Abstract
Melatonin (MEL) has been demonstrated to exert a protective effect against subarachnoid hemorrhage (SAH), and nitric oxide (NO) has been shown to play an important role in the pathogenesis of vasospasm. This study aims to explore the underlying molecular mechanisms of MEL in the control of vasospasm following SAH. MEL administration attenuates SAH-induced vasospasm and neurobehavioral deficits. Expressions of H19, eNOS, and miR-675 are low in the SAH group, while expressions of miR-138 and HIF1α are high in the SAH group. Also, MEL treatment upon SAH rats completely restores the dysregulation of H19, eNOS, miR-675, miR-138, and HIF1α to their normal levels. Moreover, MEL dose dependently increases the luciferase activity of H19 promoter and hence the expression of H19. Additionally, H19 directly targets miR-675 and miR-138 to increase miR-675 expression and inhibit miR-138 expression. As virtual target genes of miR-675 and miR-138, respectively, HIF1α and eNOS are also regulated by the treatment with MEL. In particular, MEL treatment increases the expression of miR-675 and eNOS level while decreasing the expression of miR-138 and HIF1α in a dose dependent manner. Our study found that MEL ameliorates post-SAH vasospasm by regulating the expression of eNOS and HIF1α via the H19/miR-138/eNOS/NO and H19/miR-675/HIF1α signaling pathways.
Keywords: melatonin, eNOS, HIF1α, post-SAH vasospasm, H19, miR-138, miR-675
Introduction
Although only accounting for less than 10% of all cases of stroke, subarachnoid hemorrhage (SAH) can lead to devastating consequences.1 In fact, the annual incidence of SAH in the US is about 1 in 10,000, while the combined mortality and morbidity of SAH is higher than 50%.2 As a type of SAH, aneurysmal SAH (aSAH) is particularly dangerous because it can induce vasospasm. Statistical data showed that about 30,000 patients develop aSAH in the US every year.3 On the other hand, melatonin (MEL, also termed N-acetyl-5-methoxytryptamine) has been demonstrated to exert a protective effect against SAH in experimental animals.4 For example, Ayer et al.5 showed that MEL decreased the mortality of severe SAH, while the administration of MEL was shown to reduce SAH-induced pulmonary edema, cerebral vasospasm, neurological symptoms, and oxidative stress.4
Although the mechanisms underlying vasospasm-induced injuries remain unclear, nitric oxide (NO) has been shown to play an important role in the pathogenesis of vasospasm.6 As a potent inflammation inhibitor and vasodilator, NO regulates platelet aggregation and the proliferation of smooth muscle cells.7 In addition, the polymorphisms in the gene of endothelial NO synthase (eNOS) have been linked to an increased risk of vasospasm and injuries to the cerebral aneurysm.8 Furthermore, under hypoxic conditions, the accumulation of hypoxia-inducible factor-1α (HIF1α) in tissues causes erythropoiesis, glycolysis, and angiogenesis.9 In particular, vascular endothelial growth factor (VEGF), a target of HIF1α, acts as a strong promoter of angiogenesis and participates in the development of cerebral vasospasm.10
As an extensively studied type of non-coding RNA (ncRNA), microRNAs (miRNAs) have been shown to play critical roles in many cellular and physiological processes, such as apoptosis, growth, proliferation, and differentiation of cells.11 During its action, mature miRNA binds to RNA‐induced silencing complex (RISC) and subsequently interacts with the 3′‐untranslated region (3′‐UTR) of its target mRNAs to regulate their expression at transcriptional and post‐transcriptional levels.12 Recently, it has been shown that long ncRNAs (lncRNAs) also play important functions in many cellular processes.13 Unlike miRNAs, lncRNAs usually contain more than 200 nucleotides and hence have more complex structures.13 In general, lncRNAs can be divided into the following five types based on their positions relative to adjacent protein‐coding genes: intergenic lncRNA, intronic lncRNA, bidirectional lncRNA, antisense lncRNA, and sense lncRNA.14 In terms of SAH, it was demonstrated that the expression of NGF, miR-675, and H19 is significantly increased in SAH rats, while the treatment of SAH rats by MEL reduced the expression of NGF, miR-675, and H19, indicating that MEL can prevent SAH-induced early brain injury (EBI) by regulating the activity of the H19-let-7a-NGF-apoptosis and H19-miR-675-P53-apoptosis pathways.15
In our previous study, MEL treatment has been reported to regulate the expression of H19.15 Furthermore, H19 acts as a host gene of miR-675 and the increase in H19 expression has been associated with increased miR-675 expression.16 Meanwhile, as a competing endogenous RNA of miR-138, H19 can sponge the function of miR-138.17 Furthermore, we found that, as a target of miR-675, HIF1α is functionally associated with post-SAH vasospasm.18 In this study, we established an animal model of SAH and vasospasm. By treating SAH rats with MEL, we investigated the effect of MEL on the signaling pathways of H19/miR-138/eNOS/NO and H19/miR-675/HIF1α.
Results
MEL Administration Attenuates Neurobehavioral Deficits
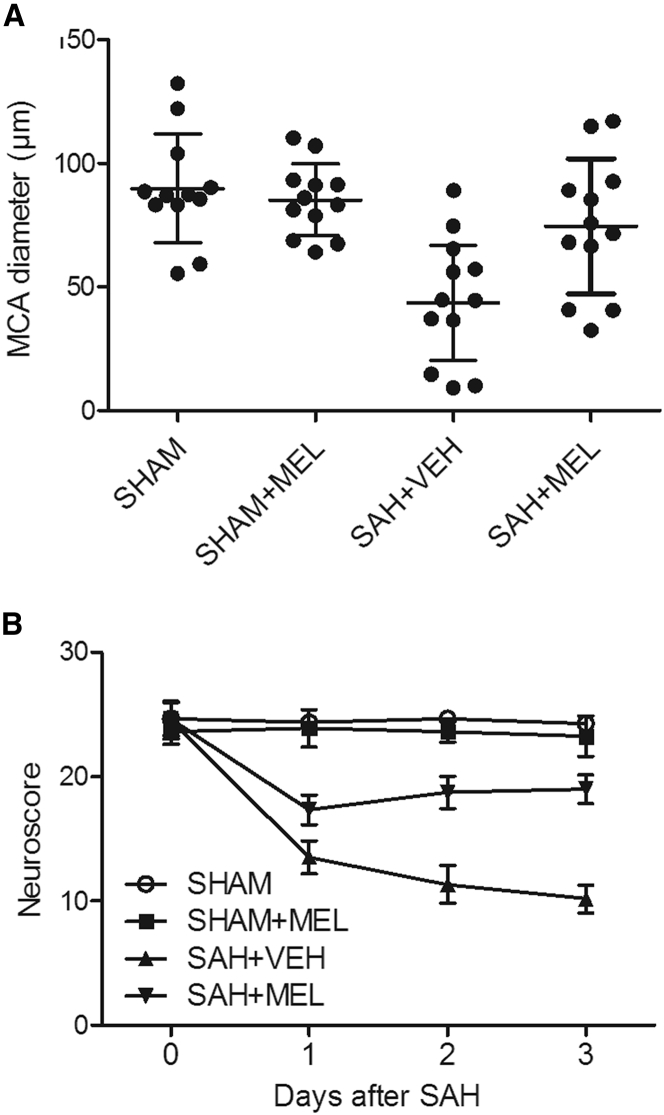
The diameter of the middle cerebral artery (MCA) in sham rats showed no obvious difference upon MEL treatment, while the rats in the SAH group showed a reduced MCA diameter, which was then significantly increased by the administration of MEL (Figure 1A). In addition, vehicle-treated SAH rats displayed significant neurobehavioral deficits, while the treatment with MEL reduced the level of neurobehavioral deficits (Figure 1B), thus validating that MEL administration could attenuate neurobehavioral deficits.
