Abstract
INTRODUCTION:
Reduced hippocampal volume is associated with late life cognitive decline, but prior studies have not determined whether this association persists after accounting for Alzheimer’s and other neuropathologies.
METHODS:
Participants were 531 deceased older adults from community-based cohort studies of aging who had undergone annual cognitive evaluations. At death, brain tissue underwent neuropathologic examination and MRI. Linear mixed models examined whether hippocampal volume measured via MRI accounted for variation in decline rate of global cognition and five cognitive domains, above and beyond neuropathologic indices.
RESULTS:
Demographics and indices of Alzheimer’s disease, cerebrovascular disease, Lewy body disease, hippocampal sclerosis, TDP-43, and atherosclerosis accounted for 42.6% of the variation in global cognitive decline. Hippocampal volume accounted for an additional 5.4% of this variation and made similar contributions in four of the five cognitive domains.
DISCUSSION:
Hippocampal volume is associated with late life cognitive decline, above and beyond contributions from common neuropathologic indices.
Keywords: Atrophy, older adults, Alzheimer’s disease, TDP-43, hippocampal sclerosis
1. INTRODUCTION
Cognitive decline is a common, costly, and feared consequence of aging [1,2]. However, individuals deteriorate at markedly different rates, with some declining precipitously, others remaining relatively stable until death, and many falling somewhere between those extremes [3,4]. While much of this variation can be accounted for by histopathologic indices of the known drivers of dementia including Alzheimer’s disease (AD), cerebrovascular disease (CVD), and Lewy body disease (LBD), about half remains unexplained by common neuropathologic indices [3]. Identifying other neurobiologic factors that contribute to this unexplained variation in the rate of cognitive decline is crucial to the development of additional interventional strategies that may effectively delay the onset of late life cognitive decline or slow its progression.
Brain volumetric measures represent one such avenue for investigation beyond neuropathologic indices derived via histopathology [4]. Since they are readily available via MRI, they provide a potentially important in vivo window to neuropathology that led to brain atrophy. Atrophy is a common correlate of several forms of dementia [5–7], suggesting that it reflects nonspecific neuronal loss resulting from a variety of upstream causes, including AD and other known neuropathologies [8–10], but also perhaps from other pathologies that have yet to be identified or well quantified. Hippocampal volume in particular has been tied to decline in multiple cognitive domains including episodic, semantic, and working memory and visuospatial ability [11–13]. These associations between hippocampal volume and cognitive decline are often attributed to underlying AD pathology, particularly neurofibrillary tangles [14]. However, clinical-pathologic data are needed to clarify these relationships; prior studies have not determined whether hippocampal volume’s association with cognitive decline persists after accounting for AD and other commonly quantified pathologies.
We approached this question by analyzing postmortem MRI and histopathologic data from 531 participants in two clinicopathologic studies of aging. Participants underwent detailed annual cognitive evaluations over many years before death, providing an estimate of their longitudinal decline trajectory. Via a series of linear mixed models, we quantified the degree to which hippocampal volume derived from MRI accounted for variation in the decline rate of global cognition and five individual cognitive domains, beyond the contributions of common neurodegenerative and vascular pathologic indices as well as total cerebral volume.
2. METHODS
2.1. Participants
Participants were drawn from two clinicopathologic studies of aging, the Rush Memory and Aging Project (MAP) and the Religious Orders Study (ROS) [15]. MAP and ROS enrollees provided written informed consent to annual interviews, including detailed cognitive evaluations, and organ donation at death, in accordance with protocols approved by the Rush University Institutional Review Board. These procedures have been harmonized between MAP and ROS to allow pooling of their data in analyses. To be included in this study, participants were required to have at least two annual cognitive evaluations, autopsy data, and postmortem brain MRI. At the time of our analyses in November of 2018, a total of 3,485 participants had completed baseline testing (2,037 in MAP and 1,448 in ROS). Since the inception of postmortem MRI in MAP and ROS in October of 2006, 1,212 participants died and 87% (1,054) of those cases came to autopsy, 671 of which underwent postmortem brain MRI. Quality control and post-processing had been performed on the first 552 of these, and we further excluded 21 participants who had only undergone one cognitive exam prior, leaving a sample of 531.
2.2. Cognitive Evaluation and Clinical Diagnosis
Trained study staff administered a battery of cognitive tests at annual study visits in MAP and ROS. The Mini-Mental State Exam was used for descriptive purposes only. As described previously, a composite score of global cognition was computed based on z-scores from 17 other tests that are common to both studies [16]. Scores representing performance in five cognitive domains were computed based on subsets of the 17 tests defined in previous work [16]. Clinical classification of dementia and cognitive impairment was carried out via a three-step process implemented at the inception of MAP and ROS. In the first step, computerized scoring of 11 of the cognitive tests generated impairment ratings in five cognitive domains [15,17]. Next, a neuropsychologist reviewed these computer-generated ratings and rendered a judgment on impairment in light of cognitive data, education, sensorimotor function, and motivation, but blinded to all other data. In the final step, an experienced clinician reviewed the cognitive data, neuropsychologist’s impairment ratings, medical history, and results of neurologic examination, and rendered a decision on whether dementia was present and its likely cause. Clinical diagnosis of Alzheimer’s dementia was based on criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA) [17,18]. These criteria are consistent with our previous work as well as with most other research on the clinical syndrome of Alzheimer’s dementia. They include a history of cognitive decline with impairment in memory and at least one other cognitive domain. Cases of cognitive impairment not meeting the criteria for dementia were classified as mild cognitive impairment (MCI) [15,18]. At death, a board-certified neurologist reviewed all available clinical data and rendered a summary diagnostic opinion of the most likely clinical diagnosis at the time of death [19].
2.3. Postmortem Brain MRI and Volumetric Measures
Upon death, the brain was removed and hemisected during rapid autopsy using previously described techniques. One cerebral hemisphere was immersed in 4% paraformaldehyde solution and refrigerated at 4 degrees Celsius in preparation for imaging and neuropathologic evaluation [15]. The other hemisphere was frozen in preparation for alternative processing, including genomic and proteomic techniques. At approximately 30 days postmortem, this specimen was warmed to room temperature and imaged in a 3-Tesla MRI scanner using techniques and pulse sequences described previously [20,21].
