Abstract
INTRODUCTION:
Leisure activities impact brain aging and may be prevention targets. We characterized how physical and cognitive activities relate to brain health for the first time in autosomal dominant frontotemporal lobar degeneration (FTLD).
METHODS:
105 mutation carriers (C9orf72/MAPT/GRN) and 69 noncarriers reported current physical and cognitive activities at baseline, and completed longitudinal neurobehavioral assessments and brain MRIs.
RESULTS:
Greater physical and cognitive activities were each associated with an estimated >55% slower clinical decline per year among dominant gene carriers. There was also an interaction between leisure activities and frontotemporal atrophy on cognition in mutation carriers. High activity carriers with frontotemporal atrophy (−1SD/year) demonstrated >2-fold better cognitive performances per year compared to less their active peers with comparable atrophy rates.
DISCUSSION:
Active lifestyles were associated with less functional decline and moderated brain-to-behavior relationships longitudinally. More active carriers “outperformed” brain volume, commensurate with a cognitive reserve hypothesis. Lifestyle may confer clinical resilience, even in autosomal dominant FTLD.
Keywords: Physical Activity, Exercise, Cognitive Activity, Cognitive Reserve, Frontotemporal dementia
1. INTRODUCTION.
Lifestyle behaviors (e.g., physical and cognitive activity) clearly impact brain aging trajectories, are associated with reduced incidence and delayed onset of cognitive decline with age[1–5] and improved outcomes in Alzheimer’s[6], vascular[7,8], and Parkinson’s[9,10] diseases, yet they have not been examined in the context of frontotemporal lobar degeneration (FTLD). Given the lack of current disease-modifying pharmacological therapies, we need to understand alternate targets that may prevent or mitigate disease trajectories and support the aging brain across neurodegenerative syndromes.
FTLD is among the most common neurodegenerative diseases in adults <65 years old, presenting with a range of behavioral, motoric, and language symptomologies, and unlike AD, up to ~40% of FTLD cases have a related family history with ~10% evidencing an autosomal dominant mode of gene inheritance (i.e., C9orf72, GRN, MAPT)[11]. The relatively high prevalence of family members and mutation carriers (MC) at-risk for adult onset disease provides a unique opportunity for early implementation of potential primary prevention approaches. Indeed, despite being autosomal dominant, recent work from the DIAN Study demonstrated that AD MC with higher physical activity levels showed better cognitive and functional outcomes, and lower pathological profiles of CSF Aβ and tau markers than their less active peers[12]. Additionally, previous works show that lifestyle-related cardiovascular factors (e.g., obesity, diabetes, smoking) are elevated in individuals with sporadic FTLD[13,14] and that higher occupational attainment is associated with longer survival time, underscoring that life exposures may importantly alter risk and/or disease trajectories in FTLD [15,16]. In the context of the large body of research in aging and AD[17], these studies provide early evidence that neurodegenerative trajectories may be modifiable through lifestyle even among autosomal dominant MC and in FTLD. Interestingly, many of the posited mechanisms underlying the relationship between leisure physical and cognitive behaviors and the brain[18–22] are also implicated in FTLD, including immune/microglial[23–25] and synaptic[26] dysregulation. Lifestyle behaviors may therefore represent a potentially modifiable intervention target to delay disease progression or even prevent dementia onset at low cost with high scalability in FTLD.
As the first study to examine lifestyle activities in the context of FTLD, we aimed to characterize the relationship between everyday physical and cognitive behaviors and brain health outcomes in a longitudinally-followed cohort of autosomal dominant FTLD MC from the ARTFL/LEFFTDS Study.
2. METHODS.
2.1. Participants.
174 family members affected by the genetic forms of FTLD enrolled in the multisite Longitudinal Evaluation of Familial Frontotemporal Dementia Subjects (LEFFTDS) or Advancing Research and Treatment in Frontotemporal Lobar Degeneration (ARTFL) studies and were included. 105 individuals carried a pathogenic variant of the autosomal dominant MAPT, GRN, or C9orf72 genes, and 69 were non-pathogenic gene carrying family members (Table 1). FTLD-modified global Clinical Dementia Rating Scale (FTLD-CDR)[27] was used as a marker of disease severity. The FTLD-CDR is a measure of disease severity and includes ratings across six functional domains captured in the traditional CDR, in addition to two new domains specific to the core clinical features of FTLD: language and behavior. Following a standardized algorithm[27], the eight domain scores were summed to create a global score (0–8), while each domain was scored on a scale from 0–3 and summed to create a more continuous measure of symptom severity (0–24). All genetic testing was completed in the same laboratory at the University of California, Los Angeles using standardized methods previously described[27]. To capture a functionally intact comparison group, noncarriers were only included in the current study if global FTLD-CDR=0 via study partner interviews.
Table 1.
Clinical and demographic characteristics of study sample.
| Pathogenic Mutation Carrier (n=105) |
Noncarrier (n=69) |
p-value | |
|---|---|---|---|
| Age, y | 50.8 (14.3) | 48.9 (13.2) | 0.37 |
| Sex, n, % F | 49 (46.7%) | 30 (43.5%) | 0.68 |
| Education, y | 15.2 (2.4) | 15.3 (2.6) | 0.68 |
| Genotype (n, % of carriers) | --- | --- | |
| MAPT | 33 (31.4%) | ||
| Visits (n, % of group) | --- | --- | |
| 3 | 6 (5.7%) | ||
| Years followed (median, IQR) | 0.84 (0, 1.06) range: 0–2 |
--- | --- |
| FTLD-CDR Sum of Boxes (median, IQR) | 1 (0, 5) | 0 (0, 0) | <0.001 |
| FTLD-CDR Global (median, IQR) | 0.5 (0, 1) | 0 (0, 0) | <0.001 |
| MoCA Total Score | 23.7 (6.9) | 27.4 (2.3) | <0.001 |
| Episodic Memory (z-score) | −0.13 | 0.30 | <0.001 |
| Trails A (seconds) (median, IQR) | 27 (21, 45) | 22 (18, 27.5) | <0.001 |
| Executive composite (z-score) | −0.29 (1.0) | 0.28 (0.62) | <0.001 |
| GDS (median, IQR) | 1 (0, 4) | 1 (0, 2) | 0.16 |
| Physical Activity Scale for the Elderly (PASE) | 138.5 (95.3) (range: 0, 436.5) |
180.7 (87.2) (range: 25, 360.9) |
0.005 |
| Cognitive Activity Scale (CAS) | 20.2 (9.0) (range: 0, 51) |
23.4 (7.9) (range: 9, 41) |
0.02 |
Note. Means and SD reported unless otherwise specified. FTLD CDR = Frontotemporal Lobar Degeneration Clinical Dementia Rating; MoCA = Montreal Cognitive Assessment; FAQ = Functional Activity Scale; GDS = Geriatric Depression Scale.
The ARTFL/LEFFTDS consortia data represent ongoing longitudinal studies. In the current study, among the MC followed longitudinally, all completed a baseline visit, 56 completed one follow-up, and 6 completed two follow-up visits in the current analyses. Participants at baseline did not demographically differ from those who completed follow-up visits (age F(2, 166)=0.68, p=0.51; sex χ2=0.94, p=0.62; education F(2, 166)=0.90, p=0.41; genotype χ2=6.8, p=0.15).
2.2. Baseline Leisure Activity Assessment.
Participants completed self-reported measures of physical (Physical Activity Scale for the Elderly (PASE)[28]) and cognitive (Cognitive Activity Scale (CAS))[2] activities. The PASE is an 11-item self-administered measure of physical activity levels over the past 7 days in three life domains: recreational, household, and work-related. Participants rated weekly frequency and daily duration for the following recreational activities: walking; light, moderate and strenuous sports; and strength training. For each activity, a score was obtained by multiplying activity frequency by a task-specific weight according to the scoring manual. Activity scores were then summed to calculate a total PASE score representing overall physical activity level, with higher values indicating greater levels of current physical activity and range from 0 to >400[29]. The PASE has been widely validated for use in older adults across cultures, and demonstrated adequate test-rest reliability (ICC 0.49 to 0.95)[30], intraclass correlation (alpha=0.65)[31], and construct validity[28,30,32].
The Cognitive Activity Scale (CAS) is a 10-item self-reported measure of engagement in mentally stimulating recreational activities in the past 12-months adapted from the Mayo group[33]. Participants rated frequency of participation from 1 (Never) to 7 (Daily) across a variety of activities, including reading, playing games or a musical instrument, artistic activities, social activities, and computer use. Activity frequencies were summed, with higher values indicating greater frequency of cognitive activity engagement and range from 0 to 70. This scale has been previously used in longitudinal studies demonstrating that late-life cognitive activity was associated with better current cognitive status[33].
