0.77 (−3%), p=0.004) and waist circumference (−4.46±6.72 cm (−4%), p=0.0097; Figure 3B, Table 1). Mixed linear model analyses showed that these changes (except for waist circumference) could not be explained by the change in weight (Supplemental Table 3). Waist circumference showed a correlation with change in weight (p=0.017), change in eating interval (p=0.005), and combined change in weight and eating interval (p=0.004) (Supplemental Table 3).
The degree of weight loss observed in our study with TRE (mean −3% from baseline, p<0.00028) is comparable to studies examining the effects of calorie restriction combined with exercise in patients with glucose intolerance, which have reported weight loss of −1% (Bhopal et al., 2014), −3% (Ackermann et al., 2015), −5% (Katula et al., 2013), and −6% (Knowler et al., 2002). These studies reported either no significant reduction in blood pressure (Ackermann et al., 2015; Bhopal et al., 2014), no significant reduction in total cholesterol (Ackermann et al., 2015), or did not report blood pressure or lipid end-points (Katula et al., 2013; Knowler et al., 2002).
TRE reduced atherogenic lipid levels and blood pressure
Major risk factors for cardiovascular disease (CVD) include elevated blood pressure and atherogenic lipids, including low-density lipoprotein cholesterol (LDL-C), and non-high-density lipoprotein cholesterol (non-HDL-C), which reflects the contribution of triglyceride-rich lipoproteins (such as very low-density lipoprotein cholesterol (VLDL-C)) to CVD risk. We also observed significant reductions in total cholesterol (−13.16±24.29 mg/dL (−7%), p=0.03), low-density lipoprotein cholesterol (LDL-C) (−11.94±19.01 mg/dL (−11%), p=0.016), and non-high-density lipoprotein cholesterol (non-HDL-C) (−11.63±22.94 mg/dL (−9%), p=0.04; Figure 3C, Table 1). A mixed linear model analysis showed that these changes were not explained by changes in weight (Supplemental Table 3). An elevation in low-density lipoprotein particle number (LDL-p) is associated with CVD risk, and LDL-p is often discordantly elevated (relative to LDL-C) in insulin resistance states, such as metabolic syndrome. There was a trend toward a reduction in LDL-p (−107.76±249.62 nmol/L (−7%), p=0.094). Change in high-density lipoprotein cholesterol (HDL-C) was not significant but had a trend toward reduction (−1.5±3.19 mg/dL (−3%), p=0.051; Figure 3C, Table 1, Supplemental Figure 2).
Loss of 5% of body weight should produce a 3-5% reduction in LDL-C (Jacobson et al., 2015); in our study, a 3% weight loss occurred with an 11% reduction in LDL-C. The baseline mean LDL-C of 104 mg/dL in our study reflects the high use of statin therapy (as baseline mean LDL-C in our 4 participants who were not on statin therapy was 146.5 mg/dL), and with TRE, mean LDL-C was reduced to 92 mg/dL (p=0.016). Treating LDL-C to a target of <100 mg/dL by adding medications to statin therapy is recommended in treating high-risk patients for primary prevention of CVD (Lloyd-Jones et al., 2010). Our ability to do this with TRE as “add-on” therapy to statins is intriguing and given its potential clinical implications warrants further investigation.
The extent of weight loss under TRE did not correlate with changes in plasma triglyceride levels (TGs). There were no significant changes in triglycerides (Figure 3C, Table 1, Supplemental Figure 2) or hs-CRP (a marker of inflammation; Table 1). Typically, weight loss is expected to promote lower TGs. Loss of 5-10% of body weight should lead to a 20% reduction in TGs (Miller et al., 2011). It is unclear why we did not observe a change in TGs with TRE despite the significant weight loss achieved. One possibility is the effect of TRE on TGs was blunted by the significant portion of participants taking statins (79%), although this did not limit our ability to detect significant reductions in TC, LDL-C, and non-HDL-C. In the setting of concomitant statin use, perhaps a shorter TRE window (e.g. 8 h) or a longer period of 10 h TRE beyond 12 weeks would lead to a decrease in TG.
We observed significant reductions in systolic and diastolic blood pressure (−5.12±9.51 mmHg (−4%), p=0.041 and −6.47±7.94 mmHg (−8%), p=0.004, respectively; Figure 3C, Table 1, Supplemental Figure 2). The reduction in blood pressure observed with TRE in our study is similar to that expected by weight loss through other means. Any degree of weight loss (from 0 to < 10%), is generally expected to result in an average BP reduction at 6 to < 12 months by 2.675 mmHg (systolic) and 1.337 mmHg (diastolic) (Zomer et al., 2016). However, a mixed linear model analysis showed no significant relationship between weight and change in blood pressure (Supplemental Table 3). The absolute mean reduction in BP observed in our study was even more than these general estimates, however, large standard deviation reflects our small sample size and we did not have a control arm. Importantly, a significant reduction in blood pressure was observed in our study in the context of 63% of patients taking anti-hypertensive therapy. Thus, similar to our observations on the effects of TRE as “add-on” therapy to statins for atherogenic lipid-lowering, TRE may also be an “add-on” therapy to anti-hypertensives in those with metabolic syndrome. As an important risk factor for cardiovascular disease, a reduction in blood pressure is a very important finding in metabolic syndrome, and the long-term impact of TRE on blood pressure lowering deserves further examination.
Metabolic syndrome is also associated with non-alcoholic fatty liver disease (NAFLD), and plasma levels of the liver transaminases (alanine aminotransferase (ALT) and aspartate aminotransferase (AST)) may be elevated in fatty liver, reflecting underlying liver pathology. Only one participant had elevated levels (≥50 units/L) of ALT at baseline and their ALT levels did not decrease with the 12-week intervention. However, within all participants, we observed a trend toward a reduction in ALT (−2.89±7.15 units/L (−10%), p=0.095) and AST (−2.42±5.98 units/L (−9%), p=0.094).
