Abstract
Purpose:
This review discusses the importance of understanding the impact of genetic factors on adolescent substance use within a developmental framework. Methods for identifying genetic factors, relevant endophenotypes and intermediate phenotypes, and gene-environment interplay effects will be reviewed.
Findings:
Prior work supports the role of polygenic variation on adolescent substance use. Mechanisms through which genes impact adolescent phenotypes consist of differences in neural structure and function, early temperamental differences, and problem behavior. Gene-environment interactions are characterized by increased vulnerability to both maladaptive and adaptive contexts.
Summary:
Developmental considerations in genetic investigations highlight the critical role that polygenic variation has on adolescent substance use. Yet, determining what to do with this information, especially in terms of personalized medicine, poses ethical and logistic challenges.
Keywords: adolescence, genes, gene x environment, substance use, intermediate phenotypes, neurogenetics
Introduction
Numerous studies supporting the role that genetic variation has on clinical syndromes, including substance use disorders (SUD), have emerged [1, 2]. However, the detection of specific genes involved in pathological outcomes, mechanisms underlying genetic effects, and replicating findings have been challenging. This is especially true in the adolescent literature. This is partly due to the reliance on binary diagnostic phenotypes (i.e., SUD yes/no), the joint contribution of genetic and environmental factors, and not framing these investigations within a developmental framework. In terms of the latter, SUD is best understood as the end result of a gradual progression through different stages of use (i.e., initiation, regular use, heavy use, and problematic use; see Figure 1), with each stage characterized by unique etiological influences [2]. Cascade models posit that early manifestations of SUD can be detected in childhood as difficult temperament (e.g., low behavioral control), which increases risk for problem behavior in early adolescence, and in turn, greater risk of substance use (SU) initiation in mid- to late-adolescence [3]. Moreover, research indicates that the relative contribution of genetic and environmental influences on SU varies across both developmental age, as well as stage of SU (see Figure 2) [4–6]. Thus, longitudinal investigations are critical when examining the etiology of adolescent SU.
Figure 1.
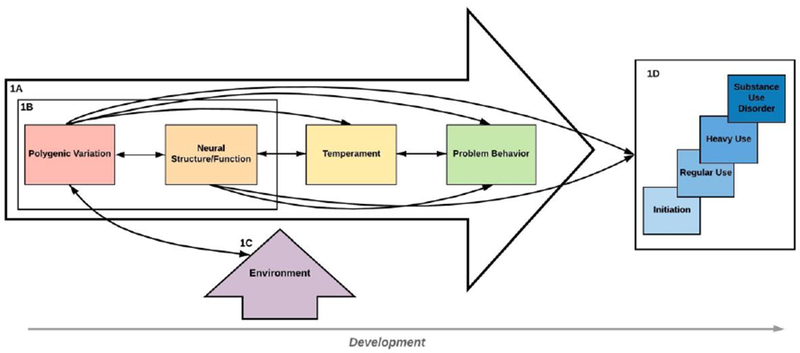
The Developmental Progression of Polygenic and Environmental Factors on Adolescent Substance Use Outcomes. (1A) Multilevel Developmental Pathways. Depicts pathways through which polygenic variation impacts adolescent substance use outcomes across development via endophenotypes (e.g., neural function) and intermediate phenotypes (e.g., temperament). (1B) Neurogenetics. Combines genetics and individual differences in brain structure/function reflecting biological mechanisms associated with substance use risk. (1C) Gene-Environment Interplay. Consists of gene-environment correlation (rGE, depicted by the double-headed arrow between polygenic variation and environmental exposures), as well as gene x environment interactions (GxE), whereby genetic factors impact an individual’s susceptibility to environmental exposures. (ID) Stage Sequential Nature of Substance Use. Substance use behavior typically progresses gradually through different stages of use with escalating severity.
Figure 2.
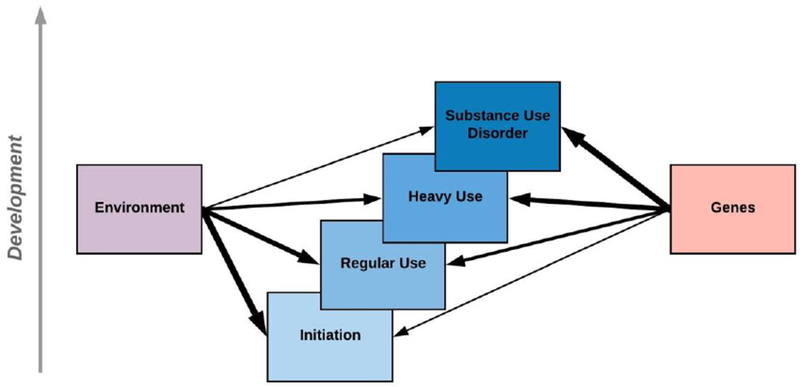
Relative Contribution of Environment and Genes on Substance Use Outcomes across Development. Environmental exposures tend to have a stronger impact on earlier stages of substance use that typically occur during early adolescence. In contrast, genes tend to have a stronger impact on later stages of use that typically arise in adulthood. The change in strength of these associations across development are depicted by the weight of each line.
This review will discuss these important developmental considerations, as well as the identification of relevant genetic factors, endophenotypes and intermediate phenotypes, and interactions with genes. The focus is on alcohol, cigarette, and marijuana use as these substances are typically initiated during adolescence [7].
Identifying Relevant Molecular Genetic Factors
Several methods exist in the identification of relevant genetic factors. Quantitative methods infer genetic and environmental contributions to adolescent SU outcomes primarily through twin and adoption studies. This review focuses on molecular genetic approaches that involve measured genetic variance (i.e., polymorphisms). Clinical syndromes that are highly heritable (e.g., schizophrenia) tend to have the greatest success in terms of gene identification [8]. Less progress has been made with regard to adolescent SU outcomes as they are less heritable, and thus require much larger samples to account for a nontrivial degree of variance [9].
