Abstract
Background
Autosomal recessive osteopetrosis is a rare skeletal disorder with increased bone density due to a failure in osteoclast bone resorption. In most cases, the defect is cell-autonomous, and >50% of patients bear mutations in the TCIRG1 gene, encoding for a subunit of the vacuolar proton pump essential for osteoclast resorptive activity. The only cure is hematopoietic stem cell transplantation, which corrects the bone pathology by allowing the formation of donor-derived functional osteoclasts. Therapeutic approaches using patient-derived cells corrected ex vivo through viral transduction or gene editing can be considered, but to date functional rescue cannot be demonstrated in vivo because a relevant animal model for xenotransplant is missing.
Methods
We generated a new mouse model, which we named NSG oc/oc, presenting severe autosomal recessive osteopetrosis owing to the Tcirg1oc mutation, and profound immunodeficiency caused by the NSG background. We performed neonatal murine bone marrow transplantation and xenotransplantation with human CD34+ cells.
Results
We demonstrated that neonatal murine bone marrow transplantation rescued NSG oc/oc mice, in line with previous findings in the oc/oc parental strain and with evidence from clinical practice in humans. Importantly, we also demonstrated human cell chimerism in the bone marrow of NSG oc/oc mice transplanted with human CD34+ cells. The severity and rapid progression of the disease in the mouse model prevented amelioration of the bone pathology; nevertheless, we cannot completely exclude that minor early modifications of the bone tissue might have occurred.
Conclusion
Our work paves the way to generating an improved xenograft model for in vivo evaluation of functional rescue of patient-derived corrected cells. Further refinement of the newly generated mouse model will allow capitalizing on it for an optimized exploitation in the path to novel cell therapies.
Keywords: Osteopetrosis, Mouse model, Transplantation
Highlights
-
•
Ex vivo corrected autologous HSCs might cure Autosomal Recessive Osteopetrosis (ARO).
-
•
There is no animal model to prove in vivo functional rescue of corrected human cells.
-
•
NSG oc/oc mice display osteoclast-rich cell-autonomous ARO and immunodeficiency.
-
•
Human CD34+ cell-transplanted NSG oc/oc mice show human cell chimerism in the BM.
-
•
Further improvements will allow in vivo evaluating corrected patient-derived cells.
1. Introduction
Osteopetrosis is a heterogeneous group of rare skeletal diseases, characterized by an increase in bone density due to defective bone resorption resulting from an alteration in osteoclast formation or function (Sobacchi et al., 2013). The autosomal recessive form (ARO) is the most severe, affecting children in early life and often leading to death if left untreated. The main clinical features of ARO are high bone density, reduced medullary space leading to pancytopenia, compression of cranial nerves, blindness and/or deafness; in addition, other multi-systemic defects (e.g. severe neurological defects) may be present (Sobacchi et al., 2013).
To date, hematopoietic stem cell transplantation (HSCT) is the only therapy (Penna et al., 2019). The outcome of this procedure is influenced by several factors: the age at the time of transplantation, the presence of secondary defects, the genetic defect and the availability of a compatible HLA donor. Regarding specifically this latter issue, in the absence of an HLA-matched donor, the probability of a successful transplant is variable and, despite substantial enhancement, HLA-haploidentical transplantation remains a procedure to be undertaken only in experienced centers (Bahr et al., 2016; Pronk et al., 2017; Neven et al., 2019; Stepensky et al., 2019). In recent years, an increasing number of ARO patients surviving until adulthood without a cure (hence classified as intermediate) have been reported (Sobacchi et al., 2014; Palagano et al., 2015; Sobacchi et al., 1993; Stattin et al., 2017). Despite a milder presentation as compared to “classical ARO”, they accumulate debilitating skeletal (and extra-skeletal, as well) complications over time, thus prompting to consider the set-up of personalized therapeutic interventions (Stepensky et al., 2019; Neri et al., 2015; Teti and Econs, 2017). In particular, transplantation of ex vivo corrected autologous HSCs might represent a valid therapeutic option (Askmyr et al., 2009a). In 2007, the feasibility and efficacy of this approach was demonstrated in the oc/oc mouse model (Johansson et al., 2007), bearing a spontaneous homozygous genomic deletion in the Tcirg1 gene (Frattini et al., 2005; Scimeca et al., 2000), which is also the most frequently mutated gene in ARO patients (Palagano et al., 2018). The Tcirg1 gene encodes the a3 subunit of the osteoclast ATP-dependent vacuolar proton pump V-ATPase, essential for the acidification of the resorption lacuna and for osteoclast resorptive function (Frattini et al., 2000). Johansson and colleagues demonstrated that neonatal intraperitoneal infusion of oc/oc fetal liver cells transduced with a retroviral vector expressing TCIRG1 and GFP. This improved the survival of transplanted oc/oc mice, ameliorated their skeletal phenotype at 8 weeks and almost completely normalized it after 18 weeks (Johansson et al., 2007). Based on these encouraging results, lentiviral-mediated correction of the genetic defect in human cells was undertaken; their functional rescue was demonstrated in vitro after differentiation in bone-resorbing osteoclasts (Moscatelli et al., 2013; Thudium et al., 2016), while in vivo transplant in immunodeficient NSG mice proved their capacity to engraft (Moscatelli et al., 2018). Overall, these observations further fueled efforts towards the development of gene therapy for ARO. At the same time, the demonstration of in vivo functional rescue and amelioration of the disease by gene-corrected human cells could not be provided due to lack of a suitable animal model.
Immunodeficient animal models are largely used in human stem cell research as they can be engrafted with human cells thus allowing the assessment of human stem cell function in vivo (Manz and Di Santo, 2009; Fujiwara, 2018). In particular, the non-obese diabetic (NOD) SCID Il2rγ−/− (NSG) mice lack the adaptive immune response due to the defect in the Prkdc gene as well as the innate immune response (NK cells) due to the disruption of the Il2rγ gene (DiSanto et al., 1995), and express a Sirpα polymorphism that enhances the binding of mouse Sirpα to human CD47, thus preventing macrophage-mediated rejection of human cells (Takenaka et al., 2007).
We took advantage of this mouse model and, through an appropriate mating strategy, introduced the Tcirg1oc mutation in the NSG background, eventually generating a new mouse model that we called NSG oc/oc, displaying osteopetrosis with immunodeficiency. Our findings set the bases for an improved xenograft model to evaluate in vivo, in a relevant murine condition, the efficacy of gene-corrected cells from ARO patients.
2. Methods
2.1. Animals
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) (Shultz et al., 2005) and B6C3Fe a/a Tcirg1 oc/J-Ly5.2 (oc/+) mice were purchased from The Jackson Laboratory (Bar Harbor, ME); of note, prior to the present work, we had fully backcrossed the oc/+ mice on the C57BL/6J background.
Animals were group-housed in a specific-pathogen-free animal facility, under a 12-hour dark/light cycle, with water and food provided ad libitum. All the procedures involving mice were performed in accordance with ethical rules of the Institutional Animal Care and Use Committee of Humanitas Clinical and Research Center and with international laws (Italian Ministry of Health, protocol n.473/2017-PR).
2.2. Mouse genotyping
Mice were genotyped by PCR using DNA extracted from a tail biopsy. The PCR conditions for the Prkdc mutation were: 95 °C for 2 min, then 35 cycles, 95 °C for 30 s, 58 °C for 30 s and 72 °C for 1 min, then 72 °C for 5 min, with forward primer 5′-TTGAGCAGACAATGCTGAGAA-3′ and reverse primer 5′-TTCTGCATTCACAAGTCTTACCA-3′. The 157 base pair (bp) amplicon obtained was sequenced with the forward primer to identify the T-to-A transversion point mutation at position c.12138 in the Prkdc gene (ENSMUST00000023352.8).
For the Il2rγ gene, the targeted allele was identified by the presence of the Neomycin cassette, using the following PCR conditions: 95 °C for 2 min, then 35 cycles, 95 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min, then 72 °C for 5 min, with forward primer 5′-GATGCTCTTCGTCCAGATCA-3′ and reverse primer 5′-CTCCTGCCGAGAAAGTATCC-3′, producing a 150 bp amplicon in the presence of the Neo cassette; the wild type allele was detected through the same protocol using forward primer 5′-TCATGGGATGCATTGTCAGT-3′ and reverse primer 5′-CCTGGAGCTGGACAACAAAT-3′, producing a 234 bp amplicon (ENSMUST00000033664.13) in the absence of the Neo cassette.
For the Tcirg1 gene, which in the oc/oc model carries a 1579 bp genomic deletion spanning from intron 1 to the 5′ of exon 3, genotyping was performed as previously described (Tondelli et al., 2009).
2.3. Tissue preparation and histology
Mice were sacrificed by CO2 asphyxiation; tissues were harvested and either fixed in 4% paraformaldehyde (PFA) or processed to obtain cell suspensions for further analysis (see below). Selected bones were decalcified in an Ion Exchange Decal Unit (Biocare Medical, LLC, Pacheco, CA), dehydrated and embedded in paraffin for Hematoxylin and Eosin (H/E) staining. Other bones, after fixation, were decalcified in 14% ethylenediaminetetraacetic acid (EDTA), pH 7.4 with Acetic Acid, then dehydrated and embedded in paraffin for TRAP staining (Sigma-Aldrich, Saint Louis, MO). Von Kossa/Van Gieson staining was performed on plastic-embedded spine, according to standard procedures. Images were acquired with an Olympus BX51 Microscope (Olympus Optical Co., Ltd., Shinjuku, Tokyo, Japan).
