Abstract
Improving real‐life functioning is the main goal of the most advanced integrated treatment programs in people with schizophrenia. The Italian Network for Research on Psychoses previously explored, by using network analysis, the interplay among illness‐related variables, personal resources, context‐related factors and real‐life functioning in a large sample of patients with schizophrenia. The same research network has now completed a 4‐year follow‐up of the original sample. In the present study, we used network analysis to test whether the pattern of relationships among all variables investigated at baseline was similar at follow‐up. In addition, we compared the network structure of patients who were classified as recovered at follow‐up versus those who did not recover. Six hundred eighteen subjects recruited at baseline could be assessed in the follow‐up study. The network structure did not change significantly from baseline to follow‐up, and the overall strength of the connections among variables increased slightly, but not significantly. Functional capacity and everyday life skills had a high betweenness and closeness in the network at follow‐up, as they had at baseline, while psychopathological variables remained more peripheral. The network structure and connectivity of non‐recovered patients were similar to those observed in the whole sample, but very different from those in recovered subjects, in which we found few connections only. These data strongly suggest that tightly coupled symptoms/dysfunctions tend to maintain each other's activation, contributing to poor outcome in schizophrenia. Early and integrated treatment plans, targeting variables with high centrality, might prevent the emergence of self‐reinforcing networks of symptoms and dysfunctions in people with schizophrenia.
Keywords: Schizophrenia, network analysis, real‐life functioning, psychopathology, personal resources, internalized stigma, recovery, functional capacity, everyday life skills
Improving real‐life functioning is the main goal of the most advanced integrated treatment programs in people with schizophrenia1, 2, 3, 4. Research has clarified that real‐life functioning in these people does not depend exclusively on psychopathology, but is influenced by a range of variables, some of which are illness‐related, while others are relevant to the personal resources of the individual, or are context‐related5, 6, 7, 8.
In order to advance knowledge on the relative impact of the above variables on real‐life functioning in people with schizophrenia, the Italian Network for Research on Psychoses carried out a large multicenter study involving 921 community‐dwelling, clinically stable patients with that diagnosis9, 10. That study (from here on referred to as the baseline study) assessed a larger number of variables as compared with all previous relevant investigations, some of them never explored before.
The interplay of 27 variables concerning the illness, personal resources, social context and real‐life functioning was investigated using network analysis. This analytical approach makes it possible to interpret the correlations among a large number of variables by providing a clear‐cut picture of the relevant links. Moreover, it provides useful insights about the most central variables in the network, which may inform clinicians about possible therapeutic targets.
In our baseline study, functional capacity and everyday life skills were the most central and interconnected nodes of the network, while psychopathological variables were more peripheral10. Social cognition, neurocognition, resilience, and the three domains of real‐life functioning of interest for community dwelling people with schizophrenia (work skills, interpersonal relationships and everyday life skills) formed highly interconnected, spatially contiguous clusters.
The Italian Network for Research on Psychoses has now completed a 4‐year follow‐up of the original sample. In the present study, we tested whether the pattern of relationships among illness‐related variables, personal resources, context‐related factors and real‐life functioning was similar at follow‐up versus baseline in patients assessed at both waves.
In addition, we aimed to compare the network structure of patients who achieved recovery at follow‐up versus those who did not recover. Based on the few previous reports on changes in network structures in remitted versus non‐remitted subjects with various diagnoses, covering psychopathological but not functional variables11, 12, 13, we expected a less interconnected network structure in recovered than in non‐recovered subjects.
METHODS
Participants
All 921 patients recruited for the baseline study by the 26 Italian university psychiatric clinics and/or mental health departments participating in the baseline study9 were asked to join the follow‐up study. Subjects were contacted by phone, e‐mail or during a routine follow‐up visit or rehabilitation session.
The inclusion criterion was a diagnosis of schizophrenia according to DSM‐IV, confirmed by the Structured Clinical Interview for DSM‐IV ‐ Patient version (SCID‐I‐P)14. Exclusion criteria were: a history of head trauma with loss of consciousness in the 4‐year interval between baseline and follow‐up; progressive cognitive deterioration possibly due to dementia or other neurological illness diagnosed in the last 4 years; a history of alcohol and/or substance abuse in the last 6 months; current pregnancy or lactation; inability to provide an informed consent; treatment modifications and/or hospitalization due to symptom exacerbation in the last 3 months.
When participants in the baseline study could not be traced or were deceased, investigators were asked to fill in an ad hoc form reporting clinical information available at the last contact and, in the relevant cases, the cause of death.
All patients were asked to sign a written informed consent to participate, after receiving a comprehensive explanation of the study procedures and goals. Approval of the study protocol was obtained from the Local Ethics Committees of the participating centers. Recruitment took place from March 2016 to December 2017.
Procedures
Enrolled patients completed the assessments in three days, with the following schedule: on day 1, in the morning, collection of socio‐demographic information, psychopathological evaluation and neurological assessment; on day 2, in the morning, assessment of neurocognitive functions, social cognition and functional capacity; on day 3 (morning or afternoon) or in the afternoon of day 1 or 2, according to the patient's preference, assessment of personal resources and perceived stigma. For real‐life functioning assessment, patient's key caregiver was invited to join one of the scheduled sessions.
Assessment tools
Illness‐related factors
With the support of all available sources of information (patients, relatives, medical records and mental health workers), a clinical form was filled in with data on disease course and treatments in the previous 4 years.
The Positive and Negative Syndrome Scale (PANSS)15 was used to assess symptom severity. In line with the baseline study10, the scores for the dimension “positive symptoms” were calculated based on the consensus 5‐factor solution proposed by Wallwork et al16. “Disorganization” was the PANSS item P2, to avoid overlap with cognitive impairment. Negative symptoms were assessed using the Brief Negative Symptom Scale (BNSS)17, which includes five negative symptom domains: anhedonia, asociality, avolition, blunted affect and alogia; for the purpose of the present paper, as already done in our previous network analysis10, we used two factors: “expressive deficit” (sum of the subscales blunted affect and alogia) and “avolition” (sum of the subscales anhedonia, asociality and avolition).
Depressive symptoms were evaluated using the Calgary Depression Scale for Schizophrenia (CDSS)18. Extrapyramidal symptoms were assessed by means of the St. Hans Rating Scale (SHRS)19, a multidimensional rating scale consisting of four subscales: hyperkinesia, parkinsonism, akathisia and dystonia.
