Abstract
Objectives
To assess the vitamin D status in postmenopausal women with osteoporotic fractures, determine its concentration by fracture site at the clinical setting, and compare the proportion of vitamin D deficiency with that reported in literature.
Methods
The prospective study included 317 postmenopausal women with osteoporotic fractures who were treated consecutively from 2016 to 2018. After obtaining informed consent for participation in the seamless treatment of osteoporosis against fractures study, which is our initiative to prevent secondary osteoporotic fractures, we registered the patients, examined bone mineral density (BMD) at the unfractured femoral neck and lumbar spine, serum 25-hydroxyvitamin D (25(OH)D) concentration, blood chemistry, and bone turnover markers.
Results
The mean age of the patients was 80.7 years. Moreover, 78% of patients of all fractures had 25(OH)D concentration < 20 ng/mL, whereas 12% of patients had 25(OH)D concentration ≥ 30 ng/mL 25(OH)D concentration in hip fractures was significantly lower than that in vertebral or distal radius fractures (P < 0.05). Multiple regression analysis revealed that 25(OH)D concentration is significantly associated with femoral neck BMD (β = 0.16; 95% confidence interval [CI], 0.78–12.17, P = 0.03) and serum albumin concentration (β = 0.21; 95% CI, 0.62–2.96, P < 0.001) in patients with 25(OH)D concentration < 30 ng/mL.
Conclusions
The results of this study show that the proportion of postmenopausal women with osteoporotic fractures who had vitamin D deficiency was higher than the proportion in previous reports that examined general postmenopausal women (35.2%–52.0%).
Keywords: 25-hydroxyvitamin D, Osteoporosis, Fracture, Postmenopausal women, Bone mineral density
1. Introduction
Vitamin D is a fat-soluble vitamin that is taken orally as a dietary supplement or produced in the skin when the skin is exposed to direct ultraviolet radiation from sunlight. Vitamin D is first hydroxylated in the liver, forming 25-hydroxyvitamin D (25(OH)D; calcidiol), and the 1α-position is subsequently hydroxylated in the kidney to form active 1,25-dihydroxyvitamin D (1,25(OH)2D; calcitriol). The serum 25(OH)D level is the most commonly used indicator of vitamin D supply by oral intake and ultraviolet-induced skin synthesis. Therefore, in the guidelines of many countries, the definitions of vitamin D deficiency and insufficiency are based on serum 25(OH)D concentrations [[1], [2], [3], [4], [5]]. Based on these foreign guidelines and domestic data, vitamin D sufficiency criteria using serum 25(OH)D concentrations have been established in Japan [5]. Serum 25(OH)D concentration of ≥ 30 ng/mL is a sufficient level of vitamin D; serum 25(OH)D concentration of < 30 ng/mL, but ≥ 20 ng/mL, is regarded as vitamin D insufficiency; and serum 25(OH)D concentration < 20 ng/mL is defined as vitamin D deficiency.
According to the longitudinal Aging Study Amsterdam, which involved 643 men and 676 women aged 65–88 years, 48.4% of the participants had serum vitamin D deficiency [6]. The cross-sectional, community-based epidemiological study in Japan revealed that, among 600 ambulatory postmenopausal women, 35.2% showed vitamin D deficiency and the mean serum 25(OH)D concentration was 22.2 ng/mL [7]. The longitudinal study in Japan revealed that, in 1470 postmenopausal women, 49.6% showed vitamin D deficiency [8]. The more recent Japanese epidemiological investigation demonstrated that, among 1211 community-dwelling women aged ≥ 50 years without early menopause or diseases affecting bone metabolism, 52.0% showed vitamin D deficiency [9]. In a Hong Kong osteoporosis study of 5276 participants (70% female) aged 20 years or above, 43.8% of participants showed vitamin D deficiency [10]. All 5 previous studies and the present one utilized the same definition of vitamin D deficiency.
It is reported that individuals with 25(OH)D concentration < 20 ng/mL showed higher prevalence of accumulated fractures in 15 years compared to those with 25(OH)D concentration ≥ 20 ng/mL and that individuals with 25(OH)D concentration < 10 ng/mL had 6.55 times higher fracture risk in 5 years than those with 25(OH)D concentration ≥ 30 ng/mL [7]. The measurement of serum 25(OH)D concentration is important for the evaluation of nutritional status and prediction of fracture risk.