2.3. Clinical Outcomes
2.3.1. Neuropsychological Assessment.
Participants underwent the following neuropsychological battery: Global cognition (Montreal Cognitive Assessment), episodic memory (California Verbal Learning Test, 2nd edition, short form, 10-minute Delayed Recall; Craft Story 20-minute Delayed Recall; and Benson Figure 10-minute Delayed Recall), processing speed (Trail Making Test, Part A, seconds), and executive functions (Trail Making Test, Part B, seconds, and Digit Span Backwards, total score). Sample-based z-score composites were calculated for episodic memory and executive functions; Trail Making Test scores were log transformed to achieve greater normality for analyses.
2.3.2. Mood.
Participants completed the Geriatric Depression Scale (GDS) as an indicator of depressive symptoms in the prior two weeks[34]. Higher scores on the GDS indicate greater mood disturbance; GDS scores were log transformed to achieve greater normality for analyses.
2.3.3. Neuroimaging.
93 participants (54 MC and 37 noncarriers) completed at least one 3-Telsa brain MRI on one of three scanners across study sites: Philips Medical Systems, Siemens, or GE Medical Systems. Participants who completed MRI at baseline did not statistically differ on demographics from those who did not complete an MRI (age t=−0.78, p=0.44; sex χ2=3.0, p=0.08; education t=−0.30, p=0.76); however, those who did not have imaging were more functionally impaired (FTLD-CDR t=−2.6, p=0.009). A standard imaging protocol was applied across centers and is managed and reviewed for quality by a core group at the Mayo Clinic, Rochester, MN. We analyzed T1 images acquired as Magnetization Prepared Rapid Gradient Echo (MP-RAGE) images using the following parameters: 240 × 256 × 256 matrix; about 170 slices; voxel size = 1.05 × 1.05 × 1.25 mm3; flip angle, TE and TR varied by vendor, and processed via a standardized SPM8 pipeline as previously described[35], using a standard parcellation atlas (Desikan et al. 2006). We summed all modulated gray matter atlas regions within the frontal and temporal lobes to create a total frontotemporal volume ROI. In baseline cross-sectional models, we regressed total intracranial volume from total frontotemporal volume and entered the resulting residual (i.e., TIV adjusted volume) into our final models.
2.4. Statistical Analyses.
Baseline Models.
First, we conducted multivariable linear regression models to examine differences in activity levels across symptomatic MC (FTLD-CDR≥1), pre-to-early symptomatic MC (FTLD-CDR<1), or non-carriers, adjusting for age, sex, and education. Similarly, within the MCs, we examined activity level differences across genotypes, adjusting for the demographic factor that the MCs differed on to a statistically significant level, age. Statistically significant omnibus models were followed-up with Tukey HSD pairwise comparisons. Unstandardized betas (b) and standardized betas (β) are reported.
Given our primary question centered on the role of lifestyle activity specifically in FTLD MCs, all subsequent analyses were conducted within the MC cohort only. First, we conducted multivariable linear regression models to examine the relationship between lifestyle activities and disease severity (FTLD-CDRsb), adjusting for age, sex, and education; next we additionally adjusted for disease severity (FTLD-CDRsb) and examined the relationship between activity levels and neuropsychological performance, mood, or frontotemporal brain volume outcomes (these brain volume imaging outcomes are adjusted for total intracranial volume (TIV)).
Next, we examined the moderating effect of lifestyle activities on the relationship between frontotemporal volumes and clinical outcomes. To do so, we conducted multivariable linear regression with activity, frontotemporal volume, and the interaction between them entered as independent variables and cognition as the dependent variable; also adjusting for age, sex, education, FTLD-CDRsb (disease severity), and TIV. For all outcomes, separate models were run for cognitive activity or physical activity.
Longitudinal Models.
In MCs, linear mixed-effects models were used to examine the relationship between baseline activity levels and changes in disease severity (FTLD-CDRsb). In addition, we examined the effect of baseline activity on neuropsychological performance, mood or frontotemporal brain volume trajectories over time, adjusting for disease severity (FTLD-CDRsb). The primary predictors were activity, time from baseline (measured as a continuous covariate), and the interaction between them. In these models, the interaction between baseline activity and study time (years) was the primary independent variable of interest (activity*time), indicating the effect of activity on the change in outcome over time. The models also adjusted for baseline age, education, sex, and TIV (imaging models only). We additionally modeled interactions between time and each covariate (baseline age, education, sex, baseline FTLD-CDRsb) to adjust for possible nonlinear temporal relationships between covariates and outcomes. To allow comparisons between effect estimates of different models, each independent and dependent variable of interest was converted into a sample-based z-score (calculated within the MC cohort at baseline) such that beta values indicate 1-SD units of change (i.e., standardized betas).
Finally, we again examined the moderating role of lifestyle activities on the relationship between frontotemporal atrophy and clinical outcomes among MC via linear mixed-effects models while adjusting for baseline demographics and FTLD-CDRsb. To do so, we decomposed frontotemporal volumes into within- (i.e., changes from baseline) and between- (i.e., baseline) subject components in order to associate purely within-subject changes in frontotemporal volumes with changes in clinical outcomes and to avoid estimation bias resulting from incorrectly assuming common within- and between-subject effects following guidelines from Neuhaus et al. [36,37]. We entered the interaction between within-person frontotemporal changes by baseline activity level as the independent parameter of interest with parallel models examining each clinical outcome (neuropsychological performance or mood), adjusting for baseline frontotemporal volume, TIV, baseline demographics (age, sex, education) and baseline FTLD-CDRsb. Again, all analyses were performed in parallel with separate models for each of cognitive and physical activity, separately.
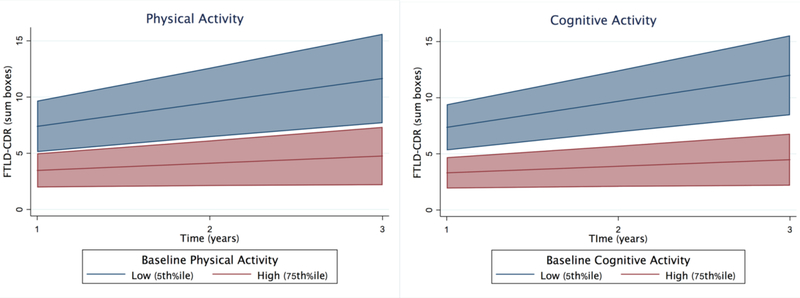
For the primary longitudinal models, effect sizes were calculated by comparing differences in beta parameters (using the reported sample-based z-score units). For illustration purposes (Figures 3–4), high (75th%ile; PASE=200.2 and CAS=26) and low (5th%ile; PASE=14.5 and CAS=6.3) activity levels were selected.
Figure 3.
Higher levels of baseline physical and cognitive activities are associated with significantly slower clinical decline over time in autosomal dominant FTLD mutation carriers (adjusted for age, sex, education). Bands represent 95% CI.
Figure 4.
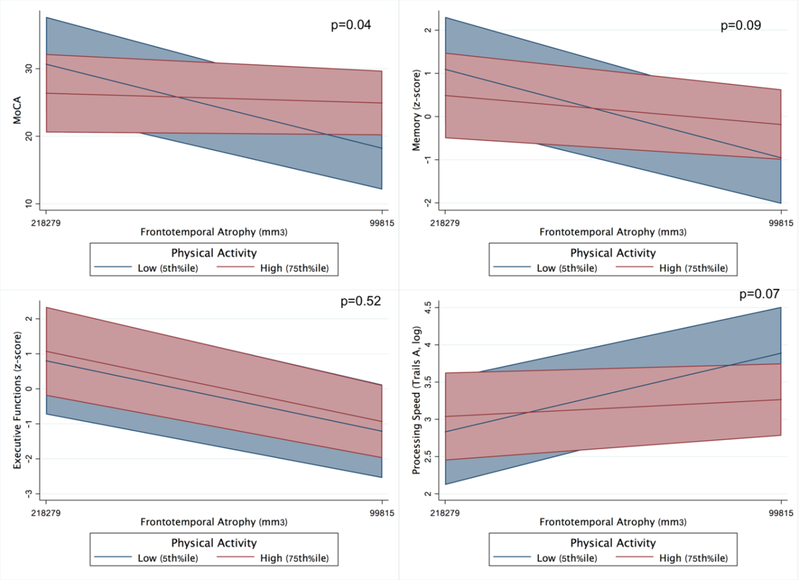
Baseline models illustrating the moderating effect of physical activity on the relationship between frontotemporal volumes and clinical outcomes in FTLD mutation carriers (adjusted for age, sex, education, and FTLD-CDR sum of boxes).
