Abstract
In this study we explored the cultivable fungal diversity in Lake Magadi and their secondary metabolite production. Isolation was done on alkaline media (Potato dextrose agar, Malt extract agar, Oatmeal agar and Sabouraud dextrose agar). A total of 52 unique isolates were recovered from the lake and were characterized using different techniques. Growth was observed at pH, temperature and salinity ranges of between 6 - 10, 25 °C - 40 °C and 0%–20% respectively. Phylogenetically, the isolates were affiliated to 18 different genera with Aspergillus, Penicillium, Cladosporium, Phoma and Acremonium being dominant. A screen for the ability to produce extracellular enzymes showed that different isolates could produce proteases, chitinases, cellulases, amylases, pectinases and lipases. Production of antimicrobial metabolites was noted for isolate 11M affiliated to Penicillium chrysogenum (99%). Cell free extracts and crude extracts from this isolate had inhibitory effects on Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Salmonella spp., Shigella spp., Candida albicans and fungal plant pathogens Schizophyllum commune, Epicoccum sorghinum strain JME-11, Aspergillus fumigatus strain EG11-4, Cladosporium halotolerans CBS 119416, Phoma destructive and Didymella glomerata). In this study we showed that different cultivation strategies can lead to recovery of more phylotypes from the extreme environments. Growth under different physiological characteristics typical of the soda lake environment (elevated temperature, pH and salts) confirmed the haloalkaliphilic nature of the fungal isolates. The use of suitable antimicrobial production media can also lead to discovery of more phylotypes producing diverse biocatalysts and bioactive metabolites.
Keywords: Microbiology, Biodiversity, Antibiotics, Mycology, Microbial genomics, Microorganism, Fungi, Extremophiles, Antibiotics, Soda lakes, Kenya
Microbiology; Biodiversity; Antibiotics; Mycology; Microbial genomics; Microorganism; Fungi; Extremophiles; Antibiotics; Soda lakes; Kenya
1. Introduction
Fungi are eukaryotic organisms having either simple unicellular or multicellular cell structures. Within the environment, fungi are mainly found in soil and rock surfaces compared to other aquatic habitats. Most fungal species inhabit soil compared to other environments as they are organotrophs forming the major group involved in the breakdown of organic compounds including decaying matter and plant material (Hasan, 2015). The occurrence of fungi in water is subtle with only around 3,000 species known to be associated with aquatic habitats, whereas about 465 species are present in marine waters (Shearer et al., 2007). Diverse fungal groups have been documented from different extreme environments such as saline liquids, hot springs, surface of dried rocks, ocean pits, dry deserts, and very low pH as well as in the polar environments (Hassan et al., 2016). Filamentous fungi harbour alkalitolerants lineages similar to those of marine habitats (Grum-grzhimaylo et al., 2016). Most fungi that inhabit extreme environments are categorized as the imperfect stage of the Ascomycota (Ndwigah et al., 2016). Different genera, including Cladosporium, Aspergillus, Penicillium, Alternaria and Acremonium have been reported to exist as either moderately or weakly alkali tolerant species in saline environments (Grum-Grzhimaylo et al., 2013). However, salinity directly affects fungal growth and sporulation. For example, at salinities above 5%, there is increased sporulation and more chlamydospores are formed while conidiogenesis is inhibited, and there are fewer hyphae (Mulder and El-Hendawy, 1999; Mandeel, 2006). High ambient salts and pH significantly stress the living organisms and, therefore, their overall biodiversity may be affected (Grum-grzhimaylo et al., 2016). To cope with the osmotic stress, fungi in extreme environments produce extremolytes and extremozymes (Raddadi et al., 2018). In cases of high osmolarity, fungi are able to counteract loss of water by the accumulation of K+ ions into their cells (Plemenitaš et al., 2014) while others accumulate osmolytes (polysols, sugars and amino acids) as compatible organic solutes (Roberts, 2005).
Fungi from extreme environments such as the soda lakes have adapted to elevated temperatures and alkaline saline conditions, which may lead to evolution and modifications of various fungal pathways (Brakhage and Schroeckh, 2011). A lot of secondary metabolites including antimicrobial agents (antibiotics), pigments and toxins are produced from the modified fungal and actinomycetes pathways (Satyanarayana et al., 2005; Brakhage and Schroeckh, 2011; Liao et al., 2015). Fungal genera that are known to produce secondary metabolites include Fusarium, Aspergillus, Acremonium and Penicillium (Wilson and Brimble, 2009). Studies on the physiology and genetics of fungi isolated from unusual habitats are important to foster the understanding of both the ecological roles and potential industrial applications (Prakash and Sharma, 2016). Due to the few numbers of fungal isolates described from the soda lake ecosystems, their diversity, function and potential to produce secondary metabolites is still not well studied. In this study, we used different media and cultivation conditions in an effort to recover novel phylotypes with the potential to produce bioactive metabolites.
2. Results
2.1. Characterization of the isolates
A total of 52 isolates were obtained with most of them being from sampling site S3 (wet sediments). The other sites (S3 Dry sediments, S2 wet sediments, S5 wet sediments, microbial mats, grassland soil, and brine sediments) had a few isolates. After plating, growth of the isolates on the respective media was monitored and recorded after a period of between 7-21 days of incubation at 30 °C. Spore formation was observed after 14 days of growth for the spore-farmers. Malt extract agar, Sabourauds dextrose agar, potato dextrose agar and oatmeal agar supported growth of most of the fungi both in high and low nutrient composition. The isolates showed varied macroscopic and microscopic characteristics in terms of colony pigmentation, surface morphology and the hyphae under the microscope. The hyphae were either septate (hyaline) or aseptate in others.
2.2. Physiological characteristics
Moderate growth was observed in all the isolates at NaCl concentration of between 0% and 5%. When the salt concentration was increased to 10%, most of the isolates showed slow growth with isolate 9M,10M, 11M, 24M, 32M, 36M, 40M, 64M, 82M and 100M growing the same as in the lower concentration. However, isolate 5M, 30M, 39M, 59M and 108M showed optimum growth at 10% while isolate 2M, 14M, 29M, 38M, 56M, 60M, 69M did not show any visible colonies on plates media (no growth). The highest salt concentration tested was 20% and only 13 isolates showed growth albeit slow while the rest did not grow (Table 1). Notable is that isolates 1M, 24M, 39M, 64M, 65M, 100M, 120M and 122M were able to grow across all the tested salt concentrations (Table 1). Different isolates showed varied growth patterns under different pH ranges. Except for pH 6 where quite a number of the isolates did not grow completely, most of the fungal isolates grew in pH 7, 8, 9 and 10. Optimum growth for majority of the fungal isolates was recorded at pH 8–10 (Table 1).
Table 1.
Growth characteristics of different isolates under varied physiological conditions (temperature, pH and NaCl).