Impact of TRE on glucose regulation
We observed trends toward improvement in fasting glucose (−5.7±13.54 mg/dL (−5%), p=0.081), fasting insulin (−3.6±8.01 uIU/mL (−21%), p=0.064), and HbA1c (−0.14±0.29% (−2%), p=0.058; Figure 3C, Table 1, Supplemental Figure 2). No significant changes were seen in CGM mean glucose (−4.94±16.32 mg/dL (−5%), p=0.194) or HOMA-IR (−1.54±3.95 (−30%), p=0.107; Figure 3C, Table 1, Supplemental Figure 2).
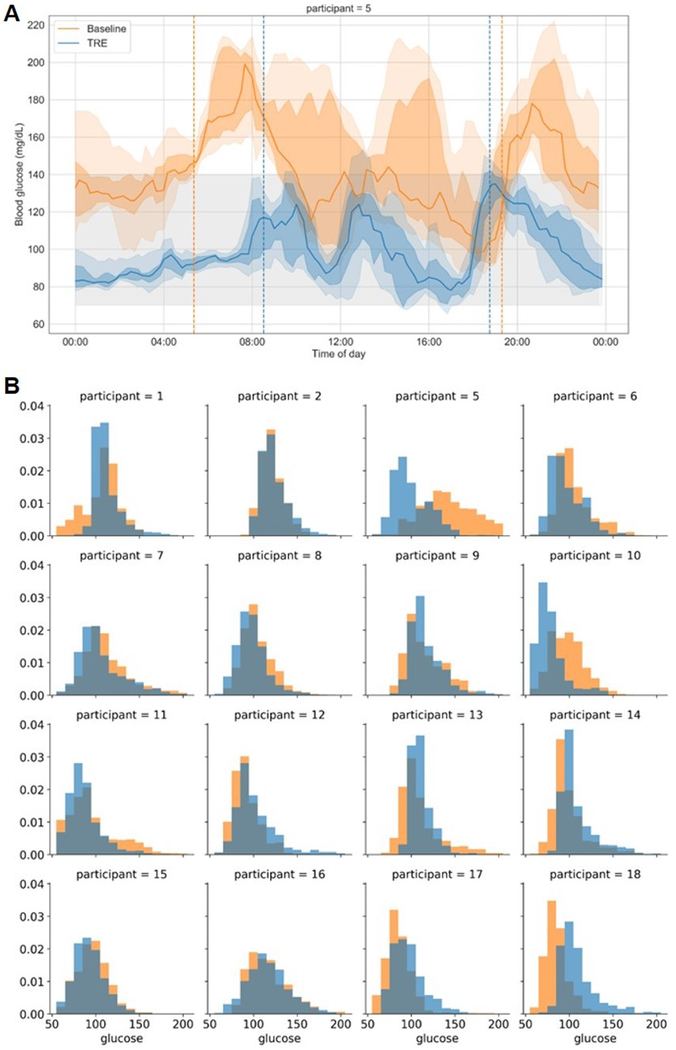
Of the 19 participants, 12 had elevated fasting glucose (≥100 mg/dL) and/or HbA1c of ≥5.7% at baseline (Supplemental Table 1). Within these participants, there was a significant reduction in HbA1c (−0.22±0.32% (3.7%), p=0.04). There were also trends toward improvement in CGM mean glucose (n=10, −10.08±16.06 mg/dL (9.4%), p=0.078) and fasting glucose from a blood draw (n=12, −8.67±16.26 mg/dL (−7.6%), p = 0.092). The reduction in blood glucose was related to the magnitude of hyperglycemia. Although most of our participants with fasting blood glucose ≥100 mg/dL at baseline had pre-diabetes, one of the participants was diagnosed with T2DM and had an HbA1c of 6.5% and fasting glucose of 167 mg/dL. At the end of intervention, this participant’s HbA1c was 5.5 (−1.0 % change) and fasting glucose was 116 mg/dL (−51 mg/dL). From the CGM data, this participant showed large improvements in mean blood glucose (−47.76 mg/dL), time in range (70-140 mg/dL; 45.76%), coefficient of variation (−0.04), and standard deviation (−9.61; Figure 4A). The distribution of time in glucose range for all participants is shown in Figure 4B.
Figure 4. Changes in glucose levels between baseline and end of intervention.
(A) Summary of 8 days of CGM data baseline (orange) and at the end of 12-weeks of TRE intervention (blue) for a participant with type 2 diabetes mellitus. Solid lines represent the median glucose, the shaded area is 25-75% interval, and the lightly shaded area is 10-90% interval. Vertical dotted lines represent the 95% eating interval at baseline and intervention. The eating window goal during intervention was 8 am to 6 pm. This participant had baseline fasting glucose of 167 mg/dL and post-TRE fasting glucose of 116 mg/dL. Baseline fasting and continuous mean glucose data were higher than other participants, and thus this is not representative of all participants, but rather shows the large change observed in an individual with type 2 diabetes mellitus. (B) Distribution of glucose assessed by CGM for 16 of the 19 participants. Three participants are not included because they did not have at least 4 days of CGM data at either time point. Summary of 8 days of CGM data at baseline (orange) and end of TRE intervention (blue) for each participant.
Our findings are consistent with previous TRE studies that also found partial changes in glucose regulation. It is not surprising as almost all studies were done in healthy or overweight participants, but not participants with elevated fasting glucose. Nine-hour TRE in adults who were overweight reported a decrease in glucose incremental Area Under the Curve in response to a mixed nutrient meal, and a decrease in mean fasting glucose based on CGM data, but no change in fasting glucose (Hutchison et al., 2019). Additionally, a 6 h TRE intervention in adults who were overweight and prediabetic found an increase in insulin sensitivity and beta-cell function, but also did not see a change in fasting glucose (Sutton et al., 2018). A recent study testing a different form of intermittent fasting (fasting-mimicking diet (FMD)) reported similar findings: fasting glucose did not change when baseline fasting glucose was ≤99 mg/dL, but a significant reduction in fasting glucose occurred among participants with baseline fasting glucose levels >99 mg/dL (Wei et al., 2017). Such observations may suggest that higher-risk patients with more severe metabolic diseases may benefit even more compared to lower-risk individuals from intermittent fasting, including TRE. However, further studies will need to be done in individuals with elevated fasting glucose, both with pre-diabetes or T2DM, to better understand the influence of TRE on glucose regulation.