There is a need for evidence regarding 25(OH)D concentrations in postmenopausal women with osteoporotic fractures to prevent subsequent osteoporotic fractures. We hypothesized that the proportion of vitamin D deficiency in postmenopausal women with osteoporotic fractures was greater than that previously reported in postmenopausal women. We consecutively registered patients with osteoporotic fractures and measured serum 25(OH)D concentration. We also investigated the association of 25(OH)D concentration with age, calcium, bone turnover markers, and bone mineral density (BMD) in the clinical setting.
2. Methods
2.1. Subjects and study design
This study was approved by the ethical review board of the University of Occupational and Environmental Health, Fukuoka, Japan (H27-190), and followed the Declaration of Helsinki. All patients agreed to participate in the study and provided written informed consent.
We have made efforts, such as the seamless treatment of osteoporosis against fractures (STOP-Fx) study, to prevent secondary osteoporotic fractures since October 2016. All patients were postmenopausal Japanese women with new osteoporotic fractures, including vertebral, hip, distal radius, and proximal humerus fractures, who were treated at our 6 nearby hospitals consecutively from October 2016 to February 2018. After obtaining informed consent from all participants in the STOP-Fx study, we registered the patients, interviewed them and their family regarding their history of fracture and residence (home, hospital, facility), and examined BMD in the unfractured femoral neck and lumbar spine, serum 25(OH)D concentration, blood chemistry, and bone turnover markers. Patients administered with agents such as supplemental vitamin D that could affect 25(OH)D concentration were excluded.
This prospective study included 317 postmenopausal women with osteoporotic fractures, including 132 new vertebral fractures, 121 hip fractures, 46 distal radius fractures, and 18 proximal humeral fractures. All fractures were caused by low impact falls, such as falls at standing height. The characteristics of all subjects are presented in Table 1. The mean age was 80.7 years (range, 45–101 years) for all patients, 80.1 years (range, 45–101 years) for those with vertebral fractures, 83.9 years (range, 49–100 years) for those with hip fractures, 76.1 years (range, 53–97 years) for those with distal radius fractures, and 75.6 years (range, 62–91 years) for those with proximal humerus fractures.
Table 1.
Patient characteristics.
| Characteristic | All | Vertebral fracture | Hip fracture | Distal radius fracture | Proximal humeral fracture | P-value |
|---|---|---|---|---|---|---|
| Number of patients | 317 | 132 | 121 | 46 | 18 | |
| Age, yr | 80.7 ± 0.54 | 80.1 ± 0.84 | 83.9 ± 0.77 | 76.1 ± 1.50 | 75.6 ± 2.07 | <0.001 |
| Albumin, g/dL | 3.94 ± 0.26 | 3.36 ± 0.06 | 3.36 ± 0.06 | 4.04 ± 0.06 | 3.61 ± 0.08 | 0.34 |
| Albumin-corrected calcium, mg/dL | 9.19 ± 0.03 | 9.22 ± 0.04 | 9.21 ± 0.07 | 9.19 ± 0.09 | 9.17 ± 0.14 | 0.63 |
| Phosphate, mg/dL | 3.37 ± 0.04 | 3.43 ± 0.04 | 3.32 ± 0.07 | 3.32 ± 0.08 | 3.25 ± 0.17 | 0.48 |
| 25-hydroxyvitamin D, ng/mL | 17.3 ± 0.82 | 16.4 ± 1.07 | 17.1 ± 1.45 | 19.5 ± 2.34 | 20.3 ± 3.90 | 0.51 |
| Tartrate-resistant acid phosphatase 5b, mU/dL | 391 ± 12.2 | 345 ± 15.0 | 449 ± 23.4 | 398 ± 29.9 | 310 ± 34.1 | <0.001 |
| Undercarboxylated osteocalcin, ng/mL | 8.41 ± 1.84 | 9.25 ± 4.00 | 9.05 ± 1.98 | 5.49 ± 0.71 | 4.14 ± 1.02 | 0.87 |
| Lumbar spine BMD, g/cm2 | 0.74 ± 0.01 | 0.71 ± 0.01 | 0.74 ± 0.02 | 0.78 ± 0.02 | 0.78 ± 0.03 | 0.07 |
| Femoral neck BMD, g/cm2 | 0.49 ± 0.01 | 0.49 ± 0.01 | 0.46 ± 0.01 | 0.57 ± 0.02 | 0.54 ± 0.03 | <0.001 |
Values are presented as mean ± standard error.
BMD, bone mineral density.
