Abstract
Objectives
The purpose of this study is to evaluate the efficacy of annual zoledronic acid treatment in Japanese patients with nonmetastatic prostate cancer during androgen deprivation therapy (ADT).
Methods
This is a single institution 12-month study. Between 2016 and 2019, patients aged 70 years or older on ADT for nonmetastatic prostate cancer had bone mineral density (BMD) measured and 10-year probability of fracture calculated using fracture risk assessment tool (FRAX). Patients who showed osteopenia or had a 10-year hip fracture risk ≥ 3% or a 10-year probability of major osteoporotic fracture ≥ 20% were offered treatment with zoledronic acid 5 mg intravenously (ZA group). The patients who did not receive treatment were set as the control group. Lumbar and hip BMD were measured 6 and 12 months after treatment in the ZA group and 12 months after baseline in the control group. The yearly BMD change of both groups was compared.
Results
The mean ages of the ZA group (n = 26) and control group (n = 12) were 80.5 ± 9.1 and 76.1 ± 6.7 years, respectively. In the ZA group, lumbar and hip BMD changes at 12 months were +2.1% and +0.8%, respectively. In the control group, lumbar and hip BMD changes were −0.9% and −4.9%, respectively. There were statistically significant differences between the 2 groups in BMD percent changes (P < 0.05).
Conclusions
Without intervention, BMD tends to continue to decrease during ADT. Our findings suggest that administration of zoledronic acid enables maintenance of BMD in the older adults.
Keywords: Androgen deprivation therapy, Bone mineral density, Osteoporosis, Prostate cancer, Zoledronic acid
1. Introduction
Prostate cancer (PCa) is the most commonly diagnosed male cancer in developed countries. In many cases, older patients diagnosed with PCa are treated with androgen deprivation therapy (ADT) [1]. Patients undergoing ADT for PCa are often at risk for age-related adverse events, such as fracture. Previous studies have indicated that ADT-associated bone loss occurs early in the course of ADT, and ranges from 2% to 4% [2,3]. Bone mineral density (BMD) reduction during ADT increases the risk of fracture and is an important health problem for older PCa patients [4,5].
The National Comprehensive Cancer Network guidelines for PCa states that patients on ADT are at greater risk for clinical fracture [6]. They recommend BMD measurement and fracture risk assessment tool (FRAX) calculation for osteoporosis screening according to the National Osteoporosis Foundation guidelines for the general population [7]. Treatment with either denosumab, zoledronic acid, or alendronate sodium is recommended when the absolute fracture risk warrants drug therapy [6]. The FRAX score developed by the World Health Organization (WHO) is a fracture risk assessment tool to predict the 10-year probability of hip and major fractures. It is a computer-based algorithm and the 10-year probability is calculated according to age, sex, BMD, and clinical risk factors [8]. We measured BMD and FRAX scores to evaluate the fracture risk of nonmetastatic PCa patients receiving ADT without intervention for osteoporosis. Additionally, we offered zoledronic acid treatment for the patients with risk of fracture.
Recent studies have shown efficacy of antiosteoporotic therapy, such as alendronate, pamidronate, risedronate, and zoledronic acid in maintaining or increasing BMD in patients on ADT [[9], [10], [11], [12]]. In particular, zoledronic acid can increase the BMD by once yearly intravenous administration [11]. We chose zoledronic acid for antiosteoporotic therapy to preserve BMD. We investigated the efficacy of zoledronic acid in preventing ADT-induced bone loss.
2. Methods
This is single institution prospective study. Between 2016 and 2019, we enrolled Japanese patients aged 70 years or older who were undergoing ADT for nonmetastatic PCa. All the patients were treated with a gonadotropin-releasing hormone (GnRH) analogue or GnRH plus bicalutamide or flutamide. None of the patients had been screened for osteoporosis. All the participants were identified histologically as having prostate adenocarcinoma. The participants were also confirmed to have no evidence of metastasis on radiographic imaging. Patients with chronic kidney stage 3 or higher, metabolic bone disease, or history of osteoporosis or osteopenia were excluded.
