Abstract
Background
Meta-analyses of observational studies associate adherence to several dietary patterns with cognitive health. However, limited evidence from full scale, randomized controlled trials precludes causal inference regarding dietary effects on cognitive function.
Methods
The Women's Health Initiative (WHI) Dietary Modification (DM) randomized trial, in 48,835 postmenopausal women, included a subset of 1,606 WHI Memory Study (WHIMS) participants >= 65 years old, to assess low-fat dietary pattern influence on global cognitive function, evaluated with annual screening (Modified Mini–Mental State Examinations [3MSE]). Participants were randomized by a computerized, permuted block algorithm, stratified by age group and center, to a dietary intervention (40%) to reduce fat intake to 20% of energy and increase fruit, vegetable and grain intake or usual diet comparison groups (60%). The study outcome was possible cognition impairment (failed cognitive function screening) through the 8.5 year (median) dietary intervention. Those failing screening received a comprehensive, multi-phase cognitive function assessment to classify as: no cognitive impairment, mild cognitive impairment, or probable dementia. Exploratory analyses examined the composite endpoint of death after possible cognitive impairment through 18.7 years (median) follow-up. The WHI trials are registered at ClinicalTrials.gov:NCT00000611.
Findings
Among the 1,606 WHIMS participants, the dietary intervention statistically significantly reduced the incidence of possible cognitive impairment (n = 126; hazard ratio [HR] 0.59 95% confidence interval [CI] 0.38–0. 91, P = 0.01) with HR for dietary influence on subsequent mild cognitive impairment of 0.65 (95% CI 0.35–1.19) and HR of 0.63 (95% CI 0.19–2.10) for probable dementia (PD). Through 18.7 years, deaths from all-causes after possible cognitive impairment were non-significantly lower in the dietary intervention group (0.56% vs 0.77%, HR 0.83 95% CI 0.35 to 2.00, P = 0.16).
Interpretation
Adoption of a low-fat eating pattern, representing dietary moderation, significantly reduced risk of possible cognitive impairment in postmenopausal women.
Funding
Several Institutes of the US National Institutes of Health.
Keywords: Cognition, Dietary modification, Low-fat dietary pattern, Randomized clinical trial, Women's Health Initiative
Research in context.
Evidence before this study
Cognitive dysfunction and dementia represent major health risks in women as they age. Observational studies have associated several dietary patterns with cognitive health. However, few randomized trials have evaluated dietary influence on cognition. The 2010 NIH Report on Preventing Alzheimer's Disease and Cognitive Decline noted a critical need for randomized trials in this area and proposed criteria for such trials. To our review, the four trials meeting these criteria have limitations of short intervention durations and absence of long term follow-up. Against this background, we provide findings from a large (n = 1606) subgroup of postmenopausal women participating in the Women's Health Initiative (WH)I Memory Study (WHIMS) who were among the 48,835 women in the WHI Dietary Modification (DM) trial evaluating the potential influence of a low-fat dietary pattern on cognitive impairment.
Added value of this study
WHIMS participants, between 65 and 79 years old, were randomized to dietary intervention with a target of fat intake reduction and increase in fruits, vegetables and grains or usual diet comparison groups. Annual cognitive function screening through 8.5 years (median) intervention was based on Modified Mini-Mental Examinations [3MSE]. The study endpoint was possible cognitive impairment (failed 3MSE screening). Those with failed screening received a comprehensive, multi-phase neuropsychological evaluation and were followed for clinical outcome. In contrast to the comparison group, women in the dietary intervention group had a significantly decreased risk of possible cognitive impairment (hazard ratio [HR] 0.59 95% confidence interval [CI] 0.38–0.91, P = 0.01). Through 18.7 years, deaths from all-causes after possible cognitive impairment were non-significantly lower in the dietary intervention group (0.56% vs 0.77%, HR 0.83 95% CI 0.35 to 2.00, P = 0.16).
Implications of the available evidence
Our current findings provide prospective, randomized clinical trial evidence that adoption of a low-fat dietary pattern, representing dietary moderation, significantly reduced risk of possible cognitive impairment in postmenopausal women. Our study design, duration of the dietary intervention and length of follow-up, substantially strengthens evidence that a dietary pattern can influence cognitive function and identifies fat intake reduction as a component of the dietary pattern.
Alt-text: Unlabelled box
1. Introduction
Late-age development of cognitive impairment and dementia represent major health risks, especially in women where about two thirds of cases are seen [1]. The World Health Organization (WHO) and others have emphasized the importance of development of prevention strategies to most effectively address this problem [2,3] while noting the critical need for randomized clinical trial evidence in this area [4,5].
The potential association between various components of diet and cognitive function have been of interest [6]. In pre-clinical models, high-fat diets have hindered rodent memory performance and injured hippocampal neurons [7,8]. High-energy and high fat intake has been associated with cognitive deficits and dementia in some, but not all, observational studies [9], [10], [11], [12]. However, a recent meta-analysis of prospective cohort studies found significant evidence of positive association between higher saturated fat intake and dementia risk [12]. The influence of a low-fat dietary pattern on global cognitive function has not previously been evaluated in a full scale, long term, randomized clinical trial setting.
The WHI DM trial provides an opportunity to evaluate whether a low-fat dietary pattern influences global cognitive function in a randomized, controlled clinical trial setting in a subset of 1606 study participants also enrolled in the Women's Health Initiative Memory Study (WHIMS) [13], where annual assessment of global cognitive assessment was performed through 8.5 years of the dietary intervention. Exploratory analyses examine subsequent risk of possible cognitive impairment followed by mortality (composite outcome) after cumulative 18.7 years (median) follow-up in WHIMS participants
2. Methods
Design details of the WHI DM trial have been described [14]. In the trial, 48,835 postmenopausal women between 50 and 79 years of age, were randomly assigned from 1993 to1998, to a dietary intervention group (40%; n = 19,541) designed to implement a low-fat dietary pattern with the primary goal of reducing dietary fat intake to 20% of energy while increasing intake of fruits, vegetables and grains or to a usual diet comparison group (60%; n = 29,294) [15]. Randomization was conducted at the WHI Clinical Coordinating Center. The protocol-specified co-primary endpoints were invasive breast cancer and colorectal cancer. Of these, 1606 study participants were also enrolled in WHIMS [13], an ancillary study to the two WHI clinical trials evaluating hormone therapy [14] where annual assessment of global cognitive function was performed during the 8.5 year (median) dietary intervention period. The WHI trials are registered at ClinicalTrials.gov:NCT00000611. Institutional Review Board (IRB) approval for each trial component was obtained at all the clinical centres and all participants provided written informed consent. The WHI Project Office at the US National Heart, Lung, and Blood Institute (NHLBI), the sponsor of this project, had no role in the preparation of this report.
For WHIMS participation, women had to be active in one of the WHI hormone therapy trials assessing estrogen plus progestin or estrogen alone, be 65 to 79 years of age, free of dementia, willing to undergo annual cognitive assessments, and willing to identify a friend or family member who could provide information about her functioning.
