Abstract
Background
Up to 30% of patients with schizophrenia are resistant to antipsychotic drug treatment, with 60% of such cases also failing to respond to clozapine. Deep brain stimulation (DBS) has been used in treatment resistant patients with other psychiatric disorders, but there is a lack of trials in schizophrenia, partly due to uncertainties over where to site the electrodes. This trial aimed to examine the effectiveness of nucleus accumbens (NAcc) and subgenual anterior cingulate cortex (subgenual ACC) targeted DBS; the primary outcome measure was PANSS total score, as assessed fortnightly.
Methods
Eight patients with schizophrenia, who met criteria for treatment resistance and were also resistant to/intolerant of clozapine, were randomly assigned using central allocation to receive DBS in the NAcc or subgenual ACC. An open stabilization phase lasting at least six months was followed by a randomized double-blind crossover phase lasting 24 weeks in those who met symptomatic improvement criteria. The primary end-point was a 25% improvement in PANSS total score. (ClinicalTrials.gov Identifier: NCT02377505; trial completed).
Findings
One implanted patient did not receive DBS due to complications of surgery. Of the remaining 7 patients, 2/3 with NAcc and 2/4 with subgenual ACC electrode placements met the symptomatic improvement criteria (58% and 86%, and 37% and 68% improvement in PANSS total score, respectively). Three of these patients entered the crossover phase and all showed worsening when the stimulation was discontinued. The fourth patient worsened after the current was switched off accidentally without her or the investigators’ knowledge. Physical adverse events were uncommon, but two patients developed persistent psychiatric adverse effects (negative symptoms/apathy and mood instability, respectively).
Interpretation
These preliminary findings point to the possibility of DBS having therapeutic effects in patients with schizophrenia who do not respond to any other treatment. Larger trials with careful attention to blinding will be necessary to establish the extent of the benefits and whether these can be achieved without psychiatric side-effects.
Research in Context.
Evidence before this study
Poor response to antipsychotic drug treatment is a well-recognized problem in schizophrenia. Deep brain stimulation (DBS) has been used in treatment resistant major depression and obsessive-compulsive disorder, but trials are so far lacking in schizophrenia. Literature review to confirm the absence of existing trials included (a) searching PubMed using the terms deep brain stimulation and schizophrenia and checking the references of all relevant publications; (b) checking ClinicalTrials.gov; and (c) personal communication with the investigators of a currently recruiting American trial.
Added value of this study
This initial trial suggests that DBS might be beneficial in some patients with treatment resistant schizophrenia. Of particular note was the near-complete disappearance of positive symptoms (delusions and hallucinations) in two patients with nucleus accumbens electrode placements.
Implications of all the available evidence
The apparent positive effects of DBS in schizophrenia in this trial need to be treated with caution given the small numbers and mainly open label evaluation. It will also be important to determine whether and to what extent clinical improvement can be obtained without psychiatric adverse effects.
Alt-text: Unlabelled box
1. Introduction
Schizophrenia is a severe and disabling mental disorder characterized by positive, negative and cognitive symptoms, affecting around 1% of the population worldwide [1]. It is estimated that around 20–30% of patients are resistant to conventional antipsychotic drug treatment [2], and less than half of such patients (40.1%, 95% confidence interval 36.8%−43.4%) respond to the most effective of the second-generation or atypical antipsychotics, clozapine [3]. Other psychopharmacological strategies, in particular the use of a range of drugs with glutamatergic actions, have so far failed to fulfil their promise, either for negative symptoms [4] or for all symptoms [5].
Deep brain stimulation (DBS) is a well-established treatment for Parkinson's disease and other movement disorders, whose use has been extended in recent years to treatment resistant psychiatric illness [6]. The technique involves high frequency stimulation of deep brain areas through electrodes implanted under stereotactic surgery. It is believed to work primarily by producing functional inhibition in the region around the electrode, but excitatory effects on local axons and more distant excitatory effects may also play a part [7]. It has additionally been argued that the intervention may act in the longer term to correct pathological brain activity in brain networks with connections to the implantation site [8]. Importantly, unlike other forms of psychosurgery, DBS is reversible, i.e., the stimulation can be turned off (and if necessary the electrodes explanted) without any permanent loss of function.
To date, DBS has been employed principally in treatment resistant patients with two psychiatric disorders, obsessive-compulsive disorder and major depression, with broadly encouraging results in both cases [9,10]. The electrode placements most frequently employed have been in or around the nucleus accumbens (NAcc) in the former disorder [9], and in this site and the subgenual anterior cingulate cortex (subgenual ACC) in the latter [10]. The potential use of DBS in treatment resistant schizophrenia is currently a topic of considerable discussion [11,12], with much of the debate focusing on where to site the electrodes. Practical experience, in contrast, is virtually non-existent. Plewnia et al. [13] reported beneficial effects of DBS on obsessions and compulsions using an NAcc electrode placement in a patient with residual schizophrenia, but this patient had no psychotic symptoms. A trial in Toronto (Clinicaltrials.gov identifier NCT01725334) aimed to use electrode placements in the NAcc or the ventral tegmental area, with the aim of improving negative symptoms; however, this trial was abandoned due to lack of recruitment. A currently recruiting trial in Baltimore (Clinicaltrials.gov indentifier NCT02361554), targets the substantia nigra pars reticulata; its rationale is that local inhibitory effects will result in disinhibition of the mediodorsal thalamic nucleus and lead to improvement in positive and cognitive symptoms.
We report the outcome of the first completed trial of DBS in treatment resistant schizophrenia. Because of the uncertainties concerning electrode placement we decided to test the effectiveness of two targets. One was in the ventral striatum, specifically the NAcc, and the other was the subgenual ACC. Choice of these two sites was driven partly by the fact that both have been employed in obsessive-compulsive disorder and/or major depression, and partly because both sites can be considered to be in some sense characterized by neuronal overactivity in schizophrenia, and so potentially susceptible to the functional inhibitory effects of DBS. In the case of the NAcc there is longstanding circumstantial evidence for a functional dopamine excess in the disorder [14], with current findings pointing to increased dopamine synthesis capacity in the striatum [15]. At a theoretical level positive symptoms (in particular delusions and hallucinations) have also been linked to ventral striatal overactivity via Kapur's [16] influential ‘aberrant salience’ proposal, of increased and inappropriate dopamine activity giving rise to abnormal reward prediction error signals. Also relevant to the decision to employ a ventral striatal electrode placement is the fact that this part of the basal ganglia receives major afferent input from the hippocampus, which has been considered to play a role in the pathophysiology of schizophrenia and has been proposed as a target for DBS [11].
