Abstract
Conjugated linoleic acid (CLA) is known for its multiple benefits including improvement of growth, increasing lean mass, and anti-carcinogenic effects. However, when used in long-term supplementations CLA does not improve semen parameters in boar and bull and reduces fertility in Japanese quails. The content of unsaturated fatty acids in dietary lipids plays a significant role in spermatogenesis owning the high proportion of unsaturated fatty acids in plasma membrane of sperms. Whether CLA plays a role in testicular tissue and epididymal fat is still unknown. Therefore, in this study we hypothesize that long-term supplementation of equal proportion of CLA isomer mix (c9,t11-CLA and t10,c12- CLA) in rabbit bucks might alter male reproductive potentials. Twelve V-Line weaned male rabbits were used in 26 weeks trial, rabbits were individually raised and randomly allocated into three dietary groups. Control group (CON) received a basal diet, a group received 0.5% CLA (CLA 0.5%), and a group received 1% CLA (CLA 1%). Rabbits were euthanized at the end of the trial and several parameters were evaluated related to growth, semen quality, and testicular and epididymal tissue histopathology and transcriptome. The long-term supplementation of CLA increased feed intake by 5% and body weight by 2–3%. CLA 1% decreased sperm progressive motility. In testicular tissue L-carnitine and α-tocopherol were decreased by CLA supplementation. In epididymal fat, CLA tended to decrease concentration of polyunsaturated fatty acids, the expression of SCD5 gene was upregulated by CLA 1% and CASP3 gene was upregulated by CLA 0.5%. Transcription of PPARG was downregulated by CLA. Feeding 1% CLA also decreased testicular epithelial thickness. Long-term supplementation of CLA modestly enhanced male rabbit growth, but negatively impacted male reproduction, especially at high dose of CLA.
Introduction
The discovery of anticancer properties made the conjugated linoleic acid (CLA) top-studied fatty acid by the scientific community [1]. Multiple studies were conducted to investigate the beneficial effects of CLA, which include enhancement of immune function and decrease inflammation in several animal models [2,3]. The nutrigenomic effects of CLA have been studied to evidence the promising nutritional properties of CLA in animal diets [4]. Additionally, the positive effect of CLA on weight loss by shifting the energy repartition in the body could explain its wide use as male dietary supplement among young athletes [5].
Despite the above-mentioned beneficial effects of CLA and the wide use of it as a dietary supplement in human and animals, the effects of long-term supplementation of CLA on body weight and male reproduction are very limited. Studies conducted to evaluate the effects of CLA on male reproduction were mostly short-term (i.e., less than 6 month) [6] and conducted after puberty looking at the effect of CLA on semen parameters either through dietary supplementation [7] or added directly to semen to extend cryopreservation [8]. Some studies reported that CLA supplementation increased fertility [9] while other studies have shown no [7,10] or limited effect on fertility based on semen evaluation only [11]. In a study on Japanese quails, CLA reduced fertility and hatchability [12].
Rabbit have high reproductive rate compared to other livestock. According to FAO [13], one rabbit buck could inseminate 15 rabbit does. Therefore, for future sustainability of rabbit production, male reproductive potential of rabbit bucks is of major concern. On the other side, dietary lipid plays a crucial role in sperm plasma membrane formation, particularly polyunsaturated fatty acids (PUFA) [14]; therefore, CLA was added in the rabbit diet to investigate whether it plays a positive or negative role in rabbit fertility. To the authors' knowledge, the effects of long-term CLA supplementation on growth and male reproductive organs in male rabbit have not been investigated. Therefore, this study hypothesized that long-term dietary CLA supplementation enhances growth but has a negative impact on male reproduction in rabbit bucks. For this purpose, semen of rabbits was evaluated and testicular and epididymal tissue samples were subjected to histopathological and transcriptomic assessments.