2.2. Serum 25(OH)D
Venous blood was obtained from all participants. Serum content was extracted using a routine centrifuge method and kept frozen at −70 °C. All serum samples were measured for 25(OH)D concentration, using the chemiluminescence immunoassay kit by LIAISON analyzer (25 OH vitamin D total assay, DiaSorin Liaison, Hitachi Chemical Diagnostics Systems Co., Ltd., Tokyo, Japan).
2.3. Serological indices
Serum albumin, calcium, phosphorus, tartrate-resistant acid phosphatase-5b (TRACP-5b), and undercarboxylated osteocalcin (ucOC) concentrations were measured.
The concentration of TRACP-5b, a bone resorption marker, was measured by a fragment-resorbing immunocapture enzyme assay, Osteolinks TRACP-5b kit (DS Pharma Biomedical Co., Ltd., Osaka, Japan); this assay can specifically measure TRACP-5b without causing a cross-reaction with TRACP-5a derived from macrophages. Serum ucOC was assayed using the Picolumi ucOC kit (Eisai Co., Ltd., Tokyo, Japan).
2.4. Bone mineral density
BMD of the lumbar spine (L1–4) and femoral neck were measured using Hologic Horizon (Hologic, Inc., Marlborough, MA, USA) with dual-energy X-ray absorption after registering of the STOP-Fx study. BMD of the femoral neck was measured at the unfractured side.
2.5. Statistical analysis
Results are expressed as means ± standard error. The differences in patient characteristics among the 4 fracture groups were evaluated using 1-way analysis of variance (ANOVA). The differences in 25(OH)D concentrations among the 4 groups were evaluated using 1-way ANOVA with a Tukey post hoc analysis and analysis of covariance (ANCOVA) with 25(OH)D as the dependent variable and age, albumin-corrected calcium, lumbar spine BMD, and femoral neck BMD as covariates in patients with 25(OH)D concentrations < 30 ng/mL (vitamin D insufficiency). Pearson correlation and multiple stepwise linear regression analyses were used to determine correlates of 25(OH)D concentration. The independent variables were age, BMD (lumbar spine and femoral neck, unfractured side), blood chemistry (albumin, albumin-corrected calcium, phosphate), and bone turnover markers (TRACP-5b, ucOC) for Pearson’s correlation analysis and age, BMD (lumbar spine and femoral neck, unfractured side), blood chemistry (albumin, albumin-corrected calcium, phosphate), bone turnover markers (TRACP-5b, ucOC), existing fragility fracture, fracture site (vertebral fracture, hip fracture, distal radius fracture, proximal humerus fracture), and residence (home, hospital, facility) for the multiple stepwise linear regression analysis. A P-value < 0.05 was considered significant. The analysis was performed using Excel version 2016 (Microsoft, Redmond, WA, USA) and IBM SPSS Statistics ver. 21.0 (IBM Japan, Tokyo, Japan).
3. Results
3.1. General patient characteristics
There were significant differences in age, TRACP-5b concentration, and femoral neck BMD among the 4 groups of fracture sites (P < 0.001) (Table 1). Patients with hip fractures had the greatest age, highest TRACP-5b concentration, and lowest femoral neck BMD among the 4 groups of fracture sites.
