Abstract
Background
Use of appropriate antibiotics for the treatment of pneumonia is integral in patients admitted to intensive care units (ICUs). Although it is recommended that empirical treatment regimens should be based on the local distribution of pathogens in patients with suspected hospital-acquired pneumonia, few studies observe patients admitted to ICUs with nursing home–acquired pneumonia (NHAP). We found factors associated with the use of inappropriate antibiotics in patients with pneumonia admitted to the ICU via the emergency room (ER).
Methods
We performed a retrospective cohort study of 83 pneumonia patients with confirmed causative bacteria admitted to ICUs via ER March 2015–May 2017. We compared clinical parameters, between patients who received appropriate or inappropriate antibiotics using the Mann-Whitney U, Pearson's chi-square, and Fisher's exact tests. We investigated independent factors associated with inappropriate antibiotic use in patients using multivariate logistic regression.
Results
Among 83 patients, 30 patients (36.1%) received inappropriate antibiotics. NHAP patients were more frequently treated with inappropriate antibiotics than with appropriate antibiotics (47.2% vs. 96.7%, p<0.001). Methicillin-resistant Staphylococcus aureus was more frequently isolated from individuals in the inappropriate antibiotics–treated group than in the appropriate antibiotics–treated group (7.5% vs. 70.0%, p<0.001). In multivariate analysis, NHAP was independently associated with the use of inappropriate antibiotics in patients with pneumonia admitted to the ICU via ER.
Conclusion
NHAP is a risk factor associated with the use of inappropriate antibiotics in patients with pneumonia admitted to the ICU via the ER.
Keywords: Nursing Home–Acquired Pneumonia, Pneumonia, Intensive Care Unit
Introduction
Pneumonia is the most serious of common infections that occur in nursing home residents and is associated with considerable morbidity and mortality1,2,3. The median incidence of nursing home-associated pneumonia (NHAP) is 1 per 1,000 patient-days, many-fold higher than that among persons residing in the community4. NHAP patients tend to be more elderly and have greater comorbidity, and severe functional impairment, as compared to community-acquired pneumonia patients5,6. In particular, critically ill patients admitted to intensive care units (ICUs) with pneumonia can progress to acute respiratory distress syndrome and acute lung injury, which are associated with a mortality rate of more than 50%7. Therefore, it is important to determine the appropriate empirical antibiotics for successful treatment of patients admitted to ICU with NHAP.
The 2016 Infectious Disease Society of America (IDSA) and the American Thoracic Society (ATS) guidelines recommend that each hospital should generate antibiograms to guide healthcare professionals on the optimal choice of antibiotics without the concept of healthcare-associated pneumonia (HCAP) including NHAP8. However, few nursing home facilities can generate antibiograms due to a number of challenges and barriers, such as lack of laboratory resources and personnel dedicated to the development and implementation of antibiograms9. Therefore, it is still important to broadly study microorganisms to facilitate the appropriate use of empirical antibiotics in NHAP patients. Several studies have shown differences in the presence of antibiotic-resistant organisms in NHAP patients, compared with community-acquired pneumonia (CAP) and hospital-acquired pneumonia patients4,5,6,10,11,12. However, few studies have been conducted on NHAP patients requiring ICU care13,14. In the present study, we aimed to find factors associated with the use of inappropriate antibiotics in patients with pneumonia who were admitted to an ICU via the emergency room (ER).
Materials and Methods
1. Study subjects
In this observational cohort study, we retrospectively reviewed the medical records of patients with pneumonia admitted to an ICU via ER at a teaching hospital between March 2015 and May 2017. We excluded patients who were transferred from other acute care hospitals or had received treatment for pneumonia within the previous 3 months.
Pneumonia was diagnosed based the presence of a new opacity development and at least two of the following four clinical criteria: fever or hypothermia (body temperature >38℃ or ≤35℃), leukocytosis or leukopenia (white blood cell count ≥10,000/µL or ≤4,000/µL), newly developed respiratory symptoms (cough, sputum, pleuritic chest pain, and dyspnea), and altered breath sounds on auscultation15. We defined NHAP as pneumonia occurring in a resident of a nursing home or a long-term care facility1. All patients were admitted to an ICU for close monitoring or after requiring mechanical ventilation with shock or acute respiratory failure in an ER.