Impact of TRE on thyroid function and blood cell counts
Thyroid stimulating hormone (TSH) was measured at baseline and at the end of TRE intervention. As specified in our exclusion criteria, patients with known thyroid disease undergoing a recent adjustment of thyroid replacement medications were excluded (STAR Methods). One participant voluntarily withdrew from the study based on lab findings of abnormal thyroid function during baseline (Figure 1B). The primary aim of measuring TSH in the current study was to screen for any thyroid dysfunction (at baseline or end of intervention) which might influence other metabolic end-points; thus, other thyroid hormones (such as free thyroxine (T4) and total triiodothyronine (T3)) were not measured. We observed a trend toward an increase in TSH with 10 h TRE (0.243±0.599 mIU/L (13%), p = 0.094). However, the post-TRE mean TSH of 2.18 mIU/L is still within normal limits (the upper limit of normal TSH is 4.0 mIU/L), and therefore the significance of this finding is unclear. Another recent study of TRE in resistance-trained men reported a significant change in total triiodothyronine with TRE compared to the control cohort, but no significant change in TSH (Moro et al., 2016). Thus, future studies of TRE in humans should measure thyroid function to explore this finding further. There was no significant change with 10 h TRE in any of the four main components of the complete blood count: white blood cell count (−0.095±1.11 1000/mm3 (−2%), p = 0.714), hemoglobin (−0.074±0.737 g/dL (0%), p = 0.668), hematocrit (+0.021±1.89% (0%), p = 0.962), and platelet count (+1.74±25.93 1000/mm3 (1%), p = 0.774; Table 1); these findings are similar to those from a recent study in humans which also did not find significant changes in blood cell counts with TRE (Gabel et al., 2018b).
Long-term follow-up
Participants were not advised to continue TRE beyond the 3-month intervention. However, to assess the rate of elective continuation of TRE, we contacted all 19 participants, ~470±124 days (~16±4 months; mean±SD) after their completion of the 3-month TRE intervention. Of the 19 participants surveyed, 5 (26.3%) were still adherent to TRE, 7 (36.8%) reported doing TRE part of the time (and in some cases using an eating window other than 10 h (range: 8-12 h)), and 7 (36.8%) were no longer doing TRE. Of the 7 participants who reported no longer doing TRE, 5 participants (71.4%) said they had continued TRE after the study ended (for an average of ~4.2 months) before stopping. To our knowledge, only one prior study has followed participants on TRE long-term, in which healthy participants continued TRE for 1 year and investigators found that all participants (n=8) were able to maintain 10-12 h TRE for this duration, and maintained benefits of weight loss, improved sleep, and subjective sense of more energy (Gill and Panda, 2015). In this study of patients with metabolic syndrome, 63.2% were engaged in some amount of TRE at ~16±4 months from the completion of the study intervention, suggesting that lasting effects of the initial intervention on behavior are feasible.
Conclusion
To our knowledge, this is a unique study of TRE in humans who have metabolic syndrome. The high level of adherence to TRE in our study, no reported adverse effects, and low dropout rate suggests that a self-selected 10 h window for TRE may be feasible for patients with metabolic syndrome to adhere to over a longer period of time. The use of a smartphone application to capture real-time data about meal timing and sleep restfulness is a unique tool to monitor and intervene the circadian aspects of lifestyle and its day-to-day variance. The use of this smartphone app will allow us to design larger studies of TRE in which participants are monitored remotely over even longer periods of time.
This study has demonstrated that a 10 h TRE intervention over 12 weeks, without an overt attempt to change physical activity or diet quality or quantity, can serve as a novel treatment for individuals with metabolic syndrome. Importantly, we observed significant decreases in systolic and diastolic blood pressure, total cholesterol, LDL-C, and non-HDL-C. These findings occurred without increased physical activity, and there was no significant correlation with changes in weight (Supplemental Table 3). However, a decrease in waist circumference was correlated with change in weight (p=0.017), a larger correlation with decreased eating interval (p=0.005), and a combined change in weight and eating interval (p=0.004) (Supplemental Table 3). Most participants were already taking stable doses of cardiovascular medications (79% and 63% of patients were taking a statin and antihypertensive therapy, respectively, Supplemental Table 2), suggesting that the observed benefits of TRE were additive to the effects of these medications. Among participants with elevated fasting blood glucose, we also observed a significant reduction in HbA1c. In this population at high risk for CVD, a significant reduction in atherogenic lipids, blood pressure, and blood glucose on top of medical therapy has important clinical implications.
Limitations of the study
This was an unblinded, single-arm pilot study with relatively small sample size. Additionally, due to the nature of metabolic syndrome and heterogeneous use of pharmacotherapy within our study population, the baseline values for participants were varied.
The myCircadianClock smartphone app is designed to capture the timing of dietary intake from food consumed in free-living conditions but does not ask for exact food portions or calorie estimates from the user. While calorie content may be retroactively estimated from photo and text entries by participants as was performed in this study, such data are less precise than mechanistic studies where participants are supplied with specific foods and the leftover food is accurately weighed after every ingestion event. Studies in mice have shown that improvement in diet quality will enhance the health benefits of TRF. More research is needed to evaluate the combined effect of TRE+nutrition quality in humans. However, our finding of an 8.62±14.47% decrease in caloric intake at the end of the 12-week TRE intervention agrees with previous findings of an estimated mean calorie reduction of 20.26% (95% CI 4.92%-35.6%) after 16-weeks of TRE without dietary counseling (n=8) (Gill and Panda, 2015). It is important to note that this change in caloric intake was achieved without a conscious effort in a group of individuals who have already unsuccessfully undergone treatment for metabolic syndrome, including recommended weight loss through calorie reduction and increased activity.
