Abstract
Insulin resistance is characterized by increased metabolic uptake of fatty acids. Accordingly, techniques to examine in vivo shifts in fatty acid metabolism are of value in both clinical and experimental settings. Partially metabolizable long chain fatty acid (LCFA) tracers have been recently developed and employed for this purpose: [9,10-3H]-(R)-2-bromopalmitate ([3H]-BROMO) and [125I]-15-(ρ-iodophenyl)-3-R,S-methylpentadecanoic acid ([125I]-BMIPP). These analogues are taken up like native fatty acids, but once inside the cell do not directly enter β-oxidation. Rather, they become trapped in the slower processes of ω and α-oxidation. Study aims were to (1) simultaneously assess and compare [3H]-BROMO and [125I]-BMIPP and (2) determine if tracer breakdown is affected by elevated metabolic demands. Catheters were implanted in a carotid artery and jugular vein of Sprague–Dawley rats. Following 5 days recovery, fasted animals (5 h) underwent a rest (n = 8) or exercise (n = 8) (0.6 mi/h) protocol. An instantaneous bolus containing both [3H]-BROMO and [125I]-BMIPP was administered to determine LCFA uptake. No significant difference between [125I]-BMIPP and [3H]-BROMO uptake was found in cardiac or skeletal muscle during rest or exercise. In liver, rates of uptake were more than doubled with [3H]-BROMO compared to [125I]-BMIPP. Analysis of tracer conversion by TLC demonstrated no difference at rest. Exercise resulted in greater metabolism and excretion of tracers with ~37% and ~53% of [125I]-BMIPP and [3H]-BROMO present in conversion products at 40 min. In conclusion, [3H]-BROMO and [125I]-BMIPP are indistinguishable for the determination of tissue kinetics at rest in skeletal and cardiac muscle. Exercise preferentially exacerbates the breakdown of [3H]-BROMO, making [125I]-BMIPP the analogue of choice for prolonged (>30 min) experimental protocols with elevated metabolic demands.
Keywords: Tracer, Kinetics, Thin layer chromatography, Uptake, Clearance
Introduction
Abnormalities in lipid trafficking and uptake are a hallmark of numerous metabolic disease states including obesity, type 2 diabetes and atherosclerosis. To assess lipid kinetics in these states, partially metabolizable long chain fatty acid (LCFA) tracers have been developed for use in vivo [1–7]. Two such tracers are [9,10-3H]-(R)-2-bromopalmitate ([3H]-BROMO) and [125I]-15-(ρ-iodophenyl)-3-R,S-methylpentadecanoic acid ([125I]-BMIPP). Both analogues are taken up by tissues like native substrates however, once inside the cell, they become trapped in various stages of ω or α-oxidation. As a result, the analogues remain in the tissue allowing their quantification by specific activity.
Developed and extensively tested by Oakes et al. [8], [3H]-BROMO shows excellent retention in the majority of tissues examined. Studies employing this tracer were the first to effectively show increased efficiency of tissue LCFA uptake in a model of dietary induced insulin resistance [9]. BMIPP was developed by Knapp et al. [10–12] and has been primarily used for cardiac imaging with an [123I] label. Using single photon emission computed tomography (SPECT) defects in fatty acid uptake by the heart are imaged and are indicative of ischemia or tissue injury [5, 13–15]. Like [3H]-BROMO, the tracer has also proven to be a powerful tool in evaluating various pharmacological treatments on cardiac metabolism [16–19].
To date, numerous studies have individually assessed the metabolism of these fatty acid tracers in vivo and some comparisons between different tracers have been reported [1]. However, studies directly comparing [125I]-BMIPP and [3H]-BROMO in specific tissues under varied metabolic conditions have not been performed. Therefore, the aim of the present study was to assess and compare [125I]-BMIPP and [3H]-BROMO in vivo. The effects of enhanced LCFA metabolism due to exercise on tissue retention of these tracers was examined. Together, these studies will provide needed information on the use of [125I]-BMIPP and [3H]-BROMO, and their applicability to the study of metabolism.
Experimental Methods
Animals
Male Sprague–Dawley rats (Harlan Industries, Indianapolis, IN) were housed individually and maintained at 23°C on a 0600–1800 hours light cycle. Rats were fed standard chow ad libitum (Purina, Nestlé, St Louis, MI) and given free access to water. The rats were housed under these conditions for ~1 week, by which time their weights had reached ~330 g. Following weight gain, rats were randomly divided into rest and exercise groups (n = 8 per group). All procedures were approved by the Vanderbilt University Animal Care and Use Subcommittee and followed National Institutes of Health guidelines for the care and use of laboratory animals.
Surgical Procedures
Surgical procedures were performed as previously described for arterial and venous catheterizations [20]. Briefly, animals were anaesthetized with a 50:5:1 vol/vol/vol mixture of ketamine, rompun, and acepromazine, and the left common carotid artery and right jugular vein were catheterized with PE50 catheters, which were exteriorized and secured at the back of the neck, filled with heparinized saline (150 U/ml), and sealed with a stainless steel plug. Immediately post-surgery, each animal received 75 mg/kg of ampicillin subcutaneously to prevent infection. After surgery, animal weights and food intake were monitored daily.