Neurocognitive functions were rated using the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB)20, 21. This battery includes tests for the assessment of seven cognitive domains: processing speed, attention/vigilance, working memory, verbal learning, visual learning, social cognition, and reasoning and problem solving.
The assessment of social cognition, partly included in the managing emotion section of the MCCB Mayer‐Salovey‐Caruso Emotional Intelligence Test (MSCEIT), was integrated by the Facial Emotion Identification Test (FEIT)22 and The Awareness of Social Inference Test (TASIT)23, which includes three sections (TASIT 1‐3), exploring emotion recognition (TASIT 1) and theory of mind (TASIT 2 and 3).
Personal resources
Resilience was evaluated by the Resilience Scale for Adults (RSA)24, a self‐administered scale including 33 items that examine intra‐ and inter‐personal protective factors thought to facilitate adaptation when facing psychosocial adversity. As described in Galderisi et al9, to avoid overlap with other measures, only the factors “perception of self” , “perception of the future” , “social competence” and “family cohesion” were included in the analysis.
The Service Engagement Scale (SES)25, an instrument including 14 items, rated on a 4‐point Likert scale (with higher scores reflecting greater levels of difficulty engaging with services), was used to assess patient's availability, cooperation, help‐seeking and treatment attitude. In the present paper, we used the total score.
Context‐related factors
The availability of a disability pension, access to family practical and financial support, and registration in the unemployment list were recorded as a count variable, ranging from 0 to 4.
The Internalized Stigma of Mental Illness (ISMI)26 was used to evaluate the experience of stigma and internalized self‐rejection.
Functional capacity and real‐life functioning
The short version of the University of California San Diego (UCSD) Performance‐based Skills Assessment Brief (UPSA‐B)27, a performance‐based instrument that assesses “financial skills” (e.g., counting money and paying bills) and “communication skills” (e.g., to dial a telephone number for emergency or reschedule an appointment by telephone) was used to assess functional capacity.
Real‐life functioning was assessed by the Specific Level of Functioning Scale (SLOF)28, a hybrid instrument that explores many aspects of functioning and is based on the key caregiver's judgment on patient's behavior and functioning. SLOF “interpersonal relationships” , “everyday life skills” and “work skills” domains were included in statistical analyses. The SLOF was administered to the key caregiver, i.e. the person most frequently and closely in contact with the patient.
Training of researchers
A centralized training of researchers was conducted two months before starting the follow‐up recruitment, to ensure comparability of data collection procedures.
For each category of variables (illness‐related factors, personal resources and context‐related factors), at least one researcher per site was trained. In order to avoid halo effects, the same researcher could not be trained for more than one category.
The inter‐rater reliability was formally evaluated by Cohen's kappa for categorical variables, and intraclass correlation coefficient (ICC) for continuous variables. For items showing a small degree of variation among patients (whose ICC would not be meaningful, since it is based on a ratio of between‐ and within‐patient variation), the percentage of perfect agreement was calculated as an alternative expression of inter‐rater reliability.
An excellent inter‐rater agreement was found for the SCID‐I‐P (Cohen's kappa=0.91). Good to excellent agreement among raters was observed for SLOF (ICC=0.58‐1.00, percentage agreement = 70‐100%), BNSS (ICC=0.74‐0.97), PANSS (ICC=0.60‐0.98, percentage agreement = 64‐100%), CDSS (ICC=0.76‐0.98) and MCCB (ICC=0.98).
Statistical analyses
Patients who participated in the 4‐year follow‐up were compared with those who did not participate on gender, age, education, and on the 27 baseline variables related to illness, personal resources, context and real‐life functioning, to determine whether they were representative of the original sample9. Between‐group comparisons were performed using the X2 test, the t‐test or Mann‐Whitney test, depending on the type of measurement and the distribution of variables. Bonferroni‐Holm correction was applied to comparisons of scale scores to control for type‐I error inflation.
To ensure pairwise comparability of baseline and follow‐up data of patients assessed at each time point, missing data were imputed using an expectation‐maximization algorithm, assuming that the pattern of missing data was random. This assumption allows estimates to be adjusted using available information. Overall, 201 values (1.1%) were imputed at baseline and 756 values (4.1%) at follow‐up. Within‐subject comparisons at baseline and follow‐up were conducted using the paired‐sample t‐test, Wilcoxon test or McNemar's test.
Patients were classified as recovered or non‐recovered at the 4‐year follow‐up according to two criteria: one based on the presence or absence of symptomatic remission according to Andreasen et al (severity criterion)29, and the other based on the presence or absence of functional recovery, defined as a weighted score of at least 76.2 on SLOF “interpersonal relationships” , “work skills” and “everyday life skills” scales. This latter cut‐off was identified through a preliminary receiver operating characteristic (ROC) analysis on Galderisi et al's sample9 using a Personal and Social Performance (PSP) score ≥71 as the gold standard30. That cut‐off identified patients with vs. without functional recovery with a sensitivity of 86.9%, a specificity of 68.5%, and an area under the curve of 0.84.
We then compared the pattern of relationships among study variables at baseline and follow‐up in the overall study population, and between recovered and non‐recovered patients at follow‐up, using network analysis.
A network is a graphical representation that includes nodes (variables) and edges (correlations among variables). The network structure of the 27 study variables at baseline and follow‐up was estimated using the statistical package JASP, version 0.10.2 (https://jasp-stats.org/). A non‐paranormal transformation was performed prior to the analysis to relax the normality assumption, because variables were not normally distributed31. The least absolute shrinkage and selection operator (LASSO)32 was used to reduce the number of false‐positive edges and to improve the interpretability of the network. This procedure applies a penalty to small edges by setting them to zero. The shrinkage parameter that optimized the number of edges was selected by minimizing the extended Bayesian information criterion (EBIC) parameter33.
The location of nodes was based on the Fruchterman‐Reingold algorithm34, that places nodes with stronger or more connections close to each other and nodes with weaker connections at the periphery of the network. We constrained the layout of the networks to be the same at baseline and follow‐up to facilitate visual comparison of the edges at the two time points. Three centrality indices of the network were calculated for all variables at baseline and follow‐up. Strength or degree centrality is the sum of the absolute values of the edges of a given node to other nodes. The two other centrality measures are betweenness, i.e. the number of times a node lies on the shortest path length between any two other nodes, and closeness, that indicates how easy it is to reach all other nodes from the node of interest. Centrality measures were standardized to facilitate comparisons.
