Abstract
In recent years, due to unhealthy dietary habits and other reasons, advanced esophageal cancer patients are on the rise, threatening human health and life safety at all times. Stents implantation as an important complementary or alternative method for chemotherapy has been widely applied in clinics. However, the adhesion and proliferation of pathological cells, such as tumor cells, fibroblasts and epithelial cells, may interfere the efficacy of stents. Further multiple implantation due to restenosis may also bring pain to patients. In this contribution, we preferred a biodegradable material Mg–Zn–Y–Nd alloy for potential application of esophageal stent. The hardness testing showed that Mg–Zn–Y–Nd alloy owned less mechanical properties compared with the commercial esophageal stents material, 317L stainless steel (317L SS), while Mg–Zn–Y–Nd displayed significantly better biodegradation than 317L SS. Cell apoptosis assay indicated Mg–Zn–Y–Nd inhibited adhesion and proliferation of tumor cells, fibroblasts and epithelial cells. Our research suggested potential application of Mg–Zn–Y–Nd alloy as a novel material for biodegradable esophageal stent.
Keywords: Esophageal stent, Biodegradable metal material, Mg–Zn–Y–Nd alloy, Hardness, Biocompatibility
Graphical abstract
Highlights
-
•
The Mg–Zn–Y–Nd alloy has less surface microharhness than 317LSS, avoiding additional pain and injury to the patients.
-
•
The Mg–Zn–Y–Nd alloy presents stable degradation characteristics, avoiding multiple operations.
-
•
The Mg–Zn–Y–Nd alloy shows better ability of inhibiting esophageal cancer cell than 317LSS.
1. Introduction
Esophageal cancer is a common digestive tract cancer. About 300,000 people die of esophageal cancer every year in the world [1]. The incidence and mortality of esophageal cancer vary greatly from country to country. The main treatment of esophageal cancer is surgical resection combined with radiotherapy and chemotherapy. However, surgical treatment for advanced esophageal cancer patients is not ideal, the 5-year survival rate of patients is only 10%–15% [2]. Some studies have found that in palliative therapy, stent implantation as an alternative treatment can significantly improve the quality of life of patients and prolong their life span [3,4]. However, the problem with this new therapy is that the stent material (317L stainless steel) has good biocompatibility, so it cannot prevent tumors, granulation and fibrous tissue overgrowth along the stent grid or from both ends to the esophagus [5]. Reciprocating instrumentation brings great pain to patients, and the high hardness of 317L SS also brings extra pain to patients. Therefore, choosing a suitable material can not only inhibit the growth of esophageal cancer cells, but also alleviate the pain of patients caused by high hardness, which has become a bottleneck restricting the development of esophageal cancer stents.
Magnesium alloy as a biodegradable material has been extensively studied in various cavity stents fields due to their good biocompatibility [6]. Their biodegradability is considered to be the best solution for repeated implantation of instruments [7]. Additionally, their alkaline degradation products can further neutralize the acidic pH environment at the site of cancer lesions, thereby inhibiting cancer cells [8]. Thus, in this study, we prefer a Mg–Zn–Y–Nd alloy, which has been studied a lot in the cardiovascular stent field [[6], [7], [8]], as the potential biodegradable material for esophageal stent.
2. Materials and method
2.1. Materials performance comparison of Mg–Zn–Y–Nd and 317L SS
Both the Mg–Zn–Y–Nd (preserved in our laboratory) and 317L SS plates (Baoji, China) were cut into small discs with the 8 mm diameter and polished as the previous reports described [9,10]. The morphology and roughness of the clear Mg–Zn–Y–Nd alloy and the 317L SS control were observed by 3D optical microscopy (NPFLEX, Bruker, Madison, USA) [11]. To investigate the mechanical property of the Mg–Zn–Y–Nd alloy and 317L SS control, surface hardness was detected as our previous work described [8]. The wettability of the Mg–Zn–Y–Nd alloy and 317L SS surface was examined by a water contact angle instrument [12]. Electrochemical tests were performed to investigate the biodegradability of the Mg–Zn–Y–Nd alloy and 317L SS control [6,7]. The surface morphologies of the Mg–Zn–Y–Nd alloy and 317L SS control were observed by scanning electron microscopy (SEM, FEI Quanta 200, Eindhoven, Holland) after immersed in the Hank's solution for 1 day, 3 days, 7 days, 10 days, 22 days and 30 days. The corrosion rates of the samples (Mg–Zn–Y–Nd alloy and 317L SS) after immersion and the pH change of Hank's solution were also determined [13].