Candidate Genes
Candidate gene association studies are based on case-control designs focusing on binary phenotypes. Candidate gene studies typically focus on factors that are purported to have functional relevance, such as those impacting the functioning of neurotransmitter systems [10, 11•]. Namely, genetic variants in the serotonergic (e.g., 5-HTTLPR) [12, 13], dopaminergic (e.g., DRD4, DRD2) [14, 15], and GABAergic (e.g., GABRA2, SLC6A1) [1, 14, 6••, 10, 16] neurotransmission, in addition to those involved in the activity of the oxytocin (e.g., OXTR) [12, 17], the encephalic opioid (e.g., OPRM1) [18–21], and nicotinic receptor (CHRNA5-CHRNA3-CHRNB4) [22] systems have been widely examined. Candidate gene studies provide an in-depth understanding of mechanisms through which these variants impact adolescent SU and how their effect may strengthen or weaken over time in comparison to environmental contexts. Yet, candidate gene studies have been subject to overwhelming criticism given difficulties with replication [9]. Furthermore, it is unlikely that only a few variants determine adolescent SU.
Genome-Wide Association Studies
Genome-wide association studies (GWAS) reflect an exploratory, hypothesis-free approach for discovering genotype-phenotype associations by scanning the entire genome. After genotyping millions of single nucleotide polymorphisms (SNPs), researchers run correlations to find which SNP is associated with a phenotype using a stringent significance threshold (e.g., p <.05 x 10−8)[2]. Accordingly, GWAS research necessitates very large samples.
Traditionally, GWAS have been conducted in adults with very few studies focused on adolescent SU [2]. One exception is a GWAS on longitudinal consumption of alcohol from childhood to early adulthood [1]. Findings support significant associations between GABA transporter 1, SLC6A1 (rs11710497, rs6778281), and LOC100129340 (rs7031417, rs17053864, rs7019589) and adolescent alcohol consumption [1]. Another study found significant associations between ATP2C2 (rs1574587) and age at first cannabis use [23]. Some researchers have used results from adult GWAS to inform hypothesis-driven analyses with adolescent samples. For example, GABRA2, a genetic factor originally identified among adults at risk for SUD [24], has been the focus of several adolescent studies [25, 6••, 10, 16], GABRA2 variation has been associated with adolescent externalizing behavior, but not problematic alcohol or drug use, suggesting that genetic risk in adolescence may be nonspecific and impact general problem behaviors [16]. This is consistent with prior research indicating that the impact of genetic influence on SU increases with age (see Figure 2), with some reporting peak effects at age 30 [26]. GWAS focused on identifying genetic factors associated with early stages of SU could have utility in extending findings outside of those derived from adult samples.
There are some limitations to GWAS, however. Genetic factors reaching genome-wide significance in one study may not replicate in another [27]. In some cases, the same SNP may be identified across studies but the risk allele differs [27]. It is possible that loci demonstrating modest associations with SU may be overlooked by the discovery GWAS [28]. Lastly, the total variance accounted for in SU by factors identified through GWAS is still low [29, 2].
Genome-Wide Polygenic Scores
Researchers have recently adopted approaches that account for various genetic factors based on findings from GWAS. First, an existing discovery GWAS is leveraged to identify SU markers. Next, a score consisting of a weighted sum or unweighted counts across top-ranking markers are calculated across participants in a replication sample. This composite index is then used to assess links between the score and SU. The term polygenic risk score was first used to describe this approach. Given that some variants may be related to traits that confer risk for adolescent SU via specific mechanisms, but are not problematic per se, a more general term, genome-wide polygenic scores (GPSs), has been adopted (see Figure 1, 1B) [30].
A recent GPS study sought to determine whether genetic factors discovered in an adult smoking GWAS were associated with the progression of cigarette use from childhood to adulthood [4]. The GPS, comprised of multiple variants from the CHRNA5-CHRNA3-CHRNB4 and CYP2A6 gene clusters, was unrelated to smoking initiation. Yet, those with a higher GPS were more likely to convert to daily smoking as teenagers, progressed more rapidly from initiation to heavy use, were persistent heavy users, developed dependence more frequently, and reported more failed cessation attempts [4]. Another study focused on a GPS derived from an adult smoker GWAS that included similar variants found support for developmental moderation [31]. The GPS was associated with cigarettes smoked per day at ages 20 and 24, but not during adolescence. These studies support the use of GPSs to investigate polygenic effects on the developmental progression of SU.
There are, however, some limitation to the GPS approach. This approach assumes additivity and neglects interactions between genes. GPSs do not address underlying molecular mechanisms that may inform preventative interventions. GPSs only show modest improvement in explaining phenotypic variance [32, 31]. Lastly, GPSs are constrained by both the phenotypes of interest and the sample sizes in discovery samples. Thus, most GPSs may not be relevant to adolescents given that most GWAS focus on adult samples.
Genome-Wide Complex Trait Analysis
It is estimated that the heritability of most quantitative traits is approximately 50% [2, 21]. Approaches discussed thus far only explain a small degree of phenotypic variance in adolescent SU. The discrepancy between the phenotypic variance explained by quantitative genetics and variance explained using molecular approaches is known as the missing heritability problem [2]. Genome-wide complex trait analysis (GCTA) estimates heritability by considering the additive effect of all SNPs simultaneously as a random effect in a mixed linear model [33,34]. A genetic relatedness matrix is then used to predict the similarity in phenotypic relatedness. One study demonstrated that SNPs collectively explained 25% of the variance in marijuana use initiation using GCTA [35]. Another study demonstrated that the aggregate SNP effect accounts for 21% (Alcohol Dependence), 36% (Nicotine Use/Dependence), 38% (Alcohol Consumption), and 45% (Drug Dependence) of the heritable variance in these traits using GCTA [36]. These results indicate that most of the heritability in adolescent SU may be “hiding” rather than “missing” as the effect sizes needed to reach genome-wide significance for individual SNPs using traditional methods may be too small [34].