At the time of osteoporotic screening, lumbar and hip BMD were measured by dual-energy X-ray absorptiometry fan-beam bone densitometer (Lunar Prodigy; GE Healthcare, Chicago, IL, USA) and the 10-year fracture probability was calculated. The FRAX tool (available at http://www.shef.ac.uk/FRAX/tool.jsp?lang=en) was used to calculate the 10-year probabilities of major osteoporotic and hip fractures. Calculations were performed with each patient’s body mass index. The WHO BMD T-score criteria were used for classifying osteoporosis and osteopenia [13] (osteoporosis, T-score ≤ − 2.5; osteopenia, −2.5 < T-score < −1.0; and normal, T-score ≥ − 1.0). The T-score was calculated from a Japanese male reference database [14]. All patients with osteoporosis were considered to have secondary osteoporosis due to PCa and ADT. Treatment intervention was indicated for patients with a BMD T-score lower than −1.0. Patients with a 10-year probability of major osteoporotic fracture risk ≥ 20% or a hip fracture risk ≥ 3% were also offered treatment, regardless of BMD T-score. The patients who consented to treatment were administered zoledronic acid 5 mg intravenously.
The patients treated with zoledronic acid (ZA group) had lumbar and hip BMD measured at 6 and 12 months after treatment. Patients who did not receive zoledronic acid (control group) had their BMD measured at 12 months after screening.
The background of patients in both groups and the BMD changes between the 2 groups were evaluated by the Mann-Whitney U test. BMD change from baseline to 12 months was examined with the paired t-test with a 2-sided significance level set at P = 0.05.
This study was approved by the Institutional Review Boards at Toyohashi Municipal Hospital (TMH-1864). This study was conducted in accordance with the 1964 Declaration of Helsinki for research involving human subjects. Informed consent was obtained from all the participants before BMD screening and treatment intervention.
3. Results
Sixty patients had their BMD measured and FRAX score calculated. Thirty-three patients (55%) had osteopenia or osteoporosis (BMD T-score < −1.0). The 10-year major osteoporotic fracture rate of 14 patients (23%) exceeded 20%. The 10-year hip fracture rate of 58 patients (97%) exceeded 3%. After osteoporotic screening, 26 patients agreed to receive zoledronic acid (ZA group). Among them, 20 patients completed BMD measurements 12 months after intervention. Twelve patients in the control group had BMD measurements 12 months after screening. The characteristics of the patients are shown in Table 1. There were significant differences between the groups in age, baseline BMD, and FRAX score. The ZA group was older than the control group and had lower baseline BMDs and higher FRAX scores.
Table 1.
Characteristics of patients at the time of osteoporotic screening.
| Characteristic | ZA group (n = 26) | Control (n = 12) | P-value |
|---|---|---|---|
| Age, yr | 80.5 ± 9.1 | 76.1 ± 6.7 | <0.05 |
| Body mass index, kg/m2 | 23.8 ± 3.5 | 23.4 ± 3.9 | NS |
| PSA level during intervention, ng/mL | 0.005 ± 0.21 | 0.004 ± 0.21 | NS |
| Duration of ADT, mo | 40.0 ± 5.5 | 40.5 ± 5.5 | NS |
| Serum corrected Calcium, mg/dL | 9.5 ± 0.20 | 9.4 ± 0.25 | NS |
| FRAX 10-year MOF rate, % | 16.0 ± 6.9 | 10.5 ± 6.5 | <0.05 |
| FRAX 10-year HF rate, % | 10.0 ± 5.7 | 4.5 ± 4.0 | <0.05 |
| Serum creatinine, mg/mL | 0.92 ± 0.3 | 0.94 ± 0.3 | NS |
| Lumbar BMD, g/cm2 | 1.034 ± 0.197 | 1.2385 ± 0.192 | <0.05 |
| Hip BMD, g/cm2 | 0.732 ± 0.245 | 0.942 ± 0.206 | <0.05 |
Values are presented as mean ± standard deviation.
