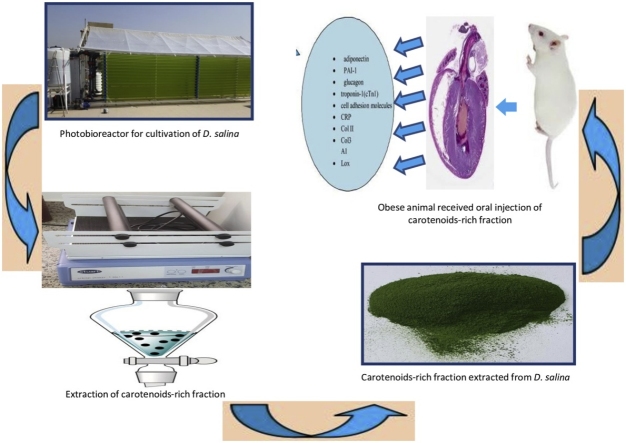
Graphical abstract
Keywords: Dunaliella salina, Adipokines, Cell adhesion molecules, Cardiac dysfunction associated inflammation, Histopathology
Highlights
-
•
The carotenoid-rich fraction of Dunaliella salina improves serum inflammatory markers.
-
•
The fraction has the ability to improve various disorders associated cardiac dysfunction in the high-fat diet treated rats.
-
•
The fraction attenuates fibrotic cardiac tissue and congestion of myocardial blood vessels.
-
•
The mentioned promising activities may be related to that fraction acts as an antioxidant and anti-inflammatory agent.
Abstract
The carotenoid rich fraction of microalgae Dunaliella salina (crf-DS) have been receiving great attention, due to they abilities to protect and improve various disorders. The objective of this study is to explore the therapeutic efficiency of crf-DS on obesity-assciated cardiac dysfunction in the high-fat diet (HFD) treated rats. These rats were orally administered with crf-DS (150 mg /kg body weight), for six consecutive weeks in comparison with reference drug(orlistat). Specific cardiac biomarkers were examined including; adiponectin, plasminogen activator inhibitor (PAI-1), glucagon, troponin-I (cTnI). The cell adhesion molecules (VCAM and ICAM), C-reactive protein (CRP), collagen type II (Col II), collagen alpha-1 (III) chain (Col3A1), lipoxygenase activity (LOX), as well as histopathological examination of cardiac tissue were investigated. Results indicated a significant reduction(P ≤ 0.05) in adiponectin and glucagon levels in serum of obese rats. However, cTnI, PAI-1, cell adhesion molecules, CRP, Col II, and Col3A1 and LOX levels declared marked increase. Histopathological examination of cardiac tissue showed fibrosis with severe congestion in the myocardial blood vessels. On the other hand, rats medicated with a crf-DS demonstrated noticeable ameliorating effect in all the measured parameters. Beside, myocardial tissue of obese rats showed no alteration. Hence, It could be concluded that, oral supplementation with crf-DS is able to attenuate cardiac dysfunction in obese rats. Further extended work is needed to exploit, the possible application of D. salina as nutraceuticals and food additives.
1. Introduction
Given the epidemic nature of obesity, much research has focused on lifestyle and pharmaceutical interventions. Weight loss remains the most effective approach for obesity and reducing the risk of related diseases; however, weight loss can be difficult to achieve and maintain [1,2]. The biggest factor leading to excess weight gain and the development of obesity is overnutrition. Overconsumption of any macronutrient can ultimately lead to fat synthesis and accumulation. Therefore, the key dietary habit to reducing obesity is twofold: manage total caloric intake and fat content within the diet [1,2].
Cardiovascular disease (CVD) broad-spectrum has linked to obesity [[1], [2], [3]]. The morbidity and mortality of CVD could be enhanced with obesity; direct effects are elicited by the effects of adipokine on vascular homeostasis and inflammation [3]. Plasminogen activator inhibitor type 1 (PAI-1) and adipokines may be considered a causal link between diabetes and CVD as they may cause the development of obesity [1,3]. Adipokines have has been postulated to play a critical effect on the regulation of appetite, energy homeostasis and participate in the development of many inflammatory diseases [3].
Energy consumption that exceeds metabolic requirements leads to lipogenesis and fat storage within white adipose tissue, the primary storage site of fat within the body [4]. Overconsumption of dietary fat can lead to weight gain relatively quickly since dietary fat is metabolized to free fatty acids, the primary substrate for triglycerides and subsequently, lipid synthesis.
A high level of PAI-1 expression in plasma may be regulated by the insulin-resistance syndrome. For heart and vascular disease, this syndrome demonsterated an intense risk factor [4]. The evidence that an increase in the expression of PAI-1 of artery wall is linked with type 2 diabetes has been ascertained [5,6]. In diabetic cardiomyopathy; the contractile protein cardiac troponin I (cTnI) has been explored to have great effect in regulation of cardiac muscle relaxation and contraction [7]. C-reactive protein (CRP) is mainly utilized as a marker of systemic inflammation, found priority evidence of myocardial infarction, in response to factors released by fat cells [8]. An increased level of inflammatory proteins such as cell adhesion molecules, CRP and PAI-1 were correlated with the increase of body mass index [9,10]. The significant effect of lipoxygenase (5LXO) in controlling adipose tissue inflammation in vascular disease was proposed [11]. The LXO involvement in the pathogenesis of atherosclerosis induced by high-fat-diet (HFD) could be connected with the LXO-mediated monocyte-endothelial cell binding and oxidation of lipoprotein [11]. The changes in the ratio of collagen type I/type III (Col I/Col III) post-HFD-initiated obesity differed from a study to another [[11], [12], [13]]. It is therefore imperative to develop novel medications for the prevention and/ or inhibition of the cardiac dysfunction progression, which consider the most common cause of mortality and morbidity in obese and diabetic populations [14].