Between 1996 and 2007, WHIMS participants were tested at enrollment and annually thereafter by centrally trained, certified examiners, blinded to randomization status, for global cognitive functioning using the Modified Mini–Mental State Examination (3MSE) [16]. A detailed description of the WHIMS comprehensive multi-phase protocol for detecting probable dementia and mild cognitive impairment has been published [13]. Briefly, the test items measured temporal and spatial orientation, immediate and delayed recall, executive function, abstract reasoning, praxis, writing, and visuoconstructional abilities (copying). The 3MSE has demonstrated good sensitivity and specificity for detecting cognitive impairment [17,18]. After initial cognitive screening with the 3MSE, women that scored below an education-adjusted cut point [16,19] were identified as having possible cognitive impairment (considered as being cognitively vulnerable). Prior study has demonstrated ability of such screening tools to identify individuals with cognitive impairment [20].
Those with possible cognitive impairment received a comprehensive multi-phase evaluation which included administration of a comprehensive and validated neuropsychological battery [21], questionnaires regarding acquired cognitive and behavioral deficits and neuropsychiatric symptoms [22] and standardized neuropsychiatric evaluation by experienced local clinical experts (commonly neurologists, geriatricians or psychiatrists), who then reviewed this information and classified the women into one of three categories – no cognitive impairment, mild cognitive impairment or probable dementia [19,23]. For those classified as probable dementia, brain computed tomography and laboratory blood tests were used to rule out possible reversible causes. Final study classification of mild cognitive impairment or probable dementia was adjudicated at the WHIMS Clinical Coordinating Center at the Wake Forest University School of Medicine by a panel of dementia experts that included two neurologists, a geriatric psychiatrist and a geriatric psychologist. A supplemental case ascertainment protocol was developed and used to ascertain outcomes for deceased and/or proxy- dependent participants [24].
Dietary intervention group sessions were led by specially trained and certified nutritionists with 18 group sessions in year one and subsequent quarterly maintenance group sessions throughout the 8.5 year (median) dietary intervention period. Subsequently, all contact with study nutritionists ended as post-intervention follow-up began. During the intervention period, the percent reduction in energy from fat (8–10% decrease) and the increase in servings of fruits and vegetables and servings of grain were all statistically significant (P < 0.001) [15]. Although caloric restriction or weight loss were not intervention targets, an early statistically significant weight loss (3%) emerged in the intervention group.
The primary study analysis was dietary modification influence on global cognitive function (failed cognitive function screening as possible cognitive impairment) in WHIMS participants through the protocol defined 8.5 year dietary intervention period. Also, in an exploratory analysis, we examined deaths from all-causes after possible cognitive impairment in WHIMS participants through 18.7 years (median) cumulative follow-up.
Cumulative follow-up (intervention plus post-intervention phases) included deaths through December 31, 2016 (median [Q1-Q3], 18.7 [14.6–19.5] years cumulatively) with mortality ascertained by regular surveillance of the cohort with deaths documented by through the National Death Index (NDI) and by Information reports of next of kin or the postal service. NDI searches were conducted at nine time points before 2018 for all participants with unknown vital status.
2.1. Statistical analysis
The current study analyses were not protocol pre-specified. The primary endpoint of possible cognitive impairment and the exploratory composite endpoint of death from all causes after possible cognitive impairment in WHIMS participants were assessed by randomly assigned DM intervention group by dividing the number of events by the corresponding person-time elapsed from randomization in each period. HRs contrasting the intervention and comparison groups by diagnosis of possible cognitive impairment (no cognitive impairment/mild cognitive impairment/probable dementia) were estimated using competing risk models that included cause-specific baseline hazard functions and hazard ratios [25]. Cumulative hazard curves were generated, and HRs and 95% CIs and P values computed using Cox regression models stratified on age group in 5-year categories, race/ethnicity, education, and randomization status in the WHI hormone therapy trials. The endpoint of possible cognitive impairment was assessed through 8.5 years follow-up during the dietary intervention period when annual assessment of global cognitive function was available. WHIMS participants contributed follow-up time until the end of DM intervention phase, date of possible cognitive impairment, loss to follow-up, or death, whichever came first. The composite endpoint of death after possible cognitive impairment was assessed from randomization through the available 18.7 year (median) follow-up period, where participants without possible cognitive impairment were censored at the end of the intervention phase or death (whichever came first), since they were no longer at risk for the composite endpoint, while those with an impairment contributed follow-up time until death or December 31, 2016 (Fig. 1), the last date covered by the NDI linkage. This type of composite endpoint has been used elsewhere in the context of breast cancer progression [26], [27], [28], described thoroughly [29], utilize usual indicator variables for event status (1 = time to composite event was observed; 0 = observation was censored), and can be fit with the usual Cox regression software implementations.
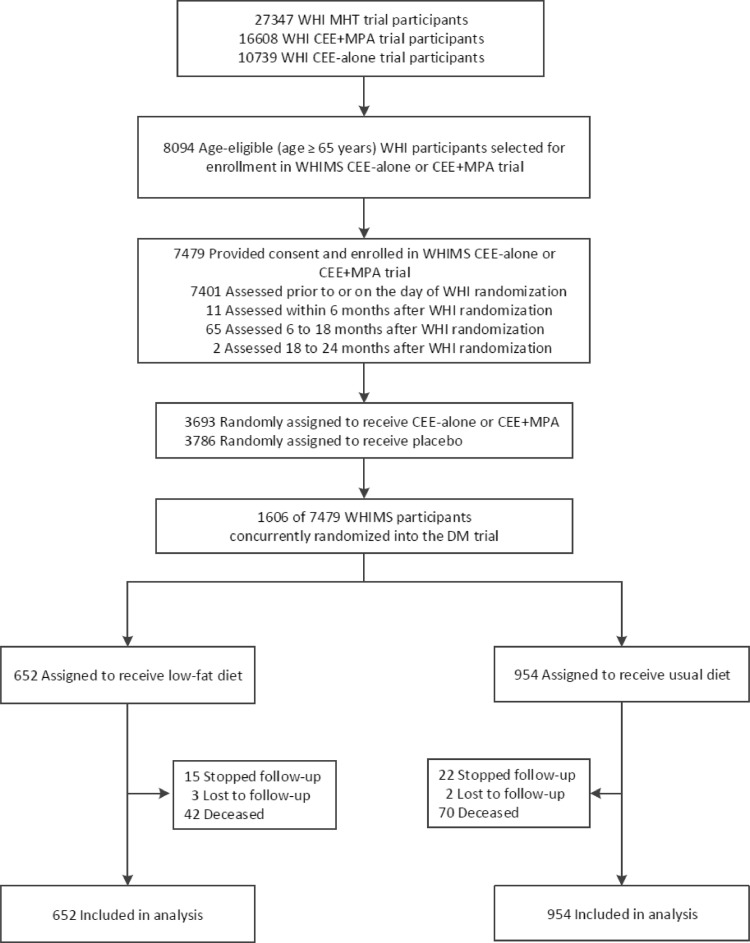
Fig. 1.
Participant flow diagram for the low-fat dietary intervention phase among the subset of DM trial participants that participated in WHIMS (n = 1606), an age eligible subset (age ≥ 65 years) of the WHI MHT trials. WHIMS = Women's Health Initiative Memory Study; MHT = menopausal hormone therapy; CEE = conjugated equine estrogen; MPA = medroxyprogesterone acetate.