3. RESULTS.
3.1. Do Leisure Activities Differ across Autosomal Dominant FTLD Mutation Carriers and Noncarriers?
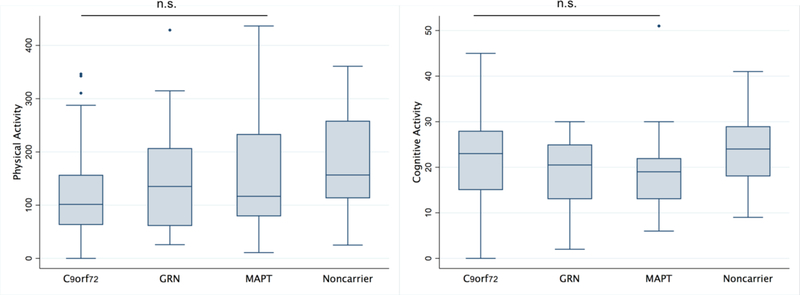
At baseline, adjusting for demographics, levels of physical and cognitive activity differed across MC and noncarriers (physical: η2=0.05, p=0.006; cognitive: η2=0.04, p=0.005). Non-carriers demonstrated higher physical activity levels than the symptomatic (Tukey HSD = 56.5, 95% confidence interval [CI] = 16.3, 96.7, P = 0.003) but not the pre-to-early–symptomatic MC (Tukey HSD = 19.7, 95% CI = −23.2, 62.7, P = 0.52). Noncarriers also demonstrated higher cognitive activity than the symptomatic (Tukey HSD=5.02, 95%CI=1.38, 8.65, p=0.004), but did not statistically differ from the pre-to-early symptomatic carriers (Tukey HSD=1.3, 95%CI= −2.56, 5.2, p=0.70). Symptomatic and pre-to-early symptomatic MC groups did statistically differ (physical: Tukey HSD=35.7, 95%CI= −12.0, 83.5, p=0.18; cognitive: Tukey HSD=3.7, 95%CI= −0.57, 7.99, p=0.10)(Figure 1). Within MCs, physical (η2=0.04, p=0.22) or cognitive (η2=0.02, p=0.37) activity levels did not statistically differ by genotype (Figure 2). Physical and cognitive activities were comparably associated within both MC (Spearman ρ=0.20, p=0.049) and noncarriers (Spearman ρ=0.17, p=0.19).
Figure 1.
Baseline levels of physical and cognitive activity are lowest in syndromic autosomal dominant FTLD mutation carriers (adjusted for age, sex, education).
Figure 2.
Baseline levels of physical and cognitive activities do not differ across FTLD genotype (adjusted for age, sex, education, and FTLD-CDR sum of boxes).
3.3. Do Leisure Activities Affect Brain Health Outcomes in Autosomal Dominant FTLD Mutation Carriers?
3.3.1. Baseline Models.
In MC, adjusting for demographics, higher physical and cognitive activity levels were associated with lower clinical disease severity (FTLD-CDRsb)(PASE: β= −0.23, b=−0.01, 95%CI=−0.02, −0.002; p=0.02; CAS β= −0.23, b=−0.12, 95%CI −0.23, −0.02, p=0.02). Covarying for demographics and FTLD-CDRsb, only higher physical activities were statistically associated with better mood (GDS β=−0.25, b= −0.002, 95%CI=−0.004, −0.0004, p=0.02). The relationship between cognitive and physical activities and other clinical outcomes (all p-values >0.15) or frontotemporal volumes (all p-values >0.20) did not reach statistical significance within this dataset.
3.3.2. Longitudinal Models.
Adjusting for demographics, higher baseline physical and cognitive activities were associated with slowed clinical disease severity (FTLD-CDsb) over time (PASE*time β=−0.11, p=0.016; CAS*time β=−0.13, p=0.003)(Table 2 and Figure 5). Each 1 SD increase in physical or cognitive activity was associated with 62.0% or 55.2% less clinical decline on the FTLD-CDsb per year, respectively. Adjusting for demographics and FTLD-CDRsb, the relationships between baseline physical or cognitive activity levels and longitudinal changes in other cognitive, mood, or brain volume outcomes not reach statistical significance (all p-values>0.30; Table 2).
Table 2.
Longitudinal mixed-effects models demonstrating the positive effect of baseline leisure activities on longitudinal clinical functioning in autosomal dominant FTLD mutation carriers.
| Outcome: | FTLD-CDRsb (N=105) | Frontotemporal Volume (N=53) | ||||
|---|---|---|---|---|---|---|
| β | SE | p-value | β | SE | p-value | |
| Baseline age | 0.29 | 0.08 | <0.001 | −0.63 | 0.11 | <0.001 |
| Sex | −0.03 | 0.16 | 0.88 | −0.15 | 0.23 | 0.52 |
| Education | 0.06 | 0.08 | 0.43 | 0.06 | 0.11 | 0.57 |
| TIV | --- | --- | --- | 0.54 | 0.09 | <0.001 |
| Time | 0.18 | 0.06 | 0.003 | −0.18 | 0.08 | 0.02 |
| Baseline Physical Activity (PA) | −0.18 | 0.08 | 0.02 | −0.01 | 0.10 | 0.89 |
| Baseline PA*time | −0.11 | 0.05 | 0.016 | 0.002 | 0.05 | 0.96 |
| Baseline age | 0.32 | 0.07 | <0.001 | −0.61 | 0.10 | <0.001 |
| Sex | −0.04 | 0.15 | 0.80 | −0.21 | 0.22 | 0.33 |
| Education | 0.06 | 0.08 | 0.46 | 0.09 | 0.10 | 0.35 |
| TIV | --- | --- | --- | 0.57 | 0.09 | <0.001 |
| Time | 0.16 | 0.15 | 0.28 | −0.41 | 0.59 | 0.49 |
| Baseline Cognitive Activity (CA) | −0.18 | 0.07 | 0.02 | 0.14 | 0.10 | 0.15 |
| Baseline CA*time | −0.13 | 0.04 | 0.003 | 0.02 | 0.05 | 0.67 |
Note. Each column represents a model. FTLD = frontotemporal lobar degeneration; CDRsb = Clinical Dementia Rating, sum of boxes; TIV = total intracranial volume.
Figure 5.
Baseline models illustrating the moderating effect of cognitive activity on the relationship between frontotemporal volumes and clinical outcomes in FTLD mutation carriers (adjusted for age, sex, education, and FTLD-CDR sum of boxes).
3.4. Do Leisure Activities Moderate Brain-Behavior Relationships in Autosomal Dominant FTLD Mutation Carriers?
3.4.1. Baseline Models.
Physical Activity.
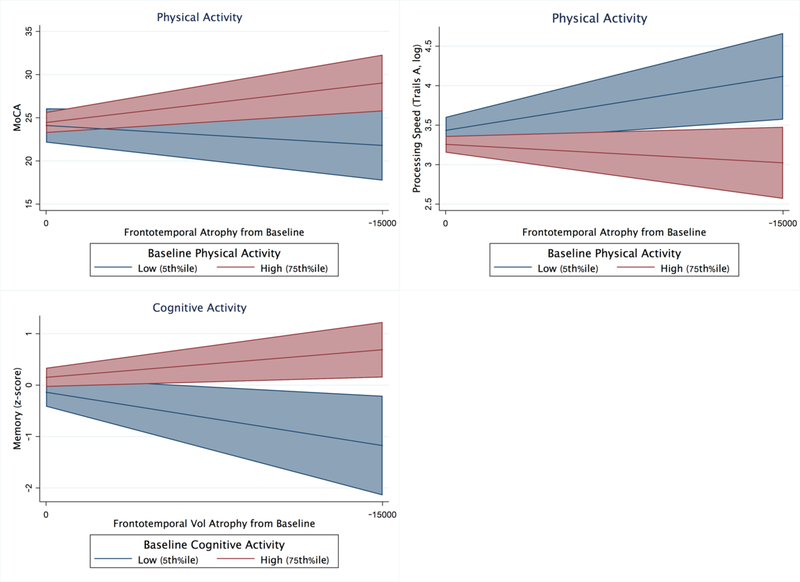
In MCs, adjusting for demographics and FTLD-CDRsb, there was a physical activity by frontotemporal volume interaction on global cognition (MoCA; PASE*volume β =−0.22, p=0.036). MCs with higher reported physical activity showed a significantly attenuated relationship between frontotemporal volumes and clinical functioning compared to their less active peers. Parallel models demonstrated a similar physical activity by volume interaction on processing speed (Trails A; PASE*volume β=0.05, p=0.07), and episodic memory (PASE*volume β=−0.21, p=0.09), though these effects did not reach α=0.05 threshold. There were no observed statistically significant interactions between physical activity and frontotemporal volumes for executive functioning or mood (all p-values>0.52; Table 3 and Figure 3).
