Abstract
The genetic diversity of Cryptosporidium spp. in Apodemus spp. (striped field mouse, yellow-necked mouse and wood mouse) from 16 European countries was examined by PCR/sequencing of isolates from 437 animals. Overall, 13.7% (60/437) of animals were positive for Cryptosporidium by PCR. Phylogenetic analysis of small-subunit rRNA, Cryptosporidium oocyst wall protein and actin gene sequences showed the presence of C. ditrichi (22/60), C. apodemi (13/60), Cryptosporidium apodemus genotype I (8/60), Cryptosporidium apodemus genotype II (9/60), C. parvum (2/60), C. microti (2/60), C. muris (2/60) and C. tyzzeri (2/60). At the gp60 locus, novel gp60 families XVIIa and XVIIIa were identified in Cryptosporidium apodemus genotype I and II, respectively, subtype IIaA16G1R1b was identified in C. parvum, and subtypes IXaA8 and IXcA6 in C. tyzzeri. Only animals infected with C. ditrichi, C. apodemi, and Cryptosporidium apodemus genotypes shed oocysts that were detectable by microscopy, with the infection intensity ranging from 2,000 to 52,000 oocysts per gram of faeces. None of the faecal samples was diarrheic in the time of the sampling.
Keywords: Epidemiology, Molecular analyses, Phylogeny, Rodentia
Graphical Abstract
Introduction
Parasites of the genus Cryptosporidium (Apicomplexa), infect epithelial cells in the microvillus border of the gastrointestinal and respiratory tract of vertebrates (Cavalier-Smith 2014; Ryan and Xiao 2014; Striepen 2013). Studies on Cryptosporidium phylogenetics and biology have revealed extensive diversity and major differences in host specificity (Kváč et al. 2014b; Ryan and Xiao 2014). Species such as C. parvum, C. baileyi, C. meleagridis and C. ubiquitum exhibit relatively broad host specificity (Fayer 2007; Li et al. 2014; Nakamura and Meireles 2015; Stenger et al. 2015; Vetterling et al. 1971), while other species are specific to one or more closely related hosts. Determining the host range of Cryptosporidium can be complicated by the occurrence of mechanical passage, whereby low numbers of oocysts pass through the animal without causing an active infection and are detected in the faeces (Graczyk et al. 1998; Kváč et al. 2012). Mechanical passage is exemplified by reports of rodent-adapted C. muris in the faeces of snakes and lizards (Crawshaw and Mehren 1987; Graczyk et al. 1996; Xiao et al. 2004), which was due to the animals ingesting infected mice (Xiao et al., 2004). Similarly, C. muris and the house mouse-adapted C. tyzzeri have been detected in pig faeces and slurry (Kváč et al. 2012), probably as a result of the pigs ingesting infected mice or food contaminated by mouse faeces (Jenkins et al. 2010; Ren et al. 2012; Xiao et al. 2006), and C. bovis, a species that is specific for cattle (Fayer et al. 2005), was detected in a fully habituated western lowland gorilla, probably because the gorilla’s environment was contaminated by grazing cattle (Sak et al. 2014).
Several Cryptosporidium spp. identified in murid rodents from the genus Apodemus are specific for other hosts (e.g. C. hominis, C. muris, C. parvum, C. scrofarum, C. suis, C. ubiquitum, Cryptosporidium chipmunk genotype I, Cryptosporidium muskrat genotype II and Cryptosporidium Naruko genotype) (Danišová et al. 2017; Hajdušek et al. 2004; Hikosaka and Nakai 2005; Kulis-Malkowska 2007; Li et al. 2014; Murakoshi et al. 2013; Perec-Matysiak et al. 2015; Song et al. 2015). Čondlová et al. (2018) recently described C. apodemi and C. ditrichi in species of Apodemus and presented evidence from experimental and field studies that these species are host specific. Given the limited scope of the original study, the present study aimed at describing the occurrence of C. apodemi and C. ditrichi across 16 European countries.
Material and Methods
Ethics statement
Traps were checked frequently and handling time was minimized to reduce animal stress, and all applicable international, national and institutional guidelines for the care and use of animals were followed. The research was conducted under ethical protocols approved by the Institute of Parasitology, Biology Centre and Central Commission for Animal Welfare, the Czech Republic.
Sample collection and parasitological examination
From May to September in 2016 and 2017, wild Apodemus spp. were trapped in Austria, Belgium, Bosnia and Herzegovina, Bulgaria, Czech Republic, Finland, France, Germany, Hungary, Latvia, Lithuania, Netherlands, Poland, Romania, Serbia and Slovakia (Table 1). Animals were captured with sterile live- or snap-traps baited with smoked cheese and were examined to determine the species. For live-trapped animals, faecal samples were collected directly from the trap or from the animal during handling and the animal was subsequently released. For snap-trapped animals, faecal samples were collected from the colon after animal dissection. Each faecal sample was stored in a separate screw-cap container, transported to the laboratory and screened for the presence of Cryptosporidium oocysts by brightfield microscopy (Olympus BX51, Tokyo, Japan), at 1,000× magnification, following aniline-carbol-methyl violet staining (Miláček and Vítovec 1985). The infection intensity was determined as the number of oocysts per gram (OPG) of faeces in accordance with Kváč et al. (2007).
Table 1.
Cryptosporidium species and genotypes based on amplification of the small subunit ribosomal rRNA (SSU), actin, Cryptosporidium oocyst wall protein (COWP) and 60kDa glycoprotein (gp60) genes by PCR in samples of striped field mice (Apodemus agrarius), yellow-necked mice (Apodemus flavicollis) and wood mice (Apodemus sylvaticus) from Austria, Belgium, Bosnia and Herzegovina, Bulgaria, Czech Republic, Finland, France, Germany, Hungary, Latvia, Lithuania, Netherlands, Poland, Romania, Serbia and Slovakia.