However, although GCTA is able to more effectively address missing heritability, it does not inform mechanistic pathways [29, 2]. Furthermore, even with GCTA, a majority of heritable variance remains unexplained [36] and the remaining genetic variance may be due in part to gene x environment (GxE) or gene x gene interactions [32]. There has also been some debate regarding the reliability of GCTA heritability estimates [37].
Endophenotypes and Intermediate Phenotypes
Once genetic factors have been identified, a critical step is determining developmental pathways linking genes with SU. Integral to this investigation is not only understanding that SU progresses through several sequential stages (see Figure 1, 1D), but recognizing that SUD represents an endpoint of developmental processes, including neurobiology, individual differences in sensitivity to the effects of substances, and heritable temperamental traits [2]. Cascade models outline a systematic progression from genetic factors, to neurobiological differences, to childhood temperament, to adolescent problem behaviors, to more problematic behaviors in adulthood, such as illicit SU (see Figure 1, 1A) [3]. Recent investigations characterizing genetic effects on downstream risk for adolescent SU have made important contributions to the field by moving beyond atemporal conceptualizations and binary syndromes. This work is characterized by endophenotype and intermediate phenotype approaches. Endophenotypes are heritable biologically-based phenotypes that underlie and predict clinical syndromes but is not part of the syndrome itself [38]. A new area of endophenotype investigation is neurogenetics (Figure 1, 1B), used to identify differences in the brain related to genetic variants and SU [39•, 25, 40]. Intermediate phenotypes are traits or behaviors that do not reflect a specific endogenous trait or biological process [2]. Intermediate phenotypes often represent more observable traits and often bear some resemblance to the outcome of interest. An emerging investigation in the field of adolescent SU is examining childhood temperament (e.g., impulsivity), as well as adolescent externalizing (e.g., aggression) and internalizing (e.g., depression) problems as intermediaries in the link between genetic variance and SU [41].
Neurogenetics
The field of neurogenetics combines genetics and neurobiological testing (e.g., functional magnetic resonance imaging [fMRI]) to link how genetic and neural differences influence clinical syndromes. Neurogenetics is oftentimes referred to as “imaging genetics.” Although neuroimaging, such as fMRI, makes up a large part of neurogenetic research, other forms of neurobiological testing (e.g., electrophysiology) are also examined. Thus, the term neurogenetics is a more accurate representation of current practices [40].
Neurogenetics allows researchers to more accurately map biological pathways of adolescent SU to ultimately improve current intervention methods [42••]. One study demonstrated a conditional pathway to problematic alcohol use among youth homozygous for the corticotropin-releasing hormone receptor 1 (CRHR1) G allele [39•]. These youth had greater right ventrolateral prefrontal cortex (rVLPFC) engagement during a negative emotional word task compared to A allele carriers, which in turn led to lower levels of negative emotionality. Moreover, this pathway was only significant among those without a history of childhood stress [39•]. Interdisciplinary methods such as these help to identify unique mechanisms leading to SU.
Neurogenetic research has focused primarily on clinical syndromes in adults [40]. Fewer studies combine neurogenetics and development. Understanding neurobiological processes related to SU risk in adolescence could have significant utility given that the brain is not fully matured, especially in terms of executive control and the incentive-motivation system that tend to impact risk-taking behavior [15]. For example, one study examined the impact of GABRA2 on nucleus accumbens (NAcc) activation during a monetary reward task spanning childhood to adulthood [25]. Findings indicate that individuals carrying the minor G allele demonstrated greater NAcc activation specifically during adolescence. Moreover, NAcc activation mediated the effect of GABRA2 on later alcohol problems [25] indicating that developmental examinations of the neural mechanisms through which genetic factors influence SUD has direct clinical implications.
Sensitivity to Substance Use
Sensitivity to substances is heritable and can increase or decrease risk for SUD [2]. Prior work has focused on genetic variants in alcohol dehydrogenase (ADH1B) and aldehyde dehydrogenase (ALDH2) that are overrepresented among East Asian populations [43]. Youth with variants of ADH1B and ALDH2 report flushing, nausea, and headaches when drinking alcohol given the accumulation of acetaldehyde and reduced enzyme activity. Studies indicate that these variants are largely protective, as these youth tend to report lower rates of binge drinking and alcohol consumption [44], which may be due in part to lower positive drinking expectancies [45]. Other studies indicate that adolescents carrying the 5HTTLPR S allele are at increased risk of SUD as this variant is associated with lower responses to alcohol (i.e., needing to drink more to feel the effects of alcohol) [13]. Carriers of the G allele of the OPRM1 receptor gene (rs1799971) also reported increased rates of drinking to enhance positive affect compared to those without the variant, and these motives mediated the effect between genetic risk and alcohol-related problems [19]. A separate study demonstrated that adolescents with the same OPRM1 variant were at risk for SU due to reports of greater subjective feelings of intoxication, stimulation and sedation, and increases in positive mood with alcohol administration [20].
This work has been extended to nicotine sensitivity. Specifically, a SNP located upstream from CHRNB2 (rs2072658) was associated with the initial subjective response to both nicotine and alcohol, whereby those with the rare allele reported increased subjective response [46]. Similarly, youth carrying the CHRNB2 (rs2072660) T allele were less likely to report dizziness or nausea shortly after their first use of tobacco compared to those with the C allele [46]. Findings supporting genetic associations on initial level of response to substances is of critical importance as sensitivity to substances is one of the strongest predictors of future SUD [13, 47].