The Institutional Review Board of Inje University Ilsan Paik Hospital approved the study design, including the review and publishing of information obtained from patient records (IRB No. 2017-12-018). The requirement for informed consent was waived for the use of patient medical data because all personally identifying information was removed before analysis.
2. Measurement
Patient medical records were reviewed to obtain data on clinical characteristics, clinical parameters, laboratory findings, clinical outcomes, and isolated microorganisms. Comorbidities including diabetes mellitus, degenerative nerve disease, chronic obstructive pulmonary disease, malignant neoplasm, and chronic renal disease were reviewed. Clinical parameters included the CURB-65 (confusion, urea, respiratory rate, blood pressure, age more than 65 years) for assessing the severity of pneumonia16, use of vasopressors, and need of mechanical ventilator. Prior antibiotics use within 90 days was arbitrarily defined as use of antibiotics within 90 days for reasons other than pneumonia.
3. Microbiology
Microbiological studies were conducted using two sets of blood cultures, gram staining and culture using sputum, tracheal aspirate, and bronchial washing fluids that were obtained by bronchoscopy. Respiratory specimens were cultured in a semi-quantitative manner, and pathogens were identified when a predominant microorganism was detected from group 4 or 5 sputum, according to Murray and Washington's grading system. Blood cultures were considered to be positive if pathogens were present, there were no other infection sources that could explain a positive culture, and the possibility of contamination was excluded. Urinary antigen test for Streptococcus pneumoniae was also considered to indicate etiological pathogens. The antibiotic sensitivity of all isolated pathogens was identified using a disc diffusion method. Multidrug-resistant gram-negative bacteria (MDRGNB) for Pseudomonas aeruginosa, Enterococcus species, Enterobacteriaceae, and Acinetobacter species was defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories17.
4. Antibiotic therapy
All patients were initially administered broad empirical antibiotics according to the ATS/IDSA guideline18. The detailed antibiotic regimen complied with the attending physician's choice, taking into consideration any risk factors of the patient. In this study, the use of inappropriate antibiotics was defined as the use of empirical antibiotics which were not effective or which were unnecessarily broad against the identified pathogens based on in vitro susceptibility testing6.
5. Statistical analysis
The data are presented as medians and interquartile ranges for continuous variables and as numbers (%) for categorical variables. The data were compared using the Mann-Whitney U test for continuous variables and Pearson's chi-square test or Fisher exact test for categorical variables. Multivariate logistic regression analysis was used to determine independent factors associated with the use of inappropriate antibiotics in patients with HCAP. All tests were 2-sided and a p-value <0.05 was considered significant. All statistical analyses were performed using IBM SPSS Statistics for Windows version 22.0 (IBM Corp., Armonk, NY, USA).
