Abstract
Antipsychotics are effective in preventing relapses of schizophrenia, but it is generally believed that their long‐term use is harmful for patients’ physical well‐being. However, there are no long‐term studies which have verified this view. This nationwide, register‐based cohort study aimed to assess the risk of hospitalization due to physical health problems, as a marker for severe physical morbidity, and the risk of all‐cause mortality, as well as of cardiovascular and suicidal death, associated with antipsychotic use in all patients treated for schizophrenia in inpatient care between 1972 and 2014 in Finland (N=62,250), with up to 20 years of follow‐up (median: 14.1 years). The use of antipsychotic drugs (i.e., use of any antipsychotic compared with non‐use) and the use of specific antipsychotics were investigated, and outcomes were somatic and cardiovascular hospitalization, and all‐cause, cardiovascular and suicide death. Hospitalization‐based outcomes were analyzed by a within‐individual design to eliminate selection bias, comparing use and non‐use periods in the same individual by stratified Cox model. Mortality outcomes were assessed by traditional between‐individual Cox multivariate models. The adjusted hazard ratios (aHRs) for any somatic hospitalization and cardiovascular hospitalization were 1.00 (95% CI: 0.98‐1.03) and 1.00 (95% CI: 0.92‐1.07) during use of any antipsychotic compared to non‐exposure periods within the same individual. The aHRs were 0.48 (95% CI: 0.46‐0.51) for all‐cause mortality, 0.62 (95% CI: 0.57‐0.67) for cardiovascular mortality, and 0.52 (95% CI: 0.43‐0.62) for suicide mortality during use vs. non‐use of any antipsychotic. The most beneficial mortality outcome was associated with use of clozapine in terms of all‐cause (aHR=0.39, 95% CI: 0.36‐0.43), cardiovascular (aHR=0.55, 95% CI: 0.47‐0.64) and suicide mortality (aHR=0.21, 95% CI: 0.15‐0.29). The cumulative mortality rates during maximum follow‐up of 20 years were 46.2% for no antipsychotic use, 25.7% for any antipsychotic use, and 15.6% for clozapine use. These data suggest that long‐term antipsychotic use does not increase severe physical morbidity leading to hospitalization, and is associated with substantially decreased mortality, especially among patients treated with clozapine.
Keywords: Schizophrenia, antipsychotic treatment, physical morbidity, hospitalization, all‐cause mortality, cardiovascular mortality, suicide, clozapine
Antipsychotics are effective in preventing relapses in schizophrenia, according to both randomized controlled trials (RCTs)1 and observational studies representing real‐world patients with long follow‐up periods2. However, antipsychotic use is associated with the risk of serious adverse events, such as tardive dyskinesia3, 4. In addition, adverse effects of antipsychotic drugs on physical health are numerous5, 6, 7. In short‐term treatment, the use of these medications has been associated with weight gain, dyslipidemias, glucose metabolism dysregulation, QTc prolongation and sudden cardiac death8, 9, 10, 11, and many of these adverse effects have been linked with their pharmacological action9, 11, 12. Nevertheless, there is a lack of knowledge about whether these cardiometabolic adverse effects are associated with greater physical morbidity and mortality in long‐term use13, 14.
According to a meta‐analysis based on studies from various countries, persons with schizophrenia have a 14.5 years shorter average life expectancy compared with the general population15. Our recent findings from a large, nationwide cohort study showed that the gap in longevity has remained the same during the last 30 years16. This unchanged excess mortality compared with the general population was explained by a simultaneous decrease in suicides and increase in cancer and cardiovascular deaths among persons with schizophrenia.
Persistent premature mortality in schizophrenia might also be attributed to long‐term antipsychotic use. However, recent systematic reviews and meta‐analyses of short‐term placebo‐controlled RCTs have found an about 30‐50% lower mortality risk in association to antipsychotic use compared with non‐use17, 18, although the duration of treatment was not identical for active vs. placebo arms in one of these studies18, and the statistical power was insufficient to reach a significant difference in the other17.
Large observational studies have also reported beneficial effects of antipsychotics on all‐cause mortality, which has been attributed to more healthy lifestyle behaviors, less psychosis‐related cortisol increase, and increased secondary prevention due to engagement with the medical system in antipsychotic‐treated patients19, 20, 21, 22.
Data on long‐term physical morbidity and mortality associated with antipsychotic use are lacking1, which would be crucial knowledge for a more in‐depth assessment of the long‐term risk‐benefit ratio of the use of these medications in the treatment of schizophrenia13. Thus, we aimed to study the risk of hospitalization due to physical health problems, as a marker for severe physical morbidity, and the risk of all‐cause mortality as well as cardiovascular and suicidal death associated with antipsychotic use in a nationwide cohort of persons with schizophrenia, with up to 20 years of follow‐up.
METHODS
Study population
The study population was identified based on the nationwide Hospital Discharge register, which is managed by the National Institute of Health and Welfare. The study cohort included all persons treated in inpatient hospital care due to schizophrenia in Finland during the period 1972‐20142, 23. Schizophrenia was defined by discharge diagnosis (ICD‐10 codes F20 and F25; ICD‐9 and ICD‐8 codes 295*).
The whole cohort (named as prevalent cohort) included 62,250 persons with schizophrenia, while the incident cohort (first‐episode patients) included 8,719 persons who were hospitalized for the first time due to schizophrenia in the period 1996‐2014, and who had not used antipsychotic drugs during the year preceding the index hospitalization.
The follow‐up started on January 1, 1996 for the prevalent cases, and at the first discharge from inpatient care for the incident cases. The follow‐up time ended at death or on December 31, 2015, whichever occurred first.