In the current study, we used images derived from a multi-spin echo sequence to compute the transverse relaxation rate constant (R2, the multiplicative inverse of T2) for each voxel, as previously described [22]. Images from the shortest echo time (TE) were spatially registered to a previously developed postmortem cerebral hemisphere template, using affine followed by nonlinear transformations obtained with FSL’s ‘flirt’ tool [23] and the Automated Registration Toolbox [24], respectively [21]. We produced a three-dimensional hippocampus mask based on this template by manually outlining the hippocampal formations in consecutive sagittal slices of the template, while referencing the axial and coronal planes to confirm key landmark positions, similar to our previously described techniques [25]. In the same manner, we produced three-dimensional masks of the amygdala, thalamus, caudate, putamen, globus pallidus, and nucleus accumbens [26]. We also produced a whole-hemisphere mask, which included any voxel containing a nonzero intensity in the template. These masks were back-transformed from template space into the original space of each individual brain image by applying the inverted forms of the affine and nonlinear transformations for each hemisphere, which were concatenated into a single transformation. We eliminated non-tissue-containing voxels and calculated the resultant volume of each back-transformed mask (Fig. 1). We did not normalize any of the regional volumes by total hemisphere volume because the latter is influenced to an unknown degree by generalized atrophy and could therefore interfere with the study’s goal of exploring the relationship between hippocampal volume and cognitive decline, beyond the effects stemming from generalized atrophy. Total intracranial volumes may be more appropriate for regional volume normalization, but these were not available from postmortem MRI.
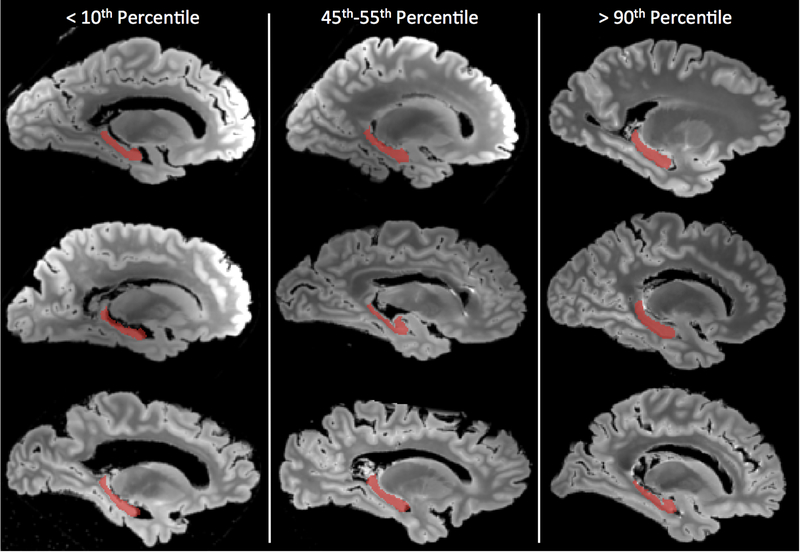
Figure 1.
Representative hippocampal segmentations (red) overlaid on sagittal slices of the shortest echo time image from a fast spin echo MRI pulse sequence carried out postmortem. Three ranges are represented: hippocampi with volume below the 10th percentile are shown in the left column, those between the 45th and 55th percentile in the middle column, and those above the 90th percentile in the right column. For each range, three cases were randomly selected for display.
2.4. Neuropathologic Indices
Following postmortem MRI, the cerebral hemisphere was sliced coronally into 1-cm thick slabs, which underwent further sectioning and staining to facilitate collection of common age-related pathologic indices. Global AD pathology burden was quantified by averaging the scaled counts of neuritic and diffuse plaques and neurofibrillary tangles derived from 1-mm2 area of greatest density on silver-stained 6-μm sections from the hippocampus and four cortical regions [15]. Visually identified chronic gross infarcts on any of the slabs were noted and coded as present upon histologic confirmation [27,28]. Similarly, chronic microinfarcts were coded as present if they were identified on hematoxylin and eosin-stained (H&E) 6-μm sections from at least 9 regions including hippocampus, five cortical regions, basal ganglia, thalamus, or midbrain [27]. Lewy bodies were coded as present if they were identified using antibodies to α-synuclein on 6-μm sections from the hippocampus or any of six cortical regions [29]. Hippocampal sclerosis marked by severe neuronal loss and gliosis identified on H&E-stained sections in CA1 or the subiculum was graded as present or absent [30]. TDP-43 pathology was semi-quantitatively graded based on immunostaining of six 6-μm sections using antibodies to a phosphorylated monoclonal TAR5P-1D3 (pS409/410; 1:100, Ascenion, Munich, Germany), with four stages corresponding to absent (Stage 0), restricted to amygdala only (Stage 1), extending also to the CA1 or dentate of the hippocampus or entorhinal cortex (Stage 2), or further extending to midfrontal or midtemporal neocortical regions (Stage 3) [30,31]. Cerebral atherosclerosis was graded on a semi-quantitative scale (none, mild, moderate, severe) based on the number of vessels in the Circle of Willis that were affected and the severity of their occlusion [32]. Arteriolosclerosis was graded on a similar four-level scale based on histologic changes in arterioles of the anterior basal ganglia [33]. Cerebral amyloid angiopathy (CAA) was also scored semi-quantitatively on a four-level scale based on beta-amyloid deposition in meningeal and parenchymal vessels as observed in sections from four neocortical regions immunostained using 4G8 (1:9000; Covance Labs, Madison, WI), 6F/3D (1:50; Dako North America Inc., Carpinteria, CA), or 10D5 (1:600; Elan Pharmaceuticals, San Francisco, CA) [34]. A board-certified neuropathologist reviewed all pathology findings.