The robustness of the network solution was assessed by estimating the accuracy of edge weights and the stability of centrality indices using non‐parametric bootstrapping procedures described by Epskamp et al35. Specifically, the accuracy of edge weights was measured by the 95% confidence intervals (CIs) obtained from 1,000 bootstrap samples drawn from the study population: the narrower the CI, the more accurate is the estimate of the edge weights. We also evaluated the stability of the centrality indices by using the node‐dropping subset bootstrap35. To this purpose, we randomly sampled a network of 26 nodes 1,000 times and repeated the procedure for networks between 25 and 2 nodes. We then estimated the mean node strength of each variable for all subset networks, to determine the extent to which the network was robust to the exclusion of some nodes.
To further examine the robustness of our findings, we compared the standard deviations (SDs) of each variable included in the networks between the two time points by means of Levene's test. If SDs change significantly, differences in the network structure might be a result of increased variation over time.
Differences in network structure and global strength between and within subjects were tested for significance using the M‐test and the S‐test included in the R‐package network comparison test (NCT), which uses permutation testing to compare networks36. The paired‐sample option was used to compare the same group at baseline and follow‐up, and independent‐sample comparisons were used when two groups were compared at the same time point.
RESULTS
Characteristics of participants
Twenty‐four out of the 26 Italian university psychiatric clinics and/or mental health departments who had contributed to the baseline study participated in the follow‐up. The two remaining centers could not join the follow‐up study due to changes in their organization. Six hundred eighteen subjects out of the 921 recruited at baseline were included in the follow‐up study.
Twenty‐four patients had been recruited at the two sites that did not participate in the follow‐up study; 19 had deceased; 10 could not be traced; 98 refused to participate; 75 were now being followed by a different psychiatrist or mental health department; 36 had changed residence and reported logistic difficulties to join the study; 24 were clinically unstable and/or had recently changed pharmacological treatment; 4 showed a significant cognitive decline, possibly due to dementia; 2 reported substance abuse in the past 6 months. In the remaining 11 individuals, reasons for not participating were not specified.
Patients who participated in the 4‐year follow‐up did not differ significantly from the rest of the sample (N=303) on baseline socio‐demographic characteristics, illness‐related variables and context‐related factors. However, follow‐up participants had significantly higher scores (i.e., better functioning) on two SLOF scales (“interpersonal relationships”: 22.8±5.9 vs. 21.3±6.3, t=3.51, p<0.001; “work skills”: 20.4±6.0 vs. 19.2±6.5, t=2.68, p=0.008) and a higher engagement with mental health services (12.2±7.5 vs. 14.4±7.9, t=–3.98, p<0.001). These mean differences in scale scores were relatively small and not clinically relevant; thus, the 618 patients participating in the follow‐up study can be considered representative of the original sample.
Socio‐demographic and clinical characteristics of the 618 patients at follow‐up are reported in Table 1. They were predominantly males (69.1%), with a mean age of 45.1 years and an average of 11.7 years of education. A moderate increase from baseline was found in the percentage of subjects with a job (from 29.2% to 34.4%; McNemar's test = 11.4, p=0.001) and with a stable affective relationship (from 14.9% to 18.9%, McNemar's test = 7.7, p=0.006).
Table 1.
Socio‐demographic and clinical variables at follow‐up (N=618)
| Gender (% males) | 69.1 |
| Age (years, mean±SD) | 45.1±10.5 |
| Married (%) | 7.4 |
| Working (%) | 34.4 |
| Education (years, mean±SD) | 11.7±3.4 |
| Stable affective relationships (%) | 18.9 |
| Current drug treatment | |
| First‐generation antipsychotics (%) | 13.1 |
| Second‐generation antipsychotics (%) | 69.3 |
| Both first‐ and second‐generation antipsychotics (%) | 15.0 |
| Antidepressants (%) | 17.6 |
| Mood stabilizers (%) | 26.0 |
| Anxiolytics (%) | 32.7 |
| Anticholinergics (%) | 9.4 |
| Polypharmacy (%) | 54.4 |
| Any psychosocial interventions (%) | 34.3 |
| Psychoeducation (%) | 3.4 |
| Cognitive training (%) | 7.9 |
| Social skills training (%) | 3.6 |
| Vocational training (%) | 4.2 |
| Leisure time activities (%) | 17.6 |
| Art therapy (%) | 5.8 |
| Self‐management (%) | 0.5 |
| Other (%) | 3.1 |
| Psychotherapy (%) | 14.9 |
| Home care (%) | 8.3 |
| Currently in a residential facility (%) | 10.1 |
| Relapse during past 4 years (%) | 43.5 |
| Substance abuse (%) | 5.0 |
| Alcohol abuse (%) | 4.9 |
| Smoking (%) | 42.1 |
| Unhealthy eating habits (%) | 25.9 |
Almost all subjects were on antipsychotic treatment (97.4%; 13.1% on first‐generation antipsychotics; 69.3% on second‐generation antipsychotics; 15.0% on both; 2.1% on no antipsychotic; for 0.5% no information was available). Polypharmacy was reported by 54.4% of patients. At least one psychosocial intervention was received by 34.3% of participants; 9.1% received two interventions, 4.1% three or more.
At least one relapse was reported in 43.5% of the sample during the previous 4 years; among patients who relapsed, the median number of relapses was 2.
Descriptive statistics of variables included in the network analysis
The mean values and SDs of all variables included in the network analysis at baseline and follow‐up are reported in Table 2.
Table 2.