| Country | Host | N | Isolate ID | Microscopical positivity (OPG) | Genotyping at the loci | |||
|---|---|---|---|---|---|---|---|---|
| SSU | actin | COWP | gp60 | |||||
| Austria | A. flavicollis | 5 | - | - | - | - | - | - |
| A. sylvaticus | 3 | - | - | - | - | - | - | |
| Belgium | A. flavicollis | 2 | 27677 | Yes (4,000) | C. ditrichi | C. ditrichi | C. ditrichi | - |
| Bosnia and Herzegovina | A. flavicollis | 1 | - | - | - | - | - | - |
| Bulgaria | A. agrarius | 5 | - | - | - | - | - | - |
| A. flavicollis | 3 | - | - | - | - | - | - | |
| A. sylvaticus | 2 | - | - | - | - | - | - | |
| Czech Republic | A. flavicollis | 153 | 14887 | Yes (28,000) | C. ditrichi | C. ditrichi | C. ditrichi | - |
| 14890 | Yes (2,000) | C. ditrichi | C. ditrichi | C. ditrichi | - | |||
| 12690 | Yes (24,000) | C. ditrichi | C. ditrichi | C. ditrichi | - | |||
| 12696 | Yes (52,000) | C. ditrichi | C. ditrichi | C. ditrichi | - | |||
| 5025 | No | C. apodemi | C. apodemi | C. apodemi | - | |||
| 12405 | Yes (4,000) | apodemus genotype I | apodemus genotype I | apodemus genotype I | XVIIa | |||
| 30891 | Yes (2,000) | apodemus genotype I | apodemus genotype I | apodemus genotype I | XVIIa | |||
| 30893 | Yes (6,000) | apodemus genotype I | apodemus genotype I | apodemus genotype I | XVIIa | |||
| 23228 | No | C. tyzzeri | C. tyzzeri | C. tyzzeri | IXaA8 | |||
| 14895 | Yes (2,000) | apodemus genotype I | apodemus genotype I | apodemus genotype I | XVIIa | |||
| 12656 | No | apodemus genotype II | apodemus genotype II | - | XVIIIa | |||
| 21523 | No | C. microti | C. microti | C. microti | - | |||
| 23009 | No | C. microti | C. microti | C. microti | - | |||
| A. sylvaticus | 23 | 12373 | Yes (8,000) | C. ditrichi | C. ditrichi | C. ditrichi | - | |
| Finland | A. flavicollis | 2 | 30329 | Yes (2,000) | C. ditrichi | C. ditrichi | C. ditrichi | - |
| France | A. flavicollis | 16 | 30357 | Yes (6,000) | C. ditrichi | C. ditrichi | - | - |
| 30358 | Yes (2,000) | C. ditrichi | C. ditrichi | - | - | |||
| A. sylvaticus | 4 | 30364 | Yes (4,000) | C. ditrichi | C. ditrichi | - | - | |
| Germany | A. flavicollis | 10 | 12058 | No | C. parvum | C. parvum | C. parvum | IIaA16GlRlb |
| 12062 | No | C. ditrichi | C. ditrichi | C. ditrichi | - | |||
| 12063 | No | C. ditrichi | C. ditrichi | C. ditrichi | - | |||
| Hungary | A. agrarius | 4 | - | - | - | - | - | - |
| Latvia | A. agrarius | 11 | 27716 | Yes (6,000) | apodemus genotype II | apodemus genotype II | - | XVIIIa |
| 27715 | Yes (4,000) | apodemus genotype II | apodemus genotype II | - | XVIIIa | |||
| 27712 | Yes (2,000) | C. ditrichi | C. ditrichi | - | - | |||
| Lithuania | A. agrarius | 3 | 27721 | Yes (2,000) | C. apodemi | C. apodemi | C. apodemi | - |
| Netherlands | A. sylvaticus | 6 | 27675 | Yes (6,000) | C. ditrichi | C. ditrichi | - | - |
| Poland | A. flavicollis | 83 | 12958 | No | C. muris | C. muris | C. muris | |
| 12957 | No | apodemus genotype II | apodemus genotype II | - | XVIIIa | |||
| 151 | No | apodemus genotype I | apodemus genotype I | apodemus genotype I | XVIIa | |||
| 111 | Yes (2,000) | apodemus genotype I | apodemus genotype I | apodemus genotype I | XVIIa | |||
| 436 | Yes (6,000) | C. ditrichi | C. ditrichi | C. ditrichi | - | |||
| 521 | Yes (4,000) | C. ditrichi | C. ditrichi | C. ditrichi | - | |||
| 361 | No | C. apodemi | C. apodemi | C. apodemi | - | |||
| Romania | A. agrarius | 2 | 30374 | Yes (2,000) | C. apodemi | C. apodemi | C. apodemi | - |
| 30375 | No | C. apodemi | C. apodemi | C. apodemi | - | |||
| A. flavicollis | 1 | - | - | - | - | - | ||
| A. sylvaticus | 1 | - | - | - | - | - | ||
| Serbia | A. agrarius | 4 | 30383 | Yes (2,000) | apodemus genotype II | apodemus genotype II | - | XVIIIa |
| A. flavicollis | 14 | 30395 | No | apodemus genotype II | apodemus genotype II | - | XVIIIa | |
| 30390 | Yes (2,000) | apodemus genotype II | apodemus genotype II | - | XVIIIa | |||
| 30387 | Yes (22,000) | C. ditrichi | C. ditrichi | C. ditrichi | - | |||
| 30394 | No | C. ditrichi | C. ditrichi | - | - | |||
| A. sylvaticus | 3 | 30389 | No | C. tyzzeri | C. tyzzeri | C. tyzzeri | IXcA6 | |
| Slovakia | A. agrarius | 33 | 10502 | No | apodemus genotype II | apodemus genotype II | - | XVIIIa |
| 11657 | No | apodemus genotype II | apodemus genotype II | - | XVIIIa | |||
| 10462 | No | C. apodemi | C. apodemi | C. apodemi | - | |||
| 4974 | Yes (2,000) | C. apodemi | C. apodemi | C. apodemi | - | |||
| 4961 | Yes (4,000) | C. apodemi | C. apodemi | C. apodemi | - | |||
| 10479 | No | C. ditrichi | C. ditrichi | C. ditrichi | - | |||
| A. flavicollis | 35 | 11985 | No | C. muris | C. muris | C. muris | ||
| 7799 | No | C. ditrichi | C. ditrichi | C. ditrichi | - | |||
| 4950 | Yes (4,000) | C. ditrichi | C. ditrichi | C. ditrichi | - | |||
| 8131 | No | C. parvum | C. parvum | C. parvum | IIaA16GlRlb | |||
| 10466 | No | C. ditrichi | C. ditrichi | C. ditrichi | ||||
| 30369 | Yes (8,000) | apodemus genotype I | apodemus genotype I | apodemus genotype I | XVIIa | |||
| 8153 | No | apodemus genotype I | apodemus genotype I | apodemus genotype I | XVIIa | |||
| 8049 | No | C. apodemi | C. apodemi | C. apodemi | - | |||
| 8060 | No | C. apodemi | C. apodemi | C. apodemi | - | |||
| 7780 | No | C. apodemi | C. apodemi | C. apodemi | - | |||
| A. sylvaticus | 8 | 30402 | Yes (4,000) | C. apodemi | C. apodemi | C. apodemi | - | |
| 30407 | No | C. apodemi | C. apodemi | C. apodemi | - | |||
Oocysts were quantified by microscopy and reported per gram of faeces (OPG); N – Number of examined samples; ID – identification; No – microscopically negative; Yes – Microscopically positive
Molecular characterisation
DNA was extracted from 100–200 mg of faeces by bead disruption for 60 s at 5.5 m/s using 0.5 mm glass beads in a Fast Prep 24 Instrument (MP Biomedicals, CA, USA), followed by isolation and purification using a commercially available kit in accordance with the manufactureŕs instructions (Exgene™ Stool DNA mini, GeneAll Biotechnology Co. Ltd, Seoul, Korea). Purified DNA was stored at −20°C prior to amplification by PCR. A nested PCR approach was used to amplify a partial region of genes encoding the small ribosomal subunit rRNA (SSU; ~830 bp; Jiang et al. 2005; Xiao et al. 1999), actin (~1066 bp; Sulaiman et al. 2002), Cryptosporidium oocyst wall protein (COWP; ~550 bp; Spano et al. 1997) and 60 kDa glycoprotein (gp60; ~850 bp; Alves et al. 2003; Li et al. 2014). The primary PCR mixtures contained 2 μl of template DNA, 2.5 U of Taq DNA Polymerase (Dream Taq Green DNA Polymerase, Thermofisher Scientific, Waltham, MA), 0.5× PCR buffer (SSU) or 1× PCR buffer (actin, COWP and gp60; Thermofisher Scientific), 6 mM MgCl2 (SSU) or 3 mM MgCl2 (actin, COWP and gp60), 200 μM each deoxynucleoside triphosphate (dNTP), 200 mM each primer and 2 μl nonacetylated bovine serum albumin (BSA; 10 mg/ml; New England Biolabs, Beverly, MA) in 50 μl reaction volume.