| Isolate | Salt tolerance |
Temperature tolerance |
pH tolerance |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0% | 5% | 10% | 20% | 25 °C | 30 °C | 35 °C | 40 °C | pH6 | pH7 | pH8 | pH9 | pH10 | |
| 1M | ++ | +++ | + | + | + | ++ | ++ | ++ | + | + | + | + | + |
| 2M | ++ | + | - | - | ++ | +++ | + | + | - | ++ | ++ | + | ++ |
| 5M | ++ | +++ | +++ | - | ++ | ++ | +++ | +++ | ++ | ++ | +++ | + | ++ |
| 9M | +++ | ++ | ++ | - | ++ | +++ | + | + | - | + | +++ | + | +++ |
| 10M | + | +++ | ++ | - | + | + | + | + | - | + | + | + | +++ |
| 11M | ++ | +++ | ++ | - | ++ | +++ | +++ | ++ | + | ++ | +++ | + | ++ |
| 13M | ++ | + | + | - | ++ | ++ | ++ | ++ | + | + | ++ | + | + |
| 14M | ++ | ++ | - | - | + | +++ | ++ | +++ | - | + | ++ | + | - |
| 15M | + | +++ | + | - | ++ | ++ | +++ | + | + | + | +++ | ++ | + |
| 16M | ++ | ++ | + | - | ++ | +++ | ++ | + | ++ | + | ++ | + | ++ |
| 18M | ++ | ++ | + | - | + | + | +++ | + | - | + | ++ | +++ | + |
| 22M | ++ | ++ | + | - | + | ++ | ++ | + | ++ | ++ | + | ++ | ++ |
| 24M | ++ | ++ | ++ | + | + | ++ | + | + | ++ | ++ | ++ | ++ | ++ |
| 25M | + | ++ | + | - | ++ | ++ | ++ | + | ++ | + | +++ | ++ | + |
| 29M | ++ | + | - | - | + | ++ | + | ++ | + | + | ++ | + | + |
| 30M | +++ | +++ | +++ | - | ++ | +++ | ++ | ++ | ++ | + | +++ | ++ | ++ |
| 31M | + | ++ | + | - | ++ | ++ | ++ | ++ | + | + | + | + | + |
| 32M | + | ++ | ++ | - | + | ++ | + | - | ++ | ++ | +++ | + | + |
| 36M | + | ++ | ++ | - | + | ++ | ++ | + | ++ | + | ++ | + | + |
| 38M | + | + | - | - | - | ++ | - | - | - | - | + | + | + |
| 39M | +++ | +++ | +++ | + | + | + | + | + | ++ | ++ | ++ | ++ | +++ |
| 40M | + | ++ | ++ | + | + | ++ | +++ | ++ | - | ++ | +++ | + | + |
| 53M | ++ | ++ | + | + | + | ++ | ++ | ++ | + | + | +++ | + | ++ |
| 56M | ++ | + | - | - | + | ++ | ++ | ++ | + | + | ++ | + | + |
| 57M | + | ++ | + | + | + | + | ++ | + | ++ | + | ++ | + | + |
| 58M | + | ++ | + | - | + | ++ | ++ | + | - | - | ++ | ++ | + |
| 59M | ++ | +++ | +++ | - | ++ | +++ | ++ | ++ | - | ++ | ++ | + | ++ |
| 60M | + | ++ | - | - | ++ | ++ | ++ | + | + | ++ | ++ | + | + |
| 64M | + | +++ | ++ | + | + | ++ | + | + | + | ++ | ++ | + | ++ |
| 65M | + | ++ | + | + | ++ | +++ | +++ | + | - | + | ++ | + | + |
| 67M | + | +++ | + | - | ++ | ++ | ++ | ++ | - | + | ++ | + | + |
| 68M | ++ | ++ | + | - | + | ++ | ++ | ++ | - | + | +++ | +++ | + |
| 69M | ++ | + | - | - | ++ | +++ | + | + | - | ++ | ++ | + | ++ |
| 70M | ++ | ++ | + | - | ++ | + | - | - | +++ | + | +++ | - | +++ |
| 71M | + | + | + | - | + | +++ | ++ | ++ | ++ | + | ++ | + | + |
| 72M | + | ++ | + | - | ++ | ++ | + | ++ | ++ | + | ++ | + | ++ |
| 73M | + | +++ | + | - | ++ | +++ | ++ | ++ | - | ++ | ++ | ++ | ++ |
| 80M | ++ | + | + | - | + | ++ | ++ | ++ | - | + | ++ | + | + |
| 82M | + | ++ | ++ | - | + | ++ | ++ | + | ++ | + | ++ | + | + |
| 87M | ++ | +++ | + | + | + | ++ | + | + | - | + | ++ | + | ++ |
| 89M | ++ | +++ | +++ | - | + | ++ | + | + | +++ | +++ | +++ | ++ | +++ |
| 90M | + | ++ | + | - | + | ++ | +++ | + | - | + | + | +++ | + |
| 94M | + | ++ | + | + | ++ | + | + | - | - | + | + | - | + |
| 95M | ++ | + | + | - | + | +++ | ++ | + | - | + | ++ | + | + |
| 100M | + | +++ | ++ | + | + | +++ | +++ | + | - | - | ++ | + | +++ |
| 108M | +++ | +++ | +++ | - | + | ++ | + | + | ++ | + | +++ | ++ | +++ |
| 111M | + | +++ | + | - | + | ++ | + | + | + | + | +++ | +++ | + |
| 113M | + | ++ | + | - | + | +++ | + | + | ++ | + | ++ | + | + |
| 114M | + | + | ++ | - | + | ++ | ++ | + | + | + | ++ | + | ++ |
| 120M | + | ++ | ++ | + | + | ++ | +++ | + | ++ | ++ | ++ | ++ | ++ |
| 122M | ++ | ++ | ++ | + | + | ++ | + | +++ | - | + | + | +++ | ++ |
| 123M | +++ | ++ | + | - | + | ++ | ++ | + | ++ | + | ++ | ++ | + |
KEY: - (no growth), + (0 – 2 mm, slight growth), ++ (2.1 – 4mm, moderate growth), +++ (> 5 mm, abundant growth).
It was observed that the optimum growth temperature for most of the isolates was 30 °C. At 25 °C, slow growth was observed in all isolates except isolate 38M which did not grow at all. When the temperature was raised to 40 °C, isolates M5, M14 and M122 exhibited fastest growth while four isolates (32M, 38M, 70M and 94M) did not grow at all at this temperature.
2.3. Screening for enzymatic activity
All the isolates were screened for their ability to produce extracellular proteases, celullases, pectinases, chitinases, amylases, xylanases, lipases and esterases by growing them on a medium with the respective substrate. Production of cellulases was observed by positive enzymatic activity on cellulose and carboxymethylcellulose in 9 and 8 isolates respectively (Table 2). Utlization of the substrate was scored as positive if there was a halo around the colony after flooding the plate with iodine solution. Eight isolates were positive for amylase production. Positive protease activity was observed in 8 of the isolates by way of a clearance zone around colony on casein agar. Pectinase and chitinase production was observed after degradation of substrates pectin and chitin by 8 and 22 isolates respectively. However, 22 isolates did not show any enzymatic activity on any of the substrates. None of the isolates showed peroxidase/laccase activity. Some isolates were able to produce more than one enzyme with a few producing up to four enzymes. Interesting isolates in terms of polyenzymatic activity were 69M, 87M, 90M, 69M, 59M and 2M. Chitinase activity was observed in 22 of the isolates. This enzyme is important as a virulence factor in entomopathogenic fungi. It was observed that most fungi recovered in this study showed low enzymatic activity as indicated by the diameter of clearing zones around the colony. Those with a clearance zone measuring up to 3mm were scored as (+). Moderate enzymatic activity was observed in isolates 1M, 2M, 39M, 69M, 87M, 94M, 100M, 123M whereby they had clearing zones of between 3.1-6mm and was recorded as (++). A few isolates 38M and 90M showed high enzymatic activity for chitinase and cellulase respectively, they recorded diameter of halo zones above 6mm which was scored as (+++) (Table 2).
Table 2.
Sumary of enzymatic activity of some of the fungal isolates. Only isolates positive for at least one substrate are shown.