Isotopic Analogues
(ρ-iodophenyl)-3-R,S-methylpentadecanoic acid was a kind gift from Oak Ridge International Laboratories (Oak Ridge, TN). Radioiodination was performed according to the manufacturer’s suggested protocol. Briefly, (ρ-iodophenyl)-3-R,S-methylpentadecanoic acid was heated in the presence of Na[125I] solution (740 MBq/200 μl), propionic acid, and copper (II) sulfate. Na2S2O3 was then added and the organic phase ether extracted and sequentially back extracted with saturated NaHCO3 and water. After evaporation, the [125I]-BMIPP was solubilized using sonication into ursodeoxycholic acid. The initial concentration of [125I]-BMIPP was 1.05 μCi/μl. However, due to the short half life of [125I]-BMIPP, the administered dose was volumetric. A dose of 100 μl was administered and the activity of the tracer was determined for each individual animal and corrected for any decay. To limit tracer decay, all studies were performed within 30 days of synthesis.
[3H]-BROMO was prepared by American Radioactive Chemicals Co (St Louis, MO) from palmitic acid as previously described [8]. The final concentration was 1 μCi/μl. On the day of the experiment, 20 μl of [3H]-BROMO was evaporated and reconstituted in 100 μl of saline containing 0.35 mg/ml rat albumin (Sigma Chemical, St Louis, MO). This solution was added to 100 μl of [125I]-BMIPP in ursodeoxycholic acid. In total, [3H]-BROMO, 19.5 μCi was administered to each animal. On the day of the experiment, 5 μl of infusate was retained for normalization of radioactivity while the remaining 195 μl was administered to the animal.
Resting Studies
On the morning of the study, rats were fasted for 5 h in a clear Tupperware (2 l) container lined with bedding. Approximately 1 h prior to the experiment, catheters were flushed with heparinized saline (10 U/ml) and connected to PE50 and Silastic tubing for infusions and sampling. Rats were then placed in the Tupperware container until the commencement of the experimental protocol. Throughout the experimental protocol rats were conscious and unrestrained. In total the experimental protocol was 40 min in duration. Prior to tracer infusions, a basal blood sample were obtained (100 μl) for the measurement of isotopic analogues, plasma glucose, insulin and nonesterified fatty acids (NEFA). To prevent declines in hematocrit, the erythrocytes taken prior to isotopic analogue infusion were washed in saline and re-infused shortly after each sample was taken. At 0 min, an instantaneous bolus of [125I]-BMIPP and [3H]-BROMO was administered and additional plasma samples (200 μl) were obtained at 2, 5, 10, 15, 25, and 40 min for the measurement of [125I]-BMIPP, [3H]-BROMO, NEFA and plasma glucose. Following the final blood sample, rats were anesthetized with pentobarbital sodium and skeletal muscle (gastrocnemius), liver, heart, brain and epididymal fat were rapidly excised, rinsed in saline to remove excess blood, freeze clamped in liquid nitrogen and frozen at −80°C until further analysis.
Exercise Studies
Two days prior to the protocol, rats in this treatment were acclimated by running on a motorized treadmill for 10 min at 0.6 mi/h. On the morning of the study, rats were fasted for 5 h in a clear Tupperware (2 l) container lined with bedding. Approximately 1 h prior to the experiment, catheters were flushed with heparinized saline (10 U/ml) and connected to PE50 and silastic tubing for infusions and sampling. Rats were then placed in the treadmill until the commencement of the experimental protocol. Following basal blood sampling, rats were run on the treadmill at 0.6 mi/h and samples were obtained as in the resting protocol. This exercise intensity is moderate for rats. At 40 min, rats were anesthetized and tissue samples were obtained as previously described.
Plasma Analysis
Plasma NEFAs were measured spectrophotometrically by an enzymatic colorimetric assay (Wako NEFA C kit, Wako Chemicals Inc., Richmond, VA). Plasma glucose concentrations were measured by the glucose oxidase method using an automated glucose analyzer (Beckman Instruments, Fullerton, CA). [125I]-BMIPP and [3H]-BROMO were measured in the same plasma sample (15 μl). Plasma was counted for [125I]-BMIPP using a Beckman Gamma 5500 counter (Beckman Instruments, Fullerton, CA). Following this, 100 μl of 50% ethanol was added to the sample and 3H radioactivity was counted after addition of fluor (10 ml; Ultimate Gold, Packard Bioscience, Boston, MA.) using a Packard Tri-Carb 2900TR Liquid Scintillation Analyzer (PerkinElmer, Boston, MA). In addition, plasma was also analyzed by thin layer chromatography (TLC) by methods that were derived from Kropp et al. [21]. Here, individual plasma time points were examined by TLC from a representative animal in each treatment group. TLC plates reflecting plasma samples were segmented into 1 cm sections and analyzed for tracer and tracer conversion products. Each plate was examined for radioactivity. Significant radioactivity appearing in minor lipid fractions over time was considered evidence of tracer breakdown, although the exact chemical nature of each product was not identified. Tracer conversion or breakdown was calculated as a ratio of total activity appearing on the plate for each time point.