The choice of a subgenual ACC placement was based on the finding of failure of de-activation in the medial frontal cortex, described by ourselves [17] and others [18]. This failure is presumed to reflect dysfunction in the so-called default mode network, a set of brain regions that are normally active at rest but which de-activate during performance of a wide range of attention-demanding tasks; the medial frontal cortex – including the pregenual and subgenual portions of the anterior cingulate cortex, but also more rostral regions – forms one of two prominent midline ‘nodes’ or ‘hubs’ of this network [19]. On these grounds, the medial frontal cortex represents a more logical cortical target for the local inhibitory effects of DBS than the dorsolateral prefrontal cortex, which is also implicated in schizophrenia but where the abnormality takes the form of hypofunction [20].
In this paper we report clinical and functional outcomes from the trial. Neuropsychological and functional imaging findings will be reported in future communications.
2. Methods
2.1. Design
The trial had a combined open (6+ month) and double-blind crossover (24 weeks) design. The 24-week crossover phase length was based on previous experience by one of the two groups of investigators with DBS for depression [21]. The aim was to recruit 8 patients who would be randomly assigned 1:1 to electrode placements in either the NAcc or the subgenual ACC. This study is registered with ClinicalTrials.gov (identifier NCT02377505).
2.2. Participants
Patients were recruited from the outpatient, inpatient and residential facilities of two mental healthcare organizations in Barcelona: FIDMAG Germanes Hospitalàries and the Hospital de la Santa Creu i Sant Pau, plus referrals by other interested clinicians. Inclusion criteria were: age 18–55 years, having a diagnosis of schizophrenia according to DSM-IV criteria, having a five-year minimum duration of illness and showing evidence of treatment resistance. For treatment resistance the following were required: (a) presence of continuing positive symptoms without remission for two years; (b) poor response to treatment with at least two different antipsychotics, including at least one atypical antipsychotic (not including clozapine) given in adequate doses for a period of at least 6 weeks; and (c) having been treated with clozapine with either no improvement at adequate dosage or poor tolerance. It was anticipated that most patients would also show negative symptoms, but presence of these was not an inclusion criteria. While not forming part of current definitions of treatment resistance, we added a requirement that the patients had been treated with ECT at some point without sustained improvement, unless this treatment was contra-indicated or not tolerated or had been refused. This was in the interests of focusing on patients in whom all other potentially effective treatment options had been tried [22].
At the time of study entry the patients were required to have a score of 4 (moderate) or greater on at least two of the Positive and Negative Symptoms Scale (PANSS) items, delusions, hallucinatory behaviour, suspiciousness, unusual thought content, or alternatively a score of 6 (severe) or greater on one symptom. They also had to score 6 or greater (severely ill) on the Clinical Global Impression (CGI) scale and to have been on stable doses of treatment for at least two months. Women of childbearing age had to have a negative pregnancy test within 72 h pre-study and had to be using contraception.
Exclusion criteria included: (a) contraindications to neurosurgery or DBS (e.g. pacemaker); (b) diagnosis of epilepsy or clozapine-induced seizures currently requiring treatment with anticonvulsants; (c) presence of suicidal or self-harming behaviour or ideation in the last two years; (d) significant cognitive impairment, as defined by WAIS III IQ <70, or performance in the severely impaired range on memory or executive function tests on more detailed neuropsychological testing; (e) presence of significant cardiovascular and/or cerebrovascular disease (defined as history of cerebrovascular events, clinical symptoms of cardiac or vascular disease, ischaemic changes on ECG, uncontrolled hypertension); (f) if female, breastfeeding; (g) history of drug or alcohol abuse/dependency in the previous two years.
Only patients who were able to fully understand the potential benefits and risks of treatment were considered for the trial. The consent process was rigorous, and included discussions with both the patient and his/her family if appropriate. Patients were also required to have a caregiver or identified responsible person (i.e., family member, social worker, case-worker, or nurse) who spent at least four hours/week with the patient and was able to provide advice and support concerning the study procedures. A committee including the patient's psychiatrist, an independent senior psychiatrist and a neurosurgeon further considered the feasibility of enrolling each patient into the study.
The patients were randomly assigned 1:1 to one of the two electrode placements using PROC PLAN of SAS (version 9). Randomization was carried out by central allocation using computer generated random numbers: an independent statistician from another department generated the list, assigned the target sequence and communicated it to one of the investigators in a sealed envelope. This investigator communicated the target to the neurosurgeons. A block randomization method was used. A similar procedure was followed for the randomization in the crossover phase (which also took into account the first randomization to ensure balanced allocation within the targets). After undergoing surgery to implant the electrodes, stimulation was started within 48–72 h and lasted until the patient was clinically stable, with a minimum period of 6 months. If the patient achieved symptomatic response (defined as an improvement ≥ 25% in PANSS total score, calculated using the formula: [PANSS baseline score – PANSS post-scores] × 100/ [PANSS baseline score-30] [23], which was maintained in at least 50% of the subsequent PANSS ratings, he or she then entered the 24-week double-blind crossover phase, and was randomized to 12 weeks with the stimulation ‘on’ followed by 12 weeks ‘off’ or vice-versa. In case of worsening, the patient could be withdrawn from the study if necessary and the stimulation turned on again (if it was currently switched off). Pharmacological treatment could not be modified throughout the study period, except for prescription of benzodiazepines or hypnotics if required.
All patients gave written informed consent. The study was in line with the Declaration of Helsinki, and was approved by the hospital ethical committee and the Spanish regulatory drug agency (Agencia Española de Medicamentos y Productos Sanitarios).
2.3. Assessments
In addition to PANSS total score, which was the primary outcome measure, and its positive and negative subscales, assessments included the Psychotic Symptom Rating Scales (PSYRATS) [24], the Scale for the Assessment of Negative Symptoms (SANS) [25], the Calgary Depression Scale for Schizophrenia (CDSS) [26] and the Global Assessment of Functioning scale (GAF) [27]. Two social functioning scales were also used, the Personal and Social Performance (PSP) Scale [28] and the Social Functioning Scale [29].