Exposure
As drug dispensing in the Prescription register data is recorded according to Anatomical Therapeutic Chemical (ATC) classification codes, we derived antipsychotics as class N05A, with exclusion of lithium. The PRE2DUP method was utilized to derive drug use periods, i.e., when drug use started and ended, based on purchase dates, amounts of drugs dispensed and personal drug use patterns24.
Outcomes
Two inpatient care‐based outcomes were defined: somatic hospitalization (all hospitalizations except psychiatric ones, i.e., excluding ICD‐10 codes F* as main diagnoses) and cardiovascular hospitalizations (ICD‐10 codes I00‐I99). Three mortality outcomes were analyzed: all‐cause mortality, cardiovascular mortality (ICD‐10 codes I00‐I99) and suicide death (X60‐X84). Follow‐up for mortality in hospital care was censored after the first seven days, due to lack of drug data during hospital care. Sensitivity analyses for all‐cause mortality were conducted without this censoring.
Statistical analyses
Hospitalization‐based outcomes (somatic and cardiovascular hospitalization) were analyzed by a within‐individual design, which is suitable for recurrent events. A stratified Cox proportional hazard regression model25 was utilized, in which each individual formed his/her own stratum, and the risk of outcome was compared between exposure and non‐exposure periods for each person. The follow‐up time for each individual was reset to zero after each outcome event. In within‐individual analyses, all time invariant covariates (such as genetics and gender) are controlled for in the design, and only time‐varying covariates are adjusted for. These covariates were the temporal order of exposures, time since cohort entry, and use of other psychotropics (antidepressants, benzodiazepines and related drugs, lithium and other mood stabilizers).
Mortality outcomes were analyzed by traditional multivariate‐adjusted Cox regression models. These analyses were adjusted for gender, age at cohort entry, year of cohort entry, time since diagnosis, number of prior psychiatric hospitalizations, temporal order of exposure to antipsychotics, other medication use, non‐adherence, prior use of long‐acting injectable (LAI) antipsychotics, prior suicide attempt, substance abuse and physical comorbidities.
Sensitivity analyses were conducted among incident patients, and by excluding the first ten years of follow‐up from the analyses for each person (long‐term survivors). Long‐term survivors were analyzed to explore longer‐term effects on all‐cause mortality after patients had passed the highest risk time for suicide26. In addition, time‐dependent Kaplan‐Meier curves for all‐cause mortality were drafted to describe the mortality hazard of antipsychotic use versus non‐use, and for the ten most commonly used antipsychotics.
Monotherapy periods of specific antipsychotics were analyzed, with all periods including more than one antipsychotic coded as “polytherapy”. Additional analyses were conducted with “any therapy” models, where use of a specific drug was assessed as “yes” or “no”, independent of concomitant use of other antipsychotics (but adjusted for this variable). The threshold of significance for p values was corrected for multiple comparisons using the Benjamini‐Hochberg false discovery rate method.
To assess cumulative exposure, sensitivity analyses on cumulative proportion of days exposed to antipsychotics during outpatient observation time were conducted in a nested case‐control design for incident patients. Days of exposure to antipsychotic drugs were divided by outpatient observation time, resulting in categorization as 0% (full non‐adherence), >0 to <80% (partial non‐adherence) and ≥80% (adherence). Outcomes were all‐cause and cardiovascular mortality.
The research project was approved by the Ethics Committee of the Finnish National Institute for Health and Welfare. Further permissions were granted by pertinent institutional authorities at the Institute, the Social Insurance Institution of Finland, and Statistics Finland.
RESULTS
At the start of follow‐up, the median age was 45.6 years (interquartile range, IQR=34.6‐57.9; mean=46.8) in the prevalent cohort (N=62,250), and 36.2 years (IQR=26.2‐52.3, mean=41.2) in the incident cohort (N=8,719). The proportion of males was 50.2% in the prevalent cohort and 56.2% in the incident cohort. The prevalence of comorbid conditions at baseline in the prevalent cohort was as follows: 4.0% for alcohol or substance abuse, 4.8% for cardiovascular disease, 5.1% for diabetes, 0.2% for liver disease, and 0.8% for renal disease.
The median follow‐up time was 14.1 years (IQR=6.9‐20.0) for the prevalent cohort and 10.1 years (IQR=5.0‐14.3) for the incident cohort. During the follow‐up, 13,889 (22.3%) persons of the prevalent cohort died, and 42,271 persons (67.9%) experienced somatic hospitalization. The corresponding figures for the incident cohort were 1,160 (13.3%) and 4,488 (51.5%). When censoring to >7 days hospitalizations, the median follow‐up time was 13.2 years (IQR 6.2‐19.3) in the prevalent cohort, and 9.4 years (IQR 4.5‐13.6) in the incident cohort.
Antipsychotic use in monotherapy was not associated with an increased risk of somatic hospitalizations (adjusted hazard ratio, aHR=1.00, 95% CI: 0.98‐1.03) compared with non‐exposure periods within the same individual (153,149 somatic hospitalizations per 579,306 person‐years of antipsychotic use vs. 49,717 somatic hospitalizations per 188,107 person‐years of non‐use).
Among specific antipsychotics, LAI fluphenazine was associated with the highest decrease of risk (HR=0.69, 95% CI: 0.56‐0.85), whereas quetiapine, olanzapine, risperidone and aripiprazole were associated with a slightly increased risk of somatic hospitalizations. Point estimates for the majority of other antipsychotics were around 1.0, with CIs crossing 1.0 (Figure 1).
Figure 1.