ZA, zoledronic acid 5 mg intravenously; PSA, prostate-specific antigen; ADT, androgen deprivation therapy; FRAX, fracture risk assessment tool; MOF, major osteoporotic fracture; HF, hip fracture; BMD, bone mineral density; NS, not significant.
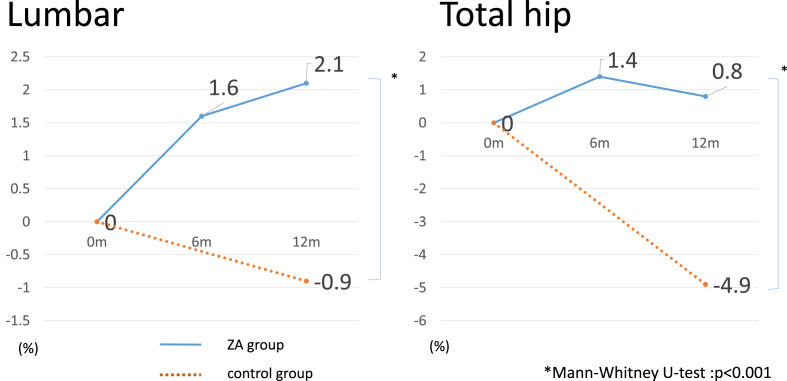
Table 2 shows changes in BMD 1 year after administration of zoledronic acid. The lumbar BMD of the ZA group increased by 1.60% (P < 0.01) from baseline to 6 months after treatment, and increased by 2.10% at 12 months after treatment (P < 0.01). The lumbar BMD of the control group had decreased by 0.85% 12 months after baseline. The hip BMD of the ZA group increased by 1.41% (P = 0.03) at 6 months, and by 0.82% (P = 0.07) at 12 months. The hip BMD of the control group decreased by 4.9% (P = 0.001) at 12 months. There was a statistically significant difference between the groups in the percent change in BMD at 12 months (Fig. 1).
Table 2.
BMD change from baseline.
| Group | Location of measurement | Baseline | 6 m | 12 m |
|---|---|---|---|---|
| ZA (n = 20) | Lumbar BMD, g/cm2 | 1.021 ± 0.203 | 1.050 ± 0.202* | 1.057 ± 0.213* |
| Change from baseline, % | 1.60 | 2.10 | ||
| Total hip BMD, g/cm2 | 0.740 ± 0.136 | 0.743 ± 0.137* | 0.738 ± 0.156 | |
| Change from baseline, % | 1.41 | 0.82 | ||
| Control (n = 12) | Lumbar BMD, g/cm2 | 1.141 ± 0.228 | – | 1.101 ± 0.249 |
| Change from baseline, % | −0.85 | |||
| Total hip BMD, g/cm2 | 0.979 ± 0.146 | – | 0.880 ± 0.154* | |
| Change from baseline, % | −4.90 |
Values are presented as mean ± standard deviation.
BMD, bone mineral density; ZA, zoledronic acid 5 mg intravenously.
Paired t-test. *P < 0.05.
Fig. 1.
Bone mineral density (BMD) % change from baseline. The BMD change rate after 1 year was statistically higher in the ZA group than the control group. ZA, zoledronic acid 5 mg intravenously.
4. Discussion
Although many studies have examined the BMD improvements with zoledronic acid during ADT, there are few reports specific to older Japanese patients with PCa. To our knowledge, this is the first report which examined the efficacy of zoledronic acid for nonmetastatic PCa patients receiving ADT. Previous studies have reported that BMD decreases by 2%–8% in the first year after ADT initiation [3,4]. However, there are no specific criteria for intervention methods or treatment for osteoporosis during ADT.