Table 3.
Baseline models illustrating the moderating effects of leisure activities on the relationship between frontotemporal volumes and clinical outcomes in autosomal dominant FTLD mutation carriers (n=53).
| Outcome: | MoCA | Processing Speed (logTrails A) | Executive Functions (z-score) | Episodic Memory (z-score) |
GDS (log) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | β | p-value | b | β | p-value | b | β | p-value | b | β | p-value | b | β | p-value | |
| PHYSICAL ACTIVITY | |||||||||||||||
| Age | 0.05 | 0.12 | 0.36 | −7.2e-5 | −0.002 | 0.99 | 0.01 | 0.13 | 0.41 | 0.0001 | 0.02 | 0.93 | −0.01 | −0.23 | 0.28 |
| Sex | 0.29 | 0.05 | 0.62 | −0.10 | −0.18 | 0.09 | 0.08 | 0.09 | 0.49 | −0.04 | −0.05 | 0.69 | −0.01 | −0.02 | 0.92 |
| Edu | 0.24 | 0.09 | 0.33 | −0.004 | −0.02 | 0.87 | 0.05 | 0.12 | 0.33 | 0.06 | 0.17 | 0.16 | −0.08 | −0.27 | 0.09 |
| FTLD-CDRsob | −1.2 | −0.73 | <0.001 | 0.10 | 0.66 | <0.001 | −0.12 | −0.44 | 0.004 | −0.12 | −0.53 | <0.01 | −0.001 | −0.02 | 0.90 |
| Frontotemporal volume | 4.1e-5 | 0.14 | 0.34 | −3.8e-6 | −0.14 | 0.37 | 1.6e-5 | 0.33 | 0.08 | 7.5e-6 | 0.20 | 0.30 | −6.7e-6 | −0.02 | 0.90 |
| Physical Activity (PA) | 0.01 | 0.10 | 0.33 | −0.001 | −0.24 | 0.03 | 0.002 | 0.16 | 0.21 | 0.0004 | 0.05 | 0.68 | −0.002 | −0.31 | 0.06 |
| FT vol*PA | −5.7e-7 | −0.22 | 0.036 | 4.7e-8 | 0.19 | 0.07 | −3.6 | −0.08 | 0.52 | −7.4e-8 | −0.21 | 0.09 | −1.0e-8 | −0.06 | 0.70 |
| COGNITIVE ACTIVITY | |||||||||||||||
| Age | 0.04 | 0.10 | 0.36 | 0.004 | 0.10 | 0.45 | 0.004 | 0.06 | 0.67 | −0.004 | −0.07 | 0.61 | −0.01 | −0.18 | 0.37 |
| Sex | 0.24 | 0.04 | 0.62 | −0.03 | −0.05 | 0.65 | 0.05 | 0.05 | 0.65 | −0.01 | −0.02 | 0.85 | −0.05 | −0.07 | 0.64 |
| Edu | 0.37 | 0.15 | 0.08 | 0.01 | 0.05 | 0.05 | 0.12 | 0.28 | 0.35 | 0.08 | 0.24 | 0.02 | −0.26 | −0.07 | 0.08 |
| FTLD-CDRsob | −1.0 | −0.63 | <0.001 | 0.10 | 0.65 | <0.001 | −0.12 | −0.44 | 0.003 | −0.09 | −0.42 | 0.001 | −0.04 | −0.16 | 0.39 |
| Frontotemporal volume | 3.5e-5 | 0.12 | 0.38 | −2.0e-6 | −0.07 | 0.65 | 1.4e-5 | 0.28 | 0.11 | 4.2e-6 | 0.11 | 0.50 | −1.1 | −0.32 | 0.20 |
| Cognitive Activity (CA) | 0.14 | 0.20 | 0.02 | 0.004 | 0.06 | 0.59 | −0.002 | −0.02 | 0.87 | 0.02 | 0.17 | 0.11 | −0.01 | −0.12 | 0.41 |
| FT vol*CA | −7.9e-6 | −0.23 | 0.009 | 5.7e-7 | 0.17 | 0.09 | −1.4e-6 | −0.25 | 0.027 | −1.7e-6 | −0.38 | <0.01 | 8.9e-7 | 0.22 | 0.12 |
Note. Each column represents a model. Frontotemporal volume represents a residual adjusted for total intracranial volume.
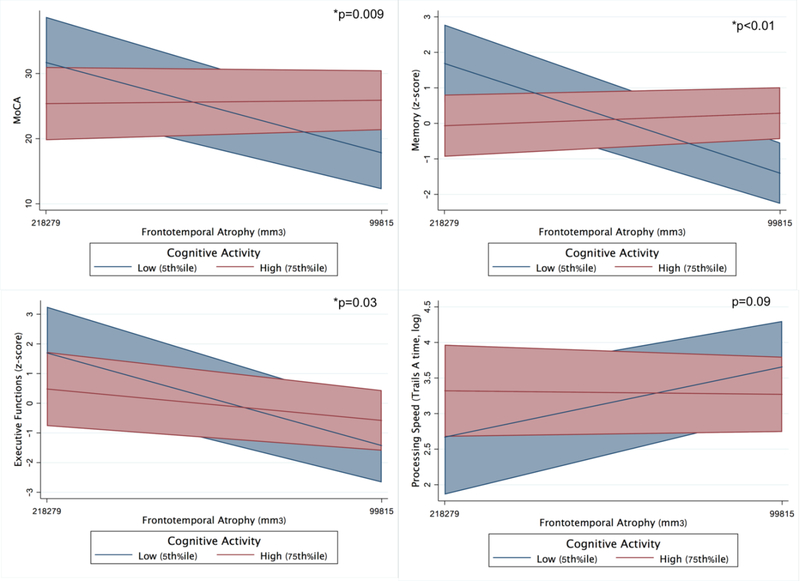
Cognitive Activity.
Similarly, there was a cognitive activity by frontotemporal volume interaction on global cognition (MoCA; CAS*volume β=−0.23, p=0.009), episodic memory (CAS*volume β=−0.38, p<0.001), and executive functioning (CAS*volume β=−0.25, p=0.03). The model examining processing speed approached, but did not reach significance (CAS*volume β=0.17, p=0.09). Again, MC who reported higher levels of cognitive activity demonstrated disproportionately weaker relationship between frontotemporal volumes and cognitive performance compared to their less cognitively active peers. There was no statistically significant cognitive activity by frontotemporal volume interaction for mood (p>0.38; Table 3 and Figure 4).
3.4.2. Longitudinal Models.
Physical Activity.
Adjusting for baseline demographics, FTLD-CDRsb, and baseline frontotemporal volumes, there was an interaction between baseline physical activity and within-person frontotemporal atrophy on changes in global cognition (MoCA) and processing speed (Trails A)(all p-values<0.03 Table 4); models examining memory approached, but did not reach statistical significance (p=0.09). MCs with higher baseline physical activity demonstrated a significant attenuation in the relationship between frontotemporal atrophy and cognition over time. High physical activity (75th%ile) MCs with 1 SD of frontotemporal volume loss demonstrated 2.5-fold better global cognition and 2.7-fold better processing speed per year compared to low physical activity (5th%ile) MC with the same amount of volume loss (Figure 4). The interaction between physical activity and frontotemporal atrophy did not reach statistical significance for executive functioning or mood (all p-values>0.62; Table 4).
Table 4.
