Abstract
BACKGROUND:
We used positron emission tomography imaging with [11C]raclopride to examine the effects of consumption of alcohol or placebo beverage by participants with alcohol use disorder (AUD) compared with healthy participants with and without family history of AUD. We sought to assess dopamine release following alcohol exposure in relation to AUD risk.
METHODS:
Three groups were enrolled: participants with AUD (n = 15) and healthy participants with family history negative (n = 34) or positive (n = 16) for AUD. Participants consumed a placebo (n = 65) or alcohol (n = 63) beverage in counterbalanced order before positron emission tomography scanning (128 scans). Binding potential (BPND) in the two drink conditions and the percent change in BPND between conditions were evaluated across striatal subregions. Subjective effects of beverage consumption were rated. Effects of group, drink order, and sex were evaluated.
RESULTS:
Alcohol resulted in greater dopamine release than did placebo in the ventral striatum (p < .001). There were no main effects of group, drink order, or sex on ventral striatum BPND or percent change in BPND. However, there was a drink order-by-group interaction (p = .02) whereby family history–positive participants who received placebo first had both lower placebo BPND and less difference between placebo and alcohol BPND than all other groups, consistent with expectation of alcohol powerfully evoking dopamine release in this group. Subjective responses showed the same order-by-group interaction.
CONCLUSIONS:
Hyper-responsivity of the dopaminergic system in family history–positive participants to expectation of alcohol may contribute to the expression of familial risk for AUD.
Keywords: Alcohol use disorder, [11C]raclopride, Dopamine, Familial risk, PET, Ventral striatum
The capacity of alcohol and alcohol-related cues to activate mesolimbic dopamine (DA) release may reinforce alcohol consumption (1,2) and contribute to the risk for alcohol use disorder (AUD). Alcohol stimulates DA release in humans, especially in the ventral striatum (VST) (3), although reports have been variable (4–7). Alcohol-stimulated DA release has been reported to differ according to risk-related traits (8) and between AUD and social drinkers (9). In individuals with a family history of AUD (family history–positive [FHP]) compared with those without this family history (family history–negative [FHN]), there is greater cue-induced DA release (10), raising the possibility that it reflects a heritable AUD risk mechanism.
However, in individuals with chronic AUD, stimulant-induced DA signaling in the VST appears to be blunted. After detoxification, individuals with chronic AUD have decreased DA D2/3 receptor availability in the striatum (11,12) as well as VST blunting of DA release after amphetamine (11) or methylphenidate (12) administration. The same markers have been investigated in individuals with family history of AUD, with findings of higher baseline receptor density than those without family history, but no difference in amphetamine-induced DA release (13). This pattern of changes is consistent with VST DA release contributing to the reinforcing effects of alcohol and learning alcohol use–related behaviors, while alcohol-seeking behaviors are represented in other brain regions and may not be dependent on VST DA release (14,15).
Across the spectrum of risk, there have been reports that individuals at risk for addictions exhibit elevated DA responses to a drink of alcohol (8) yet a diminished response to amphetamine (16,17), and that individuals with AUD release more DA on alcohol administration than social drinkers (9)—also an opposite DA abnormality to that elicited by stimulants.
The current study evaluated alcohol-induced DA release across the spectrum of AUD risk, i.e., FHN, FHP, and AUD. DA release was assessed by comparing the binding potential (BPND) of the D2/3 receptor radioligand [11C]raclopride following oral placebo to that after an alcohol beverage in a counterbalanced-order design. We sought to confirm that oral alcohol induces striatal DA release in AUD and across all participants. We investigated whether FHP and AUD participants would show altered alcohol-induced DA release in the VST relative to FHN participants.
In addition, we previously reported an order effect in a comparison of placebo drink to alcohol (6), but drink order was balanced by neither sex nor family history of participants in that study. We designed the current study with matched cells for each group and drink order, with the goal of identifying the sex and diagnostic group(s) exhibiting the order effect. To interpret the order effect we made the assumption that a placebo response of DA release to expectation of alcohol would be reflected in a lowering of BPND. Comparing expectation-induced with alcohol-induced DA release across the risk spectrum provides a fuller characterization of this measure as a possible risk factor for AUD.
METHODS AND MATERIALS
Participants
The study was approved by the New York State Psychiatric Institute/Columbia University Institutional Review Board and the Yale Human Investigation Committee, and all participants provided written informed consent. All participants in all groups were completers (underwent 2 positron emission tomography [PET] scans preceded by each of the 2 study beverages) except for 2 participants in the FHN group who underwent one PET scan preceded by the placebo beverage. Additional details on recruitment, demographics, inclusion/exclusion criteria, and safety monitoring of participants are presented in Table 1 and in the Supplement.
Table 1.
Participant Demographics
| Variable | FHP | FHN | AUD | p |
|---|---|---|---|---|
| n | 16 | 34 (2 noncompleters)a | 15 | |
| Age, Years, Mean ± SD | 25 ± 3 | 29 ± 7 | 36 ± 10 | <.05b |
| Sex, Female/Male, n | 8/8 | 17/17 | 8/7 | .97c |
| Ethnicity, AA/As/C/H/Mixed, n | 1/0/11/4/0 | 13/1/11/8/1 | 9/0/3/3/0 | .09c |
| Smokers (Currently Smoke Cigarettes), n | 1 | 4 | 6 | .02c |
AA, African American; As, Asian; AUD, alcohol use disorder; C, Caucasian; FHN, family history–negative; FHP, family history–positive; H, Hispanic; PET, positron emission tomography.