Tissue Analysis
Total [125I] radioactivity in tissues was determined on sections of whole tissue (~100 mg) using a Beckman Gamma 5500 counter (Beckman Instruments, Fullerton, CA). Following this, total lipid was extracted from the tissue using a modified Folch–Lees extraction [22]. The [125I] radioactivity in this fraction was then reassessed before 10 ml of flour was added to samples and then 3H radioactivity determined by liquid scintillation counting (Packard TRI-CARB 2900TR, Packard, Meriden, CT) with Ultima Gold (Packard) as scintillant. The relationship between gamma radioactivity and beta emissions using this specific process and counter has been previously established in our laboratory. This relationship was used to correct 3H radioactivity for beta-emissions originating from [125I] radioactivity in both tissue and plasma samples [3]. In addition, lipid was extracted from a portion of each tissue (~100 mg) from four rats in both the resting and exercise protocols using a Folch–Lees extraction [22]. Lipid fractions were then separated by TLC [23]. Plate segments were subsequently separated and individually counted. Tissues plates were separated based on lipid fraction (phospholipid, mono-diglyceride, free fatty acids and triglyceride).
Calculations
Plasma Kinetics
Identical equations were used for the determination of [125I]-BMIPP and [3H]-BROMO kinetics. Plasma tracer (p) kinetics are based on the disappearance of tracer from the plasma over time. Movement of the tracer out of the plasma pool into tissues is denoted by clearance (Kp, Eq. 1). When Kp is expressed in terms of tracee or mean LCFA concentration as measured by an enzymatic assay, the measure is termed uptake (Rp, Eq. 2). Finally, if Kp is expressed as a fraction of the original tracer dose administered (D), the resultant expression is metabolic clearance rate or MCRp. Equations 1–3 are calculated independently for [125I]-BMIPP and [3H]-BROMO. Equations employed were defined as follows where represents integral over the time between 2 and 40 min.
| (1) |
| (2) |
| (3) |
Tissue Kinetics
Rates of tissue LCFA clearance (Kt, Eq. 4) and metabolic indices (Rt, Eq. 5) were calculated from the accumulation of [125I]-BMIPP and [3H]-BROMO in tissues (t) relative to the integral of the plasma (p) concentration following the instantaneous bolus. These measurements follow from Eqs. 1–3 and have been previously described [3, 9].
| (4) |
| (5) |
A one-way repeated measures analysis of variance (ANOVA) was performed to compare differences between [125I]-BMIPP and [3H]-BROMO within specific tissues. To determine differences over time for blood glucose and NEFA, a two-way repeated measures ANOVA was performed. To establish differences within ANOVA, a Tukey post hoc test was used. Significance levels of p ≤ 0.05 were employed, and data are reported as means ± standard error of the mean (SEM).
Results
Animal Characteristics
Baseline animal characteristics for both rest and exercise experiments are outlined in Table 1. Blood glucose remained stable in the rest group (7.7 ± 0.3 mM at 40 min) while it steadily increased in the exercise group to 11.5 ± 0.6 mM at the end of the protocol (p < 0.05). Plasma NEFA levels remained stable with average values of 0.63 ± 0.06 mM and 0.56 ± 0.05 mM for rest and exercise studies at the end of the experimental protocol (p > 0.05). All animals in the exercise protocol were able to successfully complete the required 40 min of exercise.
Table 1.
Baseline characteristics of animals in the sedentary and exercise experiments
| n | Weight (g) | Recovery (days) | Glucose (mM) | NEFA (mM) | Hct 0 (%) | Hct 40 (%) | |
|---|---|---|---|---|---|---|---|
| Rest | 8 | 330 ± 10 | 7.8 ± 0.9 | 7.3 ± 0.2 | 0.76 ± 0.11 | 47 ± 1 | 43 ± 1 |
| Exercise | 8 | 340 ± 12 | 8.3 ± 0.4 | 7.5 ± 0.2 | 0.73 ± 0.11 | 46 ± 1 | 41 ± 1 |
Recovery (days) reflects the number of days between surgery and the experiment. Basal plasma glucose (glucose) and non-esterified fatty acids (NEFA) are shown along with starting and ending hematocrit (Hct) levels
Metabolic Clearance and Uptake
Whole body metabolic clearance in the resting state was set to an arbitrary value of 1.0 for each tracer for comparison to the exercise treatment. Results show comparable increments in fatty acid clearance for each tracer with exercise (Fig. 1). Tissues (skeletal muscle, heart, liver, adipose tissue) were examined for rates of fatty acid uptake (Rt, μmol/100 g/min). Absolute values are shown in Fig. 2. No quantitative difference between [125I]-BMIPP and [3H]-BROMO Rt values were noted during the resting protocol with the exception of the liver. In the liver, rates of Rt calculated using [3H]-BROMO was more than double that calculated using [125I]-BMIPP.
Fig. 1.