Materials and methods
Ethical approval
The experimental procedures for this study approved by Cairo University Institutional Animal Care and Use Committee (approval # CU/II/F/95/18). Animal number was kept to the minimum and euthanasia was carried out in accordance with AVMA (American Veterinary Medical Association) guidelines. Rabbits were purchased from the rabbit unit of the Faculty of Agriculture, Cairo University, and handled with care. Due to limited number of rabbits used in the study, rabbits were raised individually, and samples were analyzed in technical triplicates for all samples collected after euthanasia.
Experimental design, animals housing, and diet
Experimental diets were formulated to meet or exceed the NRC (National Research Council) requirements for growing rabbit [15]; 2500 Kcal digestible energy/ Kg diet, 16% crude protein, fat 2%, and crude fiber 10–12% (Table 1). Twelve V-line strain weaned male rabbits (35 d ± 5) were used in a 26 weeks experiment. Rabbits were blocked for body weight 605 g (±33.5; P = 0.99) and randomly allocated into three iso-nitrogenous-iso-caloric dietary treatments (n = 4/group) as follows: 1) CON group fed basal diet supplemented with 1% oleic acid (Techno Pharmachem, India), 2) CLA 0.5% group was fed on diet supplemented with 0.5% CLA (Lutrell Pure; BASF, Ludwigshafen, Germany; certified to contain equal proportion of c9,t11- and t10,c12-CLA; EFSA, 2016) plus 0.5% oleic acid, and 3) CLA 1% group was fed a diet supplemented with 1% CLA. Pure oleic acid was used to balance the diet for energy. Linoleic acid was not selected to balance the diet as linoleic acid was found to increase tissue accumulation of CLA contents as noticed by Garcia et al. [16].
Table 1. Diet ingredients1.
| Ingredient | (g/kg) |
|---|---|
| Barley | 230 |
| Soybean meal (44%) | 160 |
| Clover hay | 300 |
| Wheat Bran | 263 |
| Molasses | 30 |
| Limestone | 10 |
| Sodium Chloride | 4 |
| Premix2 | 3 |
| Oleic acid3 CLA4 |
Variable Variable |
1diet was formulated to provide 2500 Kcal digestible energy/kg diet according to rabbit NRC, 1977.
2The premix provides the following (per kg diet): 15,000 IU of Vit. A; 100 mg Vit. E; 21 mg Vit. K3; 10 mg Vit. B1; 40 mg Vit. B2; 15 mg Vit. B6; 0.1 mg Vit. B12; 200 mg Niacin; 100 mg Pantothenic acid; 0.5 mg Biotin; 10 mg Folic acid; 500 mg Choline Chloride; 450 mg Zn; 600 mg Mn; 0.3 mg Fe; 50 mg Cu; 250 mg I.
3Oleic acid was added at a rate of 1% on the control group diet (CON), and 0.5% in the CLA 0.5% group diet.
4Dietary CLA (Conjugated linoleic acid) was added at a dose of 0.5% in the CLA 0.5% diet and 1% in the CLA 1% diet.
Feed samples were collected from each dietary treatment and analyzed (Table 2) for chemical composition according to AOAC [17]. Rabbits were individually raised in semi-closed system and had free access to water via nipple system. Individual body weight and feed intake were recorded weekly.
Table 2. Chemical composition of the experimental diets (g/kg DM).
| Item (%) | CON1 | CLA 0.5%2 | CLA 1% 3 |
|---|---|---|---|
| Dry matter | 911.70 | 915.00 | 915.90 |
| Crude protein | 186.20 | 183.80 | 183.30 |
| Crude fat | 43.40 | 41.30 | 41.00 |
| Ash | 135.2 | 130.00 | 129.40 |
| Crude fiber | 154.40 | 147.50 | 145.50 |
1Control diet supplemented with 1% oleic acid.
2Diet supplemented with 0.5% CLA+0.5% oleic acid.
3Diet supplemented with 1% CLA.