Network variables at baseline and follow‐up (N=618)
| Baseline (mean±SD) | Follow‐up (mean±SD) | |
|---|---|---|
| PANSS positive | 9.7±4.7 | 8.4±4.3* |
| PANSS disorganization | 2.6±1.4 | 2.4±1.4* |
| BNSS avolition | 20.7±9.6 | 18.6±9.7* |
| BNSS expressive deficit | 12.7±7.9 | 12.0±7.7* |
| CDSS total score | 3.9±4.0 | 3.2±3.7* |
| RSA ‐ Perception of self | 18.1±5.3 | 15.4±4.6* |
| RSA ‐ Perception of the future | 10.7±4.2 | 10.8±4.2 |
| RSA ‐ Social competence | 19.0±5.3 | 19.0±5.3 |
| RSA ‐ Family cohesion | 20.4±5.7 | 20.5±5.3 |
| MCCB ‐ Reasoning and problem solving | 9.8±6.5 | 9.6±6.6 |
| MCCB ‐ Attention/vigilance | 1.7±0.8 | 1.6±0.9 |
| MCCB ‐ Visual learning | 16.3±8.7 | 16.0±8.1 |
| MCCB ‐ Verbal learning | 19.1±5.4 | 19.5±5.5* |
| MCCB ‐ Processing speed | 94.6±18.3 | 95.5±21.0 |
| MCCB ‐ Working memory | 11.4±3.7 | 11.2±3.8* |
| TASIT 1 | 20.1±4.9 | 20.4±4.8* |
| TASIT 2 | 37.6±10.9 | 38.6±10.2* |
| TASIT 3 | 38.4±11.0 | 38.7±9.7 |
| Facial Emotion Identification Test | 37.0±8.3 | 37.3±8.1 |
| MSCEIT | 79.0±9.0 | 90.6±14.1* |
| UPSA‐B total score | 67.3±21.6 | 68.6±23.9 |
| SLOF everyday life skills | 46.2±8.3 | 45.2±9.5* |
| SLOF interpersonal relationships | 22.8±5.9 | 21.2±6.0* |
| SLOF work skills | 20.4±6.0 | 20.1±6.1 |
| Service Engagement Scale | 12.2±7.5 | 11.5±8.0 |
| ISMI (without Stigma resistance) | 2.2±0.4 | 2.1±0.5* |
| Number of incentives | 1.8±1.1 | 1.9±1.1** |
PANSS – Positive and Negative Syndrome Scale, BNSS – Brief Negative Symptom Scale, CDSS – Calgary Depression Scale for Schizophrenia, RSA – Resilience Scale for Adults, MCCB – Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery, TASIT – The Awareness of Social Inference Test, MSCEIT – MCCB Mayer‐Salovey‐Caruso Emotional Intelligence Test, UPSA‐B – UCSD Performance‐Based Skills Assessment, SLOF – Specific Level of Functioning scale, ISMI – Internalized Stigma of Mental Illness
significant t‐test after Bonferroni‐Holm correction
significant Mann‐Whitney test after Bonferroni‐Holm correction
In the overall sample of 618 subjects participating in the follow‐up study, we found improvements in severity of positive symptoms, disorganization, avolition, expressive deficit, depression and internalized stigma. Most social cognition variables improved, while neurocognition variables were quite stable, with significant changes only for verbal learning (slightly improved) and working memory (slightly worsened). Resilience variables were also stable, and only perception of self slightly worsened at follow‐up. Everyday life skills and interpersonal relationships also slightly deteriorated. Although significant, these mean differences in scale scores were relatively small and not clinically relevant.
Network analysis of the whole sample
Figure 1 shows the baseline and follow‐up networks of the overall sample. The network structure did not change significantly from baseline to follow‐up (M‐test = 0.13, p=0.154), suggesting that links among variables were stable over time. The overall strength of the connections among variables increased slightly, but not significantly (11.18 vs. 11.75, S‐test = 0.57, p=0.196).
Figure 1.

Network showing the associations among study variables at baseline (left) and follow‐up (right). Broken edges indicate inverse associations, full edges direct correlations. The thickness of an edge reflects the magnitude of the correlation. Att – attention, Avl – avolition, Dep – depression, Dis – disorganization, ELS – everyday life skills, EnS – service engagement, ExD – expressive deficit, FC – functional capacity, FCo – family cohesion, FEI – Facial Emotion Identification Test, Inc – number of incentives, Int – interpersonal relationships, MSC – Mayer‐Salovey‐Caruso Emotional Intelligence Test, PFu – perception of the future, Pos – positive symptoms, PrS – problem solving, PSe – perception of self, PSp – processing speed, SCo – social competence, SLe – visuospatial learning, Stg – stigma, Ta – The Awareness of Social Inference Test (TASIT), VLe – verbal learning, WMe – working memory, Wrk – work skills
Visual inspection revealed broad similarities between the two networks, i.e. nodes belonging to the same construct were spatially contiguous and highly interconnected. Moreover, psychopathology variables were less interconnected than those belonging to other constructs, such as neurocognition, social cognition and resilience, consistent with the findings reported by Galderisi et al10.
Some new connections emerged at follow‐up, in particular: service engagement with SLOF scales (work skills, everyday life skills, and interpersonal relationships) and MSCEIT; attention with TASIT 1 and 3; incentives with interpersonal skills; depression with positive symptoms and FEIT; disorganization with functional capacity and spatial learning; FEIT with work skills; and processing speed with everyday life skills.
Few connections were no longer present at follow‐up, in particular: incentives with depression, positive symptoms, everyday life activities and work skills; service engagement with functional capacity; and avolition with social competence.
Notably, at both baseline and follow‐up, functional capacity and everyday life skills had high centrality, especially because they were in the pathways connecting functioning, social cognition, neurocognition and psychopathology. At both time points, working memory had the highest strength, because of its strong correlations with the other neurocognition variables. All centrality measures were similar across the two time points, except for work skills, that had a higher centrality at follow‐up, especially for betweenness (Figure 2).
Figure 2.
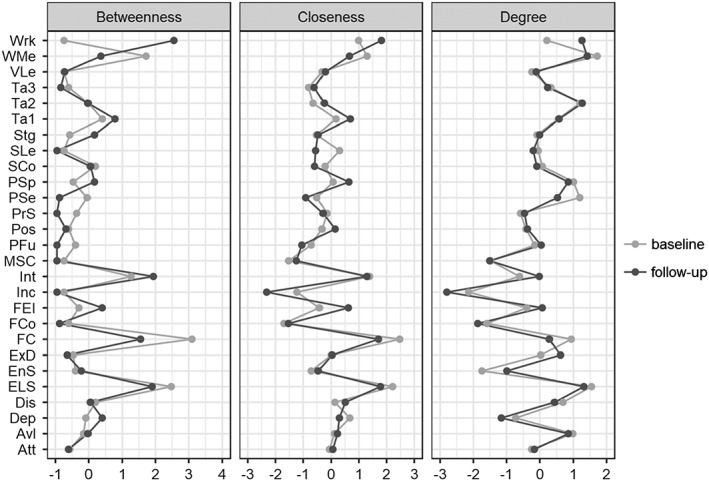
Centrality measures of the study variables at baseline and follow‐up. Att – attention, Avl – avolition, Dep – depression, Dis – disorganization, ELS – everyday life skills, EnS – service engagement, ExD – expressive deficit, FC – functional capacity, FCo – family cohesion, FEI – Facial Emotion Identification Test, Inc – number of incentives, Int – interpersonal relationships, MSC – Mayer‐Salovey‐Caruso Emotional Intelligence Test, PFu – perception of the future, Pos – positive symptoms, PrS – problem solving, PSe – perception of self, PSp – processing speed, SCo – social competence, SLe – visuospatial learning, Stg – stigma, Ta – The Awareness of Social Inference Test (TASIT), VLe – verbal learning, WMe – working memory, Wrk – work skills
The edge weight estimations were accurate at each time point, since the bootstrap mean of each edge and the original value were almost overlapping and the CIs of edge weights estimates were all narrow. As to the robustness of centrality indices, results indicate that the correlation between the strength centrality calculated on the “reduced” networks and that on the original network was >0.70 until 30% of nodes (i.e., at least 9 out of 27) were sampled. This indicates that the relationships between variables remained stable even after random elimination of some network nodes.