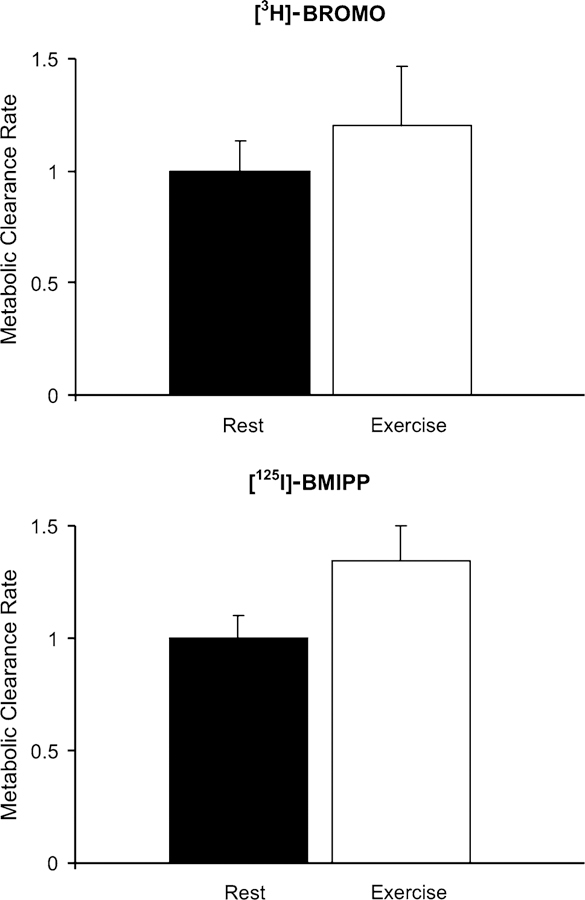
Relative change in whole body LCFA clearance rates (MCR) for [3H]-BROMO (top panel) and [125I]-BMIPP (bottom panel) from rest to exercise. Calculations are based on the measurement of radioactivity in the plasma over time. As tracer moves from the plasma into tissues, the rate of decay for each tracer can be quantified. Resting values for each tracer were set to an arbitrary value of one. Values represent means ± SEM
Fig. 2.
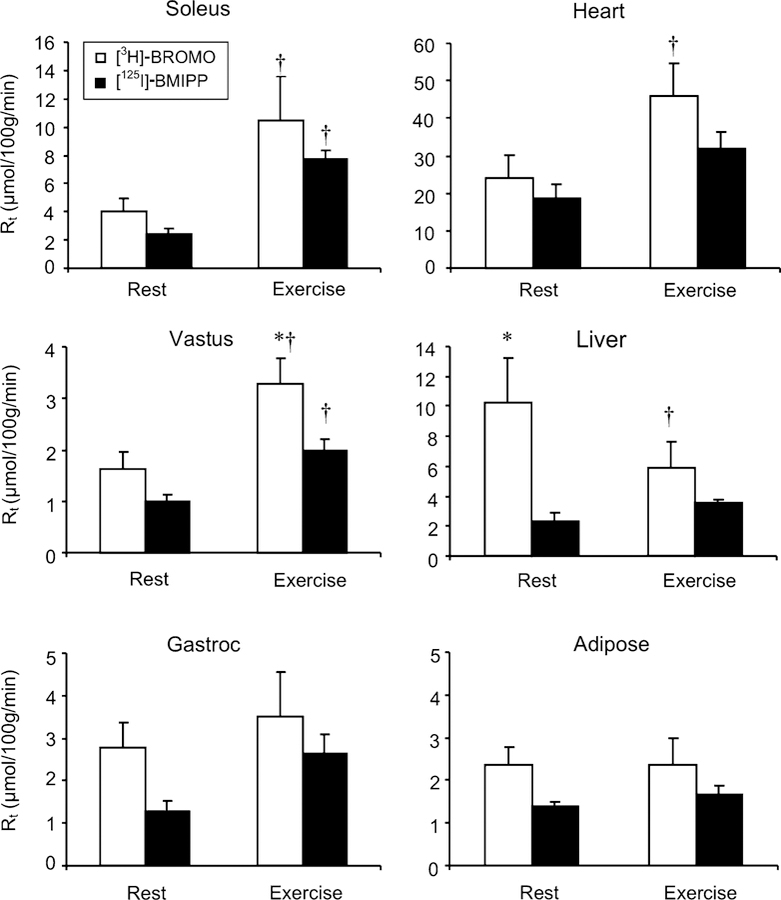
Tissue fatty acid uptake (μmol/100 g/min) for [125I]-BMIPP (filled bars) and [3H]-BROMO (empty bars). Tissues were collected following 40 min of rest or moderate intensity exercise. * Indicates a significant difference between [3H]-BROMO and [125I]-BMIPP within an experimental protocol. † Indicates a significant difference between rest and exercise for a given tracer. Values represent means ± SEM
Tracer Correlations
Correlations of tissue fatty acid uptake between [125I]-BMIPP and [3H]-BROMO are plotted in Fig. 3. R2 values for resting and exercise samples are plotted individually, and an aggregate value also presented. Results of individual muscle types for rest and exercise studies were at least moderately correlated, with aggregate R2 values of 0.39, 0.55, 0.26, and 0.56 for the soleus, vastus lateralis, gastrocnemius and heart respectively. In contrast, data from liver and adipose tissue are poorly correlated between tracers with both treatments.
Fig. 3.