Phylogenetic analysis
Secondary PCR products were separated on an agarose gel and visualized under UV illumination using ethidium bromide staining. Products were purified (Gen Elute Gel Extraction Kit, Sigma, St. Louis, MO) and sequenced in both directions with secondary primers using a Big Dye Terminator v3.1 cycle sequencing kit in an ABI Prism 3130 genetic analyzer (Applied Biosystems, Carlsbad, CA). The nucleotide sequences of each gene obtained in this study were manually edited using the program Chromas Pro 2.1.4 (Technelysium, Pty, Ltd., South Brisbane, Australia), and aligned with previously published sequences using the MAFFT version 7 online server using the Q-INS-I algorithm for SSU, actin and COWP sequences and L-INS-I algorithm for gp60 sequences (http://mafft.cbrc.jp/alignment/server/). The alignment included published sequences from Cryptosporidium species and sequences with a high similarity to study sequences using BLAST analysis (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Phylogenetic trees were inferred by maximum likelihood (ML) method, with the substitution model that best fits the alignment selected using the Bayesian information criterion. ML analysis of SSU, actin, COWP and gp60 alignments was done in the MEGA7 software. The Tamura 3-parameter model was selected for SSU, COWP and gp60 and the General Time Reversible model was used for actin alignment. All models were used under an assumption that rate variation among sites was gamma distributed with invariant sites. Bootstrap support for branching was based on 1000 replications. Phylograms were edited for style using CorelDrawX7. Sequences have been deposited in GenBank under the Accession Numbers (Acc. nos.) MH912926–MH912969 for COWP, MH912970–MH912990 for gp60, MH912991–MH913050 for SSU and MH913051–MH913110 actin gene.
Results
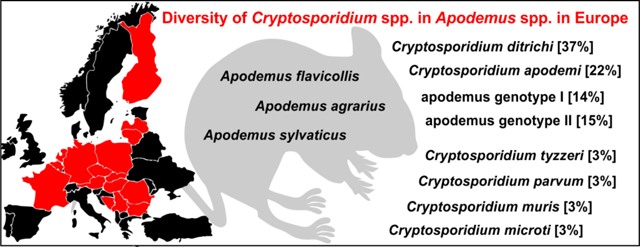
In total, 437 animals from the genus Apodemus, comprising 62 striped field mice (Apodemus agrarius), 325 yellow-necked mice (Apodemus flavicollis) and 50 wood mice (Apodemus sylvaticus), were sampled in 16 European countries (Table 1). Overall, 13.7% (60/437) of Apodemus spp. tested positive for Cryptosporidium by PCR (Table 1).
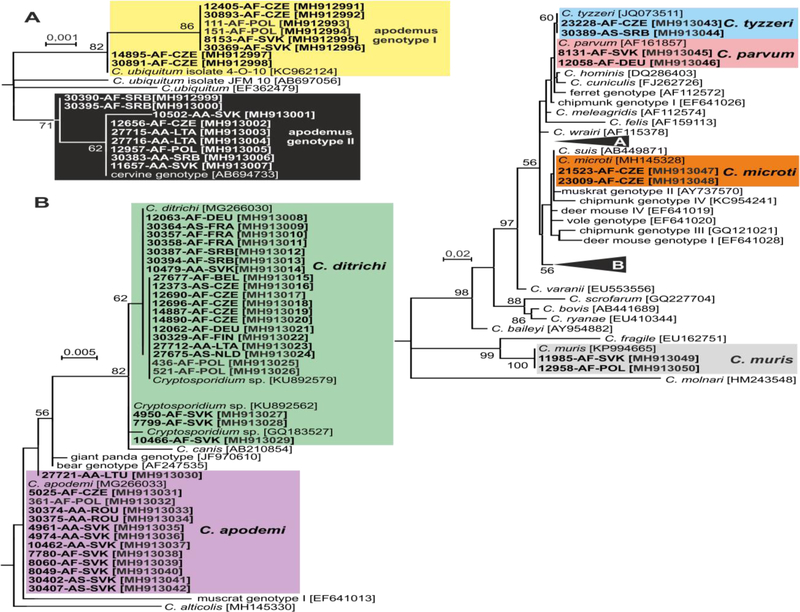
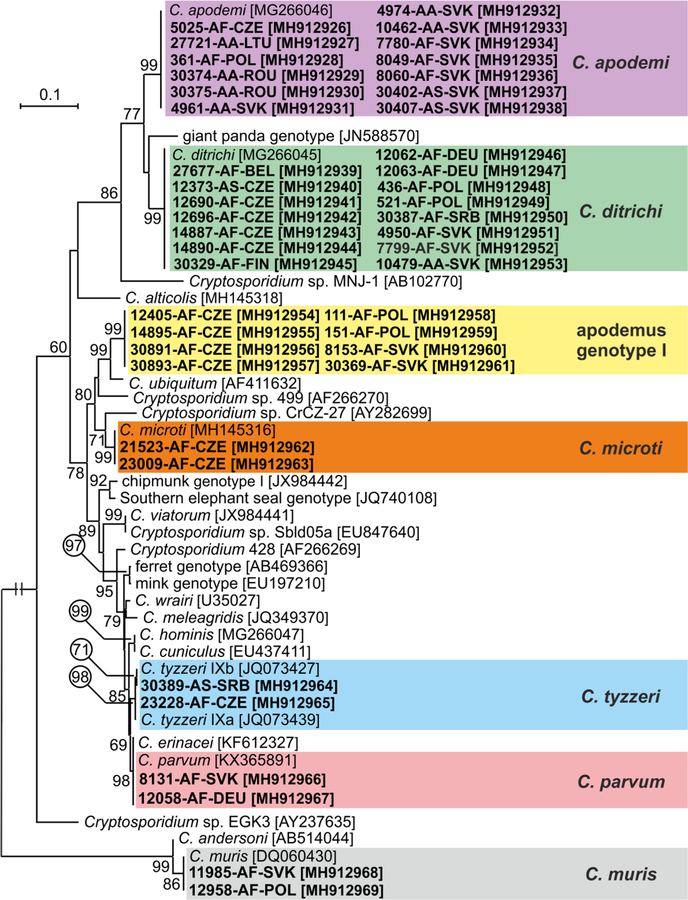
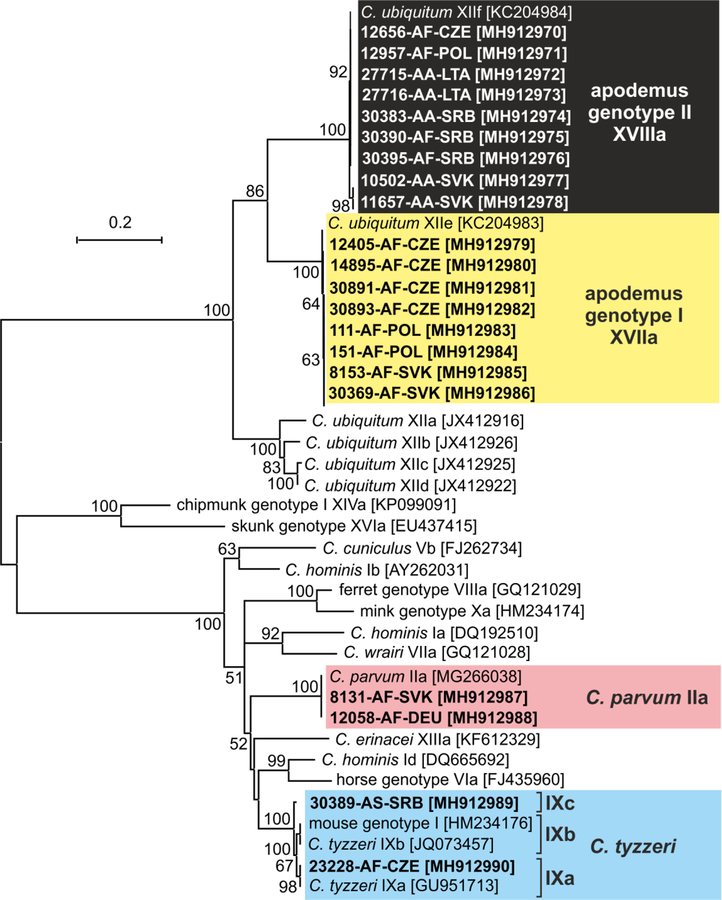
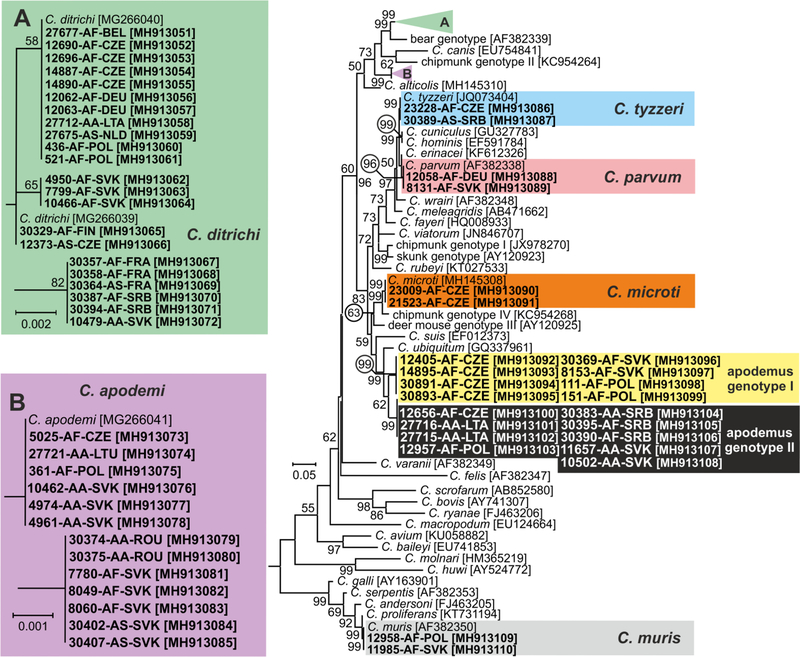
Out of 60 Cryptosporidium positive animals, 60, 60, 45 and 21 were genotyped by sequence analysis of SSU, actin, COWP and gp60 genes, respectively (Table 1). The remaining positive samples failed to amplify at COWP (n=15) and gp60 (n=39) loci. MP trees constructed from SSU, actin and COWP gene sequences in this study and representative sequences in GenBank showed the presence of eight Cryptosporidium spp. (Figs. 1–3). Cryptosporidium ditrichi (n=22) and C. apodemi (n=13), species that have been reported as Apodemus specific, were the most prevalent species in screened animals. In contrast, C. parvum (n=2), C. muris (n=2), C. tyzzeri (n=2) and C. microti (n=2), which are specific for other mammals, were detected rarely. Phylogenetic analysis of screened genes revealed the presence of two novel Cryptosporidium genotypes, which were named Cryptosporidium apodemus genotype I (n=8) and apodemus genotype II (n=9). SSU, actin and COWP sequences of these novel genotypes formed distinct groups that were closely related to each other and to C. ubiquitum. gp60 sequences of Cryptosporidium apodemus genotypes I and II clustered with isolates originally reported from Apodemus spp. and identified as C. ubiquitum XIIe and XIIf, respectively (Fig. 4). In accordance with the gp60 nomenclature established by Sulaiman et al. (2005) we named the gp60 family of Cryptosporidium apodemus genotypes I and II as novel family XVII (previously known as C. ubiquitum XIIe) and XVIII (previously known as C. ubiquitum XIIf), respectively.
Fig. 1.