For immunohistochemical staining, 3 μm thick longitudinal serial sections of formalin-fixed paraffin-embedded tissue were collected on glass slides, deparaffinized and treated for antigen retrieval, according to standard procedures. Tissue sections were incubated with 2% H2O2 for 15 min in the dark, washed with water and blocked with Rodent Block M (Biocare Medical) for 40 min at RT. Then, they were incubated overnight at 4 °C or 1 h at RT with the following primary antibodies: anti-mouse CD3 (SP7, 1:100; Thermo Fisher Scientific, Inc., Waltham, MA), anti-mouse B220 (1:300; eBioscience, Inc., San Diego, CA), anti-human CD45 (1:100; DAKO, Glostrup, Denmark), diluted in PBS 0.05% Tween 20 (PBST) (GIBCO®, Grand Island, NY, and Merck Millipore, Burlington, MA, respectively). Sections were then rinsed in PBST and incubated with Mach 1 Polymer (Biocare Medical) for 30 min at RT, rinsed in PBST, developed with 3-3'-Diaminobenzidine (DAB) and counterstained with Hematoxylin. Images were acquired by an Olympus BX51 Microscope (Olympus Optical Co., Ltd).
2.4. Flow cytometry
Cell suspensions from spleens were prepared by meshing tissues in PBS with 2% fetal bovine serum (FBS) and 1 mM EDTA (FACS buffer) on a 40 μm filter followed by red blood cell lysis, and then incubated with the following monoclonal anti-mouse fluorescent-conjugated antibodies: TER-119 (TER-119), CD335 (29A1.4), CD11b (M1/70), CD8 (53-6.7), CD4 (RM4-5), B220 (RA3-6B2), IgM (II/41), IgD (Neri et al., 2015; Teti and Econs, 2017; Askmyr et al., 2009a; Johansson et al., 2007; Frattini et al., 2005; Scimeca et al., 2000; Palagano et al., 2018; Frattini et al., 2000; Moscatelli et al., 2013; Thudium et al., 2016; Moscatelli et al., 2018; Manz and Di Santo, 2009; Fujiwara, 2018; DiSanto et al., 1995; Takenaka et al., 2007; Shultz et al., 2005), F4/80 (BM8), Ly6C (AL-21), Ly6-G (1A8). Cell suspensions from thymuses were prepared by meshing tissues in FACS buffer on a 40 μm filter and then stained with the following monoclonal anti-mouse fluorescent-conjugated antibodies: CD45 (30-F11), CD8 (53-6.7), CD4 (RM4-5).
Bone marrow (BM) cell suspensions were prepared by crushing of multiple bones as described (Crisafulli et al., 2019); in general, we had already optimized this procedure to obtain enough cells for the analysis from osteopetrotic mice, in which BM encroachment and severe growth retardation dramatically reduce the total number of BM cells (Lo Iacono et al., 2012). Staining was performed as above, using monoclonal anti-human CD45 (2D1) and CD14 (61D3) fluorescent-conjugated antibodies. The same antibody cocktail was used for splenocytes obtained from transplanted mice.
For all described tissues, dead cell discrimination was performed with LIVE/DEAD™ Fixable Dead Cell Stain Kit (Thermo Fisher Scientific), following the manufacturer's instructions.
All samples were acquired on a BD FACSCanto II or an LSR Fortessa flow cytometer (BD Biosciences, Franklin Lakes, NJ) equipped with BD FACSDIVA™ software (BD Biosciences) and analyzed with FlowJo software v10.5.3 (FlowJo LLC, BD Biosciences). Antibodies were purchased from either BD Biosciences or eBioscience, Inc.
2.5. Serological analysis
Blood was collected at necropsy by cardiac puncture in the absence of anticoagulants, according to standard procedures. After few hours, serum was separated by low-speed centrifugation and samples were stored at −20 °C until use. Total calcium and phosphorus levels were determined using a Ci16200 Architect Abbott instrument (Abbott Park, IL, USA).
2.6. In vitro osteoclast differentiation and resorption assay
In vitro osteoclastogenesis was induced following standard protocols, as previously reported (Menale et al., 2019). Briefly, 4 × 105 splenocytes/well from NSG and NSG oc/oc littermates were cultured in 96-well plate in αMEM medium (Sigma-Aldrich) supplemented with 10% FBS, 1% penicillin/streptomycin (P/S) and 1% glutamine in the presence of 25 ng/ml murine Macrophage-Colony Stimulating Factor (M-CSF), 5 ng/ml human Transforming Growth Factor β1 (TGFβ1; both reagents from Peprotech, London, UK), 1 μM Dexamethasone (Sigma-Aldrich) and with or without 100 ng/ml murine Receptor Activator of Nf-kB Ligand (RANKL) for 6 days. Mature osteoclasts were stained using the Tartrate Resistant Acid Phosphatase (TRAP) Kit (Sigma-Aldrich) following the manufacturer's instruction.
The same culture conditions were applied to achieve osteoclast formation on dentin discs (Immunodiagnostic Systems, Ltd., Scottsdale, AZ) and evaluate their resorptive capacity. After 3 weeks, dentin discs were rinsed with water, scraped to remove attached cells, stained with 1% Toluidine blue solution for 3 min and then washed with water to visualize resorption pits. Images were acquired on an EVOS XL Inverted Microscope (Thermo Fisher Scientific) for both TRAP+ mature osteoclasts and Toluidine blue-stained dentin discs.
2.7. Transplantation
For murine cell transplantation, 2–4 day old pups were irradiated with 100 centiGray (cGy) administered from a 137Caesium (137Cs) source. Four hours later, 107 total BM cells from 10 week old C57BL/6J donor mice were suspended in 30 μl PBS and injected into the temporal vein of recipient irradiated mice. After injection, the pups were returned to their cages. Mice were sacrificed by CO2 inhalation at 2 time-points: 3 weeks and 12 weeks post-transplantation.
For human cell transplantation, the radiation dose was 200 cGy, aiming at further favoring human cell engraftment. 1–2.6 × 106 CD34+ cells from mobilized peripheral blood of consenting lymphoma patients (82–92% purity), were injected into NSG and NSG oc/oc recipient mice as above described. These CD34+ cells are notoriously regarded as normal and in fact autologous hematopoietic stem cell transplantation is an established treatment for patients with hematologic malignancies (Castagna et al., 2015).
2.8. Micro-computed tomography (μCT)
MicroCT was performed using a Skyscan 1172 instrument (Bruker) at 6 μm resolution with 0.5 mm aluminum filter, source voltage 80 kV and current 100 uA. A 255 mg/ml cutoff was used for bone. Images were reconstructed using the NRecon and InstaRecon software (Bruker). Cross sectional images were obtained using Dataviewer software and three-dimensional images using the CTvox software (Bruker). Quantitative histomorphometric analysis of lumbar vertebrae and femoral bone were performed using the Bruker CTanalyser software.
2.9. Statistics
Statistical analysis was performed using Mann-Whitney test when comparing two groups. Kruskall-Wallis test was used for multiple comparisons (GraphPad Prism 6.0, GraphPad Softwares, Inc., La Jolla, CA). Statistical significance was considered when p < 0.05. All data are presented as mean ± SEM unless otherwise stated.
3. Results
3.1. Generation of the NSG oc/oc mouse model
In order to generate NSG oc/oc mice, first we bred NSG female mice with C57BL/6J oc/+ male mice. Fifty percent of the mice in the litters carried the mutations of the parental strains in the heterozygous state, on a NSG/C57BL/6J mixed background. These heterozygous mice were bred with NSG mice. In the generated litter, we selected individuals carrying the Prkdc and Il2rγ mutations in the homozygous state (hemizygous state for the Il2rγ mutation in male mice) and the Tcirg1oc mutation in the heterozygous state (hereafter indicated as NSG oc/+ on a mixed background). These were crossed with NSG mice and the same breeding scheme was applied in the following four generations. Specifically, according to the Marker-Assisted Accelerated Backcrossing (MAX-BAX®) technology (Charles River Laboratories International, Inc., Wilmington, MA), which genotypes 384 carefully selected strain-specific single nucleotide polymorphisms, mice with allelic profiles most closely matching the profile of the recipient NSG strain, were mated to NSG mice to complete backcrossing for two additional generations. At the end, the NSG oc/+ colony was maintained in the heterozygous state due to early lethality of the NSG oc/oc mice, as further detailed below.
3.2. The NSG oc/oc mouse displays immunodeficiency and severe recessive osteopetrosis
Characteristic features of the parental strains used to generate the NSG oc/oc mouse are on one hand immunodeficiency with complete lack of B, T, and NK cells (Shultz et al., 2005), and on the other osteoclast rich-osteopetrosis with severe growth retardation, defective tooth eruption and extramedullary hematopoiesis (Frattini et al., 2005). Thus, we verified that the newly generated mouse model displayed these phenotypes.
