Abstract
Activation of the antioxidant regulatory transcription factor NRF2 (Nuclear factor erythroid-derived 2) regulates cellular bioenergetics and improves neuronal health in aging. Yet how NRF2 participates in maintaining synaptic, mitochondrial and cognitive function has not been fully elucidated. This study investigates how loss of NRF2 affects neuronal metabolism, synaptic density and cognitive performance in aged mice.
Dendritic arborization as well as synaptic and mitochondrial gene expression was evaluated in hippocampal neurons isolated from mice lacking NRF2 (NRF2KO) and from wild-type (WT) C57BL6 mice. Mitochondrial function of these neurons was evaluated using the Seahorse XF platform. Additionally learning, memory and executive function were assessed in 20 month old NRF2KO and age-matched WT mice using conditioned fear response (CFR) and odor discrimination reversal learning (ODRL) tests. Hippocampal bioenergetics was profiled using mitochondria isolated from these animals and tissue was harvested for assessment of mitochondrial and synaptic genes.
NRF2KO neurons had reduced dendritic complexity and diminished synaptic gene expression. This was accompanied by impaired mitochondrial function and decreased mitochondrial gene expression. Similar mitochondrial deficits were observed in the brains of aged NRF2KO mice. These animals also had significantly impaired cognitive performance and reduced synaptic gene expression as well.
These data point to a role for NRF2 in maintaining mitochondrial and cognitive function during aging and suggest that the transcription factor may be a viable target for cognitive enhancing interventions. Because mitochondrial dysfunction and cognitive impairment also occur together in many neurodegenerative conditions there may be broad therapeutic potential of NRF2 activating agents.
Keywords: NRF2, mitochondrial function, cognition, aging
1. Introduction
The US life expectancy has increased by more than a decade in the past sixty years leading to an increase in the population of older Americans that is projected to continue, with the number of people aged 65 and older more than doubling in the coming decades [1]. This increase in the elderly population has galvanized researchers to uncover the molecular and biochemical pathways that contribute to healthy brain aging.
Deficits in memory and executive function affect the majority of older individuals [2–6]. This cognitive decline is accompanied by increased mitochondrial dysfunction and oxidative stress in the brains of aged individuals [7, 8]. Similar metabolic and cognitive deficits are observed in aged rodents [9–14] and studies have demonstrated a relationship between mitochondrial dysfunction, oxidative damage and memory impairment in rodents [15–18].
The endogenous antioxidant response pathway protects cells from oxidative stress by increasing transcription of cytoprotective genes through the binding of the transcription factor NRF2 (nuclear factor erythroid 2-related factor 2) to antioxidant response elements (AREs) in the promoters of antioxidant genes [19–21]. NRF2 has also been implicated in regulating mitochondrial function and biogenesis [22, 23]. Additionally our group and others have shown that compounds that activate NRF2 improve cognitive and mitochondrial function in aged animals [24–29]. However, the precise role of NRF2 in age related cognitive decline and mitochondrial dysfunction has not yet been clearly defined. Here we use NRF2KO mice to determine how NRF2 participates in neuronal mitochondrial dysfunction, synaptic plasticity and cognitive performance in aging.
2. Materials and Methods
2.1. Animals
NRF2KO mice were generated from homozygous NRF2KO breading pairs (on a C57BL6 background) acquired from the Jackson Laboratory (Stock# 017009). The homozygous NRF2KO line was selected because of difficulties breeding heterozygous, namely extremely small litter sizes. This strain is raised on a C57BL/6 background and so C57BL6 mice were acquired from the aged rodent colony at the National Institute of Aging to serve as wild-type (WT) controls. Mice were maintained in a climate-controlled environment with a 12-hr light/12-hr dark cycle. Diet and water were supplied ad libitum, except during behavioral testing. All procedures were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the institutional Animal Care and Use Committee of the Portland VA Medical Center. Animals used in behavioral testing were aged to 6 or 20 months prior to testing, following 3 weeks of behavioral testing animals were sacrificed and tissue harvested.
2.2. Behavioral Testing
Conditioned Fear Response (CFR): During this test mice are habituated to a 16×16×12 inch test chamber for 5 minutes and freezing behavior is recorded using AnyMaze software. Immediately following habituation, 3 one-second shocks (0.7 mA) are randomly administered over a 3 minute period, with no more than one shock per minute. Twenty four hours later animals are reintroduced to the test chamber for 5 minutes with no shock and freezing behavior is again recorded. Baseline freezing, during the habituation phase, is subtracted to quantify the change in freezing behavior induced by the test.
Odor discrimination reversal learning (ODRL): This test has three phases: shaping, acquisition and shift. In the initial shaping phase mice are introduced to a 12” x 8” x 7” testing chamber and trained to dig for a food reward in lavender scented bedding material. Mice are presented with a single bowl containing the food reward that was progressively filled with bedding in five stages, 0%, 25%, 50%, 75% and 100% filled. The animal must successfully retrieve the food reward 5 times in a row to advance to the subsequent training step.
Once the shaping phase is completed the acquisition phase is initiated in which mice are presented with two cups one containing dried beans and the other string. In every trial one digging material has a vanilla odor and the other a mint odor and the odor and material pairings were randomly alternated between trials but balanced over the acquisition phase so that each mouse is exposed to roughly equal combinations of each odor and digging material. Whether the baited cup is presented on the right or left side of the apparatus is also balanced throughout testing. In the acquisition phase the mint-scented bowl is always baited regardless of digging material. Example trials are found in Table 1. Mice are required to make 8 correct digs in any bout of 10 in order to reach criterion. The number of trials to reach criterion is recorded.
Table 1:
Example of test pairings for Odor Discrimination Reversal Learning (ODRL) test.
| Right Position | Left Position | |
|---|---|---|
| Acquisition Phase | D1+O1 | D2+O2 |
| D1+O2 | D2+O1 | |
| D2+O1 | D1+O2 | |
| D2+O2 | D1+O1 | |
| Shift Phase | D1+O1 | D2+O2 |
| D1+O2 | D2+O1 | |
| D2+O1 | D1+O2 | |
| D2+O2 | D1+O1 |
Representative combinations of odor and digging material pairings during each phase of the ODRL. D1= dried bean, D2= string, O1=vanilla, O2=mint. Italicized indicates correct trial
The shift phase is initiated immediately after a mouse reaches criterion in the acquisition phase. In this phase mice are again presented with two cups one containing dried beans and the other string. In every trial one digging material has the vanilla odor and the other the mint odor and again the odor + digging material pairings are balanced throughout the trial as is right/left location of the baited cup. In the shift phase however the cup with the dried beans is always baited regardless of odor. Again criterion is defined as 8 correct trials in any bout of 10 and trials to criterion are recorded. Mice are food restricted the night before each phase of the ODRL in order to motivate the animals.