Maximum likelihood tree (−ln = 3040.40) based on partial small subunit ribosomal RNA gene sequences of Cryptosporidium, including sequences obtained in this study (highlighted and bolded). The alignment contained 780 base positions in the final dataset. Tamura’s 3- parameter model was applied, using a discrete Gamma distribution and invariant sites. Numbers at the nodes represent the bootstrap values with more than 50% bootstrap support from 1000 pseudoreplicates. The branch length scale bar, indicating the number of substitutions per site, is given in the tree. Sequences from this study are identified by isolate number (e.g. 8131), host species (AA for Apodemus agrarius, AF for Apodemus flavicollis and AS for Apodemus sylvaticus) and region (BEL for Belgium, CZE for Czech Republic, FIN for Finland, FRA for France, DEU for Germany, LTA for Latvia, LTU for Lithuania, NLD for Nederland, POL for Poland, ROU for Romania, SRB for Serbia and SVK for Slovakia).
Fig. 3.
Maximum likelihood tree (-ln = 2652.86) based on partial sequences of gene coding Cryptosporidium oocyst wall protein (COWP), including sequences obtained in this study (highlighted and bolded). The alignment contained 455 base positions in the final dataset. Tamura’s 3- parameter model was applied, using a discrete Gamma distribution and invariant sites. Numbers at the nodes represent the bootstrap values with more than 50% bootstrap support from 1000 pseudoreplicates. The branch length scale bar, indicating the number of substitutions per site, is given in the tree. Sequences from this study are identified by isolate number (e.g. 8131), host species (AA for Apodemus agrarius, AF for Apodemus flavicollis and AS for Apodemus sylvaticus) and region (BEL for Belgium, CZE for Czech Republic, FIN for Finland, DEU for Germany, LTU for Lithuania, POL for Poland, ROU for Romania, SRB for Serbia and SVK for Slovakia).
Fig. 4.
Maximum likelihood tree (−ln = 6245.99) based on partial sequences of gene coding 60 kDa glycoprotein of Cryptosporidium (gp60), including sequences obtained in this study (highlighted and bolded). The alignment contained 947 base positions in the final dataset. Tamura’s 3- parameter model was applied, using a discrete Gamma distribution and invariant sites. Numbers at the nodes represent the bootstrap values with more than 50% bootstrap support from 1000 pseudoreplicates. The branch length scale bar, indicating the number of substitutions per site, is given in the tree. Sequences from this study are identified by isolate number (e.g. 8131), host species (AA for Apodemus agrarius, AF for Apodemus flavicollis and AS for Apodemus sylvaticus) and region (CZE for Czech Republic, DEU for Germany, LTA for Latvia, POL for Poland, SRB for Serbia and SVK for Slovakia).
A ML tree constructed using gp60 sequences obtained in this study and sequences published in GenBank revealed that the C. parvum isolates from the Czech Republic and Germany belonged to family IIa subtype A16G1R1b, C. tyzzeri from the Czech Republic belonged to family IXa and C. tyzzeri from Serbia to novel family IXc (Fig. 4).
Cryptosporidium ditrichi, C. apodemi, Cryptosporidium apodemus genotype I and Cryptosporidium apodemus genotype II were detected in ten, five, three and five out of 16 countries, respectively (Table 1 and Fig. 1).
Out of the 60 animals that tested positive for Cryptosporidium by PCR, 31 (51.7%) shed oocysts that were detectable by microscopy. The infection intensity in microscopy positive animals ranged from 2,000 to 52,000 OPG (Table 1). Only animals that were PCR positive for C. ditrichi, C. apodemi or Cryptosporidium apodemus genotypes I and II shed detectable oocysts. Out of 22 animals positive for C. ditrichi, 16 (73%) shed detectable oocysts (2,000–52,000 OPG). Similarly, 75% (6/8) of animals infected with Cryptosporidium apodemus genotype I shed detectable oocysts (2,000–8,000 OPG). In contrast, only 38% (5/13) and 44% (4/9) of animals positive for C. apodemi and Cryptosporidium apodemus genotype II, respectively, shed detectable oocysts with an infection intensity ranging from 2,000 to 4,000 OPG. None of the faecal samples was diarrhoeic at the time of sampling.