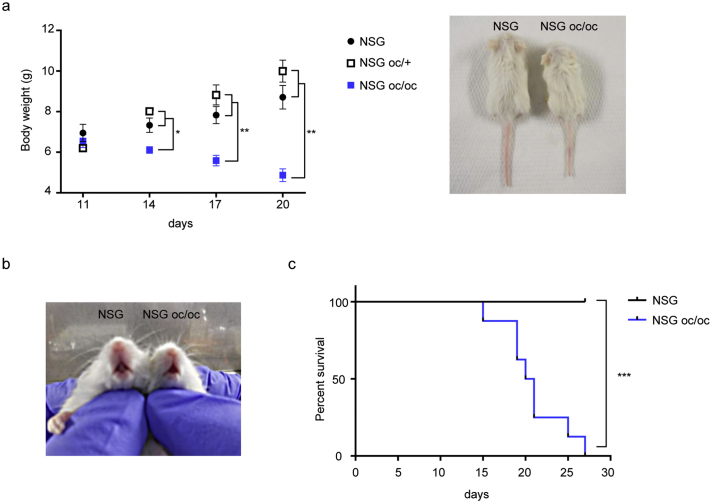
NSG oc/oc mice were born at Mendelian ratio from NSG oc/+ breeding pairs; in total, litters comprised on average 8 pups each, as expected for the NSG strain in optimal housing conditions, and were regularly nursed, differently from what occurs with the parental oc/oc strain according to our experience. NSG oc/oc mice did not display an overt phenotype at birth, but around post-natal day 14 (P14) difference in body weight became apparent as compared to NSG and NSC oc/+ littermates (Fig. 1a), and tooth eruption failed to occur (Fig. 1b). In the following days, NSG oc/oc mice started displaying the so-called “circling behavior” typical of oc/oc mice, possibly due to worsening of the skull bone defect; moreover, they gradually lost weight, likely owing to poor nutrition. The severity of the disease importantly reduced their life span: in fact, 50% of NSG oc/oc mice died before weaning, and the remaining shortly after; none of them survived longer than 4 weeks (Fig. 1c). NSG oc/+ had a normal life span and were completely indistinguishable from NSG littermates, as expected based on the recessive nature of the Tcirg1oc mutation; therefore, they were not further investigated.
Fig. 1.
The NSG oc/oc mouse model presents failure to thrive, lack of tooth eruption and reduced life span. a Left: body weight of NSG, NSG oc/+ and NSG oc/oc mice from post-natal day 11 (P11) to P20. NSG oc/+: n ≥ 3; NSG and NSG oc/oc: n ≥ 6. *p < 0.05; **p < 0.01. Right: representative photographs of NSG and NSG oc/oc littermates showing apparent reduced size in the latter. b Representative photographs of NSG and NSG oc/oc littermates showing lack of tooth eruption in the latter. c Kaplan-Mayer survival curves of NSG and NSG oc/oc mice (Log-rank test, ***p < 0.0001).
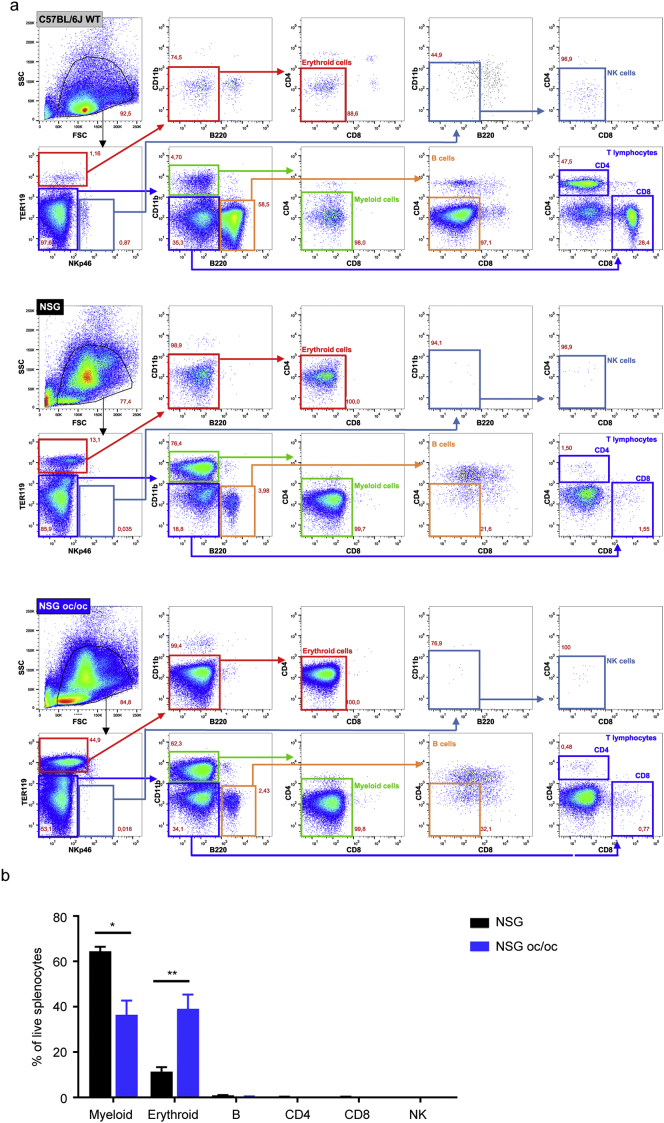
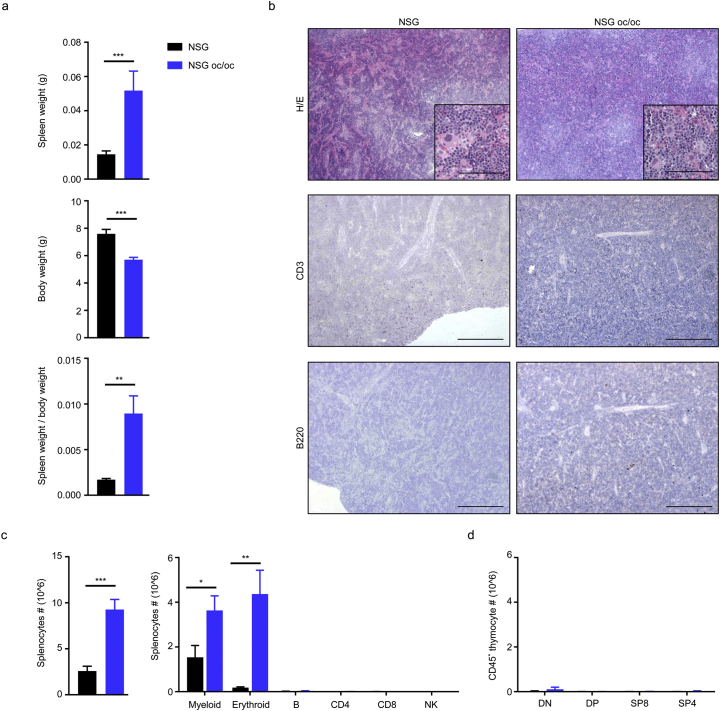
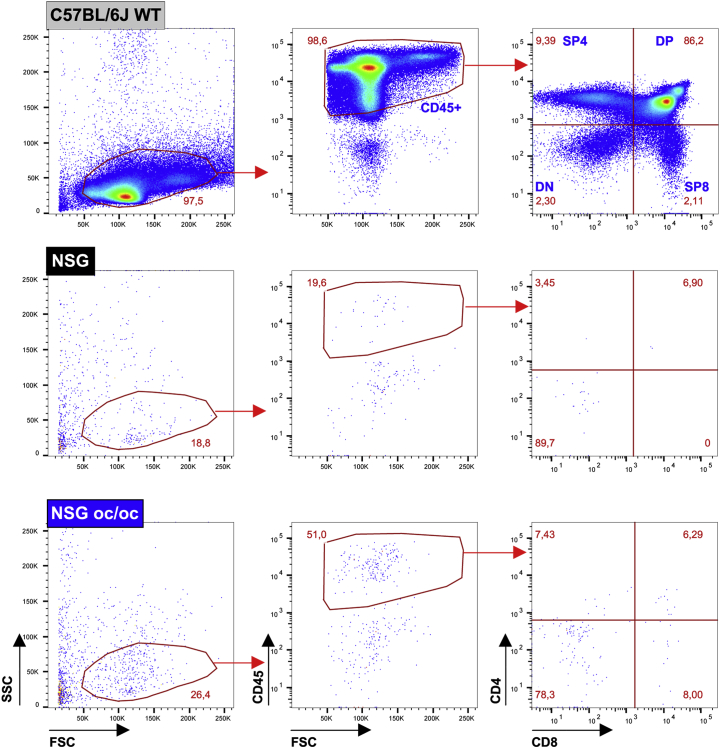
To perform a deep characterization, we sacrificed NSG oc/oc and NSG littermates at P16-18; age matched C57BL/6J wild type (WT) mice were also included as positive controls for histology (Supplementary Fig. 1) and FACS analysis of the spleen (Supplementary Fig. 2a). At necropsy, the spleen was significantly enlarged in NSG oc/oc mice as compared to controls, and, considering the marked reduction of body weight in NSG oc/oc animals, the spleen weight/body weight ratio was even more strikingly increased (Fig. 2a). Tissue histology revealed that splenic architecture was completely disrupted in both NSG and NSG oc/oc mice: Hematoxilin/Eosin (H/E) staining showed absence of separation between red and white pulp and abundance of megakaryocytes, suggestive of intense extramedullary hematopoiesis, while immunohistochemical (IHC) staining with anti-CD3 and anti-B220 antibodies did not detect any T and B cells, respectively (Fig. 2b). Accordingly, total splenic cellularity was significantly higher in NSG oc/oc mice as compared to controls (Fig. 2c). Cytofluorimetric analysis of splenic cell suspensions showed that lymphocytes were virtually absent in NSG oc/oc and NSG littermates, with negligible numbers of T, B, and NK cells, as expected (Fig. 2c). The observed higher splenic cellularity in NSG oc/oc compared to NSG mice was accounted for by increased numbers of both myeloid and erythroid cells, with a 2- and 21-fold difference, respectively, resulting in a relative expansion of the erythroid compartment over the myeloid one, as likely expected during extramedullary hematopoiesis (Supplementary Fig. 2b). The thymus was almost undetectable in NSG oc/oc and NSG littermates, in accordance with the genetic defects in the Prkdc and Il2rγ genes. Moreover, in both groups FACS analysis showed only few residual double-negative thymocytes, with almost complete absence of immature double-positive and mature single-positive T cells (Fig. 2d and Supplementary Fig. 3).