2.3. Isolation of hippocampal mitochondria
At sacrifice hippocampal mitochondria were isolated using a previously described protocol [30] with slight modifications. Briefly, isolated hippocampi were placed in cold isolation buffer containing 220 mM mannitol, 70 mM sucrose, 5 mM KH2PO4, 5 mM MgCl2, 2 mM HEPES, 1 mM EGTA and 0.5% BSA (fatty acid free) and homogenized using an Arrow Engineering JR4000 homogenizer. Homogenate was centrifuged at 500g for 5 minutes at 4C. The supernatant fraction was then isolated and centrifuged at 14,000 g for 10minutes at 4C. Then the pellet fraction was resuspended in 12% Percoll and carefully layered on top of 24% Percoll and centrifuged at 16,000 g for 20 minutes at 4C. Protein concentration was assessed by Bradford Assay.
2.4. Primary Hippocampal Culture and Morphological Analysis
Hippocampal neurons were isolated from NRF2KO and C576BL6 embryonic mice, based on the methods of Kaech and Banker [31]. Briefly, breeding pairs of NRF2KO and C57BL6 mice were acquired from Jackson Laboratories and embryos were harvested at 18 days of gestation from anesthetized females. Hippocampi were dissected, gently minced, and trypsinized to generate suspensions of dispersed neurons, which were then plated in MEM medium (GIBCO /Life Technologies), 5% FBS (Atlanta Biologicals), and 0.6% glucose (Sigma-Aldrich). After 3 hours, the medium was removed and replaced with Neurobasal Medium supplemented with 1x GlutaMAX (GIBCO/Life Technologies) and 1x Neuronal Culture Medium Supplement (Thermo).
For Sholl analyses of dendritic complexity, we plated 130,000 hippocampal neurons in 60 mm dishes in 1x MEM with 5% FBS, each dish containing 3 poly-L-lysine-coated glass coverslips with paraffin wax spacers. After 3 hours, the coverslips were flipped into 60 mm dishes containing mouse neural stem cell-derived glial cells (provided by Dr. Gary Banker, Jungers Center, OHSU) and maintained in 6 ml Neurobasal medium with GlutaMAX and Neuronal Culture Medium Supplement. Each dish was fed every week by removing 1 ml of the culture medium and adding 1 ml fresh Neurobasal medium containing GlutaMAX plus Neuronal Culture Medium Supplement, with the first feed at 5 days in vitro (DIV) containing 6 μM cytosine β-D-arabinofuranoside hydrochloride (AraC; Sigma-Aldrich). Each coverslip was fixed in 4% PFA in PBS at 19 DIV, when neurons are mature and undergoing the process of synaptogenesis and their dendritic processes are robsutly quanitfiable [31]. Coverslips were stained with Anti-MAP2B (Sigma-Aldrich #M4403; 3.3 μg/ml) and Goat anti-mouse IgG1-Cy3 (Jackson ImmunoResearch #115–165-205; 1.5μg/ml). Immunostained neurons were imaged with a Zeiss ApoTome2 microscope and blinded Sholl analyses were performed using the Fiji platform [32] with the plug-in created by Ferreira et al. [33]. Thirty isolated, non-overlapping cells were analyzed per coverslip. Arborization data was pooled across 3 independent experiments (3–5 coverslips per genotype in each experiment) providing at least 500 cells per genotype. Statistical differences between treatment groups were calculated using Student’s unpaired t-tests.
2.5. Reactive Oxygen Species quantification
Primary hippocampal neurons from E18 embryos were plated at a concentration of 75,000 cells per well in a lysine coated 96 well plate and allowed to grow in Neurobasal medium with GlutaMAX and Neuronal Culture Medium Supplement for 7 days. This time point was selected because without a feeder layer of glial cells the neurons can only survive about 10–14 days so 7 days would allow us to capture changes that occur in the cell before viability becomes an issue. Reactive oxygen species (ROS) content was assessed by a Cellular ROS Assay Kit (Abcam 113851) as per the kit’s instructions. A Bradford Assay was used to normalize the values to the total protein content of each well. Data was collected across 3 independent experiments with at least 6 wells per genotype in each experiment.
2.6. Neuronal ATP quantification
Neurons were plated on poly-L-lysine coated 12 well plates at a density of 250,000 cells per well. On the 7th day in vitro cells were lysed with 0.1% Triton X 100 in PBS and incubated with the reaction solution for 15 min at room temperature, prior to measurement. Again day 7 was chosen to ensure evaluation of cellular changes at a time point where it would not be confounded by cell death. ATP was quantified using the ATP determination kit (Life Technologies), as per the manufacturer’s instructions. Values were normalized to total protein content, as determined by Bradford Assay. Data was collected across 3 independent experiments with 6–12 wells per genotype in each experiment.
2.7. Analysis of Mitochondrial Function
Mitochondrial function was assessed using the Seahorse Bioscience XF24 Extracellular Flux Analyzer. For primary neurons, cells were plated on poly-L-lysine coated Seahorse XF culture plates (Agilent) at a density of 75,000 cells/well. As per the Agilent recommended protocol a range of cell densities was evaluated prior to choosing 75,000. Basal respiration was measured at densities of 25,000, 50,000, 75,000, 100,000, 125,000, and 150,000 cells per well and 75,000 was chosen for experimental purposes as it fell in the middle of the linear range of those densities (data not shown). On day 7 cells were rinsed in assay medium (pH 7.4) containing XF Base medium (Agilent), 5.5mM glucose and 1mM sodium-pyruvate. Cells remained in assay medium 1h at 37 C in a non-CO2 incubator prior to initializing the Seahorse24XF analysis. Using the MitoStress Kit as previously described [30], OCR was measured under varying conditions. After three initial baseline measurements of OCR, the ATP synthase inhibitor oligomycin (1 μM) was added and three subsequent measurements were taken. Next an ETC accelerator, p-trifluoromethoxy carbonyl cyanide phenyl hydrazone (FCCP at 1.5 μM), was added and after 3 measurements were taken, mitochondrial inhibitors rotenone (1 μM) and antimycin (1 μM) were added, and three final measurements were taken. Each measurement was taken at 3 minute intervals following the compound injection and 1 minute of mixing. Data were normalized to total DNA content, which was determined from each well using the CyQuant kit (Invitrogen) as per the manufacturer’s instructions. Data was collected across 4 independent experiments with at least 6 wells per genotype in each experiment.
For analysis of hippocampal bioenergetics of aged animals, isolated mitochondria were plated on Seahorse XF culture plates at a concentration of 2ug of total protein/well with 3–4 replicate wells per animal. The plate was centrifuged for 15 minutes at 2000xg and oxygen consumption rates (OCR) were measured under varying conditions using the MitoStress Kit as described above but with the addition of a saturating concentration of ADP (2mM) injection following the basal measurements as previously described [30], to ensure maximum state III respiration. As with the isolated neurons his was followed by the addition of inhibitor oligomycin (2 μM) to induce state IV respiration and three additional measurements were taken. Next FCCP (4 μM), was added to induce maximal uncoupled (state IIIu) respiration and after 3 measurements were taken, rotenone (1 μM) and antimycin (1 μM) were added, and three final measurements were taken.
2.8. Gene Expression
For gene expression analysis primary neurons were plated on poly-L-lysine coated 12 well plates at a concentration of 250,000 cells/well. On day 7 RNA was extracted using Tri-Reagent (Molecular Research Center) and reverse transcribed with the Superscript III First Strand Synthesis kit (Invitrogen) to generate cDNA as per the manufacturer’s instructions Tissue samples from the hippocampus and frontal cortex of aged animals were homogenized and RNA was extracted also using Tri-Reagent and reverse transcribed with Superscript III.