It is thought that the BMD decreases with a longer treatment period. In our institution, we calculate the fracture probability before initiating ADT and evaluate the BMD every 6–12 months for patients undergoing ADT. In the present study, the control group continued ADT for 40 months, and the BMD decreased several percents per year. Furthermore, it is clear that aging is a risk for BMD loss, and we believe that older patients need active intervention for osteoporosis. We therefore consider that all men undergoing ADT for PCa should be assessed for risk of fractures. However, the optimal method and timing of osteoporosis intervention during ADT is unclear. In this study, BMD and FRAX scores were measured to screen for osteoporosis during ADT. The FRAX score was calculated as the patients undergoing ADT were considered to have secondary osteoporosis. In this study, we set the cutoff point for intervention as a 20% risk of a major osteoporotic fracture or a 3% risk of a hip fracture, according to the American National Osteoporosis Foundation Clinician’s Guide to Prevention and Treatment of Osteoporosis [7]. Notably, almost all patients had a 10-year hip fracture risk which met treatment criteria. This tendency has been reported previously [15,16]. However, whether FRAX-derived fracture probability fits ADT risk has not been established. Further, the intervention criteria for patients with normal BMD but high fracture risk are unclear.
The ZA group patients were older and had higher FRAX scores and lower baseline BMDs than the control group. Direct comparison between the 2 groups may be difficult due to these differences. However, it is well known that older age is associated with a higher fracture probability [16]. Further, many studies have shown no correlation between baseline BMD and response to osteoporosis treatments such as zoledronate and denosumab [[16], [17], [18]]. Moreover, since aging is a factor in BMD loss, the ZA group seems to have a higher risk of BMD loss compared to the control group. Improvement of lumbar BMD and maintenance of hip BMD in the ZA group are considered to have been gained from zoledronic acid administration. We have continued to administer zoledronic acid to the patients who have shown positive effects with this treatment.
The efficacy of zoledronic acid against osteoporosis has been demonstrated in a number of studies [11,17,19]. They reported BMD increase rates of several percent per year. The BMD change of the ZA group in this study was slightly lower than the rates seen in these earlier reports. It may be harder to increase BMD in older patients. This supports the importance of maintaining BMD in older patients by early intervention.
Our study has some limitations, including small sample size and non-randomization. Bone turnover markers were not evaluated in all the participants, and it was not possible to compare these markers between the 2 groups before treatment.
5. Conclusions
Annual intravenous administration of zoledronic acid was useful in BMD maintenance for older patients undergoing ADT for nonmetastatic PCa. Osteoporotic screening should be conducted for all patients undergoing ADT treatment and interventions should be offered as early as possible. Further studies are needed to determine the optimal use of FRAX as a screening tool in this population.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
ORCID. Ippei Kojima: 0000-0001-5536-8935. Yushi Naito: 0000-0001-6256-4352. Akiyuki Yamamoto: 0000-0002-1391-8772. Yasuhiro Terashima: 0000-0001-7712-1220. Norie Sho: 0000-0002-8039-186X. Jun Nagayama: 0000-0001-6239-2917. Yurika Okada: 0000-0003-3269-0889. Tatsuya Nagai: 0000-0002-6203-8448.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Onozawa M, Hinotsu S, Tsukamoto T, Oya M, Ogawa O, Kitamura T, et al. Recent trends in the initial therapy for newly diagnosed prostate cancer in Japan. Jpn J Clin Oncol 201;44:969–981. [DOI] [PubMed]