2.2. Anti-cancer function of the Mg–Zn–Y–Nd alloy
To investigate the function of Mg–Zn–Y–Nd alloy on inhibiting esophageal cancer cells, the Eca109 (Esophageal cancer cells) cell line were seeded on the Mg–Zn–Y–Nd alloy and the 317L SS control with a concentration of 2 × 104 cells/ml, and cultured in an incubator with the environment of 37 °C and 5% CO2 for 4 h, 1 day and 3 days, respectively [14]. A cell-permeable acridine orange (AO) in combination with a plasma membrane-impermeable DNA-binding dye ethyl bromide (EB) was used to detect apoptosis or necrosis of Eca109 cells. AO and EB excite green and orange fluorescence respectively when they are intercalated into DNA. Only AO but not EB can cross the plasma membrane of normal cell [15]. The vital rates of Eca109 cells on Mg–Zn–Y–Nd and the 317L SS was counted and calculated from 15 images [16].
To further investigate the behaviors of the pathological cells related to esophageal cancer, L929 cell line (Fibroblast), Het-1A cell line (Human esophageal epithelial cells) and macrophages (Inflammatory cells) were also seeded on the Mg–Zn–Y–Nd and the 317L SS, and evaluated as described above.
2.3. Statistical analysis
The data are expressed as the mean ± standard deviation (SD). Statistical analysis was applied as one-way analysis of variance followed by Tukey test, while p values < 0.05 suggesting significant difference. The Origin Pro2018 software (OriginLab, Northampton, MA, USA) was used in the research.
3. Results and discussion
3.1. Material properties comparison of Mg–Zn–Y–Nd and 317L SS
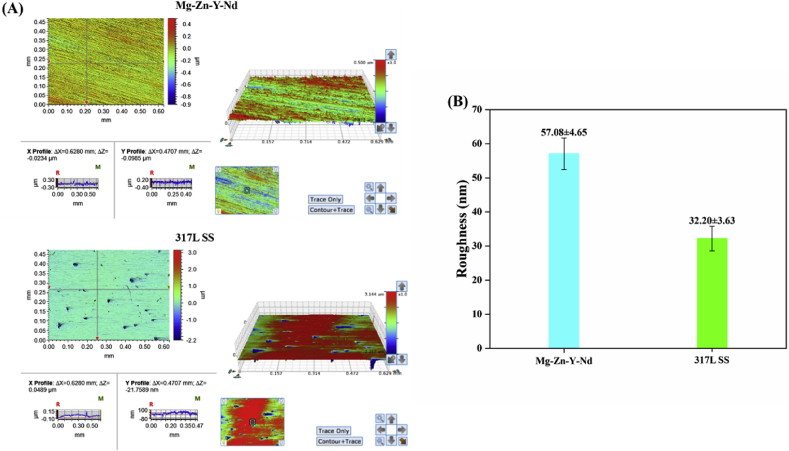
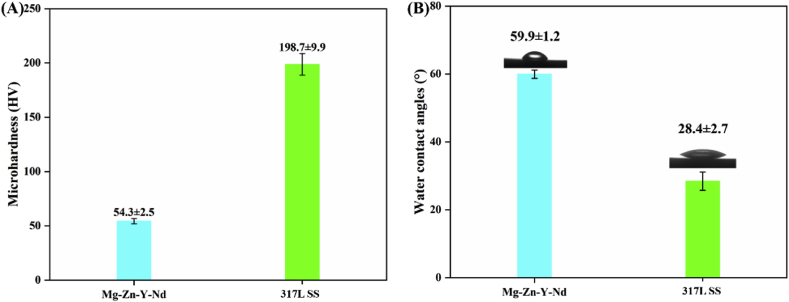
Fig. 1 displayed the morphologies and roughness of the Mg–Zn–Y–Nd and 317L SS surfaces: Both metals showed smooth surfaces with average roughness of only tens of nanometers, wherein Mg–Zn–Y–Nd had rougher surface (57.08 ± 4.05 nm) compared with 317L SS (32.20 ± 3.63 nm); the relative smooth surfaces can avoid extra pain to patients during implantation. Fig. 2A presented the surface microhardness of the Mg–Zn–Y–Nd and 317L SS: It was obvious that the Mg–Zn–Y–Nd had a lower microhardness compared to the traditional medical metal 317L SS, which might be better features to solve the problem of extra injury and pain caused by the high hardness of the existing 317L SS esophageal cancer stent. In addition, the Mg–Zn–Y–Nd showed higher water contact angle than the 317L SS (Fig. 2B), which could reduce the contact probability between pathological cells and metal surface as much as possible, so as to inhibit the adhesion of pathological cells.