| Isolate | Site | Sta. | CMC | Cas. | Pec. | Xan. | Lig. | T20 | Gt. | Chit. | Cel. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1M | mats | - | - | - | - | - | - | - | - | ++ | - |
| 2M | mats | + | + | - | + | + | - | - | - | ++ | - |
| 5M | S3S | + | - | - | - | - | - | - | - | - | - |
| 13M | S3S | - | - | - | - | - | - | - | - | + | - |
| 14M | S3S | - | - | - | - | - | - | - | - | + | - |
| 29M | S3S | - | - | + | - | - | - | + | - | - | + |
| 31M | mgs | - | - | + | - | - | - | + | - | ++ | - |
| 32M | S3S | - | - | + | - | - | - | + | - | - | - |
| 38M | mgs | - | - | - | - | - | - | - | - | +++ | - |
| 39M | S3S | - | - | - | - | - | - | - | - | ++ | - |
| 40M | S3S | - | - | + | - | - | - | + | - | + | - |
| 57M | S3S | - | - | - | - | - | - | - | - | + | - |
| 59M | mgs | + | + | - | + | + | - | - | - | - | + |
| 64M | S3S | - | - | - | - | - | - | - | - | + | - |
| 68M | S3S | - | - | - | - | - | - | - | - | + | - |
| 69M | mats | + | + | + | + | + | - | + | - | - | ++ |
| 70M | S3S | - | - | - | - | - | - | - | - | + | - |
| 71M | S3S | - | - | - | - | - | - | - | - | + | - |
| 73M | S3S | - | - | - | - | - | - | - | - | ++ | - |
| 87M | S5S | + | ++ | - | + | + | - | - | - | + | + |
| 90M | S3S | + | ++ | - | ++ | +++ | - | - | - | - | +++ |
| 94M | S3S | - | - | - | - | - | - | - | - | ++ | - |
| 95M | S3S | - | + | - | - | - | - | - | - | + | - |
| 100M | br2 | + | - | + | + | + | - | + | - | ++ | ++ |
| 108M | S3S | - | - | - | - | - | - | - | - | + | - |
| 111M | S3S | + | + | + | + | + | - | - | - | - | + |
| 113M | S3dry | + | + | + | + | + | - | - | - | - | + |
| 120M | S3S | - | - | - | - | - | - | - | - | + | + |
| 122M | S3S | - | - | - | - | - | - | - | - | + | - |
| 123M | S3S | - | - | - | - | - | - | - | - | ++ | - |
KEY: - (no activity), + (0–3 mm), ++ (3.1–6 mm), +++ (>6 mm) Iso-isolate, Sta-starch CMC- carboxymethylcellulose, Cas-casein, Pec-pectin, Xan-xanthan, Lig-lignin, T20-tween 20, Gt- Glyceryl Tributyrate, Chit-chitin, Cel-cellulose.
2.4. Phylogenetic analysis
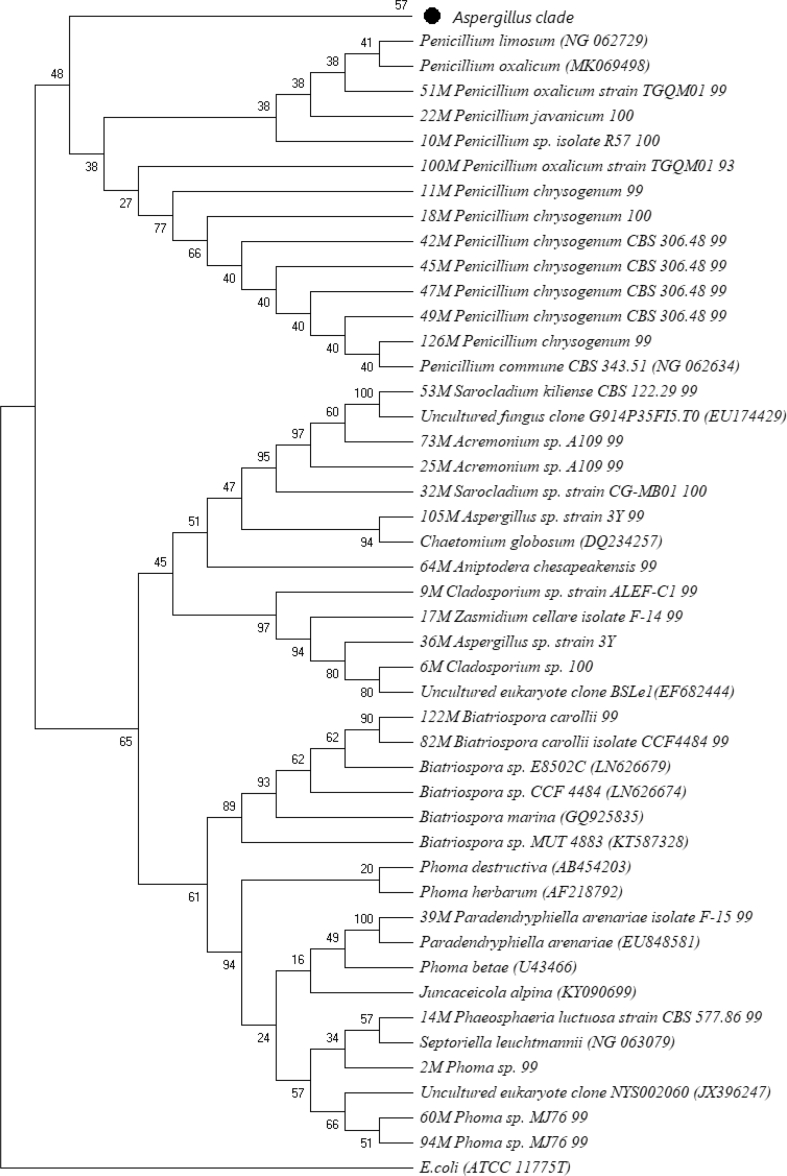
BLAST analysis was used to evaluate the phylogenetic affiliation or relatedness of the individual isolates to nearest neighbors and the results expressed as percentage similarity (Table 3). All the isolates clustered with the phylum Ascomycota and were distributed in 18 genera. Among the isolates, 15 were affiliated to the genus Aspergillus with most of them exhibiting between 99%-100% similarities. The other genera were also represented in small numbers and had close affiliation with known genera in the phylum Ascomycota. They included: Penicillium (9 isolates), Acremonium (3 isolates), Phoma (4 isolates), Cladosporium (3), Septoriella (1), Talaromyces (2), Zasmidium (1), Chaetomium (1), Aniptodera (1), Pyrenochaeta (1), Septoria (1), Juncaceicola (1), Paradendryphiella (1) Sarocladium (2) Phaeosphaeria (1) and Juncaceicola (1), Biatriospora (2). The constructed phylogenetic tree indicated the phylogenetic position of the isolates (Figure 1). The bootstrap values showed that different strains of isolates within a given genus were closely related (Figure 2). Based on bootstrap values, Zasmidium and Aspergillus are sister groups in the same clade and are therefore closely related. The genera Juncaceicola, Septoriella and Phaeosphaeria fall in the same clade and therefore have common evolutionary history. Chaetomium, Aniptodera, Sarocladium fall in the same clade with Acremonium (commonly known fungal genera). Interestingly, isolate 69M, 87M and 90M had sequence similarity of 96 %, 97% and 95% respectively therefore they could represent novel species. However, isolates 72M, 80M, 95M, 108M, 113M and 100M had sequence similarity values below 94% which means they could potentially represent new genera (Table 3).
Table 3.
BLAST analysis results of the fungal isolates from Lake Magadi and their close relative.
