Abstract
Regulatory T cells (Tregs) play a role in the induction and maintenance of tolerance, as well as in modulating aberrant immune responses. While expanded Tregs have been used in clinical trials, they are polyclonal and the frequency of specific Tregs is very low. To overcome this issue, we have endeavored to “specify” Tregs by engineering them to express receptors that can recognize a given antigen and applied this protocol in autoimmunity, hemophilia and allergy. Thus, we have used retroviral transduction of a specific T cell receptor, single-chain variable fragments (Fvs), or antigen domains in Tregs to achieve this goal. This review summarizes our steps to achieve the ultimate goal of modulating human diseases.
Main Text
CD4+ regulatory T cells (Tregs) are well characterized for their immunosuppressive functions and maintenance of immunological tolerance. The importance of Tregs in immune regulation and brokering tolerance has been robustly demonstrated,1, 2, 3 and expanded polyclonal Tregs are being developed for clinical applications.4 Tregs can be broadly grouped into two categories, either “natural” or induced (i.e., peripherally derived). Natural Tregs (nTregs) represent between approximately 4% and 8% of CD4+ T cells in healthy donor peripheral blood, whereas induced Tregs can be generated from total CD4 T cells by expansion with anti-CD3 in the presence of transforming growth factor β (TGF-β). There T cells are polyclonal and reflect the entire repertoire. Herein, we summarize studies in our laboratory designed to render polyclonal Tregs antigen-specific by transduction of specific receptors such as T cell receptors (TCRs) or single-chain variable fragments (scFvs). More recently, we engineered antigenic domains in Tregs that then can directly be recognized by, or recognize B, cell receptors (BCRs). Our laboratory has concentrated recently on efforts to expand and “specify” Tregs, as well as CD8 cytotoxic cells,2,5, 6, 7 and apply them to modulate adverse immune responses in autoimmunity, allergy, and hemophilia. Parallel work in other laboratories is summarized separately.8, 9, 10, 11
Our first efforts utilized TCRs derived from hemophilia patient clones. Hemophilia A is a monogenic X-linked bleeding disorder caused by mutations in the factor VIII (F8) gene. While severe hemophilia A results from major deletions or inversions in the F8 gene, such that these individuals have less than 1% FVIII activity, mild hemophilia occurs with missense mutations or stop codons, for example, that also may lead to significantly reduced clotting efficacy. These disorders are generally treated with recombinant or plasma-derived FVIII replacement therapy, either prophylactically or on demand. However, up to 30% of those receiving replacement FVIII develop a T cell-dependent anti-drug antibody response because they never developed tolerance to this human protein. These antibodies are referred to as “inihbitors” because they neutralize (inhibit) the therapeutic function of FVIII, rendering this important treatment ineffective. Most of the inhibitory antibodies are directed at the A2 and C2 domains of the FVIII protein, which are critical for binding to partners in the cascade. Therefore, we concentrated on designing receptors that recognize these domains. This would be important to achieve in inhibitor-positive patients or to prevent inhibitor responses, in the first place, which is of clinical importance.
The First Specific Treg: TCR-Transduced CD4 T Cells
We previously demonstrated the retroviral transduction of Fc fusions of FVIII antigenic domains into activated B cells to induce tolerance to the antigen in the associated domains in the fusion protein. In these studies, we established a role for Tregs in the B cell presentation for tolerance. Because the antibody response to FVIII is highly T cell-dependent, our first approach was to target the T cells that can recognize FVIII peptide present in major histocompatibility complex (MHC) class II. Therefore, in our first engineered Treg, we chose to clone TCR V regions from peptide-expanded T cell clones obtained from patients with mild hemophilia A,12,13 in collaboration with Dr. Kathleen Pratt. The polyclonal T cells came from healthy normal adult donors at the American Red Cross or the NIH Blood Bank.
In these experiments, Kim et al.2 in our laboratory purified CD4 fractions by magnetic cell purification, then labeled them with marker-specific antibodies and sorted them based on expression of CD4, CD25, and CD127. Thus, naive CD4 effector T cells (Teffs) were CD4+, CD25−, CD127+, and CD45RA+ whereas Tregs were CD4+, CD25high, and CD127low. Tregs isolated in this way were also Foxp3 and Helios positive, reflecting their status as nTregs. The CD25−, CD127+, and CD45RA+ T cells could then be used as Teffs.