Recently, continued education on the benefits of diet as a lifestyle intervention is important now more than ever. Several diets have proven very successful in maintaining obesity and obesity-related diseases. An abundance of bioactive natural products with pharmaceutical and industrial applications have been devoted to algae [[14], [15], [16]]. Algae have produced a system of chemical defense with the secondary metabolites to survive in a competitive environment [14]. Some of these metabolites involving a carotenoid which is a class of terpenoid pigment; has considerable attention for human health [16]. The use of algae has several advantages over the use of terrestrial plants for the production of natural carotenoids [14,16]. Carotenoids exhibited beneficial effect, especially the β-carotene astaxanthin, and lutein for the management of chronic cardiovascular disease beside, it's antioxidant, antidiabetic, and anti-obesity activities [16]. Many edible species of green algae, Umbraulva japonica, Codium fragile and Caulerpa lentillifera declared the ketocarotenoid pigment accumulation, astaxanthin which demonstrated a potential lowering of blood pressure value, attenuate cardiovascular remodeling, and protect atherosclerosis; a disease of chronicinflammation [16]. Dunaliella, a marine halotolerant unicellular eukaryotic microalga that does not produce toxins. It has already been recognized as safe food sources or authorized as additives for humans and animals and is known as a source of carotenoids [14]. The accumulation of carotenoids has led to the potent beneficial antioxidant effect help the elimination of free radicals compared to synthetic one [14]. This effect has shown promising results in prevention of inflammatory diseases and undoubtedly helpful in the prevention of cardiovascular disorders [14].
The present research demonstrated significant novelty for the first time comprise the evaluation of specific cardiac biomarkers such as adiponectin, glucagon, troponin, PAI-1, ICAM-1 and VCAM -1, CRP, Col II, Col 3A1, LOX in obese rats induced by HFD. Additionally, the medications and /or prevention of the cardiac dysfunction progression, which consider the most common cause of mortality and morbidity in obese and diabetic subjects using carotenoid fraction of D. salina microalgae which is mainly β-carotene, astaxanthin, and lutein is consider the first study till now deals with this topic.
2. Materials and methods
2.1. Cultivation of D. salina the vertical photobioreactor
Algal species (Dunaliella salina) was isolated from salt pond at Al-Fayoum governorate and were grown by using Bold media for algal isolation and purification [17]. After growing D. salina for 10 days under lab conditions then transferred to a vertical photobioreactor (PBR) with a capacity of 4000 L. Reservoir (1000 L) tank associated pipework proprietary in-line pigging systems for removal of all biofilms. In addition 10 L basket centrifuge for harvesting connected to the system. Alga Connect Data Acquisition System used for online measurements. Tap water is used for the cultivation of algae in the PBR. Water is sterilized using hypochlorite after that sodium thiosulphate was added. Chlorine test is done to ensure no residual chlorine is present. The nutrient solution of Bold was used for growing D. salina. To the culture medium, one milliliter per liter of micronutrient solution was added. To ensure the purity, samples are taken regularly and examined microscopically. The culture is left to grow until the biomass reached the maximum (2–2.5 g/L). The biomass of the alga is harvested using basket centrifuge at 2000 rpm and dried at sun dryer where the temperature reached approximately 45 °C and then grounded into a homogeneous fine powder.
2.2. Preparation of the carotenoid algal fraction
The fraction of carotenoid was extracted from algal material by maceration method under mechanical agitation with ethyl acetate and n-hexane (20:80, v/v) mixture and shacked at 150 rpm (Heidolph UNIMAX 2010 shaker), for 48 h. In order to filter the extract, a Buchner funnel and Whatman filter paper No. 4 were used. The algal residue was re-extracted for another two times using solvents of the mixture. The filtrates were concentrated and dried at 40 °C using rotary evaporatory, Heidolph-Germany [18]. The dried carotenoids rich fraction was stored at −20 °C in dark bottle for further analysis.
2.3. Biological assay
2.3.1. Chemicals
All chemicals, solvents, reagents of analytical grade and and ELISA kits were obtained from chemical companies of Sigma-Aldrich (USA) and Biodignostic (Egypt). While Orlistat standard antiobesity drug was obtained from the pharmacy (Egypt).
2.3.2. Experimental animals
Experiments were carried out in male albino rats (n = 50) weighted (150 ± 20 g) was carried out. The animals supplied from the Animal House of the National Research Centre (NRC) were allowed to acclimatize for seven days in the laboratory. They were housed under 3 °C temperature cycles (26−29 °C) with fixed light cycles. Rats were allowed free access to water ad libitum and food. Handling of the animal was performed according to the Committee of Ethics, NRC, Egypt (with ethical approval no: 33458).
2.3.3. Induction of obesity in rats
Obesity was conducted according to Adaramoye et al. [19], by feeding rats' high-fat diet (HFD, cholesterol and bile acid). A dose of cholesterol (30 mg/0.3 mL olive oil /1 kg) was orally administrated (five times per week) for 12 consecutive weeks. The HFD diet (Table 1) was mixed with lard fat and bile acid to increase the cholesterol absorption (five kg of the diet were mixed with one kg of animal lard with the addition of 2.5 g). The diet from Cairo Oil and Soap Company (Egypt) was used (4.39 kcal/g). Our previous report [7] has demonstrated the obesity by measuring the percentages of body weight gain, fecal and visceral fats.
Table 1.
Composition of the high-fat diet (HFD) used in the study (4.39 kcal/g).
| Ingredients | Percentage/Amount |
|---|---|
| Lard Fat | 20 % |
| Bile Acid | 25 % |
| Casein Purified High Nitrogen | 21–23 % |
| DL-Methionine | 0. 3 % |
| Sucrose | 60 % |
| Corn Starch | 20 % |
| Coconut Oil (Hydrogenated) | 20 % |
| Vitamin D3 | 10 MIU |
| Vitamin E | 25 MIU |
| Calcium | 0.8–1.2 % |
| Vitamin A | 10 MIU |
2.3.4. Doses and routes of administration
Orlistat (12 mg/kg b.wt.) was used as a standard drug against obesity). The drug was dissolved in distilled water for oral adminstration for 6 consecutive weeks to obese-induced rats [19]. Also, crf-DS; 150 mg/kg b.wt. was administered to obese rats for six successive weeks [20].
2.3.5. Adipokines assay
The adiponectin, an adipocyte-derived plasma protein, has a principal role in the regulation of fatty acid and glucose metabolism [21]. Adiponectin quantitative determination in serum rat was performed using ELISA kit. Glucagon peptide was estimated by a kit of enzyme immunoassay (EIA). Rat cardiac troponin I(cTn-I) and plasminogen activator inhibitor (PAI-1) were performed in serum using ELISA kit.
2.3.6. Adhesion molecules and C-reactive protein (CRP)
Serum ICAM-1, VCAM -1 and CRP were estimated by ELISA [3,8].
2.3.7. Rat collagen type II and lipoxygenase activity (LOX)
Rat collagen (Col II), collagen alpha -1(III) chain (Col 3A1) were measured in rat serum by ELISA kit. Lipoxygenase was measured in serum using the fluorometric method [11].
LOX assay substrate LOX→ Intermediate LOX Assay Probe→ Fluorescence Ex/Em500/536 nm.