2.5. Statistical Analyses
We first employed a linear mixed model to quantify the heterogeneity among study participants in terms of the total variation in rates of global cognitive decline. All data points from every participant were analyzed simultaneously such that the sample-wide average rate of decline was estimated, with person-specific factors capturing individuals’ deviations above or below this typical rate, as previously described [22]. We then expanded the model sequentially to include terms for demographics (age, sex, education), nine neuropathologic indices, hippocampal volume, total hemisphere volume, and each of six other brain region volumes from postmortem MRI. Accompanying terms for each factor’s interaction with the time variable were also included. After each stepwise addition of terms to the model, we again assessed the total variation in rate of global cognitive decline, quantifying the reduction relative to previous models as a percentage. Using this procedure, we inferred the contribution of each variable or set of variables to variation in cognitive decline, beyond that attributable to terms in the previous models. We repeated this procedure for each of five cognitive domains.
3. RESULTS
3.1. Descriptive Data
As shown in Table 1, about two-thirds of the 531-person sample were MAP participants (62.7%), with the rest coming from ROS. Most were female (71.2%) and white (98.3%). The average participant had 15.8 years of education (SD = 3.6, range = 3–30) and died at age 90.4 (SD = 6.0, range = 65.9–108.3). At baseline, 26.6% of participants had MCI, while only 5.8% met the criteria for Alzheimer’s dementia, owing to the fact that MAP and ROS enroll individuals who are free of known dementia. Their corresponding average MMSE scores and composite scores of global cognition at baseline were 27.7 (out of 30) and −0.1 normalized unit, respectively. On average, participants completed more than 9 annual cognitive evaluations (range = 2–22), declining during that time to an average MMSE of 20.3 and global cognition score of −1.0 at their last evaluation. At death, 42.0% met the criteria for Alzheimer’s dementia while the frequency of MCI remained similar to baseline at 26.0%, and the remaining 32.0% were without cognitive impairment. Neuropathologic evaluation revealed substantial AD pathology, gross and microscopic infarcts, and Lewy bodies, but also the presence of hippocampal sclerosis, TDP-43, and moderate to severe CAA, atherosclerosis, and arteriolosclerosis, as detailed in Table 1.
Table 1.
Demographic, cognitive, and pathologic characteristics of participants
| Variable | Mean (SD) or N (%) |
|---|---|
| Total Participants | 531 |
| MAP | 333 (62.7%) |
| ROS | 198 (37.3%) |
| Demographic | |
| Age at Baseline (years) | 80.4 (6.7) |
| Age at Death (years) | 90.4 (6.0) |
| Female | 378 (71.2%) |
| Education (years) | 15.8 (3.6) |
| White, Non-Hispanic | 522 (98.3%) |
| Clinical Diagnosis | |
| Cognitive Impairment at Baseline | |
| Mild Cognitive Impairment (MCI) | 141 (26.6%) |
| Alzheimer’s Dementia | 31 (5.8%) |
| Cognitive Impairment at Death | |
| Mild Cognitive Impairment (MCI) | 138 (26.0%) |
| Alzheimer’s Dementia | 223 (42.0%) |
| Cognitive | |
| Number of Annual Cognitive Evaluations | 9.3 (4.7) |
| MMSE, Baseline (score out of 30) | 27.7 (2.8) |
| MMSE, Proximate to Death | 20.3 (9.3) |
| Global Cognition, Baseline (composite of 17 z-scores) | −0.1 (0.6) |
| Global Cognition, Proximate to Death | −1.0 (1.2) |
| Global Cognition, Estimated Linear Rate of Change (per year) | −0.099 (0.004) |
| Pathologic | |
| Postmortem Interval (hours) | 8.8 (5.8) |
| AD Pathology (composite of scaled counts) | 0.78 (0.61) |
| Gross Infarcts (present) | 180 (33.9%) |
| Microscopic Infarcts (present) | 166 (31.3%) |
| Lewy Bodies (present) | 128 (24.1%) |
| Hippocampal Sclerosis (present) | 63 (11.9%) |
| TDP-43 (Stage 2–3) | 289 (35.6%) |
| Cerebral Amyloid Angiopathy (moderate to severe) | 122 (23.0%) |
| Atherosclerosis (moderate to severe) | 142 (26.7%) |
| Arteriolosclerosis (moderate to severe) | 138 (26.0%) |
3.2. Heterogeneity in Rates of Cognitive Decline
The average rate of decline in global cognition estimated via linear mixed models was −0.099 unit per year (SE = 0.004, p < 0.0001). Consistent with previous work, there was considerable heterogeneity among participants, with individuals at the 90th percentile declining much more slowly (−0.011 unit/year) than those at the 10th percentile (−0.22 unit/year). We observed similar heterogeneity in decline rates for episodic memory, semantic memory, working memory, perceptual speed, and visuospatial ability (Table 2).
Table 2.