Semen collection and sperm kinetics
Due to the large variability on the moment of puberty in rabbits (19 to 24 weeks) [18,19] semen was collected twice a week for 7 successive weeks starting from week 20 of the experiment. Semen was collected from the bucks by using an artificial vagina according to Viudes-de-Castro et al., [20]. The ejaculates were transferred immediately to the laboratory and maintained in a water bath (38.5 ºC) until evaluation. An aliquot from each sample (10 μL) was diluted 1:20 in Tris–citrate–glucose extender (250 mM tris-hydroxy methyl amino methane, 83 mM citric acid, 50 mM glucose, pH 6.8 to 7.0) and subjected to motion characteristics evaluation using a computer-assisted sperm analysis system (CASA; instrument SpermVision™ software minitube Hauptstraße 41. 84184 Tiefenbach, Germany). Ten microscopic fields were captured for each sample and the average of motility parameters were recorded including DAP (Distance Average Path, μm), DCL (Distance Curved Line, μm), DSL (Distance Straight Line, μm), VAP (Velocity Average Path, μm/s), VCL (Velocity Curved Line, μm/s), VSL (Velocity Straight Line, μm/s), STR (Straightness Track, VSL/VAP,%), LIN (Linearity, VSL/VCL,%), WOB (Wobble VAP/VCL,%), ALH (Amplitude of Lateral Head Displacement, μm), and BCF (Beat Cross Frequency, Hz).
Sperm concentration and morphology
Sperm concentration and morphology were evaluated for all samples according to Perumal et al., [21]. All chemicals used for semen processing were purchased from Sigma-Aldrich (Cairo, Egypt) unless otherwise stated.
Sperm concentration was determined using a hemocytometer and expressed as 106 sperm/ml. The proportion of live to dead spermatozoa was estimated using Eosin-Nigrosin stain. Sperm suspension smears were prepared by mixing 10 μL of semen sample with 20 μL of stain on a warm slide then allowed to dry at room temperature. Viability was assessed by phase-contrast microscope at a magnification of 1000× using immersion oil. Only sperm showing strict exclusion of stain were counted as viable, while sperm displaying partial or complete purple staining were considered non-viable. In the same fields of eosin-nigrosin stained slides, the number of spermatozoa with abnormal head morphology, cytoplasmic droplets and abnormal tails were also counted (200 sperm per slide) to determine the percentage of morphological abnormality.
For assessment of acrosomal integrity, a drop of semen was smeared on a pre-warmed slide. The slide with the drop was allowed to dry in a flow of air followed by immersion in buffered formal saline for 15 min, washed for 15–20 min, dried and immersed in the buffered Giemsa solution for 90 min then rinsed in distilled water, and allowed to dry.
The functional integrity of the sperm plasma membrane was evaluated by hypo-osmotic swelling test (HOST). The HOST assay was performed by mixing 20 μL of semen with 200 μL of a hypo-osmotic solution. The mixture was incubated for 60 min at 37° C and then a drop was smeared on a slide. A minimum of 200 sperm was observed under phase contrast microscope at 400× magnification and the percentages of sperm with swollen and curled tails were recorded for each sample.
Euthanasia and blood sampling
Rabbits were euthanized using Ketamine and Xylazine protocol as described in Simón et al., [22] and in accordance with the AVMA guidelines. Blood was collected after euthanasia for serum separation. Blood samples were allowed to clot for 20 minutes. The clot was removed by centrifugation at 2,000 ×g for 10 minutes. The serum was separated and stored at -20 ºC till analysis [23].
Serum testosterone and testicular metabolites
A testosterone ELISA kit (Cat No. 582701, Cayman Chemicals, USA) was used for serum testosterone determination according to the manufacturer instructions. The level of testosterone in serum was presented as ng/ml.