Relative gene expression was determined using TaqMan Gene Expression Master Mix (Invitrogen) and commercially available TaqMan primers (Invitrogen) for synaptophysin, post-synaptic density protein 95 (PSD95), mitochondrially encoded NADH dehydrogenase 1 (Mt-ND1), mitochondrially encoded cytochrome B (Mt-CYB), mitochondrially encoded cytochrome c oxidase 1 (Mt-CO1), mitochondrially encoded ATP synthase 6 (Mt-ATP6) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Quantitative PCR (qPCR) was performed on a StepOne Plus Machine (Applied Biosystems) and analyzed using the delta-delta Ct method normalizing to GAPDH expression.
2.9. Graphs and Statistics
All bar graphs have error bars reflecting standard error of the mean. Statistical significance was determined using t-tests for the primary neuron comparisons of WT vs NRF2KO or ANOVAs followed by post-hoc analysis with Tukey HSD pairwise comparisons for the in vivo data which included young and old mice of each genotype. Significance was defined as p ≤0.05. Analyses were performed u sing Excel or GraphPad Prism 6.
3. Results
3.1. NRF2KO neurons have impaired dendritic arborization and synaptic density
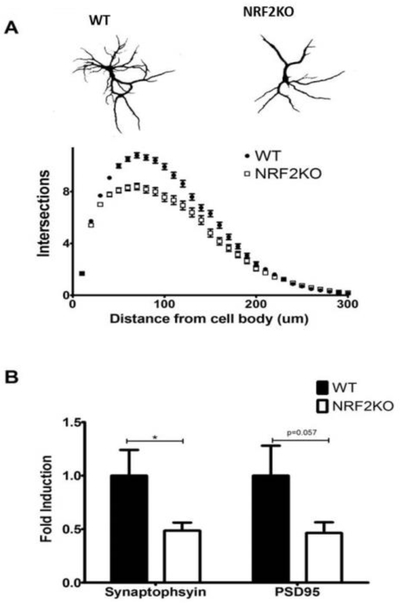
It has been previously reported that after several weeks in culture hippocampal neurons isolated from mouse models of neurodegenerative disease display a dystrophic phenotype characterized by reduced dendritic arborization and a reduction in spine density [34–36]. We observed a similar impairment in arborization in NRF2KO neurons after 3 weeks in culture (Figure 1a). There was also a reduction in the expression of the synaptic genes PSD95 and synaptophysin in NRF2KO neurons (Figure 1b).
Figure 1: NRF2KO neurons have reduced dendritic complexity and decreased synaptic gene expression.
(A) Isolated NRF2KO hippocampal neurons grown in culture for 19 days have diminished dendritic arborization relative to neurons from WT (C57BL/6) mice. n=500 cells per genotype across 3 independent experiments. (B) NRF2KO hippocampal neurons have reduced expression of synaptic genes relative to WT controls. n=12–15 wells per genotype, *p<0.05
3.2. NRF2KO neurons have increased levels of ROS
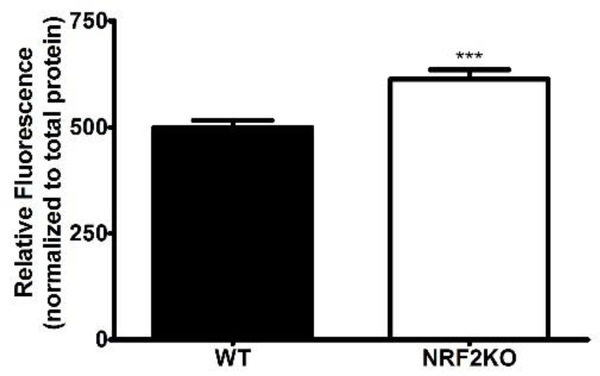
Intracellular ROS of hippocampal neurons was quantified after 7 days in vitro. There was a significant increase in ROS content in NRF2KO hippocampal neurons as compared to WT neurons (Figure 2).
Figure 2: NRF2KO neurons have increased ROS content.
Isolated NRF2KO hippocampal neurons grown in culture for 7 days have increased ROS content relative to WT controls. n=15–24 wells per genotype, across 3 independent experiments
3.3. NRF2KO neurons display deficits in mitochondrial function
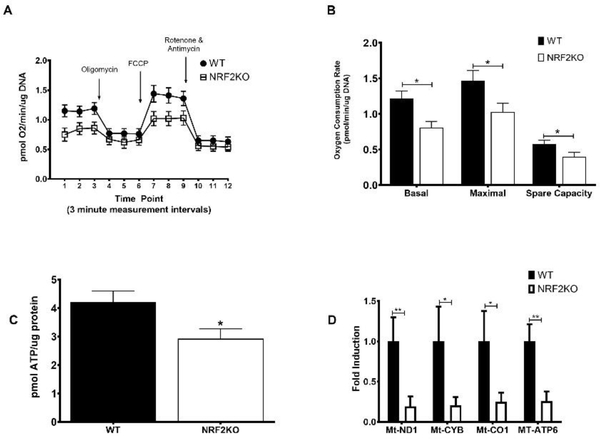
The bioenergetic profile of hippocampal neurons was determined using the Seahorse XF Analyzer (Figure 3a). NRF2KO neurons exhibited significantly reduced basal and maximal (FCCP-stimulated) respiratory rates (Figure 3b). Although the FCCP-stimulated respiratory rate was higher than basal for both WTs and KOs, it did not reach statistical significance in either genotype. This is not unsual however as different cell types respond differently to the compounds in the Seahorse assay and previous reports looking at mitochondrial function from brain-derived tissue show the OCR achieved with FCCP-administration following oligmoycin treatment is similar to basal levels [30, 37]. There was also a diminished spare capacity in NRF2KO neurons (Figure 3b). Spare capacity, or reserve capacity reflects the amount of extra ATP that can be produced by oxidative phosphorylation in case of a sudden increase in energy demand. ATP content was also reduced in NRF2KO neurons (Figure 3c). This was accompanied by a coordinate downregulation in the expression of the genes Mt-ND1, Mt-CYB, Mt-CO1 and Mt-ATP6, which encode proteins in complexes I, III, IV and V of the ETC respectively was likewise reduced in NRF2KO neurons (Figure 3d).
Figure 3: NRF2KO neurons have impaired mitochondrial function.