2.4. Experimental design
Male Wistar rats weighing between 150-170 g (mean ± SD), which is rats weight at the day received from the supplier post period of adaptation to the environment, were used. All animals were randomly allocated into five major groups (1 to 5) of 10 rats each. Group (1) was the control received distilled water by gavage and a normal diet (ND). Group (2): normal rats received normal diet and treated with 150 mg/kg b.wt. of crf from D. salina for eighteen consecutive weeks (ND/crf-DS). Group (3): rats feeding HFD for 12 weeks. Group (4): obese rats medicated for 6 weeks with 150 mg /kg b.wt. of HFD/crf-DS. Group (5): obese rats medicated for 6 weeks with the standard drug; orlistat (12 mg/kg b.wt.) (HFD/OR). Health and behavior conditions of all rats were monitored daily and no adverse events were observed throughout the study. All experiments and biochemical analysis were conducted using 50 rats with triplicate measurements.
2.5. Blood sample
At the end of treatment (weeks 12 and 18), blood collection from the sublingual vein were obtained. The animals were overnight fasting under light anesthesia with an oral adminstration of pentobarbital 30 mg/kg [22]. Separation of serum was carried out by centrifugation (4000 rpm, 10 min) and was kept at −80 °C for further analysis [22]. Post treatments (12 and 18 weeks respectively), the rats were sacrificed by decapitation and the cardiac tissue was removed for biochemical analysis. In a saline solution, a part of cardiac tissue was homogenized to yield yield 10 %. The other part was fixed in formalin (10 %), for histopathological examination.
2.6. Histological examination
Preservation of the slides of cardiac tissue in 10 % buffer formalin was carried out. Hematoxylin and eosin (H&E) stains have been used for standard paraffin sections (4 pm) [23].
2.7. Statistical analysis
SPSS computer program version 8 coupled with Co-State Computer Program were used to compared the data between the different groups. Different letters are significant at P ≤0.05.
3. Results
The data presented in Table 2 clarifying significant reduction in serum adiponectin level (−43.01%), in obese rats (P≤0.05), while medicated rats with a carotenoid-rich fraction of D. salina (crf-DS) as well as orlistat showed significant elevation in its level with a much higher percentage of improvement for crf of D. salina (20.26 %). Additionally, the level of glucagon exhibited significant reduction (P≤0.05), in obese rats (−41.425 %). However, obese rats -treated with crf-DS demonstrated significant elevation in its level in comparison with obese rats showed; higher improvement percentage for crf-DS (15.28 %) than standard drug (12.18 %). However, troponin I and PAI-1 exhibited significant enhancement in obese rats (P≤0.05), amounted +89.46 and 70.75 %, respectively. Although, obese rats- medicated with a carotenoid-rich fraction have marked lowering effect in both markers level in comparison with obese rats, demonstrating higher percentages of improvement for crf-SD (46.06 and 52.15, respectively) than orlistat (33.40 and 44.19 %).
Table 2.
Effects D. salina on adiponectin, glucagon, troponin I and PAI-1 in obese rats and therapeutic groups.
| Markers | Groups |
||||
|---|---|---|---|---|---|
| control/ND | control/ND/DS | HFD | HFD/DS | HFD/OR | |
| Adiponectin (ng/ml) | 15.25a ± 0.26 | 15.00 a ± 1.00 | 8.69b ± 0.52 | 11.78c± 0.65 | 9.54d±8.88 |
| %Change | – | 1.64 | −43.01 | −22.75 | −37.44 |
| %Improvement | – | – | – | 20.26 | 5.57 |
| Glucagon (Pg/ml) | 11. 25c ± 0.99 | 11.90 c ± 0.87 | 6.59d ± 0.20 | 8.32e ± 0.29 | 7.95e ± 0.25 |
| %Change | – | 5.77 | −41.42 | −26.04 | −29.33 |
| %Improvement | – | – | – | 15.38 | 12.18 |
| Troponin I (Pg/ml) | 42.81i ±2.88 | 40.38i ±3.86 | 81.11f±4.99 | 61.39K± 7.80 | 66.81j± 8.64 |
| %Change | – | −5.68 | +89.46 | +43.40 | +56.06 |
| %Improvement | – | – | – | 46.06 | 33.40 |
| PAI -1 (Pg/ml) | 12.58a ± 0.77 | 13.00 a ± 2.00 | 21.48b ± 0.59 | 14.92d± 1.33 | 15.92c ± 1.50 |
| %Change | – | 3.33 | +70.75 | +18.60 | +26.55 |
| %Improvement | – | – | – | 52.14 | 44.19 |
ND: normal diet, ND/DS: rats feed normal diet and treated orally with D. salina extract for 6weeks. HFD/DS: rats feed with high fat diet for 12 weeks and treated orally with D. salina for 6 weeks post induction. FHD/OR: rats feed HFD and treated orally for 6 weeks with standard drug orlistat. Statistical analysis is carried out using SPSS computer program, combined with Co-State Computer Program, where different letter is significant at P ≤ 0.05.
Significant increase in ICAM, VCAM and CRP levels (P≤0.05), was detected in obese rats reached to 163.29, 35.38 and 84.48 %, respectively (Table 3). Treatment of obese rats with a carotenoid rich fraction of D. salina modulated both CAMs levels as well as CRP and declaring higher improvement percentages (98.24, 101.90 and 41.45 %, respectively), than standard drug (84.18, 74.29 and 37.82 %, respectively). Table 4 declared a significant increase in Col II, Col 3 A1and LOX (P≤0.05), with percentages +112.52, +127.83 and 98.00 % respectively. Treatment of obese rats with a carotenoid rich fraction from D. salina markedly ameliorated Col II, Col 3A1, and LOX, recorded higher percentages of improvement (83.30, 103.42 and 76.00 %, for Col 11, Col 3A1 and LOX respectively) than standard drug.
Table 3.