Temperamental Pathways
An intermediate phenotype approach has been utilized to understand biological mechanisms underlying the association between genetic factors and adolescent SU via temperamental differences. Temperament is viewed as biologically-based early emerging individual differences in attentional, emotional, motor, and self-regulation processes that modulate reactivity [48]. In particular, temperament constructs reflecting the ability to effectively modulate control in response to environmental demands (i.e., resiliency), the ability to adaptively modulate behavior in response to immediate reward (i.e., behavioral control), and negative emotionality have been examined given their association with adolescent behavior problems (e.g., externalizing and internalizing problems) [49, 48]. In turn, internalizing (e.g., depression) [50] and externalizing (e.g., rule-breaking, aggression) [51] problems are strong precursors of adolescent SU.
One of the first genetic studies focused on intermediate phenotypes of adolescent SU found that although GABRA2 (e.g., rs279858 G allele) variants did not have a direct effect on SU outcomes, GABRA2 variants did have an indirect effect on SU via rule-breaking [16]. These findings were extended to explore neural and temperamental mechanisms linking the same GABRA2 variants and problem behaviors [52]. Carrying the GABRA2 risk variant that is associated with reduced activation to emotional stimuli had a potential tradeoff. When exposed to negative emotional stimuli, reduced activation in certain areas of the brain predicted greater resiliency. Yet, reduced brain activation in other areas of the brain interfered with experiencing pleasure, thus increasing risk for later problem behavior [52].
Other work examined how certain genetic factors are likely to impact adolescent SU via childhood temperament and adolescent problem behaviors [10, 11•]. For some, genetic risk characterized by genetic variants of GABRA6 (rs3811995) is expressed as early difficulties controlling impulses [10]. Those carrying the CC genotype had lower behavioral control; in turn, lower behavioral control predicted greater externalizing behavior, which predicted high SU. Genetic risk characterized by genetic variants of SLC6A2 (rs36021) is expressed as early difficulties modulating distress. Those carrying the AA genotype had lower resiliency; in turn, lower resiliency predicted greater externalizing behavior, which also predicted high rates of SU [10]. Findings were also extended to internalizing pathways, with evidence for the role of resiliency and depression as mediators in the association between BDNF (rs6265) and adolescent SU [11•]. A separate etiological pathway involving depression as a mediator between NPY (rs3037354) and adolescent SU also emerged [11•].
Genetic Interactions with Environmental Contexts and Interventions
Research demonstrates that gene-environment interplay is critical in the etiology of adolescent SU [53••]. One form of gene-environment interplay is evocative gene-environment correlation (rGE; Figure 1, 1c). That is, an adolescent’s genetically-influenced behavior may evoke a certain reaction from the environment. For example, adolescents with genetic propensities for low behavioral control may be challenging for parents, and evoke ineffective disciplinary practices [28, 54]; the combined effect increases SU risk. Another form of gene-environment interplay, GxE, exists when a difference in response to an environmental exposure differs by genotype. Studies related to interventions are known as gene by intervention interactions (GxI).
GxE Interactions
Various studies have examined parenting practices, peer behavior, and neighborhood contexts in combination with an adolescent’s genetic makeup on SU. For example, one study demonstrated that the association between permissive parenting on alcohol use was greater among DRD2 (rs1800497) T allele carriers [55]. Another study found that adolescents with more copies of the 5HTTLPR S allele had greater odds of smoking initiation in environments where a higher proportion of peers smoked compared to those without this genetic factor [56]. A more recent study determined that African American youth scoring high on a conduct disorder GPS and living in disadvantaged neighborhoods were more likely to meet criteria for a marijuana use disorder compared to those with a low GPS [57].
Collectively, GxE findings indicate that environmental factors and genetic makeup may have a greater combined impact on adolescent SU than when considered separately. Yet, prior work indicates that the relative contribution of genes and environmental influences on SU varies across stages. One twin study demonstrated that a majority of the variance in alcohol use initiation was accounted for by environmental influences (~65%) compared to genetic factors (~26%) [58]. In contrast, approximately half (47%) of the variance in progression to problematic use was attributable to environmental factors, while approximately one-third (35%) was due to genetic factors [58]. Thus, environmental factors tend to have a stronger impact on early stages of SU, such as initiation, while genetic factors have a greater impact on more problematic stages of SU, as depicted in Figure 2.
GxI Interactions
Studies focused on how genetic factors impact response to randomized treatments targeting adolescent SU have increased in popularity. This work may help inform why certain interventions are especially successful for some adolescents. The advantage of adopting randomized designs helps rule out alternative gene-environment effects and provides more robust causal associations. One of the first GxI studies demonstrated that adolescents carrying the 5HTTLPR S allele initiated risk behavior at higher rates than adolescents with the same genetic risk assigned to a preventive intervention condition [59]. Moreover, youth with the S allele initiated risk behavior at higher rates than adolescents with no genetic risk assigned to either condition [59]. A follow-up study that focused on the same intervention and the 7-repeat allele of the dopamine D4 receptor gene (DRD4) demonstrated not only that those carrying the risk allele assigned to the control group had a greater rate of escalation in SU compared to those assigned to the preventive intervention, but that those carrying the risk allele were also more responsive to the intervention compared to adolescents without this allele [60]. These findings indicate that in some cases, genetic variants may increase susceptibility to both maladaptive as well as adaptive environmental contexts in a “for-better-and-for-worse” manner [61], consistent with the differential susceptibility hypothesis described below.
Diathesis-Stress, Vantage Sensitivity, Differential Susceptibility
Traditionally, GxE studies have been framed within diathesis-stress frameworks. Diathesis-stress models represent what some have characterized the “dark side” of GxE interactions [62], whereby youth with a specific genetic variant are negatively affected by a maladaptive environment, while youth without this variant are unaffected. Vantage sensitivity models represent what some have termed the “bright side” of GxE interactions [63], whereby youth with a specific genetic variant are positively affected by adaptive environments (e.g., interventions), while those without this variant are unaffected. Interactions consistent with the differential susceptibility hypothesis represent youth that have increased susceptibility to environmental contexts spanning the dark and bright side continuum, while those without this variant are unaffected. There are a growing number of studies demonstrating that traditional conceptualizations of genetic variants as purely risk factors may be inaccurate and may be best conceptualized as plasticity factors [64, 59, 65, 66].