Rates of cognitive decline by domain
| Domain | Mean Decline Rate (units/year) | SE | p | 10th Percentile | 90th Percentile |
|---|---|---|---|---|---|
| Global Cognition | −0.099 | 0.004 | < 0.0001 | −0.22 | −0.011 |
| Episodic Memory | −0.098 | 0.005 | < 0.0001 | −0.24 | −0.011 |
| Semantic Memory | −0.091 | 0.005 | < 0.0001 | −0.21 | −0.012 |
| Working Memory | −0.085 | 0.004 | < 0.0001 | −0.16 | −0.023 |
| Perceptual Speed | −0.127 | 0.005 | < 0.0001 | −0.24 | −0.039 |
| Visuospatial Ability | −0.059 | 0.004 | < 0.0001 | −0.12 | −0.011 |
3.3. Variance in Rates of Cognitive Decline Accounted for by Neuropathology
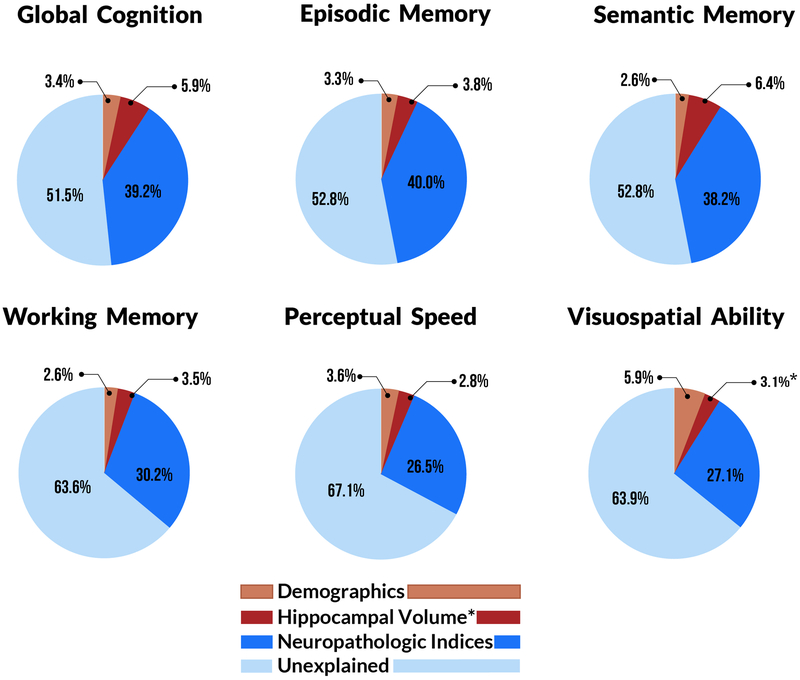
According to linear mixed models that included factors for age, sex, and education, these demographic variables accounted for 3.4% of the variation in global cognitive decline rate (Table 3, Fig. 2) and between 2.6% and 5.9% of the variation in the decline rates of the five cognitive domains (Fig. 2). To each of these six core models, we added nine pathologic indices. We found that microinfarcts, arteriolosclerosis, and CAA were not significantly associated with decline in global cognition or in any domain in this dataset, so we removed them from all subsequent analysis. The other six indices (AD, gross infarcts, LBD, hippocampal sclerosis, TDP-43, and atherosclerosis) were each associated with the rates of decline in global cognition and one or more cognitive domains. Together, they accounted for 39.2% of the variation in the rate of decline of global cognition beyond that accounted for by demographics (Table 3, Fig. 2), and between 26.5% (perceptual speed) and 40.0% (episodic memory) of the variation in decline in the cognitive domains (Fig. 2).
Table 3.
Contributions to variance in the rate of global cognitive decline from demographics, neuropathologic indices, and brain volumetric measures
| Variables | Model Identifiers | |||
|---|---|---|---|---|
| Ref | 1 | 2 | 3 | |
| Time | −0.100 (0.004)e | −0.108 (0.005)e | 0.002 (0.009) | −0.018 (0.009)a |
| Age | 0.001 (0.001) | 0.002 (0.001)d | 0.003 (0.001)e | |
| Sex | 0.024 (0.010)b | 0.009 (0.008) | −0.006 (0.008) | |
| Education | 0.003 (0.001)c | 0.002 (0.001)b | 0.002 (0.001)b | |
| AD Pathology | −0.068 (0.006)e | −0.058 (0.006)e | ||
| Gross Infarcts | −0.029 (0.008)d | −0.027 (0.008)d | ||
| Lewy Bodies | −0.026 (0.009)c | −0.025 (0.008)c | ||
| Hippocampal Sclerosis | −0.058 (0.012)e | −0.040 (0.012)d | ||
| TDP-43 | −0.009 (0.004)c | −0.006 (0.004)a | ||
| Arteriolosclerosis | −0.019 (0.005)e | −0.015 (0.005)d | ||
| Hippocampal Volume | 0.061 (0.010)e | |||
| % of Variance Accounted for by All Effects | - | 3.4% | 42.6% | 48.0% |
| % of Variance Accounted for by Effects in Boldface Only | - | 3.4% | 39.2% | 5.4% |
| Model for Comparison | - | Ref | 1 | 2 |
p < 0.1,
p < 0.05,
p < 0.01,
p < 0.001,
p < 0.0001
Figure 2.
Variation in the rates of cognitive decline that is either unexplained or accounted for by each of three different factors, for global cognition and each of five domains. In all domains, greater than 50% of the variation was unexplained, while neuropathologic indices accounted for 26.5–40.0%, and hippocampal volume accounted for up to 6.4% in the semantic memory domain. Hippocampal volume accounted for the greatest portion of variation in decline for all domains except visuospatial ability, where it accounted for 0.8% of variation. *Thus, for the visuospatial domain only, the contribution of globus pallidus volume (3.1%) rather than hippocampal volume is depicted.
3.4. Variance in Rates of Cognitive Decline Accounted for by Hippocampal and Total Hemisphere Volume
Retaining demographics and the six significant neuropathologic indices in the linear mixed models, we next added terms for hippocampal volume derived from postmortem MRI. Hippocampal volume was associated with decline in global cognition and accounted 5.4% of its variance, beyond that attributable to demographics and indices of pathology (p < 0.001) (Table 3, Fig. 2). Hippocampal volume was also associated with decline in all five individual cognitive domains examined in this work (visuospatial: p = 0.007, all others: p < 0.001). It accounted for the most variance in the decline rate for semantic memory (6.4%), which was approximately twice that of episodic memory (3.8%), working memory (3.4%), and perceptual speed (2.8%) (Table 4, Fig. 2). Hippocampal volume accounted for the least variance in the decline rate for visuospatial ability (0.8%, not shown in Fig. 2). The total variation in decline rate accounted for by the combination of demographics, neuropathologic indices, and hippocampal volume was 47.8% for global cognition and between 32.9% (perceptual speed) and 47.2% (episodic memory) for each of five cognitive domains (Fig. 2).
Table 4.