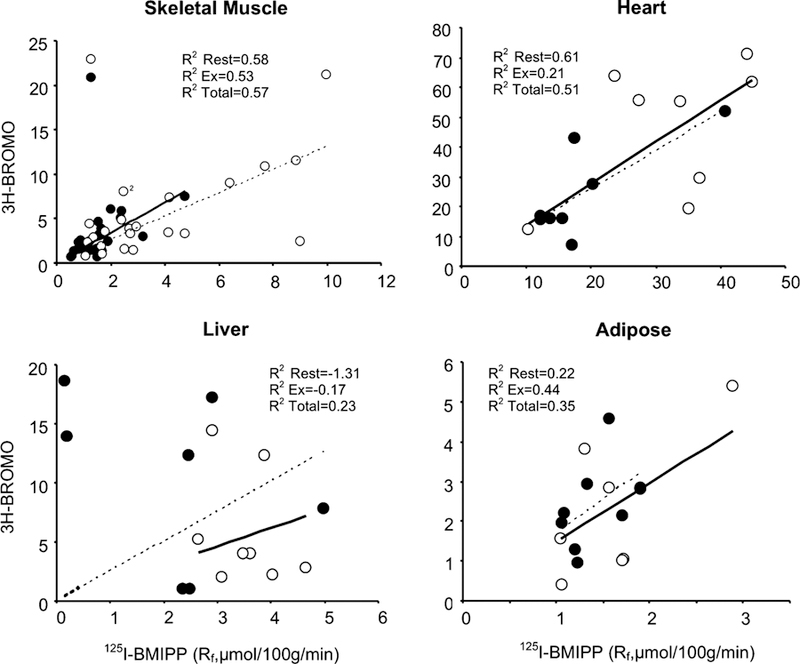
Comparison of fatty acid uptake rates between [3H]-BROMO (y axis) and [125I]-BMIPP (x axis) for individual tissue measurements. Values for resting (open circles) and exercise (closed circles) are shown along with their corresponding R2 values for rest, exercise and total aggregate are shown
Plasma Analysis
Analysis of tracer distribution in resting animals by TLC demonstrated no observable differences between [125I]-BMIPP and [3H]-BROMO at rest. Each tracer showed the expected exponential decay pattern and similar rates of fractional conversion in this state (Table 2). Analysis of fractional conversion during exercise was comparatively greater than exercise at all time points analyzed. Furthermore, results show conversion of [3H]-BROMO to exceed that of [125I]-BMIPP in the latter stages (>20 min) of the experiment (Table 2).
Table 2.
Estimated fractional tracer conversion of [3H]-BROMO and [125I]-BMIPP in plasma over time during rest and exercise
| 2 (%) | 5 (%) | 10 (%) | 15 (%) | 25 (%) | 40 (%) | |
|---|---|---|---|---|---|---|
| Rest | ||||||
| [125I]-BMIPP | 1.2 | 6.3 | 12.4 | 14.3 | 11.8 | 11.4 |
| [3H]-BROMO | 1.0 | 4.0 | 9.2 | 12.7 | 10.5 | 10.4 |
| Exercise | ||||||
| [125I]-BMIPP | 4.6 | 10.4 | 14.6 | 17.3 | 24.7 | 37.0 |
| [3H]-BROMO | 5.3 | 10.6 | 21.2 | 40.4 | 53.5 | 52.9 |
Results represent the % of radioactivity in breakdown products versus [3H]-BROMO and [125I]-BMIPP as assessed by thin layer chromatography
Tracer Tissue Distribution
The intracellular fate of tracers were analyzed by TLC. Results demonstrated tracers to reside in three distinct fractions (Table 3). The first fraction contained phospholipid, monoglyceride, and diglyceride (PL + MG + DG) while others analyzed free fatty acids (FFA), triglyceride (TG). Analysis showed the majority of radioactivity resided in the PL + MG + DG fraction followed by FFA and finally TG. Differences between tracer distributions were minimal. At rest, a lower fraction of [125I]-BMIPP was found in TG in cardiac muscle. During exercise, lower [125I]-BMIPP was found in cardiac FFA and skeletal muscle TG.
Table 3.
Incorporation of [125I]-BMIPP and [3H]-BROMO into various lipid fractions in tissues at rest (n = 5) and exercise (n = 5) as determined by thin layer chromatography
| Tissue | Lipid fraction | [3H]-BROMO | [125I]-BMIPP |
|---|---|---|---|
| Rest | |||
| Heart | PL + MG + DG | 45.5 ± 7.3# | 49.3 ± 9.6# |
| FFA | 29.9 ± 8.7# | 41.4 ± 10.1 | |
| TG | 32.7 ± 4.1 | 9.3 ± 2.3* | |
| Skeletal muscle | PL + MG + DG | 50.7 ± 10.9 | 45.0 ± 11.7 |
| FFA | 36.7 ± 7.1 | 41.2 ± 13.0 | |
| TG | 15.6 ± 6.1 | 14.0 ± 4.3 | |
| Liver | PL + MG + DG | 59.6 ± 6.3 | 37.9 ± 11.5 |
| FFA | 22.2 ± 6.3 | 49.2 ± 13.3 | |
| TG | 19.2 ± 2.5 | 12.9 ± 2.6 | |
| Exercise | |||
| Heart | PL + MG + DG | 69.7 ± 8.9 | 83.4 ± 9.5 |
| FFA | 13.0 ± 1.7 | 2.4 ± 1.0* | |
| TG | 17.3 ± 7.6 | 11.2 ± 6.6 | |
| Skeletal muscle | PL + MG + DG | 43.7 ± 6.1 | 67.4 ± 7.8 |
| FFA | 34.9 ± 5.5 | 22.6 ± 5.7 | |
| TG | 24.4 ± 5.0 | 6.3 ± 1.5* | |
| Liver | PL + MG + DG | 54.0 ± 8.6 | 49.4 ± 15.4 |
| FFA | 25.0 ± 8.7 | 39.4 ± 14.7 | |
| TG | 21.0 ± 8.8 | 11.2 ± 5.3 |
Values are expressed as a percentage of total radioactivity and represent means ± SEM.