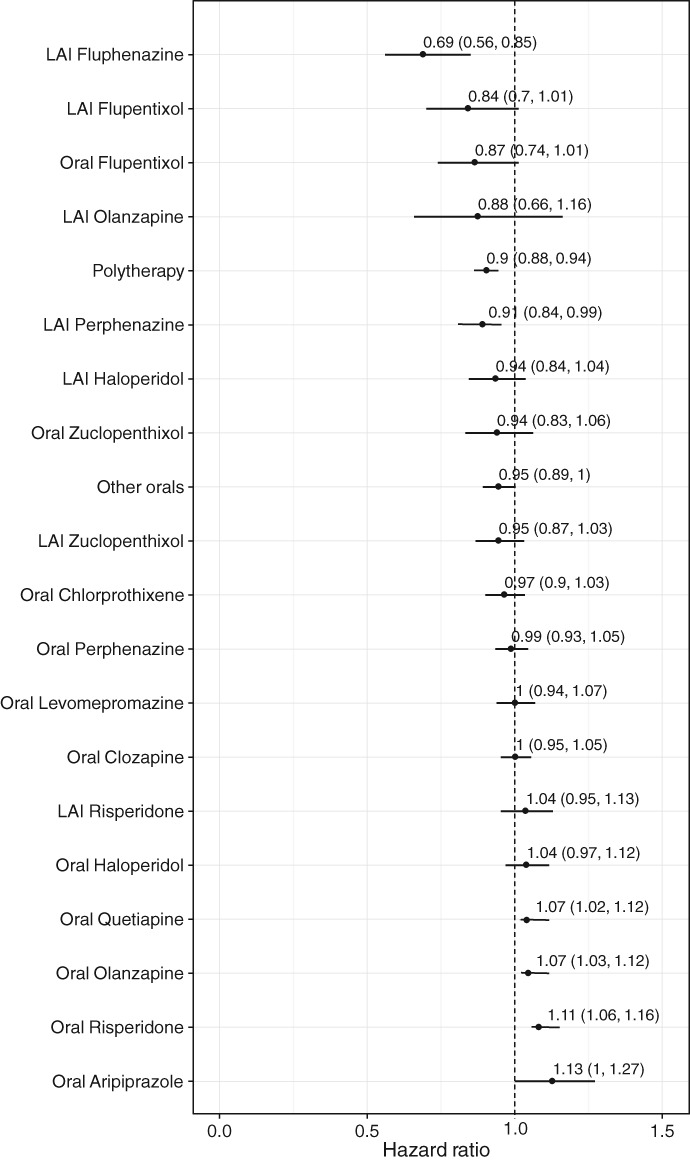
Risk of somatic hospitalization during monotherapy with specific antipsychotics compared with no use of antipsychotics in the prevalent cohort (adjusted hazard ratios with 95% CIs). LAI – long‐acting injectable
Mainly similar results were observed for specific antipsychotics used in any therapy (with or without concomitant other antipsychotics), and when within‐individual monotherapy model was additionally adjusted for use of somatic co‐medications.
Antipsychotic use was not associated with an increased risk of cardiovascular hospitalization (aHR=1.00, 95% CI: 0.92‐1.07) compared with non‐use periods within the same individual. LAI fluphenazine in monotherapy was associated with a significantly decreased risk of cardiovascular hospitalization (aHR=0.46, 95% CI: 0.32‐0.68) (Figure 2).
Figure 2.
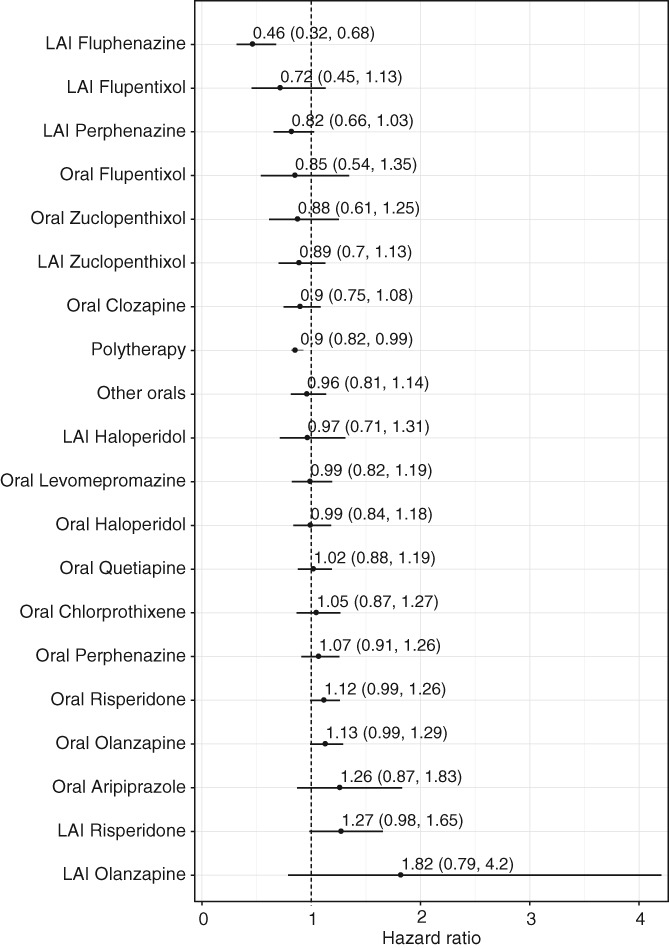
Risk of cardiovascular hospitalization during monotherapy with specific antipsychotics compared with no use of antipsychotics in the prevalent cohort (adjusted hazard ratios with 95% CIs). LAI – long‐acting injectable
All‐cause mortality was significantly lower in patients using any antipsychotic compared with those using none (Figure 3). In Kaplan‐Meier analyses, the cumulative mortality rates during follow‐up up to 20 years were 46.2% for non‐use, 25.7% for any antipsychotic use, and 15.6% for clozapine use (p<0.0001). The aHR for all‐cause mortality was 0.48 (95% CI: 0.46‐0.51) during antipsychotic use compared with non‐use in the prevalent cohort (8,264 deaths per 577,417 person‐years of antipsychotic use vs. 5,635 deaths per 187,773 person‐years of non‐use). The corresponding figure in the incident cohort was aHR=0.64 (95% CI: 0.55‐0.75) (540 deaths per 55,069 person‐years of antipsychotic use vs. 620 deaths per 25,634 person‐years of non‐use).