Another limitation of our study is the potential effect of mCC app usage on behavior, including dietary choices. However, it has been shown previously that simply using the mCC app for 3 weeks does not result in weight loss, suggesting that use of the app may not significantly influence behavior (Gill and Panda, 2015). The CGM and actigraphy devices may also have served as “reminders” to the participants that they were logging for a health study, and therefore might have altered behavior. However, the absence of improvement in activity level supports the notion that participating in the study did not overtly attempt to improve healthy behavior. Finally, as seen with all self-reported dietary intake, we cannot confirm that the data logged by participants represent a complete picture of their diet, as it is possible that participants had dietary intake that they did not log. It has been shown previously that the false-negative rate (i.e. food-consumed but not logged) using the mCC app is ~10% (Gill and Panda, 2015). Controlling for unlogged dietary events is very challenging, and the ideal control which is expensive and difficult in humans involves direct observation of all dietary events. This strategy may be feasible in the short-term but is unlikely to be a sustainable approach for a 3-month intervention such as was tested in our study of TRE.
The absence of a significant correlation between changes in weight and metabolic improvements raises the possibility that TRE might have improved the efficacy of pharmacotherapy. The participants were consistent in their medication usage at baseline and they did not change medication dosage during the intervention period. However, recent studies have suggested that the targets of the majority of drugs used to treat metabolic syndrome, including statins and anti-hypertensives, exhibit circadian rhythms (Cederroth et al., 2019). Sustaining a consistent daily rhythm in feeding and fasting may improve molecular rhythms in relevant pathways. Therefore, it is possible that TRE helps to improve circadian rhythms and thus improve the efficacy of pharmacotherapy. Further studies will need to be done to test this directly.
STAR Methods
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and data should be directed to and will be fulfilled by the Lead Contact, Dr. Satchidananda Panda (satchin@salk.edu). This study did not generate new unique reagents. For the specific request for data sharing requests, such requests will require a Material Transfer Agreement and/or a Data Use Agreement and will be managed by the Salk Institute or UC, San Diego. Both Institutes abide by the Uniform Biological Material Transfer Agreement (UBMTA).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
This study was conducted with approvals from the Institutional Review Board at UC San Diego (UCSD) and The Salk Institute for Biological Studies. Clinical trial registered as “The Impact of Time-Restricted Feeding (TRF) in Improving the Health of Patients with Metabolic Syndrome”. ClinicalTrials.gov .
All participants provided written informed consent. Participants were recruited from UCSD clinics, were diagnosed with metabolic syndrome (using AHA/National Heart, Lung, and Blood Institute cut points for waist circumference) (Alberti et al., 2009) and a self-reported eating window of ≥14 h per day. One participant with a calculated eating window <14 h at baseline was included as they admitted to starting TRE prior to the end of the baseline period and confirmed a prior eating interval of ~ 14.5 hours. One participant with an eating interval of 13.5 hours at baseline was included as well as they were very close to 14 hours. Thirty-five participants were enrolled. After screening to meet eligibility criteria, 25 began the TRE intervention, and 19 were included in the final analysis; 13 men and 6 women, 63% non-Hispanic white, age 59±11.14 (mean±SD) years. All participants met 3 or more metabolic syndrome criteria at the time of enrollment and majority of them were on medications (Supplemental Table 1, 2).
Inclusion Criteria:
-
Metabolic syndrome (i.e. presence of three or more of the following):
Waist circumference ≥ 102 cm (Men) or ≥ 88 cm (Women).
Triglycerides ≥ 150 mg/dL (or on drug treatment for elevated triglycerides).
Reduced HDL-C < 40 mg/dL (Men), < 50 mg/dL (Women) (or on drug treatment for reduced HDL-C).
Elevated blood pressure, systolic blood pressure ≥ 130 mmHg and/or diastolic blood pressure ≥ 85 mmHg (or treatment with an antihypertensive drug with a history of hypertension).
Elevated fasting glucose ≥ 100 mg/dL (or drug treatment of elevated blood glucose).
Age ≥ 18 years
If patients are on cardiovascular medications (HMG CoA reductase inhibitors (statins), other lipid-modifying drugs (including over the counter drugs such as red yeast rice and fish oil), anti-hypertensive, anti-diabetes drugs), no dose adjustments will be allowed during the study period.
Own and able to use a Smartphone with Apple iOS or Android OS.
Baseline eating period is ≥ 14 h per day (as estimated by 95% eating interval).
Exclusion Criteria
Pregnant or breast-feeding.
Caregiver for a dependent requiring frequent nocturnal care/sleep interruptions. Shift workers with variable (e.g. nocturnal) hours.
Prolonged international travel during the study period.
Active tobacco abuse or illicit drug use or history of treatment for alcohol abuse.
Known inflammatory and/or rheumatologic disease.
History of a major adverse cardiovascular event within the past 1 year (acute coronary syndrome (ACS), percutaneous coronary intervention, coronary artery bypass graft surgery, hospitalization for congestive heart failure, stroke/transient ischemic attack (TIA)).
Uncontrolled arrhythmia (i.e. rate-controlled atrial fibrillation/atrial flutter are not exclusion criteria).
History of thyroid disease requiring dose titration of thyroid replacement medication(s) within the past 3 months (i.e. hypothyroidism on a stable dose of thyroid replacement therapy is not an exclusion).
History of adrenal disease.
History of malignancy undergoing active treatment, except non-melanoma skin cancer.
Known history of type I diabetes,
History of an eating disorder.
History of cirrhosis
History of stage 4 or 5 chronic kidney disease or requiring dialysis
History of HIV/AIDs
Currently enrolled in a weight-loss or weight-management program,
On a special or prescribed diet for other reasons (e.g. Celiac disease),
Currently taking any medication that is meant for, or has a known effect on, appetite,
Any history of surgical intervention for weight management,
Uncontrolled psychiatric disorder (including a history of hospitalization for psychiatric illness).
(HDL-C, high-density lipoprotein cholesterol, HMG CoA, 3-hydroxy-3-methyl-glutaryl-coenzyme A, OS, operating system, HIV, human immunodeficiency virus, AIDS, acquired immunodeficiency syndrome.)