Figure 1.
MEL Administration Attenuated Neurobehavioral Deficits
(A) Rats in the SAH group showed a reduced MCA diameter, but the administration of MEL in SAH rats significantly increased MCA diameter. (B) SAH rats exhibited obvious neurobehavioral deficits, while the administration of MEL in SAH rats obviously reduced the severity of neurobehavioral deficits.
Differential Expressions of H19, miR-675, and miR-138 among Various Groups
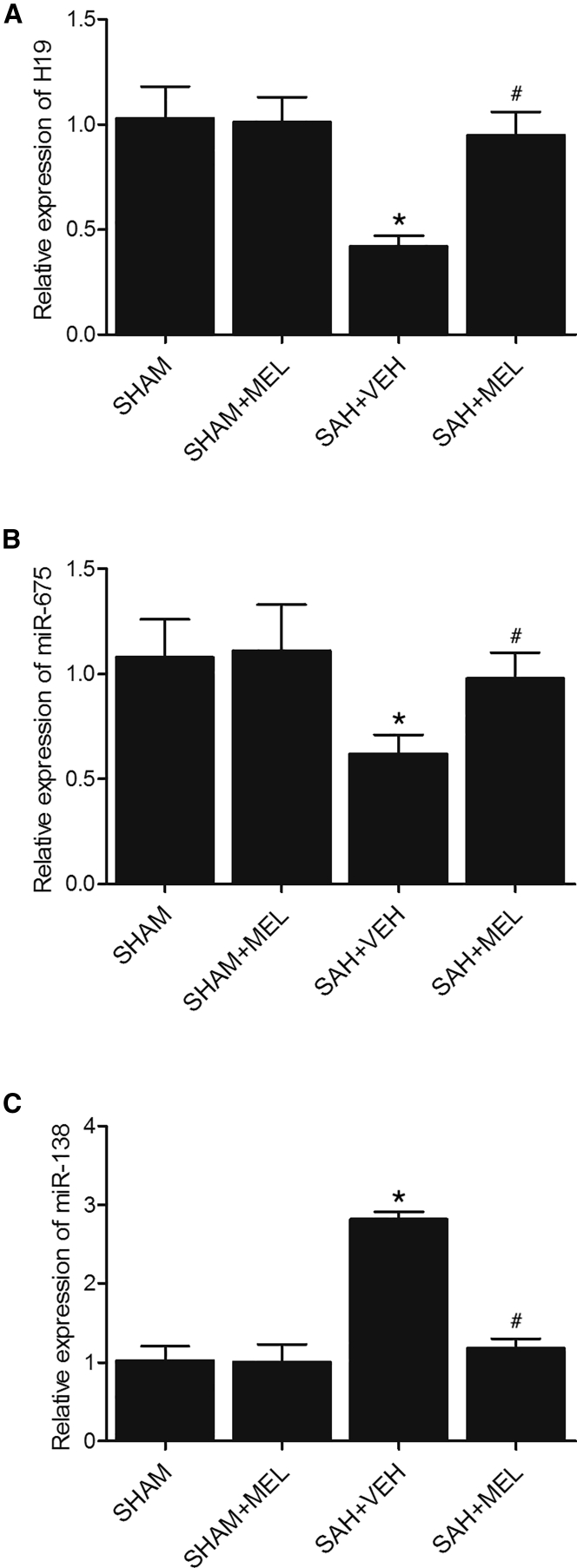
Real-time PCR was carried out to compare the expression of H19, miR-675, and miR-138 among the SHAM, SHAM+MEL, SAH+VEH, and SAH+MEL groups. As shown in Figure 2, H19 (Figure 2A) and miR-675 (Figure 2B) expression in the SHAM group were comparable with that in the SHAM+MEL group. However, the expression of H19 and miR-675 was much lower in the SAH+VEH group, but the treatment with MEL obviously increased the expression of H19 and miR-675. In contrast, the rats in the SAH+VEH group showed a much higher level of miR-138 (Figure 2C) than the rats in the SHAM group, but the treatment with MEL obviously decreased the expression of miR-138. Therefore, H19, miR-675, and miR-138 were all differentially expressed upon the onset of SAH and further treatment of MEL among SAH rats.
Figure 2.
H19, miR-675, and miR-138 Were Differentially Expressed among Various Groups
(A) H19 expressions in SHAM and SHAM+MEL rats were similar. H19 expression in SAH rats was much lower, while the administration of MEL in SAH rats obviously increased H19 expression (*p < 0.05, versus SHAM group; #p < 0.05, versus SAH+VEH group). (B) miR-675 expressions in SHAM and SHAM+MEL rats were similar. miR-675 expression in SAH rats was much lower, while the administration of MEL in SAH rats obviously increased miR-675 expression (*p < 0.05, versus SHAM group; #p < 0.05, versus SAH+VEH group). (C) miR-138 expressions in SHAM and SHAM+MEL rats were similar. miR-138 expression in SAH rats was much higher, while the administration of MEL in SAH rats obviously decreased miR-138 expression (*p < 0.05, versus SHAM group; #p < 0.05, versus SAH+VEH group).