Supplementary Fig 1.
Representative images of spleen of C57BL/6J mice stained with H/E, anti-mouse CD3 or anti-mouse B220 antibody, as technical control of the same stainings in NSG and NSG oc/oc mice. Scale bar: 500 μm.
Supplementary Fig 2.
Immunophenotypic analysis of splenocytes from NSG oc/oc and control mice. a Representative FACS analysis of splenocytes from C57BL/6J adult WT mice, serving as technical control (top panels), from NSG mice (middle panels) and from NSG oc/oc mice (bottom panels), after red blood cells lysis. Erythroid cells are defined as TER119+NKp46-CD11b-B220-CD4-CD8- (highlighted in red); NK cells as TER119-NKp46+CD11b-B220-CD4-CD8- (light blue); myeloid cells as TER119-NKp46-CD11b+B220-CD4-CD8- (green); B cells as TER119-NKp46-CD11b-B220+CD4-CD8- (orange); CD4 and CD8 T lymphocytes as TER119-NKp46-CD11b-B220-, CD4+CD8- or CD4-CD8+, respectively (dark blue). b Histogram showing mean percentage of splenic subpopulations depicted in a in the spleen of NSG and NSG-oc/oc mice. *p < 0.05; **p < 0.01.
Fig. 2.
The NSG oc/oc mouse model displays marked splenomegaly and severe immunodeficiency. a Spleen weight, body weight and spleen weight over body weight ratio in NSG and NSG oc/oc mice (n ≥ 7 per genotype). b Representative images of formalin-fixed paraffin-embedded spleen sections from NSG and NSG oc/oc mice, stained with Hematoxylin/Eosin (H/E), anti-mouse CD3 or anti-mouse B220 antibody; positive controls are shown in Supplementary Fig. 1. Scale bar: 500 μm in the low magnification images, 100 μm in the inset. c Left: number of total splenocytes in NSG and NSG oc/oc mice (n ≥ 8 per genotype). Right: number of cells of all main splenic cell subpopulations (CD11b+ myeloid cells, TER119+ erythroid cells, B220+ B cells, CD4+ and CD8+ T lymphocytes, NKp46+ NK cells) as determined by FACS analysis, following the gating strategy depicted in Supplementary Fig. 2 (n ≥ 5 per genotype). d Number of double negative (DN), double positive (DP) and CD8, CD4 single-positive (SP8 and SP4) thymocytes after gating on CD45+ hematopoietic cells, as described in Supplementary Fig. 3 (n ≥ 3 per genotype). **p <0.01; ***p < 0.001.
The NSG oc/oc mouse model displays marked splenomegaly and severe immunodeficiency. a Spleen weight, body weight and spleen weight over body weight ratio in NSG and NSG oc/oc mice (n ≥ 7 per genotype). b Representative images of formalin-fixed paraffin-embedded spleen sections from NSG and NSG oc/oc mice, stained with Hematoxylin/Eosin (H/E), anti-mouse CD3 or anti-mouse B220 antibody; positive controls are shown in Supplementary Fig. 1. Scale bar: 500 μm in the low magnification images, 100 μm in the inset. c Left: number of total splenocytes in NSG and NSG oc/oc mice (n ≥ 8 per genotype). Right: number of cells of all main splenic cell subpopulations (CD11b+ myeloid cells, TER119+ erythroid cells, B220+ B cells, CD4+ and CD8+ T lymphocytes, NKp46+ NK cells) as determined by FACS analysis, following the gating strategy depicted in Supplementary Fig. 2 (n ≥ 5 per genotype). d Number of double negative (DN), double positive (DP) and CD8, CD4 single-positive (SP8 and SP4) thymocytes after gating on CD45+ hematopoietic cells, as described in Supplementary Fig. 3 (n ≥ 3 per genotype). **p <0.01; ***p < 0.001.
Supplementary Fig 3.
Immunophenotypic analysis of thymocytes from NSG oc/oc and control mice.
Representative FACS analysis of thymocytes from C57BL/6J adult WT mice, serving as technical control (top panels), from NSG mice (middle panels) and from NSG oc/oc mice (bottom panels). After gating on the hematopoietic cell fraction based on the expression of the pan-leukocyte marker CD45, the four main thymus subpopulation are defined as follows: DP: double positive CD4+CD8+; SP4: single positive CD4+CD8-; SP8: single positive CD4-CD8+; DN: double negative CD4-CD8-.
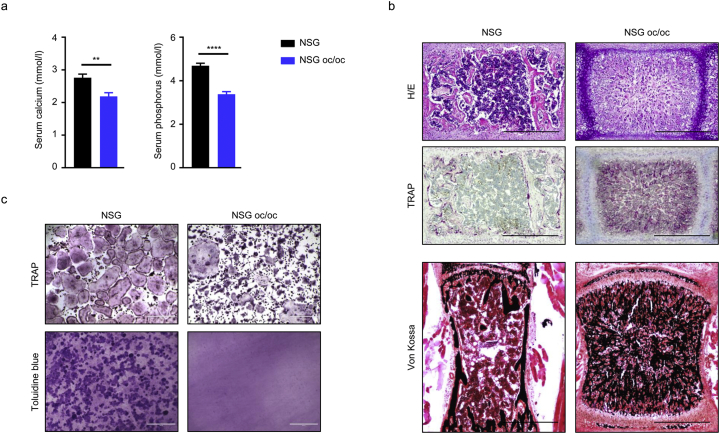
Serological analysis revealed significantly reduced calcium and phosphate levels in NSG oc/oc mice as compared to controls (Fig. 3a). Derangements in calcium homeostasis point to altered bone metabolism. Indeed, H/E staining of decalcified paraffin-embedded bone sections showed very dense tissue containing cartilage remnants, loss of corticomedullary differentiation and extremely limited marrow space in NSG oc/oc mice as compared to controls, demonstrating a typical osteopetrotic appearance (Fig. 3b). TRAP staining was increased in NSG oc/oc mice, indicating that they displayed an osteoclast-rich form of osteopetrosis. Finally, von Kossa staining on non-decalcified resin-embedded tissue sections demonstrated an abnormal pattern of mineralization typical of rickets (Fig. 3b); this peculiar feature was previously described in the oc/oc mouse model and ascribed to defective gastric acidification in the absence of Tcirg1 gene expression in gastric parietal cells, and reduced dietary calcium absorption (Schinke et al., 2009). Overall, the findings in NSG oc/oc mice mirrored those described in the oc/oc model (Scimeca et al., 2000; Schinke et al., 2009; Marks Jr. et al., 1985).
Fig. 3.
NSG oc/oc mice present osteoclast-rich osteopetrosis. a Calcium and phosphorus serum levels in NSG and NSG oc/oc mice (n ≥ 8 per genotype). **p < 0.01, ****p < 0.0001. b Representative images of decalcified formalin-fixed paraffin-embedded vertebral sections of NSG and NSG oc/oc mice stained with H/E (upper panels) and TRAP (middle panels); undecalcified formalin-fixed resin-embedded vertebral sections stained with von Kossa/van Gieson staining (lower panels). Scale bar: 500 μm. c In vitro osteoclastogenesis from splenic precursors of NSG and NSG oc/oc mice: representative images of TRAP staining on plastic (upper panels) and of Toluidine blue staining of resorption pits on dentin discs (lower panels). Scale bars: 400 μm.
Finally, we evaluated in vitro osteoclast formation and bone resorption from NSG oc/oc and NSG splenic precursors according to standard protocols. In both cases, osteoclasts formed, but those generated from NSG oc/oc mice were mostly smaller and irregularly shaped (Fig. 3c), as previously observed in oc/oc osteoclasts (Nakamura et al., 1997), in line with the involvement of the V-ATPase in vesicle trafficking, in addition to its essential proton-pumping activity (Sobacchi et al., 2013). When differentiated on dentin, NSG osteoclasts generated well-developed resorption pits, while no sign of resorption was found in the presence of NSG oc/oc differentiated cells, thus demonstrating a complete functional impairment (Fig. 3c).
In conclusion, the NSG oc/oc mouse model displayed immunodeficiency and severe recessive osteoclast-rich osteopetrosis.