All statistical tests are 2-sided and nominal P values of 0.05 or less are regarded as statistically significant. P-values do not adjust for multiple outcomes. All statistical analyses were conducted using SAS software version 9.4 (SAS Institute Inc.) and R software version 3.4 (R Foundation for Statistical Computing). As dietary intervention effects were examined in 11 subgroup analyses, less than one statistically significant interaction was expected by chance alone (≤ 11 × 0.05) [30].
3. Results
Baseline characteristics of WHIMS participants, including demographic, medical history, and dietary variables were well balanced between randomization groups (Table 1). Briefly, women were, on average, 69.9 years of age, somewhat overweight with median BMI of 29.5 kg/m2 and 29% had a college degree or higher. Characteristics associated with risk of cognitive impairment and dementia including age, education, smoking status, stroke history, diabetes, hormone use, waist circumference, blood pressure, body mass index, and aspirin use were also well-balanced between the two groups. Participant flow through cumulative follow-up is outlined in Fig. 1. After one year of dietary intervention, changes in the 1606 participants with cognitive function assessment reflected findings in the overall group, namely statistically significant reduction in percent calories fat, and increasing from and vegetable intake and, although not protocol target decreased weight and waist circumference (Table 2).
Table 1.
Baseline characteristics of DM trial participants that participated in WHIMS (n = 1606).
| No. and% of Participants1 |
||||
|---|---|---|---|---|
| Intervention (N = 652) | Comparison (N = 954) | |||
| Characteristic | ||||
| Age at screening (y), mean, SD | 69.9 | 3.7 | 69.9 | 3.6 |
| Age group at screening, y | ||||
| 60–69 | 343 | 52.6 | 501 | 52.5 |
| 70–79 | 309 | 47.4 | 453 | 47.5 |
| Race/ethnicity | ||||
| White | 557 | 85.4 | 819 | 85.8 |
| Black | 53 | 8.1 | 90 | 9.4 |
| Hispanic | 25 | 3.8 | 20 | 2.1 |
| American Indian | 0 | 0.0 | 3 | 0.3 |
| Asian/Pacific Islander | 6 | 0.9 | 10 | 1.0 |
| Unknown | 11 | 1.7 | 12 | 1.3 |
| Education | ||||
| < High school | 51 | 7.8 | 67 | 7.0 |
| High school diploma/GED | 134 | 20.6 | 221 | 23.2 |
| School after high school | 266 | 40.8 | 400 | 41.9 |
| College degree or higher | 201 | 30.8 | 266 | 27.9 |
| Smoking | ||||
| Never | 346 | 53.9 | 540 | 57.1 |
| Past | 254 | 39.6 | 354 | 37.5 |
| Current | 42 | 6.5 | 51 | 5.4 |
| Stroke | 9 | 1.4 | 18 | 1.9 |
| Diabetes | 61 | 9.4 | 93 | 9.7 |
| Hormone use | ||||
| Never | 437 | 67.0 | 662 | 69.4 |
| Past | 169 | 25.9 | 233 | 24.4 |
| Current | 46 | 7.1 | 59 | 6.2 |
| Waist circumference (cm), mean, SD | 92.0 | 13.2 | 92.7 | 13.0 |
| Systolic BP (mm Hg), mean, SD | 132.8 | 17.3 | 133.3 | 17.9 |
| Diastolic BP (mm Hg), mean, SD | 75.3 | 9.0 | 76.0 | 8.9 |
| Body mass index2, median (Q1-Q3), IQR | 29.3 | 25.7–33.4 | 29.8 | 26.3–33.8 |
| 3MSE score, median (Q1-Q3), IQR | 96.0 | 94.0–98.0 | 96.0 | 93.0–98.0 |
| Hypertensive (Self-report or high BP) | 329 | 50.6 | 538 | 56.7 |
| Aspirin use (≥ 80 mg for ≥30 days) | 136 | 20.9 | 233 | 24.4 |
| MHT randomization group | ||||
| CEE-alone | 119 | 18.3 | 218 | 22.9 |
| CEE-alone placebo | 126 | 19.3 | 218 | 22.9 |
| CEE+MPA | 188 | 28.8 | 265 | 27.8 |
| CEE+MPA placebo | 219 | 33.6 | 253 | 26.5 |
| Percent energy from total fat (%), mean, SD | 37.8 | 5.0 | 38.3 | 5.3 |
| Percent energy from protein (%), mean, SD | 16.4 | 3.0 | 16.5 | 3.0 |
| Percent energy from carbohydrates (%), mean, SD | 45.9 | 6.3 | 45.1 | 6.4 |
| Fruit and vegetable intake (med serv/day), mean, SD | 3.8 | 2.0 | 3.7 | 1.9 |
Values are reported as No. and% unless otherwise indicated.
Calculated as weight in kilograms divided by height in meters squared.
Table 2.
Change characteristics of DM trial participants that participated in WHIMS (n = 1606).