All participants in all groups were completers (underwent 2 PET scans preceded by each of the 2 study beverages) except for 2 participants in the FHN group who underwent one PET scan preceded by the placebo beverage.
Two-group t test, all 2-way comparisons: FHP vs. FHN; FHN vs. AUD; FHP vs. AUD.
χ2 test.
AUD participants were between 21 and 55 years of age and met DSM-IV criteria for alcohol abuse or dependence. They were enrolled regardless of their family history of AUD and were not treatment seeking. Participants at risk for withdrawal symptoms were admitted to an inpatient unit for monitoring and treatment as needed.
FHP and FHN participants were recruited as healthy volunteers who were moderate drinkers (so they could tolerate the study beverage). Family history was ascertained through questionnaires presented in a neutral way to all candidates on intake.
FHP participants were between 21 and 30 years of age and had a family history of AUD in a biological parent and at least one additional first- or second-degree relative. FHP participants were by inclusion criteria younger than the AUD group, as they were an at-risk group and thus could not exceed the age of risk, in contrast to the AUD group, whose greater age helped to establish their diagnosis (see Table 1 for age comparisons). In case of maternal AUD, we screened out fetal alcohol syndrome by using the Fetal Alcohol Syndrome Pre-Screen (18).
FHN participants were age and sex matched in two cohorts, one to the FHP participants and the other to the AUD participants. The FHN participants had no family history of substance dependence or abuse (including alcohol or any other substance) in any first-degree relatives.
FHN and FHP participants had a history of consuming at least 10 standard drinks per week (with no upper limit but no history of AUD) and a history of having consumed three to four drinks within a 3- to 4-hour period at least once within the past month, to ensure that they would tolerate the alcohol beverage administration during the protocol.
Smoking status for all participants was a binary evaluation of current cigarette smoking.
Procedures
Each participant underwent two scans at least 2 weeks apart, with each scan preceded by one of the two study beverages in a counterbalanced-order design (except for the 2 FHN non-completers, as noted). Participants were informed that they would receive both alcohol and placebo in blinded counterbalanced order. Participants were asked what they thought they received and accurately identified the drink content across all groups, effectively unblinding participants to drink composition for the second drink. Ten minutes before initiation of the [11C]raclopride dose administration, research staff provided the beverage, which was consumed over the intervening 10-minute period. The placebo drink consisted of 500 mL of tonic water and cranberry juice in a 3:1 ratio, with the top of the container doused in alcohol. The alcohol drink was designed to deliver 0.75 g alcohol per kilogram of body water (19–21), the equivalent of three standard drinks, using 100-proof vodka, with the remainder of the 500-mL drink consisting of tonic water and cranberry juice in a 3:1 ratio. Participants were asked to refrain from alcohol consumption for 48 hours before each PET scan, smoking tobacco for 2 hours before the scan, and consuming food for 6 hours before the scan. Participants underwent screening for substances of abuse including alcohol (AlcoTester breath alcohol detector; Testing Solutions LLC, Macomb Township, MI) on the first day of screening and on scan days.
PET Data Acquisition.
[11C]Raclopride was administered as a bolus plus constant infusion over an 80-minute period as previously described (6,22,23), with emission scanning beginning at 40 minutes and proceeding in eight successive 5-minute frames. Data were acquired using identical ECAT EXACT HR+ scanners (CTI-Siemens, Knoxville, TN) at Yale and Columbia for the FHP and matched FHN participants or a Biograph mCT scanner (Siemens Healthcare, Malvern, PA) at Columbia for the AUD and their FHN control subjects. Blood samples for alcohol level assays were drawn at 25, 40, 55, and 70 minutes after beginning the drink consumption.
Subjective Effects of Alcohol.
Participants completed the Subjective Effects of Alcohol Scale (SEAS) (24) after initiation of the [11C]raclopride infusion, immediately on completion of beverage consumption. The SEAS is a 15-item self-rated questionnaire with ratings ranging from 0 (not at all) to 10 (extremely) on subjective feelings of differing valence and arousal characteristics. Instructions were, “The following adjectives describe feelings that are sometimes produced by drinking alcohol. On a scale of 1–10, please rate the extent to which the drink has produced these feelings in you at the present time,” followed by a listing of the adjectives. Factor analysis has shown that the 15 scale items group into four factors corresponding to the four quadrants defined by high and low arousal and by positive and negative valence.
Magnetic Resonance Imaging.
Each participant underwent a structural magnetic resonance imaging (MRI) scan (GE MR 750 3T scanner, GE Healthcare, Port Washington, NY), in some cases on a separate day, for coregistration and region-of-interest analysis.
Discharge Criteria.
Participants were discharged from the PET scan procedure when blood pressure, heart rate, electrocardiography, physical and mental status exam, and field sobriety test were within normal limits and when breath alcohol concentration of <0.04 mg/dL was attained. In addition, participants were free of symptoms of alcohol withdrawal. AUD participants were informed that referrals for treatment would be provided on request at any point if they chose to leave the study for treatment, or on study completion.
PET Data Analysis
Image analysis was performed as previously described (22). In brief, PET data were coregistered to the structural MRI images using SPM8 software (Wellcome Trust Centre for Neuroimaging, London, United Kingdom) (25). Regions of interest were drawn on each individual’s MRI and applied to the coregistered PET images. Regions of interest included the striatal subregions, precommissural dorsal caudate and putamen, postcommissural caudate and putamen, and VST (23), with the cerebellum as a reference region. The BPND (unitless) was given by (region-of-interest activity/cerebellum activity – 1) during steady state, which was attained during the latter 40 minutes of the constant infusion. The primary outcome measure was the percent change in BPND (ΔBPND) between scan (drink) conditions, calculated as
This outcome measure has become standard in the field of PET measurement of induced neurotransmitter release (9,12,26) because of its quantitative relationship to magnitude of release or receptor occupancy (27) by neurotransmitter.