METHOD DETAILS
Visit Protocol
Study visits were conducted at the Altman Clinical and Translational Research Institute of UCSD. At visit 1, baseline anthropometric measurements, blood pressure, and fasting blood tests were obtained. Participants were fitted with a continuous glucose monitor (CGM) and a wrist-worn actigraphy device. They were trained to use the mCC app to log food, beverage, sleep, and exercise data.
At the end of the first week of the baseline surveillance period, participants returned for visit 2 to return their CGM and actigraphy device, with additional teaching on app use if needed. At the end of the 2-week baseline surveillance period, adherent participants (using the app at least 5 days/week, with a minimum of 3 entries/day) with a 14 h/day eating window received a phone call to begin the 12-week intervention period. Upon entering the 12-week intervention period, each participant self-selected a 10 h daily eating window and were instructed that all dietary intake other than water should occur within their 10 h window. During the intervention, data from the app was used to assess participant adherence, eating window, and sleep. TRE was the only intervention, and participants were not instructed to change their physical activity or the quality, quantity, or caloric content of their diet in any way. Eleven weeks into the intervention period (week 13 of the study), participants returned for visit 3 to have the CGM and actigraphy device placed again. During visit 4 (week 14 of the study), the CGM and wrist actigraphy device were removed and participants underwent the same testing performed at visit 1 (Figure 1A).
Blood measurements
The following fasting blood tests were performed by standard venipuncture: comprehensive metabolic panel, complete blood count, thyroid stimulating hormone, hemoglobin A1c (HbA1c), lipid panel, lipoprofile by nuclear magnetic resonance, coagulation parameters (INR/PT and PTT), insulin, and high-sensitivity C-reactive protein (hs-CRP).
Body composition
To analyze body composition, the following parameters were obtained with the Tanita Scale DC 430U (Tokyo, Japan): body mass index (BMI), body fat percentage, and visceral fat. Visceral fat is estimated using bioelectrical impedance analysis, with a Tanita Visceral Fat Score > 12 considered excessive (Tanita Corporation of America Inc., 2013).
Continuous Glucose Monitors (CGMs)
We used the Abbott Freestyle Libre Pro CGM which has been validated for 14 days of continuous use with factory calibration (Bailey et al., 2015). The devices were applied at the first and third study visits and worn for an average of 6.95 (±1.52) days at baseline and 8.27 (±1.99) days at the end of the intervention. The users were blinded to their CGM data during the entire study.
Sleep Analysis
Sleep was analyzed through 3 methods: Pittsburgh Sleep Quality Index (PSQI) questionnaire (1st and last visit), self-reported daily sleep satisfaction on the mCC app, and actigraphy.
Actigraphy
We used the Philips Respironics Actiwatch Plus (van Wouwe et al., 2011) to assess sleep and activity for 7-10 days while participants were simultaneously wearing the CGM. Philips Actiware 6 software was used to analyze all data. Default measures were used for assessing activity and the duration, quality, and timing of sleep. Sleep efficiency is determined as =total time asleep-time when active during sleep (wake after sleep onset (WASO)). Total activity per day recorded on the Actiwatch was used to assess changes in activity between baseline and end of the intervention.
Eating pattern monitoring
The HIPAA compliant mCC app which has been validated for monitoring habitual dietary behavior (Gill and Panda, 2015) was used to assess eating patterns through the 14-week study. Participants used the camera feature of the app to take a picture of the food and/or beverage that they were about to consume or use textual data entries. Even when participants submitted photographic data, they also provided text data describing and/or quantifying their intake. Entries and metadata (e.g. timestamp) were immediately transmitted to a HIPAA compliant server where it was stored for analysis. During the 2-week baseline surveillance period, participants did not receive feedback from the study team or app. Each entry was associated with a time-stamp, which was used to calculate various parameters related to the eating pattern. During the intervention period, 2-3 times a week the mCC app sent push notifications to provide tips and information about TRE and metabolic health. Twelve of the 19 participants were contacted, on average 2 times throughout the 14 weeks study, to remind them to log and/or address potential issues with app use.
QUANTIFICATION AND STATISTICAL ANALYSIS
Adherence to TRE
Participant logging in the mCC app was monitored weekly for adherence. If participants did not log or showed inconsistent logging for 2 or more days, participants were contacted over the telephone. Logging adherence was determined by a minimum of two caloric entries over a minimum of 5 h for a given day. Adherence to a designated eating window was determined by the number of days in which a participant was adherent to logging and all caloric food items were contained within a 15-minute buffer on each side of the self-selected 10 h eating window.
Caloric Intake Analysis
To conduct caloric intake assessment, 3 days were randomly chosen (using a random number generator) from baseline, and 3 from the last 12 days of intervention to use as representative samples of dietary intake for each participant. Each item and portion size was identified by the annotation and photo (when available) provided by the participant. Caloric estimates were made using the Calorie King database. If the information on a specific food was not available, calorie estimates were taken directly from the website of the brand name food or restaurant that was logged.
Eating window calculation.
Eating window was calculated as the 95% interval of all caloric entries on the mCC app during a designated interval (Baseline = 13.84±2.48 days; Intervention = 60±16.72 good logging days), and as previously described (Gill and Panda, 2015). To compare the variance of first calorie and last calorie, independently and relative to sleep (Figure 2), two weeks of baseline and the last two weeks of intervention were used. Medications and water were not included in calculating the eating interval. We also calculated the time interval between the first and last calorie of the day for adherent days to calculate the daily eating window.
Breakfast analyses
To test whether participants skipped breakfast, we analyzed the morning food choices at baseline and intervention. Among the first two food entries recorded before 11 am on each good logging day for all participants, frequency counts were collected for each food category (e.g. banana, strawberries, papaya were grouped into “fruit” food category; omelet, hard-boiled egg, raw egg were grouped into “egg” category). A breakfast score of 0, 0.5, or 1 was assigned to each food category, with a higher breakfast score representing a higher likelihood of being consumed during breakfast. For example, bagel, egg, oatmeal, cereal, muffin, or donut were scored 1.0, sandwich or burritos were scored 0.5 as they can be consumed either as breakfast or lunch in San Diego area, and pasta, salmon, burger, or rice was scored 0 as they are most likely consumed during lunch.