| Isolate Code | Closest Relative (BLAST) | Identity |
|---|---|---|
| 1M | Aspergillus sp. strain 3Y | 100% |
| 2M | Phoma herbarum strain BZYB-1 | 99% |
| 5M | Aspergillus fumigatus strain EG11-4 | 98% |
| 9M | Cladosporium cladosporioides | 98% |
| 10M | Penicillium oxalicum strain TGQM01 | 100% |
| 11M | Penicillium chrysogenum CBS 306.48 | 99% |
| 13M | Cladosporium halotolerans CBS 119416 | 99% |
| 14M | Phaeosphaeria luctuosa strain CBS 577.8 | 99% |
| 15M | Aspergillus fumigatus strain EG11-4 | 99% |
| 16M | Aspergillus versicolor isolate F | 100% |
| 18M | Penicillium commune CBS 343.51 | 100% |
| 22M | Penicillium limosum CBS 339.97 | 100% |
| 24M | Pyrenochaeta nobilis CBS 407.76 | 96% |
| 25M | Acremonium roseolum strain CBS 289.62 | 99% |
| 29M | Aspergillus versicolor isolate CWJ2 | 100% |
| 30M | Uncultured eukaryote clone NYS002060 | 98% |
| 31M | Aspergillus versicolor strain MPE9 | 99% |
| 32M | Sarocladium sp. strain CG-MB01 | 100% |
| 36M | Zasmidium cellare isolate F-14 | 100% |
| 38M | Chaetomium globosum strain F0909 | 99% |
| 39M | Paradendryphiella arenariae isolate F-15 | 99% |
| 40M | Acremonium sclerotigenum CBS 124.42 | 99% |
| 53M | Sarocladium kiliense CBS 122.29 | 99% |
| 56M | Aspergillus keveii strain CBS 209.92 28S | 99% |
| 57M | Aspergillus glaucus JCM 1575 | 98% |
| 58M | Penicillium citrinum strain IITG_KP1 | 99% |
| 59M | Aspergillus flavipes NRRL 302 | 98% |
| 60M | Phoma destructiva | 99% |
| 64M | Aniptodera chesapeakensis | 99% |
| 65M | Phoma sp. LF617 | 99% |
| 67M | Juncaceicola alpina CBS 456.84 | 99% |
| 68M | Septoriella leuchtmannii CBS 459.84 | 99% |
| 69M | Cladosporium velox | 96% |
| 70M | Aspergillus versicolor strain MF557 | 99% |
| 71M | Aspergillus sp. strain DX4H | 99% |
| 72M | Penicillium viridicatum CBS 390.48 | 81% |
| 73M | Acremonium roseolum strain CBS 289.62 | 99% |
| 80M | Uncultured fungus clone 42_Wound2L | 80% |
| 82M | Biatriospora carollii | 99% |
| 87M | Aspergillus keveii strain CBS 209.92 28S | 97% |
| 89M | Talaromyces marneffei strain Tm-HIV | 92% |
| 90M | Uncultured eukaryote clone BSLe1 | 95% |
| 94M | Phoma destructiva isolate: MUCC0064 | 99% |
| 95M | Aspergillus terreus strain AZM03 | 84% |
| 100M | Penicillium oxalicum strain TGQM01 | 93% |
| 108M | Aspergillus flavipes NRRL 302 | 83% |
| 111M | Aspergillus sp. strain AON1 | 98% |
| 113M | Talaromyces marneffei strain Tm-HIV | 92% |
| 114M | Penicillium polonicum CBS 222.28 | 98% |
| 120M | Septoria senecionis CBS 102366 | 98% |
| 122M | Biatriospora carollii CCF4484 | 99% |
| 123M | Penicillium citrinum strain IITG_KP1 | 98% |
Figure 1.
Unrooted Phylogenetic tree created using Neighbor-joining method based on a comparison of the 18S ribosomal DNA sequences of Lake Magadi isolates and their closest phylogenetic relatives. Percentages of bootstrap sampling derived from 1000 replications are indicated by the numbers on the tree.
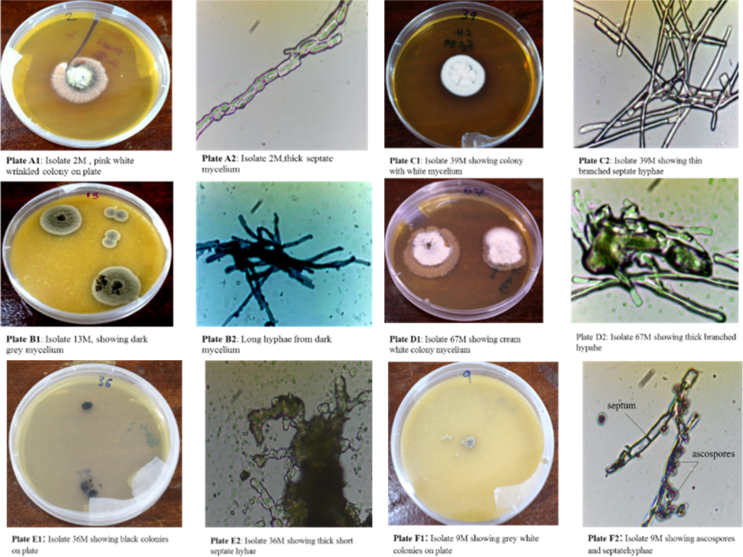
Figure 2.
A representative sample of the 52 isolates based on colony and cell characteristics (Plate A1: colony on plates) and (Plate A2: under a compound microscope magnification x40).
2.5. Antimicrobial screening
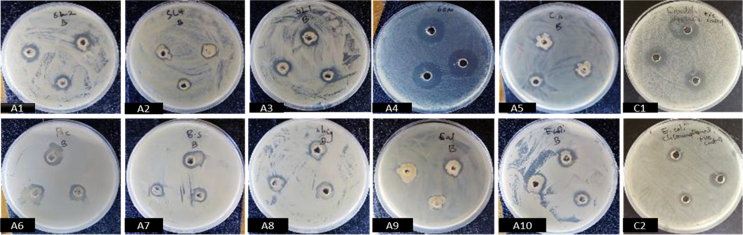
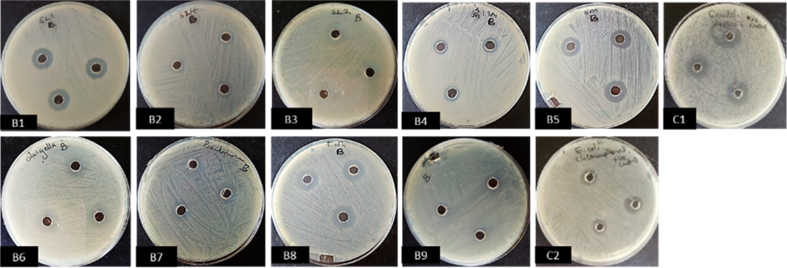
In primary screening, one isolate (11M) had positive antimicrobial activity against human enteric pathogens Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Salmonella spp., Shigella spp. and fungal human pathogen Candida albicans. The isolate also inhibited fungal plant pathogens Alternaria tenuissima and Didymella glomerata. Positive antimicrobial activity was qualitatively indicated by presence of inhibition zones. Both crude filtrates and cell free extracts from isolate 11M showed antimicrobial effects against several test pathogens as compared to the results observed in primary screening. Cell free extracts gave larger inhibition zones as compared to zones observed from inhibition by crude extracts. Extracts from production media (PM3) showed positive results for inhibition against 13 different test pathogens. Production media YESD and YPSS however did not give good inhibition results as their extracts showed positive results against few pathogens (Cladosporium. halotolerans, Phoma destructiva, Dickeya dianthicola, Phoma destructiva, Schizophyllum commune isolate ScGD28) pathogens. Crude extracts from PM3 media showed inhibition zones ranging between 11.33 ± 0.03–14.33 ± 0.03 and were active against Shigella sp, Pseudomonas aeruginosa, Escherichia coli and Staphylococcus aureus as the only human pathogens. However, five plant pathogenic fungi were inhibited by cell free extracts from PM3. Crude extracts from YESD media showed activity against Aspergillus fumigatus strain EG11 (422.33 ± 0.03), Cladosporium halotolerans CBS 119416 (22.33 ± 0.09), Phoma destructiva (20.67 ± 0.03) while cell free extracts had inhibitory effect against Schizophyllum commune isolate ScGD28 (9.67 + 0.03). Finally, crude extracts from YPSS media and cell free extracts showed inhibitory effects against Cladosporium halotolerans CBS 119416 (21.67 ± 0.03) and Schizophyllum commune isolate ScGD28 (9.33 ± 0.03) respectively. Positive controls using broad spectrum antimicrobial drugs chloramphenicol and nystatin showed inhibition zones ranging closer to 13.00 ± 0.06 and 15.00 ± 0.06 respectively (see Figures 3 and 4).
Figure 3.
Antimicrobial activity (inhibition zones) of crude extract from isolate 11M screened against test organisms using agar well diffusion method on plates. A1- Didymella glomerata; A2- Schizophyllum commune isolate ScGD28; A3- Epicoccum sorghinum strain JME-11; A4- Phoma destructive; A5- Candida albicans; A6- Pseudomonas aeruginosa; A7- Bacillus subtilis; A8- Shigella spp.; A9- Salmonella typhi; A10- Escherichia coli; C1- Positive control with Nystatin; C2- Positive control with Chloramphenicol.
Figure 4.
Antimicrobial activity (inhibition zones) of cell free extract from isolate 11M screened against test organisms using agar well diffusion method on plates. B1- Epicoccum sorghinum; B2- Schizophyllum commune isolate ScGD28; B3- Didymella glomerata; B4- Cladosporium halotolerans CBS 119416; B5- Aspergillus fumigatus strain EG11-4; B6- Shigella spp.; B7- Pseudomonas aeruginosa; B8- Escherichia coli; B9- Staphylococcus aureus; C1- Positive control with Nystatin; C2- Positive control with Chloramphenicol.