Testicular oxidative stress metabolites malondialdehyde (MDA), 8-hydroxy-2-deoxyguanosine (8-OHDG), L-carnitine and α-tocopherol were assessed with HPLC (Agilent HP 1200 series; USA). The analytical column was Supelcosil C18 (5 μm particle and 80 Ao pore size) (250 × 4.6 ID). Testicular MDA level (nmol/g tissue) and the 8-OHDG expressed as pg/g tissue were determined as described by Ahmed-Farid et al., [24]. L-carnitine concentration was detected as described by Alcorn et al., [25] and vitamin E (α-tocopherol) was determined by the method of McMurray et al., [26].
Epididymal fatty acid profile
Epididymal fatty acids were extracted according to Abdelatty et al., [27], briefly, lipids were extracted with chloroform: methanol mixture (2:1), a standard fatty acid mixture was purchased from Sigma‐Aldrich (Sigma, St. Louis, MO). Fatty acids were determined according to O'fallon et al., [28]. Gas chromatography (GC) was done with an Agilent 7890A. Hydrogen was the carrier gas with a column head pressure of 10 psi. Total flow rate at the split vent was 50 ml/min, the flow rate through the column was 2.5 ml/min and the septum purge was 2.5 ml/min. One μl was injected with the injector set at 220°C using the split-less injection mode. The temperature gradient started with a 70°C initial temperature, a linear increase to 170°C at a rate of 20°C/min, a slower linear increase to 170°C at a rate of 0.8°C/min to separate closely eluting fatty acids, followed by an increase to reach 220°C at 20°C/min, and a final 2.5 min hold. The total run time was 20.1 minutes. Fatty acid desaturation indices were calculated according to Sirriet al., [29].
Histomorphometry
Tissue samples from testis and epididymis were collected immediately after euthanasia and fixed in 10% neutral formalin buffer. Tissue samples were then processed using the paraffin embedding technique and sectioned using a microtome (Leica 2135, Germany) into 3–5 micron sections. Tissue sections were then stained with routine hematoxylin and eosin stain and Masson’s trichrome stain. Fixed epididymal tissue samples were also sectioned using cryostat in order to stain fat using Sudan Black stain. Stained tissue sections were examined using a light microscope and photographed (Olympus XC30 camera, Japan). The thickness of the epithelium lining seminepherous tubules was measured from the basement membrane to the lumen in 10 tubules/testis at angle of 90 degrees using TS view software in order to calculate the mean of epithelial thickness.
Histopathological changes were evaluated using Johnsen's scoring system in 10 seminepherous tubules in each testicular tissue. Score 1 indicated no seminiferous epithelium; Score 2: presence of Sertoli cells only and no germinal cells; Score 3: presence of spermatogonia only; Score 4: few spermatocytes with no spermatozoa or spermatids; Score 5: many spermatocytes with no spermatozoa or spermatids; Score 6: few early spermatids with no spermatozoa and no late spermatids; Score 7: many early spermatids with no spermatozoa and no late spermatids; Score 8: few late spermatids and less than five spermatozoa per tubule; Score 9: many late spermatids, disorganized epithelium indicating slightly impaired spermatogenesis; Score 10: full spermatogenesis and perfect tubules [30].
Immunohistochemistry
Paraffin-embedded tissue sections of testis and epididymis were immunohistochemically stained using caspase-3 primary antibody (Novus Biologicals, USA) as a hallmark of apoptosis. Level of caspase-3 was quantified using avidin-biotin-peroxidase (Novus Biologicals, USA). All procedures were carried out according to the manufacturer protocol. For color development 3,3′-diaminobenzidine stain was used and hematoxylin was used as counterstain. The percent area of positively stained testicular tissue was measured by using Image J software in three fields/testis.