Fig. 1.
(A) 3D optical microscopy images and (B) surface roughness of Mg–Zn–Y–Nd and 317L SS (mean ± SD, n = 3).
Fig. 2.
(A) Microhardness and (B) water contact angles of Mg–Zn–Y–Nd and 317L SS surfaces (mean ± SD, n = 6).
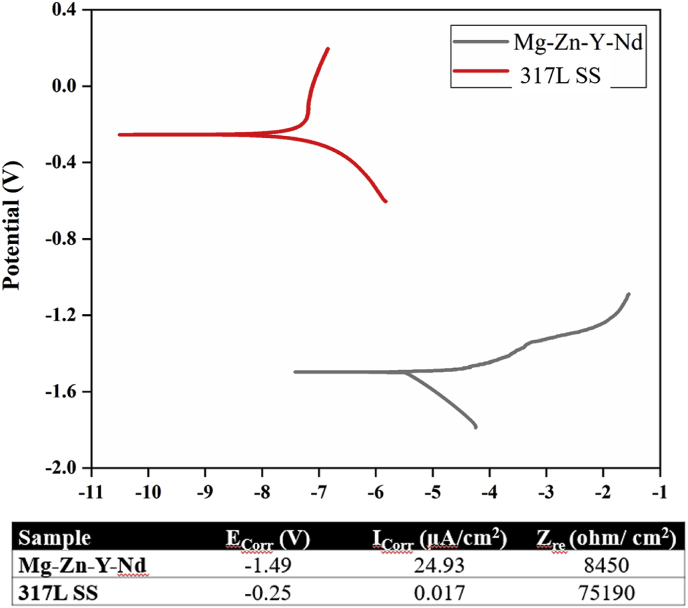
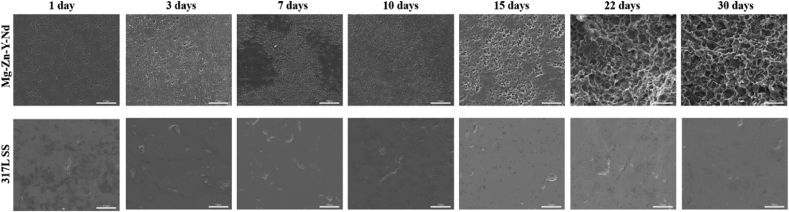
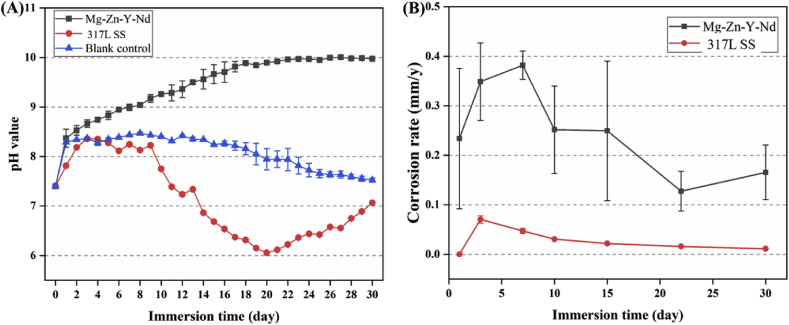
Electrochemical test is a typical method to evaluate the metals' degradability. Fig. 3 presented the typical polarization curves of the 317L SS and Mg–Zn–Y–Nd in simulated body fluid (SBF). Corrosion potential (Ecorr) and corrosion current density (Icorr) were also summarized in Fig. 3. The metals' degradability was determined by their corrosion current density and resistance. From Fig. 3, The Ecorr (−1.49 V) of Mg–Zn–Y–Nd was much lower than that of 317L SS (−0.25 V), indicating that Mg–Zn–Y–Nd was easier to degrade; The Icorr of Mg–Zn–Y–Nd (24.93 μA/cm2) was three orders of magnitude higher than that of 317L SS (0.017 μA/cm2), indicating that the degradation rate of Mg–Zn–Y–Nd was much higher than that of 317 LSS. The Zre value (8450 Ω/cm2) of Mg–Zn–Y–Nd was 88.76% lower than that of 317L SS (75,190 Ω/cm2), which also showed that the corrosion resistance of Mg–Zn–Y–Nd was weaker than that of 317L SS. All the results indicated that Mg–Zn–Y–Nd was a highly degradable metal compared with 317L SS. In order to observe the biodegradation of the Mg–Zn–Y–Nd directly, Mg–Zn–Y–Nd and 317L SS were immersed in Hank's solution and kept at 37 °C, then taken out at the 1st day, 3rd day, 7th day, 10th day, 15th day, 22nd day and 30th day, respectively, and their morphologies were observed by SEM. The Mg–Zn–Y–Nd showed an obviously porous surface, and as time went on, more and bigger porous structures appeared on the surface due to degradation, while the 317L SS showed a flat surface (Fig. 4), which indicated the Mg–Zn–Y–Nd had better Biodegradability than 317L SS. The corrosion rate showed consistent results with the SEM images: Mg–Zn–Y–Nd had markedly higher corrosion rate compared to 317L SS (Fig. 5B). In addition, the pH values of Hank's solution from different groups varied significantly: the pH of 317L SS decreased with time, and the value reached to pH 7.0 at the 14th day, and still kept under this value till the 30th day, suggesting a weak acid environment; the pH of Mg–Zn–Y–Nd was always above 7.0, and it gradually showed an upward trend within 16 days, and remained at the highest value until the 30th day (Fig. 5A). The focus of advanced esophageal cancer is in acidic environment for a long time due to inflammation and canceration. Thus, implants can create alkaline environment through direct contact and biodegradation in order to ameliorate the harsh acidic environment, and promote the treatment of esophageal cancer. At this point, Mg–Zn–Y–Nd had a unique advantage over 317L SS.