| Intervention (N = 652) |
Comparison (N = 954) |
||||
|---|---|---|---|---|---|
| Post-randomization variables | Mean | SD | Mean | SD | P-Value |
| Change in percent energy from: | |||||
| Total fat (%) | −12.3 | 7.8 | −1.6 | 6.2 | <0.001 |
| Saturated fat (%) | −4.3 | 3.3 | −0.6 | 2.6 | <0.001 |
| Polyunsaturated fat (%) | −2.3 | 2.1 | −0.2 | 2.1 | <0.001 |
| Monounsaturated fat (%) | −4.9 | 3.3 | −0.6 | 2.8 | <0.001 |
| Trans-fat (%) | −1.0 | 1.2 | −0.1 | 1.2 | <0.001 |
| Carbohydrates (%) | 11.7 | 9.0 | 1.6 | 6.9 | <0.001 |
| Protein (%) | 1.3 | 3.2 | 0.1 | 2.9 | <0.001 |
| Animal protein (%) | 0.3 | 3.6 | 0.0 | 3.1 | 0.12 |
| Vegetable protein (%) | 1.0 | 1.2 | 0.1 | 1.0 | <0.001 |
| Alcohol (%) | 0.1 | 2.4 | 0.1 | 2.1 | 0.83 |
| Change in other dietary characteristics: | |||||
| Vegetable and fruit (med serv/day) | 1.3 | 2.3 | 0.2 | 1.8 | <0.001 |
| Grains (med serv/day) | 0.0 | 2.6 | −0.6 | 2.3 | <0.001 |
| Dietary fiber (g) | 2.2 | 6.9 | −0.5 | 5.7 | <0.001 |
| Cholesterol (mg) | −92.1 | 147.7 | −26.5 | 132.4 | <0.001 |
| Change in participant characteristics: | |||||
| Weight (kg) | −2.1 | 11.9 | 0.1 | 10.0 | <0.001 |
| Waist (cm) | −1.7 | 5.8 | −0.4 | 6.1 | <0.001 |
| Systolic BP (mm Hg) | −3.6 | 15.0 | −2.1 | 17.7 | 0.10 |
| Diastolic BP (mm Hg) | −2.8 | 8.7 | −2.0 | 9.2 | 0.07 |
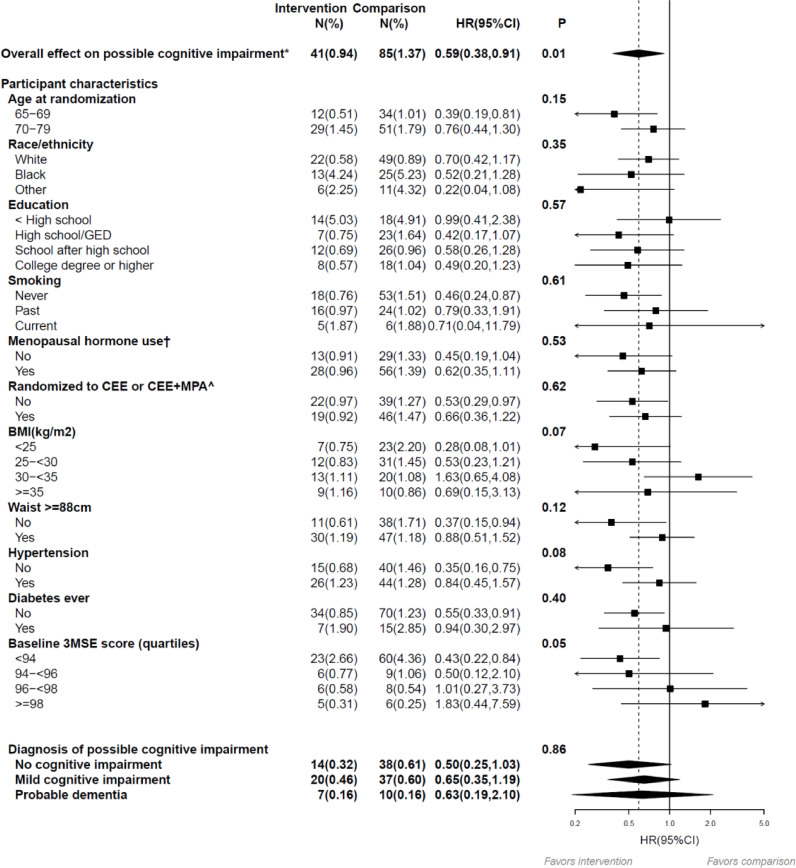
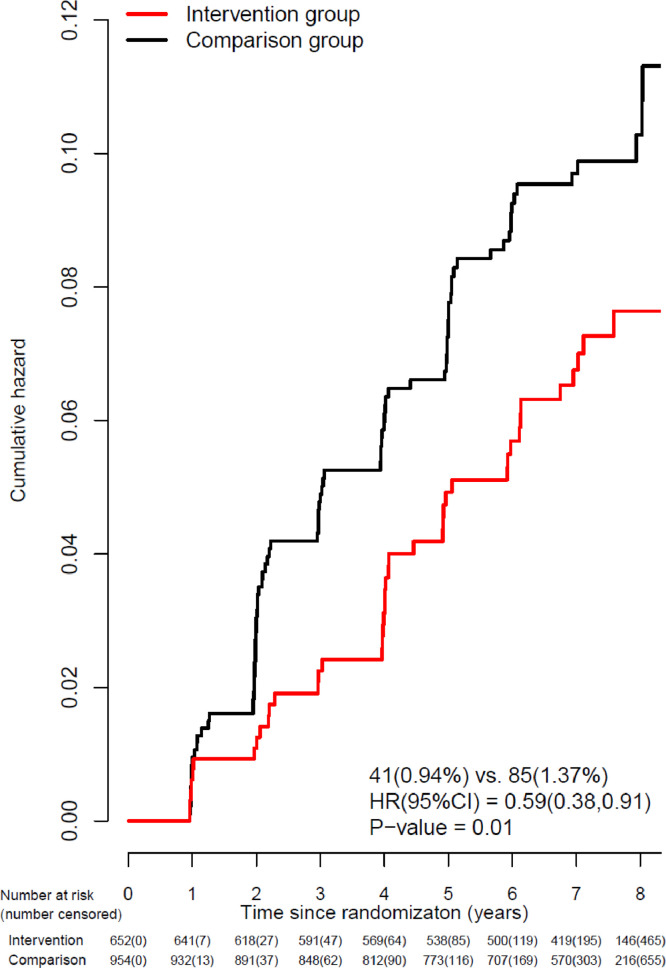
At entry, the distribution of baseline 3MSE scores (0 worst to 100 best) was notably left (negative)-skewed with median (Q1-Q3) = 96.0 (94.0, 98.0), but well balanced between groups. During median (Q1-Q3) follow-up of 7.2 (6.0–8 0) years following WHIMS enrollment, while annual global cognitive function assessment was ongoing during the dietary intervention period, there were 126 incident cases of possible cognitive impairment identified with 41 (0.94%) in the intervention group and 85 (1.37%) in the comparison group; the dietary intervention resulted in a statistically significant reduction in the incidence of possible cognitive impairment with a HR 0.59 (95%CI 0.38–0.91, P = 0.01) (Fig. 2).
Fig. 2.
Overall influence of the low-fat dietary intervention on possible cognitive impairment* during the intervention phase among the subset of DM trial participants that participated in WHIMS (n = 1606). The 7-year follow-up is over the dietary intervention phase of the trial (median, 7.2 [interquartile range {IQR}, 6.0–8.0] years). Summary statistics computed from Cox regression models stratified by 5-year age group, race/ethnicity, education, and randomization status in the WHI hormone therapy trials, using time from randomization as the time-scale. Subgroups were investigated, one at a time, by including an interaction term between randomization arm and subgroup with additional stratification of the baseline hazard by corresponding subgroup. P values corresponds to a two-sided score (log-rank) test of the dietary intervention's overall influence, or for the subgroup analysis, a test of interaction between the randomization group and corresponding subgroup. Percentages are annualized. * Possible cognitive impairment defined as transition to Phase 2 of the WHIMS protocol. After completion of Phases 2 and 3, typically within 3 months possible cognitive impairment, participants were classified as probable dementia, mild cognitive impairment, or no cognitive impairment. † Ever used menopausal hormone therapy or randomized to CEE or CEE+MPA arm of WHI hormone trial. ^ Among women randomized to either WHI hormone therapy trial. WHIMS = Women's Health Initiative Memory Study; HR = hazard ratio; CI = confidence interval.
Kaplan-Meier estimates of the cumulative hazard curves for possible cognitive impairment during the intervention period are depicted in Fig. 3. As seen, in the comparison group, there is a year-to-year increase in the risk of exceeding the target threshold for cognitive performance in 3MSE resulting in a designation of possible cognitive impairment of about 1.5% per year, with early separation (by year 2) with lower risk seen in the intervention group. Of the 126 participants identified with possible cognitive function impairment, after further comprehensive neurological assessment, 59% were categorized as having mild cognitive impairment or probable dementia; for findings in the dietary intervention versus comparison groups, HR was 0.65 (95% CI 0.35–1.19) for mild cognitive impairment and HR of 0.63 (95% CI 0.19–2.10) for probable dementia (bottom panel of Fig. 2) while the remaining women were categorized as having no cognitive impairment. Combining confirmation of mild cognitive impairment or probable dementia (27 v 47 events) yields an HR of 0.65 (95%CI 0.38–1.11).