Levels of HIF1α and eNOS among Various Groups
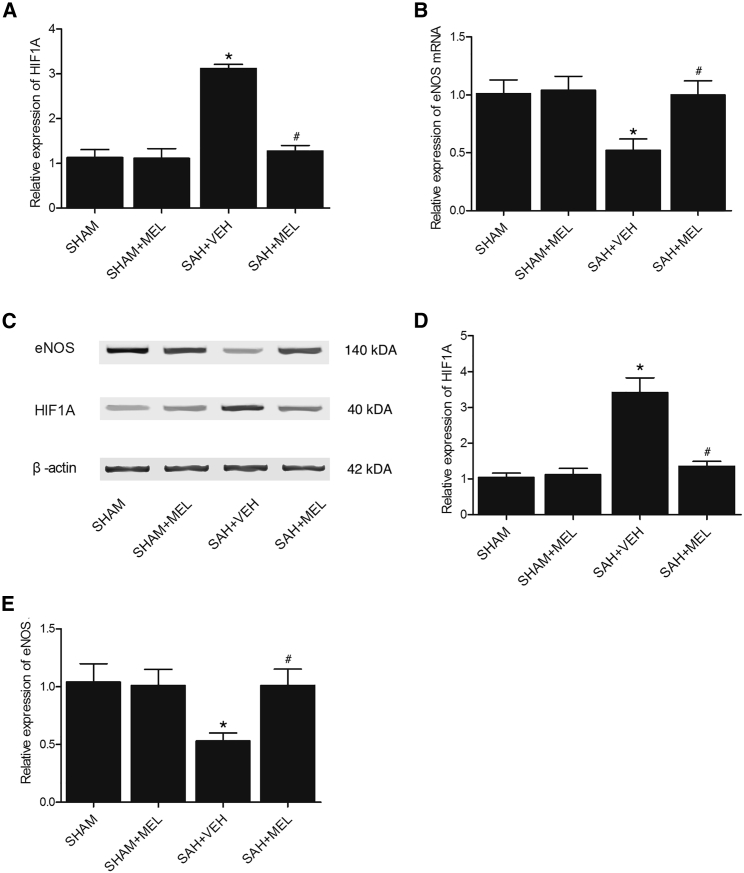
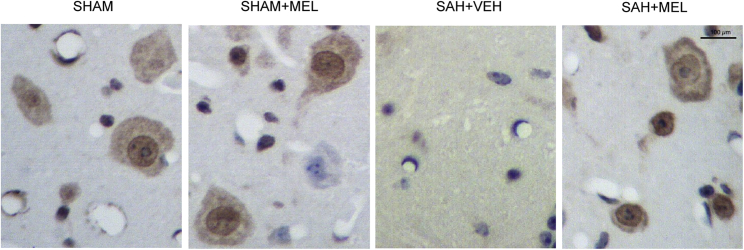
Real-time PCR and western blot analysis were performed to examine the expression of HIF1α and eNOS among the SHAM, SHAM+MEL, SAH+VEH, and SAH+MEL groups. As shown in Figure 3, the rats in the SAH+VEH group showed evidently increased expression of HIF1α mRNA (Figure 3A) and protein (Figures 3C and 3D), while the mRNA and protein levels of HIF1α in the SAH+MEL group were similar to those in the SHAM and SHAM+MEL groups. On the other hand, the rats in the SAH+VEH group showed evidently decreased expression of eNOS mRNA (Figure 3B) and protein (Figures 3C and 3E), while the mRNA and protein levels of eNOS in the SAH+MEL group were similar to those in the SHAM and SHAM+MEL groups. Furthermore, an immunohistochemistry (IHC) assay was used to compare the protein levels of HIF1α and eNOS among the four groups. As shown in Figure 4, the protein level of HIF1α in the SHAM group was similar to that in the SHAM+MEL group, but the protein level of HIF1α in the SAH+VEH group was much higher than that in the SHAM group. However, the treatment of SAH rats by MEL significantly reduced the protein level of HIF1α. Meanwhile, the level of eNOS protein (Figure 5) in the SHAM group was comparable with that in the SHAM+MEL group, while the level of eNOS protein in the SAH+VEH group was significantly lower. In addition, the treatment of SAH rats by MEL significantly increased the protein level of eNOS. Therefore, the mRNA and protein expressions of HIF1α and eNOS were all differentially expressed upon the onset of SAH and further treatment of MEL among SAH rats.
Figure 3.
Expression of HIF1α and eNOS Was Compared among Various Groups Using Real-Time PCR and Western Blot Analysis
(A) Expressions of HIF1α mRNA in the SHAM and SHAM+MEL groups were similar, while the expression of HIF1α mRNA in SAH rats was much higher, and the administration of MEL in SAH rats obviously decreased the expression of HIFIA mRNA (*p < 0.05, versus SHAM group; #p < 0.05, versus SAH+VEH group). (B) Expressions of eNOS mRNA in the SHAM and SHAM+MEL groups were similar, while the expression of eNOS mRNA in SAH rats was much lower, and the administration of MEL in SAH rats obviously increased the expression of eNOS mRNA (*p < 0.05, versus SHAM group; #p < 0.05, versus SAH+VEH group). (C) Expression of HIF1α protein was increased in SAH rats, while expression of eNOS protein was decreased in SAH rats compared with the SHAM and SHAM+MEL groups, and administration of MEL in SAH rats decreased the expression of HIF1α protein and increased the expression of eNOS protein. (D) Expressions of HIF1α protein in the SHAM and SHAM+MEL group were similar, while the administration of MEL in SAH rats decreased the upregulated expression of HIF1α protein in SAH rats (*p < 0.05, versus SHAM group; #p < 0.05, versus SAH+VEH group). (E) Expressions of eNOS protein in the SHAM and SHAM+MEL group were similar, while the administration of MEL in SAH rats increased the downregulated expression of eNOS protein in SAH rats (*p < 0.05, versus SHAM group; #p < 0.05, versus SAH+VEH group).
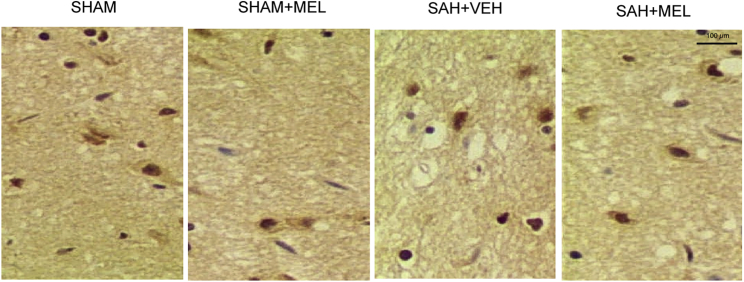
Figure 4.
Expressions of HIF1α Was Determined among Various Groups Using IHC
Expression of HIF1α was determined among various groups using IHC, and it was found that evident increase in HIF1α protein expression was observed in SAH group, while it was decreased by MEL treatment (magnification, 200×).
Figure 5.
Expressions of eNOS Was Compared among Various Groups Using IHC
Expression of eNOS was compared among various groups using IHC, and the results showed that the protein expression of eNOS was evidently decreased in the SAH group, while the administration of MEL in SAH rats obviously increased the protein expression of eNOS (magnification, 200×).