Discussion
Microscopy-based tools used in early studies on Cryptosporidium from Apodemus were poor at distinguishing among species and genotypes. Hence, oocysts that were morphometrically similar to C. parvum were identified as C. parvum and those that were similar to C. muris were identified as that gastric species (Bednarska et al. 2007; Chalmers et al. 1997; Torres et al. 2000; Webster and Macdonald 1995a, b). When molecular tools were used in later studies, C. parvum was found to be an infrequent parasite of Apodemus (Čondlová et al. 2018; Murakoshi et al. 2013; Perec-Matysiak et al. 2015; Song et al. 2015) and other rodents such as mice, rats, voles and squirrels (Horčičková et al. 2018; Lv et al. 2009; Ng-Hublin et al. 2013; Prediger et al. 2017; Saki et al. 2016; Stenger et al. 2015). Consistent with those studies, we detected C. parvum in only two out of 60 animals that were positive for Cryptosporidium. The gp60 subtype of both isolates, IIaA16G1R1, and the subtype reported by Danišová et al. (2017) in Apodemus, IIaA18G3R1, are common in domesticated ruminants worldwide (Del Coco et al. 2014; Imre et al. 2013; Ng et al. 2012; Trotz-Williams et al. 2006; Wielinga et al. 2008). These findings suggest that Apodemus species are not major hosts of C. parvum and are probably only transiently infected following exposure to contaminated manure from ruminants. This is supported by our experimental studies, which showed that C. parvum is poorly infective for A. sylvaticus, infecting only one of three inoculated animals (unpublished data).
We found C. muris in only two animals (0.5%), consistent with the low prevalence reported in previous studies in the Czech Republic, Slovakia and Poland (Čondlová et al. 2018; Danišová et al. 2017; Perec-Matysiak et al. 2015). A higher prevalence was reported in studies in the UK (5%; Chalmers et al. 1997), Spain (9.7%; Torres et al. 2000), Japan (13.3%; Murakoshi et al. 2013) and Korea (5.2–7.1%; Song et al. 2015). In Europe, all C. muris isolates that were genotyped were identified as RN66, a strain that is found predominantly in rats and house mice worldwide (Backhans et al. 2013; Iseki et al. 1989; Kváč et al. 2012; Rhee et al. 1995; Satoh et al. 2003; Xiao et al. 1999). In contrast, all isolates from Japan and Korea were identified as the Japanese field mouse genotype (Murakoshi et al. 2013; Song et al. 2015), which has only been found in Apodemus spp. in Japan and Korea.
This is the first report of C. tyzzeri in Apodemus. However, the low prevalence (0.5%) and the reported specificity of C. tyzzeri for the house mouse (Kváč et al. 2013), suggests that Apodemus spp. are minor hosts. Kváč et al. (2013) identified two major gp60 subtypes (IXa and IXb) of C. tyzzeri and showed that they had different natural host specificities – IXa was restricted to the house mouse subspecies Mus musculus musculus (Mmm) and IXb was restricted to Mus musculus domesticus (Mmd). In the present study, one of the C. tyzzeri isolates was the IXa subtype, and, consistent with the previous work by Kváč et al. (2013), it was recovered from an animal trapped in the Czech Republic, where only Mmm are found. The C. tyzzeri isolate from Serbia, an area that also only has Mmm (de Bellocq et al. 2015), had a novel gp60 subtype, IXc.
Cryptosporidium microti was detected in two animals in the present study. Given that this species is specific for voles and is not infectious for Apodemus spp. under experimental conditions (Horčičková et al. 2018), its presence here may be the result of passive passage of oocysts ingested from a contaminated environment in an area of overlapping Apodemus and vole (Microtus spp.) habitats.
Cryptosporidium ditrichi and C. apodemi were the most prevalent species in this study and were found throughout Europe (12/16 countries). This is consistent with a previous study by Čondlová et al. (2018), who found that these were the most prevalent species in Apodemus in the Czech Republic and Slovakia and showed that they were specifically infective for Apodemus under experimental conditions. Remarkably, only one other study of Apodemus reported a similar genotype. Song et al. (2015) identified Cryptosporidium sp. isolate KSFM, which shares 99.1% identity with C. apodemi at the SSU locus, from A. agrarius in South Korea. In the present study, C. ditrichi and C. apodemi were distributed in countries throughout Europe (12/16 countries), including Poland and Slovakia, where previous studies have failed to detect these species in Apodemus (Danišová et al. 2017; Perec-Matysiak et al. 2015). Although Cryptosporidium prevalence in host populations can fluctuate across locations, seasons and years (Bajer et al. 2002; Perec-Matysiak et al. 2015; Petersen et al. 2015), the absence of these species from previous studies is striking.
After C. ditrichi and C. apodemi, Cryptosporidium apodemus genotypes I and II were the next most frequently isolated Cryptosporidium from Apodemus spp. in the present study. Cryptosporidium apodemus genotype I was isolated previously from A. flavicollis in Poland Perec-Matysiak et al. (2015) and identified as C. ubiquitum 4-O-10 [GenBank Acc. No. KC962124]. Cryptosporidium apodemus genotype II was detected previously in surface water in Japan and also identified as C. ubiquitum [GenBank Acc. No. AB694733; reported as the cervine genotype, which is the previous name for C. ubiquitum]. At the gp60 locus, Cryptosporidium apodemus genotypes I and II were identical to C. ubiquitum XIIe and XIIf, respectively, which were previously reported from Apodemus agrarius and A. flavicollis in Slovakia (Li et al. 2014). However, phylogenetic analysis based on SSU, actin and COWP gene sequences show that these genotypes are distinct from C. ubiquitum. Therefore, in accordance with the gp60 subtyping nomenclature (Lv et al. 2009; Sulaiman et al. 2005), we propose to rename the gp60 family XVIIa for Cryptosporidium apodemus genotype I and family XVIIIa for Cryptosporidium apodemus genotype II.
Consistent with most reports describing natural and experimental infections with Cryptosporidium spp. in wild animals (Castro-Hermida et al. 2011; Čondlová et al. 2018; Holubová et al. 2016; Horčičková et al. 2018; Kváč et al. 2014a; Li et al. 2015; Prediger et al. 2017), Apodemus spp. shed low numbers of oocysts, often below the detection limit of microscopy, and showed no clinical signs of cryptosporidiosis. This is in contrast to the high oocyst shedding and diarrhea caused by species such as C. parvum (in humans and neonatal livestock), C. hominis (in humans) and C. meleagridis (in humans and poultry) (Bouzid et al. 2013; Fayer et al. 1998; Jacobson et al. 2016; Papanikolopoulou et al. 2018; Pedraza-Diaz et al. 2001; Tůmová et al. 2002). The different outcomes of Cryptosporidium-host interactions are probably the result of coevolutionary adaptations, but the reason for the differences in infection intensity of Cryptosporidium in different hosts is not currently known.
Conclusions
Apodemus spp. in Europe are frequently infected with four Cryptosporidium species/genotypes – C. apodemi, C. ditrichi, Cryptosporidium apodemus genotype I and Cryptosporidium apodemus genotype II. Data from this and previous studies show that Apodemus spp. are minor hosts of C. parvum, C. muris, C. tyzzeri, C. microti, C. ubiquitum, C. scrofarum, C. suis, C. hominis, Cryptosporidium muskrat genotype II or Cryptosporidium chipmunk genotype I.
Fig. 2.