3. Discussion
The present study focused on the isolation of fungi from Lake Magadi, and new fungal diversity was determined using various identification techniques. Modification of the culture dependent techniques in combination with molecular analysis using 18S rRNA gene aided in the search for new diversity in the soda lake. Fungal isolation using commercial media prepared both in high and low nutrient composition and diluted with soda lake water is a modification specific to this study. High and low nutrient media prepared from Malt extract agar, Oatmeal agar, Sabouraud dextrose agar and Potato dextrose agar showed varied results in terms of the number of isolates obtained. For example, more isolates (14) were obtained using MEA-L than MEA-H whereby only five isolates were recovered. The dominant genera found in this study were present and includes: Penicillium, Acremonium, Phoma, and Cladosporium. The same scenario was observed for PDA and oat meal agar. It is therefore clear that nutrient fluctuations within different sites in the lake may have little contribution to the diversity of fungal communities.
Unique morphological characteristics specific to haloalkaliphilic fungi identified in this study was useful in the description of potential new species and genera isolated from Lake Magadi and other soda lakes. Morphologically based characterization was an important parameter in determining various cell features that are specific to the entire fungi kingdom. Specific cell structures for example the nature of hyphae/mycelium justified the occurrence of the isolates in group Ascomycota. Septate hyphae and oval spores (ascospores) is mainly attributed to phylum Ascomycota (Raja and Shearer, 2007). However, unique features including the thick mycelium observed in Phoma herbarum strain BZYB-1 –isolate 2M are important in stress tolerance. Thick mycelium has been studied in Wallemia ichthyophaga, the most halotolerant fungi known and it is known to have 3-fold cell wall thickening as a specific feature to withstand high NaCl concentrations (Gostinčar et al., 2009; Palková and Váchová, 2006). The pigmented fungi isolated for example Zasmidium cellare isolate F-14 (isolate 36M) and others that are dark pigmented (Aspergillus keveii-56M, Cladosporium velox-69M) is a feature that enable such yeasts to thrive in the harsh environments, such as the surface of stone monuments (Liu et al., 2018). The hyphae produces melanin which brings about the black coloration in the fungi, an important feature to stress survival (Plemenitaš et al., 2008). The black coloration was also observed on most stone monuments, like Liu et al. (2018) reported. Cladosporium species for example is known to be a halophilic species dominant in black yeasts (Nazareth, 2014).
Slight differences of the sampling sites in terms of biotic and abiotic factors (pH, salts, moisture, vegetation cover and trace elements) indicated little difference in the fungal communities present. However, the highest level of fungal diversity was found in communities from S3 sediments, the common genera being Penicillium, Acremonium, Phoma, Cladosporium, Zasmidium, Aniptodera, Pyrenochaeta, Septoria, Paradendryphiella and Biatriospora. This may be attributed to the composition of various physiochemical parameters especially the pH, Na+, K+ ions and trace elements which differed from the other sampling sites. S3 sediments recorded the highest amounts of K+ and Na+ therefore the microorganisms present probably adopted the salt in strategy to survive the salt stress. The salt-tolerant yeast D. hansenii for example is known to maintain a relatively high internal sodium concentration to cope with salt stress (Prista et al., 1997, 2005). In addition, sediments in parts of the lake are considered to have high nutrient levels because of the continuous erosion of the surroundings which eventually are deposited as silts (Blomqvist et al., 2004).
The complex effects of physiological parameters (pH, salt concentration and temperature) are also vital in shaping the composition of fungal species within Lake Magadi (Table 1). It was revealed in this study that the majority of fungi isolated from Lake Magadi are alkaliphiles since the pH of the lake had a range of pH 9–10. It is however interesting that there are moderately halophilic fungal species which grow well in salt concentrations of up to 5%. In addition, halotolerant strains which grew well in up to 20% salts were also reported from the lake. The specific species that were recovered from 20% salts included Aniptodera chesapeakensis, Phoma sp. and Aspergillus sp. Their ability to tolerate salt stress is a unique characteristic for such species especially in biotechnology. There was no specific temperature requirement for most of the fungi recovered from Lake Magadi because good growth was observed to range between 25 - 45 °C. Most halophilic microorganism are known to grow optimally between 25 – 45 °C (Oren, 2016). It is evident from this study that some of the fungal isolates recovered are polyextremophiles because they are able to colonize environments having more than one extreme condition (Dhakar and Pandey, 2016). In these recent studies, such polyextremophiles have attracted great attention because of their possible application to biotechnology and also in aspects relevant to ecology (Dhakar and Pandey, 2016). Enzymatic activity of specific fungal isolates indicated their ability to produce at least more than one enzyme type. Interesting isolates for industrial enzymes included: 2M, 31M, 40M, 59M, 69M, 87M, 90M, 100M and 111M (Table 2). Their ability to produce proteases, amylase, lipases, esterase cellulases and chitinases makes them important extremophiles for industrial applications (Sheridan, 2004).
Lake Magadi has phylogenetic diversity composed of fungal communities with various dominant taxa in addition to species exhibiting spatial and temporal variations at low frequencies. Sequence analysis indicates a total of 18 different genera that were recovered in this study and all belong to the phylum Ascomycota except for 3 isolates which were grouped as unclassified fungi. These findings are similar to the results from Salano et al. (2017) who found the phylum Ascomycota as the dominant fungal group from Lake Magadi. Members of the same phylum were also found in large proportions in Tundra soils after sequencing 125 cloned fungi (Schadt et al., 2003). Fungal communities from other hypersaline environments studied by Santini et al. (2015) indicated that phylum Ascomycota was dominant with a score of 73% of the total OTUs. In contrary, reports from other hypersaline environments indicated that phylum Basidiomycota was the dominant fungal group from deep-sea environments (Singh et al. 2011; Bass et al., 2007).
The different fungal genera that were dominant in this study were Aspergillus, Penicillium, Cladosporium, Phoma, and Acremonium. Most of these genera have been recovered from saline habitats, Aspergillus for example was found to be the dominant species in the sediments of Lake Magadi as well as species of Phaeosphaeria (Kambura, 2016). Hypersaline waters of salterns have also been previously studied and Penicillium and Aspergillus species were present in diverse levels (Gunde-Cimerman et al., 2005). Several strains of genus Cladosporium have also been isolated from Caspian Sea waters (Sadati et al., 2015). Moreover, a similar study by Salano (2011) indicated the presence of genera Aspergillus, Penicillium, Cladosporium, Talaromyces, and Acremonium from Lake Magadi. The presence of dominant species from different fungal genera is a suggestion that they are highly adaptable to the extreme conditions of the soda lake. The above genera commonly found together in saline environments share ecological preferences to extreme conditions.
This study reports the presence of new diversity of fungi thriving in the sediments and soils of Lake Magadi which has not been previously reported. New fungal genera and different species have been recovered in this study including: Septoriella leuchtmannii, Phoma sp., Zasmidium cellare, Chaetomium globosum, Aniptodera chesapeakensis, Pyrenochaeta nobilis, Septoria senecionis, Paradendryphiella arenariae, Sarocladium kiliense, Juncaceicola alpina and Biatriospora carollii. which has been isolated from other different saline environments (Georgieva et al., 2012; Bonugli-santos et al., 2015). This is the first report on the occurrence of this new genera isolated from Lake Magadi. Studies on Dead Sea, saline habitats of Wadi El-Natrun, Egypt however was able to isolate Chaetomium globosum (Perl et al., 2018). Ndwigah (2017) isolated a fungal strain from Kenyan saline Lake Sonachi that had 100% alignment with Sarocladium kiliense (HQ232198). Grum-grzhimaylo et al. (2016) also recovered the same isolate in soda soil. There are limited reports on the recovery of Biatriospora sp. from saline environments however it is known to be a potential producer of potent antibiotics and a diverse set of metabolites (Kolařík et al., 2017). The marine fungus Paradendryphiella arenariae is a marine fungus and has been isolated from Thailand; investigations have indicated that it produces bioactive secondary metabolites (Yoiprommarat et al., 2015). Septoriella leuchtmannii, Zasmidium cellare, Aniptodera chesapeakensis, Pyrenochaeta nobilis, Septoria senecionis, and Juncaceicola alpine are common causes of human and plant diseases.