The TCR V regions from two of these clones, termed 17195 and 171911, recognized a human leukocyte antigen (HLA) class II (DR1) peptide in the C2 region of FVIII.12 The specific Teffs proliferated and produced multiple cytokines in response to C2 peptide and antigen-presenting cells (APCs). The transduced Tregs were expanded as described by Kim et al.2 with irradiated peripheral blood mononuclear cells (PBMCs) as APCs, and further expanded with peptide plus random oligonucleotides to maintain Treg properties (namely Foxp3 and Helios). In fact, when they were cultured with antigen, the levels of FoxP3, Helios, as well as GARP and LAP, increased, typical of activated Tregs, but they did not produce significant levels of interleukin 2 (IL-2) and interferon γ (IFN-γ).
These expanded FVIII-specific Tregs were then mixed with cell proliferation dye (CPD)-labeled FVIII-specific Teffs in various ratios plus FVIII peptide. The engineered Tregs suppressed the FVIII-specific proliferative response of Teffs even when the effector cells were cultured at an 8:1 ratio to Tregs.2 Cytokine production was also suppressed.
The antibody response to FVIII in hemophilia A patients is highly T cell-dependent.14, 15, 16 Hence, targeting the (helper) Teffs should lead to modulation of the anti-FVIII immunoglobulin G (IgG) response. This was tested in spleen cells from FVIII-deficient (E16) mice as described by Hausl et al.17 Despite being a xenogeneic system, we found that the in vitro recall antibody response to FVIII was suppressed by TCR-engineered Tregs more efficiently than by polyclonal Tregs.
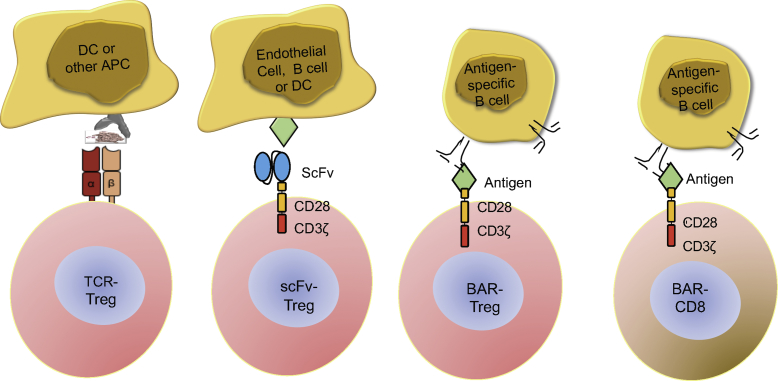
Importantly, the engineered Tregs were able to suppress the antibody response to FVIII in vivo! Amazingly, although the engineered TCR recognizes a single peptide in a large protein, the antibody response to other major epitopes of FVIII was also suppressed. This indicates that bystander suppression of other T (and B) cells had occurred, an observation that was later confirmed with responses to unrelated antigen. Thus, we had engineered specificity into expanded human Tregs and showed that they could suppress the antibody response to FVIII effectively, presumably by directly interacting and modulating the antigen-presenting function of dendritic cells or other APCs (see Figure 1). While the mechanism is not fully understood, we know that Tregs are stimulated more in the presence of activated effector cells producing IL-2 (see Parvathaneni and Scott18). This is a potential benefit when many antigens are targeted.
Figure 1.
Scheme for Design of Engineered Specific T Cells
Human CD25+, CD127low Tregs were retrovirally transduced with constructs for TCR or scFv (CAR) recognizing an HLA class II-restricted FVIII peptide or antigen domain, respectively. The BAR T cells (Tregs or CD8) expressed FVIII domains or ovalbumin.