Fig. 3.
Polarization curves and values from the polarization curves of the 317L SS and Mg–Zn–Y–Nd in SBF.
Fig. 4.
SEM images of the 317L SS and Mg–Zn–Y–Nd surfaces after immersed in the Hank's solution for 1 day, 3 days, 7 days, 10 days, 15 days, 22 days and 30 days.
Fig. 5.
(A) pH values of the Hank's solution and (B) corrosion rates from different metals groups for 1 day, 3 days, 7 days, 10 days, 15 days, 22 days and 30 days (mean ± SD, n = 3).
3.2. Anti-cancer function of the Mg–Zn–Y–Nd alloy
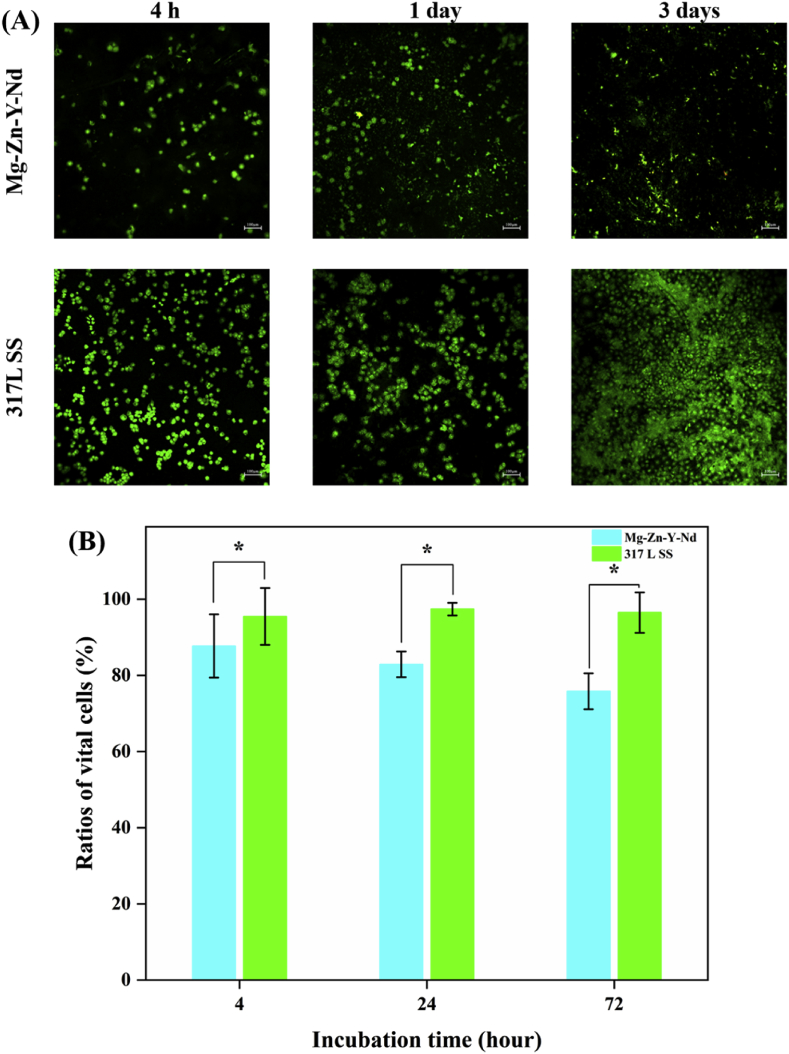
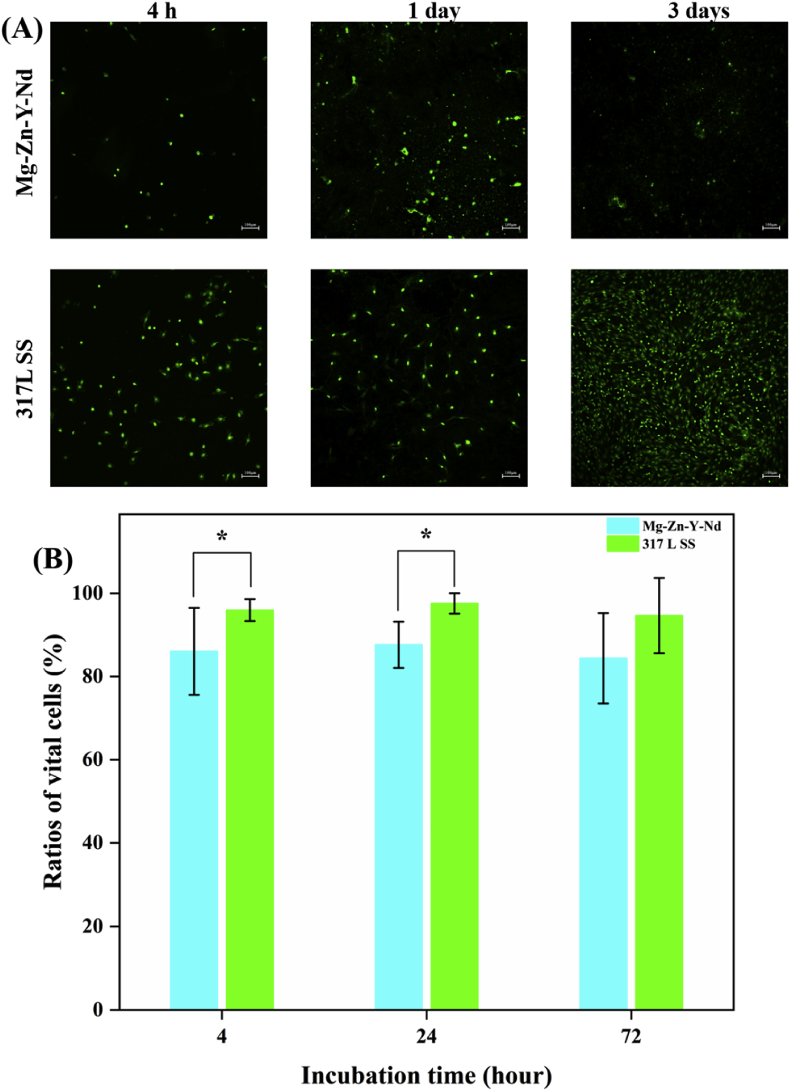
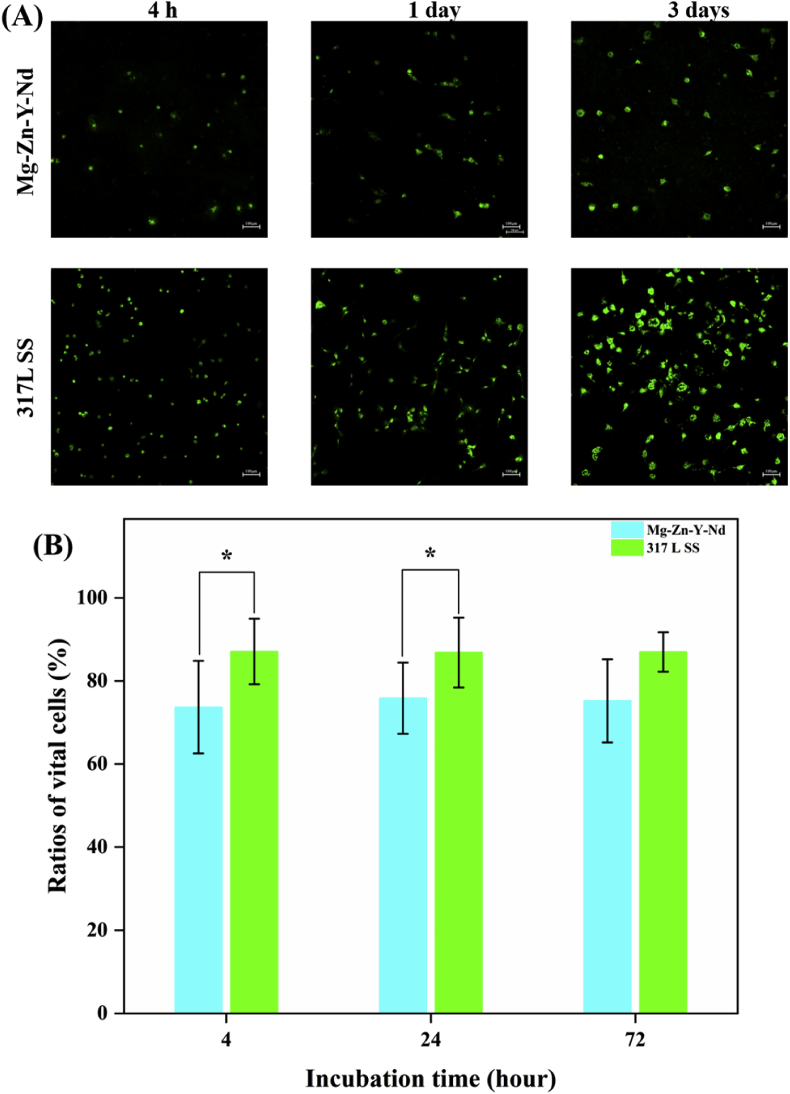