Figure 3.
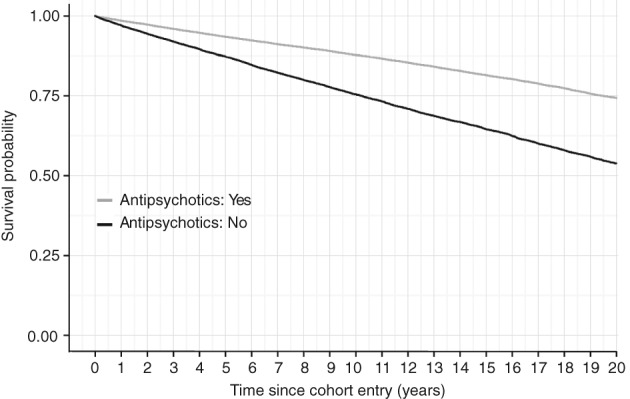
All‐cause mortality in patients using any antipsychotic versus those who used none in the prevalent cohort
Most of the specific antipsychotics in monotherapy were associated with a lower risk of death (Figure 4), with similar results in any therapy analyses.
Figure 4.
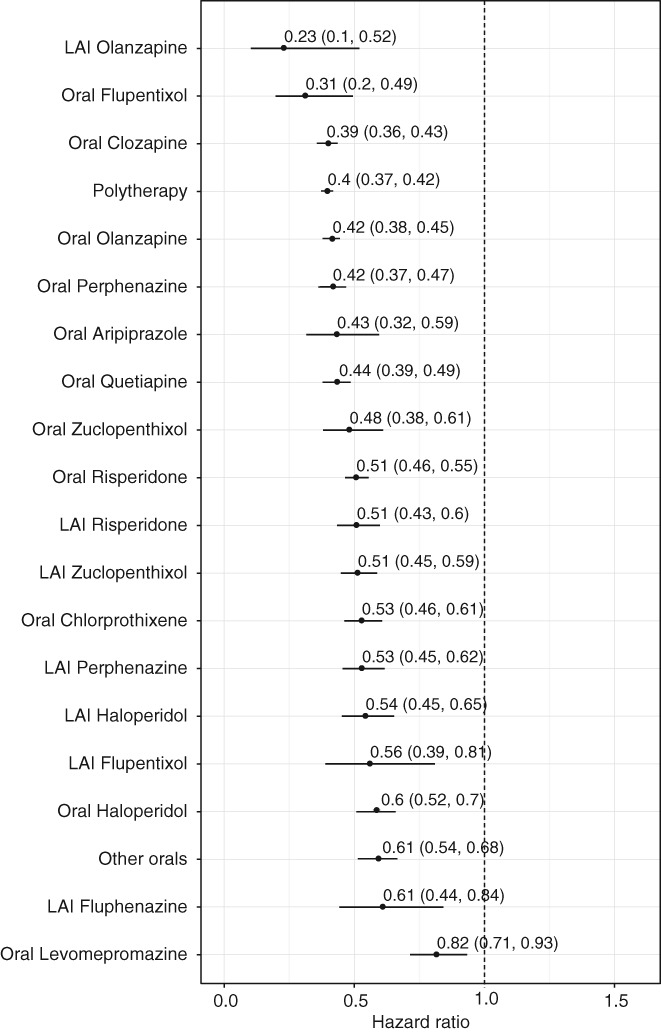
All‐cause mortality in patients receiving monotherapy with specific antipsychotics compared to those who received none in the prevalent cohort with censoring to >7 days hospitalizations (adjusted hazard ratios with 95% CIs). LAI – long‐acting injectable
Cardiovascular mortality was also significantly lower (aHR= 0.62, 95% CI: 0.57‐0.67) during any antipsychotic use compared with non‐use in the prevalent cohort. The corresponding figure in the incident cohort was aHR=0.83 (95% CI: 0.63‐1.09). No specific antipsychotic was associated with an increased risk. Instead, several antipsychotics were associated with a significantly reduced cardiovascular death risk compared with no use (aHR=0.14, 95% CI: 0.02‐1.01 for LAI olanzapine; aHR=0.24, 95% CI: 0.11‐0.54 for oral flupentixol) (Figure 5).
Figure 5.