3.3. Neonatal murine bone marrow transplantation corrects the pathology in NSG oc/oc mice
Osteopetrosis in the oc/oc mouse derives from an osteoclast cell-autonomous defect, and in fact can be corrected by WT bone marrow transplantation (BMT) (Frattini et al., 2005; Johansson et al., 2006; Askmyr et al., 2009b; Flores et al., 2010). Therefore, we verified whether the same occurred in our NSG oc/oc mouse. In detail, NSG oc/oc and NSG neonates (P2-P4, n = 6 per group) were transplanted with BM cells from adult C57BL/6J WT mice after receiving a radiation dose of 100 cGy, and were sacrificed 3 or 12 weeks after transplant. In particular, the first time-point (i.e. 3 weeks post-BMT) allowed comparing the bone and spleen phenotype with that of untreated NSG oc/oc mice, which did not survive further, as mentioned above.
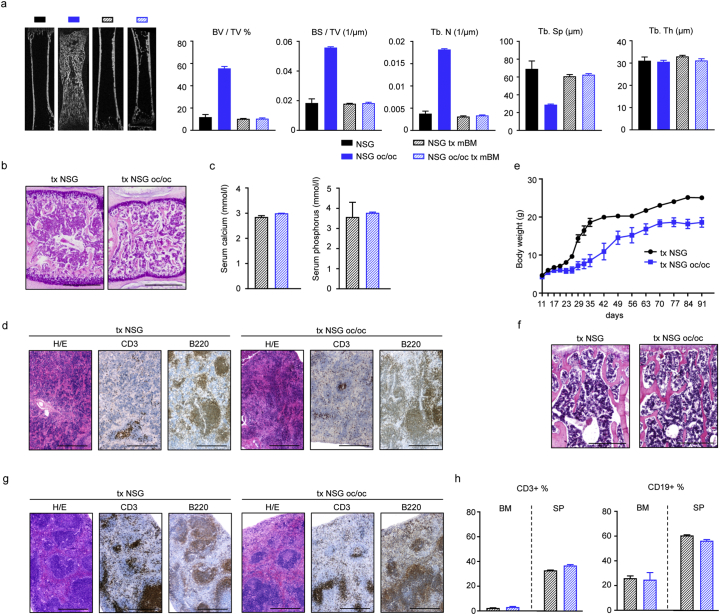
As early as 3 weeks after-BMT, μCT analysis of long bones showed complete normalization of the skeletal phenotype in transplanted NSG oc/oc mice (Fig. 4a). Of note, most of the measurements (i.e. bone volume over total volume ratio, BV/TV; bone surface over total volume ratio, BS/TV; trabecular number, Tb.N, and trabecular spacing, Tb.Sp) in transplanted NSG oc/oc mice overlapped those of untreated NSG and transplanted NSG mice. On the other hand, trabecular thickness (Tb.Th) did not differ among the different genotype and treatment groups (Fig. 4a). In parallel, axial bone histology showed substantial improvement of the bone defect in transplanted NSG oc/oc mice, with clear enlargement of the BM cavity and increase in BM cellularity (Fig. 4b). Accordingly, serological analysis showed no difference in calcium and phosphate levels in transplanted NSG oc/oc as compared to transplanted NSG mice (Fig. 4c), and normalization of these parameters in the former group as compared to untreated NSG oc/oc mice (see Fig. 3a). Finally, H/E staining of the spleen demonstrated an initial attempt to restore red and white pulp separation, and IHC staining with specific antibodies documented the presence of partially compartmentalized T and B cells, both in NSG oc/oc and in NSG mice (Fig. 4d).
Fig. 4.
Neonatal murine bone marrow transplantation corrects the pathology in NSG oc/oc mice. a MicroCT analysis of the femur of untreated NSG and NSG oc/oc mice, and murine BM-transplanted NSG and NSG oc/oc mice (n = 3 in all groups but transplanted NSG, where n = 2). b Representative images of decalcified formalin-fixed paraffin-embedded vertebral sections of transplanted (tx) NSG and NSG oc/oc mice 3 weeks after transplantation, stained with H/E. Scale bar: 500 μm. c Calcium and phosphorus serum levels in NSG and NSG oc/oc mice 3 weeks after transplantation (n = 2 per genotype; bars indicate the range). d Representative images of spleen of tx NSG and tx NSG oc/oc mice 3 weeks after transplantation, stained with H/E, anti-mouse CD3 or anti-mouse B220 antibody. Scale bar: 500 μm. e Growth curve of NSG and NSG oc/oc mice after transplantation, during the 3-month follow-up. f Representative images of decalcified formalin-fixed paraffin-embedded vertebral sections of transplanted (tx) NSG and NSG oc/oc mice 3 months after transplantation, stained with H/E. Scale bar: 500 μm. g Representative images of spleen of tx NSG and tx NSG oc/oc mice 3 months after transplantation, stained with H/E, anti-mouse CD3 or anti-mouse B220 antibody. Scale bar: 500 μm. h Histograms from FACS analysis of bone marrow (BM) and spleen (SP) cell suspensions from NSG and NSG oc/oc mice 3 months after transplantation (n = 3 per genotype).
Mice that were kept under observation for 3 months after transplantation, gained weight over time, despite remaining smaller than control littermates (Fig. 4e) and in good health; of note, none of the transplanted NSG oc/oc mice showed the “circling behavior”. At this time point, histological analysis showed no difference between transplanted NSG and NSG oc/oc mice neither in the bone (Fig. 4f) nor in the spleen structure (Fig. 4g), which acquired a canonical pattern of tissue organization and cell distribution. Accordingly, cytofluorimetric analysis confirmed similar proportion of B and T cells in BM and spleen cell suspensions, in NSG and NSG oc/oc transplanted mice (Fig. 4h).
Overall, these data demonstrated that all the cell-autonomous defects (and specifically, osteoclast resorptive function) of the new mouse model could be rescued by BM transplantation, as previously observed for the oc/oc mouse (Frattini et al., 2005; Johansson et al., 2006; Askmyr et al., 2009b; Flores et al., 2010), highlighting the potential usefulness of our model to test novel cell therapy approaches for curing osteopetrosis, including preclinical studies with transduced murine progenitors.
3.4. NSG oc/oc mice are able to host human cells in their bone
NSG mice are widely used as models for xenotransplants, based on the capacity to support engraftment and development of human cells, conferred by their specific genetic background that prevents rejection. We speculated our new mouse model might serve as a xenograft model for the functional evaluation of gene-corrected human cells. To test this hypothesis, we performed human cell transplantation in neonate NSG oc/oc mice, owing to their severe phenotype impeding survival into adulthood. As donor cells, we exploited human CD34+ cells from mobilized peripheral blood, as these are a relevant source for cell and gene therapy approaches to human osteopetrosis (Penna et al., 2019). In line with the above described experiments, here we irradiated NSG oc/oc mice and NSG controls (n = 7 and 2, respectively; age: P2-P4) sublethally with 100 cGy dose and infused CD34+ cells in the temporal vein. Necropsy was performed on average 2 weeks later, after appearance of signs of suffering typical for the pathology (i.e. circling behavior and weight loss). Cytofluorimetric analysis of BM cell suspensions showed the presence of human CD45+ cells in the NSG oc/oc (data not shown), indicating that, despite their severely compromised bone microenvironment, NSG oc/oc mice were able in principle to host human cells. However, the very low chimerism detected in the BM at this early time point (mean 1.65% ± 2.04 of human CD45+ cells, median 0.6%; for the NSG controls, mean 2.46% ± 3.46, median 2.46% for the NSG oc/oc) was not sufficient for affecting the bone phenotype in such a short timeframe.
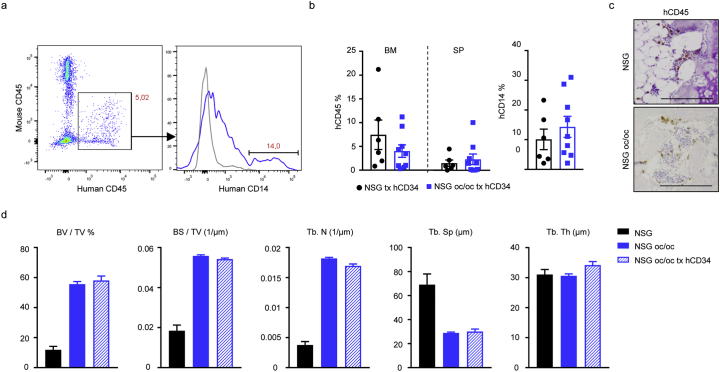
Thus, we decided to raise the radiation dose to 200 cGy in order to favor human cell engraftment by further depleting residual host cells occupying the BM or spleen stem cell niches. Nine NSG oc/oc and 6 NSG littermates were treated with this protocol; each mouse received on average 2 × 106 CD34+ cells (range: 106–2.6 × 106 cells). Actually, also in this setting NSG oc/oc mice started displaying the pathology at about 2 weeks of age; sacrifice was performed 2–3 weeks after transplantation. FACS analysis of the BM revealed an increased chimerism as compared to the previous experimental setting (human CD45+ cells in NSG oc/oc BM: mean 4.01% ± 1.31, median 3.46%; in NSG BM: mean 7.45% ± 3.1, median 5%), with some NSG oc/oc recipient mice showing up to 9–11% of human cells in their BM (Fig. 5a and b). Human cells were also present in the spleen (Fig. 5b). Importantly, human CD14+ cells were detected in the BM of both NSG and NSG oc/oc mice (Fig. 5a and b), suggesting differentiation of human cells towards the myeloid lineage in the murine bone microenvironment, which is relevant in our disease model. Human CD45+ cells could be detected also by IHC staining on bone (Fig. 5c; relevant controls for the IHC are shown in Supplementary Fig. 4). On the other hand, μCT analysis of bone demonstrated that the bone of transplanted NSG oc/oc mice remained osteopetrotic with no amelioration as compared to untreated NSG oc/oc mice (Fig. 5d), even though we cannot exclude that minor early modifications of the bone tissue might have occurred.