Transcriptomic analysis of testicular and epididymal fat
Extraction of RNA
Samples of right testis and epididymal fat were collected, snap frozen in liquid nitrogen, and stored at -80 ºC till analysis. Samples were then homogenized in 1ml Qiazol (Qiagen, Valencia, CA) by using TissueLyser (TissueLyser LT; QIAGEN; Cat No./ID: 85600). Homogenization was achieved by 5-mm steel bead shaking at 50 Hz for 5 min according to Sun et al., [31]. The homogenate was used for RNA extraction using Qiagen RNeasy lipid tissue mini-kits according to the manufacturer’s instructions (Qiagen, Valencia, CA). Contaminant genomic DNA was removed with a DNase enzyme (RNase-free DNase set, Qiagen, Valencia, CA). RNA was then collected in 30 μL RNase free water and stored at -80 ºC till cDNA synthesis.
The RNA concentration and purity were measured by Nano-Drop 2000C spectrophotometer (Thermo Scientific, USA). The RNA purity (absorbance ratio, A260/A280) for all samples was ≥ 1.9. The integrity of RNA was verified on 2% agarose gel using gel electrophoresis image (Gel Doc. BioRad) according to Norollahi et al., [32].
Synthesis of cDNA template
For the cDNA synthesis 300 ng of RNA per 20 μl reaction were used. cDNA was synthesized using SensiFast cDNA synthesis kits (Bioline, UK) according to the manufacturer’s instructions using a thermal cycler (SensoQuest, GmbH, D-37085).
Primer designing and quantitative PCR
Primer pairs for selected target and reference genes are summarized in Table 3. Primer pairs not previously published were designed using Primer Express 3, as previously described by Bionaz and Loor [33]. Primer pairs were purchased from Genwez (New Jersey, USA). Desalted lyophilized primers were reconstituted using RNase free water (Invitrogen, USA).
Table 3. Gene names, primer sequences, accession#, and product size of the used genes.
| Gene1 | Accession # | Primers sequences (5’→3’)2 | bp3 | Reference |
|---|---|---|---|---|
| UXT | XM_008272555 | F: GCGGGACTTGCGAAAGGT | 100 | Designed |
| R: AGCTTCCTGGAGTCGTTCAATG | ||||
| RPS15A | XM_008257787 | F: AGCATGGTTACATTGGCGAGTT | 100 | Designed |
| R:CTGATCACTCCGCACTTGTTTAA | ||||
| HPRT1A | XM_008267147 | F: GCAGACCTTGCTTTCCTTGGT | 100 | [32] |
| R: GCAGGCTTGCGACCTTGAC | ||||
| SCD5 | XM_008267676.2 |
F:CAAGCATCCAGATGTCATCGA R:CGCTGATCTTATAGTACTTTCTCTGGAA |
101 | Designed |
| PPARG | NM_001082148.1 |
F:GAGCCTTCCAACTCCCTCATG R:GAAACCCTTGCATCCTTCACA |
102 | Designed |
| FABP4 | XM_002710655.3 | F:AGTCAAGAGCATCATAACCCTAGATG | 100 | Designed |
| R:TTTATCGCCCTCCCGTTTTC | ||||
| LPL | NM_001177330.1 | F:CAGGAGAGACTCAGAAAAAGGTGAT | 100 | Designed |
| R:TTGTCGTGGCATTTCACAAAC | ||||
| BAX | XM_008252361.2 | F:CCCGCGAGGTCTTTTTCC | 113 | Designed |
| R:CAGGGCCTTGAGTACCAGCTT | ||||
| BCL2 | XM_008261439.2 | F:GGCTGGGATGCCTTCGT | 186 | Designed |
| R:TTTCGTGAACTGTTTGCATATCTG | ||||
| CASP3 | NM_001082117.1 |
F:GACAGTGGCATCGAGACAGACA R:GAATAGTAACCAGGTGCTGTGGAA |
110 | Designed |
| GPX1 | NM_001085444.1 |
F:CAGTTTGGGCATCAGGAGAAC R:GCATGAAGTTGGGCTCGAA |
94 | Designed |
1UXT, ubiquitously-expressed, prefoldin-like chaperone; RPS15A, Ribosomal Protein S15a; HPRT1A, Hypoxanthine phosphoribosyl transferase 1; SCD5, Stearoyl-CoA desaturase 5;PPARG, Peroxisome Proliferator Activated Receptor gamma; FABP4, Fatty Acid Binding Protein 4; LPL, lipoprotein lipase; BAX, BCL2 Associated X; BCL2, B-cell lymphoma 2; CASP3, Caspase 3; GPX1, Glutathione Peroxidase 1.