Contributions to variance in the rate of cognitive decline in five domains from the hippocampal volumetric measure
| Variables | Model Outcome (Cognitive Domain) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Episodic Memory | Semantic Memory | Working Memory | Perceptual Speed | Visuospatial Ability | ||||||
| Time | 0.024 (0.011)b | 0.004 (0.011) | 0.008 (0.010) | −0.018 (0.011) | −0.015 (0.009)a | −0.030 (0.010)c | −0.048 (0.011)e | −0.065 (0.011)e | −0.008 (0.009) | −0.017 (0.009)a |
| Age | 0.002 (0.001)b | 0.002 (0.001)c | 0.002 (0.001)c | 0.003 (0.001)e | 0.002 (0.001)c | 0.002 (0.001)d | 0.002 (0.001)c | 0.003 (0.001)d | 0.002 (0.001)b | 0.002 (0.001)c |
| Sex | 0.005 (0.009) | −0.012 (0.010) | 0.013 (0.009) | −0.003 (0.010) | 0.002 (0.008) | −0.008 (0.009) | 0.015 (0.010) | −0.001 (0.010) | 0.018 (0.007)b | 0.011 (0.008) |
| Education | 0.003 (0.001)c | 0.003 (0.001) | 0.001 (0.001) | 0.000 (0.001) | 0.002 (0.001)b | 0.002 (0.001)b | 0.002 (0.001) | 0.002 (0.001) | 0.000 (0.001) | 0.000 (0.001) |
| AD Pathology | −0.078 (0.007)e | −0.069 (0.008)e | −0.085 (0.007)e | −0.072 (0.007)e | −0.045 (0.005)e | −0.039 (0.006)e | −0.052 (0.007)e | −0.043 (0.008)e | −0.040 (0.006)e | −0.036 (0.006)e |
| Gross Infarcts | −0.035 (0.009)d | −0.034 (0.009)d | −0.022 (0.009)b | −0.019 (0.009)b | −0.032 (0.008)e | −0.031 (0.008)e | −0.018 (0.009)a | 0.018 (0.009)a | −0.005 (0.007) | −0.004 (0.007) |
| Lewy Bodies | −0.010 (0.010) | −0.009 (0.010) | −0.018 (0.010)a | −0.017 (0.010)a | −0.022 (0.009)c | −0.022 (0.008)b | 0.047 (0.010)e | −0.047 (0.010)e | −0.021 (0.008)b | −0.020 (0.008)b |
| Hippocampal Sclerosis | −0.075 (0.014)e | −0.058 (0.014)e | −0.073 (0.014)e | −0.050 (0.014)d | −0.026 (0.012)b | −0.014 (0.012) | −0.045 (0.014)c | −0.029 (0.015)a | −0.023 (0.012)b | −0.015 (0.012) |
| TDP-43 | −0.016 (0.004)d | −0.013 (0.004)c | −0.006 (0.004) | −0.003 (0.004) | −0.002 (0.004) | −0.001 (0.004) | −0.004 (0.004) | −0.001 (0.004) | −0.004 (0.004) | −0.002 (0.004) |
| Arteriolosclerosis | −0.022 (0.005)e | −0.019 (0.005)d | −0.009 (0.005)a | −0.005 (0.005) | −0.014 (0.005)c | −0.011 (0.005)b | −0.017 (0.006)c | −0.014 (0.005)c | −0.013 (0.004)c | −0.012 (0.004)c |
| Hippocampal Volume | 0.067 (0.015)e | 0.071 (0.015)e | 0.044 (0.013)d | 0.060 (0.016)e | 0.029 (0.013)b | |||||
| % of Variance Accounted for by All Effects | 43.3% | 47.1% | 40.8% | 47.2% | 32.8% | 36.4% | 30.1% | 32.9% | 33.1% | 33.8% |
| % of Variance Accounted for by Hippocampal Volume | - | 3.8% | - | 6.4% | - | 3.5% | - | 2.8% | - | 0.8% |
p < 0.1,
p < 0.05,
p < 0.01,
p < 0.001,
p < 0.0001
Since the associations of hippocampal volume with cognitive decline could be driven by generalized brain atrophy, we repeated the previous models while further controlling for whole hemisphere volume, which was moderately correlated with hippocampal volume (Pearson’s r = 0.57, p < 0.0001). We did not observe significant associations between hemisphere volume and cognitive decline. Of particular note, the associations between hippocampal volume and decline were essentially unchanged by the addition of whole hemisphere volume to the models.
3.5. Variance in Rates of Cognitive Decline Accounted for by Other Brain Region Volumes
We next examined the associations between cognitive decline and six other deep brain regions (amygdala, thalamus, caudate, putamen, globus pallidus, and nucleus accumbens), which exhibited mild to moderate correlations with hippocampal volume (p < 0.0001 for all six volumes, Pearson’s r ranging from 0.44 for the nucleus accumbens to 0.77 for the amygdala). We entered terms for each these regional volumes, one at a time, in the models for global cognition and each cognitive domain. There were two instances in which these additional volumes were significantly associated with the rate of decline after controlling for hippocampal volume. The volume of the amygdala was associated with the rate of decline in global cognition, though it accounted for less than 1% of the variance in decline rate and was quite weakly associated in comparison to hippocampal volume (p = 0.037 for amygdala volume vs. p = 0.001 for hippocampal volume). The volume of the globus pallidus, however, was not related to decline in global cognition but was more strongly associated than hippocampal volume with the rate of decline in visuospatial ability (p = 0.007 for globus pallidus volume vs. p = 0.13 for hippocampal volume), and accounted for 3.1% of the variance in decline, as reflected in Fig. 2.
4. DISCUSSION
In a group of more than 500 older adults, we examined the associations between hippocampal volume derived from postmortem MRI and the rate of late life cognitive decline estimated from up to 22 annual cognitive evaluations, after controlling for common neurodegenerative and vascular brain pathologies and generalized brain atrophy. Hippocampal volume was associated with the rate of decline in global cognition and five cognitive domains, after controlling for the effects of nine common age-related pathologies, including AD, as well as total cerebral volume. In particular, hippocampal volume accounted for 5.4% of the variation in the decline rate of global cognition and up to 6.4% in individual cognitive domains, beyond the percentages attributed to available neuropathologic indices. These findings suggest that in addition to the known neuropathologic indices that were accounted for in this study, late life cognitive abilities are also influenced by other factors that are associated with hippocampal volume loss but were either not included in this study or have not yet been identified. Future studies based on this work that incorporate volumetric measures from other brain regions and additional imaging-based indicators of brain integrity might provide valuable clues for identifying targetable determinants of cognitive decline. Such studies are highly warranted in light of the looming public health crisis created by the combination of high incidence of cognitive impairment among older persons and the rapid growth of our older population.