Fig. 5.
Analysis of mice transplanted with human CD34+ cells. a FACS analysis of the BM of a representative NSG oc/oc mouse 2 weeks after transplantation. Human CD45+ leukocytes derived from transplanted CD34+ cells were gated (left pseudocolor plot) and analyzed for the expression of the human CD14 monocyte marker (histogram on the right, blue line; gray line represents the Fluorescent-Minus-One control). b Scatter plots showing (left) the percentage of human CD45+ leukocytes within the BM and the spleen of NSG and NSG oc/oc mice, and (right) the percentage of human CD14+ cells within the BM. c Representative immunohistochemical staining of vertebral bone of transplanted mice two weeks after transplantation, showing the presence of human CD45+ cells. Scale bar: 200 μm. d MicroCT analysis of the femur of NSG and NSG oc/oc mice (n = 3 per group) and NSG oc/oc mice transplanted with human cells (n = 5).
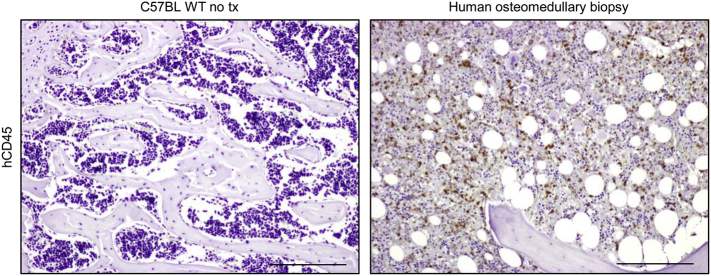
Supplementary Fig 4.
Immunohistochemical staining with anti-human CD45 antibody. Left: negative control. Representative section of formalin-fixed paraffin-embedded decalcified bone from a wild type untreated C57BL/6J mouse stained with anti-human CD45 antibody; as expected, no positive cells are found. Right: positive control. Representative section of a formalin-fixed paraffin-embedded decalcified human osteomedullary biopsy. Scale bars: 200 μm.
4. Discussion
Here we described the generation of a new mouse model of autosomal recessive osteopetrosis, which we named NSG oc/oc. This model carried the same genetic mutation as the well-known oc/oc mouse, since it was generated through a specific breeding strategy, and faithfully reproduced the key pathologic features of the parental strain. In addition, the NSG oc/oc mice displayed a complete lack of T, B and NK cells, which was the distinctive characteristic of the NSG strain.
To the best of our knowledge, there are no reports in literature of the very same approach (i.e. backcross of a disease-causing mutation into the NSG background for human hematopoietic stem cell transfer) carried out in the context of other disease models. For the sake of completeness, here we mention Brehm and colleagues, which generated the spontaneously diabetic NRG-Akita mouse model by backcrossing the Ins2Akita mutation into the NOD-Rag1null IL2rγnull background (Brehm et al., 2010). However, owing to the rapid disease course and short life-span of mice homozygous for the Ins2Akita mutation, the authors performed all studies in mice heterozygous for the Ins2Akita allele.
The original purpose of our work was to provide the scientific community with an osteopetrotic mouse that might be used as a xenotransplant model to test in vivo the functional rescue of gene-edited patient-derived cells. In fact, since several years, studies are ongoing to develop a gene therapy approach to human osteopetrosis, with particular respect to the TCIRG1-deficient form that is the most common one (Johansson et al., 2007; Moscatelli et al., 2013; Thudium et al., 2016; Moscatelli et al., 2018); moreover, in recent years other tools for gene editing and cell therapy have been implemented (Maeder and Gersbach, 2016; Doss and Sachinidis, 2019). On the other hand, at a preclinical level demonstration of a functional rescue has been limited to in vitro assays (Moscatelli et al., 2013; Thudium et al., 2016; Moscatelli et al., 2018), while in vivo studies could demonstrate only the capacity of human corrected cells to engraft and differentiate in the host, not their resorbing capacity. Therefore, we intended to provide a missing tool in preclinical research.
Since the rapid and fatal progression of the disease impeded survival of transplanted NSG oc/oc mice, correction of the skeletal pathology could not be tested in the framework of the present work. Indeed, the major obstacle was represented by the severity of the disease: all NSG oc/oc mice generated in the framework of this study became very sick and had a rapid progression of their pathology, similarly to what observed in oc/oc mice (Scimeca et al., 2000). In this respect, the Tcirg1oc mutation was invariably associated with a severe phenotype in the two different genetic backgrounds. Interestingly, in a previous work, oc/oc mice on a mixed (B6xC3x129) background were generated and, while the vast majority died at a mean age of 25 days, some of them survived up to 90 days (Blin-Wakkach et al., 2006). It is possible that, if our mouse model had had an extended life span, this could have been more compatible with the timing of human cell differentiation, which notoriously takes more time than murine cell differentiation (Abkowitz et al., 2000), and thus it could have possibly allowed achieving a benefit to the mouse from the transplanted human cells. However, by anticipating the xenotransplantation to the prenatal period, it could be possible to give transplanted cells more time to differentiate into functional osteoclasts, or at least into myeloid mature cells able to fuse with resident osteoclasts thereby rescuing their functional defect (Wakkach et al., 2008; Jacome-Galarza et al., 2019). In this regard, by performing in utero HSCT, we were able to rescue the osteopetrotic phenotype of oc/oc mice (Frattini et al., 2005; Tondelli et al., 2009); on the other hand, this kind of approach with human cells would be technically more challenging than with murine cells, requiring some refinement of the published protocol.
The transplant conditions we used, specifically the conditioning regimen, might raise some concern. Actually, despite being completely immunodeficient, NSG mice usually undergo myeloablative protocols prior to human cell transplantation, both when treated at the adult age and at the neonatal stage (Ishikawa et al., 2007; Cuddihy et al., 2012; Ko et al., 2017; Pang et al., 2019), and the radiation doses we used were in those ranges. In addition, the osteopetrotic bone is known to be a poorly permissive environment for cell engraftment, due to the lack of a proper niche, therefore a stronger host cell depletion is likely required to allow donor cell engraftment (Mansour et al., 2012). Accordingly, osteopetrotic patients are usually subjected to strong pretransplant conditioning, and only recent papers reported encouraging results in terms of outcome using reduced intensity conditioning (Bahr et al., 2016; Tolar et al., 2006; Shadur et al., 2018).
Last, osteoclast differentiation is known to crucially depend on the presence of RANKL and M-CSF. Indeed, mouse RANKL is known to be effective both on mouse and on human progenitors, at variance with mouse M-CSF which has been reported to be species specific (Gow et al., 2012). Therefore, it could be hypothesized that, if NSG oc/oc mice had survived longer, lack of human M-CSF might have anyway impeded human osteoclast formation in the murine bone. We considered this concern and conducted a pilot experiment in which together with xenotransplantation mice received repeated doses of human M-CSF; no improvement was observed in this experiment (data not shown), so this approach was not further pursued. Of note, other authors evaluated the possibility to induce ectopic expression of human M-CSF in CD34+ cells, but this strategy blunted osteoclastogenesis, at least in vitro (Montano Almendras et al., 2017).
Humanized mice expressing human M-CSF are reported in the literature (Rathinam et al., 2011) and we might have considered the possibility to use that strain instead of NSG to generate our xenograft osteopetrotic mouse model. We did not undertake this strategy, so we do not know whether in that case the short lifespan of the model and severity of the disease might have still constituted an insuperable hurdle.
5. Conclusion
In summary, this work describes the generation and characterization of the NSG oc/oc mouse model of osteoclast-rich cell-autonomous Autosomal Recessive Osteopetrosis together with immunodeficiency. We show human cell chimerism in the bone marrow of NSG oc/oc mice transplanted with human CD34+ cells. Exploitation of this new mouse model in the context of xenotransplants will likely require more attempts to set up experimental conditions, starting from our results, including for example infusion of human cytokines to foster differentiation, or further crossing with another genetic background. This notwithstanding, efforts would be likely worth the case, based on the importance of testing the functionality of human corrected cells in a relevant, live organism.
The following are the supplementary data related to this article.