Characteristics of recovered and non‐recovered patients
At the 4‐year follow‐up, 124 patients met criteria for recovery (20.1%) and 494 (79.9%) were non‐recovered. Table 3 shows that, compared with patients who did not recover, those who recovered were significantly younger, more educated, more likely to be working and to have a stable affective relationship. Moreover, substance abuse was more common among recovered patients, and they were less likely to live in a residential facility.
Table 3.
Socio‐demographic and clinical variables in non‐recovered and recovered patients
| Non‐recovered (N=494) | Recovered (N=124) | p | |
|---|---|---|---|
| Gender (% males) | 70.0 | 65.3 | 0.309 |
| Age (years, mean±SD) | 45.9±10.4 | 41.8±10.1 | <0.001 |
| Married (%) | 6.9 | 9.7 | 0.289 |
| Working (%) | 26.2 | 67.2 | <0.001 |
| Education (years, mean±SD) | 11.5±3.3 | 12.7±3.4 | <0.001 |
| Stable affective relationships (%) | 15.1 | 34.4 | <0.001 |
| Current drug treatment | |||
| First‐generation antipsychotics (%) | 14.2 | 8.9 | 0.118 |
| Second‐generation antipsychotics (%) | 66.8 | 79 | 0.008 |
| Both first‐ and second‐generation antipsychotics (%) | 17.0 | 7.3 | 0.007 |
| Antidepressants (%) | 17.6 | 17.5 | 0.975 |
| Mood stabilizers (%) | 26.6 | 23.3 | 0.459 |
| Anxiolytics (%) | 36.7 | 16.7 | <0.001 |
| Anticholinergics (%) | 11.3 | 1.7 | <0.001 |
| Polypharmacy (%) | 57.6 | 41.7 | 0.002 |
| Any psychosocial intervention (%) | 39.7 | 40.3 | 0.895 |
| Psychoeducation (%) | 3.8 | 1.6 | 0.22 |
| Cognitive training | 6.9 | 12.1 | 0.055 |
| Social skills training (%) | 3.6 | 3.2 | 0.822 |
| Vocational training (%) | 3.0 | 8.9 | 0.004 |
| Leisure time activities (%) | 19.0 | 12.1 | 0.07 |
| Art therapy (%) | 5.3 | 8.1 | 0.234 |
| Self‐management (%) | 0.6 | 0 | 0.384 |
| Other (%) | 3.6 | 0.8 | 0.102 |
| Psychotherapy (%) | 9.7 | 14.5 | 0.122 |
| Home care (%) | 9.3 | 4.0 | 0.056 |
| Currently in a residential facility (%) | 11.5 | 4.8 | 0.029 |
| Relapse during past 4 years (%) | 45.5 | 36.6 | 0.074 |
| Substance abuse (%) | 4.0 | 8.9 | 0.028 |
| Alcohol abuse (%) | 5.5 | 2.4 | 0.157 |
| Smoking (%) | 43.1 | 38.1 | 0.331 |
| Unhealthy eating habits (%) | 26.2 | 24.2 | 0.621 |
Concerning treatments, the proportion of patients receiving any psychosocial intervention was similar in the two groups. However, patients who recovered were receiving vocational training more frequently. Pharmacological treatment with antipsychotics was provided to almost all patients. Treatment with second‐generation antipsychotics was more common in recovered individuals, while treatment with both first‐ and second‐generation antipsychotics was more common in non‐recovered individuals. Polypharmacy, i.e. prescription of drugs of two different classes, was more common among non‐recovered patients, who more often received treatment with anxiolytics and anticholinergic drugs.
Network analysis of recovered and non‐recovered patients
Figure 3 shows the follow‐up network structure of patients who recovered and those who did not recover. The network structure and connectivity of non‐recovered patients were similar to those observed in the whole sample, but very different from those found in recovered subjects. Actually, in these latter individuals, only few connections were found: positive symptoms and disorganization were connected to each other, as well as avolition and expressive deficit; neurocognitive (with working memory showing the highest betweenness), resilience and social cognition variables remained interconnected within and between domains. Instead, the three domains of real‐life functioning were not interrelated and were disconnected from the other domains. Incentives, engagement with services, depression, family cohesion and MSCEIT were isolated from the rest of the network.
Figure 3.