Longitudinal models illustrating the moderating effects of baseline leisure activities on the relationship between within-person frontotemporal atrophy and clinical outcomes in autosomal dominant FTLD mutation carriers (n=53).
| Outcome: | MoCA | Processing Speed, time (Trails A) | Executive Functions (z-score) | Episodic Memory (z-score) |
GDS (log) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | p-value | β | SE | p-value | β | SE | p-value | β | SE | p-value | β | SE | p-value | |
| PHYSICAL ACTIVITY | |||||||||||||||
| Baseline age | 0.18 | 0.13 | 0.17 | 0.03 | 0.14 | 0.81 | 0.14 | 0.16 | 0.40 | 0.02 | 0.17 | 0.89 | −0.17 | 0.21 | 0.43 |
| Sex | 0.25 | 0.22 | 0.26 | 0.35 | 0.24 | 0.14 | −0.10 | 0.28 | 0.74 | 0.22 | 0.28 | 0.44 | 0.14 | 0.36 | 0.70 |
| Education | 0.11 | 0.10 | 0.22 | −0.04 | 0.11 | 0.73 | 0.12 | 0.12 | 0.33 | 0.17 | 0.12 | 0.15 | −0.26 | 0.15 | 0.08 |
| Baseline FTLD-CDRsb | −0.92 | 0.15 | <0.001 | −0.09 | 0.17 | 0.60 | −0.55 | 0.20 | 0.006 | −0.67 | 0.19 | <0.001 | −0.01 | 0.32 | 0.97 |
| TIV | −0.27 | 0.13 | 0.036 | −0.05 | 0.14 | 0.73 | −0.17 | 0.17 | 0.32 | −0.11 | 0.17 | 0.53 | 0.008 | 0.22 | 0.97 |
| Baseline FT vol | 0.13 | 0.15 | 0.39 | −0.09 | 0.17 | 0.60 | 0.35 | 0.20 | 0.07 | 0.13 | 0.19 | 0.51 | −0.15 | 0.26 | 0.60 |
| Within-person FT vol | −0.15 | 0.07 | 0.039 | 0.06 | 0.11 | 0.59 | −0.13 | 0.12 | 0.28 | 0.10 | 0.10 | 0.33 | 0.05 | 0.17 | 0.78 |
| Physical Activity (PA) | 0.12 | 0.09 | 0.18 | −0.36 | 0.10 | 0.009 | −.13 | 0.12 | 0.27 | −0.07 | 0.11 | 0.51 | −0.30 | 0.15 | 0.04 |
| Within-person FT vol*PA | −0.20 | 0.09 | 0.03 | 0.39 | 0.14 | 0.004 | −0.17 | 0.15 | 0.25 | 0.26 | 0.14 | 0.06 | 0.12 | 0.23 | 0.62 |
| COGNITIVE ACTIVITY | |||||||||||||||
| Baseline age | 0.13 | 0.11 | 0.21 | 0.12 | 0.13 | 0.36 | 0.09 | 0.14 | 0.54 | −0.01 | 0.14 | 0.09 | 0.003 | 0.19 | 0.99 |
| Sex | 0.19 | 9.20 | 0.35 | 0.23 | 0.23 | 0.32 | −0.04 | 0.26 | 0.88 | 0.12 | 0.26 | 0.64 | 0.22 | 0.36 | 0.55 |
| Education | 0.11 | 0.08 | 0.18 | 0.03 | 0.10 | 0.72 | 0.09 | 0.11 | 0.41 | 0.17 | 0.10 | 0.08 | −0.22 | 0.13 | 0.11 |
| Baseline FTLD-CDRsb | 0.11 | 0.14 | 0.41 | 0.89 | 0.17 | <0.001 | −0.59 | 0.18 | 0.001 | −0.58 | 0.17 | 0.001 | −0.13 | 0.30 | 0.67 |
| TIV | −0.22 | 0.12 | 0.07 | −0.11 | 0.13 | 0.43 | −0.15 | 0.15 | 0.33 | −0.08 | 0.15 | 0.62 | −0.15 | 0.22 | 0.49 |
| Baseline FT vol | 0.11 | 0.14 | 0.41 | −0.04 | 0.16 | 0.78 | 0.32 | 0.18 | 0.07 | 0.13 | 0.17 | 0.44 | −0.09 | 0.25 | 0.73 |
| Within-person FT vol | −0.06 | 0.06 | 0.31 | −0.13 | 0.10 | 0.22 | −0.06 | 0.10 | 0.57 | −0.02 | 0.07 | 0.75 | −0.05 | 0.11 | 0.64 |
| Cognitive Activity (CA) | 0.10 | 0.09 | 0.26 | 0.07 | 0.10 | 0.51 | −0.06 | 0.11 | 0.58 | 0.20 | 0.10 | 0.049 | −0.13 | 0.15 | 0.36 |
| Within-person FT vol*CA | 0.06 | 0.10 | 0.53 | −0.10 | 0.16 | 0.53 | 0.01 | 0.16 | 0.96 | −0.25 | 0.11 | 0.029 | −0.001 | 0.19 | 0.99 |
Note. Each column represents a model. MoCA = Montreal Cognitive Assessment; GDS = Geriatric Depression Scale (higher indicates greater depressive symptoms); FAQ = Functional Activity Questionnaire (higher indicates greater impairment). All models evaluated the interaction between baseline nuisance covariates (baseline age, sex, education, and FTLD-CDRsb) and time in study (years), none of which reached significance (ps>0.05).
Cognitive Activity.
In parallel models, there was an interaction between baseline cognitive activity and within-person frontotemporal atrophy on episodic memory changes (p=0.29; Table 4). High cognitive activity (75th%ile) MCs with 1 SD of frontotemporal atrophy demonstrated 2.3-fold better memory performances per year compared to low cognitive activity (5th%ile) MCs with comparable volume loss (Figure 6). The interaction between cognitive activity and brain atrophy did not reach statistical significance for other clinical outcomes (all p-values>0.19; Table 4 and Figure 6).
Figure 6.
Baseline physical and cognitive activities significantly moderate the relationship between frontotemporal atrophy and longitudinal clinical outcomes in autosomal dominant FTLD mutation carriers (adjusted for baseline age, sex, education, and FTLD-CDR sum of boxes). Bands represent 95% CI.
4. Discussion
We examined the direct and moderating effects of having physically and cognitively active lifestyles for brain and cognitive health in autosomal dominant FTLD MCs. Overall, physical and cognitive activities were lower in syndromically impaired MCs, and higher baseline activity levels were associated with slower clinical decline (FTLD-CDR) in MCs over time. Once adjusting for clinical severity, we did not observe strong direct relationships between activity engagement and cognitive or brain outcomes; however, there were significant moderating effects of leisure activities on the relationship between frontotemporal brain volumes and cognition both at baseline and beginning to extend longitudinally. Autosomal dominant MC who engaged in more physical or cognitive activities at baseline demonstrated disproportionately better neuropsychological performances given their frontotemporal atrophy compared to their less active peers. These data suggest that: 1) lifestyle behaviors may play an important role in the clinical presentation of genetic disease, conferring resilience even in autosomal dominant FTLD, and 2) highlight the importance of expanding the extant aging and AD literature examining environmental factors for cognitive health trajectories into FTLD.
Our pattern of results supports a potential “cognitive reserve” hypothesis in genetic FTLD. While greater engagement in physical and cognitive behaviors were associated with less overall clinical severity both cross-sectionally and longitudinally, activities were not significantly associated with frontotemporal volumes, particularly once adjusting for clinical severity. These latter null findings may indicate 1) a small effect of lifestyle activities on brain structure that we were not powered to detect (e.g., n=53 with imaging), and/or 2) that leisure activities do not directly affect brain structure. Given the autosomal dominant nature of the disease, perhaps it is less surprising that activities may have less of a direct effect on brain structure; instead, it appears that lifestyle activities moderate how changes in brain structure present clinically in the context of an ongoing genetic disorder. In other words, perhaps the “protective mechanism” behind leisure activities does not directly affect brain structure but instead affects other mechanisms that can influence how existing brain structure functions (e.g., neuroinflammatory regulation, synaptic facilitation, glymphatic clearance, etc.). Regarding this significant moderation effect, “cognitive reserve” refers to the dynamic processes that support cognition and day-to-day functioning despite brain changes or damage, and is operationalized as any factor that therefore interacts with or modifies the brain-behavior relationship[38,39]. Though a latent construct and inherently difficult to measure, “reserve” provides a useful framework to model and test factors that contribute to the clinical heterogeneity of brain diseases and help identify potential intervention points. In the context of our findings, our data suggest that lifestyle activities may help maintain or support cognition despite, and particularly among, MCs demonstrating atrophy; in other words, more active MC appeared to be defying traditional brain-behavior relationships and “outperforming” what their brain volumes would otherwise predict, consistent with a host of prior literature examining this activity-related cognitive reserve phenomenon in aging, AD, PD, HIV, and MS, among others[40–43].
The important modifying role of lifestyle on brain development with age is becoming more apparent across the spectrum of neurodegenerative diseases and converging evidence demonstrates these effects even in autosomal dominant human diseases. Decades of large-scale epidemiological works and growing clinical trials have demonstrated benefits of physical and cognitive enrichment for the brain in “typical” aging and, most extensively, in the AD spectrum[1,4,17,44–49]. In both the DIAN Study and Columbian (PSEN1 E280A mutation) cohorts of autosomal dominant AD, greater physical activity and educational attainment were associated with delayed estimated clinical onset, and in the DIAN cohort, lower markers of AD pathology[12,50]. We are extending these findings into the FTLD spectrum with autosomal dominant inheritance. Autosomal dominant neurodegenerative disorders provide a powerful framework to test basic questions regarding disease pathophysiology in-vivo. Now, data across disparate autosomal dominant neurodegenerative disorders demonstrate that lifestyle behaviors play a role in disease manifestation, supporting the proof-of-principle that lifestyle is a candidate factor influencing the development of neurodegeneration in humans.