Results
1. Baseline characteristics
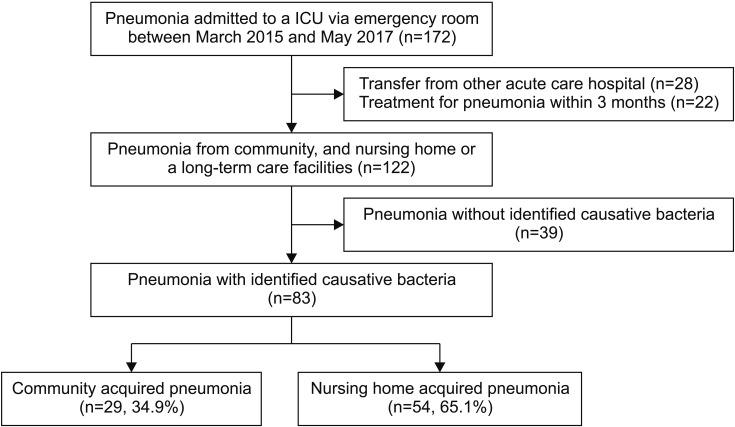
From the medical records of 172 patients with pneumonia admitted to an ICU via ER, after excluding 28 patients who were transferred from other acute care hospitals and 22 patients who had received treatment for pneumonia within the previous 3 months, a total of 122 patients with CAP and NHAP were included. Among 122 patients, 83 with identified causative bacteria were included in the present study; 39 patients were excluded because a causative agent could not be identified. Of the 83 patients, 29 patients had CAP (34.9%) and 54 had NHAP (65.1%) (Figure 1). In this study, 50 patients (63.9%) and 30 patients (36.1%) received appropriate and inappropriate antibiotics, respectively. Among the total 88 patients, respiratory viral real-time polymerase chain reaction was performed in 48 (57.8%) and 16 (19.3%) patients were confirmed to have bacterial and viral co-infections. The clinical characteristics of the study subjects are summarized in Table 1. NHAP patients were more frequently treated with inappropriate antibiotics rather than appropriate antibiotics (47.2% [25/53] vs. 96.7% [29/30], p<0.001). A CURB-65 score ≥3 was more common in the inappropriate antibiotic-treated group than in the appropriate antibiotic-treated group (25.8% [31/53] vs. 70.0% [21/30], p=0.046). There were no significant differences between the clinical parameters, laboratory parameters, and identified microorganisms between the two groups. The differences in clinical outcomes, including duration of overall admission, duration of ICU admission, and 30-day mortality, between the two groups were not significant.
Figure 1. Flow chart for study enrollment. ICU: intensive care unit.
Table 1. Baseline characteristics and treatment outcomes of 83 patients admitted to intensive care units with pneumonia.
| Characteristic | Appropriate antibiotics (n=53) | Inappropriate antibiotics (n=30) | p-value |
|---|---|---|---|
| Age, yr | 77 (65−86) | 74 (64−83) | 0.977 |
| Male | 33 (62.3) | 19 (63.3) | 0.923 |
| Lifetime nonsmoker | 31 (58.5) | 18 (60.0) | 0.893 |
| Comorbidity | |||
| Diabetes mellitus | 13 (24.5) | 12 (40.0) | 0.140 |
| Cardiovascular disease | 17 (32.1) | 12 (40.0) | 0.467 |
| Degenerative nerve disease | 16 (30.2) | 10 (33.3) | 0.767 |
| COPD | 12 (22.6) | 6 (20.0) | 0.779 |
| Malignant neoplasms | 8 (15.1) | 2 (6.7) | 0.257 |
| Chronic renal disease | 3 (5.7) | 4 (13.3) | 0.227 |
| Prior antibiotics use within 90 days | 12 (22.6) | 12 (40.0) | 0.094 |
| NHAP | 25 (47.2) | 29 (96.7) | <0.001 |
| Clinical parameter | |||
| CURB-65 score | 3 (2−4) | 3 (3−4) | 0.291 |
| CURB-65 score ≥3 | 31 (58.5) | 24 (80.0) | 0.046 |
| Use of vasopressors | 35 (66.0) | 21 (70.0) | 0.711 |
| Mechanical ventilation | 28 (52.8) | 20 (66.7) | 0.220 |
| Laboratory finding | |||
| White blood cell, ×1,000/mm3 | 10.18 (5.64−14.93) | 9.69 (5.49−15.32) | 0.504 |
| Procalcitonin, mg/dL | 4.2 (0.5−15.7) | 2.1 (0.5−10.1) | 0.502 |
| C-reactive protein, mg/dL | 13.7 (4.7−21.5) | 12.5 (3.4−24.3) | 0.824 |
| Platelet, ×1,000/mm3 | 207 (143−264) | 211 (152−276) | 0.837 |
| Creatinine | 1.1 (0.7−1.8) | 1.1 (0.6−1.5) | 0.633 |
| Clinical outcome | |||
| Duration of admission, day | 14 (6−24) | 11 (7−23) | 0.627 |
| Duration of ICU admission, day | 4 (2−9) | 7 (2−9) | 0.422 |
| 30-Day mortality | 14 (26.4) | 6 (20.0) | 0.511 |
Values are presented as median (interquartile range) or number (%).