Statistical Analysis
The overall effect of alcohol for the full sample and for each diagnostic group was evaluated by t test across all participants and within each group to test whether ΔBPND differed from zero. Effects of drink order, sex, group, and smoking status (determined as currently smoking cigarettes) on ΔBPND were assessed with a univariate general linear mixed-effects model with age included as a covariate. The two FHN cohorts were included as distinct groups, but the model showed no impact of pooling them into a single FHN group, which was then adopted for the summary and presentation. A two-tailed probability value of p < .05 was chosen as statistically significant.
We analyzed the SEAS data by focusing on the eight positive-valence items (24), noting the number of these that exhibited the same group and order response pattern as the changes in PET BPND. To protect against multiple-comparisons risk we used a permutation test to determine whether this number was greater than expected by chance. In this permutation test, data were randomly reshuffled by condition and group on each of 100,000 iterations to assess statistical significance at p < .05.
RESULTS
Drinking History
The Timeline Followback Interview (28,29) was used to assess recent drinking history (past 30 days) for all participants. On average AUD participants consumed 8.1 ± 8.6 drinks per drinking occasion, FHN participants consumed 3.5 ± 1.5 drinks per drinking occasion, and FHP participants consumed 3.9 ± 2.0 drinks per drinking occasion. Age when participants began regular drinking was 21 ± 3 years for FHP, 21 ± 4 years for FHN, and 22 ± 6 years for AUD, who had 15 ± 11 years of drinking history. AUD participants were ascertained under DSM-IV criteria, but all met DSM-5 AUD criteria: 3 met two criteria, 8 met three criteria, and 4 met four criteria. In the AUD group there were several participants who began regular drinking relatively late (2 in their mid-20s and 2 over 30 years of age), and the median in this group was 20 years.
Alcohol-Induced DA Release (ΔBPND)
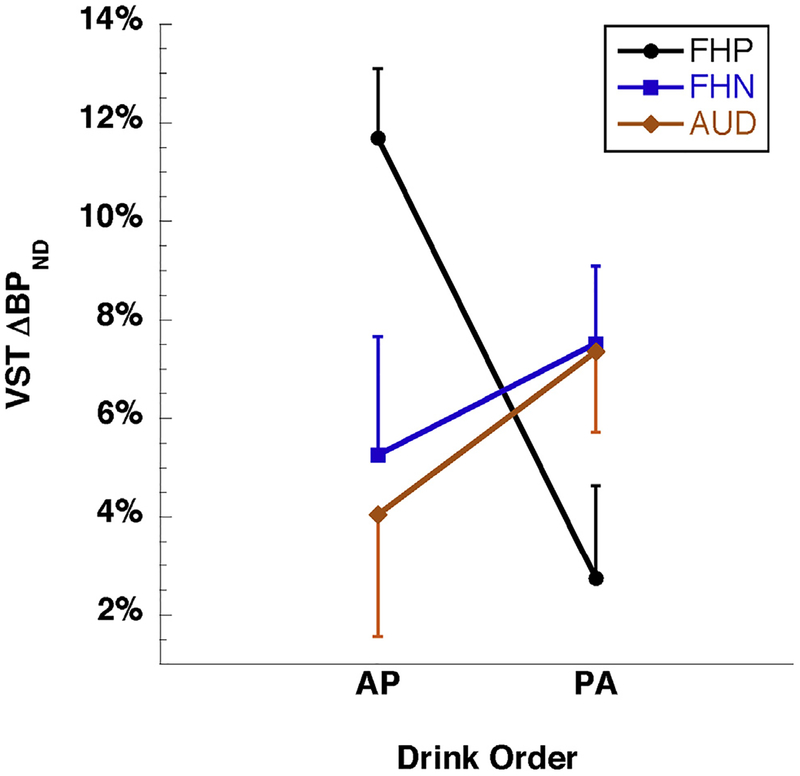
ΔBPND, the percent reduction in BPND from the placebo scan to the alcohol scan, was greatest in VST (Table 2). VST BPND following alcohol was significantly lower than following the placebo drink across all participants (ΔBPND < 0, p < .001, two-tailed t test). The same comparison was significant within each group (p < .01, two-tailed t test). PET scanning characteristics (injected dose, mass) did not contribute to the difference between alcohol and placebo scans (Supplemental Table S2). There were no main effects of group, drink order, or sex on VST ΔBPND, nor was age a significant covariate. Years of drinking history did not correlate with VST ΔBPND. Cigarette smoking showed a trend-level impact of enhancing ΔBPND. There was a significant group-by-drink order interaction (p = .02) in which post hoc analysis showed that ΔBPND was significantly different from zero in both FHN groups, FHP participants who consumed alcohol first (FHP-AP), and AUD participants who consumed placebo first (AUD-PA), but not FHP participants who consumed placebo first (FHP-PA) or AUD participants who consumed alcohol first (AUD-AP). Among the FHP participants, those who consumed alcohol first exhibited a much greater ΔBPND (11.7 ± 4.0%) than those who consumed placebo first (2.7 ± 5.4%, p = 0.002) (Figure 1; Supplemental Table S3).
Table 2.