Single-Chain Fv-Transduced CD4 T cells
These TCR-transduced Tregs, although highly effective, are nonetheless MHC class II restricted, thus limiting their eventual utility to patients sharing the same MHC globally. Therefore, in collaboration with Anja Naumann Schmidt and Christoph Königs in Frankfurt, we developed a second approach to engineer specificity, namely a scFv, as in the chimeric antigen receptor (CAR) T cells used in cancer therapy. Dr. Schmidt then created a scFv using the V regions from a phage display library.19,20 ANS8, a chimeric receptor that recognized the A2 domain of FVIII, was incorporated into our retroviral CAR vector and used to transduce both CD4 effectors and Tregs. These scFv-transduced cells recognized free FVIII but responded to membrane or plate-bound FVIII more effectively, reflecting the recognition of the A2 domain under these conditions by the scFv. When ANS8 human Tregs, generated under the same conditions as the 17195 Tregs, were mixed with spleen cells from FVIII-primed mice, the antibody response to FVIII was suppressed in vitro.5 Notably, the degree of suppression was similar to that found with 17195 TCR-transduced Tregs.
Suppression of the antibody response to FVIII by these human Tregs in vivo also was observed, and this effect lasted up to 8 weeks, even though the human Tregs were rejected within 2–3 weeks!5
These data indicate that the scFv CAR Tregs that recognize conformation epitopes in the A2 region of FVIII and the TCR Tregs that recognize a C2 peptide are able to suppress the antibody response to multiple epitopes in FVIII. That is, engineered Tregs that recognize different B cell and T cell domains of FVIII can be suppressive against multiple epitopes of this large immunogenic protein.
These data provide evidence for bystander suppression by engineered Tregs. Further evidence for bystander suppression comes from another system in our laboratory, namely expression of a myelin basic protein (MBP)-specific TCR in human Tregs and their suppression of active experimental allergic encephalomyelitis in mice.18,21
Antigen-Expressing CD4 Tregs and Cytotoxic T Cells
The antibody response to FVIII is highly T cell-dependent, leading to B cell activation and IgG secretion. To directly target B cells, we designed and engineered two additional Tregs that expressed the cognate antigen that B cells recognize via their IgM and IgD receptors. We refer to these as BAR Tregs and BAR CD8 (cytotoxic) T cells, since they are recognized by the B cell antibody receptor.
The Payne group7 at the University of Pennsylvania designed a similar CAR targeting an autoantigen in pemphigus and called it a chimeric autoantibody receptor, or CAAR. We prefer BAR as the acronym, as it is more generic and can be used for any antigen (see below).
Therefore, Kalpana Parvathaneni, then a PhD student in my laboratory, applied the principle of engineered cytotoxic CAR T cells to directly target FVIII-specific B cells. She engineered immunodominant B cell domains of FVIII into both expanded cytotoxic CD8 and regulatory CD4 T cells (see Figure 1). When specific B cells encounter engineered BAR T cells, for example, they would bind them to form a synapse and would receive a putative negative signal from these Tregs or cytotoxic CD8 T cells, resulting in suppression of the B cell response or cell death, in the latter case. For a simple demonstration of this principle, she employed polyclonal stimulation of naive B cells with lipopolysaccharide (LPS), which generates antibody secretion reflecting the total B cell repertoire. Thus, BAR CD8 T cells killed B cells that were FVIII specific but did not affect ovalbumin (OVA)-specific B cells, for example.18 This effect requires both A2-expressing and C2-expressing BARs, reflecting target specificity and the lack of bystander effect.
In addition, Zhang et al.6 showed that BAR-expressing Tregs suppressed the response to FVIII in vivo when given prophylactically. Mixtures of B and T cells from tolerant mice demonstrated that B cells and not T cells were the targets. Presumably, this is due to anergy rather than cytotoxicity of targeted B cells.
Most recently, we tested the BAR Treg concept in a model of allergy, namely the IgE response to OVA. When mice are immunized with OVA mixed with aluminum hydroxide (alum adjuvant), they make IgG and IgE responses that can lead to anaphylaxis when challenged. The OVA-expressing BAR Tregs could potentially interact with OVA-specific B cells and IgE anti-OVA bound to mast cells. Upon challenge with a high dose of OVA, these mice undergo a rapid drop in temperature that reflects an anaphylactic response. Abdeladhim et al.22 in our laboratory demonstrated that injection of OVA BAR Tregs (but not FVIII BAR Tregs) 48 h before high-dose OVA protected these mice from a significant temperature drop This not only occurred in actively immunized mice, but it also was seen in passively sensitized mice (that is, naive mice given IgE anti-OVA the day before). This suggests that the BAR Tregs must be interacting with and suppressing mast cells directly. Moreover, these data suggest that circulating antibody did not block the effect of the BAR Tregs, a result verified in the FVIII model.