Maximum likelihood tree (−ln = 7878.45) based on partial sequences of gene coding actin of Cryptosporidium, including sequences obtained in this study (highlighted and bolded). The alignment contained 695 base positions in the final dataset. The General Time Reversible model was applied, using a discrete Gamma distribution and invariant sites. Numbers at the nodes represent the bootstrap values with more than 50% bootstrap support from 1000 pseudoreplicates. The branch length scale bar, indicating the number of substitutions per site, is given in the tree. Sequences from this study are identified by isolate number (e.g. 8131), host species (AA for Apodemus agrarius, AF for Apodemus flavicollis and AS for Apodemus sylvaticus) and region (BEL for Belgium, CZE for Czech Republic, FIN for Finland, FRA for France, DEU for Germany, LTA for Latvia, LTU for Lithuania, NLD for Nederland, POL for Poland, ROU for Romania, SRB for Serbia and SVK for Slovakia).
Highlights.
Eight Cryptosporidium species detected in mice of the genus Apodemus
Apodemus spp. in Europe are frequently infected with four Cryptosporidium spp.
Cryptosporidium apodemi and C. ditrichi are most prevalent in Apodemus spp. Mice
Novel Cryptosporidium apodemus genotype I and II were described in Apodemus spp.
Apodemus spp. are minor hosts of C. parvum, C. muris, C. tyzzeri and C. microti
Acknowledgements
The authors thank all collaborators involved in the project for providing biology material for our research. This study was funded by grants from the Ministry of Education, Youth and Sports of the Czech Republic (LTAUSA17165) and the Grant Agency of the University of South Bohemia (project No. 002/2016/Z) to MK and by a grant from NIH-NIAID to JM (1R15AI122152-01A1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alves M, Xiao LH, Sulaiman I, Lal AA, Matos O, Antunes F, 2003. Subgenotype analysis of Cryptosporidium isolates from humans, cattle, and zoo ruminants in Portugal. J. Clin. Microbiol 41, 2744–2747, 10.1128/JCM.41.6.2744-2747.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhans A, Jacobson M, Hansson I, Lebbad M, Lambertz ST, Gammelgard E, Saager M, Akande O, Fellstrom C, 2013. Occurrence of pathogens in wild rodents caught on Swedish pig and chicken farms. Epidemiol. Infect 141, 1885–1891, 10.1017/S0950268812002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajer A, Bednarska M, Pawelczyk A, Behnke JM, Gilbert FS, Sinski E, 2002. Prevalence and abundance of Cryptosporidium parvum and Giardia spp. in wild rural rodents from the Mazury Lake District region of Poland. Parasitology 125, 21–34. [DOI] [PubMed] [Google Scholar]
- Bednarska M, Bajer A, Sinski E, Girouard AS, Tamang L, Graczyk TK, 2007. Fluorescent in situ hybridization as a tool to retrospectively identify Cryptosporidium parvum and Giardia lamblia in samples from terrestrial mammalian wildlife. Parasitol. Res 100, 455–460, 10.1007/s00436-006-0276-y. [DOI] [PubMed] [Google Scholar]
- Bouzid M, Hunter PR, Chalmers RM, Tyler KM, 2013. Cryptosporidium pathogenicity and virulence. Clin. Microbiol. Rev 26, 115–134, 10.1128/Cmr.00076-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Hermida JA, Garcia-Presedo I, Gonzalez-Warleta M, Mezo M, 2011. Prevalence of Cryptosporidium and Giardia in roe deer (Capreolus capreolus) and wild boars (Sus scrofa) in Galicia (NW, Spain). Vet. Parasitol 179, 216–219, 10.1016/j.vetpar.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T, 2014. Gregarine site-heterogeneous 18S rDNA trees, revision of gregarine higher classification, and the evolutionary diversification of Sporozoa. Eur. J. Protistol 50, 472–495, 10.1016/j.ejop.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Chalmers RM, Sturdee AP, Bull SA, Miller A, Wright SE, 1997. The prevalence of Cryptosporidium parvum and C. muris in Mus domesticus, Apodemus sylvaticus and Clethrionomys glareolus in an agricultural system. Parasitol. Res 83, 478–482. [DOI] [PubMed] [Google Scholar]
- Crawshaw GJ, Mehren KG, 1987. Cryptosporidiosis in zoo and wild animals., Erkrankungen der Zootiere. Verhandlungsbericht des 29 Internationalen Symposiums Uber die Erkrankungen der Zootiere. Akademie-Verlag, Berlin, Cardiff, pp. 353–362. [Google Scholar]
- Čondlová S, Horčičková M, Sak B, Květoňová D, Hlásková L, Konečný R, Stanko M, McEvoy J, Kváč M, 2018. Cryptosporidium apodemi sp. n. and Cryptosporidium ditrichi sp. n. (Apicomplexa: Cryptosporidiidae) in Apodemus spp. Eur. J. Protistol 63, 1–12, 10.1016/j.ejop.2017.12.006. [DOI] [PubMed] [Google Scholar]
- Danišová O, Valenčáková A, Stanko M, Luptaková L, Hatalová E, Canady A, 2017. Rodents as a reservoir of infection caused by multiple zoonotic species/genotypes of C. parvum, C. hominis, C. suis, C. scrofarum, and the first evidence of C. muskrat genotypes I and II of rodents in Europe. Acta Trop 172, 29–35, 10.1016/j.actatropica.2017.04.013. [DOI] [PubMed] [Google Scholar]
- de Bellocq JG, Baird SJE, Albrechtova J, Sobekova K, Piálek J, 2015. Murine Cytomegalovirus Is Not Restricted to the House Mouse Mus musculus domesticus: Prevalence and Genetic Diversity in the European House Mouse Hybrid Zone. J. Virol 89, 406–414, 10.1128/Jvi.02466-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Coco VF, Cordoba MA, Bilbao G, de Almeida Castro AP, Basualdo JA, Fayer R, Santin M, 2014. Cryptosporidium parvum GP60 subtypes in dairy cattle from Buenos Aires, Argentina. Res. Vet. Sci 96, 311–314, 10.1016/j.rvsc.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Fayer R, 2007. General Biology, in: Fayer R, Xiao L (Eds.), Cryptosporidium and cryptosporidiosis, 2 ed CRC Press, Boca Raton, FL, pp. 1–42. [Google Scholar]
- Fayer R, Gasbarre L, Pasquali P, Canals A, Almeria S, Zarlenga D, 1998. Cryptosporidium parvum infection in bovine neonates: dynamic clinical, parasitic and immunologic patterns. Int. J. Parasitol 28, 49–56. [DOI] [PubMed] [Google Scholar]
- Fayer R, Santín M, Xiao L, 2005. Cryptosporidium bovis n. sp. (Apicomplexa: Cryptosporidiidae) in cattle (Bos taurus). J. Parasitol 91, 624–629, 10.1645/GE-3435. [DOI] [PubMed] [Google Scholar]
- Graczyk TK, Cranfield MR, Fayer R, 1998. Oocysts of Cryptosporidium from snakes are not infectious to ducklings but retain viability after intestinal passage through a refractory host. Vet. Parasitol 77, 33–40. [DOI] [PubMed] [Google Scholar]
- Graczyk TK, Fayer R, Cranfield MR, 1996. Cryptosporidium parvum is not transmissible to fish, amphibians, or reptiles. J. Parasitol 82, 748–751. [PubMed] [Google Scholar]