2Underline denotes exon-exon junction.
3Product size; base pair.
The cDNA was diluted 1:20 using molecular grade RNase free water (Invitrogen, USA). Quantitative PCR reaction was performed using Maxima SYBR Green/ROX qPCR master mix (2x) (Thermo Scientific, USA). The reaction was performed in 0.1 ml qPCR strip tubes with optical caps (Gunster Biotech Co., Taiwan). The reaction consisted of 12.5 μL of SYBR green Master Mix, 11 μL of diluted cDNA, and 0.75 μL of each forward and reverse primer pair to reach final volume of 25 μL. Each sample was performed in triplicate. A non-template control (NTC) was run to check for primer dimer and RNA contamination. The reaction was performed in AriaMx Real Time PCR (Agilent Technologies, USA) using a two steps protocol: initial denaturation at 95 ºC for 10 min, then 40 cycles of denaturation at 95 ºC for 15 s followed by annealing/extension at 60 ºC for 60 s. A melting curve protocol was run at the end of the PCR by heating at 95 ºC for 30 s followed by a 65 ºC for 30 s and 95 ºC for 30 s.
Fluorescent amplification curves data was downloaded from the AriaMx PCR System and analyzed using LinReg PCR software [34]. Three reference genes were tested including ubiquitously expressed transcript (UXT), small ribosomal protein subunit 15 (RPS15A), and hypoxanthine phosphoribosyl transferase 1 (HPRT1A). The reliability of reference genes was determined using NormFinder [35]. The stability value of the combination of the two best reference genes (UXT and RPS15A) was 0.19. The normalization factor was calculated as the geometrical mean of the two best reference genes and final data of target genes were obtained by dividing the RTqPCR values obtained by LinRegPCR to the normalization factor.
Statistical analysis
The MIXED procedure of SAS 9.4 (SAS Institute Inc., Cary, NC, USA) was used for repeated measures analysis of feed intake, body weight, and sperm kinetics, viability, and concentration. Both treatment and time and their interaction were considered as fixed factors in the model and rabbit was considered as random effect.
Shapiro-Wilks test was used to evaluate normality for all parameters before performing the statistical analysis to make sure that P-value for Shapiro-Wilks test is > 0.05, meaning that the data were normally distributed for the 12 rabbits.
The UNIVARIATE procedure of SAS 9.4 was used for testing the difference between groups of serum testosterone, testicular metabolites, epididymal fatty acids, gene expression, IHC area % testicular lesion scoring and testicular epithelial thickness. Significance was determined at P ≤ 0.05 whereas tendencies were determined at P ≤ 0.10.
Results
Growth performance
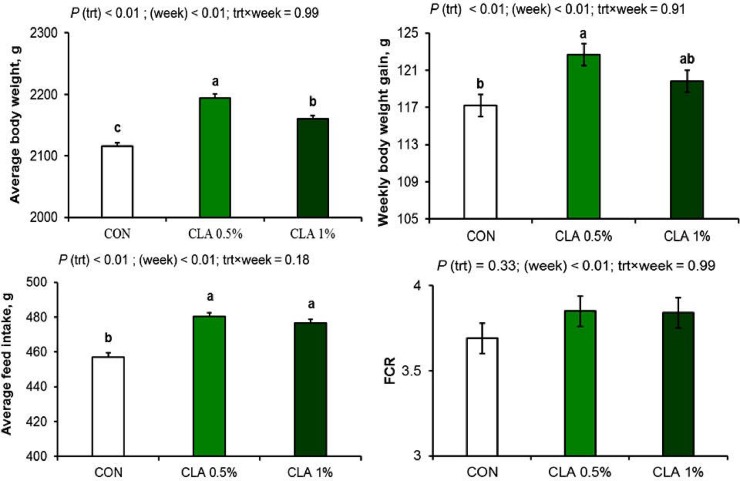
The effect of long-term dietary CLA supplementation on growth performance indices is shown in Fig 1. Despite the significant increase in feed intake of CLA fed rabbits (P < 0.01), the effect of long-term dietary supplementation of CLA on feed intake was minimal, where feed intake increased only by 5% in both CLA fed groups than the control group. Additionally, body weight was significantly affected by CLA supplementation (P < 0.01); however, the increase was 2.1% and 3.7% in CLA 1% and CLA 0.5% groups, respectively in comparison to the control group. Additionally, body weight gain was increased only in CLA 0.5% group by 4.6%.