[11C]Raclopride BPND for Striatal Subregions in Placebo and Alcohol Conditions and Percent Change in BPND (ΔBPND) for FHP, FHN, and AUD Groups
| ROI | FHP (n = 16) | FHN (n = 32) | AUD (n = 15) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BPND Placebo | BPND Alcohol | ΔBPND (%) | pa | BPND Placebo | BPND Alcohol | ΔBPND (%) | pa | BPND Placebo | BPND Alcohol | ΔBPND (%) | pa | |
| VST | 2.30 ± 0.25 | 2.13 ± 0.24 | −7.2 ± 6 | <.01 | 2.43 ± 0.28 | 2.25 ± 0.26 | −6.5 ± 8 | <.01 | 2.37 ± 0.27 | 2.23 ± 0.21 | −5.6 ± 6 | <.01 |
| PreDCA | 2.44 ± 0.23 | 2.33 ± 0.26 | −4.7 ± 7 | .01 | 2.77 ± 0.37 | 2.62 ± 0.36 | −3.7 ± 8 | <.01 | 2.76 ± 0.30 | 2.62 ± 0.20 | −4.6 ± 7 | .02 |
| PreDPU | 3.00 ± 0.31 | 2.88 ± 0.34 | −4.0 ± 7 | .02 | 3.30 ± 0.37 | 3.14 ± 0.35 | −3.5 ± 7 | <.01 | 3.27 ± 0.27 | 3.16 ± 0.21 | −3.2 ± 6 | >.05 |
| PostCA | 1.84 ± 0.40 | 1.78 ± 0.36 | −2.4 ± 10 | >.05 | 1.89 ± 0.34 | 1.78 ± 0.31 | −4.1 ± 14 | .03 | 1.92 ± 0.29 | 1.75 ± 0.28 | −5.0 ± 8b | .04 |
| PostPU | 3.21 ± 0.33 | 3.10 ± 0.27 | −3.0 ± 7 | >.05 | 3.34 ± 0.32 | 3.21 ± 0.33 | −3.0 ± 6 | .01 | 3.33 ± 0.42 | 3.24 ± 0.30 | −2.1 ± 8 | >.05 |
Values represent mean ± SD.
AUD, alcohol use disorder; BPND, binding potential; FHN, family history–negative; FHP, family history–positive; PostCA, postcommissural caudate; PostPU, postcommissural putamen; PreDCA, precommissural dorsal caudate; PreDPU, precommissural dorsal putamen; ROI, region of interest; VST, ventral striatum.
Paired t test; note that p < .001 for each striatal subregion when all subjects’ data are pooled across groups.
After removal of one outlier in the AUD group with PostCA ΔBPND more than 3 SDs above the mean.
Figure 1.
Alcohol-induced change in ventral striatal D2/3 receptor binding (ventral striatum [VST] percent change in binding potential [ΔBPND], the percent decrease from placebo day to alcohol day) by group and drink order. The family history–positive (FHP) group showed an opposite response to the drink conditions compared with the family history–negative (FHN) and alcohol use disorder (AUD) groups (p = .02, group-by-order interaction). Plot symbols indicate group mean and error bars indicate SEM. Participant group sizes were FHP: n = 8 alcohol beverage at first scan (AP) and n = 8 alcohol beverage at second scan (PA); FHN: n = 15 AP and n = 17 PA; AUD: n = 8 AP and n = 7 PA (data shown in Supplemental Table S3).
Binding Potential
A secondary analysis for both VST BPND placebo and VST BPND alcohol, pooled across drink order, found that there was no difference between groups, and there was no main effect of drink order or sex.
Subjective Effects of Alcohol
Ratings on the positive valence items of the SEAS (24) similarly showed a unique pattern for the FHP participants in contrast to the other two participant groups as observed for ΔBPND. To test this, a set of three criteria was defined that described this pattern: 1) in the FHP group, the difference between the effect of placebo when it was administered on the first day versus the second day was greater than the difference between the effect of alcohol when it was administered on the second day versus the first day (i.e., there was an interaction between drink-content group and order of administration); 2) in the FHP group, the smallest effect across group and order was that for placebo when it was administered on the second day; and 3) the interaction effect described by criterion 1 was greater for FHP than for the average of the other two groups (FHN and AUD). Five of eight items (mellow, relaxed, secure, lively, talkative) met all three criteria describing the BPND pattern, a proportion that was above the chance level (p = .012, permutation test). Thus, mirroring DA release, positive subjective experiences were more accentuated for alcohol in both drink orders and the placebo-first condition than for the placebo-second condition, and this pattern was more pronounced in the FHP group than in the other two.
Blood Alcohol Levels
Participants became notably but not heavily intoxicated, with only 1 of 65 enrollees unable to proceed to the PET scan following the drink because of nausea and emesis. Mean blood levels (assessed at 25, 40, 55, and 70 minutes) reached their maximum at 55 minutes following the drink (1.04 ± 0.31 mg/mL) (Supplemental Figure S1), plateaued at that level (1.04 ± 0.25 mg/mL) at 70 minutes, and did not differ between groups at any time point. Blood alcohol levels and VST ΔBPND were not correlated at any of the time points.
DISCUSSION
The main finding of this relatively large (n = 128 PET scans) study was a highly significant DA-releasing effect of alcohol compared with placebo across all participants and all striatal subregions, with greatest effect observed in VST (Table 2), confirming the observations that alcohol consumption induces DA release. However, there were no differences in ΔBPND across the FHP, FHN, or AUD groups, indicating similar magnitude of alcohol-induced DA release across all three groups. There were also no significant effects of drink order, sex, or smoking status on BPND or ΔBPND. Additionally, we found significantly lower BPND compared with the other groups in the placebo condition in FHP when placebo was consumed first, consistent with a DA-releasing effect of placebo when placebo was consumed first.