Symptomatic changes were evaluated every two weeks with the PANSS until the end of the trial. The remaining clinical scales were administered at the main study points (i.e., baseline, immediately before the crossover phase and at termination). Adverse events were assessed in detail monthly by the member of the trial team performing the psychiatric and social evaluations. Following termination from the trial patients continued to be followed up by members of the trial team or their treating clinicians.
In the stabilization phase the assessors were blind to which electrode placement each patient had and to any changes in stimulation parameters that were made. Both the patients and the clinicians were unaware of the patients’ stimulation status during the crossover phase. Measures to assess the success of blinding were not employed.
2.4. Surgical procedure
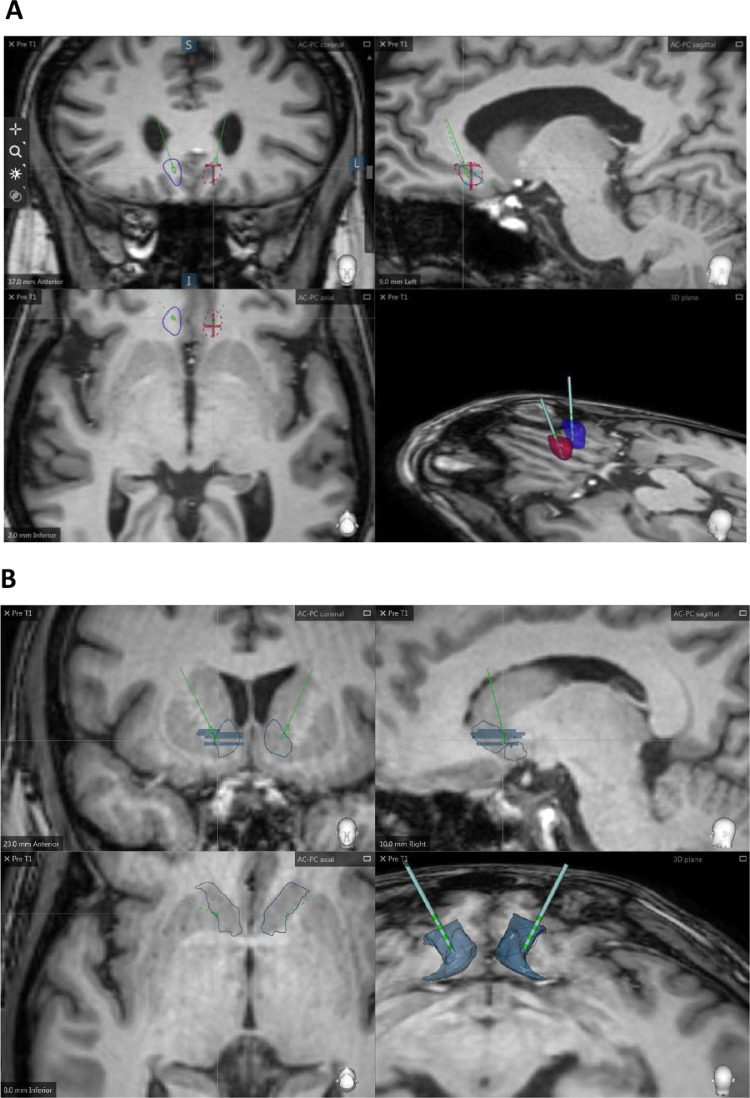
Electrode implantation was in the white matter adjacent to the Cg25 region (subgenual ACC) and in the NAcc. The DBS pulse-generating device was implanted abdominally. Prior to surgery, a Leksell G stereotactic frame (Elekta Instruments, Atlanta, GA, USA) was fitted to the patient's head. Using the StealthStation 7 (Medtronic Inc., Minneapolis, MN, USA) the CT scan with the stereotactic frame was fused to the MRI image to calculate the surgical targets. The target subgenual ACC white matter was delimited as follows: in a midline T2 sagittal image the cingulate gyrus inferior to the genu of the corpus callosum was identified; next, a line was traced from this point of the corpus callosum to the anterior commissure and the mid-point was identified; an image was then taken of the T2 coronal section corresponding to the plane of the mid-point and the definitive coordinates were calculated for the transition area between the white and grey matter for BA 25. The NAcc target was determined measuring the distances from the anterior commissure (AC) and the posterior commissure (PC) with the following coordinates: x = 6–8 mm lateral to midpoint, y = 1–3 mm anterior to the AC, Z = 4 mm inferior to the AC line (see Fig. 1 for location of the NAc and subgenual ACC electrode implantations in subjects N5 and N7, respectively). In the operating room, with the patient under general anaesthesia, a burr hole was drilled in front of the coronal suture and laterally at a variable distance from the midline seeking to avoid the ventricles. DBS electrodes (Medtronic model 3387, Medtronic Inc., Minneapolis, MN, USA) were implanted bilaterally. Each of the four electrode contacts was tested intraoperatively at maximal voltage (9.0 V). During the same surgical procedure, a programmable internal pulse generator (Activa PC, Medtronic Inc., Minneapolis, MN, USA) was implanted subcutaneously in abdominal wall tissue. The procedure was carried out under general anaesthesia.
Fig 1.
Location of the deep brain stimulation leads in the (A) nucleus accumbens (NAcc) and in the (B) subgenual anterior cingulate cortex (subgenual ACC) in right and left hemispheres. The upper row of images corresponds to coronal and sagittal slices; the lower images correspond to axial section in 2D and 3D views. The 3D plane shows a volumetric reconstruction of both targets (bilateral NAcc in metallic blue; left subgenual ACC in purple, right subgenual ACC in blue. Images were generated using the SureTune software (Medtronic Eindhoven Design centre, MEDC).
Stimulation was started 48–72 h after surgery using unilateral left stimulation, (contact anode, case cathode), with the initial following parameters: 2.5 V, 60 microseconds (µs) pulse width and 130 Hertz (Hz) of frequency; these were chosen based on prior experience by our group (Sant Pau) with DBS in patients with major depression. Two contacts with good levels of impedance were selected taking into account the position of the electrode within the selected area (e.g., patient N1 showed had a contact outside the NAcc and so this was not used). After a week bilateral stimulation was begun, again based on previous clinical experience with DBS in psychiatric populations.