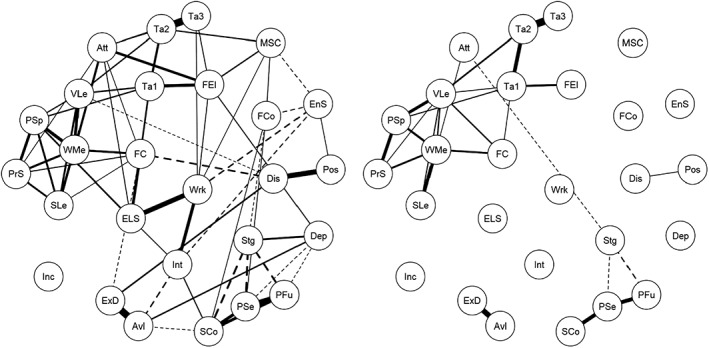
Network showing the associations among study variables among non‐recovered (left) and recovered (right) patients. Broken edges indicate inverse associations, full edges direct correlations. The thickness of an edge reflects the magnitude of the correlation. Att – attention, Avl – avolition, Dep – depression, Dis – disorganization, ELS – everyday life skills, EnS – service engagement, ExD – expressive deficit, FC – functional capacity, FCo – family cohesion, FEI – Facial Emotion Identification Test, Inc – number of incentives, Int – interpersonal relationships, MSC – Mayer‐Salovey‐Caruso Emotional Intelligence Test, PFu – perception of the future, Pos – positive symptoms, PrS – problem solving, PSe – perception of self, PSp – processing speed, SCo – social competence, SLe – visuospatial learning, Stg – stigma, Ta – The Awareness of Social Inference Test (TASIT), VLe – verbal learning, WMe – working memory, Wrk – work skills
The strength of connections was significantly higher in non‐recovered than in recovered patients (S‐test = 9.156, p<0.001) and the network structure was remarkably different between the two subgroups (M‐test = 0.371, p=0.002). Concerning centrality measures, only strength (degree) could be compared between the two groups, because it is the sum of edges connecting each node to the others, while closeness was always zero among non‐recovered individuals, as some nodes were disconnected, and betweenness was irrelevant given the sparsity of the network (Figure 4). We found that everyday life skills and disorganization had a higher strength among non‐recovered patients.
Figure 4.
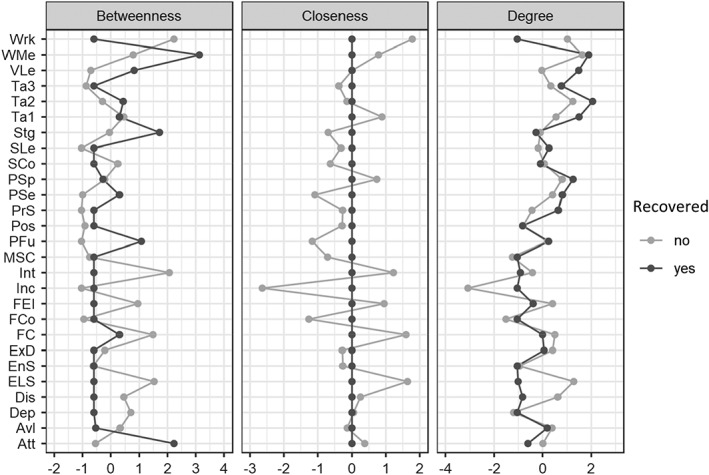
Centrality measures among non‐recovered and recovered patients. Because some nodes are disconnected among recovered patients, closeness is always 0 by definition in this group. Att – attention, Avl – avolition, Dep – depression, Dis – disorganization, ELS – everyday life skills, EnS – service engagement, ExD – expressive deficit, FC – functional capacity, FCo – family cohesion, FEI – Facial Emotion Identification Test, Inc – number of incentives, Int – interpersonal relationships, MSC – Mayer‐Salovey‐Caruso Emotional Intelligence Test, PFu – perception of the future, Pos – positive symptoms, PrS – problem solving, PSe – perception of self, PSp – processing speed, SCo – social competence, SLe – visuospatial learning, Stg – stigma, Ta – The Awareness of Social Inference Test (TASIT), VLe – verbal learning, WMe – working memory, Wrk – work skills
Bootstrap tests indicated that edges were accurate in non‐recovered and less so among recovered patients, in which larger 95% CIs were obtained. The strength centrality remained stable in both patient groups until 40% (i.e., at least 11 out of 27) of nodes were sampled.
DISCUSSION
Our follow‐up study aimed at two main goals: a) to assess the long‐term stability in the pattern of relationships among illness‐related variables, personal resources, context‐related factors and real‐life functioning in subjects with schizophrenia recruited for the multicenter investigation of the Italian Network for Research on Psychoses; b) to compare the network structure of patients who were classified as recovered versus those who were non‐recovered.
Subjects participating at both time points were living in the community and stabilized on antipsychotic treatment. We could detect some significant changes from the baseline. In particular, more subjects had a job and stable affective relationships. However, on average, real‐life functioning had slightly worsened, in spite of small, not clinically significant improvements in psychopathology and social cognition.
The baseline and follow‐up networks did not show significant differences. At both time points, variables relevant to the domains of social cognition, neurocognition, resilience and real‐life functioning were spatially contiguous and highly interconnected, regardless of the use of one or more measures of the same construct. Psychopathological variables had a less interconnected pattern, with avolition/expressive deficit on one side of real‐life functioning nodes, and positive/disorganization nodes on the opposite side.
A closer look at the two networks revealed some changes, in particular for the service engagement node, that appeared more interconnected at follow‐up than at baseline, as it acquired direct connections with all real‐life domains, and with one node of the social cognition (MSCEIT). It is also worth mentioning that, in the follow‐up network, besides the indirect connection through the functional capacity, one of the social cognition nodes (FEIT) acquired a direct connection with one of the real‐life functioning nodes (work skills), and a neurocognition node (processing speed) established a direct connection with the everyday life skills node.
These direct connections between cognition nodes and real‐life functioning domains were not observed in our previous study including 921 subjects10, and were not detected in the present study at baseline; in both cases, we only found an indirect connection through functional capacity. We might hypothesize that the emergence of these direct links reflects the slight improvements observed in social cognition and neurocognition variables. However, current data do not allow firm conclusions in this respect.
All centrality measures were similar across the two time points. Work skills represented the only exception, as it showed a higher centrality at follow‐up, in particular in terms of betweenness. This might be explained by the newly established link with the cognition area, through social cognition, that at baseline was linked to the real‐functioning domains only through functional capacity, while at follow‐up acquired a direct connection with work skills through FEIT.
We also observed an increased strength for service engagement at follow‐up, reflecting its higher number of connections with other nodes, and a decreased betweenness of functional capacity, probably because neurocognitive and social cognition variables established direct connections with real‐life functioning domains. The increased centrality of service engagement might be due to the fact that more collaborative and treatment adherent patients were more likely to join the follow‐up study.
In a population of chronic patients, the good degree of stability of the network structure after a 4‐year follow‐up confirms the robustness of the baseline findings and supports the stability and replicability of network analyses. We believe that this finding is important, in the light of some recent criticisms to the network analysis approach37, 38.
In the light of the focus of the Italian Network for Research on Psychoses on the variables that influence real‐life functioning and recovery in schizophrenia, the other important goal of the study was to compare the network structure of recovered versus non‐recovered subjects. We found significant differences between the networks of the two groups, in terms of number and strengths of connections. In fact, differently from non‐recovered patients, recovered patients have a very sparse network, with real‐life functioning and psychopathology nodes disconnected, in most cases, from the remaining nodes.