The current study makes a unique contribution to our understanding of the relationship between hippocampal volume and late life cognitive decline by clarifying the degree to which this association persists after accounting for currently known and quantified neuropathologic drivers of cognitive impairment. Numerous studies investigated the link between hippocampal volume and cognition [35,36], several others explored the neuropathologic correlates of hippocampal atrophy [14,37], and our own past work elucidated the association between hippocampal volume and level of cognition proximate to death, after accounting for neuropathology [38]. However, the key finding of the current study has not been reported previously. Namely, the association of hippocampal volume with late life cognitive decline rate persists even after adjustment for a broad range of neurodegenerative and vascular neuropathologic indices, including AD. This finding partially differs from a recent study that did not find cognitive decline attributable to reduced hippocampal volume beyond brain amyloidosis, a marker of AD pathology [39]. Results of the current work instead suggest that the association between hippocampal volume and cognitive decline is indeed partially influenced by factors other than AD and other known neuropathologic drivers of cognitive decline. This has important implications for charting the course of development of therapeutic strategies to combat late life cognitive decline. For example, our findings provide evidence that in Alzheimer’s dementia clinical trials employing hippocampal volume either as part of an enrichment strategy or as an outcome measure, the apparent effectiveness of a therapy against Alzheimer’s-related hippocampal atrophy and the associated cognitive decline could be influenced by the presence of non-targeted pathologies or factors [40].
Thus, this study further emphasizes the need for investigations aimed at uncovering additional factors that contribute to late life cognitive decline. It also provides a potentially valuable clue that some of these factors exert vital influence on cognition via their effects on hippocampal volume. There are several possible mechanisms by which this could occur. For example, additional age-related neuropathologies that have not yet been identified or quantified may lead to hippocampal atrophy and cognitive impairments. Another possibility is that hippocampal neurogenesis, the subject of recent controversy as to whether it actually occurs in late life [41,42], affects the total number of neurons and thus the volume of the structure in old age, with an accompanying effect on cognition. There is also evidence that development and continued health of the hippocampi are negatively affected by chronic psychological distress due to, for example, stress hormones such as cortisol [43,44] or decreased density of dendrites and dendritic spines [45]. It is therefore possible that stressful experiences throughout life erode a potential store of cognitive resilience that might otherwise have been imparted in the form of well-developed and maintained hippocampi. As some of these factors will be more readily targetable than others, it is necessary to elucidate their relative contributions to variation in hippocampal volume and cognition in order to judiciously allocate resources to the development of different interventions.
Although the major findings of this study are based on analyses in which global cognition was the outcome, we also report results from domain-specific analyses. Of particular interest, hippocampal volume accounted for approximately twice as much variation in decline of semantic memory (6.4%) than that of episodic memory (3.5%). This was somewhat surprising, because although both forms of memory are served by the hippocampus [46], most evidence suggests that episodic memory is more tightly linked to hippocampal function [47], with semantic memory relying to a greater degree on surrounding medial temporal regions [48,49]. This apparent discrepancy can be partially explained by the fact that demographics and neuropathologic indices accounted for slightly more variation in decline of episodic memory (43.3%) than that of semantic memory (40.8%) (Fig. 2), some of which might otherwise have been attributed to hippocampal volume.
Our analyses also demonstrated that total hemisphere volume makes no appreciable contribution to the variation in decline when considered in the same models alongside hippocampal volume. Since any form of cognition involves communication among multiple regions of the brain, we assumed that generalized brain atrophy would complement hippocampal volume in accounting for variation in cognitive decline rates. This was not the case in our analyses of global cognition or in any of five cognitive domains, as the effect of total hemisphere volume was not significant, and the explained percentage of variation in decline rates remained essentially unchanged regardless of whether hemisphere volume was included in the models with hippocampal volume.
In addition, we considered the associations between the volumes of six other deep brain regions and cognitive decline. The volume of the globus pallidus was associated with the rate of decline in visuospatial ability, accounting for 3.1% of its variance and resulting in a weakening of the association between hippocampal volume and decline in that domain to a non-significant level. This appears to be a unique finding in the literature and may warrant further investigation. However, this domain-specific finding reflects the only instance in which any of the six volumes exhibited an association with decline that was stronger than hippocampal volume’s association. These results reaffirm the role of hippocampal volume as a particularly consistent determinant of late life decline in multiple cognitive domains.
More than half of the total variation in rates of decline remained unexplained (Fig. 2) after accounting for demographics, neuropathologic indices, and volumes of multiple deep brain regions derived from MRI. A number of factors could be responsible for this phenomenon, and we offer three possibilities. First, our current quantification of age-related neuropathology is incomplete; this is in part because there likely are additional, as yet unidentified pathologies that contribute to decline and also because some of our pathologies are measured via binary measures (present vs. absent) such that we do not capture the full burden of disease. Second, resilience factors that buffer against cognitive decline even in the face of accumulating pathology may also play a role. Third, because the same cognitive tests are administered annually, practice effects may differentially influence scores among individuals. Additional work is needed to further understand the basis of the unexplained decline.
The availability of both volumetric and neuropathologic data in the same study is rare because MRI is usually carried out during life, whereas most types of neuropathologic data are only available upon postmortem examination of tissue. A major strength of this work was the circumvention of this difficulty by employing postmortem brain MRI in ongoing clinicopathologic studies of aging in which cognition was assessed annually and all participants agree to brain donation as condition of enrollment. This approach facilitated the relatively rapid accumulation of cases with all three types of data required for our analyses. Although antemortem MRI is also underway in the MAP and ROS cohorts, the respective sample sizes at the time of our analyses still favored the use of postmortem MRI.