Alcohol-Induced DA Release (ΔBPND)
The highly significant DA-releasing effect of alcohol compared with placebo across all participants and all striatal subregions, greatest in the VST, confirms prior observations that alcohol consumption, combined with its expectation, induces net DA release (3–6). The absence of a main effect of group on ΔBPND indicated normal alcohol-induced DA release in those participants with AUD. This is in contrast to prior reports of blunted DA release in individuals with AUD using distinct DA releasing agents [amphetamine (11,30) and methylphenidate (12)] as well as elevated release under intravenous alcohol administration (9). This contrast may be due to lower severity of AUD in our cohort, as discussed below in connection with the BPND findings. It is also possible that this difference is related to the administration of alcohol rather than amphetamine or methylphenidate, which probe different aspects of dopaminergic transmission. Amphetamine and methylphenidate increase synaptic DA by blocking or reversing transporters and at high doses, releasing DA from vesicles (31). Mechanisms that contribute to the ability of alcohol to stimulate DA release include direct excitation of DA ventral tegmental area neurons and reduction of the afterhyperpolarization that follows spontaneous action potentials by reducing a quinidine-sensitive K+ current (32). Ventral tegmental area DA neurons from adult rats have been found to exhibit increased inhibitory tone after adolescent alcohol exposure leading to potentiation of stimulus-evoked phasic DA release, driving risky choice behavior in adulthood (33). Particular subsets of medial ventral tegmental area DA neurons have shown enhanced alcohol sensitivity, specifically attributed to adolescent drinking, over a range of alcohol concentrations associated with social drinking in humans (34,35). Furthermore, alcohol may promote DA release by a local calcium-dependent effect at the DA terminals in the striatum and accumbens (36,37). Consistent with our own findings, passively administered intravenous alcohol can stimulate DA release, while alcohol-related cues evoke additional DA release (38,39). Also consistent with the idea of distinct DA-releasing mechanisms of alcohol and stimulant challenges is the lower effect of alcohol-induced release in control subjects (FHP and FHN) than under stimulant-induced release, comparable to that seen in AUD under both types of challenge administration. It is also possible that use of an ethanol-scented placebo drink might have decreased the probability of observing a main effect of alcohol ingestion but increased the probability of observing an effect of potent cues.
Binding Potential
Our findings of similar BPND across groups contrast with prior literature reporting elevations in DA D2/3 receptors in unaffected members of AUD families (13,40), considered in those reports potentially to be a protective factor [but see Munro et al. (41)], or decreases in AUD participants (11,12). These prior measurements were made under baseline conditions with no pharmacological challenge (40) or intravenous placebo (saline) infusion (13), while our BPND measurements were made under placebo or alcohol beverage conditions. Furthermore, there were no decreases in BPND in AUD compared with the FHP and FHN groups, unlike previous findings, possibly owing to to lower severity of AUD in our cohort. Our AUD sample consumed 8 ± 9 drinks per day with a history of 15 ± 11 years of drinking, while in Martinez et al. (11) the AUD sample drank 20 ± 8 drinks per day with a history of 18 ± 7 years of drinking, and in Volkow et al. (12) these figures were 16 ± 6 drinks per day with 23 ± 8 years of drinking.
Group-by-Drink Order Interaction
The counterbalanced-order design showed that, when the two drink orders were pooled, there were no differences among the groups in BPND or ΔBPND attributable to the combined effects of alcohol consumption and the anticipation of alcohol consumption. However, tracking the effects by drink order within each group addressed differences in the effects of alcohol alone from those of expectation of alcohol.
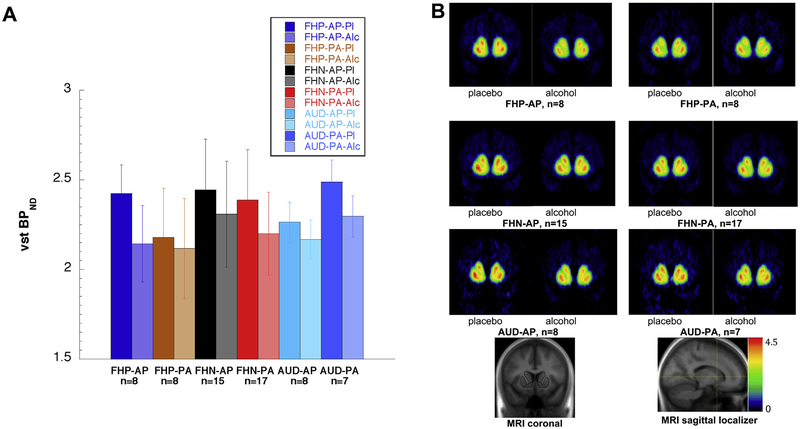
The significant group-by-drink order interaction indicates contrasting DA release responses to placebo and alcohol beverages by the different diagnostic groups, depending on which beverage was consumed first. To interpret this finding, post hoc analysis showed significantly smaller ΔBPND in the FHP-PA group (2.7 ± 5.4%) compared with in the FHP-AP group (11.7 ± 4.0%; p = .002) (Figure 1). Contributing to the small 2.7% ΔBPND in the FHP-PA group was the low BPND for FHP-PA on the placebo day (Figure 2A; see also Figure 2B for voxelwise data), similar to BPND for both FHP subgroups on their alcohol days. One potential explanation for the low BPND for FHP-PA participants on the placebo day is provided by the principle that in every [11C]raclopride measurement, BPND is proportional to receptor density but is reduced by binding to endogenous DA (42). In other words, lower BPND in Figure 2A is consistent with greater DA availability within the perisynaptic space. Thus, BPND values within each condition shown in Figure 2A can be thought of relative to a hypothetical baseline measurement (not acquired in this study) under plausible assumptions about the baseline (see Supplement for a full derivation). In short, the markedly lower ΔBPND values in the FHP-PA group than in the FHP-AP group might have resulted from as great a release of DA under placebo in the FHP-PA group as under the alcohol in either the PA or the AP order. We note as well the contribution to the high FHP-AP ΔBPND from the minimal DA release on the placebo-second day, when participants (who already received alcohol) know not to expect alcohol; however, this feature is shared with the FHN-AP group and therefore does not fully account for the group contrast. For the FHN and AUD groups, ΔBPND was moderate and comparable in both drink orders (Figure 1; Supplemental Table S3), ranging from 4% to 7%, corresponding to more modest BPND differences by order in Figure 2A. The similarity of ΔBPND for the FHN and AUD groups was not due to predominance of negative family history among the AUD group: 7 AUD participants had negative family history, 6 had positive family history, and 2 declined to specify their family history; analysis showed that the FHP and FHN AUD subgroups each showed the same behavior as the total group (greater ΔBPND in the PA order than in the AP order). This contrast with the FHP group is consistent with a recent report of greater cue-induced DA release compared with a neutral flavor stimulus in individuals with family history of AUD (10), although that study made no comparison to alcohol itself.