COPD: chronic obstructive disease; NHAP: nursing home–acquired pneumonia; ICU: intensive care unit.
2. Initial antibiotics treatment
In 29 CAP patients and 54 NHAP patients admitted to the ICU via ER, the majority of them received combination antibiotic therapy as the initial treatment (CAP 71.4%, NHAP 66.7%) (Table 2). Among the 54 NHAP patients, 25 (46.3%) received appropriate antibiotics. Patients treated with combination antibiotics that included vancomycin were more frequently encountered in the appropriate antibiotics group than in the inappropriate antibiotics group (20.0% [5/25] vs. 0% [0/29], p=0.017).
Table 2. Initial antibiotics treatment in 83 patients.
| Empirical antibiotics | CAP (n=29) | NHAP (n=54) | ||||
|---|---|---|---|---|---|---|
| Appropriate antibiotics (n=28) | Inappropriate antibiotics (n=1) | p-value | Appropriate antibiotics (n=25) | Inappropriate antibiotics (n=29) | p-value | |
| Monotherapy | 8 (28.6) | 1 (100) | 9 (36.0) | 10 (34.5) | ||
| Fluoroquinolone | 2 (7.1) | 0 (0) | >0.999 | 0 (0) | 0 (0) | N/A |
| Anti-pseudomonal β-lactamase | 5 (17.9) | 1 (100) | 0.207 | 4 (16.0) | 5 (17.2) | >0.999 |
| Carbapenem | 1 (3.6) | 0 (0) | >0.999 | 5 (20.0) | 5 (17.2) | 0.795 |
| Combination therapy | 20 (71.4) | 0 (0) | 16 (64.0) | 19 (65.5) | ||
| β-lactamase+macrolide | 4 (14.3) | 0 (0) | >0.999 | 1 (4.0) | 1 (3.4) | >0.999 |
| Anti-pseudomonal β-lactamase+fluoroquinolone | 14 (50.0) | 0 (0) | >0.999 | 10 (40.0) | 18 (62.1) | 0.106 |
| Carbapenem+fluoroquinolone | 1 (3.6) | 0 (0) | >0.999 | 0 (0) | 0 (0) | N/A |
| Carbapenem+vancomycin | 1 (3.6) | 0 (0) | >0.999 | 5 (20.0) | 0 (0) | 0.017 |
Values are presented as number (%).
CAP: community-acquired pneumonia; NHAP: nursing home–acquired pneumonia; N/A: not applicable.
3. Microorganisms identified in 83 patients
The identified microorganisms in the study subjects are summarized in Table 3. Streptococcus pneumoniae was more frequently isolated from subjects in the appropriate antibiotics group than from those in the inappropriate antibiotics group (20.8% [11/53] vs. 0% [0/30], p<0.001). Staphylococcus aureus was more frequently isolated from subjects in the inappropriate antibiotics group than from those in the appropriate antibiotics group (28.3% [15/53] vs. 70% [21/30], p<0.001). The prevalence of the other identified microorganisms was not significantly different between the two groups. Methicillinresistant Staphylococcus aureus (MRSA) was more frequently isolated from subjects in the inappropriate antibiotics group than from those in the appropriate antibiotics group (7.5% [4/54] vs. 70% [21/30], p<0.001). The prevalence of MDRGNB was not significantly different between the two groups.
Table 3. Microorganisms identified in 83 patients.
| Microgorganism | Appropriate antibiotics (n=53) | Inappropriate antibiotics (n=30) | p-value |
|---|---|---|---|
| Gram-positive bacteria* | 26 (49.1) | 21 (70.0) | 0.064 |
| Streptococcus pneumoniae | 11 (20.8) | 0 (0.0) | 0.007 |
| Staphylococcus aureus | 15 (28.3) | 21 (70.0) | <0.001 |
| Gram-negative bacteria* | 28 (52.8) | 11 (36.7) | 0.156 |
| Pseudomonas aeruginosa | 10 (18.9) | 2 (6.7) | 0.129 |
| Klebsiella pneumoniae | 6 (11.3) | 4 (13.3) | 0.787 |
| Acinetobacter baumanii | 0 (0) | 2 (6.7) | 0.057 |
| Escherichia coli | 5 (9.3) | 3 (10.0) | 0.933 |
| Haemophilus influenza | 2 (3.8) | 0 (0) | 0.281 |
| Moraxella catahallis | 2 (3.8) | 0 (0) | 0.281 |
| Other gram-negative species | 4 (7.5) | 1 (3.3) | 0.438 |
| Polymicrobial pathogens* | 1 (1.9) | 3 (10.0) | 0.097 |
| MRSA | 4 (7.5) | 21 (70.0) | <0.001 |
| MDRGNB | 4 (7.5) | 6 (20.0) | 0.094 |
| CRGNB | 4 (7.5) | 3 (10.0) | 0.699 |
Values are presented as number (%).