Hippocampal neurons isolated from NRF2KO mice grown in culture for 7 days have (A) an altered bioenergetic profile relative to WT neurons with (B) impaired basal and maximal (FCCP-stimulated) respiration as well as spare capacity. n=16–20 wells per genotype. (C) NRF2 neurons also have reduced ATP content and (D) diminished expression of genes encoding enzymes in the ETC. n=12–16 wells per genotype, across 4 experiments *p<0.05, **p<0.01
3.4. Aged NRF2KO mice have impaired learning, memory and executive function
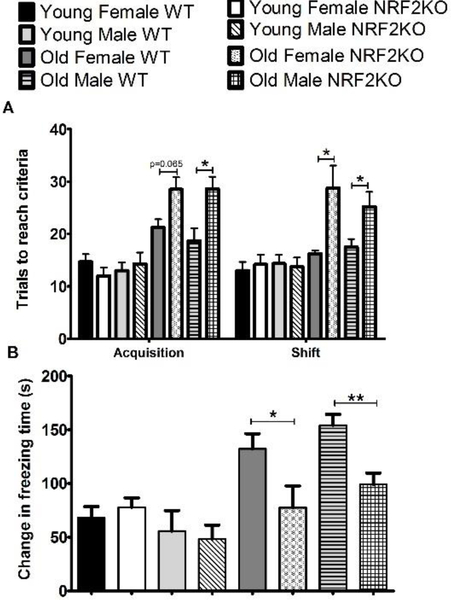
Executive function was assessed in 6 and 20 month old animals using the Odor Discrimination Reversal Learning (ODRL) test. The acquisition phase of the ODRL evaluates learning while the shift phase specifically assesses the cognitive flexibility domain of executive function, by monitoring the ability of the animal to ignore the previously learned association (odor) and start paying attention to a second discriminatory dimension (digging material). Aged male and female NRF2KO mice displayed impairments in both the acquisition and shift phases of this test relative to WTs, taking more trials to reach criterion in each phase of the test (Figure 4a). Young animals required fewer trials to learn the association than older animals but there was no difference between genotypes in either sex.
Figure 4: Aged NRF2KO mice have cognitive deficits relative to aged WT animals.
(A) In both phases of the ODRL 20 month old NRF2KO mice took more trials to reach criterion than age-matched WT mice. There was no difference due to genotype in either phase in the number of trials to reach criteria for the 6 month old mice (B) Aged NRF2KO mice froze significantly less in the CFR test than aged matched WT mice. There were no differences in freezing times between genotypes in the 6 month old animals. n=5–8 animals per condition,*p<0.05, **p<0.01
The conditioned fear response test (CFR) evaluates contextual memory. In this test the amount of time a mouse freezes when placed in a chamber where it previously received a shock is measured and a longer freezing time indicates that the mouse remembers the association of the chamber with the foot shock. We observed that both male and female aged NRF2KO mice froze significantly less than their WT age matched counterparts (Figure 4b). There were no differences between genotypes in either sex in the young animals although there was a trend toward both male and female aged WT mice freezing longer than young WT animals. (Figure 4b)
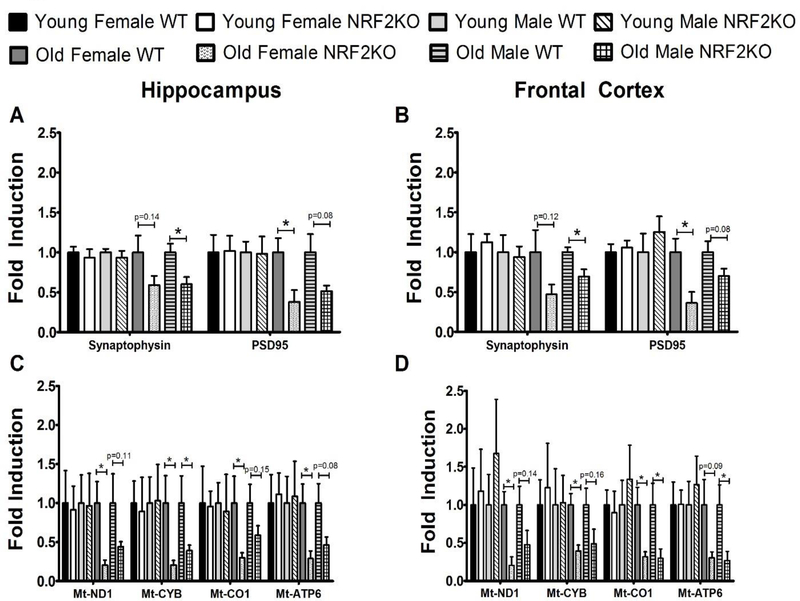
3.5. Mitochondrial and synaptic gene expression is reduced in the brains of aged NRF2KO mice
The expression of the synaptic genes PSD95 and synaptophysin were reduced in the hippocampus and frontal cortex of both male and female NRF2KO mice (Figure 5a and 5b). A similar reduction was observed in the expression of mitochondrial genes in these animals (Figure 5c and 5d). No differences were observed between the genotypes in young mice of either sex for any of the genes measured (Figure 5a–5d).
Figure 5: Aged NRF2KO animals have reduced hippocampal and cortical expression of synaptic and mitochondrial genes.
The expression of synaptic genes reduced in the (A) hippocampus and (B) frontal cortex on aged NRF2KO mice as compared to age matched WT mice but no changes were observed in young animals. A trend toward reduced expression of ETC genes was likewise observed in the (C) hippocampus and (D) frontal cortex of aged NRF2KO mice relative to their age matched controls but again no effect was seen in young animals. All gene expression shown is fold induction relative to the WT animals of that age and gender. n=5–8 mice of each gender per genotype, *p<0.05.
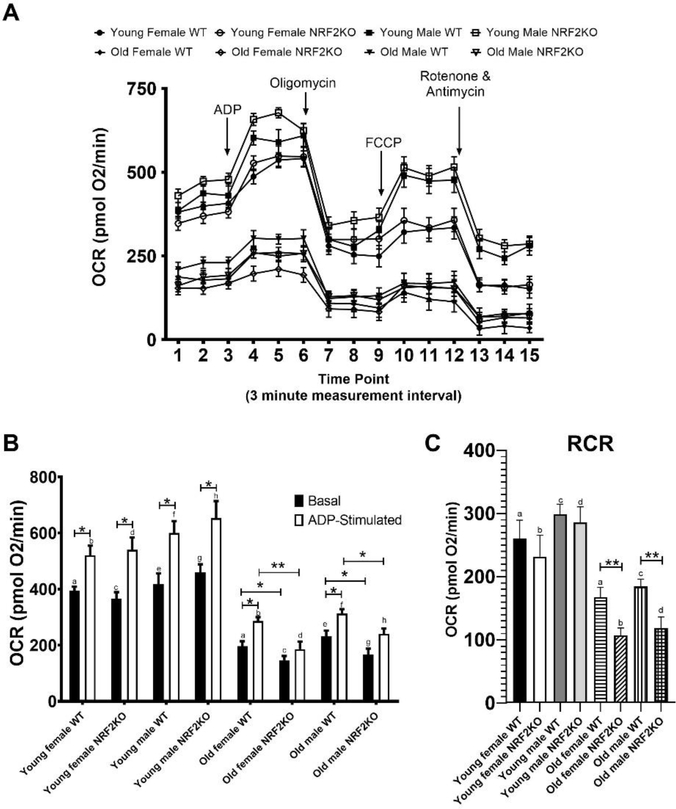
3.6. Hippocampal mitochondrial function is impaired in aged NRF2KO mice
Aged NRF2KO mice displayed a deficit in hippocampal mitochondrial bioenergetics that was apparent in younger mice (Figure 6a). Mitochondria isolated from the hippocampus of aged male and female NRF2KO mice had reductions in both basal and ADP-stimulated, state III, respiration relative to WT mice (Figure 6b). A diminished respiratory control ratio (RCR, the difference between state III and oligomycin induced state IV respiration) was also observed in aged NRF2KO animals compared to the aged WT animals (Figure 6c). There were no differences in mitochondrial function in the young animals of either genotype although the respiratory rate was significantly higher for all young animals as compared to their older counterparts regardless of sex or genotype. Consistent with previous reports in isolated brain mitochondria the maximum respiratory state was achieved following ADP administration and there was only a modest increase in uncoupled or state IIIu respiration following FCCP stimulated in all ages and genotypes [30].
