How does muscle regenerate?
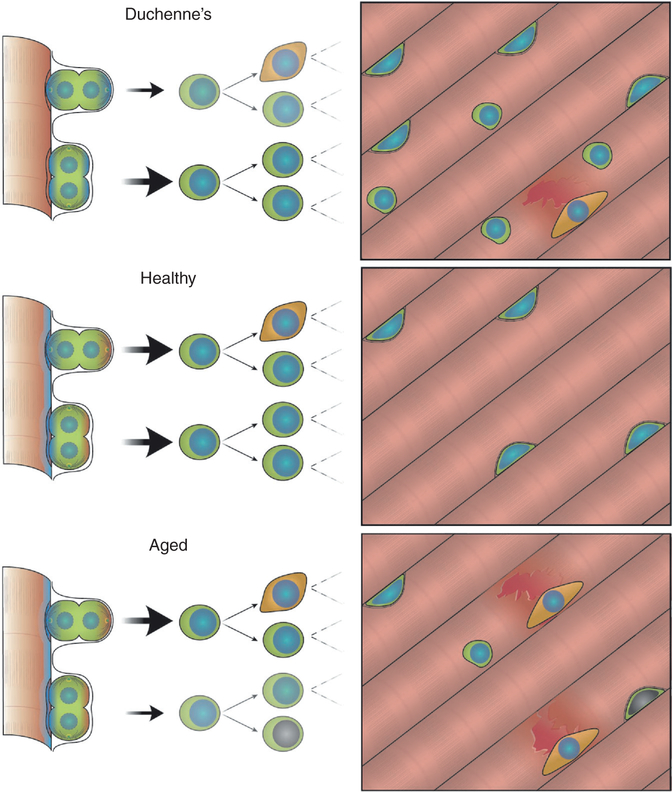
Wear on our bodies requires continuous repair, facilitated by stem cells resident in each tissue. Muscle stem cells represent a subset of the ‘satellite cell’ population, which are responsible for muscle growth and repair throughout life. Upon activation from damage, muscle stem cells divide asymmetrically to generate a stem cell and a progenitor (stem cell maintenance) or divide symmetrically to generate two stem cells (stem cell expansion). Maintaining muscle mass with age requires a careful balance in adequate numbers of muscle stem cells and myogenic progenitors for efficient regeneration and to resist muscle wasting caused by exhaustion of the muscle stem cell pool.
How are self-renewal and commitment regulated?
An activated satellite cell exists in a specific niche juxtaposed to a myonucleus and endothelial cell and positioned between the myofiber and basal lamina, where it receives an array of spatially discrete and temporal cues. The accumulation of different signals on the basal and apical surface of the cell results in polarized intracellular signalling and is the basis of satellite cell polarity. A polarized satellite cell may undergo cell division where the orientation of the mitotic spindle (apico–basal vs. planar) dictates if both daughters will interact with the myofiber and basal lamina equally (symmetric) or unequally, having one daughter in contact with the basal lamina and one with the myofiber (asymmetric). Fate determinants that were sequestered in a polarized manner in the cell can thus be inherited in a symmetric or asymmetric manner, leading to formation of two new stem cells or one stem cell and one cell permissive to differentiation (Figure 1). Cell-fate determinants consist of epigenetic regulators, transcription factors and RNA-binding proteins that can act to either promote a stem cell fate or modify a cell to undergo commitment. Changes in the satellite cell niche and intracellular signalling can lead to an imbalance in polarity and division orientation, causing inefficient muscle commitment or early exhaustion of the satellite cell pool.
Figure 1. Satellite cell self-renewal and commitment are critical for muscle regeneration.
Regeneration is impaired during age and disease due to altered cell signalling and changes to the satellite cell niche. In Duchenne muscular dystrophy (DMD), loss of dystrophin protein results in myofiber fragility and constant damage in DMD muscle. This results in increased numbers of satellite cells that inefficiently differentiate, resulting eventually in satellite cell exhaustion. With age, accumulation of DNA damage, altered niche signalling and an increasingly stiff myofiber results in precocious myogenic commitment as well as cellular senescence. The resulting muscle has an inability to repair due to exhaustion of the satellite cell pool.
How are satellite cells affected by age and disease?
With age and disease, disproportionate growth factor signalling in muscle (FGF2, IL-6, TGFβ), increased myofiber stiffness, DNA damage, cell senescence, inflammation, and altered cell polarity all contribute to loss of balance in self-renewal and commitment, resulting in precocious activation, decline in the satellite cell pool and eventually muscle wasting. In specific muscle disease such as Duchenne muscular dystrophy (DMD), loss of dystrophin protein leads to perturbation in satellite cell polarity establishment as well as structural weakness in differentiated myofibers. The result is a cycle of continual damage and regeneration exacerbated by altered cell polarity and inefficient generation of progenitors. This leads to an accumulation of satellite stem cells and inefficient muscle regeneration, eventually resulting in cardiac and respiratory failure.
How can we improve satellite cell function?
Understanding the mechanisms involved in self-renewal and commitment provides avenues to intervene and promote symmetric expansion or asymmetric differentiation of the satellite stem cell pool to augment stem cell creation and efficient regeneration. Specifically, treatment of aged satellite cells with JAK/STAT inhibitors or the ligand Wnt7a stimulates satellite stem cell self-renewal, increasing satellite cell numbers and improving muscle regeneration. Promoting specific signalling in the endogenous satellite cell is a means to promote whole body muscle repair. However, in cases such as DMD, restoring dystrophin expression will be required to reorganize satellite cell polarity and rescue intracellular signalling but also restore myofiber integrity.
Where do we go from here?
Muscle provides an exciting opportunity to explore the molecular mechanisms underlying cell fate and polarity. Specifically, emerging questions involve exploring signalling involved in establishment of spindle orientation, as this is the causal driver of asymmetric division following polarity establishment; defining the hierarchy of fate determinants sequestered in a polarized satellite cell and their function post-mitosis to identify drug-able targets involved in satellite cell commitment; and exploring multifaceted approaches promoting stem cell self-renewal as well as commitment to augment long-term muscle regeneration with age and disease.
Where can I find out more?
Brack, A.S., and Munoz-Canoves, P. (2016). The ins and outs of muscle stem cell aging. Skel. Musc. 6, 1.
Chang, N.C., Chevalier, F.P., and Rudnicki, M.A. (2016). Satellite cells in muscular dystrophy - lost in polarity. Trends Mol. Med. 22, 479–496.
Dumont, N.A., Wang, Y.X., and Rudnicki, M.A. (2015). Intrinsic and extrinsic mechanisms regulating satellite cell function. Development 142,1572–1581.
Hwang, A.B, and Brack, A.S. (2018). Muscle stem cells and aging. Curr. Top. Dev. Biol. 126, 299–322.