Abbreviations
- ARO
Autosomal Recessive Osteopetrosis
- BM
Bone Marrow
- BMT
Bone Marrow Transplantation
- BS/TV
Bone Surface/Total Volume
- BV/TV
Bone Volume/Total Volume
- cGy
centiGray
- Cs
Caesium
- DAB
3-3'-Diaminobenzidine
- EDTA
ethylenediaminetetraacetic acid
- FBS
Fetal Bovine Serum
- GFP
Green Fluorescent Protein
- H/E
Hematoxylin/Eosin
- HLA
Human Leukocyte Antigen
- HSCT
Hematopoietic Stem Cell Transplantation
- IHC
immunohistochemical
- M-CSF
Macrophage-Colony Stimulating Factor
- μCT
Micro-computed tomography
- NSG
NOD Scid Gamma common chain
- PBS
Phosphate-buffered saline
- PCR
Polymerase chain reaction
- PFA
paraformaldehyde
- P/S
penicillin/streptomycin
- RANKL
Receptor Activator of Nf-kB Ligand
- Tb.N
Trabecular Number
- Tb.Sp
Trabecular Spacing
- Tb.Th
Trabecular Thickness
- TGFβ
Transforming Growth Factor β
- TRAP
Tartrate Resistant Acid Phosphatase
- WT
wild type
Acknowledgments
Acknowledgements
We thank Fondazione Damiano per l'Ematologia and Fondazione Nicola del Roscio for their support to SM and LC, respectively (both, fellowship's assignees). We thank Dr. Ambra Rizzo (Laboratory of Clinical Pathology and Medical Genetics, Fondazione IRCCS Istituto Neurologico Carlo Besta) for the technical assistance.
Authors' contributions
PV conceived the project. PV, AV and HCB provided financial support. CS and FF designed and supervised the experiments. EP, SM, LC, ILT, DS, SM, EF and MM performed the experiments and measurements. SLL and CCS collected the human samples. EM, HCB, CS and FF analyzed the data. CS and FF wrote the manuscript. All authors reviewed and approved the final manuscript.
Funding
This work was partially supported by the EC Seventh Framework Programme (FP7/2007-2013, SYBIL Project, n. 602300) to AV, by the Ministry of Health, Italy (Grant n. PE-2011-02347329) to PV, and by the Department of Veterans Affairs (USA) grant I01BX002490 and the National Institutes of Health (USA) grant AR065407 to HCB.
Ethics approval
The study was approved by the Institutional Animal Care and Use Committee of Humanitas Clinical and Research Center and by the Italian Ministry of Health, protocol n.473/2017-PR.
Consent for publication
Not applicable.
Availability of data and materials
The data that support the findings of this study are included in this article or available from the corresponding author upon request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abkowitz J.L., Golinelli D., Harrison D.E., Guttorp P. In vivo kinetics of murine hemopoietic stem cells. Blood. 2000;96(10):3399–3405. [PubMed] [Google Scholar]
- Askmyr M., Flores C., Fasth A., Richter J. Prospects for gene therapy of osteopetrosis. Current Gene Therapy. 2009;9(3):150–159. doi: 10.2174/156652309788488613. [DOI] [PubMed] [Google Scholar]
- Askmyr M., Holmberg J., Flores C., Ehinger M., Hjalt T., Richter J. Low-dose busulphan conditioning and neonatal stem cell transplantation preserves vision and restores hematopoiesis in severe murine osteopetrosis. Exp. Hematol. 2009;37(2):302–308. doi: 10.1016/j.exphem.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Bahr T.L., Lund T., Sando N.M., Orchard P.J., Miller W.P. Haploidentical transplantation with post-transplant cyclophosphamide following reduced-intensity conditioning for osteopetrosis: outcomes in three children. Bone Marrow Transplant. 2016;51(11):1546–1548. doi: 10.1038/bmt.2016.180. [DOI] [PubMed] [Google Scholar]
- Blin-Wakkach C., Breuil V., Quincey D., Bagnis C., Carle G.F. Establishment and characterization of new osteoclast progenitor cell lines derived from osteopetrotic and wild type mice. Bone. 2006;39(1):53–60. doi: 10.1016/j.bone.2005.12.078. [DOI] [PubMed] [Google Scholar]
- Brehm M.A., Bortell R., Diiorio P., Leif J., Laning J., Cuthbert A. Human immune system development and rejection of human islet allografts in spontaneously diabetic NOD-Rag1null IL2rgammanull Ins2Akita mice. Diabetes. 2010;59(9):2265–2270. doi: 10.2337/db10-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna L., Carlo-Stella C., Mazza R., Santoro A. Current role of autologous and allogeneic stem cell transplantation for relapsed and refractory hodgkin lymphoma. Mediterranean Journal of Hematology and Infectious Diseases. 2015;7(1) doi: 10.4084/MJHID.2015.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisafulli L., Muggeo S., Uva P., Wang Y., Iwasaki M., Locatelli S. MicroRNA-127-3p controls murine hematopoietic stem cell maintenance by limiting differentiation. Haematologica. 2019;104(9):1744–1755. doi: 10.3324/haematol.2018.198499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuddihy A.R., Suterwala B.T., Ge S., Kohn L.A., Jang J., Andrade J. Rapid thymic reconstitution following bone marrow transplantation in neonatal mice is VEGF-dependent. Biology of Blood and Marrow Transplantation: Journal of the American Society for Blood and Marrow Transplantation. 2012;18(5):683–689. doi: 10.1016/j.bbmt.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSanto J.P., Muller W., Guy-Grand D., Fischer A., Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc. Natl. Acad. Sci. U. S. A. 1995;92(2):377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doss M.X., Sachinidis A. Current challenges of iPSC-based disease modeling and therapeutic implications. Cells. 2019;8(5) doi: 10.3390/cells8050403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores C., de Vries T.J., Moscatelli I., Askmyr M., Schoenmaker T., Langenbach G.E. Nonablative neonatal bone marrow transplantation rapidly reverses severe murine osteopetrosis despite low-level engraftment and lack of selective expansion of the osteoclastic lineage. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research. 2010;25(9):2069–2077. doi: 10.1002/jbmr.90. [DOI] [PubMed] [Google Scholar]
- Frattini A., Orchard P.J., Sobacchi C., Giliani S., Abinun M., Mattsson J.P. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat. Genet. 2000;25(3):343–346. doi: 10.1038/77131. [DOI] [PubMed] [Google Scholar]
- Frattini A., Blair H.C., Sacco M.G., Cerisoli F., Faggioli F., Cato E.M. Rescue of ATPa3-deficient murine malignant osteopetrosis by hematopoietic stem cell transplantation in utero. Proc. Natl. Acad. Sci. U. S. A. 2005;102(41):14629–14634. doi: 10.1073/pnas.0507637102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S. Humanized mice: a brief overview on their diverse applications in biomedical research. J. Cell. Physiol. 2018;233(4):2889–2901. doi: 10.1002/jcp.26022. [DOI] [PubMed] [Google Scholar]
- Gow D.J., Garceau V., Kapetanovic R., Sester D.P., Fici G.J., Shelly J.A. Cloning and expression of porcine Colony Stimulating Factor-1 (CSF-1) and Colony Stimulating Factor-1 Receptor (CSF-1R) and analysis of the species specificity of stimulation by CSF-1 and Interleukin 34. Cytokine. 2012;60(3):793–805. doi: 10.1016/j.cyto.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa F., Yoshida S., Saito Y., Hijikata A., Kitamura H., Tanaka S. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat. Biotechnol. 2007;25(11):1315–1321. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- Jacome-Galarza C.E., Percin G.I., Muller J.T., Mass E., Lazarov T., Eitler J. Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature. 2019;568(7753):541–545. doi: 10.1038/s41586-019-1105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M., Jansson L., Ehinger M., Fasth A., Karlsson S., Richter J. Neonatal hematopoietic stem cell transplantation cures oc/oc mice from osteopetrosis. Exp. Hematol. 2006;34(2):242–249. doi: 10.1016/j.exphem.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Johansson M.K., de Vries T.J., Schoenmaker T., Ehinger M., Brun A.C., Fasth A. Hematopoietic stem cell-targeted neonatal gene therapy reverses lethally progressive osteopetrosis in oc/oc mice. Blood. 2007;109(12):5178–5185. doi: 10.1182/blood-2006-12-061382. [DOI] [PubMed] [Google Scholar]
- Ko Y., Jeong Y.H., Lee J.A. Transplantation of human umbilical cord blood CD34(+) cells into the liver of newborn NOD/SCID/IL-2Rgamma null (NSG) mice after busulfan conditioning. Blood Research. 2017;52(4):316–319. doi: 10.5045/br.2017.52.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Iacono N., Blair H.C., Poliani P.L., Marrella V., Ficara F., Cassani B. Osteopetrosis rescue upon RANKL administration to Rankl(−/−) mice: a new therapy for human RANKL-dependent ARO. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research. 2012;27(12):2501–2510. doi: 10.1002/jbmr.1712. [DOI] [PubMed] [Google Scholar]