Figure 6: Aged NRF2KO mice have impaired hippocampal mitochondria function.
(A) Isolated hippocampal mitochondria from aged NRF2KO animals show an altered bioenergetic profile relative to mitochondria isolated from aged WT mice. Young mice showed increased oxygen consumption relative to aged mice regardless of sex and genotype. (B) Significant reductions in basal and ADP stimulated respiration as well as (C) respiratory control ratio (RCR) were apparent in hippocampal mitochondria from both aged male and female NRF2KO mice. These parameters were all reduced in aged mice relative to young mice but there was no difference between WT and NRF2KO mice at the young age n=5–8 of each gender per genotype. *p<0.05, pairs of letters indicate statistical significance p<0.001
4. Discussion
As the brain ages free radicals accumulate damaging cellular macromolecules while at the same time the brain’s ability to counterbalance this increase with endogenous antioxidant defenses is diminished [38]. The resulting increase in oxidative stress is believed to contribute to the energetic and synaptic decline that also occurs during aging [39]. This has led to a growing interest in targeting the NRF2 regulated endogenous antioxidant response pathway for cognitive enhancing interventions. Several studies have shown that dietary inventions with compounds known to activate NRF2 improve cognitive function in older adults [26, 40–42]. A similar relationship between NRF2 activation and cognitive improvements has been observed in rodent models of aging as well [43–45]. In fact our own lab has shown that a NRF2 activating extract of the plant Centella asiatica improved learning, memory and executive function in aged mice and this improvement was accompanied by enhanced brain mitochondrial function [28, 29]. The effects of NRF2 activation on mitochondrial function has been similarly demonstrated in other mouse models systems as well [46, 47] yet the exact relationship between NRF2, mitochondrial function and cognition during aging remains unknown. In the study, we explore the role of NRF2 in mitochondrial function as well as learning, memory and executive function during aging.
We found that neurons isolated from NRF2KO mice had reduced dendritic complexity as well as decreased expression of synaptic genes suggesting the involvement of NRF2 in synaptic plasticity. Although to our knowledge this is the first report of morphological changes in isolated neurons lacking NRF2, this finding is in line with other studies, including some from our own lab, showing that compounds that activate NRF2 increase dendritic arborization and spine density [34, 48, 49]. Because synaptic density is so tightly correlated with cognitive function, our findings in NRF2KO neurons suggest that if similar changes in synaptic density occur in vivo then cognitive impairments may be evident in NRF2KO animals as well.
Interestingly we did see changes in both synaptic gene expression as well as cognitive function but only in the aged animals. Although our results in isolated embryonic neurons made us thing we might also observe an effect of the loss of NRF2 in young animals as well, this was not the case. We observed a reduction in hippocampal and cortical expression of synaptic genes in 20 month old NRF2KO mice relative to their age-matched WT counterparts. This suggests potential alterations in synaptic density although future experiments evaluating dendritic complexity and spine density are necessary to definitely confirm this. However, this change in gene expression was accompanied by significant impairments in learning, memory and executive function in these animals. Executive function includes elements like impulse control, attention, planning, cognitive flexibility and problem solving. It is mediated by the prefrontal cortex and is very sensitive to age-related decline [2, 50]. The Wisconsin Card Sorting Test (WCST) is one of a number of widely used tests to assess executive function in humans. In this task subjects are required to adapt behavioral responses to choose the “correct” stimulus array based on sudden rule changes across multiple modalities [51]. Performance in this task is tightly controlled by the prefrontal cortex and declines with age [52]. The ODRL, also called attention set shifting task, is a parallel test that has been developed for rats and, more recently, mice [53, 54]. Like the WCST the ODRL requires paying attention to relevant stimuli while ignoring irrelevant stimuli and subsequently shifting the attention, either within dimensions or between dimensions of the test stimuli, and thus reflects the cognitive flexibility aspects of executive functioning of the animal [53]. Also like the WCST performance in the ODRL declines with age [12, 55]. Aged male and female NRF2KO mice performed significantly worse in both the acquisition and shift phases of ODRL test indicating impairments in learning as well as executive function while no differences were seen in the young NRF2KO animals compared to the young WT animals.
Aged NRF2KO mice also performed more poorly in the CFR test indicating impaired contextual memory relative to age matched WT animals. Although there was no effect of the knockout in young animals, surprisingly, young WT mice of both sexes tended to freeze less than old mice, implying that the contextual memory was improved with age in WT, but not NRF2KO, animals. This unexpected finding is consistent with previous reports that freezing decreases with age [56] and could be related to greater total activity in the young animals or altered sensitivity to pain in the older animals. Future work is needed to control for these factors and determine if in fact performance in this test is not diminished with age.
Performance in CFR is regulated by inputs from both the hippocampus and amygdala suggesting that the cognitive consequences of loss of NRF2 are not limited to any one brain region. Our gene expression data support this as we saw comparable reductions in synaptic gene expression in both the hippocampus and frontal cortex of aged NRF2KO animals.
A similar exacerbation of cognitive impairment and reduction in synaptic density has been observed in response to RNAi induced downregulation of NRF2 in the senescence-accelerated mouse prone 8 (SAMP8) mouse model of aging. In these animals down-regulation of NRF2 resulted in decreased protein expression of PSD95 and synaptophysin and impaired spatial and recognition memory [57]. Conversely, activation of NRF2 has been shown to improve cognitive performance. The NRF2 activating compounds lycopene, naringenin, apigen and quercetin as well as a NRF2 activating extraqct of Centella asiatica have all been shown to improve cognitive function in rodent models of aging [28, 58–64] further supporting a role for NRF2 in maintaining cognitive function during aging.
We also observed significant bioenergetic deficits in isolated NRF2KO neurons. These cells had impaired basal and maximal respiration, reduced spare capacity and diminished ATP content relative to WT cells. The expression of genes encoding enzymes in the ETC was also reduced in the NRF2KO neurons possibly reflecting a decrease in mitochondrial number, although further experiments using electron microscopy or monitoring citrate synthase activity would be necessary in order to confirm an effect on mitochondrial number. Taken together the results presented here indicate a potential role for NRF2 in regulating mitochondrial function and biogenesis. This is consistent with other reports investigating the effects of loss of NRF2 in other cell types. Embryonic fibroblasts isolated from NRF2KO mice have similarly been shown to have decreased basal and maximal respiration as well as reduced ATP content [65]. Moreover activation of NRF2 has been shown to improve mitochondrial function and induce mitochondrial biogenesis in murine cardiomyocytes and primary neurons as well as in human fibroblasts [66–69].