*Both gram-positive and gram-negative bacteria were identified in four patients, and two gram-negative bacteria were identified in one patient.
MRSA: methicillin-resistant Staphylococcus aureus; MDRGNB: multidrug-resistant gram-negative bacteria; CRGNB: carbapenem-resistant gram-negative bacteria.
4. Factors associated with the use of inappropriate antibiotics
Candidate variables for logistic regression analysis included prior antibiotics use within 90 days, NHAP, identification of polymicrobial pathogens, and MDRGNB, which were objective variables with p<0.1 when the appropriate antibiotics group and inappropriate antibiotics group were compared (Table 4). In multivariate analysis, identification of NHAP (adjusted odds ratio, 28.66; 95% confidence interval, 3.45??38.12; p=0.002) was independently associated with the use of inappropriate antibiotics.
Table 4. Factors associated with the use of inappropriate antibiotics in 83 patients admitted to the intensive care units with pneumonia.
| Characteristic | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Prior antibiotics use within 90 days | 2.28 (0.86−6.03) | 0.097 | 1.02 (0.34−3.08) | 0.979 |
| NHAP | 32.48 (4.12−256.15) | 0.001 | 28.66 (3.45−238.12) | 0.002 |
| Polymicrobial pathogens | 5.79 (0.57−58.23) | 0.137 | 2.72 (0.26−23.13) | 0.401 |
| Identification of MDRGNB | 3.06 (0.79−11.89) | 0.106 | 1.39 (0.33−5.47) | 0.686 |
OR: odds ratio; CI: confidence interval; NHAP: nursing home–acquired pneumonia; MDRGNB: multi-drug resistant gram-negative bacteria.
Discussion
In the present study, we showed that the use of inappropriate antibiotics was more frequent in NHAP patients. Streptococcus pneumoniae was more frequently isolated from subjects in the appropriate antibiotics group, but Staphylococcus aureus was more frequently encountered in the inappropriate antibiotics group. The identification of MRSA was more frequently seen in the inappropriate antibiotics group than in the appropriate antibiotics group. In addition, we found that NHAP was a risk factor for the use of inappropriate antibiotics in patients with pneumonia admitted to ICUs via ER.
In this study, we found that the most common pathogen was Staphylococcus aureus (50.0%) in NHAP patients admitted to the ICU via ER. Also, the identification of Staphylococcus aureus was associated with inappropriate antibiotics use. Some previous studies that enrolled all cases of NHAP reported that Streptococcus pneumoniae was the most common pathogen among NHAP patients, which was similar to CAP patients1,4,10,11,12,19,20. However, studies that enrolled severe cases of NHAP, including our study, consistently showed that the most common pathogen was Staphylococcus aureus13,14, suggesting that antibiotics for targeting Staphylococcus aureus may be considered in NHAP patients admitted to the ICU. From this result, it can be inferred that MRSA is the most common pathogen in ICU admitted patients. In this study, MRSA was identified more frequently in NHAP patients (44.4%) than in CAP patients (3.3%).