Fig. 3.
Kaplan-Meier estimates of the cumulative hazard for possible cognitive impairment* during the intervention phase among the subset of DM trial participants that participated in WHIMS (n = 1606). The 7-year follow-up is over the dietary intervention phase of the trial (median, 7.2 [interquartile range {IQR}, 6.0–8.0] years). Summary statistics computed from a Cox regression model stratified by 5-year age group, race/ethnicity, education, and randomization status in the WHI hormone therapy trials, using time from randomization as the time-scale. P-value corresponds to a two-sided score (log-rank) test of the dietary intervention's overall influence. Percentages are annualized. * Possible cognitive impairment defined as transition to Phase 2 of the WHIMS protocol. After completion of Phases 2 and 3, typically within 3 months of possible cognitive impairment, participants were classified as probable dementia, mild cognitive impairment, or no cognitive impairment. WHIMS = Women's Health Initiative Memory Study; HR = hazard ratio; CI = confidence interval.
The dietary influence on possible cognitive impairment was most evident among women in the lowest quartile of 3MSE score at WHIMS enrollment, identifying those with lower global cognitive function (HR 0.43 95% CI 0.22–0.84) as the influence diminished with increasing 3MSE score (P interaction = 0.05) (Fig. 2). This result was explored further by assessing the dietary influence on year-to-year mean change in 3MSE score as a measure of global cognitive function stratified by baseline 3MSE quartiles. Although the overall influence of the dietary intervention on 3MSE change score was not significant, mean (95% CI) = 0.1 (−0.2 to 0.4) with P = 0.47, there was a significant interaction between randomization group and baseline 3MSE score (quartile; P = 0.02, based on test of linear-trend of group means (Supplemental Figure). Specifically, dietary intervention resulted in an increase in 3MSE score beginning in year two among women with low baseline 3MSE score corroborating the favorable effect of dietary intervention seen on possible cognitive impairment in the same low 3MSE score subgroup; mean change score during follow-up was mean (95% CI) = 0.7(0.1 to 1.3). In contrast, the mean change scores for increasing quartiles did not suggest a difference; mean (95% CI) = 0.2(−0.5, 0.9), −0.3(−0.9, 0.3) and −0.04(−0.5, 0.4), respectively. Suggestions of a heterogeneous influence for diet was not observed in any of the remaining subgroups explored (Fig. 2); none of the corresponding interactions were statistically significant.
The clinical significance of screening positive for possible cognitive impairment was examined for subsequent mortality risk (death from all causes) through 18.7 years median follow-up by cognitive function category: normal (never failed 3MSE screening, n = 1480), no cognitive impairment (but failed screening as possible cognitive impairment, n = 52); mild cognitive impairment (n = 57); and probable dementia (n = 17). Subsequent mortality by group was 46%, 58%, 61%, and 100%, respectively. Thus, the designation of possible cognitive impairment likely identifies a population of clinical relevance. Exploratory analyses found deaths from all-causes after possible cognitive impairment was non-significantly lower for the intervention versus comparison group (0.56% vs 0.77%, HR 0.83 95% CI 0.35 to 2.00, P = 0.16).
4. Discussion
In the WHI Dietary Modification randomized clinical trial evaluating a low-fat dietary pattern, in a subgroup of 1606 postmenopausal women also enrolled in the WHI Memory Study (WHIMS) having annual assessment of global cognitive function, adoption of a low-fat dietary pattern significantly reduced the risk of possible cognitive impairment based on the findings from serial 3MSE results. Subsequent neurological assessment of those with possible cognitive impairment diagnosed 59% with either mild cognitive impairment (45%) or probable dementia (14%). As exploratory analyses were suggestive of a higher mortality risk in women with possible cognitive impairment, even in the absence of mild cognitive impairment or probable dementia, compared to women without such findings, those failing 3MSE screening likely identifies a population of clinical relevance.
The significant reduction in possible cognitive impairment (HR 0.59 95% CI 0.38–0.91, P = 0.01) in the dietary intervention group was followed by a smaller non-significant reduction in deaths after possible impairment (HR = 0.83), suggest that a post-impairment survival benefit is unlikely [29]. These results are consistent with the hypothesis offered by Feart and colleagues [31] in response to their cohort study finding that higher Mediterranean diet adherence was associated with slower cognitive decline but not with dementia. They suggested that the Mediterranean diet may have a beneficial effect during the prodromal phase of dementia, rather than the last few years preceding diagnosis, after which pathophysiological processes cannot be reversed by diet. Such findings suggest lifestyle interventions may have most effect when begun by individuals without cognitive impairment.
The favorable dietary effect of reducing the incidence of possible cognitive impairment was greater in women with lower, less favorable, baseline 3MSE scores, a finding supported by serial year-to-year analyses of global cognitive function where a statistically significant interaction suggests more favorable diet effect in the group with lowest baseline 3MSE scores. Comparatively less favorable results among women having higher baseline 3MSE scores may represent a limitation of the 3MSE assessment. For example, the top quartile of women have scores ≥ 98 leaving little/no room for improvement against an upper 3MSE score limit of 100. Subgroup analyses should be interpreted with caution because of multiple comparisons.
Review of observational study findings has associated higher adherence to the Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean -DASH Intervention for Neuro- degenerative Delay (MIND) diets with less cognitive decline [32,33]. However, few randomized trials have evaluated nutrition based interventions in relation to cognition and most have been limited in size and duration [6].
The 2010 National Institutes of Health Evidence Report on Preventing Alzheimer's Disease and Cognitive Decline [34] proposed criteria for future randomized clinical trials which included intervention duration of at least one year and size of ≥500 participants. Using these criteria, we identified four prior randomized trials assessing a dietary intervention with or without other intervention components with design and findings outlined in Table 3. Three [35], [36], [37] of four trials entered participants with cognitive dysfunction or those at high risk for cognitive dysfunction or vascular disease. Intervention durations were 1 year [38], 2 years [36], 3 years [37] and 6.5 years [35]. Each of the four trials used different cognitive function endpoints. All presented baseline and final endpoint assessments precluding determination of trajectory of cognitive dysfunction over time. None of the trials evaluated a low-fat eating pattern.
Table 3.