This finding is consistent with data reported by van Rooijen et al12, who found that the network observed in remitted psychotic patients had fewer connections than that found in non‐remitted subjects. It is also in line with the study by van Borkulo et al11, who reported that depressed patients showing persistent symptoms at 2‐year follow‐up exhibited a more densely connected network at baseline than remitters.
All these findings are consistent with the network theory assumption that a strongly interconnected network, possibly due to tightly coupled symptoms/dysfunctions that tend to maintain each other's activation, might play an important role in the persistence of mental disorder39. Our data also suggest that the same mechanism may drive the poor functional outcome of the disorder.
The present study has many strengths, in particular the large sample size and the assessment of variables that are core aspects of the recovery process, in addition to the traditional psychopathological ones. However, the relatively small size of the recovered subgroup requires replication in a larger sample.
In conclusion, in our follow‐up study, the network structure did not change significantly from baseline in the overall sample and in non‐recovered patients. Functional capacity and everyday life skills had high betweenness and closeness, as they had at baseline, whereas psychopathological variables remained more peripheral. However, the network structure was very different in recovered subjects, in which we found few connections only. Early and integrated treatment plans, targeting variables with high centrality, might prevent the emergence of self‐reinforcing networks of symptoms/dysfunctions in people with schizophrenia.
APPENDIX 1.
Members of the Italian Network for Research on Psychoses who participated in this study include: Francesco Catapano, Giuseppe Piegari, Carmen Aiello, Francesco Brando, Luigi Giuliani, Daria Pietrafesa (University of Campania “Luigi Vanvitelli”, Naples); Marco Papalino, Giovanni Mercadante, Piergiuseppe Di Palo (University of Bari); Stefano Barlati, Giacomo Deste, Paolo Valsecchi (University of Brescia); Federica Pinna, Benedetta Olivieri, Daniela Manca (University of Cagliari); Maria Salvina Signorelli, Laura Fusar Poli (University of Catania); Domenico De Berardis, Silvia Fraticelli, Mariangela Corbo (University of Chieti); Stefano Pallanti (University of Florence); Mario Altamura, Raffaella Carnevale, Stefania Malerba (University of Foggia); Pietro Calcagno, Domenico Zampogna, Alessandro Corso (University of Genoa); Laura Giusti, Anna Salza, Donatella Ussorio, Dalila Talevi, Valentina Socci, Francesca Pacitti (University of L'Aquila); Andrea de Bartolomeis (University of Naples Federico II); Carla Gramaglia, Eleonora Gambaro, Eleonora Gattoni (University of Eastern Piedmont, Novara); Angela Favaro, Elena Tenconi, Paolo Meneguzzo (University of Padua); Matteo Tonna, Paolo Ossola, Maria Lidia Gerra (University of Parma); Claudia Carmassi, Ivan Cremone, Barbara Carpita (University of Pisa); Nicoletta Girardi, Marianna Frascarelli, Antonio Buzzanca, Roberto Brugnoli, Anna Comparelli, Valentina Corigliano (Sapienza University of Rome); Giorgio Di Lorenzo, Cinzia Niolu, Michele Ribolsi (Tor Vergata University of Rome); Giulio Corrivetti, Giammarco Cascino, Gianfranco del Buono (Department of Mental Health, Salerno); Simone Bolognesi, Andrea Fagiolini, Arianna Goracci (University of Siena); Silvio Bellino, Cristiana Montemagni, Claudio Brasso (University of Turin).
Contributor Information
Italian Network for Research on Psychoses:
Francesco Catapano, Giuseppe Piegari, Carmen Aiello, Francesco Brando, Luigi Giuliani, Daria Pietrafesa, Marco Papalino, Giovanni Mercadante, Piergiuseppe Di Palo, Stefano Barlati, Giacomo Deste, Paolo Valsecchi, Federica Pinna, Benedetta Olivieri, Daniela Manca, Maria Salvina Signorelli, Laura Fusar Poli, Domenico De Berardis, Silvia Fraticelli, Mariangela Corbo, Stefano Pallanti, Mario Altamura, Raffaella Carnevale, Stefania Malerba, Pietro Calcagno, Domenico Zampogna, Alessandro Corso, Laura Giusti, Anna Salza, Donatella Ussorio, Dalila Talevi, Valentina Socci, Francesca Pacitti, Andrea de Bartolomeis, Carla Gramaglia, Eleonora Gambaro, Eleonora Gattoni, Angela Favaro, Elena Tenconi, Paolo Meneguzzo, Matteo Tonna, Paolo Ossola, Maria Lidia Gerra, Claudia Carmassi, Ivan Cremone, Barbara Carpita, Nicoletta Girardi, Marianna Frascarelli, Antonio Buzzanca, Roberto Brugnoli, Anna Comparelli, Valentina Corigliano, Giorgio Di Lorenzo, Cinzia Niolu, Michele Ribolsi, Giulio Corrivetti, Giammarco Cascino, Gianfranco del Buono, Simone Bolognesi, Andrea Fagiolini, Arianna Goracci, Silvio Bellino, Cristiana Montemagni, and Claudio Brasso
REFERENCES
- 1. Fleischhacker WW, Arango C, Arteel P et al. Schizophrenia – time to commit to policy change. Schizophr Bull 2014;40(Suppl. 3):S165‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harvey PD, Strassnig M. Predicting the severity of everyday functional disability in people with schizophrenia: cognitive deficits, functional capacity, symptoms, and health status. World Psychiatry 2012;11:73‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boden R, Sundstrom J, Lindstrom E et al. Association between symptomatic remission and functional outcome in first‐episode schizophrenia. Schizophr Res 2009;107:232‐7. [DOI] [PubMed] [Google Scholar]
- 4. Green MF, Horan PW, Lee J. Nonsocial and social cognition in schizophrenia: current evidence and future directions. World Psychiatry 2019;18:146‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bromley E, Brekke JS. Assessing function and functional outcome in schizophrenia. Curr Top Behav Neurosci 2010;4:3‐21. [DOI] [PubMed] [Google Scholar]
- 6. Lambert M, Karow A, Leucht S et al. Remission in schizophrenia: validity, frequency, predictors, and patients' perspective 5 years later. Dialogues Clin Neurosci 2010;12:393‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. San L, Ciudad A, Alvarez E et al. Symptomatic remission and social/vocational functioning in outpatients with schizophrenia: prevalence and associations in a cross‐sectional study. Eur Psychiatry 2007;22:490‐8. [DOI] [PubMed] [Google Scholar]