We showed that the use of inappropriate antibiotics was significantly different between CAP patients and NHAP patients. This result could be associated with the presence of resistant pathogens in NHAP patients. Shorr et al.21 already showed that residence in a nursing home could be an independent factor associated with resistant infection. NHAP patients have poor performance status, presence of a nasogastric tube, swallowing difficulties, difficulty with oropharyngeal secretions, increased confusion, and increased agitation22,23,24,25. Debilitated nursing home residents with a high risk for aspiration are most likely to develop pneumonia1. Because nursing home residents are previously exposed to the use of antibiotics for symptomatic urinary infections, lower respiratory infections, wound infections, and infections at other sites23,24, NHAP patients who aspirated oral resident flora with antibiotics resistance would have drug-resistant pathogens.
We demonstrated that NHAP was a risk factor for the use of inappropriate antibiotics in patients with pneumonia admitted to the ICU. In particular, NHAP was 29 times for use of inappropriate antibiotics in multivariate analysis. These results were associated with the identification of MRSA in this study. Because MRSA pneumonia is associated with all-cause mortality of 55.5%, rapid institution of appropriate antibiotic therapy is crucial26. MRSA burden and transmission were associated with nursing home care for more residents with chronic illness or indwelling devices27. Also, a recent increase in frequent, prolonged ventilatory support of an aging, often chronically ill, population has resulted in a large increase in MRSA pneumonia cases in nursing homes26. Therefore, physicians may consider the use of antibiotics against MRSA for the treatment of NHAP patients admitted to ICU.
In this study, interestingly, the identification of MDRGNB was not associated with the use of inappropriate antibiotics. The 2005 ATS/IDSA guidelines recommend the use of antibiotics against MRSA for initial empirical antibiotics therapy for HCAP including NHAP18. The prescription according to these guidelines is not likely to be associated with inappropriate antibiotics. However, because these guidelines only recommended that additional anti-MRSA antibiotics, including linezolid or vancomycin, be used as additional coverage for patients with MRSA risk factors18, physicians may tend not to consider vancomycin or linezolid, which have common side effects, such as nephrotoxicity, for initial empirical therapy. The 2016 ATS/IDSA guidelines recommend anti-MRSA antibiotics in (1) patients who received intravenous antibiotics during the prior 90 days and (2) in units where the prevalence of MRSA among Staphylococcus aureus isolated is not known or is >20% without the concept of NHAP8. Therefore, as recommended by these guidelines8, physicians should consider antibiotics against MRSA in patients who are at high risk for mortality, such as patients admitted to ICU.
There were several limitations to the present study. First, this was a retrospective study conducted at a single center ICU. Patents who are admitted to the ICU via ER could vary widely by region or country. However, our center is located in a new city between urban and rural areas, suggesting that our sample reflected various conditions of nursing homes. Second, gold standard examination for confirming pathogens is not equal to all patients. Two patients were confirmed through urine antigens, which cannot be performed via drug sensitivity tests28. Although 35% of Streptococcus pneumoniae are reported as MDR Streptococcus pneumoniae in Korea, the resistance rates to cephalosporins (10%) and fluoroquinolones (0.6%) are relatively low29. In the present study, two positive urine antigens for Streptococcus pneumoniae were considered as drug-sensitive pathogens. Third, we did not perform molecular technique-based tests for the detection of Panton-Valentine leucocidin (PVL). It is known that PVL is a toxin that causes leukocyte destruction and tissue necrosis commonly associated with community-associated MRSA and severe pneumonia26. This study focused on evaluating clinical factors associated with the use of inappropriate antibiotics in NHAP patients. Fourth, this study did not enroll many patients with NHAP admitted to the ICU. Larger population studies, such as multicenter or epidemiological studies are needed.
In conclusion, this study demonstrated that NHAP was a risk factor for the use of inappropriate antibiotics in patients with pneumonia admitted to the ICU via the ER. Identification of MRSA was found in patients who received inappropriate antibiotics. Therefore, physicians may consider antibiotics against MRSA as initial empirical antibiotics in NHAP patients who are admitted to ICUs via the ER.
Footnotes
- Conceptualization: Kang HK.
- Methodology: Kim DH, Kang HK.
- Formal analysis: Kim DH, Koo HW, Kang HK.
- Data curation: Kim DH, Kim HJ, Bae W, Park SH, Koo HK, Park HK, Lee SS, Kang HK.
- Software: Kim DH, Koo HW, Kang HK.