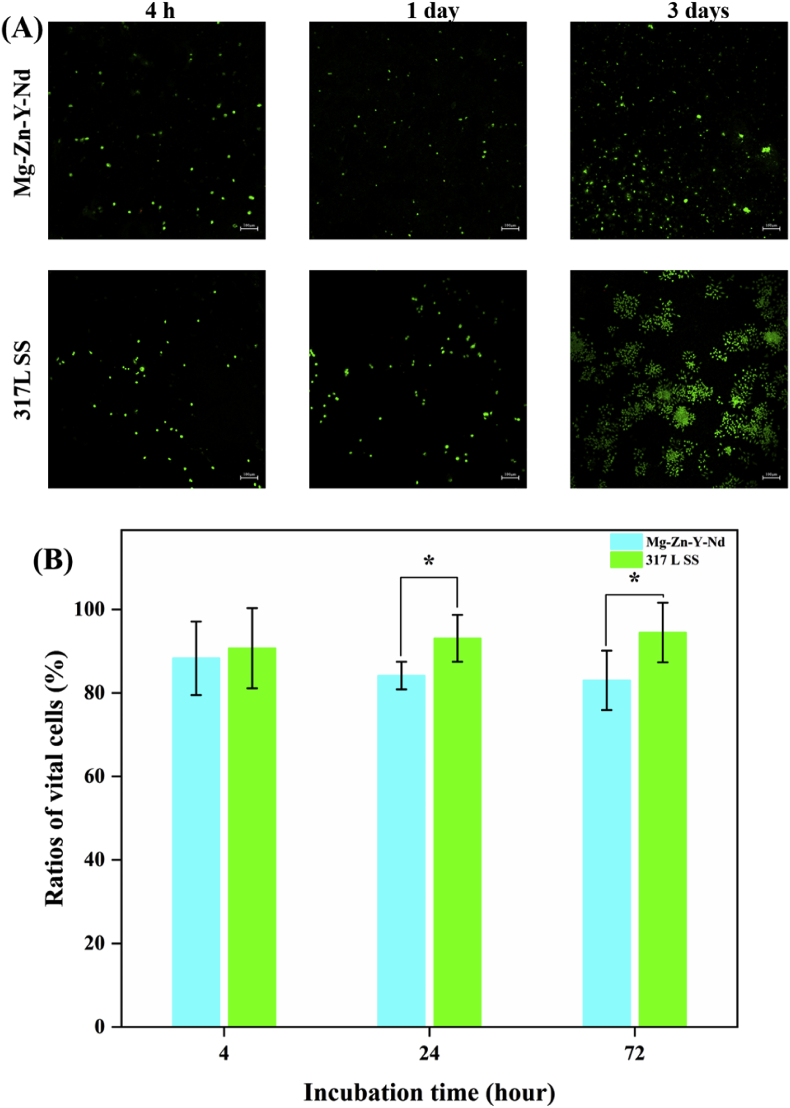
The AO/EB staining images showed that there were obviously fewer Eca109, L929, Het-1A and macrophages on the Mg–Zn–Y–Nd alloy surface than those on the 317L SS (Fig. 6A, Fig. 7A, Fig. 8A and Fig. 9A). In addition, with the passage of time, the numbers of pathological cells on the surface of Mg–Zn–Y–Nd alloy decreased gradually or maintained at a lower value, while the number of pathological cells on the surface of 317LS increased rapidly. This phenomenon indicated that the traditional 317L SS metal had no additional inhibitory effect on esophageal cancer related pathological cells, while Mg–Zn–Y–Nd significantly inhibited those cells. The vital ratios showed consistent results with the fluorescence images: Mg–Zn–Y–Nd had significantly lower vital ratios of Eca109, L929, Het-1A and macrophages than the 317L SS. The esophageal cancer cells (Eca109), myofibroblasts (L929) and esophageal squamous cells (Het-1A) participate restenosis in esophageal stent, and macrophages are the main pathological cells involved in the inflammatory response [17,18]. All the data indicated that choosing Mg–Zn–Y–Nd as the novel biodegradable metal had effective inhibition on these cells.