PL phospholipid, MG monoglyceride, DG diglyceride, FFA free fatty acids, TG triglyceride.
Represents a significant difference between [125I]-BMIPP and [3H]-BROMO within an experimental protocol
Represents a significant difference with [125I]-BMIPP or [3H]-BROMO between rest and exercise protocols
Discussion
The purpose of this study was to assess and compare [3H]-BROMO and [125I]-BMIPP during rest and a state of accelerated fatty acid metabolism (moderate exercise). Tracers were simultaneously administered so that direct comparisons of tissue fatty acid uptake could be made. Results show good agreement between [3H]-BROMO and [125I]-BMIPP for cardiac and skeletal muscle during rest and exercise. In contrast, liver and epididymal fat pads showed poor correlations under both conditions. At rest, rates of liver [3H]-BROMO uptake were more than double those of [125I]-BMIPP.
Conceptually, both agents are regarded as ‘‘generic’’ long chain fatty acid tracers. The close agreement between these tracers in muscle tissue is reassuring in terms of this usual simplification. There may be tissue specific differences related to precise molecular structure of both fatty acids and fatty acid tracers that are reflected in the differences in adipose and liver. An increase in hepatic extraction and breakdown of [3H]-BROMO compared to [125I]-BMIPP may also play a role. Evidence of increased hepatic [3H]-BROMO breakdown has been previously reported [8]. Intravenous administration of [3H]-BROMO to rodents found liver to have a retention rate of only 77% compared to skeletal muscle that retained over 90% of tracer during 16 min of infusion [8]. Given these and the present findings, it is reasonable to hypothesize that [125I]-BMIPP retention rates may most closely reflect actual liver LCFA uptake. For epididymal fat, we show absolute rates of fatty acid uptake to be similar between tracers. However, correlation of individual animals between [3H]-BROMO and [125I]-BMIPP for this tissue yielded poor results. This discrepancy is likely due to the low rate of fatty acid uptake and tracer detection in this tissue.
To examine fractional conversion of each tracer by tissue, detailed analysis of tracer distribution in plasma over time was conducted by TLC. A known conversion product for [3H]-BROMO is 3H2O. Previous analysis of the tracer show conversion to be ~5% of the dose at 16 min in sedentary rats [8]. This value compares favorably with the present study that estimates a conversion of ~4% at 15 min at rest. Like [3H]-BROMO, [125I]-BMIPP becomes trapped in α-oxidation [24]. End products of this reaction yield CO2, a fatty acid shortened by one carbon and a methyl substitution at the second carbon, which in the case of [123I]-BMIPP and [131I]-BMIPP is 2-(ρ-iodophenyl) acetic acid (IPC2) [21]. Previous analysis of fractional conversion of [123I]-BMIPP show ~11% of injected dose is released at 60 min in humans while perfusion of isolated rat hearts have a conversion of ~12% following 3 h. In the present study, we show conversion of BMIPP to be ~12% of total radioactivity at 40 min. Comparison of [125I]-BMIPP and [3H]-BROMO during rest, show comparable levels of conversion over time. Given this, either tracer is suitable for resting studies.
Elevation of metabolic demands by moderate exercise resulted in increases fatty acid uptake in skeletal and cardiac muscle for both [125I]-BMIPP and [3H]-BROMO. Generally, tissue extraction of fatty acids doubled with exercise. This finding confirms previous observations of Oakes and colleagues [8] who have shown [3H]-BROMO is not affected by metabolic (oxidative vs. nonoxidative) status in skeletal muscle. Of note, differences in liver fatty acid uptake at rest were not observed during exercise. This may be due to a diversion of blood flow away from this organ during exercise. In addition to increasing tracer tissue uptake, exercise also resulted in greater tissue excretion of tracers at all time points measured. At 40 min, 37 and 53% of the initial tracer dose were present in conversion products for [125I]-BMIPP and [3H]-BROMO respectively. This excretion is due to elevated α and ω-oxidation of fatty acids with exercise or simply back diffusion. However, despite increasing fractional conversion with exercise, we show good agreement between tracers in cardiac and skeletal muscle.
From a technical perspective, [3H]-BROMO has advantages primarily related to the label-induced limitations of [125I]-BMIPP. These include its shorter half-life, elevated biological risk and synthesis. [125I]-BMIPP has a half life of 60 days compared to [3H]-BROMO which is 12 years. Given this, corrections for tracer decay with [125I]-BMIPP need to be considered, when studies extend over days to weeks. In the present study, a single batch of [125I]-BMIPP was employed and studies occurred over a 1-month period. All results were scaled to include corrections for tracer decay. Another consideration involves the biological risk involved with working these isotopes. [125I]-BMIPP is a gamma and X-ray emitter and although used in low doses, still poses a greater hazard than [3H]-BROMO which is a lower energy beta emitter. Finally, the labelling for [3H]-BROMO is complex but the compound is currently commercially available, while [125I]-BMIPP is straightforward to label but requires specific precautions related to iodination, and currently must be performed in an institutional setting. Finally, in studies where multiple tracers are employed, there may be specific limitations related to other radioactive labeled compounds in concurrent use. These issues must be considered prior to commencing studies with either tracer.