GxE Interactions Across Development
An important next step for GxE research is to include developmental considerations given that some interactions may be age-specific (e.g., GxExD) [6••, 2]. Progress in this area has been hampered by a lack of statistical methods able to capture the inherent complexity of GxE over time. A new methodological approach, time-varying effect modeling (TVEM), allows the effect of interest, such as GxE, to vary as a complex function of age [33]. One study demonstrated that a specific intervention reduced alcohol misuse among youth carrying the GABRA2 (rs279845) TT genotype from ages 12.5 and 17 but did not reduce alcohol misuse among youth without genetic risk at any age using TVEM [6••]. These findings indicate not only which adolescents may benefit most from preventive interventions; they help pinpoint periods when youth may be most receptive to treatment gains.
Conclusion
Understanding the emergence of adolescent SU requires a multilevel perspective spanning across genes, neurobiology, temperament, problem behavior, and key socialization factors. Interdisciplinary collaborations, improved genotyping technologies, and cutting-edge statistical methods have advanced the adolescent SU field. Mechanistic characterizations reflecting pathways through which genes impact adolescent SU via neurobiological differences, early childhood temperament, and problem behaviors may improve the nosology and prevention of adolescent SU. Prior work has also demonstrated that genetic factors can impact the degree to which adolescents are susceptible to both maladaptive and adaptive socialization contexts. The impact of genes on SU outcomes is developmentally-specific with stronger effects on mature phenotypes (e.g., SUD), compared to environmental exposures that have the strongest impact on early stages of use (e.g., initiation). Moreover, statistical advances (e.g., TVEM) have helped identify critical periods where youth carrying specific genetic variants may be most susceptible to environmental contexts.
Despite these developments, we propose several future directions. First, work thus far has largely focused on European samples [67]. Additional work with more diverse racial/ethnic groups is necessary to determine whether findings generalize. Second, given that sample size often impacts the effectiveness of gene identification methods there is a need to create open-access data repositories across sites, such as the National Institute of Drug Abuse-sponsored Gene, Environment, and Development Initiative (GEDI) [2]. Lastly, a larger discussion regarding how to use results gleaned from genetic studies to inform adolescent SU prevention is necessary. Although findings provide information suggestive of who, when, and how individuals are most at risk for SU, the use of universal genetic screening raises logistical and ethical concerns. Not only is genotyping expensive, the use of genetic screening to identify subgroups for interventions may stigmatize children and lead to genetic discrimination [9]. Moreover, it is unclear what procedures should be in place regarding incidental findings as genome-wide screens will also provide information regarding risk for medical problems outside of SUD. Perhaps targeting a host of modifiable intermediate phenotypes (e.g., behavioral disinhibition, aggression) identified through various objective and subjective methods and combined via machine learning [68] could inform personalized treatment with fewer ramifications.
Acknowledgements
This publication was supported by the National Institute on Minority Health and Health Disparities (U54 MD012393 to E. M. Trucco) and the National Institute on Alcohol Abuse and Alcoholism (K08 AA023290 to E. M. Trucco) of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
Elisa M. Trucco, Brigitte Madan, and Michelle Villar declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Elisa M. Trucco, Florida International University, Psychology Department, Center for Children and Families, 11200 SW 8th Street, AHC-1, Miami, FL 33199
Brigitte Madan, Florida International University, Center for Children and Families, 11200 SW 8th Street, AHC-4, Miami, FL 33199
Michelle Villar, Florida International University, Center for Children and Families, 11200 SW 8th Street, AHC-1, Miami, FL 33199
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Adkins DE, Clark SL, Copeland WE, Kennedy M, Conway K, Angold A et al. Genome-wide meta-analysis of longitudinal alcohol consumption across youth and early adulthood. Twin Res Hum Genet 2015; 18(4):335–47. Doi: 10.1017/thg.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trucco EM, Schlomer GL, Hicks BM. Alcohol use disorders In: Fitzgerald HE, Puttler LI, editors. Alcohol use disorders. New York, NY, US: Oxford University Press; 2018. p. 49–68. [Google Scholar]
- 3.Dodge KA, Malone PS, Lansford JE, Miller S, Pettit GS, Bates JE et al. A dynamic cascade model of the development of substance-use onset. Monogr Soc Res Child Dev 2009;74(3):119 Doi: 10.1111/j.1540-5834.2009.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belsky DW, Moffitt TE, Baker TB, Biddle AK, Evans JP, Harrington H et al. Polygenic risk and the developmental progression to heavy, persistent smoking and nicotine dependence: evidence from a 4-decade longitudinal study. JAMA Psychiatry. 2013;70(5):534–42. Doi: 10.1001/jamapsychiatry.2013.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Drinking or abstaining at age 14? A genetic epidemiological study. Alcohol Clin Exp Res 2001. ;25(11): 1594–604. Doi: 10.1111/j.1530-0277.200l.tb02166.x. [DOI] [PubMed] [Google Scholar]
- 6.••.Russell MA, Schlomer GL, Cleveland HH, Feinberg ME, Greenberg MT, Spoth RL et al. PROSPER intervention effects on adolescents’ alcohol misuse vary by GABRA2 genotype and age. Prev Sci 2018;19(1):27–37. Doi: 10.1007/s11121-017-0751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study is among the first to test gene x environment x development effects on adolescent alcohol misuse. A cutting-edge methodological approach, time-varying effect modeling is used to pinpoint periods during which differences in intervention effects by GABRA2 genotype are most pronounced across adolescence.