Although the mechanisms underlying this relationship cannot be determined from our study design, several candidate pathways may be implicated for future works. Decades of animal studies have demonstrated the important, causal role of physical and cognitive enrichment on synaptogenesis, including increased density of dendritic spines and arborization, and development of more complex, perforated post-synaptic densities[18,51–53]. Perhaps these behaviors help support synaptic homeostasis and resilience, promoting functioning even in the context of disease. Additionally, converging evidence suggests that immune functioning may be at the crux, causally and/or secondarily involved, in the pathological development of at least some forms of FTLD[23–25]. Engagement in more physically and also cognitively enriched environments appears to directly modulate immune functioning in the brain, including increased number of glial cells, astrocytic hypertrophy and ensheathement of neurons, reduced microglial reactivity, and promotion of glymphatic clearance[19–21]. Perhaps more active and “enriched” lifestyles help prevent or mitigate FTLD-related immune and glial dysregulation, thereby supporting functioning. Future works modeling the effects of lifestyle in system models of FTLD, as well as utilization of novel molecular biofluid markers in humans may help tease these mechanisms apart, inform our pathophysiological understanding of FTLD, and help identify risk stratification and monitoring tools for future behavioral interventions.
There are several limitations to our data. First, it is important to note that our design was observational and the contribution of reverse causality must be considered (more impaired individuals are engaging in fewer activities). We statistically adjusted for overall disease severity (FTLD-CDRsb) in all primary models to help mitigate this effect and the longitudinal component and nature of the interaction help support the high clinical relevance, but future experimental interventions would be necessary to determine causality. Additionally, only a subset of MC had available neuroimaging and ~50% had longitudinal time-points. Several outcomes that reached significance in our baseline models did not hold longitudinally, and the relationship between lifestyle activities and brain volumes did not reach significance; given our sample size limitations, it is difficult to determine if these are “true” null results or represent Type II error. Nonetheless, we demonstrated a pattern of significant baseline models that we began to expand longitudinally, and utilized mixed-effects analyses to leverage all available data. However, larger cohorts and additional longitudinal time-points are needed to replicate these findings and better estimate effect sizes. Additionally, the clinical heterogeneity of genetic FTLD spans from motor neuron disease to behavioral variant FTD and aphasia syndromes. Given our relatively small sample sizes, we were unable to tease apart the impact of lifestyle on any specific genotype or emerging clinical syndrome; this would be an important area of future inquiry and may help support potential mechanistic pathways. Lastly, our measures of lifestyle activities were self-report, which carry inherent social desirability and other potential biases, especially in disease states[54].
Ours are among the first data supporting a potentially protective role of lifestyle behaviors in FTLD and, in the absence of pharmacological interventions, underscore the need to study these environmental factors across the neurodegenerative spectrum. Lifestyle behaviors may be a contributing factor to the highly variable clinical onset and trajectory observed in genetic FTLD, which has very relevant implications for clinical trials in search of predictive algorithms and reliable endpoints. Potentially combining both pharmacologic and lifestyle into holistic treatment approaches may represent a more powerful tool to promote brain health and delay disease in MC. Future works are needed to: 1) further develop mechanistic and other biomarker monitoring tools to understand how (and in whom) lifestyle affects neurobehavior in FTLD, 2) integrate objective measures of lifestyle (e.g., activity monitors), and 3) manipulate lifestyle behaviors via experimental designs to better estimate effect sizes and support causality.
Research In Context.
Systematic review: We reviewed the literature using traditional sources (e.g., PubMed, GoogleScholar). Although more physically and cognitively demanding lifestyles are consistently associated with better brain health outcomes in typical aging and Alzheimer’s disease, its role in development of frontotemporal lobar degeneration (FTLD) has been largely neglected. Some preliminary works linked occupational complexity to outcomes in FTLD; we extend to examine current lifestyle activities and test the veracity of this relationship in individuals with autosomal dominant FTLD mutations.
Interpretation: Our results suggest that more active lifestyles are associated with better clinical outcomes and greater clinical resilience in FTLD, consistent with the Alzheimer’s literature. Importantly, these are the first data to support the proof-of-principle that lifestyle appears to be important even in autosomal dominant FTLD.
Future directions: These findings call for more in-depth work to parse out: 1) the molecular mechanisms driving these effects, 2) objective lifestyle monitoring, and 3) experimental interventions to determine causality of lifestyle on FTLD outcomes.
Highlights.
Can lifestyle modify development of autosomal dominant FTLD?
More active lifestyles were associated with >55% slower decline in FTLD carriers
High activity carriers had >2x better clinical outcomes than predicted by atrophy
Low-cost lifestyle changes may modify autosomal dominant neurodegenerative outcomes
Acknowledgements
This study was supported by the following NIH-NIA grants, Longitudinal Evaluation of Familial Frontotemporal Dementia Subjects (LEFFTDS) (U01AG045390) and Advancing Research and Treatment in Frontotemporal Lobar Degeneration (ARTFL; U54NS092089), K23AG058752 and L30AG057123 (PI: Casaletto). Our work was also supported by the Larry L. Hillblom Fellowship (2017-A-004-FEL; Casaletto).
Disclosures
Casaletto, K.B.- receives research support from the NIH and Larry L. Hillblom Foundation.
Staffaroni, A.M. – receives research support from the NIH and Larry L. Hillblom Foundation.
Wolf, A – nothing to disclose
Appleby, B – receives research support from CDC
Brushaber, D – nothing to disclose
Coppola, G – receives research support from the NIH, the Tau Consortium, the Adelson Medical Research Foundation, Takeda Pharmaceutical Company Ltd., the John Douglas French Alzheimer’s Foundation, the Friedreich’s Ataxia Research Alliance, the CHDI foundation, the Hillblom Foundation, the Eleanor Leslie Chair in Innovative Brain Research from the Brain Research Institute, and the Semel Institute for Neuroscience and Human Behavior at the University of California Los Angeles.
Dickerson, B – receives research support from NIH and royalties from Oxford University Press and Cambridge University; consults for Biogen, Merch, Lilly, Wave Lifesciences and Arkuda; and is paid by Elsevier for editorial activity
Domoto-Reilly, K – Has served as an investigator for clinical trials sponsored by Avid Radiopharmaceuticals, Biogen, Janssen Pharmaceuticals. Has served as Advisory Board consultant for Biogen. Receives research support from NIH
Elahi, F.M. – nothing to disclose
Fields, J – receives research support from NIH
Fong J – nothing to disclose
Forsberg, L.K. – nothing to disclose
Ghoshal, N - has participated or is currently participating in clinical trials of anti-dementia drugs sponsored by the following companies: Bristol Myers Squibb, Eli Lilly/Avid Radiopharmaceuticals, Janssen Immunotherapy, Novartis, Pfizer, Wyeth, SNIFF (The Study of Nasal Insulin to Fight Forgetfulness) study, and A4 (The Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease) trial. She receives research support from Tau Consortium and Association for Frontotemporal Dementia and is funded by the NIH.
Graff-Radford N – receives royalties from UpToDate, has participated in multicenter therapy studies by sponsored by Biogen, TauRx, AbbVie, Novartis and Lilly. He receives research support from NIH.
Grossman M - receives grant support from NIH, Avid and Piramal; participates in clinical trials sponsored by Biogen, TauRx, and Alector; serves as a consultant to Bracco and UCB; and serves on the Editorial Board of Neurology
Heuer, H.W. – nothing to disclose
Hsiung G-Y – has served as an investigator for clinical trials sponsored by AstraZeneca, Eli Lilly, and Roche / Genentech. He receives research support from Canadian Institutes of Health Research and the Alzheimer Society of British Columbia.
Huey E – receives research support from NIH
Irwin D – receives support from NIH, Brightfocus Foundation and Penn Institute on Aging.
Kantarci K - served on the Data Safety Monitoring Board for Takeda Global Research & Development Center, Inc.; data monitoring boards of Pfizer and Janssen Alzheimer Immunotherapy; research support from the Avid Radiopharmaceuticals, Eli Lilly, the Alzheimer’s Drug Discovery Foundation and NIH
Kaufer D - Has served as an investigator for clinical trials sponsored by Abbvie, Axovant, Janssen Research & Development, Navidea Biopharmaceuticals, and TauRx. He has consulted for Abbvie, Axovant, Janssen Research & Development, Takeda/Zinfandel. He serves on the Scientific Advisory Board of the Lewy Body Dementia Association. He receives research funding from the NIH, HRSA, and Bryan Family Foundation.