During the stabilization phase stimulation parameters were modified individually for each patient by an independent clinician depending on clinical state, and guided specifically by the presence and degree of positive symptoms. Patients were not informed about any changes. The sequence of changes to maximize the therapeutic effect was (1) increasing voltage up to 7.5 V, (2) increasing pulse width or frequency up to 210 µs/Hz, and (3) changing active contacts or mode.
2.5. Data analysis
Formal analysis of changes in PANSS and other ratings was considered to be of limited value due to the small sample size, and is also complicated by the fact that the endpoint criteria (i.e., progression to crossover phase or termination due to lack of effectiveness) depended on response to DBS. For completeness, however, statistical models were fitted to the PANSS data (PANSS total, PANSS positive, PANSS negative) for the sample as a whole (i.e., not separating by electrode placement), considering only the stabilization phase. Linear mixed models with repeated measures evaluated the effect of DBS by considering the PANSS scores in the visit previous to the surgical implant and all the subsequent assessments up till the end of the stabilization phase. This model also tested the effect of time (evolution of PANSS scores across time, discounting the DBS off-on effect) including also individual slopes and intercepts (i.e., the random effects part of the model). Paired t-tests were additionally carried out, considering individual PANSS scores at the baseline visit and at the last recorded visit in the stabilization phase. Statistical analyses were conducted with version 3.6.0 of the R software.
2.6. Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Patient characteristics
Slightly less than thirty patients were considered for the trial (Sant Pau N = 14; FIDMAG N = 13–15, exact numbers not recorded). The most common reason for exclusion was a lack of perceived need for treatment by the patient. Other reasons in individual cases included failure to meet diagnostic criteria, ongoing substance abuse, presence of comorbid psychiatric disorder (obsessive-compulsive disorder and autistic spectrum disorder) and failure to meet severity criteria.
Eight patients were enrolled in the study. The first patient was recruited on 28/11/2014 and the last on 20/04/2017. One patient (N3) did not proceed to stimulation; he suffered a right internal capsule haemorrhage immediately after surgery and subsequently developed infection in the device which threatened to spread to the electrodes. As a result the generator and electrodes were removed three months after surgery.
All but one of the seven remaining patients showed ultra-treatment resistant illness, according to Howes et al. [30]. In the remaining patient (N6) clozapine was not tolerated at doses higher than 100 mg/day (the dose she was taking at study entry). These seven patients’ baseline characteristics and electrode placement after first randomization are shown in Table 1. Three patients were implanted in the NAcc (N1, N5, N6) and 4 patients in the subgenual ACC (N2, N4, N7, N8). All patients were receiving treatment with clozapine augmented with different antipsychotics. Highest stimulation parameters achieved during the study period in each patient are shown in supplementary Table S1.
Table 1.
Patients’ baseline characteristics and electrode placement at baseline.
| Patient ID | Sex | Age | Illness duration (years) | Antipsychotic treatment (mg/d) | ECT | PANSS Total | PANSS Positive | PANSS Negative | PANSS General | GAF | CGI | Electrode Placement |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | F | 46 | 24 | Clozapine 350 | Refused | 97 | 27 | 24 | 46 | 40 | 6 | NAcc |
| Risperidone 6 | ||||||||||||
| N2 | M | 34 | 9 | Clozapine 500 | Non-response | 108 | 26 | 28 | 54 | 25 | 6 | Subgenual ACC |
| Haloperidol 5 | ||||||||||||
| N3 | M | 37 | 8 | Clozapine 600 | Non-response | 76 | 21 | 28 | 27 | 25 | 6 | NAcc |
| Aripiprazole 45 | ||||||||||||
| N4 | F | 53 | 23 | Clozapine 800 | Yes | 84 | 22 | 23 | 39 | 30 | 6 | Subgenual ACC |
| Olanzapine 10 | ||||||||||||
| Pimozide 10 | ||||||||||||
| N5 | M | 43 | 11 | Clozapine 400 | Intolerance (confusion) | 102 | 24 | 29 | 49 | 30 | 6 | NAcc |
| Ziprasidone 160 | ||||||||||||
| N6 | F | 35 | 10 | Clozapine 100 | Refused | 65 | 17 | 12 | 36 | 30 | 6 | NAcc |
| Paliperidone 3 | ||||||||||||
| N7 | F | 38 | 21 | Clozapine 450 | Refused | 85 | 27 | 21 | 37 | 31 | 6 | Subgenual ACC |
| Paliperidone depot 100/month | ||||||||||||
| N8 | M | 54 | 35 | Clozapine 500 | Refused | 87 | 22 | 32 | 33 | 31 | 6 | Subgenual ACC |
| Aripiprazole 20 |
PANSS – Positive and Negative Symptoms Scale; GAF – Global Assessment of Functioning scale; CGI – Clinical Gobal Impression of severity of illness.
3.2. Stabilization phase
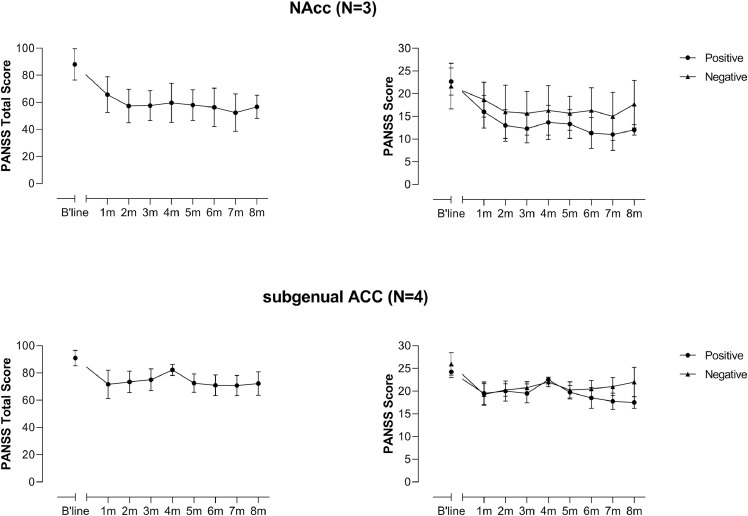
The open stabilization phase lasted between 8 and 20 (average 13) months. Average scores over first eight months of treatment (the period during which all patients remained in the stabilization phase), separated according to electrode placement, are shown in Fig. 2. Individual scores for the patients over the entire stabilization phase are shown in Supplementary Figs. S1 and S2. It is noteworthy that two of the three patients with NAcc placements showed a nearly full remission (N1) and full remission apart from intermittent recurrences (N6) of positive symptoms (which took the form exclusively of delusions and hallucinations in both cases). One of these patients (N1) has been reported previously [31].