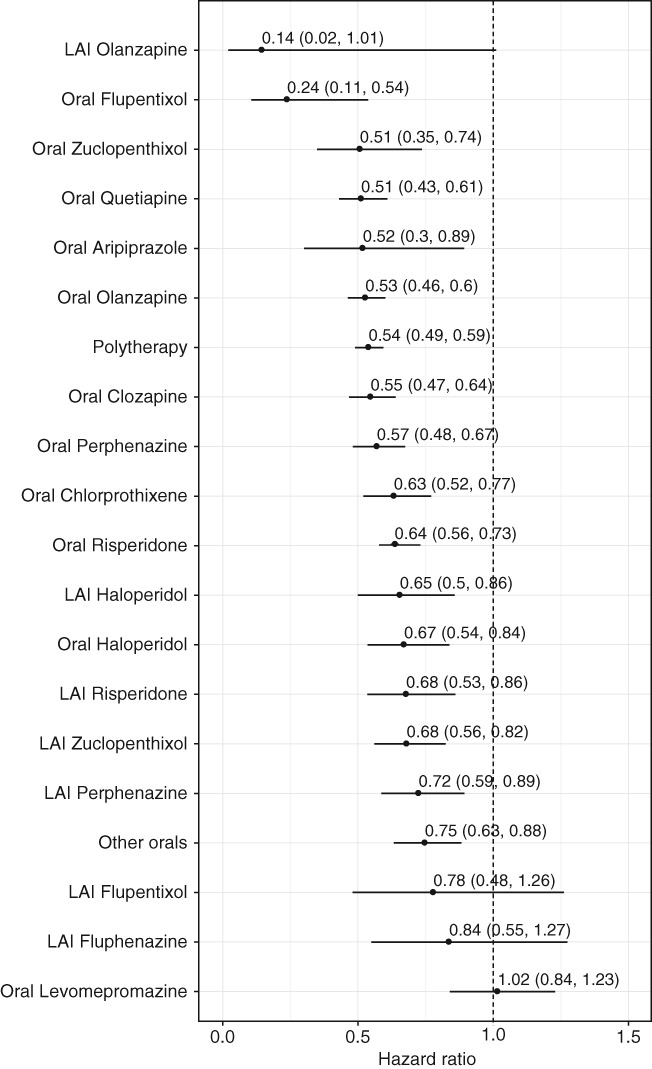
Cardiovascular mortality in patients receiving monotherapy with specific antipsychotics compared to those who received none in the prevalent cohort with censoring to >7 days hospitalizations (adjusted hazard ratios with 95% CIs). LAI – long‐acting injectable
Suicide mortality was significantly lower (aHR=0.52, 95% CI: 0.43‐0.62) during antipsychotic use compared with non‐use in the prevalent cohort. The corresponding figure in the incident cohort was aHR=0.50 (95% CI: 0.33‐0.74). Several antipsychotics were associated with a reduced suicide mortality (Figure 6).
Figure 6.
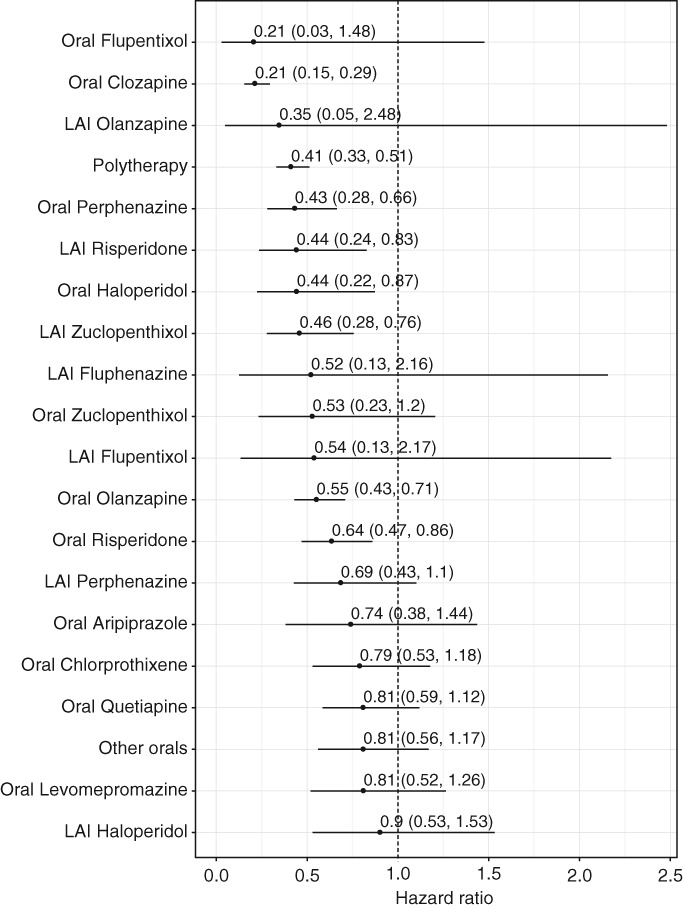
Suicide mortality in patients receiving monotherapy with specific antipsychotics compared to those who received none in the prevalent cohort with censoring to >7 days hospitalizations (adjusted hazard ratios with 95% CIs). LAI – long‐acting injectable
Overall, the most beneficial mortality outcome was associated with clozapine, considering all‐cause (aHR=0.39, 95% CI: 0.36‐0.43), cardiovascular (aHR=0.55, 95% CI: 0.47‐0.64) and suicide mortality (aHR=0.21, 95% CI: 0.15‐0.29). Clozapine was used by 14,350 (23.1%) of persons at some point during the follow‐up. The weakest mortality outcome was associated with levomepromazine, considering all‐cause (aHR=0.82, 95% CI: 0.71‐0.93), cardiovascular (aHR=1.02, 95% CI: 0.84‐1.23) and suicide mortality (aHR=0.81, 95% CI: 0.52‐1.26).
The results of the sensitivity analyses among first‐episode patients were consistent with primary analyses (clozapine monotherapy: aHR=0.42, 95% CI: 0.30‐0.59 for all‐cause mortality, and aHR=0.46, 95% CI: 0.19‐1.09 for cardiovascular mortality). In the comparison between any LAI versus an equivalent oral antipsychotic, they were associated with similar mortality risk (aHR=1.00, 95% CI: 0.93‐1.07). Analyses of all‐cause mortality in the prevalent cohort without censoring to >7 days hospitalizations yielded similar results as with censoring.