Kerwin, D has served on an Advisory Board for AbbVie and as site PI for studies funded by Roche/Genentech, AbbVie, Avid, Novartis, Eisai, Eli Lilly and UCSF.
Knopman D - serves on the DSMB of the DIAN-TU study, is a site PI for clinical trials sponsored by Biogen, Lilly and the University of Southern California, and is funded by NIH.
Kornak J - – has provided expert witness testimony for Teva Pharmaceuticals in Forest Laboratories Inc. et al. v. Teva Pharmaceuticals USA, Inc., Case Nos. 1:14-cv-00121 and 1:14-cv-00686 (D. Del. filed Jan. 31, 2014 and May 30, 2014) regarding the drug Memantine; for Apotex/HEC/Ezra in Novartis AG et al. v. Apotex Inc., No. 1:15-cv-975 (D. Del. filed Oct. 26, 2015, regarding the drug Fingolimod. He has also given testimony on behalf of Puma Biotechnology in Hsingching Hsu et al, vs. Puma Biotechnology, INC., et al. 2018 regarding the drug Neratinib. He receives research support from the NIH.
Kramer, JH – consults with Biogen Pharmaceuticals; receives research support from NIH
Litvan I– receives research support from NIH, Parkinson Study Group, Parkinson Foundation, Michael J Fox Foundation, AVID Pharmaceuticals, C2N Diagnostics/Abbvie and Bristol-Myers Squibb. She was a member of the Biogen and Bristol-Myers Squibb Advisory Boards, Biotie/Parkinson Study Group Medical Advisory Board and consultant for Toyama Pharmaceuticals. Receives salary from the University of California San Diego and as Editor in Frontiers in Neurology
Mackenzie I.R. – receives research funding from Canadian Institutes of Health Research.
Mendez M – supported by NIH (NIA) research grants and has received research support from Biogen
Miller B.L. – receives research support from NIH
Rademakers R – receives research funding from NIH and the Bluefield Project to Cure Frontotemporal dementia
Ramos EM – nothing to disclose
Rascovsky, K – nothing to disclose
Roberson ED – receives research support from NIH, Bluefield Project to Cure Frontotemporal Dementia, Alzheimer’s Association, BrightFocus Foundation, the Alzheimer’s Drug Discovery Foundation, Biogen, Alector, and owns intellectual property related to tau. He has served as a consultant for Novartis and Biogen.
Syrjanen, J.A. – nothing to disclose
Tartaglia M.C. – receives research funding from CIHR and NIH, and is an investigator on pharmaceutical studies with Biogen, Roche, Eli Lilly, and Boehringer
Weintraub S – receives research support from NIH
Boxer A – receives research support from NIH, the Tau Research Consortium, the Association for Frontotemporal Degeneration, Bluefield Project to Cure Frontotemporal Dementia, Corticobasal Degeneration Solutions, the Alzheimer’s Drug Discovery Foundation and the Alzheimer’s Association. He has served as a consultant for Aeton, Abbvie, Alector, Amgen, Arkuda, Ionis, Iperian, Janssen, Merck, Novartis, Passage BIO, Pinteon, Samumed, Toyama and UCB, and received research support from Avid, Biogen, BMS, C2N, Cortice, Eli Lilly, Forum, Genentech, Janssen, Novartis, Pfizer, Roche and TauRx.
Boeve B – has served as an investigator for clinical trials sponsored by GE Healthcare and Axovant. He receives royalties from the publication of a book entitled Behavioral Neurology Of Dementia (Cambridge Medicine, 2009, 2017). He serves on the Scientific Advisory Board of the Tau Consortium. He receives research support from NIH, the Mayo Clinic Dorothy and Harry T. Mangurian Jr. Lewy Body Dementia Program and the Little Family Foundation.
Rosen H – has received research support from Biogen Pharmaceuticals, has consulting agreements with Wave Neuroscience and Ionis Pharmaceuticals, and receives research support from NIH.
Yaffe, K – receives research support from the NIH
References
- [1].Hörder H, Johansson L, Guo X, Grimby G, Kern S, Östling S, et al. Midlife cardiovascular fitness and dementia: A 44-year longitudinal population study in women. Neurology 2018:10.1212/WNL.0000000000005290. doi: 10.1212/WNL.0000000000005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wilson RS, Boyle PA, Yu L, Barnes LL, Schneider JA, Bennett DA. Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology 2013;81:314–21. doi: 10.1212/WNL.0b013e31829c5e8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Grande G, Vanacore N, Maggiore L, Cucumo V, Ghiretti R, Galimberti D, et al. Physical activity reduces the risk of dementia in mild cognitive impairment subjects: A cohort study. J Alzheimer’s Dis 2014. doi: 10.3233/JAD-131808. [DOI] [PubMed] [Google Scholar]
- [4].Yaffe K, Barnes D, Nevitt M, Lui L-Y, Covinsky K. A Prospective Study of Physical Activity and Cognitive Decline in Elderly Women. Arch Intern Med 2001;161:1703. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- [5].Barnes DE, Yaffe K, Satariano WA, Tager IB. A Longitudinal Study of Cardiorespiratory Fitness and Cognitive Function in Healthy Older Adults. J Am Geriatr Soc 2003;51:459–65. doi: 10.1046/j.1532-5415.2003.51153.x. [DOI] [PubMed] [Google Scholar]
- [6].Lautenschlager NT, Cox KL, Flicker L, Foster JK, Van Bockxmeer FM, Xiao J, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: A randomized trial. JAMA - J Am Med Assoc 2008. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- [7].Aarsland D, Sardahaee FS, Anderssen S, Ballard C, Alzheimer’s Society Systematic Review group. Is physical activity a potential preventive factor for vascular dementia? A systematic review. Aging Ment Health 2010. doi: 10.1080/13607860903586136. [DOI] [PubMed] [Google Scholar]
- [8].Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, et al. Physical activity, APOE genotype, and dementia risk: Findings from the Cardiovascular Health Cognition Study. Am J Epidemiol 2005. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- [9].Chen H, Zhang SM, Schwarzschild MA, Hernán MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology 2005. doi: 10.1212/01.WNL.0000151960.28687.93. [DOI] [PubMed] [Google Scholar]
- [10].Thacker EL, Chen H, Patel AV., McCullough ML, Calle EE, Thun MJ, et al. Recreational physical activity and risk of Parkinson’s disease. Mov Disord 2008. doi: 10.1002/mds.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bang J, Spina S, Miller BL. Non-Alzheimer’s dementia 1: Frontotemporal dementia. Lancet 2015. doi: 10.1016/S0140-6736(15)00461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Müller S, Preische O, Sohrabi HR, Gräber S, Jucker M, Ringman JM, et al. Relationship between physical activity, cognition, and Alzheimer pathology in autosomal dominant Alzheimer’s disease. Alzheimer’s Dement 2018;0. doi: 10.1016/j.jalz.2018.06.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rasmussen Eid H, Rosness TA, Bosnes O, Salvesen Ø, Knutli M, Stordal E. Smoking and Obesity as Risk Factors in Frontotemporal Dementia and Alzheimer’s Disease: The HUNT Study. Dement Geriatr Cogn Dis Extra 2019;9:1–10. doi: 10.1159/000495607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Golimstok A, Cámpora N, Rojas JI, Fernandez MC, Elizondo C, Soriano E, et al. Cardiovascular risk factors and frontotemporal dementia: a case-control study. Transl Neurodegener 2014;3:13. doi: 10.1186/2047-9158-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Massimo L, Zee J, Xie SX, Mcmillan CT, Rascovsky K, Irwin DJ, et al. Occupational attainment influences survival in autopsy-confirmed frontotemporal degeneration. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Massimo L, Xie SX, Rennert Lior, Massimo DM, Halpin A, Placek K, et al. Occupational attainment influences longitudinal decline in behavioral variant frontotemporal degeneration. Brain Imaging Behav n.d;0:3. doi: 10.1007/s11682-018-9852-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang HX, MacDonald SWS, Dekhtyar S, Fratiglioni L. Association of lifelong exposure to cognitive reserve-enhancing factors with dementia risk: A community-based cohort study. PLoS Med 2017;14. doi: 10.1371/journal.pmed.1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pang TYC, Hannan AJ. Enhancement of cognitive function in models of brain disease through environmental enrichment and physical activity. Neuropharmacology 2013. doi: 10.1016/j.neuropharm.2012.06.029. [DOI] [PubMed] [Google Scholar]