The diverse groups of fungi present in the lake are terrestrial fungi and may have originated from the surrounding agricultural/vegetation soils. They are carried by surface run offs and deposited into various sediments in the lake either in the form of spores or fungal hyphae. Such fungi develop effective strategies to cope with the extreme conditions of salts, alkaline pH and temperatures. Further studies on their metabolite production potential will be of interest because of their ability to adapt to extreme saline environments. The discovery of fungi that are common causes of plant and animal diseases from the soda lake will ignite more research on their genome structures so as to determine their toxin pathways. Appropriate strategies to control such pathogens can thus be developed.
Antimicrobial metabolite production noted for isolate 11M affiliated to Penicillium chrysogenum CBS 306.48 (99%) is of interest because it produces active agents against both human pathogenic bacteria and plant pathogenic fungi. The use of modified production medium, in this case PM3, is an important consideration in the search for antimicrobial metabolites from haloalkaliphilic fungi. Since the discovery of Penicillium chrysogenum as the source for the first antibiotic penicillin, various studies on antimicrobial production have been done on various species of Penicillium. Halophilic and halophilic species of Penicillium are known to be producers of various polyketides including penicillic acids, antibiotics (penicillins) and amino acid derived extrolites (Frisvad, 2005). In particular, different strains of Penicillium chrysogenum have been isolated from saline environments (Nayak et al., 2012; Gunde-cimerman and Zalar, 2014; Cantrell et al., 2006). Antimicrobial agents (β-lactam antibiotics) from Penicillium chrysogenum and other Penicillium species are known to be active against most of the Gram positive pathogenic bacteria (Salo, 2016). Penicillium chrysogenum CBS 306 strain recovered in this study proved to be active against Gram negative, Gram positive, Candida albicans and also inhibited various plant pathogenic fungi (Schizophyllum commune, Epicoccum sorghinum strain JME-11, Aspergillus fumigatus strain EG11-4, Cladosporium halotolerans CBS 119416, Phoma destructive and Didymella glomerata). Similar findings on the same strain of Penicillium has not been reported in previous studies. Further purification and identification of the specific metabolites produced by Penicillium chrysogenum CBS 306 strain will be useful in agricultural and pharmaceutical systems especially when the active agents are formulated into products. Exploration of extreme environments therefore enhances the isolation of new strains of producer fungi mainly with the modification of growth and production medium. This will ignite industrial improvement programs that will enhance activities to develop high metabolite producing strains.
4. Materials and methods
4.1. Isolation of fungal strains
Soil samples used in this study were collected from the hypersaline Lake Magadi located in the East African Rift valley (2 °S and 36 °E). The lake lies about 660 m above sea level, depth ranging between 1-5 m and covers an area estimated to be 90 km2 (Behr and Röhricht, 2000). Serially diluted samples (0.1 g in 1 ml of sterile lake water) were plated onto Potato dextrose agar (PDA), Malt extract agar (MEA), Oatmeal agar and Sabouraud dextrose agar (SDA) with chloramphenicol (100 mg/L) as an antibiotic to inhibit bacterial growth. Onto each medium, 100 μl from each dilution was spread plated and the plates incubated at 28 °C until visible colonies appeared. The colonies were picked and transferred several times onto fresh medium until axenic cultures were obtained.
4.2. Physiological and biochemical characterization
Morphological features (color, pigmentation and colony surface morphology) together with cellular features (hyphae type) were used to select unique isolates from the different media. Physiological characterization was done to test the ability of the selected isolates to grow at elevated pH ranges (6, 7, 8, 9, and 10), different NaCl concentrations {(w/v) 0%, 5%, 10% and 20%} and varied temperature ranges (25 °C, 30 °C, 35 °C and 40 °C). Growth was scored in terms of the size of the colony after 48 h of growth. Growth was scored depending on the colony size. Ability of the different isolates to produce exoenzymes was tested on a basal medium (Tryptone 10g, Sodium chloride 10g, and Yeast Extract 5g) supplemented with the respective substrate as summarized in Table 4.
Table 4.
Summary of enzymatic activity using different substrates.
| Enzyme | Substrate | Duration | Temp. | Assay | Observation | Reference |
|---|---|---|---|---|---|---|
| Lipases | Tween 20 | 48 h | 28 °C | Halo | Sierra (1957) | |
| Esterase | Glyceryl Tributyrate | 48 h | 28 °C | Halo | Sierra (1957) | |
| Proteases | Casein | 24 h | 28 °C | Halo | Vieira (1999) | |
| Cellulases | Cellulose, Carboxymethylcellulose | 24 h | 28 °C | Congo red | Halo | Stamford et al., 1998 |
| Pectinases | Pectin | 24 h | 28 °C | Lugol solution | Halo | Andro et al. (1984) |
| Peeroxidases/Laccases | Lignin | 48 h | 28 °C | Halo | Bonugli-santos et al., 2015 | |
| Amylases | Starch | 24 h | 28 °C | Lugol solution | Halo | Castro et al. (1993) |
| Chitinases | 4-methylumbelliferylN-acetyl-b-D-glucosaminide solution | 30minutes | 37 °C | Phospahte buffer solution | Fluorecence under U.V light | (Chand et al., 2008) |
4.3. Molecular characterization
A sterile loop was used to aseptically scrape the mycelia/spores into 100μl sterilized resuspension buffer (50mM Tris pH 8.5, 50mM EDTA pH 8.0 and 25 % sucrose solution) in an Eppendorf tube. To it was added 400μl of the lysis buffer (10mM Tris pH 8.5, 5mM EDTA pH 8.0 and 1 % SDS), 10μl of Proteinase K (20 mg/l) followed by incubation at 65 °C for 60 min. Phase separation was achieved by addition of an equal volume of chloroform and centrifugation at 13,200 rpm for 10 min at 4 °C. The supernatant was carefully transferred to a new tube and recovered using the standard sodium acetate/isopropanol/ethanol method. An aliquot (3μl) of the recovered DNA was run on 1% agarose to confirm the presence and quality.
The 18S rDNA gene was amplified using the primer pair Fungi 683f (5′-GCTCGTAGTTGAACCTTTGG-3′) and Fungi1394r (5′-TCTGGACCTGGTGAGTTTC-3′) on a Surecycler 8800 machine (Agilent Technologies). The PCR mix consisted of 0.6μl dNTP's, 6μl of PCR buffer (×10), 1.5μl (5pmol) of FF390r reverse primer, 1.5μl (5pmol) of Fung5f forward primer, 0.5μl of template DNA, 0.1μl Taq polymerase and 18.3μl of water in a final volume of 30μl. Cycling was done as follows:36 cycles. Initial denaturation at 95 °C for 3 min followed by 30 cycles of denaturation at 95 °C for 1 min; Primer annealing at 58 °C for 45 s and extension at 72 °C for 1 min. A final extension step at 72 °C for 5 min was included. An aliquot of 5μl of the PCR products was run on a 1 % agarose gel stained with Cyber green in 1× TAE buffer and visualized under ultraviolet. The PCR products were purified using ExoSAP-IT™ (Applied Biosystems™) as described by the manufacturer. The cleaned amplicons were sent for Sequencing to Inqaba Biotech, South Africa.