Discussion and Conclusions
Engineering Tregs specific for a given antigen by transduction of a TCR, scFv, or BAR can render polyclonal Tregs more specific by increasing the number of cells that can target a given immune response,8 as well as reduce potential non-specific suppression by Tregs of irrelevant specificities (see Brunstein et al.23). Aside from our laboratory, Levings and colleagues9,24 previously reported the development of an scFv recognizing a human class I antigen, as well as the role of signaling domains in their efficacy. Interestingly, alterations of the latter did not improve the regulatory function significantly.24
We have primarily focused on human Tregs with the goal of moving to clinical translation. In lieu of a clinical translation, in vivo experiments must involve a xenogeneic transfer into mice. Despite the rejection of these Tregs by immunocompetent mice, they were effective at suppressing immune responses to FVIII or anaphylaxis to OVA in vivo, thus proving their utility. Further experiments with engineered murine Tregs (and CD8 cells) have now been successfully performed.
Circulating antibody can bind to BAR antigen and theoretically opsonize or neutralize Tregs and block the effect of the Tregs on immune responses. The lack of a significant effect of antibody on BAR Tregs was somewhat surprising, but it was previously demonstrated by Ellebrecht et al.7 in the pemphigus system. Indeed, circulating antibody may stimulate proliferation of BAR Tregs, an effect we have observed with BAR CD4 T cells (Pohida et al.,2018, ASH, abstract). Further experiments are needed to verify this effect in vivo.
The three types of engineered Tregs all have different targets, advantages and disadvantages, and potential mechanisms of action. The TCR Tregs, as mentioned above (and shown in Figure 1), must interact with APCs, perhaps to downregulate MHC class II and render them tolerogenic. The scFv CAR Tregs may act via the same mechanism but need to be able to “see” a conformational determinant on some cell surface (APCs, B cells, or endothelial cells), whereas BAR Tregs can either interact with B cells or IgE (IgG?) bound to Fc receptors.
Because TCR Tregs are MHC restricted, they would require a matching MHC, but they are highly specific and could be efficacious in disease with strong, limited MHC linkage. Alternatively, scFv CAR Tregs are not MHC restricted, but they need to recognize conformational determinants that may occur in a fluid phase but seem to be more efficient on cell surfaces. Likewise, BAR Tregs are not MHC restricted and must interact with cells bearing specific receptors.
Nonetheless, an advantage of all of these Tregs is the bystander effect, meaning that one does not need to know all of the undesirable antigens, just a few of them. This would become important as well in terms of using Tregs to treat allergic responses, since targeting the sensitized mast cells could have an additional effect.22 Alternatively, BAR CD8 T cells are effective killers that allow us to precisely target one antigen or part of one protein without suppressing responses to nearby epitopes.
Table 1 summarizes these facts and the successes of engineered Tregs with optimism toward future clinical trials.
Table 1.
Summary of Engineered T Cells and Their Efficacy
| Specific Type of Engineered T Cell | Specificity | Disease Model | References and Comments |
|---|---|---|---|
| Human TCR Tregs | FVIII peptide, MBP peptide | hemophilia A, EAE | HLA-restricted, bystander effect for suppression2,18 |
| Human scFv (CAR) Tregs | FVIII domain | hemophilia A | conformation-dependent, not HLA restricted, bystander effect for suppression5 |
| B cell antibody BAR Tregs | FVIII domains, ovalbumin | hemophilia A, allergy | suppresses B cell directly, bystander effect for suppression;6 targets mast cells in allergy22 |
| B cell antibody BAR CD8s | FVIII domains | hemophilia A | suppresses B cells directly18 |
EAE, experimental autoimmune encephalomyelitis.