Figure 2.
(A) [11C]Raclopride binding potential (BPND) for each group and drink order. Each group (family history–positive [FHP], family history–negative [FHN], alcohol use disorder [AUD]) is represented by four bars: the placebo day (Pl) and the alcohol day (Alc) for each order (alcohol beverage at first scan [AP]; alcohol beverage at second scan [PA]). In all three groups, alcohol (second bar in each pair) resulted in greater dopamine release (reduction in BPND) than placebo did. However, the FHP participants showed much smaller alcohol-induced reduction in BPND when placebo was first (FHP-PA) than when placebo was second (FHP-AP). In contrast, the FHN and AUD participants showed moderate BPND reductions between placebo and alcohol day that were similar regardless of drink order. The six within-group differences between the placebo and alcohol day for each order (as percent of placebo) are the six data points in Figure 1. Bar heights indicate group mean BPND and error bars indicate SD (data shown in Table 3). (B) [11C]Raclopride BPND for each group and drink order, voxelwise maps. These images display the BPND values shown as regional means in panel (A). Note that the greatest intensity changes from placebo to alcohol scan occur for the FHP-AP group, and the smallest changes occur for the FHP-PA group. MRI, magnetic resonance imaging; VST, ventral striatum.
Subjective Effects
Alcohol-related cues such as place-conditioned anticipation of alcohol or initial olfactory sensations have generally been found in rodents to induce DA release in the nucleus accumbens (38,43,44). In humans, alcohol-related conditioned cues induce craving and lead to consumption (45,46), although their effect on DA release is less well established.
In our study the apparently heightened release of DA under the placebo-first drink, interpreted as a response to expectation of alcohol, occurs particularly in the group at risk for AUD by family history. This pattern of enhanced response was observed for both ΔBPND and the positive subjective effects of alcohol, suggesting enhanced subjective value associated with alcohol cues in FHP individuals. Many functional MRI studies have consistently shown that the human VST encodes a subjective value signal (47) that is widely thought to depend on DA.
Limitations
One limitation of the study is the use of two scanners; however, we compensated for this by acquiring the FHP and matched FHN subjects on the same scanner (ECAT EXACT HR+). Similarly, the AUD participants and matched FHN control subjects were acquired on the same scanner (Biograph mCT). We did not detect a site or scanner effect for any of the outcome measures acquired on the Siemens HR+ scanners or the Biograph mCT. The study was also limited by the absence of baseline BPND measurements (without placebo or alcohol), which would have necessitated at least a third scan for each participant, and a fourth if a baseline measurement was acquired in association with each drink administration. This makes the interpretations based on BPND comparisons in Figure 2A tentative and dependent on our assumptions regarding baseline BPND values (see Supplement).
In summary, our study shows similar magnitude of alcohol-induced DA release in FHP, FHN, and AUD, with an exaggerated response to expectation of alcohol in FHP participants. Further investigation is needed to determine whether this exaggerated DA release under expectation of alcohol is an independent risk factor for AUD.
Supplementary Material
Table 3.
Ventral Striatal BPND by Group, Drink Order, and Beverage Day
| Group | ||||||
|---|---|---|---|---|---|---|
| FHP | FHN | AUD | ||||
| Drink Order | ||||||
| AP (n = 8) | PA (n = 8) | AP (n = 15) | PA (n = 17) | AP (n = 8) | PA (n = 7) | |
| VST BPND Placebo Day | 2.42 ± 0.16 | 2.18 ± 0.27 | 2.44 ± 0.28 | 2.39 ± 0.28 | 2.26 ± 0.24 | 2.49 ± 0.26 |
| VST BPND Alcohol Day | 2.14 ± 0.21 | 2.12 ± 0.28 | 2.31 ± 0.30 | 2.20 ± 0.23 | 2.17 ± 0.23 | 2.30 ± 0.17 |
Values represent mean ± SD.
AP, alcohol beverage at first positron emission tomography scan; AUD, alcohol use disorder; BPND, binding potential; FHN, family history–negative; FHP, family history–positive; PA, alcohol beverage at second positron emission tomography scan; VST, ventral striatum.