4.4. Screening for antimicrobial activity
Fungal isolates were grown for 14 days in broth at 30 °C in a shaker incubator at 100rpm. The crude extracts were tested for antimicrobial activity against Gam positive bacteria Staphylococcus aureus and Bacillus subtilis, Gram negative bacteria Escherichia coli, Pseudomonas aeruginosa, Salmonella spp., Shigella spp. and fungal human pathogen Candida albicans. to allow for a qualitative selection of bioactive isolates (Arora et al., 2016). The antimicrobial assay was done using the agar well diffusion method (Rajpal et al., 2016) on Muller Hinton agar (pH 8 and 5% NaCl). The fungal isolates were also tested for antagonistic effect against common fungal plant pathogens to determine their potential as biocontrol agents using agar well diffusion method. The test fungal pathogens used were Epicoccum sorghinum strain JME-11, Alternaria tenuissima, Didymella glomerata, Schizophyllum commune isolate ScGD28, Phoma destructive, Cladosporium halotolerans CBS 119416, Aspergillus fumigatus EG11-4 isolated from the lake and plant bacterial pathogen Dickeya dianthicola obtained from infected vegetable. Isolates that showed positive antimicrobial activity in primary screening were qualitatively selected for secondary screening using agar well diffusion method on Muller Hinton agar. However, for this test, the producer strain was grown on three production media (YESD, PM3 and YPSS, pH 9 and 5% NaCl) in a shaker incubator at 30 °C at 100rpm. Cell free extracts obtained after centrifugation of crude extracts at 10000 rpm for 10 min and crude extracts were used. Inhibition zones were measured as mean diameter of the wells 0.6cm plus the clearing zone in triplicates for every test organism used. Broad-spectrum antibiotic and antifungal (chloramphenicol 20 μg/ml/nystatin 5 μg/ml) were used as positive controls. The media used are:
Media A (YESD)-g/L Tryptone soya broth 30.0, yeast extract 5.0, tap water (VanderMolen et al., 2013).
Media B (PM3)-g/L glucose 0.5, glycerol- 2.5ml, oat meal 5.0, soy bean 5.0, casamino acids 2.0, yeast extract 0.5, 1ml from solutions of calcium chloride (156mg/10ml), magnesium chloride (190mg/10ml) and manganese chloride (12.58 mg/10ml) and tap water (Jose and Jebakumar, 2013).
Media C (YPSS)-g/L Yeast extract 4.0, starch 14.0, dibasic K2HPO4 1.0, MgSO4.7H2O 0.5, tap water (VanderMolen et al., 2013).
Declarations
Author contribution statement
Philemon Orwa, Romano Mwirichia: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
George Mugambi, Vitalis Wekesa: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
The work was supported by the Alexander von Humboldt Stiftung Equipment Grant, DAAD Material Resources Programme, Equipment donation by Seeding Labs, USA, The National Research Fund, Kenya and The World Academy of Sciences. Philemon Orwa was supported by the University of Embu postgraduate scholarship.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Andro T., Chambost J.-P., Kotoujansky A., Cattaneo J., Bertheau Y., Barras F. Mutants of Erwinia chrysanthemi defective in secretion of pectinase and cellulase. J. Bacteriol. 1984;160(3):1199–1203. doi: 10.1128/jb.160.3.1199-1203.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora P., Wani Z.A., Nalli Y., Ali A., Riyaz-Ul-Hassan S. Antimicrobial potential of thiodiketopiperazine derivatives produced by phoma sp., an endophyte of Glycyrrhiza glabra linn. Microb. Ecol. 2016;72(4):802–812. doi: 10.1007/s00248-016-0805-x. [DOI] [PubMed] [Google Scholar]
- Bass D., Howe A., Brown N., Barton H., Demidova M., Michelle H. Yeast forms dominate fungal diversity in the deep oceans. Proc. R. Soc. Biol. Sci. 2007;274(1629):3069–3077. doi: 10.1098/rspb.2007.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr H.-J., Röhricht C. Record of seismotectonic events in siliceous cyanobacterial sediments (Magadi cherts), Lake Magadi, Kenya. Int. J. Earth Sci. 2000;89(2):268–283. [Google Scholar]
- Blomqvist S., Gunnars A., Elmgren R. Why the limiting nutrient differs between temperate coastal seas and freshwater lakes: a matter of salt. Limnol. Oceanogr. 2004;49(6):2236–2241. [Google Scholar]
- Bonugli-santos R.C., Maria R., Vasconcelos S., Passarini M.R.Z. Marine-derived fungi: diversity of enzymes and biotechnological applications. Front. Microbiol. 2015;6 doi: 10.3389/fmicb.2015.00269. (April) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakhage A.A., Schroeckh V. Fungal secondary metabolites–strategies to activate silent gene clusters. Fungal Genet. Biol. 2011;48(1):15–22. doi: 10.1016/j.fgb.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Cantrell S.A., Casillas-Martínez L., Molina M. Characterization of fungi from hypersaline environments of solar salterns using morphological and molecular techniques. Mycol. Res. 2006;110(8):962–970. doi: 10.1016/j.mycres.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Castro G.R., Ferrero M.A., Méndez B.S., Sin‾ eriz F. Screening and selection of bacteria with high amylolytic activity. Acta Biotechnol. 1993;13(2):197–201. [Google Scholar]
- Chand R., Richa K., Dhar H., Dutt S., Gulati A. A rapid and easy method for the detection of microbial cellulases on agar plates using Gram’ s iodine. Curr. Microbiol. 2008;57:503–507. doi: 10.1007/s00284-008-9276-8. [DOI] [PubMed] [Google Scholar]
- Dhakar K., Pandey A. Wide pH range tolerance in extremophiles: towards understanding an important phenomenon for future biotechnology. Appl. Microbiol. Biotechnol. 2016;100(6):2499–2510. doi: 10.1007/s00253-016-7285-2. [DOI] [PubMed] [Google Scholar]
- Frisvad J.C. Adaptation to Life at High Salt Concentrations in Archaea, Bacteria, and Eukarya. Springer; 2005. Halotolerant and halophilic fungi and their extrolite production; pp. 425–439. [Google Scholar]
- Georgieva M.L., Lebedeva M.P., Bilanenko E.N. Mycelial fungi in saline soils of the western Transbaikal region. Eurasian Soil Sci. 2012 [Google Scholar]
- Gostinčar C., Grube M., De Hoog S., Zalar P., Gunde-Cimerman N. Extremotolerance in fungi: evolution on the edge. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Ecol. 2009;71(1):2–11. doi: 10.1111/j.1574-6941.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- Grum-grzhimaylo A.A., Georgieva M.L., Bilanenko E.N. On the diversity of fungi from soda soils, Fungal Divers. 2016;76:27–74. [Google Scholar]
- Grum-Grzhimaylo A.A., Georgieva M.L., Debets A.J.M., Bilanenko E.N. Are alkalitolerant fungi of the Emericellopsis lineage (Bionectriaceae) of marine origin? IMA Fungus. 2013;4(2):213–228. doi: 10.5598/imafungus.2013.04.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunde-Cimerman N., Frisvad J.C., Zalar P., Plemenitaš A. Halotolerant and halophilic fungi. In: Deshmukh S.K., Rai M.K., editors. Biodiversity of Fungi: Their Role in Human Life. Oxford & IBH Publishing Co. Pvt. Ltd.; New Delhi, India: 2005. [Google Scholar]
- Gunde-cimerman N., Zalar P. Extremely halotolerant and halophilic fungi inhabit brine in solar salterns around the globe. Food Technol. Biotechnol. 2014;9862(2):170–179. [Google Scholar]
- Hasan S. Screening of soil fungi for production of lytic enzymes. Ann. Appl. Bio-Sci. 2015;2(4) [Google Scholar]
- Hassan N., Rafiq M., Hayat M. Psychrophilic and psychrotrophic fungi: a comprehensive review Psychrophilic and psychrotrophic fungi: a comprehensive review. Rev. Environ. Sci. Biotechnol. 2016 (April) [Google Scholar]
- Jose P.A., Jebakumar S.R.D. Diverse actinomycetes from Indian coastal solar salterns - a resource for antimicrobial screening. J. Pure Appl. Microbiol. 2013;7(4):2569–2575. [Google Scholar]