Fig 2.
Symptomatic changes in the 7 DBS treated patients over the first 8 months of the stabilization phase.
Given the limitations of applying statistical analysis to the data, detailed results are reported in the Supplementary material. Briefly, however, testing across the whole sample (N = 7) revealed a significant effect of DBS on PANSS total, positive symptoms and negative symptoms scores (all p<0.001), when the baseline (i.e., DBS off) was compared to all remaining time points in the stabilization phase (i.e., DBS on) using a repeated measures linear mixed model. This result was replicated for PANSS total score and positive symptoms score, but not negative symptoms score, when a simpler paired t-test was performed, considering the baseline (DBS off) and last observation (DBS on) (PANSS total p = 0.007; PANSS positive p = 0.002; PANSS negative p = 0.18).
3.3. Double-blind crossover phase
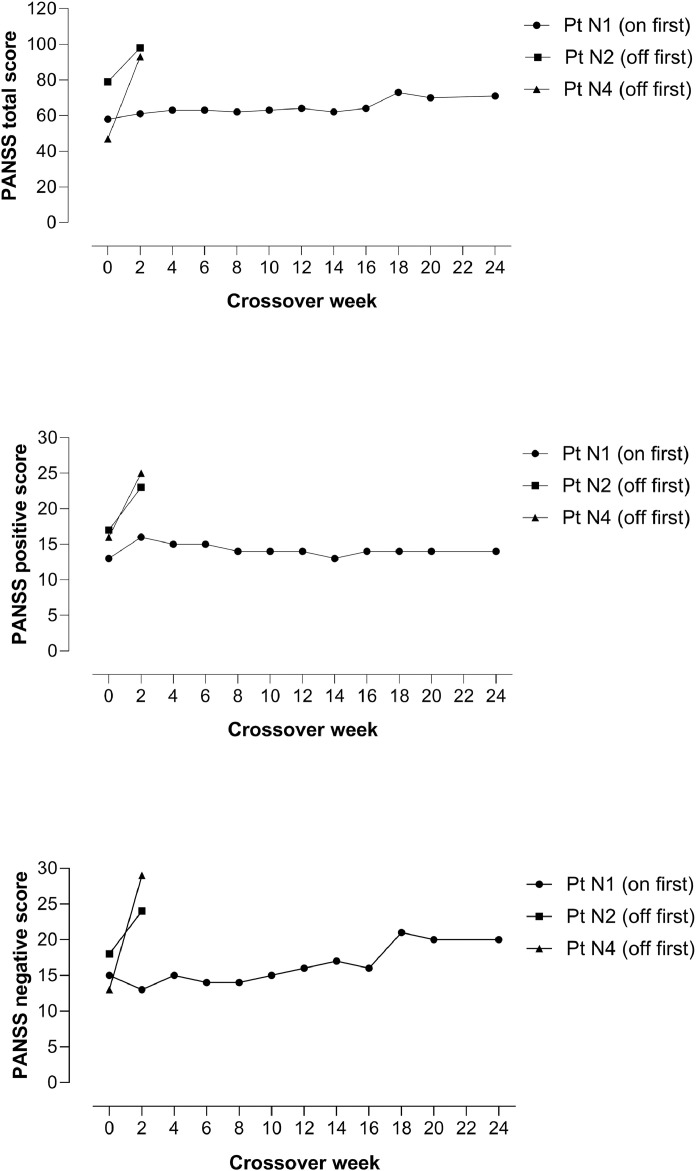
Four patients (N1, N2, N4 and N6) met the symptomatic response criteria, 2 with NAcc (N1 and N6) and 2 (N2 and N4) with subgenual ACC placements (for percentages of improvement see Supplementary Table S2). Three of these patients entered the crossover phase. Patient N1 (NAcc placement) entered in an ‘on’ phase. Six weeks after the stimulation was switched to ‘off’ a worsening in psychotic symptoms became evident, which took the form mainly of a worsening of negative symptoms [PANSS scores immediately before entering crossover and in the first switch-off visit where a worsening of symptoms was evident: total: 64 vs. 73, positive symptoms: 14 vs. 14, negative symptoms: 16 vs. 21]. This patient remained in the trial until the end of the crossover phase. Patient N2 (subgenual ACC placement) began in an ‘off’ phase. This was followed by a worsening of psychotic symptoms within a few days [PANSS scores: total: 79 vs. 98, positive symptoms: 17 vs. 23, negative symptoms: 18 vs. 24]. The patient was admitted with suicidal ideation, the stimulation was switched back on, and he was withdrawn from the study. Patient N4 (subgenual ACC placement) also began in an ‘off’ phase and also showed a worsening of psychotic symptoms, in this case two weeks later [PANSS scores: total: 47 vs. 93, positive symptoms: 16 vs. 25, negative symptoms: 13 vs. 29. The stimulation was switched back on, and the patient was withdrawn from the study. Details of the three patients’ courses during the crossover phase are summarized in Fig. 3.
Fig 3.
Changes in PANSS total, positive and negative scores in the three patients who entered the crossover phase.
The fourth patient who met the symptomatic response criteria (N6, NAcc placement) achieved excellent remission of psychotic symptoms, although these intermittently returned, requiring frequent alterations of stimulus parameters. After 10 months in total, at a time when she had been symptom free for several weeks, the stimulation was turned off unknown to the patient and the investigators. (What happened is unclear but possibilities include either that the off button had been accidentally touched when her stimulus parameters were checked during a clinic visit, or that the stimulator function was interrupted when she passed through a security scanner at a tourist attraction the same day.) Her symptoms (delusions and auditory hallucinations) returned within 24 h and persisted until the switching off event was discovered a few days later. The patient reported subjective improvement 20 min after the current was turned back on. The next day she was symptom free. The patient was given the option of formally entering the crossover phase, but declined.