Randomized trials of dietary/multidomain interventions and cognition.
| Name | Eligibility | N | Intervention | Duration | Endpoint | Outcome | |
|---|---|---|---|---|---|---|---|
| FINGER Lance 2015t | FINnish GERriatric Intervention Study to Prevent Cognitive Impairment and Disability | 60–70 years old Cardiovascular Risk Factors Aging and Dementia score (CAIDE) ≥ 6 points |
1260 46% Female |
Diet, exercise, cognitive training, vascular risk monitoring versus control | 2 years | Neuropsychological test battery (NTB) Z score |
NTB score higher in Intervention P = 0.030 |
| MAPT Lancet Neurology 2017 |
Multidomain Alzheimer Prevention Trial |
70+ years old 1) Memory complaints 2) Limitations in ADL or 3) Slow gait |
1525 65% Female |
4 arms, Multidomain (MD) +placebo; Omega 3 FA alone; MD + Omega 3 FA; placebo |
3 years | Composite Z score Combining 4 cognitive tests |
No significant effect on cognitive decline compared to placebo |
| NU-AGE Diet Frontier in Physiology 2018 |
New dietary strategies addressing the specific needs of the elderly population for healthy ageing in Europe | 67–79 years old Free of dementia relatively healthy older adults |
1279 100% Female |
NU-AGE DIET, Medi, like versus usual diet control |
1 year | Cognitive function Mini-Mental State Examination (MMSE) Plus CERAD score |
No overall difference Higher adheres Improvement in global function |
| PREDIMED-NAVARRA J Neurol Neurosurg Psychiatry 2013 |
Prevención con Dieta Mediterránea | Age 75 years (mean) High vascular risk |
522 55% Female |
3 arms, 2 MedDiets ± extra-virgin olive oil or mixed nuts versus “low fat” control** |
6.5 years | Mini-Mental State Examination (MMSE) plus Clock Drawing Test (CDT) |
MedDiets higher than control MMSE, P = 0.005 CDT, P = 0.001 |
Two of the four trials had positive findings. The FINish GERiatric Intervention Study (FINGER) was a mutidomain intervention (diet, exercise, cognitive training, and vascular risk monitoring) involving 1260 participants. The dietary component included recommendations for 10–20% of energy from protein, 25–35% of energy from fat with targets for specific fat types and energy intake reduction facilitating 5–10% body weight reduction if needed. The intervention resulted in improved or maintained cognition over the two year study duration [36]. The second study with positive findings presents a subsample report from one of 12 recruitment centres of the PREvencion con Dieta MEDiterranea (PREDIMED) where effects of a Mediterranean diet influence on cognitive function diet was assessed against a “low fat” control diet with 522 participants randomized across three interventions. As no decrease in dietary fat intake was reported in the “low fat” group, PREDIMED operationally had a usual diet control. In any event, a Mediterranean diet enhanced with either extra-virgin olive oil or nuts appeared to improve cognition compared to the control condition [35]. Of these larger randomized trials, three of four entered participants at cognitive function risk while three of four reported findings after interventions of three years or less.
The current report adds to the emerging evidence that dietary intake can influence cognitive function in a randomized trial involving relatively healthy postmenopausal women not selected for cognitive risk and followed over a long period. In this setting, findings from the WHI DM trial indicate that dietary fat intake reduction may also be a factor to be incorporated in future dietary recommendations.
The WHI DM low-fat dietary plan is somewhat similar to the Dietary Approach to Stopping Hypertension (DASH) which includes recommendations to increase fruits, vegetables and grains, use no/low fat dairy products, and reduce total fat intake [39]. While the WHI DM low-fat dietary plan does provide more emphasis on total fat reduction than DASH, it can still be best described as one of dietary moderation not requiring extreme change.
The WHI DM finding of a favorable effect of a low- fat dietary pattern on possible cognitive impairment is also similar in many respects to, and supportive of, the most recent World Health Organization (WHO) Guidelines for Risk reduction of Cognitive Decline and Dementia [40]. Their recommendation for a healthy diet includes five portions of fruits, vegetables and whole grains a day and less than 30% of total energy intake from fats. Additionally, the guideline specifically recommends the Mediterranean diet as “more effective than usual care in reducing risk/progression of cognitive decline and/or dementia” [40].
The dietary changes of the WHIMS subset in the dietary intervention reflect those achieved by women in the entire dietary intervention group. After one year, the percent energy from fat was 24.3% (mean [SD] 7.5%) [15] and remained statistically significantly different than the control group throughout the 8.5 year intervention where, at final assessment, level was 29.8% [8.3] [41]. These levels are not much different from the average US intake of postmenopausal women of 33%. While statistically significant, the increase in fruit and vegetable intake was modest with an increase from about four to five servings per day for each and achieved WHO nutritional recommendations. Although not an intervention target, a statistically significant weight loss difference of between 1.9 to 0.4 kg, compared to the control group, was maintained throughout the dietary intervention period. The modest dietary changes of the WHI Dietary Modification trial should be easily achievable by most postmenopausal women.
Study strengths include the randomized design, a population of postmenopausal women, not selected for cognitive dysfunction, dietary program adherence supported by body weight and biomarker differences [15] carefully designed and implemented procedures assessing possible cognitive impairment, annual assessment of Global cognitive function providing information on trajectory of cognitive change, and a long follow-up period to support exploratory analyses regarding implications for mortality.
Limitations include those associated with secondary analyses. These analyses are exploratory and should be viewed as hypothesis-generating, requiring reassessment in prospectively conducted confirmatory trials. While mild cognitive impairment or probable dementia would have been a more meaningful endpoint, we were limited by available sample size. The parent cohort, WHIMS (n = 7479), was designed to have 80% power to detect an influence on all-cause dementia. In addition, this study relied on the 3MSE, while other instruments such as the Montreal Cognitive Assessment (MoCA) [42] may better detect mild cognitive impairment. The WHI low-fat dietary program reduced fat and commensurately increased fruit, vegetable and grain consumption, therefore the effects of these increases cannot be separated from the effects of reduced fat. Also, social participation and support are strongly connected to good health and well-being, so the sustained cognitive engagement by nutritionist led intervention sessions could have had some positive influence independent of dietary change. However, there is insufficient evidence regarding social activity and risk-reduction for risk of cognitive decline. As linkage with Medicare provides more comprehensive assessment of dementia outcomes [43], a future analysis will examine the low-fat dietary pattern influence on dementia incidence and dementia mortality, in all 48,835 study participants.
As diabetes [44] and the metabolic syndrome have been associated with higher dementia risk [45], the favorable WHI dietary intervention effects on the course of diabetes [41], hypertension [46], other metabolic syndrome components [47] and weight management [48], represent potential mediators of the cognitive effects seen.
In summary, adoption of a low-fat dietary pattern, representing dietary moderation, significantly reduced risk of possible cognitive impairment in postmenopausal women. Subsequent mortality findings suggest the designation of possible cognitive impairment likely identifies a higher risk population of clinical relevance.
Women's Health Initiative (WHI) Data Sharing Statement:
| Item1 | WHI statement |
|---|---|
| Will individual participant data be available (including data dictionaries)? | Deidentified individual participant data is available. |
| What data in particular will be shared? | All of the deidentified participant data collected during the trial. |
| What other documents will be available? | Study protocol, study procedures, data collection forms and other documents. |
| When will data be available (start and end dates)? | Data is available through the WHI online resource, https://www.whi.org/researchers/data/Pages/Home.aspx, while the WHI remains funded2 and indefinitely through BioLINCC, https://biolincc.nhlbi.nih.gov/studies/whict/. |
| With whom? | Eligible researchers3 may download the data directly at the WHI online resource. Other researchers may download the publicly available data through BioLINCC, in accordance with NHLBI's BioLINCC guidelines. |
| For what types of analyses? | Eligible researchers3 with an approved specified purpose. Other researchers in accordance with NHLBI's BioLINCC guidelines. |
| By what mechanism will data be made available? | Data are available at the aforementioned links. |
1Items correspond to those specified in: Taichman, D.B., Sahni, P., Pinborg, A., Peiperl, L., Laine, C., James, A., Hong, S.T., Haileamlak, A., Gollogly, L., Godlee, F. and Frizelle, F.A., 2017. Data sharing statements for clinical trials: a requirement of the International Committee of Medical Journal Editors. Annals of internal medicine, 167(1), pp. 63–65.