- Kambura A.K. 2016. Metagenomic and Metatranscriptomic Analysis of Bacterial, Archaeal and Fungal Communities within the hot springs of lake Magadi in Kenya. [Google Scholar]
- Kolařík M., Spakowicz D.J., Gazis R., Shaw J., Kubátová A., Nováková A. Biatriospora (Ascomycota: Pleosporales) is an ecologically diverse genus including facultative marine fungi and endophytes with biotechnological potential. Plant Syst. Evol. 2017;303(1):35–50. [Google Scholar]
- Liao C., Liu X., Shan L. Approaches of genetic regulation in actinomycetes for antibiotic synthesis. J. Mol. Genet. Med. 2015;9(4):10–11. [Google Scholar]
- Liu X., Meng H., Wang Y., Katayama Y., Gu J.-D. Water is a critical factor in evaluating and assessing microbial colonization and destruction of Angkor sandstone monuments. Int. Biodeterior. Biodegrad. 2018;133:9–16. [Google Scholar]
- Mandeel Q.A. Biodiversity of the genus Fusarium in saline soil habitats. J. Basic Microbiol. 2006;46(6):480–494. doi: 10.1002/jobm.200510128. [DOI] [PubMed] [Google Scholar]
- Mulder J.L., El-Hendawy H. Microfungi under stress in Kuwait’s coastal saline depressions. Kuwait J. Sci. Eng. 1999;26:157–172. [Google Scholar]
- Nayak S.S., Gonsalves V., Nazareth S.W. Isolation and salt tolerance of halophilic fungi from mangroves and solar salterns in Goa - India. 2012;41(April):164–172. [Google Scholar]
- Nazareth S.W. 2014. The World of Halophilic Fungi. [Google Scholar]
- Ndwigah F.I. COHES-JKUAT; 2017. Characterization, Identification and Metabolites of Fungi from the Soda Lakes in Kenya. [Google Scholar]
- Ndwigah F.I., Boga I.H., Wanyoike W., Kachiuri R. An aspergilllus isolate and its secondary metabolites from Lake elmentaita in Kenya. 2016;17(1) [Google Scholar]
- Oren A. fourth ed. American Society of Microbiology; 2016. Life in High-Salinity Environments. In Manual of Environmental Microbiology; pp. 3–4. [Google Scholar]
- Palková Z., Váchová L. Life within a community: benefit to yeast long-term survival. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Rev. 2006;30(5):806–824. doi: 10.1111/j.1574-6976.2006.00034.x. [DOI] [PubMed] [Google Scholar]
- Perl T., Kis-papo T., Nevo E. 2018. Fungal Biodiversity in the Hypersaline Dead Sea: Extinction and Evolution; pp. 122–132. (May) [Google Scholar]
- Plemenitaš A., Lenassi M., Konte T., Kejžar A., Zajc J., Gostinčar C., Gunde-Cimerman N. Adaptation to high salt concentrations in halotolerant/halophilic fungi: a molecular perspective. Front. Microbiol. 2014;5:199. doi: 10.3389/fmicb.2014.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plemenitaš A., Vaupotič T., Lenassi M., Kogej T., Gunde-Cimerman N. Adaptation of extremely halotolerant black yeast Hortaea werneckii to increased osmolarity: a molecular perspective at a glance. Stud. Mycol. 2008;61:67–75. doi: 10.3114/sim.2008.61.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash O., Sharma R. Diversity and distribution of phenol oxidase producing fungi from soda lake and description of curvularia lonarensis sp. nov. Front. Microbiol. 2016;7:1847. doi: 10.3389/fmicb.2016.01847. (December) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prista C., Almagro A., Loureiro-Dias M.C., Ramos J. Physiological basis for the high salt tolerance of Debaryomyces hansenii. Appl. Environ. Microbiol. 1997;63(10):4005–4009. doi: 10.1128/aem.63.10.4005-4009.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prista C., Loureiro-Dias M.C., Montiel V., García R., Ramos J. Mechanisms underlying the halotolerant way of Debaryomyces hansenii. FEMS Yeast Res. 2005;5(8):693–701. doi: 10.1016/j.femsyr.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Raddadi Noura, Cherif Ameur, Daffonchio Daniele, Neifar Mohamed, Fava F. 2018. Biotechnological Applications of Extremophiles, Extremozymes and Extremolytes. [DOI] [PubMed] [Google Scholar]
- Raja H.A., Shearer C.A. Freshwater ascomycetes: aliquandostipite minuta (Jahnulales, Dothideomycetes), a new species from Florida. Mycoscience. 2007;48(6):395–398. [Google Scholar]
- Rajpal K., Aziz N., Prasad R., Varma R.G., Varma A. Extremophiles as biofactories of novel antimicrobials and cytotoxics – an assessment of bioactive properties of six fungal species inhabiting rann of Kutch, India. Indian J. Sci. Tech. 2016;9 (June) [Google Scholar]
- Roberts M.F. Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Syst. 2005;1(1):5. doi: 10.1186/1746-1448-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadati R., Barghi A., Larki R.A. Isolation and screening of lipolytic fungi from coastal waters of the Southern Caspian sea (North of Iran) Jundishapur J. Microbiol. 2015;8(4) doi: 10.5812/jjm.8(4)2015.16426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salano O.A. 2011. Isolation and Characterization of Fungi from Lake Magadi of the Kenyan Rift Valley. [Google Scholar]
- Salano O.A., Makonde H.M., Kasili R.W., Nyawira L., Nawiri M.P., Boga H.I. Diversity and distribution of fungal communities within the hot springs of soda lakes in the Kenyan rift valley. 2017;11(19):764–775. [Google Scholar]
- Salo O. University of Groningen; 2016. Secondary Metabolism by Industrially Improved Penicillium chrysogenum Strains. [Google Scholar]
- Santini T.C., Warren L.A., Kendra K.E. Microbial diversity in engineered haloalkaline environments shaped by shared geochemical drivers observed in natural analogues. Appl. Environ. Microbiol. 2015;81(15):5026–5036. doi: 10.1128/AEM.01238-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayana T., Raghukumar C., Shivaji S. Extremophilic microbes: diversity and perspectives. Curr. Sci. 2005:78–90. [Google Scholar]
- Schadt C.W., Martin A.P., Lipson D.A., Schmidt S.K. Seasonal dynamics of previously unknown fungal lineages in tundra soils. Science. 2003;301(5638):1359–1361. doi: 10.1126/science.1086940. [DOI] [PubMed] [Google Scholar]
- Shearer C.A., Descals E., Kohlmeyer B., Kohlmeyer J., Marvanová L., Padgett D. Fungal biodiversity in aquatic habitats. Biodivers. Conserv. 2007;16(1):49–67. [Google Scholar]
- Sheridan C. Nature Publishing Group; 2004. Kenyan Dispute Illuminates Bioprospecting Difficulties. [DOI] [PubMed] [Google Scholar]
- Sierra G. A simple method for the detection of lipolytic activity of micro-organisms and some observations on the influence of the contact between cells and fatty substrates. Antonie Leeuwenhoek. 1957;23(1):15–22. doi: 10.1007/BF02545855. [DOI] [PubMed] [Google Scholar]
- Singh P., Raghukumar C., Verma P., Shouche Y. Fungal community analysis in the deep-sea sediments of the Central Indian Basin by culture-independent approach. Microb. Ecol. 2011;61(3):507–517. doi: 10.1007/s00248-010-9765-8. [DOI] [PubMed] [Google Scholar]
- Stamford T.L.M., Araújo J.M., Stamford N.P. Atividade enzimática de microrganismos isolados do jacatupé (Pachyrhizus erosus L. Urban) Ciência E Tecnologia de Alimentos. 1998;18(4):1–10. [Google Scholar]
- VanderMolen K.M., Raja H.A., El-Elimat T., Oberlies N.H. Evaluation of culture media for the production of secondary metabolites in a natural products screening program. Amb. Express. 2013;3(1):71. doi: 10.1186/2191-0855-3-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J.D.G. Purificação e caracterização de uma a-amilase de Streptomyces sp. São Paulo (Tese de Doutorado) USP. 1999;116 [Google Scholar]
- Wilson Z.E., Brimble M.A. Molecules derived from the extremes of life. Nat. Prod. Rep. 2009;26(1):44–71. doi: 10.1039/b800164m. [DOI] [PubMed] [Google Scholar]
- Yoiprommarat S., Srichomthong K., Deelai S., Suetrong S., Sakayaroj J., Bunyapaiboonsri T., Unagul P. Secondary metabolites of the marine fungus Paradendryphiella arenariae BCC 17999. Bot. Mar. 2015;58(5):393–399. [Google Scholar]