- Validation: Kim DH, Kang HK.
- Investigation: Kim DH, Kang HK.
- Writing - original draft preparation: Kim DH, Kang HK.
- Writing - review and editing: Kim DH, Kim HJ, Koo HW, Bae W, Park SH, Koo HK, Park HK, Lee SS, Kang HK.
- Approval of final manuscript: all authors.
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
Funding: This study was supported by grants from Alumini Association of Division of Pulmonary, Allergy and Critical Care Medicine, Internal Medicine of Chung-Ang University Hospital (Ungye Research Funds).
References
- 1.Mylotte JM. Nursing home-acquired pneumonia. Clin Infect Dis. 2002;35:1205–1211. doi: 10.1086/344281. [DOI] [PubMed] [Google Scholar]
- 2.Mehr DR, Binder EF, Kruse RL, Zweig SC, Madsen R, Popejoy L, et al. Predicting mortality in nursing home residents with lower respiratory tract infection: The Missouri LRI Study. JAMA. 2001;286:2427–2436. doi: 10.1001/jama.286.19.2427. [DOI] [PubMed] [Google Scholar]
- 3.Muder RR, Brennen C, Swenson DL, Wagener M. Pneumonia in a long-term care facility: a prospective study of outcome. Arch Intern Med. 1996;156:2365–2370. [PubMed] [Google Scholar]
- 4.Muder RR. Pneumonia in residents of long-term care facilities: epidemiology, etiology, management, and prevention. Am J Med. 1998;105:319–330. doi: 10.1016/s0002-9343(98)00262-9. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Moragon E, Garcia Ferrer L, Serra Sanchis B, Fernandez Fabrellas E, Gomez Belda A, Julve Pardo R. Community-acquired pneumonia among the elderly: differences between patients living at home and in nursing homes. Arch Bronconeumol. 2004;40:547–552. doi: 10.1016/s1579-2129(06)60373-x. [DOI] [PubMed] [Google Scholar]
- 6.Micek ST, Kollef KE, Reichley RM, Roubinian N, Kollef MH. Health care-associated pneumonia and community-acquired pneumonia: a single-center experience. Antimicrob Agents Chemother. 2007;51:3568–3573. doi: 10.1128/AAC.00851-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Pascale G, Bello G, Tumbarello M, Antonelli M. Severe pneumonia in intensive care: cause, diagnosis, treatment and management: a review of the literature. Curr Opin Pulm Med. 2012;18:213–221. doi: 10.1097/MCP.0b013e328351f9bd. [DOI] [PubMed] [Google Scholar]
- 8.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospitalacquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furuno JP, Comer AC, Johnson JK, Rosenberg JH, Moore SL, MacKenzie TD, et al. Using antibiograms to improve antibiotic prescribing in skilled nursing facilities. Infect Control Hosp Epidemiol. 2014;35 Suppl 3:S56–S61. doi: 10.1086/677818. [DOI] [PubMed] [Google Scholar]
- 10.Koh SJ, Lee JH. Clinical characteristics of nursing home-acquired pneumonia in elderly patients admitted to a Korean teaching hospital. Korean J Intern Med. 2015;30:638–647. doi: 10.3904/kjim.2015.30.5.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polverino E, Dambrava P, Cilloniz C, Balasso V, Marcos MA, Esquinas C, et al. Nursing home-acquired pneumonia: a 10 year single-centre experience. Thorax. 2010;65:354–359. doi: 10.1136/thx.2009.124776. [DOI] [PubMed] [Google Scholar]
- 12.Carratala J, Mykietiuk A, Fernandez-Sabe N, Suarez C, Dorca J, Verdaguer R, et al. Health care-associated pneumonia requiring hospital admission: epidemiology, antibiotic therapy, and clinical outcomes. Arch Intern Med. 2007;167:1393–1399. doi: 10.1001/archinte.167.13.1393. [DOI] [PubMed] [Google Scholar]
- 13.El-Solh AA, Sikka P, Ramadan F, Davies J. Etiology of severe pneumonia in the very elderly. Am J Respir Crit Care Med. 2001;163(3 Pt 1):645–651. doi: 10.1164/ajrccm.163.3.2005075. [DOI] [PubMed] [Google Scholar]