Fig. 6.
(A) AO/EB staining images and (B) vital ratios of Eca109 on the Mg–Zn–Y–Nd and 317L SS surfaces for 4 h, 1 day and 3 days (*p < 0.05, mean ± SD, n = 3).
Fig. 7.
(A) AO/EB staining images and (B) vital ratios of L929 on the Mg–Zn–Y–Nd and 317L SS surfaces for 4 h, 1 day and 3 days (*p < 0.05, mean ± SD, n = 3).
Fig. 8.
(A) AO/EB staining images and (B) vital ratios of Het-1A on the Mg–Zn–Y–Nd and 317L SS surfaces for 4 h, 1 day and 3 days (*p < 0.05, mean ± SD, n = 3).
Fig. 9.
(A) AO/EB staining images and (B) vital ratios of macrophages on the Mg–Zn–Y–Nd and 317L SS surfaces for 4 h, 1 day and 3 days (*p < 0.05, mean ± SD, n = 3).
4. Conclusions
In summary, Mg–Zn–Y–Nd alloy is an ideal metal for potentially application of esophageal stent with the advantages of biodegradability, lower hardness, and strong inhibition of esophageal cancer related pathological cells. It can be expected that the combination of magnesium alloy and surface modification technology will greatly improve the function of esophageal cancer stent and the quality of life of patients with advanced esophageal cancer.
CRediT authorship contribution statement
Shuo Wang: Methodology, Software, Validation, Formal analysis, Data curation, Visualization. Xueqi Zhang: Validation, Formal analysis, Data curation. Jingan Li: Conceptualization, Methodology, Investigation, Writing - original draft, Writing - review & editing, Project administration. Changsheng Liu: Validation, Investigation. Shaokang Guan: Writing - review & editing, Resources, Supervision, Funding acquisition.
Declaration of competing interest
The authors declared no conflict of interest.
Acknowledgements
This work was funded by The Key Projects of the Joint Fund of the National Natural Science Foundation of China (U1804251), National Key Research and Development Program of China (2018YFC1106703, 2017YFB0702500 and 2016YFC1102403), Key Scientific and Technological Research Projects in Henan Province (grant number 182102310076), and Top Doctor Program of Zhengzhou University (grant number 32210475).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Jingan Li, Email: lijingan@zzu.edu.cn.
Shaokang Guan, Email: skguan@zzu.edu.cn.