- 7.Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME. Monitoring the Future national survey results on drug use, 1975-2018: Overview, key findings on adolescent drug use. 2019. p. 119.
- 8.Moises HW, Yang L, Kristbjamarson H, Wiese C, Byerley W, Macciardi F et al. An international two-stage genome-wide search for schizophrenia susceptibility genes. Nat Genet 1995; 11(3):321–4. Doi: 10.1038/ngl195-321. [DOI] [PubMed] [Google Scholar]
- 9.Dick DM. Commentary for special issue of prevention science “using genetics in prevention: science fiction or science fact?”. Prev Sci 2018;19(1):101–8. Doi: 10.1007/s11121-017-0828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trucco EM, Hicks BM, Villafuerte S, Nigg JT, Burmeister M, Zucker RA. Temperament and externalizing behavior as mediators of genetic risk on adolescent substance use. J Abnorm Psychol 2016; 125(4):565–75. Doi: 10.1037/abn0000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.•.Trucco EM, Villafuerte S, Hussong A, Burmeister M, Zucker RA. Biological underpinnings of an internalizing pathway to alcohol, cigarette, and marijuana use. J Abnorm Psychol 2018;127(1):79–91. Doi: 10.1037/abn0000310. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates the utility of examining intermediate phenotypes through which specific genetic risk factors impact substance use in late adolescence. Findings indicate that early difficulties coping effectively with stressors and later depression may represent one pathway through which genetic risk factors impact adolescent substance use.
- 12.Beach SRH, Lei MK, Brody GH, Philibert RA. Prevention of early substance use mediates, and variation at SLC6A4 moderates, SAAF intervention effects on OXTR methylation. Prev Sci 2018;19(1):90–100. Doi: 10.1007/s11121-016-0709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cope LM, Munier EC, Trucco EM, Hardee JE, Burmeister M, Zucker RA et al. Effects of the serotonin transporter gene, sensitivity of response to alcohol, and parental monitoring on risk for problem alcohol use. Alcohol. 2017;59:7–16. Doi: 10.1016/j.alcohol.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brody GH, Chen Yf, Beach SRH. Differential susceptibility to prevention: GABAergic, dopaminergic, and multilocus effects. J Child Psychol Psychiatry.2013;54(8):863–71. Doi: 10.1111/jcpp.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macare C, Ducci F, Zhang Y, Ruggeri B, Jia T, Kaakinen M et al. A neurobiological pathway to smoking in adolescence: TTC12-ANKK1-DRD2 variants and reward response. Eur Neuropsychopharmacol 2018;28(10):1103–14. Doi: 10.1016/j.euroneuro.2018.07.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trucco EM, Villafuerte S, Heitzeg MM, Burmeister M, Zucker RA. Rule breaking mediates the developmental association between GABRA2 and adolescent substance use. J Child Psychol Psychiatry. 2014;55(12):1372–9. Doi: 10.1111/jcpp.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarnyai Z Oxytocin as a potential mediator and modulator of drug addiction. Addict Biol 2011;16(2):199–201. Doi: 10.1111/j.1369-1600.2011.00332.x. [DOI] [PubMed] [Google Scholar]
- 18.Korucuoglu O, Gladwin TE, Baas F, Mocking RJT, Ruhé HG, Groot PFC et al. Neural response to alcohol taste cues in youth: Effects of the OPRM1 gene. Addict Biol 2017;22(6): 1562–75. Doi: 10.1111/adb.12440. [DOI] [PubMed] [Google Scholar]
- 19.Miranda R, Ray L, Justus A, Meyerson LA, Knopik VS, McGeary J et al. Initial evidence of an association between OPRM1 and adolescent alcohol misuse. Alcohol Clin Exp Res 2010;34(1): 112–22. Doi: 10.1111/j.1530-0277.2009.01073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ray LA, Hutchison KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res 2004;28(12): 1789–95. Doi: 10.1097/01.ALC.0000148114.34000.B9. [DOI] [PubMed] [Google Scholar]
- 21.van der Zwaluw CS, Otten R, Klleinjan M, Engels RCME. Different trajectories of adolescent alcohol use: testing gene-environment interactions. Alcohol Clin Exp Res 2014;38(3):704–12. Doi: 10.1111/acer.12291. [DOI] [PubMed] [Google Scholar]
- 22.Liu JZ, Tozzi F, Waterworth DM, Pillai SG, Muglia P, Middleton L et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet 2010;42(5):436–40. Doi: 10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minică CC, Verweij KJH, van der Most PJ, Mbarek H, Bernard M, van Eijk KR et al. Genome- wide association meta- analysis of age at first cannabis use. Addiction. 2018; 113(11):2073–86. Doi: 10.1111/add.14368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edenberg HJ, Dick D, Xuei X, Tian H, Almasy L, Bauer LO et al. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet 2004;74(4):705–14. Doi: 10.1086/383283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heitzeg MM, Villafuerte S, Weiland BJ, Enoch M-A, Burmeister M, Zubieta J-K et al. Effect of GABRA2 genotype on development of incentive-motivation circuitry in a sample enriched for alcoholism risk. Neuropsychopharmacology. 2014;39(13):3077–86. Doi: 10.1038/npp.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kendler KS, Gardner C, Dick DM. Predicting alcohol consumption in adolescence from alcohol-specific and general externalizing genetic risk factors, key environmental exposures and their interaction. Psychol Med 2011. ;41:1507–16. Doi: 10.1017/S003329171000190X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plomin R Child development and molecular genetics: 14 years later. Child Dev 2013;84:104–20. Doi: 10.1111/j.l467-8624.2012.01757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elam KK, Chassin L, Lemery-Chalfant K, Pandika D, Wang FL, Bountress K et al. Affiliation with substance-using peers: Examining gene-environment correlations among parent monitoring, polygenic risk, and children’s impulsivity. Dev Psychobiol 2017; 59(5):561–573. Doi: 10.1002/dev.21529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Latendresse SJ, Musci R, Maher BS. Critical issues in the inclusion of genetic and epigenetic information in prevention and intervention trials. Prev Sci 2018;19(1):58–67. Doi: 10.1007/s11121-017-0785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webb BT, Edwards AC, Wolen AR, Salvatore JE, Aliev F, Riley BP et al. Molecular genetic influences on normative and problematic alcohol use in a population-based sample of college students. Front Genet 2017;8:30 Doi: 10.3389/fgene.2017.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vrieze SI, McGue M, Iacono WG. The interplay of genes and adolescent development in substance use disorders: leveraging findings from GWAS meta-analyses to test developmental hypotheses about nicotine consumption. Hum Genet 2012; 131(6):791–801. Doi: 10.1007/s00439-012-1167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–53. Doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet 2011. ;88(1):76–82. Doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J, Lee SH, Goddard ME, Visscher PM. Genome-wide complex trait analysis (GCTA): methods, data analyses, and interpretations. Methods Mol Biol 2013;1019:215–36. Doi: 10.1007/978-1-62703-447-0_9. [DOI] [PubMed] [Google Scholar]