Two spontaneous disconnections also occurred in N5 (NAcc placement) at the beginning of treatment. These lasted less than a week on both occasions and were not obviously associated with clinical changes.
3.4. Secondary outcomes
Average PSYRATS and SANS scores from baseline to randomization or termination for all 7 patients are shown in Table 2. Mirroring the pattern with the PANSS, reductions were evident on both scales. Depression scores also decreased. Improvements were also seen on the GAF and CGI. There was evidence of improvement on one of the two social functioning scales used, the PSP (see Table 2), but not on the other, the SFS (see Supplementary Table S3).
Table 2.
Changes in other scales from baseline to randomization or termination.
| Mean score at baseline (±SD) | Mean score at randomization/termination (±SD) | |
|---|---|---|
| PSYRATS (sum of delusion and hallucination subscales | 44.71±12.65 | 32.57±21.79 |
| SANS (total score) | 55.57±12.97 | 46.29±23.43 |
| CDSS | 7.29±6.21 | 3.71±4.79 |
| GAF | 31.14±4.49 | 47.14±17.07 |
| CGI | 6 ± 0.0 | 4.14±1.68 |
| Personal and Social Performance Scale* | 30.83±8.49 | 42.67±25.91 |
Based on 6 patients, data missing at randomization/termination for 1 patient.
For scores on the Social Functioning Scale see Supplementary Table S3.
PSYRATS – Psychotic Symptom Rating Scales; SANS – Scale for the Assessment of Negative Symptoms; CDSS – Calgary Depression Scale for Schizophrenia; GAF – Global Assessment of Functioning scale; CGI – Clinical Global Impression of Severity.
3.5. Adverse events
DBS was generally well-tolerated in the 7 patients who proceeded to electrode activation. However, one patient with an NAcc placement (N1) developed akathisia after the stimulation was changed from unilateral to bilateral; this eventually responded when the stimulation was changed back to unilateral, maintaining the reduced parameters. As noted, during the crossover phase this patient developed worsening of negative symptoms from a previously mild level. This worsening has persisted, despite DBS being resumed and subsequent alterations in the stimulation parameters.
Patient N5 described an electrical sensation in his trunk and his head that occurred occasionally and depended on certain body movements. Examination, including neuroimaging, did not reveal evidence of device malfunction (e.g., damaged electrodes or wire connections to the internal pulse generator). Changing of the stimulation from unipolar to bipolar mode was followed by a resolution of the symptom.
After trial end, after 11 months of stimulation in total, patient N6 developed behavioural changes compatible with hypomania. Immediately prior to this she had taken a unilateral decision to stop taking antipsychotic medication. Stimulation parameters were initially lowered with a certain amount of improvement. However, six weeks later she was admitted in a mixed affective state, which responded to treatment with antipsychotics. Since then the patient's clinical state has been characterized by mood instability with fluctuations on a daily basis, impulsive behaviour and at times suicidal ideation. These symptoms initially proved difficult to control with mood stabilizers and changes to her DBS parameters, although her mood fluctuations improved after re-instatement of antipsychotic medication (aripiprazole). Currently she is free of prolonged periods of mood disturbance, although she continues to experience spells of depression lasting hours or days. Her psychotic symptoms (referential delusions accompanied by auditory hallucinations) have remained in remission most of the time, though they are prone to re-appear for periods of 12–36 h, often when she is under stress.
3.6. Post-operative course in the patient who did not receive DBS
After suffering a right-sided peri‑operative haemorrhage, patient N3 initially showed confusion which lasted four days. After this settled he was noted to show improvement in his previously severe active psychotic symptoms. This improvement remained evident for over seven months, but hallucinations and referential delusions ultimately re-appeared, although less severely than before the operation.
Six months after being withdrawn from the trial, the patient showed no neurological signs, though he had experienced seizures which were controlled with anticonvulsant medication. He shows a lesion in the anterior right internal capsule.
4. Discussion
To our knowledge, this is the first study reporting the effectiveness and safety of DBS in patients with treatment resistant schizophrenia, all but one of whom were also resistant to clozapine. Although the sample size was small and the variability in treatment response makes it difficult to generalize, there were indications of improvement, with the NAcc appearing to be the more promising of the two placements employed. Caution is necessary before accepting improvement observed under open conditions at face value. However, three of the four patients who showed significant improvement worsened when the current was switched off under double-blind conditions; the fourth patient also worsened abruptly when the device was accidentally switched off without her or her clinicians’ knowledge.
Two out of the three patients with NAcc targeted DBS met the symptomatic improvement criterion. In these patients the improvement in their main symptoms, delusions and hallucinations, was marked, taking the form of a near complete disappearance (patient N1) and complete absence of these symptoms most of the time (patient N6). As noted in the Introduction, the NAcc and the ventral striatum of which it forms part is a plausible target for the local functional inhibitory effects of DBS, as it is strongly suspected of being a site of functional overactivity in schizophrenia, specifically increased dopaminergic activity. Nevertheless, it should be noted that functional imaging studies in schizophrenia have uniformly found that ventral striatal activation in response to reward predictive stimuli is reduced rather than increased as the salience theory predicts [32]. On this basis it is unclear why a functional inhibition produced by DBS would have an ameliorative effect on positive symptoms. Possible explanations for this inconsistency might be in terms of the local or more distant excitatory effects of DBS [7], or possibly in terms of modulatory effects on the mesolimbic and mesocortical components of the mesotelencephalic dopamine projection [11].
In the patients with electrode placements in the subgenual ACC, improvement appeared less marked and, in the two who went on to meet improvement criteria, was more gradual (see individual graphs in Supplementary Fig. S2). Why this should be so is unclear. However, as noted by Veerakumar and Berton [8], while the motor effects of DBS in neurological disorders are immediate, the response in psychiatric disorders such as major depression is typically of the order of weeks to months, suggesting that longer-term effects on brain networks of which the implantation site forms part may be relevant.
As noted in the Introduction, our choice of sites for electrode placements was guided by a combination of evidence from its use in other psychiatric disorders and pathophysiological findings from patients with the disorder itself. Relevant findings from animal studies have also recently become available and may potentially provide further insights into the mechanism of DBS in schizophrenia. Thus, Bikovsky et al. [33] examined the effects of DBS in an animal model of schizophrenia in which pregnant females were administered a viral mimic (polyinosinic-polycytidilic acid, poly I:C). The progeny of mothers administered these or other similar agents have been found to show a variety of behavioural and neuropathological changes reminiscent of schizophrenia, which only emerge in late adolescence or early adulthood; they also show decreased prepulse inhibition and disrupted latent inhibition. DBS administered in both the NAcc and the medial prefrontal cortex to such rats in adulthood was found to reduce abnormalities in both prepulse inhibition and latent inhibition.