Limitations of the study are also noted. First, hippocampal volume was assessed at only one timepoint at the end of life. This measure is sensitive to hippocampal atrophy, but might also partially reflect maximum hippocampal volume attained earlier in life. We also cannot establish the timing of hippocampal atrophy relative to the onset of cognitive decline. The accuracy of the volumetry technique itself may also be considered a limitation, as we relied on a manual tracing on a single template that was then propagated to all individual images. Work is underway to develop a more accurate multi-atlas approach tailored for use with postmortem brain images. Neuropathologic indices were derived from only one cerebral hemisphere for each individual, a limitation in that certain pathologies such as hippocampal sclerosis may exhibit considerable asymmetry. Lastly, we note that our sample consisted of very old adults who had voluntarily participated in aging research. Generalizability to broader populations will need to be explored in future studies.
5. ACKNOWLEDGMENTS
We are grateful to the participants of the Rush Memory and Aging Project and the Religious Orders Study and to the faculty and staff of the Rush Alzheimer’s Disease Center. This work was supported by the National Institute on Aging (P30AG10161, R01AG15819, R01AG33678, R01AG34374, R01AG17917) and the Illinois Department of Public Health. More information regarding obtaining MAP and ROS data for research use can be found at the RADC Research Resource Sharing Hub (www.radc.rush.edu).
6 REFERENCES
- 1.Comas-Herrera A, Wittenberg R, Pickard L, Knapp M Cognitive impairment in older people: Future demand for long‐term care services and the associated costs. Int J Geriatr Psychiatry. 2007; 22: 1037–1045 [DOI] [PubMed] [Google Scholar]
- 2.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB et al. Prevalence of cognitive impairment without dementia in the united states. Ann Intern Med. 2008; 148: 427–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle PA, Wilson RS, Yu L, Barr AM, Honer WG, Schneider JA, Bennett DA Much of late life cognitive decline is not due to common neurodegenerative pathologies. Ann Neurol. 2013; 74: 478–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zahodne LB, Wall MM, Schupf N, Mayeux R, Manly JJ, Stern Y, Brickman AM Late-life memory trajectories in relation to incident dementia and regional brain atrophy. J Neurol. 2015; 262: 2484–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson R, Colloby SJ, Blamire AM, O’Brien JT Subcortical volume changes in dementia with lewy bodies and alzheimer’s disease. A comparison with healthy aging. Int Psychogeriatr. 2016; 28: 529–536 [DOI] [PubMed] [Google Scholar]
- 6.Westman E, Cavalin L, Wahlund L, Volumetric MRI as a Diagnostic Tool in Alzheimer’s Disease, in Ingelsson M, Lannfelt L (Eds.). Immunotherapy and Biomarkers in Neurodegenerative Disorders. Humana Press: Clifton, NJ; 2016, p. 181–198 [Google Scholar]
- 7.Whitwell JL, Boeve BF, Weigand SD, Senjem ML, Gunter JL, Baker MC et al. Brain atrophy over time in genetic and sporadic frontotemporal dementia: A study of 198 serial magnetic resonance images. Eur J Neurol. 2015; 22: 745–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frisoni GB, Galluzzi S, Pantoni L, Filippi M The effect of white matter lesions on cognition in the elderly--small but detectable. Nat Rev Neurol. 2007; 3: 620–627 [DOI] [PubMed] [Google Scholar]
- 9.Nedelska Z, Ferman TJ, Boeve BF, Przybelski SA, Lesnick TG, Murray ME et al. Pattern of brain atrophy rates in autopsy-confirmed dementia with lewy bodies. Neurobiol Aging. 2015; 36: 452–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng L, Vinters HV, Mack WJ, Weiner MW, Chui HC, IVD program project. Differential effects of ischemic vascular disease and alzheimer’s disease on brain atrophy and cognition. J Cereb Blood Flow Metab. 2016; 36: 204–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galton CJ, Patterson K, Graham K, Lambon-Ralph MA, Williams G, Antoun N et al. Differing patterns of temporal atrophy in alzheimer’s disease and semantic dementia. Neurology. 2001; 57: 216–225 [DOI] [PubMed] [Google Scholar]
- 12.Sexton CE, Mackay CE, Lonie JA, Bastin ME, Terrière E, O’Carroll RE, Ebmeier KP MRI correlates of episodic memory in alzheimer’s disease, mild cognitive impairment, and healthy aging. Psychiatry Res Neuroimaging. 2010; 184: 57–62 [DOI] [PubMed] [Google Scholar]
- 13.Zamboni G, de Jager CA, Drazich E, Douaud G, Jenkinson M, Smith AD et al. Structural and functional bases of visuospatial associative memory in older adults. Neurobiol Aging. 2013; 34: 961–972 [DOI] [PubMed] [Google Scholar]
- 14.Josephs KA, Murray ME, Tosakulwong N, Whitwell JL, Knopman DS, Machulda MM et al. Tau aggregation influences cognition and hippocampal atrophy in the absence of beta-amyloid: A clinico-imaging-pathological study of primary age-related tauopathy (PART). Acta Neuropathol. 2017; 133: 705–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA Religious orders study and rush memory and aging project. J Alzheimer’s Dis. 2018: 1–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson RS, Boyle PA, Yu L, Barnes LL, Sytsma J, Buchman AS et al. Temporal course and pathologic basis of unawareness of memory loss in dementia. Neurology. 2015; 85: 984–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF et al. Decision rules guiding the clinical diagnosis of alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006; 27: 169–176 [DOI] [PubMed] [Google Scholar]
- 18.Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002; 59: 198–205 [DOI] [PubMed] [Google Scholar]
- 19.Schneider JA, Arvanitakis Z, Bang W, Bennett DA Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007; 69: 2197–2204 [DOI] [PubMed] [Google Scholar]