Fig 1. Growth performance of male rabbits with long-term supplementation of CLA.
Dietary supplementation of CLA for 26 weeks increased feed intake, body weight, and body weight gain but not the FCR.
Sperm Kinetics
Results of sperm kinetics are summarized in Table 4. The effect of CLA on sperm kinetics was mostly associated with the dose of 1%, where progressive motility, DAP, and BCF were decreased (P ≤ 0.05) by 8.2%, 6.8%, and 6.2%, respectively. The total motile sperm tended to increase by 5.5% in the CLA 0.5% group but not the CLA 1%, compared to the CON group (P = 0.07).
Table 4. Effect of long term dietary CLA supplementation on sperm kinetics1.
| Item | CON2 | CLA 0.5%3 | CLA 1%4 | SEM | P-value |
|---|---|---|---|---|---|
| Motility, % | 79.10 | 84.65 | 79.57 | 1.62 | 0.07 |
| Progressive motility, % | 60.52a | 65.82a | 52.30b | 1.78 | <0.01 |
| DAP, μm5 | 28.81a | 29.70a | 26.86b | 0.63 | 0.04 |
| DCL, μm6 | 58.93 | 57.77 | 55.35 | 2.04 | 0.39 |
| DSL, μm7 | 22.27ab | 23.30a | 21.05b | 0.52 | 0.05 |
| VAP, μm/s8 | 65.21 | 67.58 | 62.74 | 2.02 | 0.25 |
| VCL, μm/s9 | 132.51 | 130.83 | 125.14 | 4.73 | 0.45 |
| VSL, μm/s10 | 50.55 | 53.16 | 48.73 | 1.44 | 0.13 |
| STR,%11 | 0.77 | 0.77 | 0.77 | 0.01 | 0.97 |
| LIN,%12 | 0.38 | 0.40 | 0.40 | 0.01 | 0.54 |
| WOB,%13 | 0.49 | 0.51 | 0.51 | 0.01 | 0.29 |
| ALH, μm14 | 4.78 | 4.75 | 4.77 | 0.17 | 0.99 |
| BCF, Hz15 | 31.14a | 31.05a | 29.20b | 0.52 | 0.05 |
1Values are least square mean, n = 4 samples/group, semen was collected twice/week for 7 successive weeks
2Control group receiving basal diet supplemented with 1% oleic acid
3Group receiving diet supplemented with 0.5% CLA and 0.5% Oleic acid
4Group receiving diet supplemented with 1% CLA
5 Distance average path
6distance curved line
7 distance straight line
8 velocity average path
9velocity curved line
10 velocity straight line
11 straightness track
12 linearity = VSL/VCL
13 wobble = VAP/VCL
14amplitude of lateral head displacement
15 beat cross frequency.
Different superscript letters denote P ≤ 0.05 between treatments.