- 35.Minică CC, Dolan CV, Hottenga J-J, Pool R, Fedko IO, Mbarek H et al. Heritability, SNP- and gene-based analyses of cannabis use initiation and age at onset. Behav Genet 2015;45(5):503–13. Doi: 10.1007/s10519-015-9723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vrieze SI, McGue M, Miller MB, Hicks BM, Iacono WG. Three mutually informative ways to understand the genetic relationships among behavioral disinhibition, alcohol use, drug use, nicotine use/dependence, and their co-occurrence: Twin biometry, GCTA, and genome-wide scoring. Behav Genet 2013;43(2):97–107. Doi: 10.1007/s10519-013-9584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar SK, Feldman MW, Rehkopf DH, Tuljapurkar S. Reply to Yang et al.: GCTA produces unreliable heritability estimates. Proc Natl Acad Sci USA. 2016; 113(32):E4581 Doi: 10.1073/pnas.1608425113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–45. Doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 39.•.Glaser YG, Zubieta J-K, Hsu DT, Villafuerte S, Mickey BJ, Trucco EM et al. Indirect effect of corticotropin-releasing hormone receptor 1 gene variation on negative emotionality and alcohol use via right ventrolateral prefrontal cortex. J Neurosci 2014;34(11):4099–107. doi: 10.1523/JNEUROSCI.3672-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work represents one of the few empirical studies that combines genetic, neurobiological, and social aspects of substance use risk in one model. A moderated mediation model was estimated whereby genetic risk factors to substance use behavior via brain function were examined, in addition to testing whether these associations may differ for those experiencing childhood stressors.
- 40.Hyde LW. Developmental psychopathology in an era of molecular genetics and neuroimaging: A developmental neurogenetics approach. Dev Psychopathol 2015;27(2):587–613. Doi: 10.1017/S0954579415000188. [DOI] [PubMed] [Google Scholar]
- 41.Yang J, Lee SH, Wray NR, Goddard ME, Visscher PM. GCTA-GREML accounts for linkage disequilibrium when estimating genetic variance from genome-wide SNPs. Proc Natl Acad Sci USA. 2016;113(32):E4580 Doi: 10.1073/pnas.1602743113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.••.Bogdan R, Salmeron BJ, Carey CE, Agrawal A, Calhoun VD, Garavan H et al. Imaging genetics and genomics in psychiatry: A critical review of progress and potential. Biol Psychiatry. 2017;82(3):165–75. Doi: 10.1016/j.biopsych.2016.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article provides a comprehensive review of the current state of the neurogenetics field. This review discusses progress made in neurogenetics as well as potential pitfalls of novel approaches.
- 43.Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30:5–13. [PMC free article] [PubMed] [Google Scholar]
- 44.Takeshita T, Morimoto K. Self-reported alcohol-associated symptoms and drinking behavior in three ALDH2 genotypes among Japanese university students. Alcohol Clin Exp Res 1999;23(6): 1065–9. Doi: 10.1097/00000374-199906000-00015 [DOI] [PubMed] [Google Scholar]
- 45.McCarthy MI, Wall TL, Brown S, Carr LG. Integrating biological and behavioral factors in alcohol use risk: The role of ALDH2 status and alcohol expectancies in a sample of Asian Americans. Exp Clin Psychopharmacol 2000;8(2): 168–75. Doi: 10.1037//1064-1297.8.2.168 [DOI] [PubMed] [Google Scholar]
- 46.Ehringer MA, Clegg HV, Collins AC, Corley RP, Crowley T, Hewitt JK et al. Association of the neuronal nicotinic receptor β2 subunit gene (CHRNB2) with subjective responses to alcohol and nicotine. Am J Med Genet B Neuropsychiatr Genet 2007;144B(5):596–604. Doi: 10.1002/ajmg.b.30464. [DOI] [PubMed] [Google Scholar]
- 47.Shuckit MA. Genetics of the risk for alcoholism. Am J Addict 2000;9(2): 103–12. Doi: 10.1080/10550490050173172 [DOI] [PubMed] [Google Scholar]
- 48.Rothbart MK. Temperament, development, and personality. Curr Dir Psychol Sci 2007;16(4):207–12. Doi: 10.1111/j.l467-8721.2007.00505.x [DOI] [Google Scholar]
- 49.Ellingson JM, Richmond-Rakerd LS, Statham DJ, Martin NG, Slutske WS. Most of the genetic covariation between major depressive and alcohol use disorders is explained by trait measures of negative emotionality and behavioral control. Psychol Med 2016; 46(2919-2930). Doi: 10.1017/S0033291716001525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hussong AM, Jones DJ, Stein GL, Baucom DH, Boeding S. An internalizing pathway to alcohol use and disorder. Psychol Addict Behav 2011;25:390–404. Doi: 10.1037/a0024519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zucker RA, Hicks BM, Heitzeg MM. Alcohol use and the alcohol use disorders over the life course: A cross-level developmental review In: Cicchetti D, editor. Developmental psychopathology: Maladaptation and Psychopathology. Hoboken, NJ: John Wiley & Sons; 2016. p. 833–97. [Google Scholar]
- 52.Trucco EM, Cope LM, Burmeister M, Zucker RA, Heitzeg MM. Pathways to youth behavior: The role of genetic, neural, and behavioral markers. J Res Adolesc 2018;28(1):26–39. Doi: 10.1111/jora.12341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.••.Hines L, Morley KI, Mackie C, Lynskey M. Genetic and environmental interplay in adolescent substance use disorders. Curr Addict Rep 2015;2(2): 122–9. Doi: 10.1007/s40429-015-0049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article provides a comprehensive review of the interplay between genetic and environmental influences in the etiology of adolescent substance use. Moreover, the importance of incorporating a stage-sequential conceptualization of substance use in models when testing the independent and combined effects of genes and the environment are discussed.