Effect of MEL on the Expression of H19, miR-675, miR-138, HIF1α, and eNOS
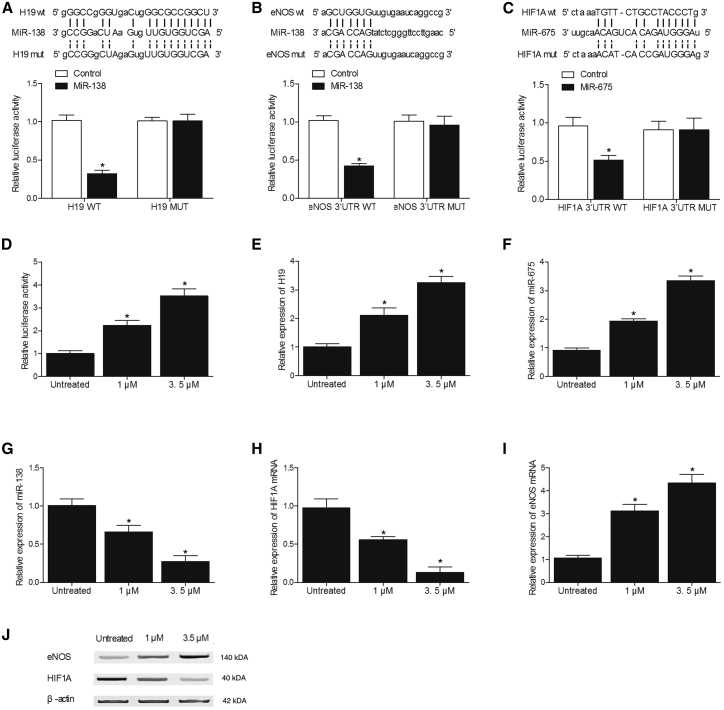
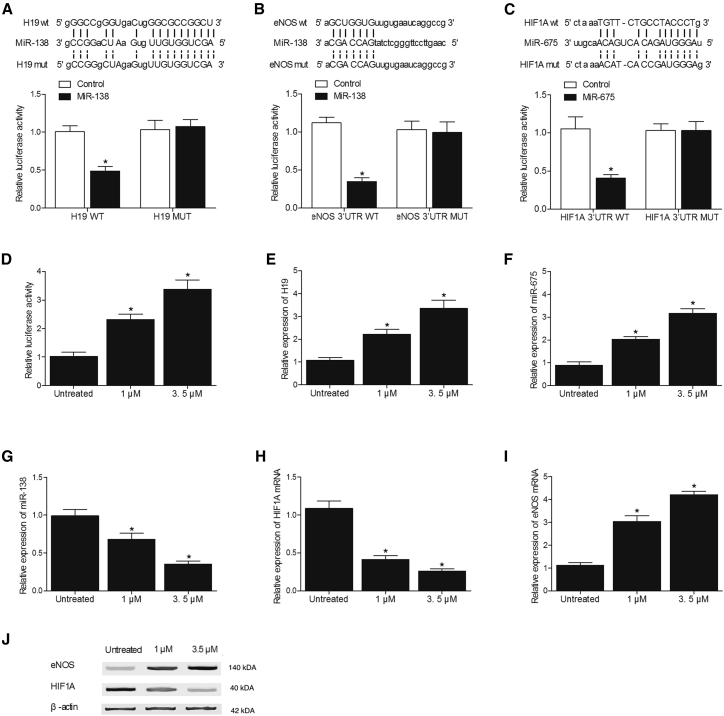
Effects of MEL on the expression of H19, miR-675, miR-138, HIF1α, and eNOS were investigated in SH-SY5Y (Figure 6) and U251 (Figure 7) cells using luciferase assay, real-time PCR, and western blot analysis. As shown in Figures 6A and 7A, the co-transfection of SH-SY5Y and U251 cells with H19 and miR-138 suppressed the luciferase activity of H19, validating H19 to sponge miR-138 in a cellular model. In addition, the transfection of SH-SY5Y (Figure 6B) and U251 (Figure 7B) cells with wild-type 3′ UTR of eNOS significantly reduced the luciferase activity of miR-138, indicating eNOS as a potential target of miR-138. Meanwhile, SH-SY5Y (Figure 6C) and U251 (Figure 7C) cells co-transfected with wild-type 3′ UTR of HIF1α and miR-675 mimic showed a reduced luciferase activity, leading to the conclusion that miR-675 could target HIF1α. Furthermore, the luciferase activity of H19 showed a concentration-dependent increase over an increasing concentration of MEL (Figures 6D and 7D), and MEL also upregulated the expression of H19 (Figures 6E and 7E) and miR-675 (Figures 6F and 7F) while downregulating the expression of miR-138 (Figures 6G and 7G) in SH-SY5Y and U251 cells in a concentration-dependent manner. Moreover, the administration of MEL dose dependently decreased the mRNA (Figures 6H and 7H) and protein (Figures 6J and 7J) expression of HIF1α while increasing the mRNA (Figures 6I and 7I) and protein expression of eNOS in SH-SY5Y and U251 cells. All these results suggested that MEL increased H19 expression, which in turn increased the expression of miR-675 and decreased the expression of miR-138, thus affecting the expression of targets of miR-675 or miR-138, HIF1α, and eNOS.
Figure 6.
Effect of MEL on the Expression of H19, miR-675, miR-138, HIF1α, and eNOS in SH-SY5Y Cells
(A) Co-transfection of the cells with H19 and miR-138 mimics reduced the luciferase activity of the cells (*p < 0.05, versus control group). (B) Transfection of the cells with wild-type 3′ UTR of eNOS significantly decreased the luciferase activity of miR-138 (*p < 0.05, versus control group). (C) Transfection of the cells with miR-675 mimics significantly decreased the luciferase activity of wild-type 3′ UTR of HIF1α (*p < 0.05, versus control group). (D) MEL dose dependently increased the luciferase activity of the H19 promoter (*p < 0.05, versus untreated cells). (E) MEL dose dependently increased the expression of H19 (*p < 0.05, versus untreated cells). (F) MEL dose dependently increased the expression of miR-675 (*p < 0.05, versus untreated cells). (G) MEL dose dependently decreased the expression of miR-138 (*p < 0.05, versus untreated cells). (H) MEL dose dependently downregulated the expression of HIF1α mRNA (*p < 0.05, versus untreated cells). (I) MEL dose dependently upregulated the expression of eNOS mRNA (*p < 0.05, versus untreated cells). (J) MEL dose dependently downregulated the expression of HIF1Α protein while upregulating the expression of eNOS protein.
Figure 7.