Effects D. salina on the cell adhesion molecules (ICAM and VCAM) and CRP in obese rats and therapeutic groups.
| Markers | Groups |
||||
|---|---|---|---|---|---|
| control/ND | control/ND/DS | HFD | HFD/DS | HFD/OR | |
| ICAM (ng/ml) | 213.5a ± 9.10 | 237.00 a ± 7.88 | 562.14b ± 7.99 | 352.39c± 11.60 | 382.41d±12.55 |
| %Change | – | 11.00 | – | +65.05 | +79.11 |
| %Improvement | – | – | 163.29 | 98.24 | 84.18 |
| VCAM (Pg/ml) | 1.05c ± 0.05 | 1.00c ± 0.07 | 3.11d± 0.89 | 2.04f ± 0.21 | 2.33g ± 0.29 |
| %Change | – | 4.76 | +35.38 | +94.29 | +121.90 |
| %Improvement | – | – | – | 101.90 | 74.29 |
| CRP (Pg/ml) | 8.25i ±0.96 | 8.30i ±0.65 | 15.22f±1.20 | 11.80j± 1.26 | 12.10j± 0.60 |
| %Change | – | −0.60 | +84.48 | +43.03 | +46.67 |
| %Improvement | – | – | – | 41.45 | 37.82 |
ND: normal diet, ND/DS: rats feed normal diet and treated orally with D. salina extract for 6weeks. HFD/DS: rats feed with high fat diet for 12 weeks and treated orally with D. salina for 6 weeks post induction. FHD/OR: rats feed HFD and treated orally for 6 weeks with standard drug orlistat. Statistical analysis is carried out using SPSS computer program, combined with Co-State Computer Program, where different letter is significant at P ≤ 0.05.
Table 4.
Effects D. salina on Col II, Col 3 A1 and LOX in obese rats and therapeutic groups.
| Parameters | Groups |
||||
|---|---|---|---|---|---|
| control/ND | control/ND/DS | HFD | HFD/DS | HFD/OR | |
| Col II (ng/ml) | 20.36a ± 1.64 | 19.00 a ± 1.23 | 43.27b ± 1.74 | 26.31g ±1.64 | 27.21g± 1.19 |
| %Change | – | −6.67 | +112.52 | +29.22 | +33.64 |
| %Improvement | – | – | – | 83.30 | 78.88 |
| Col 3A1(ng/ml) | 12. 29c ± 1.01 | 12.00 c ± 1.00 | 28.00 d ±0.96 | 15.29e ± 0.96 | 18.92h ± 7.20 |
| %Change | – | −2.3 | +127.83 | +24.41 | 53.95 |
| %Improvement | – | – | – | 103.42 | 73.88 |
| LOX (ng/ml) | 0.50i ±0.03 | 0.54i ±0.02 | 0.99f±0.04 | 0.61j± 1.66 | 0.72h± 0.86 |
| %Change | – | −8.00 | +98.00 | +22.00 | +44.00 |
| %Improvement | – | – | – | 76.00 | 54.00 |
ND: normal diet, ND/DS: rats feed normal diet and treated orally with D. salina extract for 6weeks. HFD/DS: rats feed with high fat diet for 12 weeks and treated orally with D. salina for 6 weeks post induction. FHD/OR: rats feed HFD and treated orally for 6 weeks with standard drug orlistat. Statistical analysis is carried out using SPSS computer program, combined with Co-State Computer Program, where different letter is significant at P ≤ 0.05.
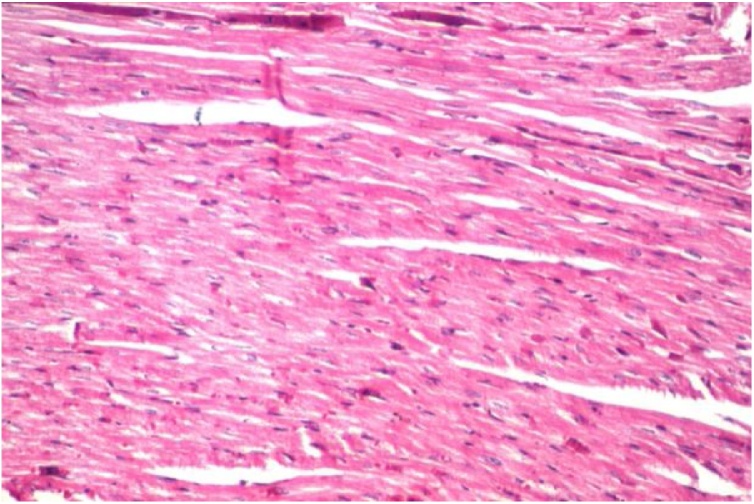
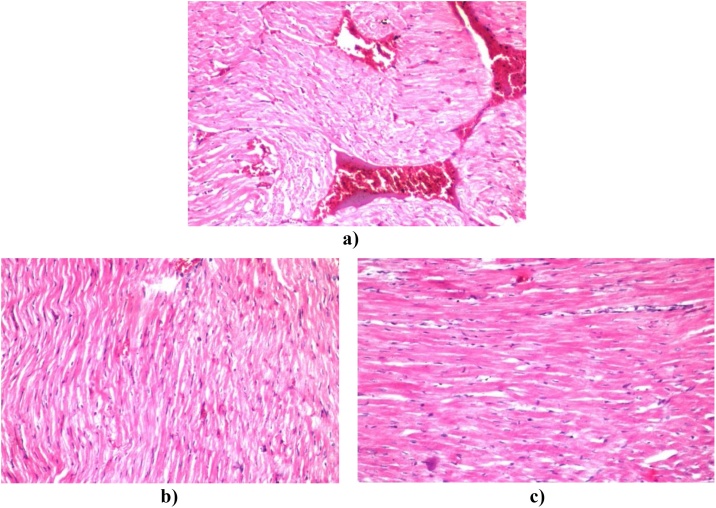
Histopathological examination of rat cardiac tissue of control-treated carotenoid rich fraction of D. salina (crf-DS) showed no histopathological alteration and normal histological structure of the myocardium bundles compared to control (Fig. 1). While photomicrograph of cardiac obese rats induced by high-fat diet demonstrated fibrosis and severe congestion in the myocardial blood vessels (Fig. 2a). Medicated obese rats with either carotenoid rich fraction from the green microalgae D. salina or orlistat declared no histopathological alterations in cardiac tissue (Fig. 2b, c).
Fig. 1.
Photomicrograph of cardiac control rats stained with H & E and treated with the carotenoid rich fraction showed no histopathological alteration.
Fig. 2.
a): Photomicrograph of cardiac obese rats induced by high fat diet stained with H & E showed sever congestion and fibrosis in the myocardial blood vessels. b): Photomicrograph of cardiac obese rats stained with H & E treated with the carotenoid rich fraction for 6 weeks showed no histopathological alteration. c): Photomicrograph of cardiac obese rats stained with H & E and treated with standard drug for 6 weeks and showed no histopathological alteration.