- Hajdušek O, Ditrich O, Šlapeta J, 2004. Molecular identification of Cryptosporidium spp. in animal and human hosts from the Czech Republic. Vet. Parasitol 122, 183–192, 10.1016/j.vetpar.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Nakai Y, 2005. A novel genotype of Cryptosporidium muris from large Japanese field mice, Apodemus speciosus. Parasitol. Res 97, 373–379, 10.1007/s00436-005-1459-7. [DOI] [PubMed] [Google Scholar]
- Holubová N, Sak B, Horčičková M, Hlásková L, Květoňová D, Menchaca S, McEvoy J, Kváč M, 2016. Cryptosporidium avium n. sp. (Apicomplexa: Cryptosporidiidae) in birds. Parasitol. Res 115, 2243–2251, 10.1007/s00436-016-4967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horčičková M, Čondlová S, Holubová N, Sak B, Květoňová D, Hlásková L, Konečný R, Sedláček F, Clark M, Giddings C, McEvoy J, Kváč M, 2018. Diversity of Cryptosporidium in common voles and description of Cryptosporidium alticolis sp. n. and Cryptosporidium microti sp. n. (Apicomplexa: Cryptosporidiidae). Parasitology, 1–14, 10.1017/S0031182018001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imre K, Luca C, Costache M, Sala C, Morar A, Morariu S, Ilie MS, Imre M, Darabus G, 2013. Zoonotic Cryptosporidium parvum in Romanian newborn lambs (Ovis aries). Vet. Parasitol 191, 119–122, 10.1016/j.vetpar.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Iseki M, Maekawa T, Moriya K, Uni S, Takada S, 1989. Infectivity of Cryptosporidium muris (strain RN 66) in various laboratory animals. Parasitol. Res 75, 218–222. [DOI] [PubMed] [Google Scholar]
- Jacobson C, Williams A, Yang R, Ryan U, Carmichael I, Campbell AJ, Gardner GE, 2016. Greater intensity and frequency of Cryptosporidium and Giardia oocyst shedding beyond the neonatal period is associated with reductions in growth, carcase weight and dressing efficiency in sheep. Vet. Parasitol 228, 42–51, 10.1016/j.vetpar.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Jenkins MB, Liotta JL, Lucio-Forster A, Bowman DD, 2010. Concentrations, viability, and distribution of Cryptosporidium genotypes in lagoons of swine facilities in the Southern Piedmont and in coastal plain watersheds of Georgia. Appl. Environ. Microbiol 76, 5757–5763, 10.1128/AEM.00434-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Alderisio KA, Xiao L, 2005. Distribution of Cryptosporidium genotypes in storm event water samples from three watersheds in New York. Appl. Environ. Microbiol 71, 4446–4454, 10.1128/AEM.71.8.4446-4454.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulis-Malkowska K, 2007. [The impact of nematode invasions on the pattern of Cryptosporidium parvum infection in wild rodents]. Wiad Parazytol 53, 251–252. [PubMed] [Google Scholar]
- Kváč M, Hofmannová L, Hlásková L, Květoňová D, Vitovec J, McEvoy J, Sak B, 2014a. Cryptosporidium erinacei n. sp. (Apicomplexa: Cryptosporidiidae) in hedgehogs. Vet. Parasitol 201, 9–17, 10.1016/j.vetpar.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Kváč M, Kestřánová M, Květoňová D, Kotková M, Ortega Y, McEvoy J, Sak B, 2012. Cryptosporidium tyzzeri and Cryptosporidium muris originated from wild West-European house mice (Mus musculus domesticus) and East-European house mice (Mus musculus musculus) are non-infectious for pigs. Exp. Parasitol 131, 107–110, 10.1016/j.exppara.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Kváč M, McEvoy J, Loudová M, Stenger B, Sak B, Květoňová D, Ditrich O, Rašková V, Moriarty E, Rost M, Macholán M, Piálek J, 2013. Coevolution of Cryptosporidium tyzzeri and the house mouse (Mus musculus). Int. J. Parasitol 43, 805–817, 10.1016/j.ijpara.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kváč M, McEvoy J, Stenger B, Clark M, 2014b. Cryptosporidiosis in other vertebrates, in: Cacciò SM, Widmer G (Eds.), Cryptosporidium: Parasite and Disease, 1st ed Springer, Wien, pp. 237–326. [Google Scholar]
- Kváč M, Ondráčková Z, Květoňová D, Sak B, Vítovec J, 2007. Infectivity and pathogenicity of Cryptosporidium andersoni to a novel host, southern multimammate mouse (Mastomys coucha). Vet. Parasitol 143, 229–233, 10.1016/j.vetpar.2006.08.031. [DOI] [PubMed] [Google Scholar]
- Li N, Xiao L, Alderisio K, Elwin K, Cebelinski E, Chalmers R, Santin M, Fayer R, Kváč M, Ryan U, Sak B, Stanko M, Guo Y, Wang L, Zhang L, Cai J, Roellig D, Feng Y, 2014. Subtyping Cryptosporidium ubiquitum, a zoonotic pathogen emerging in humans. Emerg. Infect. Dis 20, 217–224, 10.3201/eid2002.121797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Pereira M, Larsen R, Xiao C, Phillips R, Striby K, McCowan B, Atwill ER, 2015. Cryptosporidium rubeyi n. sp. (Apicomplexa: Cryptosporidiidae) in multiple Spermophilus ground squirrel species. Int. J. Parasitol. Parasites Wildl 4, 343–350, 10.1016/j.ijppaw.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv C, Zhang L, Wang R, Jian F, Zhang S, Ning C, Wang H, Feng C, Wang X, Ren X, Qi M, Xiao L, 2009. Cryptosporidium spp. in wild, laboratory, and pet rodents in china: prevalence and molecular characterization. Appl. Environ. Microbiol 75, 7692–7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miláček P, Vítovec J, 1985. Differential staining of cryptosporidia by aniline-carbol-methyl violet and tartrazine in smears from feces and scrapings of intestinal mucosa. Folia Parasitol 32, 50. [PubMed] [Google Scholar]
- Murakoshi F, Fukuda Y, Matsubara R, Kato Y, Sato R, Sasaki T, Tada C, Nakai Y, 2013. Detection and genotyping of Cryptosporidium spp. in large Japanese field mice, Apodemus speciosus. Vet. Parasitol 196, 184–188, 10.1016/j.vetpar.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Nakamura AA, Meireles MV, 2015. Cryptosporidium infections in birds--a review. Rev. Bras. Parasitol. Vet 24, 253–267, 10.1590/S1984-29612015063. [DOI] [PubMed] [Google Scholar]
- Ng-Hublin JS, Singleton GR, Ryan U, 2013. Molecular characterization of Cryptosporidium spp. from wild rats and mice from rural communities in the Philippines. Infect. Genet. Evol 16, 5–12, 10.1016/j.meegid.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Ng JS, Eastwood K, Walker B, Durrheim DN, Massey PD, Porigneaux P, Kemp R, McKinnon B, Laurie K, Miller D, Bramley E, Ryan U, 2012. Evidence of Cryptosporidium transmission between cattle and humans in northern New South Wales. Exp. Parasitol 130, 437–441, 10.1016/j.exppara.2012.01.014. [DOI] [PubMed] [Google Scholar]
- Papanikolopoulou V, Baroudi D, Guo Y, Wang Y, Papadopoulos E, Lafi SQ, Abd El-Tawab MM, Diakou A, Giadinis ND, Feng Y, Xiao L, 2018. Genotypes and subtypes of Cryptosporidium spp. in diarrheic lambs and goat kids in northern Greece. Parasitol. Int 67, 472–475, 10.1016/j.parint.2018.04.007. [DOI] [PubMed] [Google Scholar]