Effect of MEL on the Expression of H19, miR-675, miR-138, HIF1Α, and eNOS in U251 Cells
(A) Co-transfection of the cells with H19 and miR-138 mimics reduced the luciferase activity of the cells (*p < 0.05, versus control group). (B) Transfection of the cells with wild-type 3′ UTR of eNOS significantly decreased the luciferase activity of miR-138 (*p < 0.05, versus control group). (C) Transfection of the cells with miR-675 mimics significantly decreased the luciferase activity of wild-type 3′ UTR of HIF1α (*p < 0.05, versus control group). (D) MEL dose dependently increased the luciferase activity of H19 promoter (*p < 0.05, versus untreated cells). (E) MEL dose dependently increased the expression of H19 (*p < 0.05, versus untreated cells). (F) MEL dose dependently increased the expression of miR-675 (*p < 0.05, versus untreated cells). (G) MEL dose dependently decreased the expression of miR-138 (*p < 0.05, versus untreated cells). (H) MEL dose dependently downregulated the expression of HIF1α mRNA (*p < 0.05, versus untreated cells). (I) MEL dose dependently upregulated the expression of eNOS mRNA (*p < 0.05, versus untreated cells). (J) MEL dose dependently downregulated the expression of HIF1α protein while upregulating the expression of eNOS protein.
Discussion
In mammalians, MEL acts as an essential constituent and a free radical scavenger in the defense against oxidants and hence can effectively protect cells against oxidative damage.19 MEL may play its anti-oxidative role via several routes, i.e., by increasing the activity of endogenous antioxidant enzymes such as superoxide dismutase (SOD), by protecting the functions of endogenous antioxidant enzymes such as catalase, and by reducing the level of free radicals in the body.20 In fact, both MEL and its metabolites, including N1-acetyl-5-methoxykynuramine and N1-acetyl-N2-formyl-5-methoxykynuramine, are potent scavengers of free radicals.21 Furthermore, MEL can cross cell membranes easily and will not be reduced by redox cycling.22 Several previous studies have shown that MEL alleviates the severity of SAH by reducing the severity of focal cerebellum injury by decreasing the magnitude of vasospasm and endothelial cell apoptosis in blood vessels, by reducing the rate of mortality, and by reducing the symptoms of EBI and neurological disorders.4,5,23, 24, 25 MEL has also been reported to affect the expression of H19 lncRNA, a host of miR-675 and a negative regulator of p53 expression.24 In this study, we found that the administration of MEL attenuated SAH-induced vasospasm and neurobehavioral deficits. In addition, H19 and miR-675 were lowly expressed in the SAH rats, along with highly expressed miR-138, while the treatment with MEL restored the expression of H19, miR-675, and miR-138 in SAH rats.
It has been shown that, by regulating the expression of let-7a and miR-675, H19 is implicated in post-SAH brain injury by mediating nerve growth factor (NGF)- and P53-induced apoptosis.15 In fact, H19 plays its role by harboring a hairpin that serves as the precursor of miR-675-3p and miR-675-5p4.26 In addition, individuals suffering from post-SAH EBI usually show significantly increased levels of cerebral vasospasm, apoptosis, oxidative stress, and inflammatory responses.27 Recently, a pre-clinical study on glioma also demonstrated the presence of a loop between miR-675-5p and HIF1α.28 In addition, it was shown that cerebral vasospasm can be caused by the impairment to the blood-brain barrier via dysregulated VEGF expression and excessive apoptosis of endothelial cells.29,30 Furthermore, in a rat model of SAH, the expression and activity of HIF1α are elevated in the cortex and hippocampus, suggesting the presence of a direct correlation between apoptosis and the expression of HIF1α.31 In this study, we found that the expression of HIF1α in the SAH+MEL group was much higher than that in the control group, along with a much lower level of eNOS expression.
By directly interacting with the 3′ UTR of Ca2+ binding protein S100A1 and downregulating its expression, miR-138 can reduce eNOS activity in the body.32 Subsequently, the reduced level of eNOS limits the amount of NO available in the regulation of vascular tone by decreasing the amount of L-arginine metabolized into citrulline and NO. As a result, upon the development of SAH, the immunoreactivity of eNOS in cerebrovascular endothelia is decreased.33 On the other hand, the treatment of SAH by simvastatin, a compound that can increase the expression of eNOS in cerebrovascular tissues, delayed the occurrence of cerebral vasospasm.28 Using a canine model of SAH, Khurana et al.34 showed that vasospasm can be alleviated with intrathecal delivery of eNOS mRNA, while other results indicated that the increase in eNOS expression may reduce the severity of cerebral vasospasm. A large amount of evidence also suggested that various factors can induce the onset of post-SAH cerebral vasospasm.35 For example, several in vitro and in vivo studies showed that oxyhemoglobin and its metabolites can trigger the synthesis of free radicals, which subsequently generate oxidative stress and induce vasospasm.36 In summary, critical vasodilators, such as NO and peroxynitrite, which is generated by a rapid reaction between NO and superoxide radical (O2−), may lead to the imbalance between cerebral vasoconstrictors and vasodilators, eventually causing cerebral vasospasm.37,38 In this study, we found that MEL dose dependently increased H19 expression, which in turn increased the expression of miR-675 and decreased the expression of miR-138, thus affecting the expression of target genes of miR-675 or miR-138.
In this study, our results suggested that MEL could ameliorate post-SAH vasospasm by regulating the expression of eNOS and HIF1α via H19/miR-138/eNOS/NO and H19/miR-675/HIF1α signaling pathways.
Materials and Methods
Animal Grouping and Treatment
In this study, a total of 32 male SD rats with an average body weight of 400 g were randomly allocated into four weight-matched groups, i.e., a sham group (SHAM group, surgery without the induction of SAH, N = 8), a sham+MEL group (SHAM+MEL group, sham-operated rats treated with MEL, N = 8), a SAH+vehicle group (SAH+VEH group, SAH rats treated with saline, N = 8), and a SAH+MEL group (SAH+MEL group, SAH rats treated with MEL, N = 8). First, the rats in SAH+VEH and SAH+MEL groups underwent a previously described surgical operation to induce the onset of SAH.12 In brief, after an incision of 4 cm in length was made over the anterior midline on the neck, the internal carotid artery (ICA) and right external carotid artery were separated. Subsequently, a sharpened wire was inserted into the stump of the right external carotid artery and advanced to the intracranial ICA. Upon the proper placement of the wire, a laser doppler flowmetry (AD Instruments, Colorado Springs, CO, USA) should show that the signal of ipsilateral core binding factor (CBF) was reduced. In the next step, the wire was pushed inward 3 mm to puncture the cerebral ICA at the bifurcation. Among the three experimental groups set up in this study, the rats in the SHAM group underwent all surgical procedures except the step of suture perforation, while the rats in the other two groups underwent the entire surgical procedure. After the surgical operation, all animals were housed in single cages and had free access to food and water until dissection. All animal experiments were performed in line with internationally recognized guidelines for the care and use of laboratory animals, and the institutional animal ethics committee approved this study.