In conclusion, this study directly compares isotopic analogs for the measurement of fatty acid kinetics in vivo. Results show both analogues are effective for the measurement of FFA uptake and clearance in plasma. We showed a high correlation between tracers for cardiac and skeletal muscle. However, in both the liver and adipose tissue, derived rates of uptake diverged depending on the specific tracer. As a result, studies employing these or other fatty acid tracers in these tissues must be interpreted with caution. Generally, technical considerations argue for [3H]-BROMO. However, when studies are prolonged (>20 min) and employ experimental manipulations requiring elevated metabolism, we show the preferable analog to be [125I]-BMIPP due to its lower rates of tissue excretion.
Acknowledgments
This work would not have been possible with the contribution of BMIPP from Dr. Russ Knapp, Nuclear Medicine Program, Oak Ridge National Laboratory, TN. The authors gratefully acknowledge his generosity and technical advice. JS holds salary support awards from the Alberta Heritage Foundation for Medical Research, the Heart and Stroke Foundation and the Canadian Diabetes Association. This work is supported by the CIHR (JS), Genome Canada (JS), NIDDK (DK-54902, U24-DK-59637). The authors wish to acknowledge the technical contributions of Wanda Sneed, Angela Slater, Carla Harris and Freyja James.
Abbreviations
- [125I]-BMIPP
[125I]-15-(ρ-Iodophenyl)-3-R,S-methylpentadecanoic acid
- ([3H]-BROMO)
[9,10–3H]-(R)-2-Bromopalmitate
- Kt
Tissue long chain fatty acid clearance
- LCFA
Long chain fatty acid
- NEFA
Nonesterified fatty acid
- Rt
Index of tissue long chain fatty acid uptake
- TLC
Thin layer chromatography
Contributor Information
Jane Shearer, Department of Molecular Physiology and Biophysics, Vanderbilt University, Nashville, TN, USA.
Kimberly R. Coenen, Department of Molecular Physiology and Biophysics, Vanderbilt University, Nashville, TN, USA
R. Richard Pencek, Department of Molecular Physiology and Biophysics, Vanderbilt University, Nashville, TN, USA.
Larry L. Swift, Department of Pathology, Vanderbilt University, Nashville, TN, USA
David H. Wasserman, Department of Molecular Physiology and Biophysics, Vanderbilt University, Nashville, TN, USA The Mouse Metabolic Phenotyping Center, Vanderbilt University, Nashville, TN, USA.
Jeffrey N. Rottman, Department of Cardiology, Vanderbilt University, Nashville, TN, USA The Mouse Metabolic Phenotyping Center, Vanderbilt University, Nashville, TN, USA.
References
- 1.Renstrom B, Rommelfanger S, Stone CK, DeGrado TR, Carlson KJ, Scarbrough E, Nickles RJ, Liedtke AJ, Holden JE (1998) Comparison of fatty acid tracers FTHA and BMIPP during myocardial ischemia and hypoxia. J Nucl Med 39(10):1684–1689 [PubMed] [Google Scholar]
- 2.Stone CK, Pooley RA, DeGrado TR, Renstrom B, Nickles RJ, Nellis SH, Liedtke AJ, Holden JE (1998) Myocardial uptake of the fatty acid analog 14-fluorine-18-fluoro-6-thia-heptadecanoic acid in comparison to beta-oxidation rates by tritiated palmitate. J Nucl Med 39(10):1690–1696 [PubMed] [Google Scholar]
- 3.Rottman JN, Bracy D, Malabanan C, Yue Z, Clanton J, Wasserman DH (2002) Contrasting effects of exercise and NOS inhibition on tissue-specific fatty acid and glucose uptake in mice. Am J Physiol Endocrinol Metab 283(1):E116–E123 [DOI] [PubMed] [Google Scholar]
- 4.Coburn CT, Knapp FF Jr, Febbraio M, Beets AL, Silverstein RL, Abumrad NA (2000) Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem 275(42):32523–32529 [DOI] [PubMed] [Google Scholar]
- 5.Zhao C, Shuke N, Okizaki A, Yamamoto W, Sato J, Ishikawa Y, Ohta T, Hasebe N, Kikuchi K, Aburano T (2003) Comparison of myocardial fatty acid metabolism with left ventricular function and perfusion in cardiomyopathies: by 123I-BMIPP SPECT and 99mTc-tetrofosmin electrocardiographically gated SPECT. Ann Nucl Med 17(7):541–548 [DOI] [PubMed] [Google Scholar]
- 6.Fujibayashi Y, Yonekura Y, Kawai K, Yamamoto K, Tamaki N, Konishi J, Yokoyama A, Torizuka K (1988) [Basic studies on I-123-beta-methyl-p-iodophenylpentadecanoic acid (BMIPP) for myocardial functional diagnosis: effect of beta-oxidation inhibitor]. Kaku Igaku 25(10):1131–1135 [PubMed] [Google Scholar]
- 7.Verberne HJ, Sloof GW, Beets AL, Murphy AM, van Eck-Smit BL, Knapp FF (2003) 125I-BMIPP and 18F-FDG uptake in a transgenic mouse model of stunned myocardium. Eur J Nucl Med Mol Imaging 30(3):431–439 [DOI] [PubMed] [Google Scholar]