- Pedraza-Diaz S, Amar C, Iversen AM, Stanley PJ, McLauchlin J, 2001. Unusual Cryptosporidium species recovered from human faeces: first description of Cryptosporidium felis and Cryptosporidium ‘dog type’ from patients in England. J. Med. Microbiol 50, 293–296. [DOI] [PubMed] [Google Scholar]
- Perec-Matysiak A, Bunkowska-Gawlik K, Zalesny G, Hildebrand J, 2015. Small rodents as reservoirs of Cryptosporidium spp. and Giardia spp. in south-western Poland. Ann. Agr. Env. Med 22, 1–5, 10.5604/12321966.1141359. [DOI] [PubMed] [Google Scholar]
- Petersen HH, Jianmin W, Katakam KK, Mejer H, Thamsborg SM, Dalsgaard A, Olsen A, Enemark HL, 2015. Cryptosporidium and Giardia in Danish organic pig farms: Seasonal and age-related variation in prevalence, infection intensity and species/genotypes. Vet. Parasitol 214, 29–39, 10.1016/j.vetpar.2015.09.020. [DOI] [PubMed] [Google Scholar]
- Prediger J, Horčičková M, Hofmannová L, Sak B, Ferrari N, Mazzamuto MV, Romeo C, Wauters LA, McEvoy J, Kváč M, 2017. Native and introduced squirrels in Italy host different Cryptosporidium spp. Eur. J. Protistol 61, 64–75, 10.1016/j.ejop.2017.09.007. [DOI] [PubMed] [Google Scholar]
- Ren X, Zhao J, Zhang L, Ning C, Jian F, Wang R, Lv C, Wang Q, Arrowood MJ, Xiao L, 2012. Cryptosporidium tyzzeri n. sp. (Apicomplexa: Cryptosporidiidae) in domestic mice (Mus musculus). Exp. Parasitol 130, 274–281, 10.1016/j.exppara.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Rhee JK, Yook SY, Park BK, 1995. Oocyst production and immunogenicity of Cryptosporidium muris (strain MCR) in mice. Korean J. Parasitol 33, 377–382. [DOI] [PubMed] [Google Scholar]
- Ryan U, Xiao L, 2014. Taxonomy and Molecular Taxonomy, in: Cacciò SM, Widmer G (Eds.), Cryptosporidium: parasite and disease, 1 ed Springer, pp. 3–42. [Google Scholar]
- Sak B, Petrzelkova KJ, Květoňová D, Mynářová A, Pomajbíková K, Modrý D, Cranfield MR, Mudakikwa A, Kváč M, 2014. Diversity of microsporidia, Cryptosporidium and Giardia in mountain gorillas (Gorilla beringei beringei) in Volcanoes National Park, Rwanda. PLoS One 9, e109751, 10.1371/journal.pone.0109751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saki J, Foroutan-Rad M, Asadpouri R, 2016 Molecular Characterization of Cryptosporidium spp. in Wild Rodents of Southwestern Iran Using 18s rRNA Gene Nested-PCR-RFLP and Sequencing Techniques. J. Trop. Med 2016, 6834206, 10.1155/2016/6834206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M, Hikosaka K, Sasaki T, Suyama Y, Yanai T, Ohta M, Nakai Y, 2003. Characteristics of a novel type of bovine Cryptosporidium andersoni. Appl. Environ. Microbiol 69, 691–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Kim CY, Chang SN, Abdelkader TS, Han J, Kim TH, Oh H, Lee JM, Kim DS, Kim JT, Oh HS, Hur M, Suh JH, Park JH, 2015. Detection and molecular characterization of Cryptosporidium spp. from wild rodents and insectivores in South Korea. Korean J. Parasitol 53, 737–743, 10.3347/kjp.2015.53.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano F, Putignani L, McLauchlin J, Casemore DP, Crisanti A, 1997. PCR-RFLP analysis of the Cryptosporidium oocyst wall protein (COWP) gene discriminates between C. wrairi and C. parvum, and between C. parvum isolates of human and animal origin. FEMS Microbiology Letters 150, 209–217. [DOI] [PubMed] [Google Scholar]
- Stenger BL, Clark ME, Kváč M, Khan E, Giddings CW, Prediger J, McEvoy JM, 2015. North American tree squirrels and ground squirrels with overlapping ranges host different Cryptosporidium species and genotypes. Infect. Genet. Evol 36, 287–293, 10.1016/j.meegid.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Striepen B, 2013. Parasitic infections: Time to tackle cryptosporidiosis. Nature 503, 189–191. [DOI] [PubMed] [Google Scholar]
- Sulaiman IM, Hira PR, Zhou L, Al-Ali FM, Al-Shelahi FA, Shweiki HM, Iqbal J, Khalid N, Xiao L, 2005. Unique endemicity of cryptosporidiosis in children in Kuwait. J. Clin. Microbiol 43, 2805–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman IM, Lal AA, Xiao LH, 2002. Molecular phylogeny and evolutionary relationships of Cryptosporidium parasites at the actin locus. J. Parasitol 88, 388–394, 10.1645/0022-3395(2002)088[0388:MPAERO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Torres J, Gracenea M, Gomez MS, Arrizabalaga A, Gonzalez-Moreno O, 2000. The occurrence of Cryptosporidium parvum and C. muris in wild rodents and insectivores in Spain. Vet. Parasitol 92, 253–260. [DOI] [PubMed] [Google Scholar]
- Trotz-Williams LA, Martin DS, Gatei W, Cama V, Peregrine AS, Martin SW, Nydam DV, Jamieson F, Xiao L, 2006. Genotype and subtype analyses of Cryptosporidium isolates from dairy calves and humans in Ontario. Parasitol. Res 99, 346–352. [DOI] [PubMed] [Google Scholar]
- Tůmová E, Skřivan M, Marounek M, Pavlásek I, Ledvinka Z, 2002. Performance and oocyst shedding in broiler chickens orally infected with Cryptosporidium baileyi and Cryptosporidium meleagridis. Avian Dis 46, 203–207, 10.1637/0005-2086(2002)046[0203:PAOSIB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Vetterling JM, Jervis HR, Merrill TG, Sprinz H, 1971. Cryptosporidium wrairi sp. n. from the guinea pig Cavia porcellus, with an emendation of the genus. J. Protozool 18, 243–247. [DOI] [PubMed] [Google Scholar]
- Webster JP, Macdonald DW, 1995a. Cryptosporidiosis reservoir in wild brown rats (Rattus norvegicus) in the UK. Epidemiol. Infect 115, 207–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JP, Macdonald DW, 1995b. Parasites of wild brown rats (Rattus norvegicus) on UK farms. Parasitology 111, 247–255. [DOI] [PubMed] [Google Scholar]
- Wielinga PR, de VA, van der Goot TH, Mank T, Mars MH, Kortbeek LM, van der Giessen JW, 2008. Molecular epidemiology of Cryptosporidium in humans and cattle in The Netherlands. Int. J. Parasitol 38, 809–817. [DOI] [PubMed] [Google Scholar]
- Xiao L, Escalante L, Yang C, Sulaiman I, Escalante AA, Montali RJ, Fayer R, Lal AA, 1999. Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Appl. Environ. Microbiol 65, 1578–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Moore JE, Ukoh U, Gatei W, Lowery CJ, Murphy TM, Dooley JS, Millar BC, Rooney PJ, Rao JR, 2006. Prevalence and identity of Cryptosporidium spp. in pig slurry. Appl. Environ. Microbiol 72, 4461–4463, 10.1128/AEM.00370-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Ryan UM, Graczyk TK, Limor J, Li L, Kombert M, Junge R, Sulaiman IM, Zhou L, Arrowood MJ, Koudela B, Modrý D, Lal AA, 2004. Genetic diversity of Cryptosporidium spp. in captive reptiles. Appl. Environ. Microbiol 70, 891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]