Assessment of Vasospasm
The assessment of vasospasm in rats was performed on day 3 after the surgery using cerebrovascular casting.27, 28, 29 In brief, the rats were anesthetized using isoflurane and then perfused transcardially using PBS containing 4% of formalin and 3% of gelatin-India ink. Subsequently, the rats were dissected to collect the brain tissue, and the severity of SAH was graded by inspecting the morphology of blood vessels under a microscope in conjunction with a high-definition medical image analysis system (HMIAP-2000, Tongji Medical University, Wuhan, China).27, 28, 29 In addition, the smallest diameter within the MCA outside the lateral sulcus was measured to evaluate the degree of vasospasm.
RNA Isolation and Real-Time PCR
The total RNA from tissue and cell samples was extracted using a Trizol kit (Invitrogen, Carlsbad, CA, USA), followed by the determination of the concentration and purity of extracted RNA using a DU-640 spectrophotometer (Beckman, San Jose, CA, USA). A ratio of A260/A280 between 1.8 and 2.0 was considered as acceptable purity for extracted RNA to be used in subsequent experiments. In the next step, the total extracted RNA was reversely transcribed into cDNA in accordance with the instruction of a PrimeScript RT reagent kit (Takara, Tokyo, Japan) using the following reaction conditions: reverse transcription at 37°C for 15 min and reverse transcriptase inactivation at 85°C for 5 s. Subsequently, real-time PCR was performed on an ABI7500 instrument (Applied Biosystems, Foster City, CA, USA) using a SYBR premix EX Taq kit (Takara, Tokyo, Japan) and the following reaction conditions: pre-denaturation at 95°C for 10 min and 40 cycles of denaturation at 95°C for 15 s and annealing at 60°C for 1 min. The real-time PCR reaction system contained 9.0 μL of SYBR mix, 0.5 μL of forward primer, 0.5 μL of reverse primer, 2.0 μL of cDNA template, and 8.0 μL of RNase-free dH2O, with a total volume of 20 μL. The relative expression of H19 (forward, 5′-TGCTGCACTTTA CAACCACTG-3′; reverse, 5′-ATGGTGTCTTTGATGTTGGGC-3′), miR-675 (forward, 5′-TGGTGCGGAGAGGGCCCACAGTG-3′; reverse, 5′-GCGAGCACAGAATTAATACGAC-3′), miR-138 (forward, 5′-GGTGTCGTGGAGTCGGCAA-3′; reverse, 5′-AACTTCACAACACCAGCTTA-3′), HIF1α mRNA (forward, 5′-CGGGATCCTCTCTAGTCTCACGAGGGGTTTCC-3′; reverse, 5′-GCTCTAGAGATGCTACTGCAATGCAATGGTT-3′), and eNOS mRNA (forward, 5′-GCCAATGCAGTGAAGATC-3′; reverse, 5′-GCACAGAAGTGCGGGTAT-3′) was calculated using the 2−ΔΔCt method, while the expression of U6 (forward, 5′-CGCTTCGGCAGCACATATACTA-3′; reverse, 5′-TGCTGTAGCCAAATTCGTTG-3′), and GAPDH (forward, 5′-TCAGTGGTGGACCTGACCTG-3′; reverse, 5′-TGCTGTAGCCAAATTCGTTG-3′) were used as the internal reference.
Cell Culture and Transfection
SH-SY5Y and U251 cells were cultured at 37°C and 5% CO2 in a 95% humidity incubator. For transfection experiments, the cells were maintained in a DMEM culture medium supplemented with 10% fetal bovine serum (Gibco, Thermo Fisher Scientific, Waltham, MA, USA). When the cells reached a density of 85%, they were trypsinized using 0.25% trypsin and passaged. Subsequently, the cells in the logarithmic phase of growth were seeded into 96-well plates at a density of 2 × 104 cells/well and cultured overnight. On the next day, the cells were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following the instruction of the manufacturer. After transfection, the cells were cultured at 37°C for 48 h before they were collected for subsequent analyses. In addition, for experiments of MEL treatment, the cells were treated with different concentrations of MEL for 24 h prior to the measurement of expression of various target genes.
Vector Construction, Mutagenesis, and Luciferase Assay
To investigate the regulatory relationship of H19/miR-138, miR-138/eNOS, and miR-675/HIF1α, as well as to investigate the effects of MEL treatment on the activity of H19 promoter, full-length sequences of H19, miR-138, and miR-675, as well as the 3′ UTR sequences of HIF1α and eNOS containing the binding sites for miR-675 and miR-138, respectively, were cloned into pcDNA luciferase vectors (Promega, Madison, WI, USA). At the same time, site-directed mutagenesis was carried out in the site of interaction of H19/miR-138, miR-138/eNOS, and miR-675/HIF1α to create mutant vectors of H19, miR-138, miR-675, HIF1α, and eNOS. Subsequently, SH-SY5Y and U251 cells were co-transfected with wild-type and mutant vectors of genes of interest, and the luciferase activity of transfected cells was measured using a dual-luciferase reporter gene assay kit (Promega, Madison, WI, USA) at 48 h after transfection on a luminescence plate reader (Promega, Madison, WI, USA).
Western Blot Analysis
Cell and tissue samples were harvested and lysed in an SDS lysis buffer to extract sample proteins, which were then heated to 100°C for 5 min and loaded (20 μL per sample) onto a 10% polyacrylamide gel electrophoresis (PAGE) gel. Subsequently, the resolved proteins were transferred onto a polyvinylidene fluoride (PVDF) membrane at a voltage of 48 V, and the membrane was incubated with 5% bovine serum albumin for 2 h at room temperature to block endogenous antibodies. In the next step, after being rinsed in tris-buffered saline and Tween 20 (TBST), the membrane was incubated overnight at 4°C with anti-HIF1α (cat. no. #3716S, 1:500 dilution, Cell Signaling Technologies, Beverly, MA, USA) and anti-eNOS primary antibodies (cat. no. #9572S, 1:500 dilution, Cell Signaling Technologies, Beverly, MA, USA). After the incubation with primary antibodies, the membrane was washed with 1× TBST and then incubated with horseradish peroxidase (HRP)-labeled secondary antibodies (cat. no. sc2357, 1:2,000 dilution, Santa Cruz Biotechnology, Santa Cruz, CA, USA). After the incubation, the membrane was washed with TBST again and then developed in enhanced chemiluminescence reagents (Thermo Fisher Scientific, Waltham, MA, USA). The relative protein expression of HIF1α and eNOS was then calculated using GAPDH as the internal control.