A weighted group breakfast score ranging between 0 to 1 was calculated for baseline and the last two weeks of intervention period using the formula (Σ frequency count * score)/Σ frequency count). The first two items logged before 11 am were analyzed with matched number of days analyzed in each condition. In baseline, the breakfast measuring score was 0.8102, and the score of the intervention period was slightly higher at 0.8282.
A limitation of this approach is that what constitutes breakfast food is defined by the research team based on the common food habits in the local area and on the assumption that people eat different food types for breakfast and lunch. However, as the analysis was limited to the first two items logged before 11 am, we can assume that this was their morning meal and not their afternoon meal.
The distribution of average daily breakfast food entries was normal for both baseline and intervention, with no significant outliers present in the data. In order to test for the existence of statistically significant behavioral changes in consuming breakfast, a paired two-sample t-test was conducted to assess the difference in the average daily breakfast food consumption. With a p-value of 0.84, the t-statistic was not significant at the 0.05 critical alpha level.
Statistical analysis
SPSS software was used to test for normal distribution for each measure and paired samples t-tests were used to compare baseline to post-intervention values. A two-tailed p < 0.05 was considered statistically significant. For our final analysis, we sought to have data from at least 15 participants since by using the paired samples t-test, a sample size of N = 15 would provide 80% power at the 0.05 significance level to detect an effect size of 0.8. The variance was assessed using Levene’s Test for equality of variances.
Excluded Particiant or data.
The following participant or data were excluded. The participant IDs and the reasons for exclusion are shown below.
| 20 | Reported significant changes in diet and poor logging |
| 21 | Poor logging |
| 22 | Lost to follow-up |
| 18 | Removed from blood pressure analysis. Participant changed anti-hypertensive medication near the end of the intervention |
| 17 | Removed from hs-CRP analysis. Had significantly elevated hs-CRP due to underlying inflammatory disease |
| 9 | Removed from blood pressure analysis. Did not take anti-hypertensive medication the day of coming in for the final visit |
Missing Data:
Participant ID, missing data and the reason for missing data are indicated below.
| 14 | Baseline LDL-p, hs-CRP. Post-intervention LDL-p. Lab error |
| 3 | Post-intervention CGM and actiwatch. Participant refused to place actiwatch and CGM. |
| 7 | Post-intervention LDL-p. Lab error |
| 4 | Post-intervention LDL-C. Unable to calculate LDL-C given triglycerides in the 400s |
| 19 | Post-intervention CGM. Data saving error |
| 3 ,5, 6 | Baseline and post-intervention sleep reported on mCC app. Participants did not log sleep on the mCC app |
Relationship between change in body weight and cardiometabolic risk factors:
A mixed linear model was used to assess the relationship between weight and eating interval with health outcomes. Neither eating interval nor weight could account for all changes in cardiometabolic health. Changes in weight and eating interval did have a significant relationship with a change in waist circumference.
Each outcome variable of interest was fitted with a mixed linear model of the form:
with a random slope and intercept fitted per study participant and a repeated measure of experimental condition (Condition ∈ [Baseline,Intervention]). Weight is the participant’s weight at the end of that experimental condition in pounds, and EatingInterval is the participant’s 95% eating interval in h calculated over the 10 h TRE intervention period. The model for each outcome variable was fit using the maximum likelihood algorithm in statsmodels v. 0.10.1. The results of these fits can be seen in Supplemental Table 3.
DATA AND CODE AVAILABILITY
The published article and supplemental information include all relevant datasets generated or analyzed during this study.
ADDITIONAL RESOURCES:
None.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| None | ||
| Bacterial and Virus Strains | ||
| None | ||
| Biological Samples | ||
| Human Blood Samples | This paper | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| None | ||
| Critical Commercial Assays | ||
| Lipoprofile by NMR | ARUP (Salt Lake City, UT) | Cat#2013716, Ascend 600 instrument (Bruker; Billerica, Massachusetts) |
| Insulin | ARUP (Salt Lake City, UT) | Cat#0070022, chemiluminescent immunoassays on an ADVIA Centaur (Siemens Corporation; Washington, DC) |
| Glycated Hemoglobin | UCSD Jacobs Medical Center (JMC) (La Jolla, CA) | Cat#Cobas8000 (Roche; Indianapolis, IN) |
| Complete Blood Count | JMC/Center for Advanced Laboratory Medicine (CALM) (La Jolla, CA) | Cat#XN-10 (Sysmex; Chuo-ku, Japan) |
| Prothrombin time | JMC/CALM (La Jolla, CA) | Cat#ACLTOP700 (Instrumentation Laboratory; Bedford, MA) |
| Partial thromboplastin time | JMC/CALM (La Jolla, CA) | Cat#ACLTOP700 (Instrumentation Laboratory; Bedford, MA) |
| Comprehensive Metabolic Panel | CALM (La Jolla, CA) | Cat#Cobas8000 (Roche; Indianapolis, IN) |
| Thyroid Stimulating Hormone | CALM (La Jolla, CA) | Cat#Cobas8000 (Roche; Indianapolis, IN) |
| High sensitivity C-reactive protein | CALM (La Jolla, CA) | Cat#Cobas8000 (Roche; Indianapolis, IN) |
| Deposited Data | ||
| None | ||
| Experimental Models: Cell Lines | ||
| None | ||
| Experimental Models: Organisms/Strains | ||
| None | ||
| Oligonucleotides | ||
| None | ||
| Recombinant DNA | ||
| None | ||
| Software and Algorithms | ||
| Extracting glucose data: Libre View Device Drivers v.3.2.1 | Abbott | https://www2.libreview.com/Dashboard/UploadMeter |
| Analyzing Actiwatch Data: Actiware Software v.6.0.9 – Software CD | Philips Respironics | Cat#1104775 |
| SPSS Statistics | IBM | https://www.ibm.com/products/spss-statistics |
| Prism 8 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| Other | ||
| Pittsburgh Sleep Quality Index | Buysse DJ et al. (1989) | https://www.cmu.edu/common-cold-project/measures-by-study/health-practices/sleep-habits/psqi_rev.pdf |
| Continuous Glucose Monitor Sensor: FreeStyle Libre 14 day Flash Glucose Monitoring Systems | Abbott | Cat#71562-01 |
| Continuous Glucose Monitor Reader: FreeStyle Libre 14 day Flash Glucose Monitoring Systems | Abbott | Cat#71687-01 |
| DC-430U Dual Frequency Total Body Composition Analyzer | Tanita (Tokyo, Japan) | Cat#DC-430U |
| Actiwatch Spectrum Plus | Philips Respironics | Cat#1101894 |
Highlights.