DBS was physically well-tolerated in the seven patients who proceeded to electrode activation. The only major physical adverse event was akathisia in one patient with a NAcc placement which appeared after stimulation was changed from unilateral to bilateral; this improved when the stimulation was changed back to unilateral [31]. On the other hand, lasting psychiatric adverse effects were prominent in the two patients with NAcc placements whose positive symptoms improved. One (N1) developed an amotivational state during the crossover phase that has since proved resistant to all interventions. The features of this state are consistent with negative schizophrenic symptoms, but apathy is also a recognized side-effect of DBS in Parkinson's disease [34]. The other patient (N6) developed mood instability that has so far lasted over a year, though with a gradually improving course. Depression and mood elevation, which can sometimes be prolonged, are also recognized complications of DBS for Parkinson's disease with a variety of different electrode placements [34]. It may also be relevant that while this patient was given a diagnosis of schizophrenia before trial entry, lifetime structured psychiatric interview at that time revealed a previous one-month episode when, after her antipsychotic medication was changed, she developed erotomanic ideation and showed flight of ideas; psychotic symptoms remained present during this period. It seems possible, therefore that her diagnosis may actually be schizoaffective disorder.
In conclusion, this small initial trial of DBS in schizophrenia provides grounds for considering that it may be useful in some treatment resistant and ultra-treatment resistant patients. There were suggestions that the NAcc placement had greater beneficial effects than that in the subgenual ACC, though this finding could easily be overturned in a larger trial. NAcc-targeted DBS also seemed to be particularly effective in patients with a clinical picture characterized mainly by delusions and hallucinations. One of the eight patients implanted suffered a serious complication (internal capsule haemorrhage), though fortunately this was without serious long-term sequelae. This frequency has to be contrasted with experience from DBS in neurological disorders, where the risk of neurosurgical complications, including infection and haemorrhage, have been estimated to be 1–3% [35]. Additionally, in the two patients in whom marked improvement was seen, this came at a cost of significant psychiatric complications. The main limitation of this trial is its small sample size. Additionally, the 24 week duration of the crossover phase may have been insufficient to fully dissipate carryover effects. The fact that, out of the three patients who entered the double-blind crossover phase, the two randomized to ‘off first’ both received subgenual ACC DBS could cast doubt on the findings in this part of the study.
5. Disclosures
Dr. Corripio has received research grants and served as consultant, advisor or speaker for the companies Otsuka and Ferrer. Dr. Pérez has been a consultant to or has received honoraria or grants from AB·Biotics, AstraZeneca, Bristol-Myers-Squibb, CIBERSAM, ISCIII, Janssen Cilag, Lundbeck, Otsuka, Servier and Pfizer. Dr. Álvarez has received consulting and educational honoraria from Eli Lilly, Lundbeck, Pfizer, Sanofi-Aventis and Otsuka; has participated as principal local investigator in clinical trials sponsored by Eli Lilly, Bristol-Myrers Squibb, Sanofi-Aventis and AB·Biotics; and has served as national coordinator of clinical trials sponsored by Servier and Lundbeck. All these collaborations are not related to DBS project. All the other authors declare no competing interests.
Funding/financial support
Trial registration: clinicaltrials.gov NCT02377505, co-ordinating PI: E. Álvarez.
This work was supported by the Catalonian Government (2017-SGR-01271 to E.P.-C and 2017-SGR-1343 to M. J. P.) and several grants from the Plan Nacional de I+D+i and co-funded by the Instituto de Salud Carlos III and the European Regional Development Fund (FEDER) “Investing in your future”: Miguel Servet research contracts (CPII16/00018 to E.P.-C. and CPII16/00020 to M.J.P.) and Research Projects (PI12/00042 to E.A., PI12/00686 to S.S and PI18/00880 to P.J.M.). The project was also supported by Ona Corporation (AMA DABLAM Research Project, IIBSP-AMA-2016-99).
Acknowledgements
The trial was funded by the Instituto de Salud Carlos III (PI12/00042 and PI12/00686). We thank Raymond Salvador for his statistical analysis of the data. The authors are grateful to the patients who consented to participate, and their caregivers, for their huge contribution to making this project possible.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2019.11.029.