Immunohistochemistry
Tissue samples were fixed in 10% formaldehyde, dehydrated in gradient alcohol, embedded in paraffin, and sliced into 4-μm sections. The sections were then dewaxed and dehydrated by gradient alcohol (95%, 80%, and 75%; 1 min each step). After being washed by running water for 1 min, the sections were incubated with 3% H2O2 at 37°C for 30 min, followed by a PBS rinse. In the next step, the sections were boiled in 0.01 M citrate buffer at 95°C for 20 min. After being cooled down to room temperature, the sections were washed again by PBS. Subsequently, the sections were blocked with normal goat serum at 37°C for 10 min and incubated with anti-HIF1α and anti-eNOS monoclonal antibodies (1:100 dilution, Abcam, Cambridge, MA, USA) overnight at 4°C. Following the incubation, the sections were washed with PBS for 2 min and incubated with HRP-conjugated secondary antibodies (1:1,000 dilution, Abcam, Cambridge, MA, USA) at room temperature for 30 min. Afterward, the sections were developed with diaminobenzidine (DAB), counterstained with hematoxylin, and mounted. In the next step, 10 view fields were randomly selected on each section and observed under a microscope to determine the percentage of cells positive for the expression of target genes.
H&E Staining
The collected tissue samples were dissected and fixed overnight at 4°C with 4% paraformaldehyde. These samples were then embedded in paraffin and sectioned for H&E staining, which was carried out according to the instruction of an H&E staining kit (Beyotime Biotechnology, Shanghai, China).
TUNEL Assay
Collected tissues were stained according to the instructions of a TUNEL kit (Beyotime Biotechnology, Shanghai, China) to observe the status of cell apoptosis in different samples. In brief, the tissue samples were made into paraffin sections, dewaxed by a conventional xylene treatment, rehydrated by gradient alcohol, blocked with 3% H2O2 solution at room temperature for 10 min, and incubated with the reagents in the kit following the manufacturer’s instructions. Finally, the sections were observed and photographed under a fluorescence microscope (BX53, Olympus, Tokyo, Japan) to analyze the apoptotic profile of the samples.
Statistical Analysis
All results were analyzed by SPSS statistical software version 21.0. Each experiment was repeated in triplicate. All measurement data were expressed as mean ± SD. The t test was used for comparing two groups, while a one-way ANOVA was used for comparing multiple groups, with a Scheffé test being used as a post hoc test. A p value of <0.05 was considered statistically significant.
Ethics Approval and Consent to Participate
All animal experiments were performed in line with internationally recognized guidelines for the care and use of laboratory animals.
Availability of Data and Material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author Contributions
G.H. and H.C. planned the study; G.H., H.C., Y.Y., Y.P., and F.J. collected and analyzed the data; G.H. and Y.P. composed the manuscript, and all the other co-authors approved the final manuscript.
Conflicts of Interest
The authors declare no competing interests.
Contributor Information
Yaohua Pan, Email: neucrosis@yeah.net.
Xiaohua Zhang, Email: daoguac30363696@163.com.
Feng Jia, Email: projiafeng@163.com.
References
- 1.van Gijn J., Kerr R.S., Rinkel G.J. Subarachnoid haemorrhage. Lancet. 2007;369:306–318. doi: 10.1016/S0140-6736(07)60153-6. [DOI] [PubMed] [Google Scholar]
- 2.King J.T., Jr. Epidemiology of aneurysmal subarachnoid hemorrhage. Neuroimaging Clin. N. Am. 1997;7:659–668. [PubMed] [Google Scholar]
- 3.Ingall T., Asplund K., Mähönen M., Bonita R. A multinational comparison of subarachnoid hemorrhage epidemiology in the WHO MONICA stroke study. Stroke. 2000;31:1054–1061. doi: 10.1161/01.str.31.5.1054. [DOI] [PubMed] [Google Scholar]
- 4.Ersahin M., Toklu H.Z., Cetinel S., Yüksel M., Yeğen B.C., Sener G. Melatonin reduces experimental subarachnoid hemorrhage-induced oxidative brain damage and neurological symptoms. J. Pineal Res. 2009;46:324–332. doi: 10.1111/j.1600-079X.2009.00664.x. [DOI] [PubMed] [Google Scholar]
- 5.Ayer R.E., Sugawara T., Chen W., Tong W., Zhang J.H. Melatonin decreases mortality following severe subarachnoid hemorrhage. J. Pineal Res. 2008;44:197–204. doi: 10.1111/j.1600-079X.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz A.Y., Sehba F.A., Bederson J.B. Decreased nitric oxide availability contributes to acute cerebral ischemia after subarachnoid hemorrhage. Neurosurgery. 2000;47:208–214. doi: 10.1097/00006123-200007000-00042. discussion 214–215. [DOI] [PubMed] [Google Scholar]
- 7.Moncada S., Palmer R.M., Higgs E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 8.Khurana V.G., Sohni Y.R., Mangrum W.I., McClelland R.L., O’Kane D.J., Meyer F.B., Meissner I. Endothelial nitric oxide synthase gene polymorphisms predict susceptibility to aneurysmal subarachnoid hemorrhage and cerebral vasospasm. J. Cereb. Blood Flow Metab. 2004;24:291–297. doi: 10.1097/01.WCB.0000110540.96047.C7. [DOI] [PubMed] [Google Scholar]
- 9.Semenza G.L., Agani F., Feldser D., Iyer N., Kotch L., Laughner E., Yu A. Hypoxia, HIF-1, and the pathophysiology of common human diseases. Adv. Exp. Med. Biol. 2000;475:123–130. doi: 10.1007/0-306-46825-5_12. [DOI] [PubMed] [Google Scholar]
- 10.Bhardwaj A. SAH-induced cerebral vasospasm: unraveling molecular mechanisms of a complex disease. Stroke. 2003;34:427–433. [PubMed] [Google Scholar]