3.2. 25(OH)D concentration
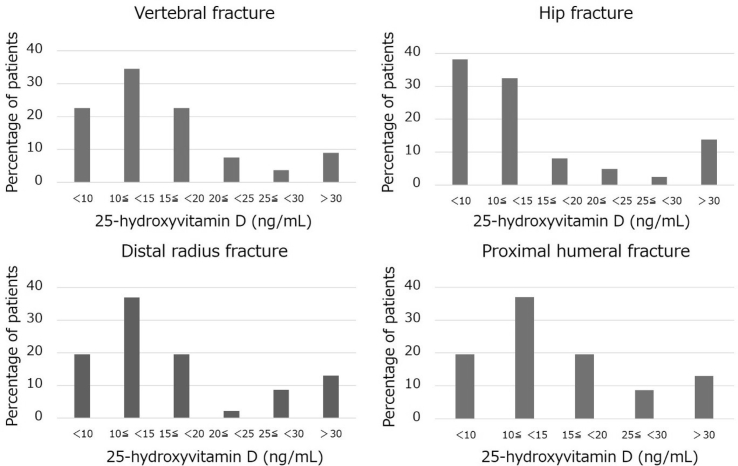
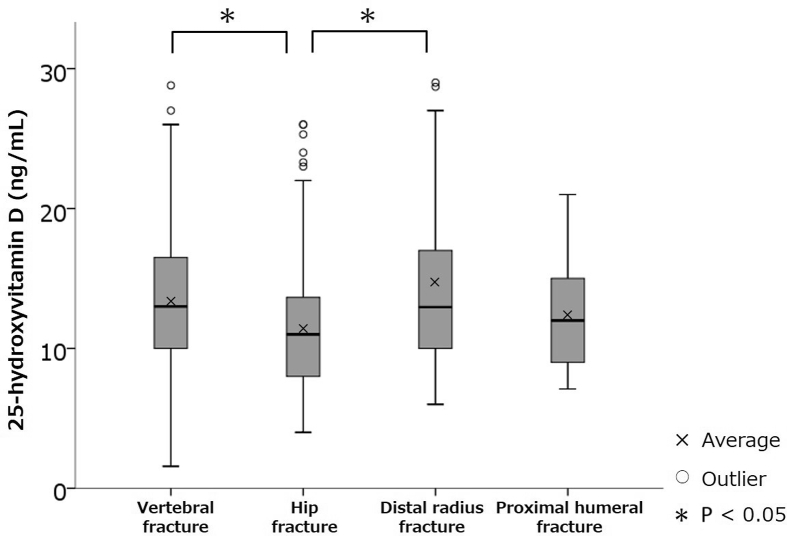
In this study, 78% of patients with 4 osteoporotic fractures including vertebral fractures, hip fractures, distal radial fractures, and proximal humeral fractures, 80% with vertebral fractures, 79% with hip fractures, 76% with distal radius fractures, and 72% with proximal humerus fractures had 25(OH)D concentration < 20 ng/mL (vitamin D deficiency), while 12% of patients with all fractures, 9% with vertebral fractures, 14% with hip fractures, 13% with distal radius fractures, and 22% with proximal humerus fractures had 25(OH)D concentration ≥ 30 ng/mL (vitamin D sufficiency) (Fig. 1). For patients with 25(OH)D concentrations < 30 ng/mL (vitamin D insufficiency), 1-way ANOVA with a Tukey post hoc analysis revealed that 25(OH)D concentrations in hip fractures were significantly lower than those in vertebral and distal radial fractures (P < 0.05) (Fig. 2). ANCOVA revealed that 25(OH)D concentrations in hip fractures were significantly lower than those in vertebral fractures (P < 0.05).
Fig. 1.
Percentage of patients by serum 25-hydroxyvitamin D concentration in each osteoporotic fracture site.
Fig. 2.
Comparison of 25-hydroxyvitamin D (25(OH)D) serum concentrations in each osteoporotic fracture site in patients with 25(OH)D concentrations < 30 ng/mL (vitamin D insufficiency).
3.3. Significant factors associated with 25(OH)D concentration
Simple linear regression and multiple linear stepwise regression analyses revealed that lower 25(OH)D concentration was significantly associated with higher serum albumin-corrected calcium concentration (P = 0.02 and P = 0.04, respectively) (Table 2).
Table 2.
Simple linear regression analysis and multiple linear stepwise regression analysis (n = 317).
| Variable | 25-hydroxyvitamin D (ng/mL) |
||||
|---|---|---|---|---|---|
| Simple linear regression analysis |
Multiple linear stepwise regression analysis |
||||
| R | P-value | β (95% CI) | t-value | P-value | |
| Age, yr | 0.00 | 0.50 | - | −0.10 | 0.92 |
| Lumbar spine BMD, g/cm2 | −0.04 | 0.30 | - | −0.53 | 0.60 |
| Femoral neck BMD, g/cm2 | −0.02 | 0.40 | - | −0.07 | 0.95 |
| Albumin, g/dL | 0.10 | 0.08 | - | 0.97 | 0.34 |
| Albumin-corrected calcium, mg/dL | −0.14 | 0.02 | −0.137 (−6.55 to −0.10) | −2.03 | 0.04 |
| Phosphate, mg/dL | −0.04 | 0.27 | - | −0.32 | 0.75 |
| Tartrate-resistant acid phosphatase 5b, mU/dL | 0.04 | 0.29 | - | 0.58 | 0.56 |
| Undercarboxylated osteocalcin, ng/mL | −0.09 | 0.10 | - | −1.15 | 0.25 |
| Existing fragility fracture | - | - | - | 0.06 | 0.96 |
| Fracture site | |||||
| Vertebral fracture | - | - | - | −0.29 | 0.77 |
| Hip fracture | - | - | - | −0.96 | 0.34 |
| Distal radius fracture | - | - | - | 1.08 | 0.28 |
| Proximal humerus fracture | - | - | - | 0.97 | 0.33 |
| Place of residence | |||||
| Home | - | - | - | −0.88 | 0.38 |
| Hospital | - | - | - | 0.56 | 0.57 |
| Facility | - | - | - | 0.67 | 0.51 |
R = correlation coefficient; β = standardized correlation coefficients (95% confidence interval [CI]); BMD, bone mineral density.