In sensitivity analysis excluding the first 10 years of follow‐up, the most favorable outcome for all‐cause mortality was observed with LAI olanzapine (aHR=0.13, 95% CI: 0.03‐0.53) and oral flupentixol (aHR=0.36, 95% CI: 0.16‐0.81), whereas clozapine ranked as 7th among the 19 most frequently used antipsychotics (aHR=0.47, 95% CI: 0.41‐0.54).
The results of sensitivity analyses with a nested case‐control design on associations between cumulative antipsychotic exposure and all‐cause and cardiovascular mortality were also consistent with the primary analyses. Compared with non‐use of antipsychotics, use for ≥80% of outpatient observation time was associated with a decreased risk of all‐cause mortality (adjusted odds ratio, aOR=0.73, 95% CI: 0.60‐0.88). For cardiovascular mortality, antipsychotic use was also associated with a decreased risk, but confidence intervals were wide, resulting in non‐significant findings (aOR=0.80, 95% CI: 0.57‐1.12). Results for clozapine use were similar to those for any antipsychotic use, concerning both all‐cause mortality (aOR=0.61, 95% CI: 0.44‐0.86) and cardiovascular mortality (aOR=0.54, 95% CI: 0.21‐1.39).
DISCUSSION
In this non‐randomized, observational, nationwide sample with up to 20 years of follow‐up (median 14.1 years), we found that antipsychotic use was not associated with an increased risk of hospitalization due to somatic or cardiovascular reasons in patients with schizophrenia. Furthermore, antipsychotic use was associated with a decreased risk of all‐cause, cardiovascular and suicide mortality, also in terms of cumulative antipsychotic exposure. Among specific antipsychotics, clozapine was associated with the most beneficial outcome concerning reduced mortality.
Our results on the association between antipsychotic use and mortality are consistent with previous observational studies19, 20, 21, 22, 27. However, the present investigation uniquely adds to the literature with the largest cohort ever, allowing meaningful analyses regarding specific antipsychotics, plus truly long‐term follow‐up of up to 20 years compared with 5‐11 years in previous studies. Thus, we were able to assess longer‐term outcomes, which is important due to the life‐long duration of schizophrenia and the occurrence of adverse events as a function of long‐term cumulative exposure.
Regarding physical morbidity, periods of antipsychotic use were not associated with an increased risk of somatic or cardiovascular hospitalizations. These findings on long‐term outcomes may appear inconsistent with the adverse effects of short‐term antipsychotic use, including weight gain and obesity, impaired glucose tolerance, dyslipidemias and cardiovascular events, which are all intermediate risk factors for cardiovascular morbidity and mortality9, 11, 12, 14. An explanation for this disconnect is likely to be the improved control of psychiatric symptoms associated with antipsychotic use, which in turn may lead to improved adherence to healthy lifestyle behaviors and utilization of health care services for physical illnesses13.
Persons with schizophrenia have a greater prevalence of sedentary lifestyle, obesity and smoking6, 28, 29, are less likely to receive adequate pharmacotherapy for hypertension and dyslipidemias30, 31, and are seldom tested for glucose and lipid alterations32, 33. This problematic general reduction in adequate secondary prevention of cardiovascular morbidity and mortality is likely aggravated in individuals with schizophrenia not taking antipsychotics.
There are several other possible mechanisms explaining the decreased mortality in patients receiving antipsychotic treatment. Antipsychotics reduce symptoms of schizophrenia, and this may be a major factor for decreased suicide mortality20. Relief of stress may also have a beneficial effect on cardiovascular mortality. Smoking and high blood pressure are among the most important risk factors for cardiovascular death34, and antipsychotics, especially clozapine, decrease blood pressure and possibly also the rate of smoking35, 36.
The results of our study are consistent with previous results from Sweden21. However, some differences in the comparative effectiveness of specific antipsychotics emerged. In the Swedish study, LAIs were associated with a 33% lower risk of all‐cause mortality compared with equivalent oral antipsychotics21, while in the current study no significant difference between LAIs and oral antipsychotics was observed. The superiority of LAIs in Sweden may be related to the fact that those preparations were used more frequently (29% of all antipsychotic use person‐years) during the observation period in that country (2006‐2013)21, compared to this study in Finland (8.5% of antipsychotic use person‐years in the period 1996‐2015).
We found that clozapine was associated with the lowest mortality, in line with the meta‐analysis by Vermeulen et al37, which reported that continuous clozapine treatment is associated with an about 40% lower all‐cause mortality compared to other antipsychotics. Clozapine is recommended for use after two other antipsychotics have been ineffective38 and, therefore, is generally initiated later in the illness course than other antipsychotics. Since mortality may be particularly high in the early phase of the illness39, the later use of clozapine could introduce a survival bias. Therefore, we conducted sensitivity analyses by excluding the first ten years of follow‐up. In these analyses, clozapine was indeed associated with a slightly lower comparative effectiveness, ranking as 7th among the 19 most frequently used antipsychotics in monotherapy. This finding suggests that survival bias related to early phase mortality may affect the rank order of antipsychotics to some extent.
To our knowledge, this is the largest cohort with the longest follow‐up to study morbidity and mortality during antipsychotic treatment in people with schizophrenia or any diagnosis. The results may be particularly generalizable to countries with a state‐funded health care system, where antipsychotics are provided for patients with no or very small co‐payments. Antipsychotic use was modelled with the PRE2DUP method24, which produces reliable estimates of drug exposure and performs better than other previously used modelling methods for register‐based data40.