10h time-restricted eating (TRE) in metabolic syndrome (MetS) promotes weight loss.
TRE in MetS reduces waist circumference, percent body fat, and visceral fat.
TRE in MetS lowers blood pressure atherogenic lipids, and glycated hemoglobin.
Benefits of TRE are “add-on” to statin and anti-hypertensive medications.
Context and Significance.
People with metabolic syndrome are at risk for diabetes and heart disease. Current treatment requires weight loss and lifestyle changes that are challenging, thus new behavioral interventions are needed. Ten-hour time-restricted eating (TRE) involves limiting daily dietary intake to a consistent 10-hour window, creating a 14-hour fast each night. Researchers studied whether TRE for 12-weeks in people with metabolic syndrome receiving standard medical care (including medications to lower cholesterol and blood pressure) led to improved markers of health. TRE led to weight loss, healthier body composition (including decreased waist circumference), lower blood pressure and levels of cardiovascular disease-promoting lipids (i.e. “bad cholesterol” levels), and more restful sleep. TRE could be an effective dietary intervention to help those with metabolic syndrome.
Acknowledgements
The authors thank Christiana Stark, Alma Fregoso, and Savannah Pipp for their contributions to this research. Funding: This study was funded by the University of California San Diego Public Health Pilot Grant. MJW’s role in this study was supported by an American College of Cardiology (ACC)/Merck Research Fellowship Award. ENCM is supported by the Larry L. Hillblom Foundation Postdoctoral Fellowship and Salk Women in Science Fellowship. PRT is supported by NIH grant DK118278. SP is supported by NIH grant DK115214, Department of Homeland Security (EMW-2016-FP-00788) and the Department of Defense (W81XWH1810645), The Leona M. and Harry B. Helmsley Charitable Trust grant #2012-PG-MED002, and Robert Wood Johnson Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
All authors have no disclosures related to this manuscript. Pam R. Taub is a consultant for Sanofi/Regeneron, Novo-Nordisk, Boehringer-Ingleheim, Janssen, Pfizer, and Amgen. She is a stockholder of Cardero Therapeutics. S. Panda is the author of “The Circadian Code” for which he collects a nominal author royalty.
References
- Ackermann RT, Liss DT, Finch EA, Schmidt KK, Hays LM, Marrero DG, and Saha C (2015). A Randomized Comparative Effectiveness Trial for Preventing Type 2 Diabetes. Am J Public Health 105, 2328–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, and Smith SC Jr. (2009). Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120, 1640–1645. [DOI] [PubMed] [Google Scholar]
- Antoni R, Robertson TM, Robertson MD, and Johnston JD (2018). A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects. Journal of Nutritional Science 7. [Google Scholar]
- Asher G, and Sassone-Corsi P (2015). Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell 161, 84–92. [DOI] [PubMed] [Google Scholar]
- Bailey T, Bode BW, Christiansen MP, Klaff LJ, and Alva S (2015). The Performance and Usability of a Factory-Calibrated Flash Glucose Monitoring System. Diabetes Technol Ther 17, 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhopal RS, Douglas A, Wallia S, Forbes JF, Lean ME, Gill JM, McKnight JA, Sattar N, Sheikh A, Wild SH, et al. (2014). Effect of a lifestyle intervention on weight change in south Asian individuals in the UK at high risk of type 2 diabetes: a family-cluster randomised controlled trial. Lancet Diabetes Endocrinol 2, 218–227. [DOI] [PubMed] [Google Scholar]
- Cederroth CR, Albrecht U, Bass J, Brown SA, Dyhrfjeld-Johnsen J, Gachon F, Green CB, Hastings MH, Helfrich-Forster C, Hogenesch JB, et al. (2019). Medicine in the Fourth Dimension. Cell Metab 30, 238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A, Zarrinpar A, Miu P, and Panda S (2014). Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 20, 991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H, Chou W, Sears DD, Patterson RE, Webster NJ, and Ellies LG (2016). Time-restricted feeding improves insulin resistance and hepatic steatosis in a mouse model of postmenopausal obesity. Metabolism 65, 1743–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervin RB (2009). Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003-2006. Natl Health Stat Report, 1–7. [PubMed] [Google Scholar]
- Gabel K, Hoddy KK, Haggerty N, Song J, Kroeger CM, Trepanowski JF, Panda S, and Varady KA (2018a). Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr Healthy Aging 4, 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel K, Hoddy KK, and Varady KA (2018b). Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab. [DOI] [PubMed] [Google Scholar]
- Gill S, Le HD, Melkani GC, and Panda S (2015). Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science 347, 1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, and Panda S (2015). A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab 22, 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta NJ, Kumar V, and Panda S (2017). A camera-phone based study reveals erratic eating pattern and disrupted daily eating-fasting cycle among adults in India. PLoS One 12, e0172852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, Hazen N, Herman J, Adams Hillard PJ, Katz ES, et al. (2015). National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health 1, 233–243. [DOI] [PubMed] [Google Scholar]
- Hutchison AT, Regmi P, Manoogian ENC, Fleischer JG, Wittert GA, Panda S, and Heilbronn LK (2019). Time-Restricted Feeding Improves Glucose Tolerance in Men at Risk for Type 2 Diabetes: A Randomized Crossover Trial. Obesity (Silver Spring) 27, 724–732. [DOI] [PubMed] [Google Scholar]
- Jacobson TA, Maki KC, Orringer CE, Jones PH, Kris-Etherton P, Sikand G, La Forge R, Daniels SR, Wilson DP, Morris PB, et al. (2015). National Lipid Association Recommendations for Patient-Centered Management of Dyslipidemia: Part 2. J Clin Lipidol 9, S1–122 e121. [DOI] [PubMed] [Google Scholar]