In patients with 25(OH)D concentration < 30 ng/mL (vitamin D deficiency and insufficiency), simple linear regression analysis revealed that lower 25(OH)D concentration was significantly associated with lower femoral neck BMD (P < 0.001) and lower serum albumin concentration (P < 0.001) (Table 3). Moreover, multiple linear stepwise regression analysis revealed that lower 25(OH)D concentration was significantly associated with lower femoral neck BMD (P = 0.03) and lower serum albumin concentration (P < 0.001) (Table 3).
Table 3.
Simple linear regression analysis and multiple linear stepwise regression analysis (25-hydroxyvitamin D < 30 ng/mL) (n = 279).
| Variable | 25-hydroxyvitamin D (ng/mL) |
||||
|---|---|---|---|---|---|
| Simple linear regression analysis |
Multiple linear stepwise regression analysis |
||||
| R | P-value | β (95% CI) | t-value | P-value | |
| Age, yr | −0.12 | 0.05 | - | 0.41 | 0.68 |
| Lumbar spine BMD, g/cm2 | 0.06 | 0.19 | - | −0.62 | 0.54 |
| Femoral neck BMD, g/cm2 | 0.20 | <0.001 | 0.16 (0.78–12.17) | 2.24 | 0.03 |
| Albumin, g/dL | 0.24 | <0.001 | 0.21 (0.62–2.96) | 3.01 | <0.001 |
| Albumin-corrected calcium, mg/dL | 0.04 | 0.29 | - | 1.21 | 0.23 |
| Phosphate, mg/dL | 0.07 | 0.15 | - | 0.98 | 0.33 |
| Tartrate-resistant acid phosphatase 5b, mU/dL | −0.15 | 0.02 | - | −1.37 | 0.17 |
| Undercarboxylated osteocalcin, ng/mL | −0.08 | 0.12 | - | −0.86 | 0.39 |
| Existing fragility fracture | - | - | - | −1.56 | 0.12 |
| Fracture site | |||||
| Vertebral fracture | - | - | - | 1.57 | 0.12 |
| Hip fracture | - | - | - | −1.67 | 0.10 |
| Distal radius fracture | - | - | - | 0.17 | 0.87 |
| Proximal humerus fracture | - | - | - | −0.32 | 0.75 |
| Place of residence | |||||
| Home | - | - | - | −0.27 | 0.79 |
| Hospital | - | - | - | −0.83 | 0.41 |
| Facility | - | - | - | 0.78 | 0.44 |
R = correlation coefficient; β = standardized correlation coefficients (95% confidence interval [CI]); BMD, bone mineral density.
4. Discussion
This study demonstrated that 78% of the Japanese postmenopausal women with osteoporotic fractures had vitamin D deficiency, while only 12% of them had vitamin D sufficiency. Lower serum 25(OH)D concentration is significantly associated with lower femoral neck BMD in patients with vitamin D deficiency or insufficiency. The percentage of vitamin D deficiency in Japanese postmenopausal women with osteoporotic fractures (78%) was higher than those in Japanese ambulatory postmenopausal women (35.2%) [7], Japanese postmenopausal women (49.6%) [8], and Japanese community-dwelling women aged ≥ 50 years without metabolic bone disease or premature menopause (52.0%) [9]. The percentage of vitamin D sufficiency in Japanese postmenopausal women with osteoporotic fractures (12%) was similar to that in Japanese community-dwelling women aged ≥ 50 years without early menopause or diseases affecting bone metabolism (10%) [9]. Thus, we can consider that vitamin D insufficiency in postmenopausal women leads to vitamin D deficiency, thus increasing fracture risks.