- 11.Shivdasani R.A. MicroRNAs: regulators of gene expression and cell differentiation. Blood. 2006;108:3646–3653. doi: 10.1182/blood-2006-01-030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 13.Hung T., Chang H.Y. Long noncoding RNA in genome regulation: prospects and mechanisms. RNA Biol. 2010;7:582–585. doi: 10.4161/rna.7.5.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao Q., Liu C., Yuan X., Kang S., Miao R., Xiao H., Zhao G., Luo H., Bu D., Zhao H. Large-scale prediction of long non-coding RNA functions in a coding-non-coding gene co-expression network. Nucleic Acids Res. 2011;39:3864–3878. doi: 10.1093/nar/gkq1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang S., Tang W., He Y., Wen L., Sun B., Li S. Long non-coding RNA and microRNA-675/let-7a mediates the protective effect of melatonin against early brain injury after subarachnoid hemorrhage via targeting TP53 and neural growth factor. Cell Death Dis. 2018;9:99. doi: 10.1038/s41419-017-0155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu C., Chen Z., Fang J., Xu A., Zhang W., Wang Z. H19-derived miR-675 contributes to bladder cancer cell proliferation by regulating p53 activation. Tumour Biol. 2016;37:263–270. doi: 10.1007/s13277-015-3779-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang W.C., Fu W.M., Wong C.W., Wang Y., Wang W.M., Hu G.X., Zhang L., Xiao L.J., Wan D.C., Zhang J.F., Waye M.M. The lncRNA H19 promotes epithelial to mesenchymal transition by functioning as miRNA sponges in colorectal cancer. Oncotarget. 2015;6:22513–22525. doi: 10.18632/oncotarget.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan J., Chen C., Lei J., Yang L., Wang K., Liu J., Zhou C. 2-methoxyestradiol reduces cerebral vasospasm after 48 hours of experimental subarachnoid hemorrhage in rats. Exp. Neurol. 2006;202:348–356. doi: 10.1016/j.expneurol.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Reiter R.J. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991;12:151–180. doi: 10.1210/edrv-12-2-151. [DOI] [PubMed] [Google Scholar]
- 20.Olayaki L.A., Alagbonsi I.A., Abdulrahim A.H., Adeyemi W.J., Bakare M., Omeiza N. Melatonin prevents and ameliorates lead-induced gonadotoxicity through antioxidative and hormonal mechanisms. Toxicol. Ind. Health. 2018;34:596–608. doi: 10.1177/0748233718773508. [DOI] [PubMed] [Google Scholar]
- 21.Onuki J., Almeida E.A., Medeiros M.H., Di Mascio P. Inhibition of 5-aminolevulinic acid-induced DNA damage by melatonin, N1-acetyl-N2-formyl-5-methoxykynuramine, quercetin or resveratrol. J. Pineal Res. 2005;38:107–115. doi: 10.1111/j.1600-079X.2004.00180.x. [DOI] [PubMed] [Google Scholar]
- 22.Luchetti F., Canonico B., Betti M., Arcangeletti M., Pilolli F., Piroddi M., Canesi L., Papa S., Galli F. Melatonin signaling and cell protection function. FASEB J. 2010;24:3603–3624. doi: 10.1096/fj.10-154450. [DOI] [PubMed] [Google Scholar]
- 23.Aydin M.V., Caner H., Sen O., Ozen O., Atalay B., Cekinmez M., Altinors N. Effect of melatonin on cerebral vasospasm following experimental subarachnoid hemorrhage. Neurol. Res. 2005;27:77–82. doi: 10.1179/016164105X18331. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Cruz F., Espinar A., Pozo D., Osuna C., Guerrero J.M. Melatonin prevents focal rat cerebellum injury as assessed by induction of heat shock protein (HO-1) following subarachnoid injections of lysed blood. Neurosci. Lett. 2002;331:208–210. doi: 10.1016/s0304-3940(02)00884-4. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi M., Zhou C., Nanda A., Zhang J.H. Ras protein contributes to cerebral vasospasm in a canine double-hemorrhage model. Stroke. 2004;35:1750–1755. doi: 10.1161/01.STR.0000129898.68350.9f. [DOI] [PubMed] [Google Scholar]
- 26.Cai X., Cullen B.R. The imprinted H19 noncoding RNA is a primary microRNA precursor. RNA. 2007;13:313–316. doi: 10.1261/rna.351707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sehba F.A., Hou J., Pluta R.M., Zhang J.H. The importance of early brain injury after subarachnoid hemorrhage. Prog. Neurobiol. 2012;97:14–37. doi: 10.1016/j.pneurobio.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGirt M.J., Lynch J.R., Parra A., Sheng H., Pearlstein R.D., Laskowitz D.T., Pelligrino D.A., Warner D.S. Simvastatin increases endothelial nitric oxide synthase and ameliorates cerebral vasospasm resulting from subarachnoid hemorrhage. Stroke. 2002;33:2950–2956. doi: 10.1161/01.str.0000038986.68044.39. [DOI] [PubMed] [Google Scholar]
- 29.Zhou C., Yamaguchi M., Kusaka G., Schonholz C., Nanda A., Zhang J.H. Caspase inhibitors prevent endothelial apoptosis and cerebral vasospasm in dog model of experimental subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2004;24:419–431. doi: 10.1097/00004647-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Kusaka G., Ishikawa M., Nanda A., Granger D.N., Zhang J.H. Signaling pathways for early brain injury after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2004;24:916–925. doi: 10.1097/01.WCB.0000125886.48838.7E. [DOI] [PubMed] [Google Scholar]
- 31.Li Y., Zhou C., Calvert J.W., Colohan A.R., Zhang J.H. Multiple effects of hyperbaric oxygen on the expression of HIF-1 alpha and apoptotic genes in a global ischemia-hypotension rat model. Exp. Neurol. 2005;191:198–210. doi: 10.1016/j.expneurol.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 32.Most P., Lerchenmüller C., Rengo G., Mahlmann A., Ritterhoff J., Rohde D., Goodman C., Busch C.J., Laube F., Heissenberg J. S100A1 deficiency impairs postischemic angiogenesis via compromised proangiogenic endothelial cell function and nitric oxide synthase regulation. Circ. Res. 2013;112:66–78. doi: 10.1161/CIRCRESAHA.112.275156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pluta R.M., Thompson B.G., Dawson T.M., Snyder S.H., Boock R.J., Oldfield E.H. Loss of nitric oxide synthase immunoreactivity in cerebral vasospasm. J. Neurosurg. 1996;84:648–654. doi: 10.3171/jns.1996.84.4.0648. [DOI] [PubMed] [Google Scholar]
- 34.Khurana V.G., Smith L.A., Baker T.A., Eguchi D., O’Brien T., Katusic Z.S. Protective vasomotor effects of in vivo recombinant endothelial nitric oxide synthase gene expression in a canine model of cerebral vasospasm. Stroke. 2002;33:782–789. doi: 10.1161/hs0302.103735. [DOI] [PubMed] [Google Scholar]
- 35.Grasso G. An overview of new pharmacological treatments for cerebrovascular dysfunction after experimental subarachnoid hemorrhage. Brain Res. Brain Res. Rev. 2004;44:49–63. doi: 10.1016/j.brainresrev.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Aladag M.A., Turkoz Y., Sahna E., Parlakpinar H., Gul M. The attenuation of vasospasm by using a sod mimetic after experimental subarachnoidal haemorrhage in rats. Acta Neurochir. (Wien) 2003;145:673–677. doi: 10.1007/s00701-003-0052-z. [DOI] [PubMed] [Google Scholar]
- 37.Toda N., Ayajiki K., Tanaka T., Okamura T. Preganglionic and postganglionic neurons responsible for cerebral vasodilation mediated by nitric oxide in anesthetized dogs. J. Cereb. Blood Flow Metab. 2000;20:700–708. doi: 10.1097/00004647-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Mori T., Nagata K., Town T., Tan J., Matsui T., Asano T. Intracisternal increase of superoxide anion production in a canine subarachnoid hemorrhage model. Stroke. 2001;32:636–642. doi: 10.1161/01.str.32.3.636. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.