- Katula JA, Vitolins MZ, Morgan TM, Lawlor MS, Blackwell CS, Isom SP, Pedley CF, and Goff DC Jr. (2013). The Healthy Living Partnerships to Prevent Diabetes study: 2-year outcomes of a randomized controlled trial. Am J Prev Med 44, S324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, and Nathan DM (2002). Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England journal of medicine 346, 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCheminant JD, Christenson E, Bailey BW, and Tucker LA (2013). Restricting night-time eating reduces daily energy intake in healthy young men: a short-term cross-over study. Br J Nutr 110, 2108–2113. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. (2010). Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 121, 586–613. [DOI] [PubMed] [Google Scholar]
- Lunn RM, Blask DE, Coogan AN, Figueiro MG, Gorman MR, Hall JE, Hansen J, Nelson RJ, Panda S, Smolensky MH, et al. (2017). Health consequences of electric lighting practices in the modern world: A report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci Total Environ 607-608, 1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Allison DB, Fontana L, Harvie M, Longo VD, Malaisse WJ, Mosley M, Notterpek L, Ravussin E, Scheer FA, et al. (2014). Meal frequency and timing in health and disease. Proc. Natl. Acad. Sci. U. S. A. 111, 16647–16653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, et al. (2011). Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 123, 2292–2333. [DOI] [PubMed] [Google Scholar]
- Mohebbi I, Shateri K, and Seyedmohammadzad M (2012). The relationship between working schedule patterns and the markers of the metabolic syndrome: comparison of shift workers with day workers. International journal of occupational medicine and environmental health 25, 383–391. [DOI] [PubMed] [Google Scholar]
- Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, Palma A, Gentil P, Neri M, and Paoli A (2016). Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med 14, 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S (2016). Circadian physiology of metabolism. Science 354, 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pot GK, Almoosawi S, and Stephen AM (2016). Meal irregularity and cardiometabolic consequences: results from observational and intervention studies. Proc Nutr Soc 75, 475–486. [DOI] [PubMed] [Google Scholar]
- Puttonen S, Harma M, and Hublin C (2010). Shift work and cardiovascular disease - pathways from circadian stress to morbidity. Scand J Work Environ Health 36, 96–108. [DOI] [PubMed] [Google Scholar]
- Sierra-Johnson J, Unden AL, Linestrand M, Rosell M, Sjogren P, Kolak M, De Faire U, Fisher RM, and Hellenius ML (2008). Eating meals irregularly: a novel environmental risk factor for the metabolic syndrome. Obesity (Silver Spring) 16, 1302–1307. [DOI] [PubMed] [Google Scholar]
- Sperling LS, Mechanick JI, Neeland IJ, Herrick CJ, Despres JP, Ndumele CE, Vijayaraghavan K, Handelsman Y, Puckrein GA, Araneta MR, et al. (2015). The CardioMetabolic Health Alliance: Working Toward a New Care Model for the Metabolic Syndrome. J Am Coll Cardiol 66, 1050–1067. [DOI] [PubMed] [Google Scholar]
- St-Onge MP, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris-Etherton P, and Varady K (2017). Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement From the American Heart Association. Circulation 135, e96–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulli G, Manoogian ENC, Taub PR, and Panda S (2018). Training the Circadian Clock, Clocking the Drugs, and Drugging the Clock to Prevent, Manage, and Treat Chronic Diseases. Trends Pharmacol Sci 39, 812–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, and Peterson CM (2018). Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab 27, 1212–1221 e1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanita Corporation of America Inc., T. (2013). <VisceralFatMeasurmentp1.pdf>.
- Tinsley GM, Forsse JS, Butler NK, Paoli A, Bane AA, La Bounty PM, Morgan GB, and Grandjean PW (2017). Time-restricted feeding in young men performing resistance training: A randomized controlled trial. Eur J Sport Sci 17, 200–207. [DOI] [PubMed] [Google Scholar]
- van Wouwe NC, Valk PJ, and Veenstra BJ (2011). Sleep monitoring: a comparison between three wearable instruments. Mil Med 176, 811–816. [DOI] [PubMed] [Google Scholar]
- Wei M, Brandhorst S, Shelehchi M, Mirzaei H, Cheng CW, Budniak J, Groshen S, Mack WJ, Guen E, Di Biase S, et al. (2017). Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci Transl Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinsier RL, Wilson LJ, and Lee J (1995). Medically safe rate of weight loss for the treatment of obesity: a guideline based on risk of gallstone formation. Am J Med 98, 115–117. [DOI] [PubMed] [Google Scholar]
- Wennberg M, Gustafsson PE, Wennberg P, and Hammarstrom A (2016). Irregular eating of meals in adolescence and the metabolic syndrome in adulthood: results from a 27-year prospective cohort. Public Health Nutr 19, 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Zhu C, Wang M, Mo F, Du W, and Ma X (2018). Night Shift Work Increases the Risks of Multiple Primary Cancers in Women: A Systematic Review and Meta-analysis of 61 Articles. Cancer Epidemiol Biomarkers Prev 27, 25–40. [DOI] [PubMed] [Google Scholar]
- Zarrinpar A, Chaix A, and Panda S (2016). Daily Eating Patterns and Their Impact on Health and Disease. Trends Endocrinol Metab 27, 69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomer E, Gurusamy K, Leach R, Trimmer C, Lobstein T, Morris S, James WP, and Finer N (2016). Interventions that cause weight loss and the impact on cardiovascular risk factors: a systematic review and meta-analysis. Obes Rev 17, 1001–1011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article and supplemental information include all relevant datasets generated or analyzed during this study.