Most patients have serum vitamin D deficiency independent of osteoporotic fracture sites. Regarding the 4 sites of fractures, 25(OH)D concentration in patients with hip fractures was significantly lower than that in those with vertebral and distal radius fractures. Tanaka et al. [8] reported that serum 25(OH)D concentration < 25 ng/mL was significantly associated with long bone fractures (P < 0.01) and moderately associated with hip fractures (P = 0.07), but not associated with vertebral fractures (P = 0.88) in 1470 Japanese postmenopausal women. Bischoff-Ferrari et al. [11] reported that individuals with 25(OH)D concentration > 24 ng/mL had low risk of hip fractures (hazard ratio, 0.63; 95% confidence interval, 0.46–0.87) (P = 0.02) compared with those with 25(OH)D concentration < 12 ng/mL and aged > 65 years. It has been reported that when serum 25(OH)D concentration is high, the cortical bone of the femoral neck becomes thick and the medullary volume decreases [12]. The e-ADVANCED study compared the use of alendronate 35 mg/wk combined with eldecalcitol 0.75 μg/d (eldecalcitol is a strong analogue of vitamin D) and the use of plain vitamin D 400 IU/d combined with calcium 610 mg/d; the results of that study showed a significant increase in femoral neck BMD in the group receiving eldecalcitol. However, lumbar BMD did not significantly differ between the 2 groups [13]. The serum 25(OH)D concentration and exogenous application of vitamin D are closely associated with fractures, bone structure, and BMD in the femoral neck.
There was a significant difference in age among the 4 groups of fracture sites, while 25(OH)D concentration was not associated with age. Intestinal calcium absorption gradually decreased with age in women and men [14]. To maintain homeostasis of serum calcium, it is necessary to increase serum 25(OH)D concentration. In elderly individuals, increase in 25(OH)D concentration after sunlight exposure was slower compared to that in the young adults [15], and intestinal calcium absorption was increased after supplementation with active vitamin D or eldecalcitol, compared to supplementation with plain vitamin D [16]. Thus, in elderly individuals, it is difficult for 25(OH)D concentration to increase after sunlight exposure and to affect intestinal calcium absorption.
Miu and Lam [17] reported that 79% of patients with hip fractures are at risk of malnutrition, and serum albumin concentration significantly decreased in the malnourished group compared with that in the well-nourished group. Bohl et al. [18] reported that the prevalence of hypoalbuminemia (serum albumin concentration < 3.5 g/dL) was 45.9% in patients with geriatric hip fractures. Hypoalbuminemia is also a risk factor of vertebral fracture [19]. In this study, the average serum albumin concentration of vertebral and hip fractures was >3.5 g/dL, and it was considered that malnutrition may be involved in both fractures. However, 25(OH)D concentrations in hip fractures were significantly lower than that in vertebral fractures (P < 0.05). A decrease in vitamin D concentration in patients with hip fractures may be caused by lesser sunlight exposure time due to decline in activities of daily living, in addition to malnutrition.
The measurement of serum 25(OH)D concentration is greatly important, and it is essential to determine the status of dietary habits and lifestyle of patients, presume the response to bisphosphonate [20,21], and predict fracture risk [8,9,11]. Thus, we have to measure serum 25(OH)D concentration at the start of osteoporosis treatment. In individuals with a 25(OH)D concentration < 30 ng/mL, osteoid volume per bone volume, osteoid surface per bone surface, and osteoid thickness were increased [22]. Mineralization was impaired histologically in those with 25(OH)D concentration < 30 ng/mL. Bisphosphonate has affinity to the mineralized surface of the bone but does not have affinity to the osteoid surface. In this study, because approximately 88% of patients had 25(OH)D concentration < 30 ng/mL, they seem to show increased osteoid tissue (so-called osteoporomalacia) histologically. These patients with osteoporotic fractures must be treated with bisphosphonate together with or after improving calcium metabolic status appropriately.
This study had 2 limitations. First, we recruited patients throughout the year and did not consider seasonal variation. The serum 25(OH)D concentration varies seasonally [23]. Synthesis of vitamin D by the skin upon exposure to ultraviolet light directly affects serum 25(OH)D deficiency [24]. However, the difference in serum 25(OH)D concentration between summer and winter is only about 7 ng/mL [23], which is considered to have a small effect in this study. Second, physical performance was not routinely assessed in this study. There is a positive correlation between serum 25(OH)D concentration and body performance, such as grip strength and short physical performance battery (SPPB) [25,26]. In this study, we evaluated the residence at the time of injury, and it was considered that physical performance of the home residents was better than that of facility and hospital residents. However, the evaluation of physical performance was considered insufficient in the evaluation of only the place of residence.