As with the cognitive endpoints, we observed metabolic deficits in isolated hippocampal mitochondria from NRF2KO mice but these impairments were only evident in the brains of aged and not young NRF2KO mice. Again based on our results in isolated embryonic neurons we would have expected mitochondrial respiration to be reduced in the young animals as well. Our extraction technique did not allow us to differentiate between neuronal mitochondria and those from other cell types so it is possible there is some sort of compensatory response from non-neuronal cell types in the young brain. Although more experiments are needed to determine exactly why a decrease was not observed in the young mouse brain these findings are in accordance with previous work by our group and others indicating that primary hippocampal neurons in culture show similar synaptic and bioenergetics phenotypes as the is seen in the brains of aged animals [29, 34, 35, 69, 70].
We observed reduced ETC gene expression was in the hippocampus and cortex of both male and female aged NRF2KO mice relative to age-matched WT controls. Isolated hippocampal mitochondria these animals similarly displayed impaired basal and ADP-stimulated respiration as well as a reduced RCR. These results are consistent with previous reports where loss of NRF2 has showed to impair mitochondrial function in a variety of tissue types including the brain, liver and heart of NRF2KO mice [65] and reduced mitochondrial number in skeletal muscle [71]. Conversely, the NRF2 activating compound dimethyl fumarate was shown to improve mitochondrial function and induce biogenesis in the brain, muscle and liver of treated mice [66] and our lab has likewise shown that a NRF2 activating extract of Centella asiatica can increase expression of ETC genes in the brains of aged mice [28, 29]. While our results suggest a role for NRF2 in maintaining proper mitochondrial function during aging, future experiments including quantification of ATP and ROS in different brain regions would certainly strengthen this conclusion. Work is ongoing in our lab exploring additional ages of mice to determine when the deficit in brain mitochondrial function becomes apparent and these endpoints will be included in those analyses.
5. Conclusions
The findings from this study demonstrate that the transcription factor NRF2 plays an important role in maintaining neuronal mitochondrial function, synaptic plasticity and cognitive performance during aging. Loss of NRF2 has profound effects on mitochondrial function both in vitro and in vivo in aged animals and results in significantly impaired learning, memory and executive function in aged mice. While these findings suggest that increased oxidative damage contributes to these deteriorations, and in fact we demonstrated that NRF2KO cells in culture do have an increased production in ROS relative to WT cells, further experiments verifying increased oxidative damage in vivo are needed to confirm this fact. Inflammatory endpoints will also be important to investigate in future work since inflammation is also known to increase with aging [72] and because of the complex interplay between inflammatory pathways, oxidative stress and mitochondrial function. Nevertheless, these results suggest that targeting NRF2 pharmacologically could be an effective cognitive enhancing strategy. It would be interesting in future work to investigate whether loss of NRF2 accelerates age-related cognitive and metabolic deficiencies and also to explore whether long-term activation of NRF2 beginning in young animals could slow or prevent age-related metabolic and cognitive decline. Experiments are currently underway in our lab to address both of these questions. Additionally because increased oxidative stress, mitochondrial dysfunction and cognitive impairment are common features of many neurodegenerative conditions, including Alzheimer’s disease, Parkinson’s disease and multiple sclerosis, it is possible that NRF2 activation may be a more broadly relevant therapeutic strategy beyond healthy aging.
Highlights.
Neurons lacking NRF2 have reduced synaptic density and dendritic arborization.
Neurons lacking NRF2 have increased oxidative stress and mitochondrial dysfunction.
Aged NRF2KO mice display deficits in learning, memory and executive function.
No differences in cognitive function are observed in young NRF2KO mice.
Acknowledgements
This work was funded by NIH-NCCIH grant R00AT008831 (Gray), NIH-NCCIH grant R01AT008099 (Amala Soumyanath), and a Department of Veterans Affairs Merit Review grant awarded to Joseph Quinn.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bureau PR, Aging in the United States. Population Bulletin, 2015. 70(2): p. 325–373. [Google Scholar]
- 2.Buckner R, Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron, 2004. 44(1): p. 195–208. [DOI] [PubMed] [Google Scholar]
- 3.Cansino S, Episodic memory decay along the adult lifespan: a review of behavioral and neurophysiological evidence. Int J Psychophysiol, 2009. 71(1): p. 64–9. [DOI] [PubMed] [Google Scholar]
- 4.Johnson S, Sacks PK, Turner SM, Gaynor LS, Ormerod BK, Maurer AP, Bizon JL, Burke SN., Discrimination performance in aging is vulnerable to interference and dissociable from spatial memory. Learn Mem, 2016. 23(7): p. 339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Methqal I, Provost JS, Wilson MA, Monchi O, Amiri M, Pinsard B, Ansado J, Joanette Y, Age-Related Shift in Neuro-Activation during a Word-Matching Task. Front Aging Neurosci., 2017. 9: p. 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park D, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK, Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002. 17(2): p. 299–320. [PubMed] [Google Scholar]
- 7.Haider S, Saleem S, Perveen T, Tabassum S, Batool Z, Sadir S, Liaquat L, Madiha S, Age-related learning and memory deficits in rats: role of altered brain neurotransmitters, acetylcholinesterase activity and changes in antioxidant defense system. Age, 2014. 36(3): p. 9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Atamna H, Kuratsune H, Ames BN, Delaying brain mitochondrial decay and aging with mitochondrial antioxidants and metabolites. Ann N Y Acad Sci, 2002. 959(133–66). [DOI] [PubMed] [Google Scholar]
- 9.Burke S, Wallace JL, Nematollahi S, Uprety AR, Barnes CA, Pattern separation deficits may contribute to age-associated recognition impairments. Behav Neurosci, 2010. 124(5): p. 559–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalley J, Cardinal RN, Robbins TW, Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Res, 2004. 28(7): p. 771–84. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert P, Pirogovsky E, Brushfield AM, Luu TT, Tolentino JC, Renteria AF, Age-related changes in associative learning for olfactory and visual stimuli in rodents. Ann NY Acad Sci, 2009. 1170: p. 718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young J, Powell SB, Geyer MA, Jeste DV, Risbrough VB, The mouse attentional set-shifting task: A method for assaying successful cognitive aging? Cogn Affect Behav Neurosci, 2010. 10(2): p. 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McQuail J, Nicolle MM, Spatial reference memory in normal aging Fischer 344 × Brown Norway F1 hybrid rats. Neurobiol Aging, 2015. 36(1): p. 323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimizu C, Oki Y, Mitani Y, Tsuchiya Y, Nabeshima T, Moderate-dose Regular Lifelong Alcohol Intake Changes the Intestinal Flora, Protects against Aging, and Keeps Spatial Memory in the Senescence-accelerated Mouse Prone 8 (SAMP8) Model. J pharm Pharm Sci, 2016. 19(4): p. 430–447. [DOI] [PubMed] [Google Scholar]