Acknowledgments
The research progress summarized in this review was supported in part by grants from the NIH (NHLBI, RO1 HL126727), as well as a Pfizer ASPIRE grant. I thank Drs. Kate Pratt, Anja Schmidt, Kai Wucherpfennig, and Christoph Königs for specific receptors; Drs. Ed Mitre and Laura Kropp for help with allergy experiments; and members of the Scott laboratory (Patrick Adair, Maha Abdeladhim, Yongchan Kim, Kalpana Parvathaneni, Shiva Venkatesha, Jeongheon Yoon, and Aihong Zhang) for their devotion and contributions to this research. The views expressed herein are those of the author, not those of the Department of Defense or US government.
References
- 1.Sakaguchi S., Wing K., Miyara M. Regulatory T cells—a brief history and perspective. Eur. J. Immunol. 2007;37(Suppl 1):S116–S123. doi: 10.1002/eji.200737593. [DOI] [PubMed] [Google Scholar]
- 2.Kim Y.C., Zhang A.H., Su Y., Rieder S.A., Rossi R.J., Ettinger R.A., Pratt K.P., Shevach E.M., Scott D.W. Engineered antigen-specific human regulatory T cells: immunosuppression of FVIII-specific T- and B-cell responses. Blood. 2015;125:1107–1115. doi: 10.1182/blood-2014-04-566786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bluestone J.A. Regulatory T-cell therapy: is it ready for the clinic? Nat. Rev. Immunol. 2005;5:343–349. doi: 10.1038/nri1574. [DOI] [PubMed] [Google Scholar]
- 4.Bluestone J.A., Buckner J.H., Fitch M., Gitelman S.E., Gupta S., Hellerstein M.K., Herold K.C., Lares A., Lee M.R., Li K. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl. Med. 2015;7:315ra189. doi: 10.1126/scitranslmed.aad4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon J., Schmidt A., Zhang A.H., Königs C., Kim Y.C., Scott D.W. FVIII-specific human chimeric antigen receptor T-regulatory cells suppress T- and B-cell responses to FVIII. Blood. 2017;129:238–245. doi: 10.1182/blood-2016-07-727834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang A.-H., Parvathaneni K., Yoon J., Kim Y., Scott D.W. Targeting antigen-specific B cells using BAR-transduced cytotoxic and regulatory T cells. J. Immunol. 2016;196(Suppl 1) 70.7. [Google Scholar]
- 7.Ellebrecht C.T., Bhoj V.G., Nace A., Choi E.J., Mao X., Cho M.J., Di Zenzo G., Lanzavecchia A., Seykora J.T., Cotsarelis G. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353:179–184. doi: 10.1126/science.aaf6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sicard A., Levings M.K., Scott D.W. Engineering therapeutic T cells to suppress alloimmune responses using TCRs, CARs, or BARs. Am. J. Transplant. 2018;18:1305–1311. doi: 10.1111/ajt.14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacDonald K.N., Piret J.M., Levings M.K. Methods to manufacture regulatory T cells for cell therapy. Clin. Exp. Immunol. 2019;197:52–63. doi: 10.1111/cei.13297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azimi C.S., Tang Q., Roybal K.T., Bluestone J.A. NextGen cell-based immunotherapies in cancer and other immune disorders. Curr. Opin. Immunol. 2019;59:79–87. doi: 10.1016/j.coi.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira L.M.R., Muller Y.D., Bluestone J.A., Tang Q. Next-generation regulatory T cell therapy. Nat. Rev. Drug Discov. 2019;18:749–769. doi: 10.1038/s41573-019-0041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ettinger R.A., James E.A., Kwok W.W., Thompson A.R., Pratt K.P. HLA-DR-restricted T-cell responses to factor VIII epitopes in a mild haemophilia A family with missense substitution A2201P. Haemophilia. 2010;16:44–55. doi: 10.1111/j.1365-2516.2008.01905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ettinger R.A., James E.A., Kwok W.W., Thompson A.R., Pratt K.P. Lineages of human T-cell clones, including T helper 17/T helper 1 cells, isolated at different stages of anti-factor VIII immune responses. Blood. 2009;114:1423–1428. doi: 10.1182/blood-2009-01-200725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian J., Borovok M., Bi L., Kazazian H.H., Jr., Hoyer L.W. Inhibitor antibody development and T cell response to human factor VIII in murine hemophilia A. Thromb. Haemost. 1999;81:240–244. [PubMed] [Google Scholar]