5. Conclusions
In this study, 78% of the postmenopausal women with osteoporotic fractures showed serum vitamin D deficiency. The proportion of vitamin D deficiency in postmenopausal women with osteoporotic fractures was greater than that in postmenopausal women reported by previous literatures. Serum vitamin D concentration was significantly associated with femoral neck BMD in patients with vitamin D deficiency or insufficiency. We should be aware that almost all postmenopausal patients with osteoporotic fractures have vitamin D deficiency or insufficiency independent of the fracture site.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
We would like to thank all doctors and staff who contributed to the STOP-Fx study. We also would like to express our gratitude to Ms. Noriko Fukuda for managing the data. ORCID. Yoshiaki Yamanaka: 0000-0001-9382-0849. Kunitaka Menuki: 0000-0003-3985-3636. Yukichi Zenke: 0000-0002-5354-105X. Satoshi Ikeda: 0000-0001-9202-3290. Eiji Hatakeyama: 0000-0001-7616-6347. Kimiaki Kawano: 0000-0002-3656-3193. Satoshi Nishida: 0000-0002-5151-8104. Hiroaki Tanaka: 0000-0001-7737-3452. Keiichi Yumisashi: 0000-0002-3827-1434. Akinori Sakai: 0000-0002-6023-5536.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Cosman F., de Beur S.J., LeBoff M.S., Lewiecki E.M., Tanner B., Randall S. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dawson-Hughes B., Mithal A., Bonjour J.P., Boonen S., Burckhardt P., Fuleihan G.E. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21:1151–1154. doi: 10.1007/s00198-010-1285-3. [DOI] [PubMed] [Google Scholar]
- 3.Hanley D.A., Cranney A., Jones G., Whiting S.J., Leslie W.D., Cole D.E. Vitamin D in adult health and disease: a review and guideline statement from Osteoporosis Canada. CMAJ (Can Med Assoc J) 2010;182:E610–E618. doi: 10.1503/cmaj.080663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 5.Okazaki R., Ozono K., Fukumoto S., Inoue D., Yamauchi M., Minagawa M. Assessment criteria for vitamin D deficiency/insufficiency in Japan: proposal by an expert panel supported by the research program of intractable diseases, ministry of health, labour and welfare, Japan, the Japanese society for bone and mineral research and the Japan endocrine society [opinion] J Bone Miner Metab. 2017;35:1–5. doi: 10.1007/s00774-016-0805-4. [DOI] [PubMed] [Google Scholar]
- 6.Kuchuk N.O., Pluijm S.M., van Schoor N.M., Looman C.W., Smit J.H., Lips P. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab. 2009;94:1244–1250. doi: 10.1210/jc.2008-1832. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura K., Tsugawa N., Saito T., Ishikawa M., Tsuchiya Y., Hyodo K. Vitamin D status, bone mass, and bone metabolism in home-dwelling postmenopausal Japanese women: Yokogoshi Study. Bone. 2008;42:271–277. doi: 10.1016/j.bone.2007.09.056. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka S., Kuroda T., Yamazaki Y., Shiraki Y., Yoshimura N., Shiraki M. Serum 25-hydroxyvitamin D below 25 ng/mL is a risk factor for long bone fracture comparable to bone mineral density in Japanese postmenopausal women. J Bone Miner Metab. 2014;32:514–523. doi: 10.1007/s00774-013-0520-3. [DOI] [PubMed] [Google Scholar]
- 9.Tamaki J., Iki M., Sato Y., Kajita E., Nishino H., Akiba T. Total 25-hydroxyvitamin D levels predict fracture risk: results from the 15-year follow-up of the Japanese Population-based Osteoporosis (JPOS) Cohort Study. Osteoporos Int. 2017;28:1903–1913. doi: 10.1007/s00198-017-3967-6. [DOI] [PubMed] [Google Scholar]
- 10.Leung R.Y., Cheung B.M., Nguyen U.S., Kung A.W., Tan K.C., Cheung C.L. Optimal vitamin D status and its relationship with bone and mineral metabolism in Hong Kong Chinese. Bone. 2017;97:293–298. doi: 10.1016/j.bone.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 11.Bischoff-Ferrari H.A., Willett W.C., Orav E.J., Lips P., Meunier P.J., Lyons R.A. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367:40–49. doi: 10.1056/NEJMoa1109617. [DOI] [PubMed] [Google Scholar]