2Currently through 2020.
3See https://www.whi.org/researchers/data/Pages/Home.aspx for eligibility.
Declaration of competing interest
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Chlebowski reported being a consultant for Novartis, AstraZeneca, Immunomedics, Amgen, Puma and Genentech.
Acknowledgments
Funding/support
The WHI program is supported by the National Heart, Lung and Blood Institute, National Institutes of Health, Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.
Program office
(National Heart, Lung, and Blood Institute, Bethesda, MD) Jacques Rossouw, Shari Ludlum, Dale Burden, Joan McGowan, Leslie Ford, and Nancy Geller.
Additional information
A full list of all the investigators who have contributed to Women's Health Initiative science appears at: https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Inv estigator%20Long%20List.pd.
Trial registration
Clinicaltrials.gov Identifier: NCT00000611.
The research protocol
Available at: https://www.whi.org/about/SitePages/Dietary%20Trial.aspx.
Role of the funding source
Representatives from National Heart, Lung and Blood Institute (NHLBI) had a role in the design and conduct of the study but played no role in data interpretation, writing of the report or the decision to submit for publication. RTC had full access to all study data and had final responsibility for the decision to submit for publication.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2019.100240.
Appendix. Supplementary materials
References
- 1.Prince M., Ali G.C., Guerchet M., Prina A.M., Albanese E., Wu Y.T. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther. 2016;8(1):23. doi: 10.1186/s13195-016-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization and Alzheimer's Disease International. Dementia: A Public Health Priority. Geneva: World Health Organization; 2012. pp. 92-93. http://www.who.int/mental_health/publications/dementia_report_2012/en/
- 3.Frankish H., Horton R. Prevention and management of dementia: a priority for public health. Lancet. 2017;390(10113):2614–2615. doi: 10.1016/S0140-6736(17)31756-7. [DOI] [PubMed] [Google Scholar]
- 4.Williams J.W., Plassman B.L., Burke J., Benjamin S. Preventing alzheimer's disease and cognitive decline. Evid Rep Technol Assess (Full Rep) 2010;193:1–727. [PMC free article] [PubMed] [Google Scholar]
- 5.Lindenberger U. Human cognitive aging: corriger la fortune? Science. 2014;346(6209):572–578. doi: 10.1126/science.1254403. [DOI] [PubMed] [Google Scholar]
- 6.Klimova B., Valis M. Nutritional interventions as beneficial strategies to delay cognitive decline in healthy older individuals. Nutrients. 2018;10(7) doi: 10.3390/nu10070905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wahl D., Coogan S.C., Solon-Biet S.M., de Cabo R., Haran J.B., Raubenheimer D. Cognitive and behavioral evaluation of nutritional interventions in rodent models of brain aging and dementia. Clin Interv Aging. 2017;12:1419–1428. doi: 10.2147/CIA.S145247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ledreux A., Wang X., Schultzberg M., Granholm A.C., Freeman L.R. Detrimental effects of a high fat/high cholesterol diet on memory and hippocampal markers in aged rats. Behav Brain Res. 2016;312:294–304. doi: 10.1016/j.bbr.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okereke O.I., Rosner B.A., Kim D.H., Kang J.H., Cook N.R., Manson J.E. Dietary fat types and 4-year cognitive change in community-dwelling older women. Ann Neurol. 2012;72(1):124–134. doi: 10.1002/ana.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnard N.D., Bunner A.E., Agarwal U. Saturated and trans fats and dementia: a systematic review. Neurobiol Aging. 2014;35 Suppl(2):S65–S73. doi: 10.1016/j.neurobiolaging.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 11.Beilharz J.E., Maniam J., Morris M.J. Diet-Induced cognitive deficits: the role of fat and sugar, potential mechanisms and nutritional interventions. Nutrients. 2015;7(8):6719–6738. doi: 10.3390/nu7085307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding B., Xiao R., Ma W., Zhao L., Bi Y., Zhang Y. The association between macronutrient intake and cognition in individuals aged under 65 in china: a cross-sectional study. BMJ Open. 2018;8(1) doi: 10.1136/bmjopen-2017-018573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shumaker S.A., Reboussin B.A., Espeland M.A., Rapp S.R., McBee W.L., Dailey M. The women's health initiative memory study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials. 1998;19(6):604–621. doi: 10.1016/s0197-2456(98)00038-5. [DOI] [PubMed] [Google Scholar]
- 14.Anderson G.L., Manson J., Wallace R., Lund B., Hall D., Davis S. Implementation of the women's health initiative study design. Ann Epidemiol. 2003;13(9 Suppl):S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 15.Prentice R.L., Caan B., Chlebowski R.T., Patterson R., Kuller L.H., Ockene J.K. Low-fat dietary pattern and risk of invasive breast cancer: the women's health initiative randomized controlled dietary modification trial. JAMA. 2006;295(6):629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 16.Shumaker S.A., Legault C., Rapp S.R., Thal L., Wallace R.B., Ockene J.K. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the women's health initiative memory study: a randomized controlled trial. JAMA. 2003;289(20):2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 17.Teng E.L.C.H., Gong A. Comparisons between the mini-mental state exam (MMSE) and its modified version: the 3MS test. In: Hasegawa K.H.A., editor. Psychogeriatrics biomedical and social advances. Excerpta Medica; Tokyo, Japan: 1990. pp. 189–192. [Google Scholar]
- 18.Bland R.C., Newman S.C. Mild dementia or cognitive impairment: the modified mini-mental state examination (3MS) as a screen for dementia. Canadian Journal of Psychiatry Revue Canadienne de Psychiatrie. 2001;46(6):506–510. doi: 10.1177/070674370104600604. [DOI] [PubMed] [Google Scholar]
- 19.Shumaker S.A., Legault C., Kuller L., Rapp S.R., Thal L., Lane D.S. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: women's health initiative memory study. JAMA. 2004;291(24):2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 20.Livingston G., Sommerlad A., Orgeta V., Costafreda S.G., Huntley J., Ames D. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 21.Morris J.C., Heyman A., Mohs R.C., Hughes J.P., van Belle G., Fillenbaum G. The consortium to establish a registry for alzheimer's disease (CERAD). part I. clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 22.Espeland M.A., Rapp S.R., Shumaker S.A., Brunner R., Manson J.E., Sherwin B.B. Conjugated equine estrogens and global cognitive function in postmenopausal women: women's health initiative memory study. JAMA. 2004;291(24):2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 23.Bowen J., Teri L., Kukull W., McCormick W., McCurry S.M., Larson E.B. Progression to dementia in patients with isolated memory loss. Lancet. 1997;349(9054):763–765. doi: 10.1016/S0140-6736(96)08256-6. [DOI] [PubMed] [Google Scholar]