ACKNOWLEDGMENTS AND DISCLOSURES
This study was supported by National Institute on Alcohol Abuse and Alcoholism Grant No. P50-AA-012870 (to JHK).
We thank Thomas B. Cooper, Director of the Analytical Psychopharmacology Laboratory at the Nathan S. Kline Institute, Orangeburg, NY, for the alcohol blood level assays.
LSK has received research support from Amgen. JHK has stock or stock options in Spring Health, Biohaven Medical Sciences, ArRETT Neuroscience, Inc., Blackthorn Therapeutics, and Luc Therapeutics; he consults broadly to the pharmaceutical industry, but his annual income over the past year did not exceed $5,000 for any organization; he receives over $5,000 in income from the Society of Biological Psychiatry for editing the journal Biological Psychiatry. AA-D receives compensation from The Society of Biological Psychiatry and from ACNP for editorial roles on their journals. Over the past year she received honoraria from Otsuka for lectures, and from Roche for consultation. She received research support from Neurocrine. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsc.2018.03.018.
REFERENCES
- 1.Fibiger HC (1978): Drugs and reinforcement mechanisms: A critical review of the catecholamine theory. Annu Rev Pharmacol Toxicol 18:37–56. [DOI] [PubMed] [Google Scholar]
- 2.Samson HH, Tolliver GA, Haraguchi M, Hodge CW (1992): Alcohol self-administration: Role of mesolimbic dopamine. Ann N Y Acad Sci 654:242–253. [DOI] [PubMed] [Google Scholar]
- 3.Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, et al. (2003): Alcohol promotes dopamine release in the human nucleus accumbens. Synapse 49:226–231. [DOI] [PubMed] [Google Scholar]
- 4.Yoder KK, Constantinescu CC, Kareken DA, Normandin MD, Cheng TE, O’Connor SJ, et al. (2007): Heterogeneous effects of alcohol on dopamine release in the striatum: A PET study. Alcohol Clin Exp Res 31:965–973. [DOI] [PubMed] [Google Scholar]
- 5.Yoder KK, Morris ED, Constantinescu CC, Cheng TE, Normandin MD, O’Connor SJ, et al. (2009): When what you see isn’t what you get: Alcohol cues, alcohol administration, prediction error, and human striatal dopamine. Alcohol Clin Exp Res 33:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urban NB, Kegeles LS, Slifstein M, Xu X, Martinez D, Sakr E, et al. (2010): Sex differences in striatal dopamine release in young adults after oral alcohol challenge: A positron emission tomography imaging study with [11C]raclopride. Biol Psychiatry 68:689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volkow ND, Wiers CE, Shokri-Kojori E, Tomasi D, Wang GJ, Baler R (2017): Neurochemical and metabolic effects of acute and chronic alcohol in the human brain: Studies with positron emission tomography. Neuropharmacology 122:175–188. [DOI] [PubMed] [Google Scholar]
- 8.Setiawan E, Pihl RO, Dagher A, Schlagintweit H, Casey KF, Benkelfat C, et al. (2014): Differential striatal dopamine responses following oral alcohol in individuals at varying risk for dependence. Alcohol Clin Exp Res 38:126–134. [DOI] [PubMed] [Google Scholar]
- 9.Yoder KK, Albrecht DS, Dzemidzic M, Normandin MD, Federici LM, Graves T, et al. (2016): Differences in IV alcohol-induced dopamine release in the ventral striatum of social drinkers and nontreatment-seeking alcoholics. Drug Alcohol Depend 160:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, Albrecht DS, Yoder KK, et al. (2013): Beer flavor provokes striatal dopamine release in male drinkers: Mediation by family history of alcoholism. Neuropsychopharmacology 38:1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, et al. (2005): Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry 58:779–786. [DOI] [PubMed] [Google Scholar]
- 12.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, et al. (2007): Profound decreases in dopamine release in striatum in detoxified alcoholics: Possible orbitofrontal involvement. J Neurosci 27:12700–12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvanzo AA, Wand GS, Kuwabara H, Wong DF, Xu X, McCaul ME (2015): Family history of alcoholism is related to increased D2/D3 receptor binding potential: A marker of resilience or risk? Addict Biol 22:218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barker JM, Taylor JR (2014): Habitual alcohol seeking: Modeling the transition from casual drinking to addiction. Neurosci Biobehav Rev 47:281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gremel CM, Lovinger DM (2017): Associative and sensorimotor cortico-basal ganglia circuit roles in effects of abused drugs. Genes Brain Behav 16:71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casey KF, Benkelfat C, Cherkasova MV, Baker GB, Dagher A, Leyton M (2014): Reduced dopamine response to amphetamine in subjects at ultra-high risk for addiction. Biol Psychiatry 76:23–30. [DOI] [PubMed] [Google Scholar]
- 17.Leyton M (2017): Altered dopamine transmission as a familial risk trait for addictions. Curr Opin Behav Sci 13:130–138. [Google Scholar]
- 18.Michigan Department of Community Health (2008): Fetal Alcohol Spectrum Disorders Program. Fetal alcohol syndrome (FAS) pre-screen. Available at: https://www.michigan.gov/documents/mdch/FASD_Prescreen_form_Feb-10_314457_7.pdf. Accessed October 21, 2013.