- 12.Martin E.N., Haney E.M., Shannon J., Cauley J.A., Ensrud K.E., Keaveny T.M. Femoral volumetric bone density, geometry, and strength in relation to 25-hydroxy vitamin D in older men. J Bone Miner Res. 2015;30:562–569. doi: 10.1002/jbmr.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakai A., Ito M., Tomomitsu T., Tsurukami H., Ikeda S., Fukuda F. Efficacy of combined treatment with alendronate (ALN) and eldecalcitol, a new active vitamin D analog, compared to that of concomitant ALN, vitamin D plus calcium treatment in Japanese patients with primary osteoporosis. Osteoporos Int. 2015;26:1193–1202. doi: 10.1007/s00198-014-2991-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bullamore J.R., Wilkinson R., Gallagher J.C., Nordin B.E., Marshall D.H. Effect of age on calcium absorption. Lancet. 1970;2:535–537. doi: 10.1016/s0140-6736(70)91344-9. [DOI] [PubMed] [Google Scholar]
- 15.Holick M.F., Matsuoka L.Y., Wortsman J. Age, vitamin D, and solar ultraviolet. Lancet. 1989;2:1104–1105. doi: 10.1016/s0140-6736(89)91124-0. [DOI] [PubMed] [Google Scholar]
- 16.Uenishi K., Tokiwa M., Kato S., Shiraki M. Stimulation of intestinal calcium absorption by orally administrated vitamin D3 compounds: a prospective open-label randomized trial in osteoporosis. Osteoporos Int. 2018;29:723–732. doi: 10.1007/s00198-017-4351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miu K.Y.D., Lam P.S. Effects of nutritional status on 6-month outcome of hip fractures in elderly patients. Ann Rehabil Med. 2017;41:1005–1012. doi: 10.5535/arm.2017.41.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bohl D.D., Shen M.R., Hannon C.P., Fillingham Y.A., Darrith B., Della Valle C.J. Serum albumin predicts survival and postoperative course following surgery for geriatric hip fracture. J Bone Joint Surg Am. 2017;99:2110–2118. doi: 10.2106/JBJS.16.01620. [DOI] [PubMed] [Google Scholar]
- 19.Finigan J., Greenfield D.M., Blumsohn A., Hannon R.A., Peel N.F., Jiang G. Risk factors for vertebral and nonvertebral fracture over 10 years: a population-based study in women. J Bone Miner Res. 2008;23:75–85. doi: 10.1359/jbmr.070814. [DOI] [PubMed] [Google Scholar]
- 20.Ishijima M., Sakamoto Y., Yamanaka M., Tokita A., Kitahara K., Kaneko H. Minimum required vitamin D level for optimal increase in bone mineral density with alendronate treatment in osteoporotic women. Calcif Tissue Int. 2009;85:398–404. doi: 10.1007/s00223-009-9295-x. [DOI] [PubMed] [Google Scholar]
- 21.Peris P., Martinez-Ferrer A., Monegal A., Martinez de Osaba M.J., Muxi A., Guanabens N. 25 hydroxyvitamin D serum levels influence adequate response to bisphosphonate treatment in postmenopausal osteoporosis. Bone. 2012;51:54–58. doi: 10.1016/j.bone.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 22.Priemel M., von Domarus C., Klatte T.O., Kessler S., Schlie J., Meier S. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res. 2010;25:305–312. doi: 10.1359/jbmr.090728. [DOI] [PubMed] [Google Scholar]
- 23.Rajakumar K., Holick M.F., Moore C.G., Cohen E., Olabopo F., Haralam M.A. Impact of seasonal flux on 25-hydroxyvitamin D and bone turnover in pre- and early pubertal youth. Pediatr Int. 2014;56:35–42. doi: 10.1111/ped.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bours P.H., Wielders J.P., Vermeijden J.R., van de Wiel A. Seasonal variation of serum 25-hydroxyvitamin D levels in adult patients with inflammatory bowel disease. Osteoporos Int. 2011;22:2857–2867. doi: 10.1007/s00198-010-1484-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houston D.K., Tooze J.A., Hausman D.B., Johnson M.A., Nicklas B.J., Miller M.E. Change in 25-hydroxyvitamin D and physical performance in older adults. J Gerontol A Biol Sci Med Sci. 2011;66:430–436. doi: 10.1093/gerona/glq235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalliokoski P., Bergqvist Y., Lofvander M. Physical performance and 25-hydroxyvitamin D: a cross-sectional study of pregnant Swedish and Somali immigrant women and new mothers. BMC Pregnancy Childbirth. 2013;13:237. doi: 10.1186/1471-2393-13-237. [DOI] [PMC free article] [PubMed] [Google Scholar]