Appendix. Supplementary materials
References
- 1.Kahn R.S., Sommer I.E., Murray R.M. Schizophrenia. Nat Rev Dis Primers. 2015;1:15067. doi: 10.1038/nrdp.2015.67. [DOI] [PubMed] [Google Scholar]
- 2.Elkis H. Treatment-resistant schizophrenia. Psychiat Clin N Am. 2007;30:511–533. doi: 10.1016/j.psc.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Siskind D., Siskind V., Kisely S. clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62:772–777. doi: 10.1177/0706743717718167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchanan R.W., Javitt D.C., Marder S.R. The cognitive and negative symptoms in schizophrenia trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry. 2007;164:1593–1602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- 5.Kinon B.J., Zhang L., Millen B.A. A multicenter, inpatient, phase 2, double-blind, placebo-controlled dose-ranging study of LY2140023 monohydrate in patients with dsm-iv schizophrenia. J Clin Psychopharmacol. 2011;31:349–355. doi: 10.1097/JCP.0b013e318218dcd5. [DOI] [PubMed] [Google Scholar]
- 6.Krack P., Hariz M.I., Baunez C., Guridi J., Obeso J.A. Deep brain stimulation: from neurology to psychiatry. Trends Neurosci. 2010;33:474–484. doi: 10.1016/j.tins.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Deniau J.M., Degos B., Bosch C., Maurice N. Deep brain stimulation mechanisms: beyond the concept of local functional inhibition. Eur J Neurosci. 2010;32:1080–1091. doi: 10.1111/j.1460-9568.2010.07413.x. [DOI] [PubMed] [Google Scholar]
- 8.Veerakumar A., Berton O. Cellular mechanisms of deep brain stimulation: activity-dependent focal circuit reprogramming. Curr Opin Behav Sci. 2015;4:48–55. doi: 10.1016/j.cobeha.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kisely S., Hall K., Siskind D., Frater J., Olson S., Crompton D. Deep brain stimulation for obsessive-compulsive disorder: a systematic review and meta-analysis. Psychol Med. 2014;44:3533–3542. doi: 10.1017/S0033291714000981. [DOI] [PubMed] [Google Scholar]
- 10.Kisely S., Li A., Warren N., Siskind D. A systematic review and meta-analysis of deep brain stimulation for depression. Depress Anxiety. 2018;35:468–480. doi: 10.1002/da.22746. [DOI] [PubMed] [Google Scholar]
- 11.Mikell C.B., Sinha S., Sheth S.A. Neurosurgery for schizophrenia: an update on pathophysiology and a novel therapeutic target. J Neurosurg. 2016;124:917–928. doi: 10.3171/2015.4.JNS15120. [DOI] [PubMed] [Google Scholar]
- 12.Gault J.M., Davis R., Cascella N.G. Approaches to neuromodulation for schizophrenia. J Neurol Neurosurg Psychiatry. 2018;89:777–787. doi: 10.1136/jnnp-2017-316946. [DOI] [PubMed] [Google Scholar]
- 13.Plewnia C., Schober F., Rilk A. Sustained improvement of obsessive-compulsive disorder by deep brain stimulation in a woman with residual schizophrenia. Int J Neuropsychopharmacol. 2008;11:1181–1183. doi: 10.1017/S1461145708009188. [DOI] [PubMed] [Google Scholar]
- 14.Howes O.D., Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCutcheon R., Beck K., Jauhar S., Howes O.D. Defining the locus of dopaminergic dysfunction in schizophrenia: a meta-analysis and test of the mesolimbic hypothesis. Schizophr Bull. 2018;44:1301–1311. doi: 10.1093/schbul/sbx180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 17.Pomarol-Clotet E., Salvador R., Sarro S. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network. Psychol Med. 2008;38:1185–1193. doi: 10.1017/S0033291708003565. [DOI] [PubMed] [Google Scholar]
- 18.Hu M.L., Zong X.F., Mann J.J. A review of the functional and anatomical default mode network in schizophrenia. Neurosci Bull. 2017;33:73–84. doi: 10.1007/s12264-016-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 20.Tan H.Y., Callicott J.H., Weinberger D.R. Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cereb Cortex. 2007;17(1):i171–i181. doi: 10.1093/cercor/bhm069. Suppl. [DOI] [PubMed] [Google Scholar]
- 21.Puigdemont D., Portella M., Perez-Egea R. A randomized double-blind crossover trial of deep brain stimulation of the subcallosal cingulate gyrus in patients with treatment-resistant depression: a pilot study of relapse prevention. J Psychiat Neurosci. 2015;40:224–231. doi: 10.1503/jpn.130295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinclair D.J., Zhao S., Qi F., Nyakyoma K., Kwong J.S., Adams C.E. Electroconvulsive therapy for treatment-resistant schizophrenia. Cochrane Database Syst Rev. 2019;3 doi: 10.1002/14651858.CD011847.pub2. CD011847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leucht S., Davis J.M., Engel R.R., Kissling W., Kane J.M. Definitions of response and remission in schizophrenia: recommendations for their use and their presentation. Acta Psychiatr Scand Suppl. 2009:7–14. doi: 10.1111/j.1600-0447.2008.01308.x. [DOI] [PubMed] [Google Scholar]
- 24.Haddock G., McCarron J., Tarrier N., Faragher E.B. Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS) Psychol Med. 1999;29:879–889. doi: 10.1017/s0033291799008661. [DOI] [PubMed] [Google Scholar]
- 25.Andreasen N.C. Negative symptoms in schizophrenia. definition and reliability. Arch Gen Psychiatry. 1982;39:784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- 26.Addington D., Addington J., Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3:247–251. doi: 10.1016/0920-9964(90)90005-r. [DOI] [PubMed] [Google Scholar]
- 27.Association AP . 4th ed., text rev. American Psychiatric Association; Washington, DC: 2000. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 28.Morosini P.L., Magliano L., Brambilla L., Ugolini S., Pioli R. Development, reliability and acceptability of a new version of the DSM-IV social and occupational functioning assessment scale (SOFAS) to assess routine social functioning. Acta Psychiat Scand. 2000;101:323–329. [PubMed] [Google Scholar]
- 29.Birchwood M., Smith J., Cochrane R., Wetton S., Copestake S. The social functioning scale. the development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. Br J Psychiatry. 1990;157:853–859. doi: 10.1192/bjp.157.6.853. [DOI] [PubMed] [Google Scholar]
- 30.Howes O.D., McCutcheon R., Agid O. Treatment-resistant schizophrenia: treatment response and resistance in psychosis (trrip) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry. 2017;174:216–229. doi: 10.1176/appi.ajp.2016.16050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corripio I., Sarro S., McKenna P.J. Clinical improvement in a treatment-resistant patient with schizophrenia treated with deep brain stimulation. Biol Psychiatry. 2016;80:e69–e70. doi: 10.1016/j.biopsych.2016.03.1049. [DOI] [PubMed] [Google Scholar]
- 32.Radua J., Schmidt A., Borgwardt S. Ventral striatal activation during reward processing in psychosis: a neurofunctional meta-analysis. JAMA Psychiatry. 2015;72:1243–1251. doi: 10.1001/jamapsychiatry.2015.2196. [DOI] [PubMed] [Google Scholar]
- 33.Bikovsky L., Hadar R., Soto-Montenegro M.L. Deep brain stimulation improves behavior and modulates neural circuits in a rodent model of schizophrenia. Exp Neurol. 2016;283:142–150. doi: 10.1016/j.expneurol.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voon V., Kubu C., Krack P., Houeto J.L., Troster A.I. Deep brain stimulation: neuropsychological and neuropsychiatric issues. Mov Disord. 2006;21(14):S305–S327. doi: 10.1002/mds.20963. Suppl. [DOI] [PubMed] [Google Scholar]
- 35.Breit S., Schulz J.B., Benabid A.L. Deep brain stimulation. Cell Tissue Res. 2004;318:275–288. doi: 10.1007/s00441-004-0936-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.