- 8. Couture SM, Granholm EL, Fish SC. A path model investigation of neurocognition, theory of mind, social competence, negative symptoms and real‐world functioning in schizophrenia. Schizophr Res 2011;125:152‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galderisi S, Rossi A, Rocca P et al. The influence of illness‐related variables, personal resources and context‐related factors on real‐life functioning of people with schizophrenia. World Psychiatry 2014;13:275‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Galderisi S, Rucci P, Kirkpatrick B et al. Interplay among psychopathologic variables, personal resources, context‐related factors, and real‐life functioning in individuals with schizophrenia: a network analysis. JAMA Psychiatry 2018;75:396‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Borkulo C, Boschloo L, Borsboom D et al. Association of symptom network structure with the course of [corrected] depression. JAMA Psychiatry 2015;72:1219‐26. [DOI] [PubMed] [Google Scholar]
- 12. van Rooijen G, Isvoranu AM, Kruijt OH et al. A state‐independent network of depressive, negative and positive symptoms in male patients with schizophrenia spectrum disorders. Schizophr Res 2018;193:232‐9. [DOI] [PubMed] [Google Scholar]
- 13. Segal A, Wald I, Lubin G et al. Changes in the dynamic network structure of PTSD symptoms pre‐to‐post combat. Psychol Med (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. First MB, Spitzer R, Gibbon M et al. Structured clinical interview for DSM‐IV‐TR axis I disorders, research version, patient edition (SCID‐I/P). New York: Biometrics Research, New York State Psychiatric Institute, 2002. [Google Scholar]
- 15. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261‐76. [DOI] [PubMed] [Google Scholar]
- 16. Wallwork RS, Fortgang R, Hashimoto R et al. Searching for a consensus five‐factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res 2012;137:246‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kirkpatrick B, Strauss GP, Nguyen L et al. The Brief Negative Symptom Scale: psychometric properties. Schizophr Bull 2011;37:300‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Addington D, Addington J, Maticka‐Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry 1993;163(Suppl. 22):39‐44. [PubMed] [Google Scholar]
- 19. Gerlach J, Korsgaard S, Clemmesen P et al. The St. Hans Rating Scale for extrapyramidal syndromes: reliability and validity. Acta Psychiatr Scand 1993;87:244‐52. [DOI] [PubMed] [Google Scholar]
- 20. Nuechterlein KH, Green MF, Kern RS et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry 2008;165:203‐13. [DOI] [PubMed] [Google Scholar]
- 21. Kern RS, Nuechterlein KH, Green MF et al. The MATRICS Consensus Cognitive Battery, part 2: co‐norming and standardization. Am J Psychiatry 2008;165:214‐20. [DOI] [PubMed] [Google Scholar]
- 22. Kerr SL, Neale JM. Emotion perception in schizophrenia: specific deficit or further evidence of generalized poor performance? J Abnorm Psychol 1993;102:312‐8. [DOI] [PubMed] [Google Scholar]
- 23. McDonald S, Bornhofen C, Shum D et al. Reliability and validity of The Awareness of Social Inference Test (TASIT): a clinical test of social perception. Disabil Rehabil 2006;28:1529‐42. [DOI] [PubMed] [Google Scholar]
- 24. Friborg O, Hjemdal O, Rosenvinge JH et al. A new rating scale for adult resilience: what are the central protective resources behind healthy adjustment? Int J Methods Psychiatr Res 2003;12:65‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tait L, Birchwood M, Trower P. A new scale (SES) to measure engagement with community mental health services. J Ment Health 2002;11:191‐8. [DOI] [PubMed] [Google Scholar]
- 26. Boyd Ritsher J, Otilingam PG, Grajales M. Internalized stigma of mental illness: psychometric properties of a new measure. Psychiatry Res 2003;121:31‐49. [DOI] [PubMed] [Google Scholar]
- 27. Mausbach BT, Harvey PD, Goldman SR et al. Development of a brief scale of everyday functioning in persons with serious mental illness. Schizophr Bull 2007;33:1364‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mucci A, Rucci P, Rocca P et al. The Specific Level of Functioning Scale: construct validity, internal consistency and factor structure in a large Italian sample of people with schizophrenia living in the community. Schizophr Res 2014;159:144‐50. [DOI] [PubMed] [Google Scholar]
- 29. Andreasen NC, Carpenter WT Jr, Kane JM et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry 2005;162:441‐9. [DOI] [PubMed] [Google Scholar]
- 30. Nasrallah H, Morosini P, Gagnon DD. Reliability, validity and ability to detect change of the Personal and Social Performance scale in patients with stable schizophrenia. Psychiatry Res 2008;161:213‐24. [DOI] [PubMed] [Google Scholar]
- 31. Liu H, Han F, Yuan M et al. The nonparanormal SKEPTIC. Proceedings of the 29th International Conference on Machine Learning, ICML 2012;2:1415‐22. [Google Scholar]
- 32. Costantini G, Epskamp S, Borsboom D et al. State of the art personality research: a tutorial on network analysis of personality data. J Res Pers 2015;54:13‐29. [Google Scholar]
- 33. Foygel R, Drton M. Extended Bayesian information criteria for Gaussian graphical models. Adv Neural Inf Process Syst 2010;23:604‐12. [Google Scholar]
- 34. Fruchterman TMJ, Reingold EM. Graph drawing by force‐directed placement. Soft Pract Exper 1991;21:1129‐64. [Google Scholar]
- 35. Epskamp S, Borsboom D, Fried EI. Estimating psychological networks and their accuracy: a tutorial paper. Behav Res Methods 2018;50:195‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Borkulo C, Boschloo L, Kossakowski J et al. Comparing network structures on three aspects: a permutation test Working Paper, 2017. [DOI] [PubMed] [Google Scholar]
- 37. Borsboom D, Robinaugh DJ, The Psychosystems Group et al. Robustness and replicability of psychopathology networks. World Psychiatry 2018;17:143‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Forbes MK, Wright AGC, Markon KE et al. The network approach to psychopathology: promise versus reality. World Psychiatry 2019;18:272‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Borsboom D. A network theory of mental disorders. World Psychiatry 2017;16:5‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]