4. Discussion
In the present work, significant reduction in serum adiponectin level was recorded in obese rats (P≤0.05), while medicated rats with a carotenoid rich fraction of D. salina (crf-DS) as well as orlistat showed significant elevation in its level with a much higher percentage of improvement for crf of D. salina. Additionally, the level of glucagon recorded significant reduction, in obese rats. However, obese rats -treated with crf-DS demonstrated significant elevation in its level in comparison with obese rats showed; higher improvement percentage for crf-DS than standard drug. The current study demonstrated that, the cardiac tissue was directly affected by HFD, the mechanism and /or mechanisms implicated are proposed to include the participation of fibrosis, hypertrophy, inflammation and contractile dysfunction [24]. Dysregulation of lipids and adipocytokines strongly connected with perturbations in glucose/insulin metabolism [25]. Our study proposed that the low serum level of adiponectin combined well with HFD, hence, it may be a critical point determine insulin resistance [26]. The association between the risk of cardiovascular disease's and adiponectin level, dismiss other factors proposes that the intermediate effects of adiponectin either directly on the vascular tissue health, or indirect through the sensitivity of insulin and diabetic status. Further, it has been speculating that adiponectin is a classic anti-inflammatory agent reducing inflammation, hence, inverse relationship between the levels of adiponectin and the levels of inflammatory cytokines in serum were suggested [10]. Our data claryfing that, troponin I and PAI-1 exhibited significant enhancement in obese rats (P≤0.05), while, obese rats- medicated with a carotenoid rich fraction have marked lowering effect in both markers level in comparison with obese rats. Carotenoids are considered as a promising natural class of pigments that are stored in several human tissues especially in the liver and adipose tissue. It could be assumed that the increased expression level of PAI-1 in obese rats in the current study may be related to adipose tissue development. The PAI-1 overexpression in insulin resistance could adjust the mass of fat by lowering adiposity. Adiposity was decreased in mice with leptin deficiency and the genetic cause of obesity was displayed mainly through PAI-1 gene disruption [4]. It was illustrated that PAI-1 expression level was reduced in adiposity joined with the loss of weight [4], and the alterations in the expression level of PAI-1 are relying on visceral fat areas which display greater PAI-1 than the abdominal fat, subcutaneous or femoral tissues [4]. Risk factors for developing heart disease include the high level of PAI-1 [27], eliciting lesion development of thrombosis and atherosclerotic [28].
In the present study, significant increase in ICAM, VCAM and CRP levels (P≤0.05), was detected in obese rats while treatment of obese rats with a carotenoid rich fraction of D. salina modulated both CAMs levels as well as CRP higher than standard drug. The elevated level of CRP was related with nearly all the important risk factors, including metabolic syndrome/insulin resistance syndrome/pre-diabetes, cardiovascular disease and dyslipidemia [29,30]. C-reactive protein has been induced endothelial PAI-1 activation/dysfunction. Elevated level of many circulating cytokines in obese models causes activation of endothelial tissue, leading to reduced rats of platelet activation, prostaglandin secretion, and plug formation. Thus, the high levels of clotting factor and the activation of platelet in obese rats are associated with the low fibrinolysis rate and assignable to PAI-1 high level, which considers a hypercoagulable condition, which may be related to atherogenesis via high platelets and fibrous deposition resulting in the development of plaques [31,32]. The prolonged feeding of HFD promoting hypertrophy of myocardial tissue and fibrosis [31]. It was reported that CRP has been detected to activate adhesion molecules; ICAM-1, VCAM-1 and angiotensin type 1 receptor [10]. Our results showed that the endothelial dysfunction markers VCAM-1 and ICAM-1 levels were higher in obese rats than the healthy group, which are quite similar to other studies in diabetes [9,36], and cardiovascular disease [11,35] In this study, we found a correlation between obesity and intercellular adhesion molecules levels leading us to conclude that obesity has a role in the inflammatory state (high CRP levels) observed in the obese rats under study.
Significant increase in Col II, Col 3 A1and LOX in obese rats was detected in this study, however, treatment of obese rats with a carotenoid rich fraction from D. salina markedly ameliorated Col II, Col 3A1, and LOX, recorded higher percentages of improvement than standard drug. In disagreement with the present results, da Silva et al. [13], found that the exposure time to obesity- induced by an unsaturated HFD affected the expression of myocardial collagen type I, but had no effect on myocardial collagen type III.In a parallel line with the present results Leopoldo et al. [36], declared that at 12 weeks after adult male rabbits fed with HFD, damage to the coronary arteries, fibrosis as well as interstitial accumulation of collagen deposition were reported. These suggested that the higher concentration collagen is connected with the insulin metabolism abnormalities. Activation of the endothelin-1 and renin-angiotensin-aldosterone system may have a role in the development of cardiac fibrosis in models of obesity [37]. Previously, Quilliot et al. [38], explained that glucose in itself may be implicated in the enhancement of collagen synthesis. Also, the present work has demonstrated a significant elevation in LOX in obese rats. This may be explained on the basis of 5-lipoxygenase-derived leukotriene B4 (LTB4) levels in the adipose tissues were significantly increased. In concomitant with the present results, Chakrabarti et al. [39], suggested increase the activity of lipoxygenases 5- and 12-LOX in visceral adipocytes tissue samples from obese Zucker rat as well as the level of mRNA. Also, the immunohistochemistry and quantification in these tissues have showed increased number of cells expressing the 12- and 5-lipoxygenase enzymes, monocyte chemoattractant protein-1 mRNA and IL-6 [38,39]. Chakrabarti et al. [39], Hernandez-Perez et al. [40], and El-Baz et al. [41], evidence for the up-regulation of 5- and 12-LOX concomitant with increased the pro-inflammatory cytokine/chemokine expression levels in adipocytes and adipose tissue of obese rats.
Histopathological examination of rat cardiac tissue of obese rats induced by high-fat diet demonstrated fibrosis and severe congestion in the myocardial blood vessels. While, medicated obese rats with carotenoid rich fraction from the green microalgae D. salina declared no histopathological alterations in cardiac tissue. Carotenoids can play a crucial role on health promotion and human disease prevention e.g., cardiovascular disorders, rheumatoid arthritis and atherosclerosis [14]. The impact of antioxidants and antiinflammatory agents such as carotenoids on cardiovascular disorders were explored. Dunaliella is known for it's carotenoid’s accumulation, having considerable applications in the health and nutritional benefits [14,16]. A combination of various carotenoids, including lutein, astaxanthin, astaxanthin esters, zeaxanthin, all-trans and 9-cis β-carotene, and α-carotene possessing different chemical characteristics can be used to prevent oxidative damage and its impact on obesity-related characteristics [41,42]. Carotenoids are effective antioxidants that can quench singlet oxygen (O2), suppress lipid peroxidation, and prevent oxidative damage [15,35]. Many investigations have also demonstrated that various carotenoids have good hepatoprotective, anti-inflammatory and antioxidant activities. These findings run in same line with several studies suggested that an extract that containing high carotenoids content is considered as an important scavenger against generation of several free radicals including ROS [16,43].