- 20.Dawe RJ, Bennett DA, Schneider JA, Vasireddi SK, Arfanakis K Postmortem MRI of human brain hemispheres: T2 relaxation times during formaldehyde fixation. Magn Reson Med. 2009; 61: 810–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawe RJ, Bennett DA, Schneider JA, Leurgans SE, Kotrotsou A, Boyle PA, Arfanakis K Ex vivo T 2 relaxation: Associations with age-related neuropathology and cognition. Neurobiol Aging. 2014; 35: 1549–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dawe RJ, Yu L, Leurgans SE, Schneider JA, Buchman AS, Arfanakis K et al. Postmortem MRI: A novel window into the neurobiology of late life cognitive decline. Neurobiol Aging. 2016; 45: 169–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkinson M, Smith S A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001; 5: 143–156 [DOI] [PubMed] [Google Scholar]
- 24.Ardekani BA, Guckemus S, Bachman A, Hoptman MJ, Wojtaszek M, Nierenberg J Quantitative comparison of algorithms for inter-subject registration of 3D volumetric brain MRI scans. J Neurosci Methods. 2005; 142: 67–76 [DOI] [PubMed] [Google Scholar]
- 25.Dawe RJ, Bennett DA, Schneider JA, Arfanakis K Neuropathologic correlates of hippocampal atrophy in the elderly: A clinical, pathologic, postmortem MRI study. PLoS ONE. 2011; 6: e26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Lee DY, Bailey E, Hartlein JM, Gado MH, Miller MI, Black KJ Validity of large-deformation high dimensional brain mapping of the basal ganglia in adults with tourette syndrome. Psychiatry Research: Neuroimaging. 2007; 154: 181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arvanitakis Z, Leurgans SE, Wang Z, Wilson RS, Bennett DA, Schneider JA Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann Neurol. 2011; 69: 320–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider JA, Bienias JL, Wilson RS, Berry-Kravis E, Evans DA, Bennett DA The apolipoprotein E ε4 allele increases the odds of chronic cerebral infarction detected at autopsy in older persons. Stroke. 2005; 36: 954–959 [DOI] [PubMed] [Google Scholar]
- 29.Schneider JA, Arvanitakis Z, Yu L, Boyle PA, Leurgans SE, Bennett DA Cognitive impairment, decline and fluctuations in older community-dwelling subjects with lewy bodies. Brain. 2012; 135: 3005–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nag S, Yu L, Capuano AW, Wilson RS, Leurgans SE, Bennett DA, Schneider JA Hippocampal sclerosis and TDP‐43 pathology in aging and alzheimer disease. Ann Neurol. 2015; 77: 942–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu WT, Josephs KA, Knopman DS, Boeve BF, Dickson DW, Petersen RC, Parisi JE Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in alzheimer disease. Acta Neuropathol. 2008; 116: 215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arvanitakis Z, Capuano AW, Leurgans SE, Buchman AS, Bennett DA, Schneider JA The relationship of cerebral vessel pathology to brain microinfarcts. Brain Pathol. 2017; 27: 77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buchman AS, Leurgans SE, Nag S, Bennett DA, Schneider JA Cerebrovascular disease pathology and parkinsonian signs in old age. Stroke. 2011; 42: 3183–3189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boyle PA, Yu L, Nag S, Leurgans S, Wilson RS, Bennett DA, Schneider JA Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology. 2015; 85: 1930–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, Sutherland RJ The aging hippocampus: Cognitive, biochemical and structural findings. Cereb Cortex. 2003; 13: 1344–1351 [DOI] [PubMed] [Google Scholar]
- 36.Jack CR, Petersen RC, Xu Y, O’brien PC, Smith GE, Ivnik RJ et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000; 55: 484–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jagust WJ, Zheng L, Harvey DJ, Mack WJ, Vinters HV, Weiner MW et al. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol. 2008; 63: 72–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kotrotsou A, Schneider JA, Bennett DA, Leurgans SE, Dawe RJ, Boyle PA et al. Neuropathologic correlates of regional brain volumes in a community cohort of older adults. Neurobiol Aging. 2015; 36: 2798–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burnham SC, Bourgeat P, Doré V, Savage G, Brown B, Laws S et al. Clinical and cognitive trajectories in cognitively healthy elderly individuals with suspected non-alzheimer’s disease pathophysiology (SNAP) or alzheimer’s disease pathology: A longitudinal study. Lancet Neurol. 2016; 15: 1044–1053 [DOI] [PubMed] [Google Scholar]
- 40.Hill DL, Schwarz AJ, Isaac M, Pani L, Vamvakas S, Hemmings R et al. Coalition against major diseases/european medicines agency biomarker qualification of hippocampal volume for enrichment of clinical trials in predementia stages of alzheimer’s disease. Alzheimers Dement. 2014; 10: 429. e3. [DOI] [PubMed] [Google Scholar]
- 41.Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018; 22: 599. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018; 555: 377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lebedeva A, Sundström A, Lindgren L, Stomby A, Aarsland D, Westman E et al. Longitudinal relationships among depressive symptoms, cortisol, and brain atrophy in the neocortex and the hippocampus. Acta Psychiatr Scand. 2018; 137: 491–502 [DOI] [PubMed] [Google Scholar]
- 44.Lupien SJ, De Leon M, De Santi S, Convit A, Tarshish C, Nair NPV et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998; 1: 69–73 [DOI] [PubMed] [Google Scholar]
- 45.Soetanto A, Wilson RS, Talbot K, Un A, Schneider JA, Sobiesk M et al. Association of anxiety and depression with microtubule-associated protein 2–and synaptopodin-immunolabeled dendrite and spine densities in hippocampal CA3 of older humans. Arch Gen Psychiatry. 2010; 67: 448–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr Opin Neurobiol . 2006; 16: 179–190 [DOI] [PubMed] [Google Scholar]
- 47.Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL et al. Episodic memory loss is related to hippocampal-mediated β-amyloid deposition in elderly subjects. Brain. 2008; 132: 1310–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997; 277: 376–380 [DOI] [PubMed] [Google Scholar]
- 49.Tulving E, Hayman CA, Macdonald CA Long-lasting perceptual priming and semantic learning in amnesia: A case experiment. J Exp Psychol Learn Mem Cogn. 1991; 17: 595. [DOI] [PubMed] [Google Scholar]