References
- 1.Chen M.F., Yang Y.H., Lai C.H. Outcome of patients with esophageal cancer: a nationwide analysis. Ann. Surg. Oncol. 2013;20(9):3023–3030. doi: 10.1245/s10434-013-2935-4. [DOI] [PubMed] [Google Scholar]
- 2.Mulligan C.R., Jr. Multidisciplinary management of esophageal cancer. Surg. Oncol. Clin. N. Am. 2013;22(2):217–246. doi: 10.1016/j.soc.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Vleggaar F.P., Siersema P.D. Expandable stents for malignant esophageal disease. Gastrointest. Endosc. Clin. N. Am. 2011;21(3):377–388. doi: 10.1016/j.giec.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Yuan T., Yu J., Cao J., Gao F., Zhu Y., Cheng Y., Cui W. Fabrication of a delaying biodegradable magnesium alloy-based esophageal stent via coating elastic polymer. Materials. 2016;9:384. doi: 10.3390/ma9050384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J., Wang Z., Wu K., Li J., Chen W., Shen Y., Guo S. Paclitaxel or 5-fluorouracil/esophageal stent combinations as a novel approach for the treatment of esophageal cancer. Biomaterials. 2015;53:592–599. doi: 10.1016/j.biomaterials.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Chen L., Li J.A., Chang J.W., Jin S.B., Wu D., Yan H.H., Wang X.F., Guan S.K. Mg-Zn-Y-Nd coated with citric acid and dopamine by layer-by-layer self-assembly to improve surface biocompatibility. Sci. China Technol. Sci. 2018;61(8):1228–1237. [Google Scholar]
- 7.Chen L., Li J.A., Wang S., Zhu S.J., Zhu C., Zheng B.Y., Yang G., Guan S.K. Surface modification of the biodegradable cardiovascular stent material Mg-Zn-Y-Nd alloy via conjugating REDV peptide for better endothelialization. J. Mater. Res. 2018;33(23):4123–4133. [Google Scholar]
- 8.Wang S., Zhu S.J., Zhang X.Q., Li J.A., Guan S.K. Effects of degradation products of biomedical magnesium alloys on nitric oxide release from vascular endothelial cells. Med. Gas Res. 2019;9(3):153–159. doi: 10.4103/2045-9912.266991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J.A., Wu F., Zhang K., He Z.K., Zou D., Luo X., Fan Y.H., Yang P., Zhao A.S., Huang N. Controlling molecular weight of hyaluronic acid conjugated on amine-rich surface: towards better multifunctional biomaterials for cardiovascular implants. ACS Appl. Mater. Interfaces. 2017;9:30343–30358. doi: 10.1021/acsami.7b07444. [DOI] [PubMed] [Google Scholar]
- 10.Wu F., Li J.A., Zhang K., He Z.K., Yang P., Zou D., Huang N. Multi-Functional coating based on hyaluronic acid and dopamine conjugate for potential application on surface modification of cardiovascular implanted devices. ACS Appl. Mater. Interfaces. 2016;8(1):109–121. doi: 10.1021/acsami.5b07427. [DOI] [PubMed] [Google Scholar]
- 11.Cai H.M., Xu X.Y., Lu J., Lichtman J., Yung S.P., Wong S.T.C. Using nonlinear diffusion and mean shift to detect and connect cross-sections of axons in 3D optical microscopy images. Med. Image Anal. 2008;12(6):666–675. doi: 10.1016/j.media.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Li J., Zhang K., Wu F., He Z., Yang P., Huang N. Constructing bio-functional layers of hyaluronan and type IV collagen on titanium surface for improving endothelialization. J. Mater. Sci. 2015;50:3226–3236. [Google Scholar]
- 13.Feng Y., Zhu S., Wang L., Chang L., Hou Y., Guan S. Fabrication and characterization of biodegradable Mg-Zn-Y-Nd-Ag alloy: microstructure, mechanical properties, corrosion behavior and antibacterial activities. Bioact. Mater. 2018;3(3):225–235. doi: 10.1016/j.bioactmat.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang K., Bai Y.X., Wang X.F., Li Q., Guan F.X., Li J.A. Surface modification of esophageal stent materials by a polyethylenimine layer aiming at anti-cancer function. J. Mater. Sci. Mater. Med. 2017;28:125. doi: 10.1007/s10856-017-5939-y. [DOI] [PubMed] [Google Scholar]
- 15.Bai Y.X., Zhang K., Xu R., Liu H.T., Guan F.X., Liu H.W., Chen Y., Li J.A. Surface modification of esophageal stent materials by a drug-eluting layer for better anti-restenosis function. Coatings. 2018;8(6):215. [Google Scholar]
- 16.Li J., Zhang K., Chen H., Liu T., Yang P., Zhao Y., Huang N. A novel coating of type IV collagen and hyaluronic acid on stent material-titanium for promoting smooth muscle cells contractile phenotype. Mater. Sci. Eng. C. 2014;38:235–243. doi: 10.1016/j.msec.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Li J., Zou D., Zhang K., Luo X., Yang P., Jing Y., Zhang Y., Cui G., Huang N. Strong multi-functions based on conjugating chondroitin sulfate on amine-rich surface direct vascular cells fate for cardiovascular implanted devices. J. Mater. Chem. B. 2017;5:8299–8313. doi: 10.1039/c7tb02162c. [DOI] [PubMed] [Google Scholar]
- 18.Han C., Luo X., Zou D., Li J., Zhang K., Yang P., Huang N. Nature-inspired extracellular matrix coating produced by micro-patterned smooth muscle and endothelial cells endows cardiovascular materials better biocompatibility. Biomater. Sci. 2019;7:2686–2701. doi: 10.1039/c9bm00128j. [DOI] [PubMed] [Google Scholar]