- 24.Gaussoin S.A., Espeland M.A., Absher J., Howard B.V., Jones B.M., Rapp S.R. Ascertaining dementia-related outcomes for deceased or proxy-dependent participants: an overview of the women's health initiative memory study supplemental case ascertainment protocol. Int J Geriatr Psychiatry. 2012;27(2):205–214. doi: 10.1002/gps.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prentice R.L., Kalbfleisch J.D., Peterson A.V., Jr., Flournoy N., Farewell V.T., Breslow N.E. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34(4):541–554. [PubMed] [Google Scholar]
- 26.Chlebowski R.T., Anderson G.L., Gass M., Lane D.S., Aragaki A.K., Kuller L.H. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304(15):1684–1692. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson G.L., Chlebowski R.T., Aragaki A.K., Kuller L.H., Manson J.E., Gass M. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the women's health initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13(5):476–486. doi: 10.1016/S1470-2045(12)70075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chlebowski R.T., Aragaki A.K., Anderson G.L., Thomson C.A., Manson J.E., Simon M.S. Low-Fat dietary pattern and breast cancer mortality in the women's health initiative randomized controlled trial. J Clin Oncol: Off J Am Soc Clin Oncol. 2017;35(25):2919–2926. doi: 10.1200/JCO.2016.72.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cespedes Feliciano E.M., Prentice R.L., Aragaki A.K., Neuhouser M.L., Banack H.R., Kroenke C.H. Methodological considerations for disentangling a risk factor's influence on disease incidence versus postdiagnosis survival: the example of obesity and breast and colorectal cancer mortality in the women's health initiative. Int J Cancer. 2017;141(11):2281–2290. doi: 10.1002/ijc.30931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang R., Lagakos S.W., Ware J.H., Hunter D.J., Drazen J.M. Statistics in medicine — reporting of subgroup analyses in clinical trials. New Engl J Med. 2007;357(21):2189–2194. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 31.Feart C., Samieri C., Rondeau V., Amieva H., Portet F., Dartigues J.F. Adherence to a mediterranean diet, cognitive decline, and risk of dementia. JAMA. 2009;302(6):638–648. doi: 10.1001/jama.2009.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Brink A.C., Brouwer-Brolsma E.M., Berendsen A.A.M., van de Rest O. The mediterranean, dietary approaches to stop hypertension (DASH), and mediterranean-dash intervention for neurodegenerative delay (MIND) diets are associated with less cognitive decline and a lower risk of alzheimer's disease-a review. Adv Nutr (Bethesda, MD) 2019 doi: 10.1093/advances/nmz054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X., Maguire B., Brodaty H., O'Leary F. Dietary patterns and cognitive health in older adults: a systematic review. J Alzheimer's Dis: JAD. 2019;67(2):583–619. doi: 10.3233/JAD-180468. [DOI] [PubMed] [Google Scholar]
- 34.Daviglus M.L., Bell C.C., Berrettini W., Bowen P.E., Connolly E.S., Jr., Cox N.J. National institutes of health state-of-the-science conference statement: preventing alzheimer disease and cognitive decline. Ann Intern Med. 2010;153(3):176–181. doi: 10.7326/0003-4819-153-3-201008030-00260. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Lapiscina E.H., Clavero P., Toledo E., Estruch R., Salas-Salvado J., San Julian B. Mediterranean diet improves cognition: the predimed-navarra randomised trial. J. Neurol. Neurosurg. Psychiatr. 2013;84(12):1318–1325. doi: 10.1136/jnnp-2012-304792. [DOI] [PubMed] [Google Scholar]
- 36.Ngandu T., Lehtisalo J., Solomon A., Levalahti E., Ahtiluoto S., Antikainen R. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 37.Andrieu S., Guyonnet S., Coley N., Cantet C., Bonnefoy M., Bordes S. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 2017;16(5):377–389. doi: 10.1016/S1474-4422(17)30040-6. [DOI] [PubMed] [Google Scholar]
- 38.Marseglia A., Xu W., Fratiglioni L., Fabbri C., Berendsen A.A.M., Bialecka-Debek A. Effect of the nu-age diet on cognitive functioning in older adults: a randomized controlled trial. Front Physiol. 2018;9:349. doi: 10.3389/fphys.2018.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sacks F.M., Svetkey L.P., Vollmer W.M., Appel L.J., Bray G.A., Harsha D. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. DASH-Sodium collaborative research group. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 40.WHO Guidelines Approved by the Guidelines Review Committee . Vol. 2019. World Health Organization (c) World Health Organization; Geneva: 2019. (Risk reduction of cognitive decline and dementia: who guidelines). [PubMed] [Google Scholar]
- 41.Howard B.V., Aragaki A.K., Tinker L.F., Allison M., Hingle M.D., Johnson K.C. A low-fat dietary pattern and diabetes: a secondary analysis from the women's health initiative dietary modification trial. Diabet Care. 2018;41(4):680–687. doi: 10.2337/dc17-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nasreddine Z.S., Phillips N.A., Bedirian V., Charbonneau S., Whitehead V., Collin I. The montreal cognitive assessment, moca: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Ptacek S., Kareholt I., Cermakova P., Rizzuto D., Religa D., Eriksdotter M. Causes of death according to death certificates in individuals with dementia: a cohort from the swedish dementia registry. J Am Geriatr Soc. 2016;64(11):e137–ee42. doi: 10.1111/jgs.14421. [DOI] [PubMed] [Google Scholar]
- 44.Espeland M.A., Bryan R.N., Goveas J.S., Robinson J.G., Siddiqui M.S., Liu S. Influence of type 2 diabetes on brain volumes and changes in brain volumes: results from the women's health initiative magnetic resonance imaging studies. Diabetes Care. 2013;36(1):90–97. doi: 10.2337/dc12-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ng T.P., Feng L., Nyunt M.S., Feng L., Gao Q., Lim M.L. Metabolic syndrome and the risk of mild cognitive impairment and progression to dementia: follow-up of the singapore longitudinal ageing study cohort. JAMA Neurol. 2016;73(4):456–463. doi: 10.1001/jamaneurol.2015.4899. [DOI] [PubMed] [Google Scholar]
- 46.Allison M.A., Aragaki A.K., Ray R.M., Margolis K.L., Beresford S.A., Kuller L. A randomized trial of a low-fat diet intervention on blood pressure and hypertension: tertiary analysis of the whi dietary modification trial. Am J Hypertens. 2016;29(8):959–968. doi: 10.1093/ajh/hpv196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neuhouser M.L., Howard B., Lu J., Tinker L.F., Van Horn L., Caan B. A low-fat dietary pattern and risk of metabolic syndrome in postmenopausal women: the women's health initiative. Metab Clin Exp. 2012;61(11):1572–1581. doi: 10.1016/j.metabol.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howard B.V., Manson J.E., Stefanick M.L., Beresford S.A., Frank G., Jones B. Low-fat dietary pattern and weight change over 7 years: the women's health initiative dietary modification trial. JAMA. 2006;295(1):39–49. doi: 10.1001/jama.295.1.39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.