Hospitalization‐based outcomes were analyzed by within‐individual analyses. All time‐invariant factors are controlled for in this design, which therefore is superior to other analyses in adjusting for fixed and even unmeasured characteristics such as diet, exercise and genetic factors. The underlying severity of disease is also controlled for in this design, which is an advantage over traditional observational studies, and the impact of co‐medications was adjusted for.
One theoretical source of bias is that a patient may experience a side effect and discontinue medication, be hospitalized a few days or weeks later, and counted as a non‐user of antipsychotic drugs. However, in Finland, each prescription dispensing lasts typically 90 days. The exact timing of discontinuation of use is not known, and drug use modelling assumes that all medications dispensed are used. In addition, the utilized modelling method adds some days of extra duration after the calculated antipsychotic drug use has ended, in order to ensure that the end of drug use is correctly assigned if some down‐titration happens after long drug use. These design features ensure that rapid antipsychotic discontinuations provoked by adverse effects are assigned to the antipsychotic exposure instead of non‐exposure period, and that no major misclassification of “past users” occurs.
Mortality analyses were traditional between‐individual models and were adjusted for comorbid conditions associated with survival. The analyses were also adjusted for time‐dependent use of other medications, which aimed at better control for emergence and progression of the comorbid conditions during long follow‐up. Temporal order of antipsychotics was adjusted for in all analysis, by taking into account that clozapine is initiated later. Sensitivity analyses aimed at analyzing possible sources of bias, and the results of these analyses did not change the overall interpretation of data.
As in all observational studies, residual confounding may exist, especially in between‐individual analyses. We lacked information on important lifestyle behaviors, such as smoking and diet. Somatic comorbidities were based only on diagnoses in hospital care and were, therefore, likely under‐reported. The impact of this potential bias was reduced by updating data on comorbid conditions in a time‐dependent fashion in the between‐individual analyses. Survival bias related to prevalent cases was reduced by conducting analyses separately among incident cases, and the results were similar, although the sample size and number of deaths was reduced, which limited statistical power.
In conclusion, in this nationwide observational study, long‐term antipsychotic use was not associated with increased severe physical morbidity among persons with schizophrenia when comparing exposure and non‐exposure periods in the same person. In addition, compared to no antipsychotic use, long‐term antipsychotic use was associated with substantially lower all‐cause, cardiovascular and suicide mortality in people with schizophrenia. These results indicate that excess mortality in schizophrenia may not be attributable to antipsychotics, but at least partially and to a relevant degree to non‐use of antipsychotics.
ACKNOWLEDGEMENTS
This study was funded by the Finnish Ministry of Social Affairs and Health through the developmental fund for Niuvanniemi Hospital. H. Taipale was funded by the Academy of Finland (grants 315969 and 320107). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
REFERENCES
- 1. Leucht S, Tardy M, Komossa K et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta‐analysis. Lancet 2012;379:2063‐71. [DOI] [PubMed] [Google Scholar]
- 2. Taipale H, Mehtälä J, Tanskanen A et al. Comparative effectiveness of antipsychotic drugs for rehospitalization in schizophrenia – a nationwide study with 20‐year follow‐up. Schizophr Bull 2017;44:1381‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carbon M, Kane JM, Leucht S et al. Tardive dyskinesia risk with first‐ and second‐generation antipsychotics in comparative randomized controlled trials: a meta‐analysis. World Psychiatry 2018;17:330‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rummel‐Kluge C, Komossa K, Schwarz S et al. Second‐generation antipsychotic drugs and extrapyramidal side effects: a systematic review and meta‐analysis of head‐to‐head comparisons. Schizophr Bull 2012;38:167‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Correll CU, Detraux J, De Lepeleire J et al. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry 2015;14:119‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Firth J, Siddiqi N, Koyanagi A et al. The Lancet Psychiatry Commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatry 2019;6:675‐712. [DOI] [PubMed] [Google Scholar]
- 7. Papola D, Ostuzzi G, Gastaldon C et al. Antipsychotic use and risk of life‐threatening medical events: umbrella review of observational studies. Acta Psychiatr Scand 2019;140:227‐43. [DOI] [PubMed] [Google Scholar]
- 8. De Hert M, Detraux J, Van Winkel R et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 2012;8:114‐26. [DOI] [PubMed] [Google Scholar]
- 9. Henderson DC, Vincenzi B, Andrea NV et al. Pathophysiological mechanisms of increased cardiometabolic risk in people with schizophrenia and other severe mental illnesses. Lancet Psychiatry 2015;2:452‐64. [DOI] [PubMed] [Google Scholar]
- 10. Ray WA, Chung CP, Murray KT et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009;360:225‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vancampfort D, Correll CU, Galling B et al. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large scale meta‐analysis. World Psychiatry 2016;15:166‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vancampfort D, Stubbs B, Mitchell AJ et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta‐analysis. World Psychiatry 2015;14:339‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Correll CU, Rubio JM, Kane JM. What is the risk‐benefit ratio of long‐term antipsychotic treatment in people with schizophrenia? World Psychiatry 2018;17:149‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Correll CU, Solmi M, Veronese N et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large‐scale meta‐analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 2017;16:163‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hjorthøj C, Stürup AE, McGrath JJ et al. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta‐analysis. Lancet Psychiatry 2017;4:295‐301. [DOI] [PubMed] [Google Scholar]