- 15.Masiero E, Sandri M Autophagy inhibition induces atrophy and myopathy in adult skeletal muscles. Autophagy, 2010. 6(2): p. 307–309. [DOI] [PubMed] [Google Scholar]
- 16.Forster M, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS, Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. PNAS, 1996. 93(10): p. 4765–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perrig W, Perrig P, Stähelin HB, The relation between antioxidants and memory performance in the old and very old. J Am Geriatr Soc, 1997. 1997(45): p. 6. [DOI] [PubMed] [Google Scholar]
- 18.Olsen R, Johnson LA, Zuloaga DG, Limoli CL, Raber J, Enhanced hippocampus-dependent memory and reduced anxiety in mice over-expressing human catalase in mitochondria. J Neurochem, 2013. 25(2): p. 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Q and He X, Molecular basis of electrophilic and oxidative defense: promises and perils of Nrf2. Pharmacol Rev, 2012. 64(4): p. 1055–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y, An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun, 1997. 236(2): p. 313–22. [DOI] [PubMed] [Google Scholar]
- 21.Motohashi H, Yamamoto M, Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med, 2004. 10(11): p. 549–57. [DOI] [PubMed] [Google Scholar]
- 22.Vomhof-Dekrey EE and Picklo MJ Sr., The Nrf2-antioxidant response element pathway: a target for regulating energy metabolism. J Nutr Biochem, 2012. 23(10): p. 1201–6. [DOI] [PubMed] [Google Scholar]
- 23.Dinkova-Kostova A, Abramov AY, The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med, 2015. 88(PtB): p. 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong F, Wang S, Wang Y, Yang X, Jiang J, Wu D, Qu X, Fan H, Yao R, Quercetin ameliorates learning and memory via the Nrf2-ARE signaling pathway in d-galactose-induced neurotoxicity in mice. Biochem Biophys Res Commun. 2017. 491(3): p. 636–41. [DOI] [PubMed] [Google Scholar]
- 25.Matsui N, Takahashi K, Takeichi M, Kuroshita T, Noguchi K, Yamazaki K, Tagashira H, Tsutsui K, Okada H, Kido Y, Yasui Y, Fukuishi N, Fukuyama Y, Akagi M, Magnolol and honokiol prevent learning and memory impairment and cholinergic deficit in SAMP8 mice. Brain Res, 2009. 1305: p. 108–117. [DOI] [PubMed] [Google Scholar]
- 26.Poulose S, Miller MG, Scott T, Shukitt-Hale B, Nutritional Factors Affecting Adult Neurogenesis and Cognitive Function. Adv Nutr, 2017. 8(6): p. 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carey A, Gomes SM, Shukitt-Hale B Blueberry supplementation improves memory in middle-aged mice fed a high-fat diet. J Agric Food Chem, 2014. 62(18): p. 3972–3978. [DOI] [PubMed] [Google Scholar]
- 28.Gray N, Harris CJ, Quinn JF, Soumyanath A, Centella asiatica modulates antioxidant and mitochondrial pathways and improves cognitive function in mice. J Ethnopharmacol, 2016. 180: p. 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray N, Zweig JA, Caruso M, Martin MD, Zhu JY, Quinn JF, Soumayanath A, Centella asiatica increases hippocampal synaptic density and improves memory and executive function in aged mice. Brain and Behavior, 2018. 93: p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iuso A, Repp B, Biagosch C, Terrile C, Prokisch H Assessing Mitochondrial Bioenergetics in Isolated Mitochondria from Various Mouse Tissues Using Seahorse XF96 Analyzer. Methods Mol Biol, 2017. 1567: p. 217–30. [DOI] [PubMed] [Google Scholar]
- 31.Kaech S and Banker G, Culturing hippocampal neurons. Nat Protoc, 2006. 1(5): p. 2406–15. [DOI] [PubMed] [Google Scholar]
- 32.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A Fiji: an open-source platform for biological-image Nature Methods, 2012. 9: p. 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferreira T, Blackman AV, Oyrer J, Jayabal S, Chung AJ, Watt AJ, Sjostrom PJ, van Meyel DJ Neuronal morphometry directly from bitmap images. Nature Methods, 2014. 11: p. 982–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gray N, Zweig JA, Murchison C, Caruso M, Matthews DG, Kawamoto C, Harris CJ, Quinn JF, Soumyanath A, Centella asiatica attenuates Aβ-induced neurodegenerative spine loss and dendritic simplification. Neursci Lett, 2017. 646: p. 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu H, Hudry E, Hashimoto T, Kuchibhotla K, Rozkalne A, Fan Z, Spires-Jones T, Xie H, Arbel-Ornath M, Grosskreutz CL, Bacskai BJ, Hyman BT, Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci, 2010. 30(7): p. 2636–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gray N, Zweig JA, Kawamoto C, Quinn JF, Copenhaver PF, STX, a Novel Membrane Estrogen Receptor Ligand, Protects Against Amyloid-β Toxicity. J Alzheimer’s Dis, 2016. 51(2): p. 391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fried NT, et al. , Functional mitochondrial analysis in acute brain sections from adult rats reveals mitochondrial dysfunction in a rat model of migraine. Am J Physiol Cell Physiol, 2014. 307(11): p. C1017–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franceschi C and Campisi J, Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci, 2014. 69 Suppl 1: p. S4–9. [DOI] [PubMed] [Google Scholar]
- 39.Raz N, Daugherty AM, Pathways to Brain Aging and Their Modifiers: Free-Radical-Induced Energetic and Neural Decline in Senescence (FRIENDS) Model - A Mini-Review. Gerontology, 2018. 64(1): p. 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anton S, Ebner N, Dzierzewski JM, Zlatar ZZ, Gurka MJ, Dotson VM, Kirton J, Mankowski RT, Marsiske M, Manini TM, Effects of 90 Days of Resveratrol Supplementation on Cognitive Function in Elders: A Pilot Study. J Altern Complement Med, 2018. 24(7): p. 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whyte A, Cheng N, Fromentin E, Williams CM, A Randomized, Double-Blinded, Placebo-Controlled Study to Compare the Safety and Efficacy of Low Dose Enhanced Wild Blueberry Powder and Wild Blueberry Extract (ThinkBlue™) in Maintenance of Episodic and Working Memory in Older Adults. Nutrients, 2018. 10(6): p. E660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McNamara R, Kalt W, Shidler MD, McDonald J, Summer SS, Stein AL, Stover AN, Krikorian R, Cognitive response to fish oil, blueberry, and combined supplementation in older adults with subjective cognitive impairment. Neurobiol Aging, 2018. 64: p. 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bensalem J, Dudonné S, Gaudout D, Servant L, Calon F, Desjardins Y, Layé S, Lafenetre P, Pallet V, Polyphenol-rich extract from grape and blueberry attenuates cognitive decline and improves neuronal function in aged mice. J Nutr Sci, 2018. 7: p. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shih P, Chan YC, Liao JW, Wang MF, Yen GC, Antioxidant and cognitive promotion effects of anthocyanin-rich mulberry (Morus atropurpurea L.) on senescence-accelerated mice and prevention of Alzheimer’s disease. J Nutr Biochem, 2010. 21(7): p. 598–605. [DOI] [PubMed] [Google Scholar]