- 14.Lee H, Park JY, Lee T, Lee YJ, Lim HJ, Park JS, et al. Intermediate risk of multidrug-resistant organisms in patients who admitted intensive care unit with healthcare-associated pneumonia. Korean J Intern Med. 2016;31:525–534. doi: 10.3904/kjim.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carratala J, Fernandez-Sabe N, Ortega L, Castellsague X, Roson B, Dorca J, et al. Outpatient care compared with hospitalization for community-acquired pneumonia: a randomized trial in low-risk patients. Ann Intern Med. 2005;142:165–172. doi: 10.7326/0003-4819-142-3-200502010-00006. [DOI] [PubMed] [Google Scholar]
- 16.Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 18.American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 19.Lim WS, Macfarlane JT. A prospective comparison of nursing home acquired pneumonia with community acquired pneumonia. Eur Respir J. 2001;18:362–368. doi: 10.1183/09031936.01.00204401. [DOI] [PubMed] [Google Scholar]
- 20.Mills K, Graham AC, Winslow BT, Springer KL. Treatment of nursing home-acquired pneumonia. Am Fam Physician. 2009;79:976–982. [PubMed] [Google Scholar]
- 21.Shorr AF, Zilberberg MD, Micek ST, Kollef MH. Prediction of infection due to antibiotic-resistant bacteria by select risk factors for health care-associated pneumonia. Arch Intern Med. 2008;168:2205–2210. doi: 10.1001/archinte.168.20.2205. [DOI] [PubMed] [Google Scholar]
- 22.Loeb M, McGeer A, McArthur M, Walter S, Simor AE. Risk factors for pneumonia and other lower respiratory tract infections in elderly residents of long-term care facilities. Arch Intern Med. 1999;159:2058–2064. doi: 10.1001/archinte.159.17.2058. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez S, Shell CG, Woolley TW, Berk SL, Smith JK. Nosocomial infections in long-term facilities. J Gerontol. 1988;43:M9–M17. doi: 10.1093/geronj/43.1.m9. [DOI] [PubMed] [Google Scholar]
- 24.Magaziner J, Tenney JH, DeForge B, Hebel JR, Muncie HL, Jr, Warren JW. Prevalence and characteristics of nursing home-acquired infections in the aged. J Am Geriatr Soc. 1991;39:1071–1078. doi: 10.1111/j.1532-5415.1991.tb02871.x. [DOI] [PubMed] [Google Scholar]
- 25.Harkness GA, Bentley DW, Roghmann KJ. Risk factors for nosocomial pneumonia in the elderly. Am J Med. 1990;89:457–463. doi: 10.1016/0002-9343(90)90376-o. [DOI] [PubMed] [Google Scholar]
- 26.Rubinstein E, Kollef MH, Nathwani D. Pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46 Suppl 5:S378–S385. doi: 10.1086/533594. [DOI] [PubMed] [Google Scholar]
- 27.Murphy CR, Quan V, Kim D, Peterson E, Whealon M, Tan G, et al. Nursing home characteristics associated with methicillin-resistant Staphylococcus aureus (MRSA) Burden and Transmission. BMC Infect Dis. 2012;12:269. doi: 10.1186/1471-2334-12-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcos MA, Jimenez de Anta MT, de la Bellacasa JP, Gonzalez J, Martinez E, Garcia E, et al. Rapid urinary antigen test for diagnosis of pneumococcal community-acquired pneumonia in adults. Eur Respir J. 2003;21:209–214. doi: 10.1183/09031936.03.00058802. [DOI] [PubMed] [Google Scholar]
- 29.Kim SH, Bae IK, Park D, Lee K, Kim NY, Song SA, et al. Serotype Distribution and antimicrobial resistance of Streptococcus pneumoniae isolates causing invasive and noninvasive pneumococcal diseases in Korea from 2008 to 2014. Biomed Res Int. 2016;2016:6950482. doi: 10.1155/2016/6950482. [DOI] [PMC free article] [PubMed] [Google Scholar]