- 16. Tanskanen A, Tiihonen J, Taipale H. Mortality in schizophrenia: 30‐year nationwide follow‐up study. Acta Psychiatr Scand 2018;138:492‐9. [DOI] [PubMed] [Google Scholar]
- 17. Schneider‐Thoma J, Efthimiou O, Huhn M et al. Second‐generation antipsychotic drugs and short‐term mortality: a systematic review and meta‐analysis of placebo‐controlled randomised controlled trials. Lancet Psychiatry 2018;5:653‐63. [DOI] [PubMed] [Google Scholar]
- 18. Khan A, Faucett J, Morrison S et al. Comparative mortality risk in adult patients with schizophrenia, depression, bipolar disorder, anxiety disorders, and attention‐deficit/hyperactivity disorder participating in psychopharmacology clinical trials. JAMA Psychiatry 2013;70:1091‐9. [DOI] [PubMed] [Google Scholar]
- 19. Tiihonen J, Lönnqvist J, Wahlbeck K et al. 11‐year follow‐up of mortality in patients with schizophrenia: a population‐based cohort study (FIN11 study). Lancet 2009;374:620‐7. [DOI] [PubMed] [Google Scholar]
- 20. Tiihonen J, Mittendorfer‐Rutz E, Torniainen M et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow‐up study. Am J Psychiatry 2016;173:600‐6. [DOI] [PubMed] [Google Scholar]
- 21. Taipale H, Mittendorfer‐Rutz E, Alexanderson K et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res 2018;197:274‐80. [DOI] [PubMed] [Google Scholar]
- 22. Crump C, Winkleby MA, Sundquist K et al. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry 2013;170:324‐33. [DOI] [PubMed] [Google Scholar]
- 23. Tiihonen J, Tanskanen A, Taipale H. 20‐year nationwide follow‐up study on discontinuation of antipsychotic treatment in first‐episode schizophrenia. Am J Psychiatry 2018;175:765‐73. [DOI] [PubMed] [Google Scholar]
- 24. Tanskanen A, Taipale H, Koponen M, et al. From prescription drug purchases to drug use periods – a second generation method (PRE2DUP). BMC Med Inform Decis Mak 2015;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lichtenstein P, Halldner L, Zetterqvist J et al. Medication for attention deficit‐hyperactivity disorder and criminality. N Engl J Med 2012;367:2006‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ventriglio A, Gentile A, Bonfitto I et al. Suicide in the early stage of schizophrenia. Front Psychiatry 2016;7:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vermeulen J, Van Rooijen G, Doedens P et al. Antipsychotic medication and long‐term mortality risk in patients with schizophrenia; a systematic review and meta‐analysis. Psychol Med 2017;47:2217‐28. [DOI] [PubMed] [Google Scholar]
- 28. Stubbs B, Vancampfort D, Hallgren M et al. EPA guidance on physical activity as a treatment for severe mental illness: a meta‐review of the evidence and position statement from the European Psychiatric Association (EPA), supported by the International Organization of Physical Therapists in Mental Health (IOPTMH). Eur Psychiatry 2018;54:124‐44. [DOI] [PubMed] [Google Scholar]
- 29. Vancampfort D, Firth J, Schuch FB et al. Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: a global systematic review and meta‐analysis. World Psychiatry 2017;16:308‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lahti M, Tiihonen J, Wildgust H et al. Cardiovascular morbidity, mortality and pharmacotherapy in patients with schizophrenia. Psychol Med 2012;42:2275‐85. [DOI] [PubMed] [Google Scholar]
- 31. Correll CU, Druss BG, Lombardo I et al. Findings of a U.S. national cardiometabolic screening program among 10,084 psychiatric outpatients. Psychiatr Serv 2010;61:892‐8. [DOI] [PubMed] [Google Scholar]
- 32. Mitchell AJ, Delaffon V, Vancampfort D et al. Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta‐analysis of screening practices. Psychol Med 2012;42:125‐47. [DOI] [PubMed] [Google Scholar]
- 33. Morrato EH, Druss B, Hartung DM et al. Metabolic testing rates in 3 state Medicaid programs after FDA warnings and ADA/APA recommendations for second‐generation antipsychotic drugs. Arch Gen Psychiatry 2010;67:17‐24. [DOI] [PubMed] [Google Scholar]
- 34. Sisti LG, Dajko M, Campanella P et al. The effect of multifactorial lifestyle interventions on cardiovascular risk factors: a systematic review and meta‐analysis of trials conducted in the general population and high risk groups. Prev Med 2018;109:82‐97. [DOI] [PubMed] [Google Scholar]
- 35. Yuen JWY, Kim DD, Procyshyn RM et al. Clozapine‐induced cardiovascular side effects and autonomic dysfunction: a systematic review. Front Neurosci 2018;12:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abela AR, Li Z, Lê AD et al. Clozapine reduces nicotine self‐administration, blunts reinstatement of nicotine‐seeking but increases responding for food. Addict Biol 2019;24:565‐76. [DOI] [PubMed] [Google Scholar]
- 37. Vermeulen JM, van Rooijen G, van de Kerkhof MPJ et al. Clozapine and long‐term mortality risk in patients with schizophrenia: a systematic review and meta‐analysis of studies lasting 1.1‐12.5 years. Schizophr Bull 2019;45:315‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Howes OD, McCutcheon R, Agid O et al. Treatment‐resistant schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group consensus guidelines on diagnosis and terminology. Am J Psychiatry 2017;174:216‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bitter I, Czobor P, Borsi A et al. Mortality and the relationship of somatic comorbidities to mortality in schizophrenia. A nationwide matched‐cohort study. Eur Psychiatry 2017;45:97‐103. [DOI] [PubMed] [Google Scholar]
- 40. Tanskanen A, Taipale H, Koponen M et al. Drug exposure in register‐based research – an expert‐opinion based evaluation of methods. PLoS One 2017;12:e0184070. [DOI] [PMC free article] [PubMed] [Google Scholar]