- Maeder M.L., Gersbach C.A. Genome-editing technologies for gene and cell therapy. Molecular Therapy: The Journal of the American Society of Gene Therapy. 2016;24(3):430–446. doi: 10.1038/mt.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A., Abou-Ezzi G., Sitnicka E., Jacobsen S.E., Wakkach A., Blin-Wakkach C. Osteoclasts promote the formation of hematopoietic stem cell niches in the bone marrow. J. Exp. Med. 2012;209(3):537–549. doi: 10.1084/jem.20110994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz M.G., Di Santo J.P. Renaissance for mouse models of human hematopoiesis and immunobiology. Nat. Immunol. 2009;10(10):1039–1042. doi: 10.1038/ni1009-1039. [DOI] [PubMed] [Google Scholar]
- Marks S.C., Jr., Seifert M.F., Lane P.W. Osteosclerosis, a recessive skeletal mutation on chromosome 19 in the mouse. The Journal of Heredity. 1985;76(3):171–176. doi: 10.1093/oxfordjournals.jhered.a110059. [DOI] [PubMed] [Google Scholar]
- Menale C., Campodoni E., Palagano E., Mantero S., Erreni M., Inforzato A. MSC-seeded biomimetic scaffolds as a factory of soluble RANKL in Rankl-deficient osteopetrosis. Stem Cells Transl. Med. 2019;8(1):22–34. doi: 10.1002/sctm.18-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano Almendras C.P., Thudium C.S., Lofvall H., Moscatelli I., Schambach A., Henriksen K. Forced expression of human macrophage colony-stimulating factor in CD34(+) cells promotes monocyte differentiation in vitro and in vivo but blunts osteoclastogenesis in vitro. Eur. J. Haematol. 2017;98(5):517–526. doi: 10.1111/ejh.12867. [DOI] [PubMed] [Google Scholar]
- Moscatelli I., Thudium C.S., Flores C., Schulz A., Askmyr M., Gudmann N.S. Lentiviral gene transfer of TCIRG1 into peripheral blood CD34(+) cells restores osteoclast function in infantile malignant osteopetrosis. Bone. 2013;57(1):1–9. doi: 10.1016/j.bone.2013.07.026. [DOI] [PubMed] [Google Scholar]
- Moscatelli I., Lofvall H., Schneider Thudium C., Rothe M., Montano C., Kertesz Z. Targeting NSG mice engrafting cells with a clinically applicable lentiviral vector corrects osteoclasts in infantile malignant osteopetrosis. Hum. Gene Ther. 2018;29(8):938–949. doi: 10.1089/hum.2017.053. [DOI] [PubMed] [Google Scholar]
- Nakamura I., Takahashi N., Udagawa N., Moriyama Y., Kurokawa T., Jimi E. Lack of vacuolar proton ATPase association with the cytoskeleton in osteoclasts of osteosclerotic (oc/oc) mice. FEBS Lett. 1997;401(2–3):207–212. doi: 10.1016/s0014-5793(96)01454-8. [DOI] [PubMed] [Google Scholar]
- Neri T., Muggeo S., Paulis M., Caldana M.E., Crisafulli L., Strina D. Targeted gene correction in osteopetrotic-induced pluripotent stem cells for the generation of functional osteoclasts. Stem Cell Reports. 2015;5(4):558–568. doi: 10.1016/j.stemcr.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neven B., Diana J.S., Castelle M., Magnani A., Rosain J., Touzot F. Haploidentical HSCT with post-transplant CY for primary immunodeficiencies and inherited disorders in children. Biology of Blood and Marrow Transplantation: Journal of the American Society for Blood and Marrow Transplantation. 2019;25(7):1363–1373. doi: 10.1016/j.bbmt.2019.03.009. [DOI] [PubMed] [Google Scholar]
- Palagano E., Blair H.C., Pangrazio A., Tourkova I., Strina D., Angius A. Buried in the middle but guilty: intronic mutations in the TCIRG1 gene cause human autosomal recessive osteopetrosis. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research. 2015;30(10):1814–1821. doi: 10.1002/jbmr.2517. [DOI] [PubMed] [Google Scholar]
- Palagano E., Menale C., Sobacchi C., Villa A. Genetics of osteopetrosis. Current Osteoporosis Reports. 2018;16(1):13–25. doi: 10.1007/s11914-018-0415-2. [DOI] [PubMed] [Google Scholar]
- Pang W.W., Czechowicz A., Logan A.C., Bhardwaj R., Poyser J., Park C.Y. Anti-CD117 antibody depletes normal and myelodysplastic syndrome human hematopoietic stem cells in xenografted mice. Blood. 2019;133(19):2069–2078. doi: 10.1182/blood-2018-06-858159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna S., Capo V., Palagano E., Sobacchi C., Villa A. One disease, many genes: implications for the treatment of osteopetroses. Front. Endocrinol. 2019;10:85. doi: 10.3389/fendo.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronk C.J., Turkiewicz D., Vult von Steyern K., Ehinger M., Dykes J., Toporski J. Transplantation of haploidentical TcRass-depleted hematopoietic cells allows for optimal timing and sustained correction of the metabolic defect in children with infantile osteopetrosis. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research. 2017;32(1):82–85. doi: 10.1002/jbmr.2921. [DOI] [PubMed] [Google Scholar]
- Rathinam C., Poueymirou W.T., Rojas J., Murphy A.J., Valenzuela D.M., Yancopoulos G.D. Efficient differentiation and function of human macrophages in humanized CSF-1 mice. Blood. 2011;118(11):3119–3128. doi: 10.1182/blood-2010-12-326926. [DOI] [PubMed] [Google Scholar]
- Schinke T., Schilling A.F., Baranowsky A., Seitz S., Marshall R.P., Linn T. Impaired gastric acidification negatively affects calcium homeostasis and bone mass. Nat. Med. 2009;15(6):674–681. doi: 10.1038/nm.1963. [DOI] [PubMed] [Google Scholar]
- Scimeca J.C., Franchi A., Trojani C., Parrinello H., Grosgeorge J., Robert C. The gene encoding the mouse homologue of the human osteoclast-specific 116-kDa V-ATPase subunit bears a deletion in osteosclerotic (oc/oc) mutants. Bone. 2000;26(3):207–213. doi: 10.1016/s8756-3282(99)00278-1. [DOI] [PubMed] [Google Scholar]
- Shadur B., Zaidman I., NaserEddin A., Lokshin E., Hussein F., Oron H.C. Successful hematopoietic stem cell transplantation for osteopetrosis using reduced intensity conditioning. Pediatr. Blood Cancer. 2018;65(6) doi: 10.1002/pbc.27010. [DOI] [PubMed] [Google Scholar]
- Shultz L.D., Lyons B.L., Burzenski L.M., Gott B., Chen X., Chaleff S. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 2005;174(10):6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- Sobacchi C., Villa A., Schulz A., Kornak U. In: CLCN7-Related Osteopetrosis. Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Stephens K., editors. GeneReviews((R)); Seattle (WA): 1993. [PubMed] [Google Scholar]
- Sobacchi C., Schulz A., Coxon F.P., Villa A., Helfrich M.H. Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat. Rev. Endocrinol. 2013;9(9):522–536. doi: 10.1038/nrendo.2013.137. [DOI] [PubMed] [Google Scholar]
- Sobacchi C., Pangrazio A., Lopez A.G., Gomez D.P., Caldana M.E., Susani L. As little as needed: the extraordinary case of a mild recessive osteopetrosis owing to a novel splicing hypomorphic mutation in the TCIRG1 gene. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research. 2014;29(7):1646–1650. doi: 10.1002/jbmr.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stattin E.L., Henning P., Klar J., McDermott E., Stecksen-Blicks C., Sandstrom P.E. SNX10 gene mutation leading to osteopetrosis with dysfunctional osteoclasts. Sci. Rep. 2017;7(1):3012. doi: 10.1038/s41598-017-02533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepensky P., Grisariu S., Avni B., Zaidman I., Shadur B., Elpeleg O. Stem cell transplantation for osteopetrosis in patients beyond the age of 5 years. Blood Advances. 2019;3(6):862–868. doi: 10.1182/bloodadvances.2018025890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka K., Prasolava T.K., Wang J.C., Mortin-Toth S.M., Khalouei S., Gan O.I. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat. Immunol. 2007;8(12):1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- Teti A., Econs M.J. Osteopetroses, emphasizing potential approaches to treatment. Bone. 2017;102:50–59. doi: 10.1016/j.bone.2017.02.002. [DOI] [PubMed] [Google Scholar]
- Thudium C.S., Moscatelli I., Lofvall H., Kertesz Z., Montano C., Bjurstrom C.F. Regulation and function of lentiviral vector-mediated TCIRG1 expression in osteoclasts from patients with infantile malignant osteopetrosis: implications for gene therapy. Calcif. Tissue Int. 2016;99(6):638–648. doi: 10.1007/s00223-016-0187-6. [DOI] [PubMed] [Google Scholar]
- Tolar J., Bonfim C., Grewal S., Orchard P. Engraftment and survival following hematopoietic stem cell transplantation for osteopetrosis using a reduced intensity conditioning regimen. Bone Marrow Transplant. 2006;38(12):783–787. doi: 10.1038/sj.bmt.1705533. [DOI] [PubMed] [Google Scholar]
- Tondelli B., Blair H.C., Guerrini M., Patrene K.D., Cassani B., Vezzoni P. Fetal liver cells transplanted in utero rescue the osteopetrotic phenotype in the oc/oc mouse. Am. J. Pathol. 2009;174(3):727–735. doi: 10.2353/ajpath.2009.080688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakkach A., Mansour A., Dacquin R., Coste E., Jurdic P., Carle G.F. Bone marrow microenvironment controls the in vivo differentiation of murine dendritic cells into osteoclasts. Blood. 2008;112(13):5074–5083. doi: 10.1182/blood-2008-01-132787. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are included in this article or available from the corresponding author upon request.