The microalgae Dunaliella sp. extract exerting the high antioxidant activity compared with other algae when screen extracts obtained from nine microalgae strains [42]. In the global market, the major types of commercially used terpenoid pigments are β-carotene, lutein, and zeaxanthin [16]. The prevention of cardiovascular disease is one of the uses of these carotenoids produced by the microalga Dunaliella. Zeaxanthin and lutein are xanthophylls have –OH groups in their structures. They are the most prominent and commercially important carotenoid pigments and are well known to protect lipid membranes against free radical attack [14,16].
The crf from the green alga D. salina might have an effect in peroxidation by inhibiting free radical attacks on bio-membranes. An inhibition of colon lipid peroxidation and increase antioxidant biomarkers were exerted due to the protective effect of this alga against inflammation-related disease such as ulcerative colitis in rats [46]. Our previous study showed oxidative stress suppression and alteration in the expression of inflammation-related genes induced by streptozotocin as the main reported effect of total crude alcoholic extract of D. salina in diabetic rats [44]. Carotenoids exhibited hyperlipidemic and hypercholesterolemic effects [45]. Several reports revealed that lutein and carotene extracted from Dunaliella sp. showed high levels of bioavailable 9-cis, which provided verification of a lower incidence of many degenerative disorders [16,45].
Other important carotenoids exhibiting strong antioxidant activities are astaxanthin, zeaxanthin, lutein and carotene [42]. Phytoene and phytofluene rich alga Dunaliella species were reported with strong antioxidant activity [14].
The anti-hypertensive effect of astaxanthin in spontaneously hypertensive rats was reported [34]. The contractions of thoracic aorta of these rats were enhanced in astaxanthin-treated groups as compared to the non-treated groups. Improvement of the level of vascular elastin has been reported in the arterial wall thickness and aorta of the rats treated with this carotenoid [16]. Another study reported the higher contractility indices in the group of mice (female BALB/c) treated for eight weeks with a known carotenoid; astaxanthin (800 mg/kg) than the mice in a placebo group [16,45] and also the mitochondrial membrane potentials.
Carotenoids such as astaxanthin and lutein have been suggested to increase HDL-c, decrease LDL-c, triglycerides, as well as lipid peroxidation.They also lower CRP which has an important contribution in cardiovascular problems [14,44].
In our present study the supplementation of carotenoid rich fraction from the green microalga D. salina (crf-DS) attenuates inflammation and oxidative stress biomarkers. These biomarkers are established processes contributing to cardiovascular disease [16,36]. Systolic blood pressure was lower in a certain carotenoid type-treated group (from the first week of treatment) than in the control group and a significant reduction of left ventricular hypertrophy index was reported [3]. The carotenoid effect was suggested to be related to the improvement of endothelial function on resistance arteries and accompanied by a decrease in oxidative stress and improvements in NO bioavailability [14,16].
The presence of high levels of antioxidants and anti-inflammatory compounds, such as carotenoids, β-carotene, and phycocyanin indicating the possible pharmaco-therapeutic utility [14] of this green microalga. Other organic compounds in the Dunaliella biomass such as cyanovirin, fatty acid (oleic acid, linolenic acid, and palmitoleic acid) chlorophylls and vitamins (vitamin E and B12) and phycocyanin, may suggest a synergistic effect together with carotenoids in inducing antihypertensive bronchodilatory, muscle relaxant, antioxidant, and anti-inflammatory capacities, with the potential for the reduction and prevention of diseases related to cardiac dysfunction [14]. These compounds together with the presence of protein, carbohydrates, fibers, and lipids may have amelioration effect in obesity-induced cardiac dysfunction and help reverse diet-induced metabolic syndrome [46].
The previous study, the fat mass of the abdomine was decreased and the the lean mass was increased with food supplemented with 5 % dried green algae mixture [45,46]. Other effects of endothelial dysfunction and infiltration of inflammatory cells and diastolic stiffness of the heart were also reported. In our current work, the nutritional quality of the investigated fraction of D. salina might be added to the presence of many important ω-6/ ω-3 fatty acids, involved in the risks of cardiovascular disease [43]. So, the bioactivity was not attributed solely to carotenoids but also due to other trace components of the algae.
5. Conclusion
Diet with balanced nutrients that are capable of generating metabolic benefits should be considered as the primary approach for managing obesity and metabolic disease. From the presented data, it coud be concluded that, the carotenoid rich fraction of microalgae Dunaliella salina (crf-DS) has the ability to improve various disorders associated cardiac dysfunction in the high-fat diet (HFD) treated rats. Specific cardiac biomarkers such as adiponectin, plasminogen activator inhibitor (PAI-1), glucagon, troponin-I (cTnI), adhesion molecules (VCAM and ICAM), C-reactive protein (CRP), collagen type II (Col II), collagen alpha-1 (III) chain (Col3A1), lipoxygenase activity (LOX), as well as histopathological examination of cardiac tissue were investigated. Disturbances were recoded in all the detected biomarkers levels in obese rats, while medication of crf-DS to obese rats declared noticeable improvement in these cardiac markers, attenuate fibrotic cardiac tissue and congestion of myocardial blood vessels. These promising effects may be related to algal carotenoids rich fraction acts as an antioxidant and anti-inflammatory agents. Owing to these properties, the study can be further extended on a human to exploit, the possible application of D. salina as a nutraceuticals modulating different diseases, due to the high antioxidant properties of algal carotenoid.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the alliance entitled "Integrated Pharmaceutical Alliance (IPA)". This alliance is funded by the Academy of Scientific Research and Technology, Egypt with grant ID of "Integrated Pharmaceutical Alliance (IPA) -KTA- C2-2.10".