- 19.Watson PE, Watson ID, Batt RD (1980): Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr 33:27–39. [DOI] [PubMed] [Google Scholar]
- 20.Watson TE (1989): Total body water and alcohol levels: Updating the fundamentals In: Krow KE, Batt RD, editors. Human Metabolism of Alcohol. Boca Raton, FL: CRC Press, 41–66. [Google Scholar]
- 21.Curtin JJ, Fairchild BA (2003): Alcohol and cognitive control: Implications for regulation of behavior during response conflict. J Abnorm Psychol 112:424–436. [DOI] [PubMed] [Google Scholar]
- 22.Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. (2001): Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 21:1034–1057. [DOI] [PubMed] [Google Scholar]
- 23.Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, et al. (2003): Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: Amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 23:285–300. [DOI] [PubMed] [Google Scholar]
- 24.Morean ME, Corbin WR, Treat TA (2013): The Subjective Effects of Alcohol Scale: Development and psychometric evaluation of a novel assessment tool for measuring subjective response to alcohol. Psychol Assess 25:780–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashburner J (2009): Computational anatomy with the SPM software. Magn Reson Imaging 27:1163–1174. [DOI] [PubMed] [Google Scholar]
- 26.Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, et al. (1998): Increased striatal dopamine transmission in schizophrenia: Confirmation in a second cohort. Am J Psychiatry 155:761–767. [DOI] [PubMed] [Google Scholar]
- 27.Laruelle M, D’Souza CD, Baldwin RM, Abi-Dargham A, Kanes SJ, Fingado CL, et al. (1997): Imaging D2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology 17:162–174. [DOI] [PubMed] [Google Scholar]
- 28.Maisto SA, Sobell LC, Cooper AM, Sobell MB (1982): Comparison of two techniques to obtain retrospective reports of drinking behavior from alcohol abusers. Addict Behav 7:33–38. [DOI] [PubMed] [Google Scholar]
- 29.Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E (1986): The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: Utility for alcohol research. Addict Behav 11:149–161. [DOI] [PubMed] [Google Scholar]
- 30.Narendran R, Mason NS, Paris J, Himes ML, Douaihy AB, Frankle WG (2014): Decreased prefrontal cortical dopamine transmission in alcoholism. Am J Psychiatry 171:881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sulzer D (2011): How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 69:628–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Appel SB, Liu Z, McElvain MA, Brodie MS (2003): Ethanol excitation of dopaminergic ventral tegmental area neurons is blocked by quinidine. J Pharmacol Exp Ther 306:437–446. [DOI] [PubMed] [Google Scholar]
- 33.Schindler AG, Soden ME, Zweifel LS, Clark JJ (2016): Reversal of alcohol-induced dysregulation in dopamine network dynamics may rescue maladaptive decision-making. J Neurosci 36:3698–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mrejeru A, Marti-Prats L, Avegno EM, Harrison NL, Sulzer D (2015): A subset of ventral tegmental area dopamine neurons responds to acute ethanol. Neuroscience 290:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avegno EM, Salling MC, Borgkvist A, Mrejeru A, Whitebirch AC, Margolis EB, et al. (2016): Voluntary adolescent drinking enhances excitation by low levels of alcohol in a subset of dopaminergic neurons in the ventral tegmental area. Neuropharmacology 110:386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell VA, Lamm MC, Taljaard JJ (1988): Effect of ethanol on [3H] dopamine release in rat nucleus accumbens and striatal slices. Neurochem Res 13:487–492. [DOI] [PubMed] [Google Scholar]
- 37.Wozniak KM, Pert A, Mele A, Linnoila M (1991): Focal application of alcohols elevates extracellular dopamine in rat brain: a microdialysis study. Brain Res 540:31–40. [DOI] [PubMed] [Google Scholar]
- 38.Doyon WM, Anders SK, Ramachandra VS, Czachowski CL, Gonzales RA (2005): Effect of operant self-administration of 10% ethanol plus 10% sucrose on dopamine and ethanol concentrations in the nucleus accumbens. J Neurochem 93:1469–1481. [DOI] [PubMed] [Google Scholar]
- 39.Howard EC, Schier CJ, Wetzel JS, Duvauchelle CL, Gonzales RA (2008): The shell of the nucleus accumbens has a higher dopamine response compared with the core after non-contingent intravenous ethanol administration. Neuroscience 154:1042–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, et al. (2006): High levels of dopamine D2 receptors in unaffected members of alcoholic families: Possible protective factors. Arch Gen Psychiatry 63:999–1008. [DOI] [PubMed] [Google Scholar]
- 41.Munro CA, McCaul ME, Oswald LM, Wong DF, Zhou Y, Brasic J, et al. (2006): Striatal dopamine release and family history of alcoholism. Alcohol Clin Exp Res 30:1143–1151. [DOI] [PubMed] [Google Scholar]
- 42.Laruelle M (2000): Imaging synaptic neurotransmission with in vivo binding competition techniques: A critical review. J Cereb Blood Flow Metab 20:423–451. [DOI] [PubMed] [Google Scholar]
- 43.Gonzales RA, Weiss F (1998): Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci 18:10663–10671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doyon WM, York JL, Diaz LM, Samson HH, Czachowski CL, Gonzales RA (2003): Dopamine activity in the nucleus accumbens during consummatory phases of oral ethanol self-administration. Alcohol Clin Exp Res 27:1573–1582. [DOI] [PubMed] [Google Scholar]
- 45.Carter BL, Tiffany ST (1999): Meta-analysis of cue-reactivity in addiction research. Addiction 94:327–340. [PubMed] [Google Scholar]
- 46.Litt MD, Cooney NL, Morse P (2000): Reactivity to alcohol-related stimuli in the laboratory and in the field: Predictors of craving in treated alcoholics. Addiction 95:889–900. [DOI] [PubMed] [Google Scholar]
- 47.Bartra O, McGuire JT, Kable JW (2013): The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage 76:412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.