- 2.Bergström I., Gustafsson H., Sjöberg K., Arver S. Changes in bone mineral density differ between gonadotrophin-releasing hormone analogue- and surgically castrated men with prostate cancer–a prospective, controlled, parallel-group study. Scand J Urol Nephrol. 2004;38:148–152. doi: 10.1080/00365590310018810. [DOI] [PubMed] [Google Scholar]
- 3.Berruti A., Dogliotti L., Terrone C., Cerutti S., Isaia G., Tarabuzzi R. Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol. 2002;167:2361–2367. [PubMed] [Google Scholar]
- 4.Daniell H.W., Dunn S.R., Ferguson D.W., Lomas G., Niazi Z., Stratte P.T. Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J Urol. 2000;163:181–186. [PubMed] [Google Scholar]
- 5.Cheung A.S., Zajac J.D., Grossmann M. Muscle and bone effects of androgen deprivation therapy: current and emerging therapies. Endocr Relat Cancer. 2014;21:R371–R394. doi: 10.1530/ERC-14-0172. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network NCCN guideline on prostate cancer version 4. 2019 [internet]. Fort wathington (PA): national comprehensive cancer Network; c2018 [cited 2019 Jul 1] https://www.nccn.org/professionals/physician_gls/default.asp Available from:
- 7.Dawson-Hughes B., Tosteson A.N., Melton L.J., 3rd, Baim S., Favus M.J., Khosla S. Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int. 2008;19:449–458. doi: 10.1007/s00198-008-0559-5. [DOI] [PubMed] [Google Scholar]
- 8.Kanis J.A., Johnell O., Oden A., Johansson H., McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufman J.M., Reginster J.Y., Boonen S., Brandi M.L., Cooper C., Dere W. Treatment of osteoporosis in men. Bone. 2013;53:134–144. doi: 10.1016/j.bone.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orwoll E., Ettinger M., Weiss S., Miller P., Kendler D., Graham J. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604–610. doi: 10.1056/NEJM200008313430902. [DOI] [PubMed] [Google Scholar]
- 11.Boonen S., Reginster J.Y., Kaufman J.M., Lippuner K., Zanchetta J., Langdahl B. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med. 2012;367:1714–1723. doi: 10.1056/NEJMoa1204061. [DOI] [PubMed] [Google Scholar]
- 12.Orwoll E.S., Scheele W.H., Paul S., Adami S., Syversen U., Diez-Perez A. The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003;18:9–17. doi: 10.1359/jbmr.2003.18.1.9. [DOI] [PubMed] [Google Scholar]
- 13.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis . Vol. 843. World Health Organ Tech Rep Ser; 1994. Report of a WHO Study Group; pp. 1–129. [PubMed] [Google Scholar]
- 14.Orimo H., Hayashi Y., Fukunaga M., Sone T., Fujiwara S., Shiraki M. Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab. 2001;19:331–337. doi: 10.1007/s007740170001. [DOI] [PubMed] [Google Scholar]
- 15.James H., 3rd, Aleksic I., Bienz M.N., Pieczonka C., Iannotta P., Albala D. Comparison of fracture risk assessment tool score to bone mineral density for estimating fracture risk in patients with advanced prostate cancer on androgen deprivation therapy. Urology. 2014;84:164–168. doi: 10.1016/j.urology.2013.12.071. [DOI] [PubMed] [Google Scholar]
- 16.Kanis J.A., McCloskey E., Johansson H., Oden A., Leslie W.D. FRAX(®) with and without bone mineral density. Calcif Tissue Int. 2012;90:1–13. doi: 10.1007/s00223-011-9544-7. [DOI] [PubMed] [Google Scholar]
- 17.Eastell R., Black D.M., Boonen S., Adami S., Felsenberg D., Lippuner K. Effect of once-yearly zoledronic acid five milligrams on fracture risk and change in femoral neck bone mineral density. J Clin Endocrinol Metab. 2009;94:3215–3225. doi: 10.1210/jc.2008-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCloskey E.V., Johansson H., Oden A., Austin M., Siris E., Wang A. Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J Bone Miner Res. 2012;27:1480–1486. doi: 10.1002/jbmr.1606. [DOI] [PubMed] [Google Scholar]
- 19.Black D.M., Delmas P.D., Eastell R., Reid I.R., Boonen S., Cauley J.A. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]