- 45.Souza L, Antunes MS, Filho CB, Del Fabbro L, de Gomes MG, Goes AT, Donato F, Prigol M, Boeira SP, Jesse CR, Flavonoid Chrysin prevents age-related cognitive decline via attenuation of oxidative stress and modulation of BDNF levels in aged mouse brain. Pharmacol Biochem Behav, 2015. 134: p. 22–30. [DOI] [PubMed] [Google Scholar]
- 46.Holmstrom KM, Kostov RV, and Dinkova-Kostova AT, The multifaceted role of Nrf2 in mitochondrial function. Curr Opin Toxicol, 2016. 1: p. 80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Esteras N, Dinkova-Kostova AT, and Abramov AY, Nrf2 activation in the treatment of neurodegenerative diseases: a focus on its role in mitochondrial bioenergetics and function. Biol Chem, 2016. 397(5): p. 383–400. [DOI] [PubMed] [Google Scholar]
- 48.Codocedo J, Allard C, Godoy JA, Varela-Nallar L, Inestrosa NC, SIRT1 regulates dendritic development in hippocampal neurons. PLoS One, 2012. 7(10): p. e47073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhuiyan M, Haque MN, Mohibbullah M, Kim YK, Moon IS., Radix Puerariae modulates glutamatergic synaptic architecture and potentiates functional synaptic plasticity in primary hippocampal neurons. J Ethnopharmacol, 2017. 209: p. 100–107. [DOI] [PubMed] [Google Scholar]
- 50.Raz N, Rodrigue KM, Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev, 2006. 30(6): p. 730–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eling P, Derckx K, Maes R, On the historical and conceptual background of the Wisconsin Card Sorting Test. Brain Cogn, 2008. 67(3): p. 247–53. [DOI] [PubMed] [Google Scholar]
- 52.Ashendorf L, McCaffrey RJ, Exploring age-related decline on the Wisconsin Card Sorting Test. Clin Neuropsychol, 2008. 22(2): p. 262–72. [DOI] [PubMed] [Google Scholar]
- 53.Birrell J, Brown VJ, Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci, 2000. 20(11): p. 4320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garner J, Thogerson CM, Würbel H, Murray JD, Mench JA, Animal neuropsychology: validation of the Intra-Dimensional Extra-Dimensional set shifting task for mice. Behav Brain Res, 2006. 173(1): p. 53–61. [DOI] [PubMed] [Google Scholar]
- 55.Barense M, Fox MT, Baxter MG, Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learn Mem, 2002. 9(4): p. 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oh H, Song M, Kim YK, Bae JR, CHa SY, Bae JY, Kim Y, You M, Lee Y, Shim J, Maeng S, Age-Related Decrease in Stress Responsiveness and Proactive Coping in Male Mice. Front Aging Neurosci., 2018. 10(128): p. 10.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ren HL, et al. , Downregulated Nuclear Factor E2-Related Factor 2 (Nrf2) Aggravates Cognitive Impairments via Neuroinflammation and Synaptic Plasticity in the Senescence-Accelerated Mouse Prone 8 (SAMP8) Mouse: A Model of Accelerated Senescence. Med Sci Monit, 2018. 24: p. 1132–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh A, Naidu PS, Kulkarni SK, Reversal of aging and chronic ethanol-induced cognitive dysfunction by quercetin a bioflavonoid. Free Radic Res, 2003. 37(11): p. 1245–52. [DOI] [PubMed] [Google Scholar]
- 59.Patil C, Singh VP, Satyanarayan PS, Jain NK, Singh A, Kulkarni SK, Protective effect of flavonoids against aging- and lipopolysaccharide-induced cognitive impairment in mice. Pharmacology, 2003. 69(2): p. 59–67. [DOI] [PubMed] [Google Scholar]
- 60.Palomera-Ávalos V, Griñán-Ferré C, Izquierdo V, Camins A, Sanfeliu C, Pallàs M, Metabolic Stress Induces Cognitive Disturbances and Inflammation in Aged Mice: Protective Role of Resveratrol. Rejuvenation Res, 2017. 20(3): p. 202–217. [DOI] [PubMed] [Google Scholar]
- 61.Zhao Y, Li WF, Li F, Zhang Z, Dai YD, Xu AL, Qi C, Gao JM, Gao J, Resveratrol improves learning and memory in normally aged mice through microRNA-CREB pathway. Biochem Biophys Res Commun, 2013. 435(4): p. 597–602. [DOI] [PubMed] [Google Scholar]
- 62.Gray NE, A.M.A., Lak P, Wright KM, Quinn J, Stevens JF, Maier CS, Soumyanath A, Centella asiatica: phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochemical Reviews, 2017. 17(1): p. 161–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Liu B, Chen X, Zhang N, Li G, Zhang LH, Tan LY, Naringenin Ameliorates Behavioral Dysfunction and Neurological Deficits in a d-Galactose-Induced Aging Mouse Model Through Activation of PI3K/Akt/Nrf2 Pathway. Rejuvenation Res, 2017. 20(6): p. 462–472. [DOI] [PubMed] [Google Scholar]
- 64.Zhao B, Ren B, Guo R, Zhang W, Ma S, Yao Y, Yuan T, Liu Z, Liu X, Supplementation of lycopene attenuates oxidative stress induced neuroinflammation and cognitive impairment via Nrf2/NF-κB transcriptional pathway. Food Chem Toxicol, 2017. 109(Pt 1): p. 505–516. [DOI] [PubMed] [Google Scholar]
- 65.Holmstrom KM, et al. , Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol Open, 2013. 2(8): p. 761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hayashi G, Jasoliya M, Sahdeo S, Saccà F, Pane C, Filla A, Marsili A, Puorro G, Lanzillo R, Brescia Morra V, Cortopassi G, Dimethyl fumarate mediates Nrf2-dependent mitochondrial biogenesis in mice and humans. Hum.Mol.Genet, 2017. 26(15): p. 2864–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piantadosi CA, C.M.S., Babiker A, Suliman HB, Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res, 2008. 103: p. 1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gray N, Sampath H, Zweig JA, Quinn JF, Soumyanath A, Centella asiatica Attenuates Amyloid-β-Induced Oxidative Stress and Mitochondrial Dysfunction. Journal of Alzheimer’s Disease, 2015. 45(3): p. 933–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gray N, Zweig JA, Matthews DG, Caruso M, Quinn JF, Soumyanath A, Centella asiatica Attenuates Mitochondrial Dysfunction and Oxidative Stress in Aβ-Exposed Hippocampal Neurons. Oxid Med Cell Longev, 2017. 2017: p. 7023091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gray NE, et al. , Centella asiatica attenuates hippocampal mitochondrial dysfunction and improves memory and executive function in beta-amyloid overexpressing mice. Mol Cell Neurosci, 2018. 93: p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Merry T, Ristow M, Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and the anti-oxidant response in mice. J Physiol, 2016. 594(18): p. 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yin F, Sancheti H, Patil 2, Cadenas E, Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic Biol Med, 2016. 100: p. 108–122. [DOI] [PMC free article] [PubMed] [Google Scholar]