- [19].Markham JA, Greenough WT. Experience-driven brain plasticity: Beyond the synapse. Neuron Glia Biol 2004;1:351–63. doi: 10.1017/S1740925X05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].He X, Liu D, Zhang Q, Liang F, Dai G, Zeng J, et al. Voluntary Exercise Promotes Glymphatic Clearance of Amyloid Beta and Reduces the Activation of Astrocytes and Microglia in Aged Mice. Front Mol Neurosci 2017;10:144. doi: 10.3389/fnmol.2017.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xu H, Gelyana E, Rajsombath M, Yang T, Li S, Selkoe D. Environmental Enrichment Potently Prevents Microglia-Mediated Neuroinflammation by Human Amyloid β-Protein Oligomers. J Neurosci 2016;36:9041–56. doi: 10.1523/JNEUROSCI.1023-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cracchiolo JR, Mori T, Nazian SJ, Tan J, Potter H, Arendash GW, et al. Enhanced cognitive activity – over and above social or physical activity – is required to protect Alzheimer’s mice against cognitive impairment, reduce Aβ deposition, and increase synaptic immunoreactivity. Neurobiol Learn Mem 2007;88:277–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Krabbe G, Minami SS, Etchegaray JI, Taneja P, Djukic B, Davalos D, et al. Microglial NFκB-TNFα hyperactivation induces obsessive-compulsive behavior in mouse models of progranulin-deficient frontotemporal dementia. Proc Natl Acad Sci U S A 2017;114:5029–34. doi: 10.1073/pnas.1700477114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Broce I, Karch CM, Wen N, Fan CC, Wang Y, Hong Tan C, et al. Immune-related genetic enrichment in frontotemporal dementia: An analysis of genome-wide association studies. PLoS Med 2018. doi: 10.1371/journal.pmed.1002487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lui H, Zhang J, Makinson SR, Cahill MK, Kelley KW, Huang HY, et al. Progranulin Deficiency Promotes Circuit-Specific Synaptic Pruning by Microglia via Complement Activation. Cell 2016. doi: 10.1016/j.cell.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Boxer AL, Gold M, Huey E, Gao FB, Burton EA, Chow T, et al. Frontotemporal degeneration, the next therapeutic frontier: Molecules and animal models for frontotemporal degeneration drug development. Alzheimer’s Dement 2013. doi: 10.1016/j.jalz.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Knopman DS, Kramer JH, Boeve BF, Caselli RJ, Graff-Radford NR, Mendez MF, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain 2008;131:2957–68. doi: 10.1093/brain/awn234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The Physical Activity Scale for the Elderly (PASE): Evidence for validity. J Clin Epidemiol 1999. doi: 10.1016/S0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- [29].Bolszak S, Casartelli NC, Impellizzeri FM, Maffiuletti NA. Validity and reproducibility of the Physical Activity Scale for the Elderly (PASE) questionnaire for the measurement of the physical activity level in patients after total knee arthroplasty. BMC Musculoskelet Disord 2014. doi: 10.1186/1471-2474-15-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Washburn RA, Smith KW, Jette AM, Janney CA. The physical activity scale for the elderly (PASE): Development and evaluation. J Clin Epidemiol 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- [31].Hagiwara A, Ito N, Sawai K, Kazuma K. Validity and reliability of the Physical Activity Scale for the Elderly (PASE) in Japanese elderly people. Geriatr Gerontol Int 2008;8:143–51. doi: 10.1111/j.1447-0594.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- [32].Dinger MK, Oman F, Taylor EL, Vesely SK, Able J. Stability and convergent validity of the Physical Activity Scale for the Elderly (PASE). J Sports Med Phys Fitness 2004. [PubMed] [Google Scholar]
- [33].Vemuri P, Lesnick TG, Przybelski SA, Machulda M, Knopman DS, Mielke MM, et al. Association of lifetime intellectual enrichment with cognitive decline in the older population. JAMA Neurol 2014. doi: 10.1001/jamaneurol.2014.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- [35].Staffaroni A, Bajorek L, Casaletto K, Cobigo Y, Goh S, Wolf A, et al. Assessment of Executive Function Declines in Presymptomatic AND Mildly Symptomatic Familial Frontotemporal Dementia: NIH-EXAMINER as a potential clinical trial endpoint. Alzheimer’s Dement n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Neuhaus JM, Kalbfleisch JD. Between- and within-cluster covariate effects in the analysis of clustered data. Biometrics 1998;54:638–45. [PubMed] [Google Scholar]
- [37].Neuhaus JM, McCulloch CE. Separating between- and within-cluster covariate effects by using conditional and partitioning methods. J R Stat Soc Ser B (Statistical Methodol 2006;68:859–72. doi: 10.1111/j.1467-9868.2006.00570.x. [DOI] [Google Scholar]
- [38].Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, Belleville S, Cantilon M, Chetelat G, et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s Dement 2018. doi: 10.1016/j.jmarsys.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Stern Y Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 2012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Morgan EE, Woods SP, Smith C, Weber E, Scott JC, Grant I, et al. Lower Cognitive Reserve Among Individuals with Syndromic HIV-Associated Neurocognitive Disorders (HAND). AIDS Behav 2012;16:2279–85. doi: 10.1007/s10461-012-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Stern Y Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 2012;11:1006–12. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hindle JV, Hurt CS, Burn DJ, Brown RG, Samuel M, Wilson KC, et al. The effects of cognitive reserve and lifestyle on cognition and dementia in Parkinson’s disease-a longitudinal cohort study. Int J Geriatr Psychiatry 2016;31:13–23. doi: 10.1002/gps.4284. [DOI] [PubMed] [Google Scholar]
- [43].Modica CM, Bergsland N, Dwyer MG, Ramasamy DP, Carl E, Zivadinov R, et al. Cognitive reserve moderates the impact of subcortical gray matter atrophy on neuropsychological status in multiple sclerosis. Mult Scler J 2016;22:36–42. doi: 10.1177/1352458515579443. [DOI] [PubMed] [Google Scholar]
- [44].Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol 2014. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- [45].Hoang TD, Reis J, Zhu N, Jacobs DR, Launer LJ, Whitmer RA, et al. Effect of Early Adult Patterns of Physical Activity and Television Viewing on Midlife Cognitive Function n.d. doi: 10.1001/jamapsychiatry.2015.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mewborn CM, Lindbergh CA, Stephen Miller L. Cognitive Interventions for Cognitively Healthy, Mildly Impaired, and Mixed Samples of Older Adults: A Systematic Review and Meta-Analysis of Randomized-Controlled Trials. Neuropsychol Rev 2017;27:403–39. doi: 10.1007/s11065-017-9350-8. [DOI] [PubMed] [Google Scholar]
- [47].Rebok GW, Ball K, Guey LT, Jones RN, Kim H-Y, King JW, et al. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc 2014;62:16–24. doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med 2017:bjsports-2016–096587. doi: 10.1136/bjsports-2016-096587. [DOI] [PubMed] [Google Scholar]
- [49].Middleton LE, Manini TM, Simonsick EM, Harris TB, Barnes DE, Tylavsky F, et al. Activity Energy Expenditure and Incident Cognitive Impairment in Older Adults. Arch Intern Med 2011;171:1251. doi: 10.1001/archinternmed.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Aguirre-Acevedo DC, Lopera F, Henao E, Tirado V, Muñoz C, Giraldo M, et al. Cognitive Decline in a Colombian Kindred With Autosomal Dominant Alzheimer Disease: A Retrospective Cohort Study. JAMA Neurol 2016;73:431–8. doi: 10.1001/jamaneurol.2015.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ambrogini P, Cuppini R, Lattanzi D, Ciuffoli S, Frontini A, Fanelli M. Synaptogenesis in adult-generated hippocampal granule cells is affected by behavioral experiences. Hippocampus 2010;20:799–810. doi: 10.1002/hipo.20679. [DOI] [PubMed] [Google Scholar]
- [52].Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci U S A 1990;87:5568–72. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Cracchiolo JR, Mori T, Nazian SJ, Tan J, Potter H, Arendash GW. Enhanced cognitive activity-over and above social or physical activity-is required to protect Alzheimer’s mice against cognitive impairment, reduce Aβ deposition, and increase synaptic immunoreactivity. Neurobiol Learn Mem 2007;88:277–94. doi: 10.1016/j.nlm.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Rosenman R, Tennekoon V, Hill LG. Measuring bias in self-reported data. Int J Behav Healthc Res 2011;2:320–32. doi: 10.1504/IJBHR.2011.043414. [DOI] [PMC free article] [PubMed] [Google Scholar]