References
- 1.Botchlett R., Wu C. Diet composition for the management of obesity and obesity-related disorders. J. Diabetes Mellitus Metab. Syndr. 2018;3:10–25. doi: 10.28967/jdmms.2018.01.18002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dludla P.V., Jack B., Viraragavan A., Pheiffer C., Johnson R., Louw J. A dose-dependent effect of dimethyl sulfoxide on lipid content, cell viability and oxidative stress in 3T3-L1 adipocytes. Toxicol. Rep. 2018;5:1014–1020. doi: 10.1016/j.toxrep.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koliaki C., Liatis S., Kokkinos A. Obesity, and cardiovascular disease: revisiting an old relationship. Metabolism. 2018 doi: 10.1016/j.metabol.2018.10.011. pii: S0026-0495(18)30229-4. [DOI] [PubMed] [Google Scholar]
- 4.Lee H.J., Le B., Lee D.R., Choi B.K., Yang S.H. Cissus quadrangularis extract (CQR-300) inhibits lipid accumulation by downregulating adipogenesis and lipogenesis in 3T3-L1 cells. Toxicol. Rep. 2018;5:608–614. doi: 10.1016/j.toxrep.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sobel B.E., Woodcock-Mitchell J., Schneider D.J., Holt R.E., Marutsuka K., Gold H. Increased plasminogen activator inhibitor type 1 in coronary artery atherectomy specimens from type 2 diabetic compared with nondiabetic patients: a potential factor predisposing to thrombosis and its persistence. Circulation. 1998;97:2213–2221. doi: 10.1161/01.cir.97.22.2213. [DOI] [PubMed] [Google Scholar]
- 6.Pandolfi A., Cetrullo D., Polishuck R., Alberta M.M., Calafiore A., Pellegrini G. Plasminogen activator inhibitor type 1 is increased in the arterial wall of type II diabetic subjects. Arterioscler. Thromb. Vasc. Biol. 2001;21:1378–1382. doi: 10.1161/hq0801.093667. [DOI] [PubMed] [Google Scholar]
- 7.El-Baz F.K., Aly H.F. Potential of Dunaliella Salina microalgae to ameliorate high-fat diet-induced obesity in animals’ rodent. Asian J. Pharm. Clin. Res. 2018;11:312–318. [Google Scholar]
- 8.Reho J.J., Rahmouni K. Oxidative and inflammatory signals in obesity-associated vascular abnormalities. Clin. Sci. (Lond.) 2017;131:1689–1700. doi: 10.1042/CS20170219. [DOI] [PubMed] [Google Scholar]
- 9.Aly H.F., Abd-Alla H.I., Ali S.A., Aba-Alez R., Abu-Krisha M.T., Mamdouh M.M. Bioinformatics: inflammatory cytokines and attenuation of diabetes hypercholesterolemia-induced renal injury using morning glory and necklace pod extracts. Asian J. Pharm. Clin. Res. 2017;10:347–355. [Google Scholar]
- 10.Botchlett R., Woo S.L., Liu M., Pei Y., Guo X., Li H. Nutritional approaches for managing obesity-associated metabolic diseases. J. Endocrinol. 2017;233:145–171. doi: 10.1530/JOE-16-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sears D.D., Miles P.D., Chapman J., Ofrecio J.M., Almazan F., Thapar D. 12/15-Lipoxygenase is required for the early onset of high fat diet-induced adipose tissue inflammation and insulin resistance in mice. PLoS One. 2009;4:e7250. doi: 10.1371/journal.pone.0007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll J.F., Zenebe W.J., Strange T.B. Cardiovascular function in a rat model of diet-induced obesity. Hypertension. 2006;48:65–72. doi: 10.1161/01.HYP.0000224147.01024.77. [DOI] [PubMed] [Google Scholar]
- 13.da Silva D.C.T., Lima-Leopoldo A.P., Leopoldo A.S., de Campos D.H.S., do Nascimento A.F., de Junior S.A. Influence of term of exposure to high-fat-diet-induced obesity on myocardial collagen type I and III. Arq. Bras. Cardiol. 2014;102:157–164. doi: 10.5935/abc.20130232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riccioni G., Gammone M.A., D’Orazio N. Carotenoids and cardiovascular prevention: an update. J. Nutr. Food Sci. 2016;6:441–446. [Google Scholar]
- 15.Ahmed H.H., Hegazi M.M., Abd-Alla H.I., Eskander E.F., Ellithey M.S. Antitumour and antioxidant activity of some Red Sea seaweeds in Ehrlich ascites carcinoma in vivo, Z. Naturforsch. C – J. Biosci. 2011;66:367–376. doi: 10.1515/znc-2011-7-808. [DOI] [PubMed] [Google Scholar]
- 16.Sathasivam R., Ki J.S. A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Mar. Drugs. 2018;16 doi: 10.3390/md16010026. pii: E26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein J., editor. Culture Methods and Growth Measurements. Cambridge University Press; 1975. Handbook of phycological methods; p. 448. [Google Scholar]
- 18.Varela J.C., Pereira H., Vila M., León R. Production of carotenoids by microalgae: achievements and challenges. Photosynthesis Res. 2015;3:125. doi: 10.1007/s11120-015-0149-2. [DOI] [PubMed] [Google Scholar]
- 19.Adaramoye O., Akinatyo O., Achen J., Michel A. Lipid-lowering effects of methanolic extracts of Vernonia anygdalina leaves in rats fed on high cholesterol diet. Vasc. Health Risk Manag. 2008;4:235–241. doi: 10.2147/vhrm.2008.04.01.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shalaby H.M., Tawfek N.S., Abo-El Hussein B.K., Abd El-Ghany M.S.M. The assessment of some biochemical and immunological effects by amphetamine and orlistat on obesity in rats. Food Pub. Health. 2014;4:185–192. [Google Scholar]
- 21.Ruperez F.J., Garcia-Martinez D., Baena B., Maeso N., Cifuentes A., Barbas C. Evolution of oxidative stress parameters and response to oral vitamins E and C in streptozotocin-induced diabetic rats. J. Pharm. Pharmacol. 2008;60:871–878. doi: 10.1211/jpp.60.7.0008. [DOI] [PubMed] [Google Scholar]
- 22.Mohamed N.Z., Abd-Alla H.I., Hanan F.A., Mantawy M., Ibrahim N., Hassan S.A. CCl4-induced hepatonephrotoxicity: protective effect of nutraceuticals on inflammatory factors and antioxidative status in rat. J. Appl. Pharm. Sci. 2014;4:87–100. [Google Scholar]
- 23.Drury R.A., Wallington E.A. 4th ed. Oxford University Press; New York: 1980. Carleton’s Histology Technique; pp. 653–661. [Google Scholar]