- 8.Oakes ND, Kjellstedt A, Forsberg GB, Clementz T, Camejo G, Furler SM, Kraegen EW, Olwegard-Halvarsson M, Jenkins AB, Ljung B (1999) Development and initial evaluation of a novel method for assessing tissue-specific plasma free fatty acid utilization in vivo using (R)-2-bromopalmitate tracer. J Lipid Res 40(6):1155–1169 [PubMed] [Google Scholar]
- 9.Hegarty BD, Cooney GJ, Kraegen EW, Furler SM (2002) Increased efficiency of fatty acid uptake contributes to lipid accumulation in skeletal muscle of high fat-fed insulin-resistant rats. Diabetes 51(5):1477–1484 [DOI] [PubMed] [Google Scholar]
- 10.Knapp FF Jr., Goodman MM, Callahan AP, Kirsch G (1986) Radioiodinated 15-(p-iodophenyl)-3,3-dimethylpentadecanoic acid: a useful new agent to evaluate myocardial fatty acid uptake. J Nucl Med 27(4):521–531 [PubMed] [Google Scholar]
- 11.Knapp FF Jr., Ambrose KR, Goodman MM (1986) New radioiodinated methyl-branched fatty acids for cardiac studies. Eur J Nucl Med 12(Suppl):S39–S44 [DOI] [PubMed] [Google Scholar]
- 12.Dudczak R, Schmoliner R, Angelberger P, Knapp FF, Goodman MM (1986) Structurally modified fatty acids: clinical potential as tracers of metabolism. Eur J Nucl Med 12(Suppl):S45–S48 [DOI] [PubMed] [Google Scholar]
- 13.Noriyasu K, Mabuchi M, Kuge Y, Morita K, Tsukamoto T, Kohya T, Kitabatake A, Tamaki N (2003) Serial changes in BMIPP uptake in relation to thallium uptake in the rat myocardium after ischaemia. Eur J Nucl Med Mol Imaging 30(12):1644–1650 [DOI] [PubMed] [Google Scholar]
- 14.Fujino T, Ishii Y, Takeuchi T, Hirasawa K, Tateda K, Kikuchi K, Hasebe N (2003) Recovery of BMIPP uptake and regional wall motion in insulin resistant patients following angioplasty for acute myocardial infarction. Circ J 67(9):757–762 [DOI] [PubMed] [Google Scholar]
- 15.Narita M, Kurihara T (2003) Is I-123-beta-methyl-p-iodophenyl-methylpentadecanoic acid imaging useful to evaluate asymptomatic patients with hypertrophic cardiomyopathy? I-123 BMIPP imaging to evaluate asymptomatic hypertrophic cardiomyopathy. Int J Cardiovasc Imaging 19(6):499–510 [DOI] [PubMed] [Google Scholar]
- 16.Yamauchi S, Takeishi Y, Minamihaba O, Arimoto T, Hirono O, Takahashi H, Miyamoto T, Nitobe J, Nozaki N, Tachibana H, Watanabe T, Fukui A, Kubota I (2003) Angiotensin-converting enzyme inhibition improves cardiac fatty acid metabolism in patients with congestive heart failure. Nucl Med Commun 24(8):901–906 [DOI] [PubMed] [Google Scholar]
- 17.Ito T, Hoshida S, Nishino M, Aoi T, Egami Y, Takeda T, Kawabata M, Tanouchi J, Yamada Y, Kamada T (2001) Relationship between evaluation by quantitative fatty acid myocardial scintigraphy and response to beta-blockade therapy in patients with dilated cardiomyopathy. Eur J Nucl Med 28(12):1811–1816 [DOI] [PubMed] [Google Scholar]
- 18.Hegarty BD, Furler SM, Oakes ND, Kraegen EW, Cooney GJ (2004) PPAR activation induces tissue specific effects on fatty acid uptake and metabolism in vivo—a study using the novel PPAR {alpha}/{gamma} agonist Tesaglitazar. Endocrinology p en.2004-0260 [DOI] [PubMed]
- 19.Edgley AJ, Thalen PG, Dahllof B, Lanne B, Ljung B, Oakes ND (2006) PPAR[gamma] agonist induced cardiac enlargement is associated with reduced fatty acid and increased glucose utilization in myocardium of Wistar rats. Eur J Pharmacol 538(1–3):195. [DOI] [PubMed] [Google Scholar]
- 20.Petersen HA, Fueger PT, Bracy DP, Wasserman DH, Halseth AE (2003) Fiber type-specific determinants of Vmax for insulin-stimulated muscle glucose uptake in vivo. Am J Physiol Endocrinol Metab 284(3):E541–E548 [DOI] [PubMed] [Google Scholar]
- 21.Kropp J, Ambrose KR, Knapp FF Jr, Nissen HP, Biersack HJ (1992) Incorporation of radioiodinated IPPA and BMIPP fatty acid analogues into complex lipids from isolated rat hearts. Int J Rad Appl Instrum B 19(3):283–288 [DOI] [PubMed] [Google Scholar]
- 22.Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226(1):497–509 [PubMed] [Google Scholar]
- 23.Morrison WR, Smith LM (1964) Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J Lipid Res 53:600–608 [PubMed] [Google Scholar]
- 24.Mannaerts GP VVPP (1993) Metabolic role of mammalian peroxisomes, in peroxisomes: biology and importance in toxicology and medicine. In: Gibson G, Lake BG (eds) CRC, pp 39–50 (in press) [Google Scholar]