- 15.Qian J., Burkly L.C., Smith E.P., Ferrant J.L., Hoyer L.W., Scott D.W., Haudenschild C.C. Role of CD154 in the secondary immune response: the reduction of pre-existing splenic germinal centers and anti-factor VIII inhibitor titer. Eur. J. Immunol. 2000;30:2548–2554. doi: 10.1002/1521-4141(200009)30:9<2548::AID-IMMU2548>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 16.Bray G.L., Kroner B.L., Arkin S., Aledort L.W., Hilgartner M.W., Eyster M.E., Ragni M.V., Goedert J.J. Loss of high-responder inhibitors in patients with severe hemophilia A and human immunodeficiency virus type 1 infection: a report from the Multi-Center Hemophilia Cohort Study. Am. J. Hematol. 1993;42:375–379. doi: 10.1002/ajh.2830420408. [DOI] [PubMed] [Google Scholar]
- 17.Hausl C., Ahmad R.U., Sasgary M., Doering C.B., Lollar P., Richter G., Schwarz H.P., Turecek P.L., Reipert B.M. High-dose factor VIII inhibits factor VIII-specific memory B cells in hemophilia A with factor VIII inhibitors. Blood. 2005;106:3415–3422. doi: 10.1182/blood-2005-03-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parvathaneni K., Scott D.W. Engineered FVIII-expressing cytotoxic T cells target and kill FVIII-specific B cells in vitro and in vivo. Blood Adv. 2018;2:2332–2340. doi: 10.1182/bloodadvances.2018018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahle J., Orlowski A., Stichel D., Becker-Peters K., Kabiri A., Healey J.F., Brettschneider K., Naumann A., Scherger A.K., Lollar P. Epitope mapping via selection of anti-FVIII antibody-specific phage- presented peptide ligands that mimic the antibody binding sites. Thromb. Haemost. 2015;113:396–405. doi: 10.1160/TH14-01-0101. [DOI] [PubMed] [Google Scholar]
- 20.Naumann A., Scherger A.K., Neuwirth J., Orlowski A., Kahle J., Schwabe D., Königs C. Selection and characterisation of FVIII-specific single chain variable fragments. Hamostaseologie. 2013;33(Suppl 1):S39–S45. [PubMed] [Google Scholar]
- 21.Kim Y.C., Zhang A.H., Yoon J., Culp W.E., Lees J.R., Wucherpfennig K.W., Scott D.W. Engineered MBP-specific human Tregs ameliorate MOG-induced EAE through IL-2-triggered inhibition of effector T cells. J. Autoimmun. 2018;92:77–86. doi: 10.1016/j.jaut.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdeladhim M., Zhang A.H., Kropp L.E., Lindrose A.R., Venkatesha S.H., Mitre E., Scott D.W. Engineered ovalbumin-expressing regulatory T cells protect against anaphylaxis in ovalbumin-sensitized mice. Clin. Immunol. 2019;207:49–54. doi: 10.1016/j.clim.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunstein C.G., Blazar B.R., Miller J.S., Cao Q., Hippen K.L., McKenna D.H., Curtsinger J., McGlave P.B., Wagner J.E. Adoptive transfer of umbilical cord blood-derived regulatory T cells and early viral reactivation. Biol. Blood Marrow Transplant. 2013;19:1271–1273. doi: 10.1016/j.bbmt.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawson N.A., Lamarche C., Hoeppli R.E., Bergqvist P., Fung V.C., McIver E., Huang Q., Gillies J., Speck M., Orban P.C. Systematic testing and specificity mapping of alloantigen-specific chimeric antigen receptors in regulatory T cells. JCI Insight. 2019;4:123672. doi: 10.1172/jci.insight.123672. [DOI] [PMC free article] [PubMed] [Google Scholar]