- 24.Noyan-Ashraf M.H., Shikatani E.A., Schuiki I., Mukovozov I., Wu J., Li R.K. A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation. 2013;127:74–85. doi: 10.1161/CIRCULATIONAHA.112.091215. [DOI] [PubMed] [Google Scholar]
- 25.Khalyfa A., Carreras A., Hakim F., Cunningham J.M., Wang Y., Gozal D. Effects of late gestational high fat diet on body weight, metabolic regulation and adipokine expression in offspring. Int. J. Obes. (Lond.) 2013;37:1481–1489. doi: 10.1038/ijo.2013.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lihn A.S., Pedersen S.B., Richelsen B. Adiponectin: action, regulation, and association to insulin sensitivity. Obes. Rev. 2005;6:13–21. doi: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 27.Alessi M.C., Bastelica D., Mavri A., Morange P., Berthet B., Grino M. Plasma PAI-1 levels are more strongly related to liver steatosis than to adipose tissue accumulation. Thromb. Vasc. Biol. 2003;23:1262–1268. doi: 10.1161/01.ATV.0000077401.36885.BB. [DOI] [PubMed] [Google Scholar]
- 28.Konstantinides S., Scha¨fer K., Loskutoff D.J. Do PAI-1 and vitronectin promote or inhibit neointima formation? The exact role of the fibrinolytic system in vascular remodeling remains uncertain. Arterioscler. Thromb. Vasc. Biol. 2002;22:1943–1945. doi: 10.1161/01.atv.0000047462.65341.22. [DOI] [PubMed] [Google Scholar]
- 29.Wang W., Yang J., Qi W., Yang H., Wang C., Tan B. Lipidomic profiling of high-fat-diet-induced obesity in mice: importance of cytochrome P450-derived fatty acid epoxides. Obesity. 2017;25 doi: 10.1002/oby.21692. 132 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saito M., Ishimitsu T., Minami J., Ono H., Ohrui M., Matsuoka H. Relations of plasma high-sensitivity C-reactive protein to traditional cardiovascular risk factors. Atherosclerosis. 2003;167:73–79. doi: 10.1016/s0021-9150(02)00380-5. [DOI] [PubMed] [Google Scholar]
- 31.Davi G., Guagnano M.T., Ciabattoni G., Basili S., Falco A., Marinopiccoli M. Platelet activation in obese women: role of inflammation and oxidant stress. JAMA. 2002;288:2008–2014. doi: 10.1001/jama.288.16.2008. [DOI] [PubMed] [Google Scholar]
- 32.Wojciechowski V.V., Calina D., Tsarouhas K., Pivnik A.V., Sergievich A.A., Kodintsev V.V. A guide to acquired vitamin K coagulophathy diagnosis and treatment: the Russian perspective. Daru. 2017;25:10. doi: 10.1186/s40199-017-0175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shalaby N.M., Abd-Alla H.I., Aly H.F., Albalawy M.A., Shaker K.H., Bouajila J. Preliminary in vitro and in vivo evaluation of the antidiabetic activity of Ducrosia anethifolia Boiss. and its linear furanocoumarins. BioMed. Res. Int. 2014;2014 doi: 10.1155/2014/480545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakao R., Nelson O.L., Park J.S., Mathison B.D., Thompson P.A., Chew B.P. Effect of astaxanthin supplementation on inflammation and cardiac function in BALB/c mice. Anticancer Res. 2010;30:2721–2725. [PubMed] [Google Scholar]
- 36.Leopoldo A.S., Sugizaki M.M., Lima-Leopoldo A.P., do Nascimento A.F., de Azevedo R., Luvizotto M. Cardiac remodeling in a rat model of diet-induced obesity. Can. J. Cardiol. 2010;26:423–429. doi: 10.1016/s0828-282x(10)70440-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rondinone C.M. Adipocyte-derived hormones, cytokines, and mediators. Endocrine. 2006;29:81–90. doi: 10.1385/endo:29:1:81. [DOI] [PubMed] [Google Scholar]
- 38.Quilliot D., Alla F., Böhme P., Bruntz J.F., Hammadi M., Dousset B. Myocardial collagen turnover in normotensive obese patients: relation to insulin resistance. Int. J. Obesity. 2005;29:1321–1328. doi: 10.1038/sj.ijo.0803022. [DOI] [PubMed] [Google Scholar]
- 39.Chakrabarti S.K., Wen Y., Dobrian A.D., Cole B.K., Ma Q., Pei H. Evidence for activation of inflammatory lipoxygenase pathways in visceral adipose tissue of obese Zucker rats. Am. J. Physiol. Endocrinol. Metab. 2011;300:175–187. doi: 10.1152/ajpendo.00203.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernandez-Perez M., Chopra G., Fine J., Conteh A.M., Anderson R.A., Linnemann A.K. Inhibition of 12/15-lipoxygenase protects against β cell oxidative stress and glycemic deterioration in mouse models of type 1 diabetes. Diabetes. 2017;66:2875–2887. doi: 10.2337/db17-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Baz F.K., Aly H.F., Abd-Alla H.I., Biomy D.F. Therapeutic impact of berries (Morus alba and Morus rubra) fruit extract in the regression of high-fat-diet-induced cardiac dysfunction in rats. Asian J. Pharm. Clin. Res. 2018;11:314–320. [Google Scholar]
- 42.Christaki E., Bonos E., Giannenas I., Florou-Pan P. Functional properties of carotenoids originating from algae. J. Sci. Food Agric. 2013;93:5–11. doi: 10.1002/jsfa.5902. [DOI] [PubMed] [Google Scholar]
- 43.Abdel-Daim M.M., Farouk S.M., Madkour F.F., Azab S.S. Anti-inflammatory and immunomodulatory effects of Spirulina platensis in comparison of Dunaliella salina in acetic acid-induced rat experimental colitis. Immunopharmacol. Immunotoxicol. 2015;37:126–139. doi: 10.3109/08923973.2014.998368. [DOI] [PubMed] [Google Scholar]
- 44.El-Baz F.K., Abdel Jaleel G.A., Saleh D.O., Hussein R.A. Protective and therapeutic potentials of Dunaliella salina on aging-associated cardiac dysfunction in rats. Asian Pac. J. Trop. Biomed. 2018;8:403–410. [Google Scholar]
- 45.Wan-Loy C., Siew-Moi P. Marine algae as a potential source for anti-obesity agents. Mar. Drugs. 2016;14 doi: 10.3390/md14120222. pii: E222. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Juhan-Vague I., Alessi M., Mavri A., Morange P.E. Plasminogen activator inhibitor-1, inflammation, obesity, insulin resistance and vascular risk. J. Thromb. Haemost. 2003;1:1575–1579. doi: 10.1046/j.1538-7836.2003.00279.x. [DOI] [PubMed] [Google Scholar]