- 54.Wang FL, Chassin L, Lee M, Haller M, King K. Roles of response inhibition and gene–environment interplay in pathways to adolescents’ externalizing problems. J Res Adolesc 2017;27(2):258–77. Doi: 10.1111/jora.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pieters S, van der Zwaluw CS, Van Der Vorst H, Wiers RW, Smeets H, Lambrichs E et al. The moderating effect of alcohol-specific parental rule-setting on the relation between the dopamine D2 receptor gene (DRD2), the μ-opioid receptor gene (OPRM1) and alcohol use in young adolescents. Alcohol Alcohol. 2012;47(6):663–70. Doi: 10.1093/alcalc/ags075 [DOI] [PubMed] [Google Scholar]
- 56.Daw J, Boardman JD, Peterson R, Smolen A, Haberstick BC, Ehringer MA et al. The interactive effect of neighborhood peer cigarette use and 5HTTLPR genotype on individual cigarette use. Addict Behav 2014;39(12):1804–10. Doi: 10.1016/j.addbeh.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rabinowitz JA, Musci RJ, Milam AJ, Benke K, Uhl GR, Sisto DY et al. The interplay between externalizing disorders polygenic risk scores and contextual factors on the development of marijuana use disorders. Drug Alcohol Depend. 2018;191:365–73. Doi: 10.1016/j.drugalcdep.2018.07.016doi:10.1016/j.drugalcdep.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fowler T, Lifford K, Shelton K, Rice F, Thapar A, Neale MC et al. Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. Addiction. 2007;102(3):413–22. Doi: 10.1111/j.l360-0443.2006.01694.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brody GH, Beach SRH, Philibert RA, Chen Y-f, Murry VM. Prevention effects moderate the association of 5-HTTLPR and youth risk behavior initiation: gene x environment hypotheses tested via a randomized prevention design. Child Dev 2009;80(3):645–61. Doi: 10.1111/j.1467-8624.2009.01288.x. [DOI] [PubMed] [Google Scholar]
- 60.Beach SRH, Brody GH, Lei M-K, Philibert RA. Differential susceptibility to parenting among African American youths: Testing the DRD4 hypothesis. J Fam Psychol 2010;24(5):513–21. Doi: 10.1037/a0020835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Belsky J, Pluess M. The nature (and nurture?) of plasticity in early human development. Perspect Psychol Sci 2009;4:345–51. Doi: 10.1111/j.l745-6924.2009.01136.x [DOI] [PubMed] [Google Scholar]
- 62.Pluess M Vantage sensitivity: Environmental sensitivity to positive experiences as a function of genetic differences. J Pers 2017;85(1):38–50. Doi: 10.1111/jopy.12218 [DOI] [PubMed] [Google Scholar]
- 63.Bakermans-Kranenburg MJ, van Dzendoom MH, Pijlman FT, Mesman J, Juffer F. Experimental evidence for differential susceptibility: Dopamine D4 receptor polymorphism (DRD4 VNTR) moderates intervention effects on toddlers’ externalizing behavior in a randomized controlled trial. Dev Psychol 2008;44:293–300. Doi: 10.1037/0012-1649.44.1.293 [DOI] [PubMed] [Google Scholar]
- 64.Musci RJ, Masyn KE, Uhl G, Maher B, Kellam SG, Ialongo NS. Polygenic score × intervention moderation: an application of discrete-time survival analysis to modeling the timing of first tobacco use among urban youth. Dev Psychopathol 2015;27(1): 111–22. Doi: 10.1017/S0954579414001333. [DOI] [PubMed] [Google Scholar]
- 65.Trucco EM, Villafuerte S, Heitzeg MM, Burmeister M, Zucker RA. Susceptibility effects of GABA receptor subunit alpha-2 (GABRA2) variants and parental monitoring on externalizing behavior trajectories: Risk and protection conveyed by the minor allele. Dev Psychopathol 2016;28:15–26. Doi: 10.1017/S0954579415000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trucco EM, Villafuerte S, Burmeister M, Zucker RA. Beyond risk: Prospective effects of GABA receptor subunit alpha-2 (GABRA2) × positive peer involvement on adolescent behavior. Dev Psychopathol 2017;29:711–24. Doi: 10.1017/S0954579416000419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Popejoy AB, Fullerton SM. Genomics is failing on diversity. Nature. 2016;538:161–4. Doi: 10.1038/538161a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walsh CG, Ribeiro JD, Franklin JC. Predicting risk of suicide attempts over time through machine learning. Clin Psychol Sci 2017;5(3):457–69. Doi: 10.1177/2167702617691560 [DOI] [Google Scholar]