Sperm concentration and morphology
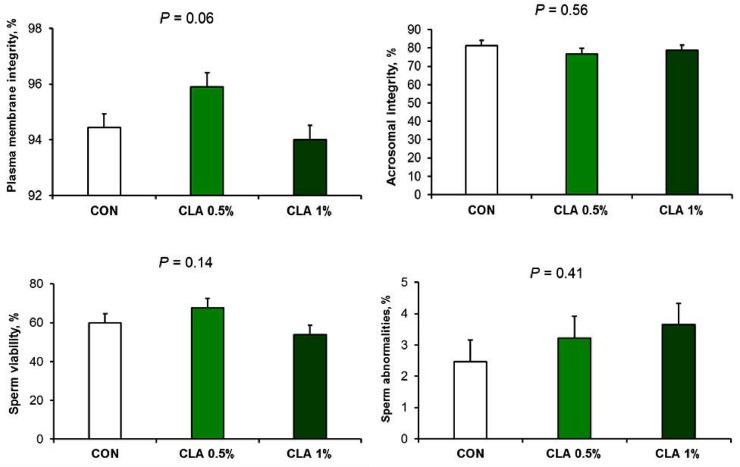
Sperm morphology (Fig 2) and concentration (Fig 3) were not affected by treatments, except for a tendency (P = 0.06) for an effect on the plasma membrane integrity due to a numerical higher value in CLA 0.5%.
Fig 2. Sperm viability of male rabbits with long-term supplementation of CLA.
Long term of dietary CLA supplementation did not improve sperm viability, abnormalities, or acrosomal integrity, however, plasma membrane integrity tended to increase in CLA 0.5% fed rabbits.
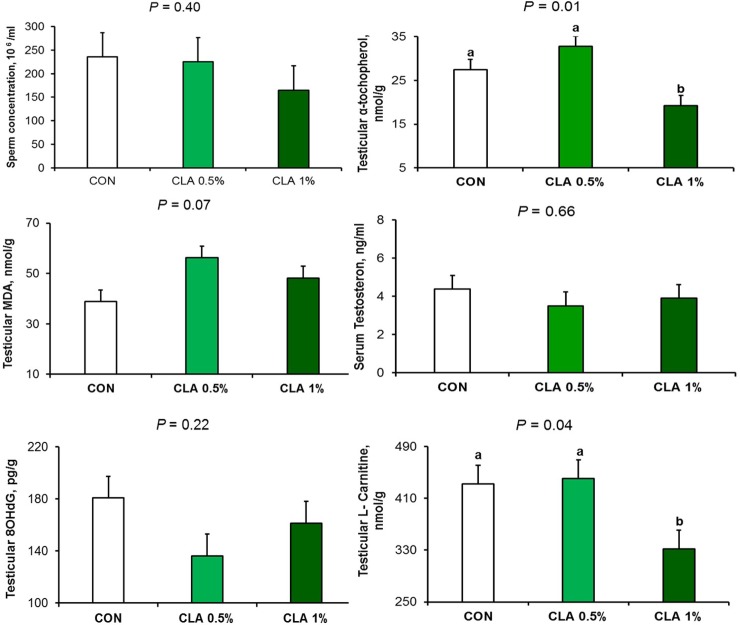
Fig 3. Sperm concentration, serum testosterone, and some testicular metabolites of CLA fed male rabbits.
Long term of dietary CLA supplementation did not affect serum testosterone level, or sperm concentration. The level of MDA tended to increase in CLA fed male rabbits. Testicular Tocopherol (Vitamin E) and L-Carnitine were decreased in CLA 1% group only.
Serum testosterone and testicular metabolites
The responses of measured testicular metabolites and serum testosterone level are presented in Fig 3. The long-term supplementation of CLA 1% decreased vitamin E (P = 0.04) and L-carnitine (P = 0.01) compared to CLA 0.5% and CON. Testicular MDA concentration tended to increase (P = 0.07) in CLA groups compared to CON.
Epididymal fatty acid profile
Fatty acid profile of epididymal fat for each group is presented in Table 5. Medium-chain fatty acids from C10 to C14 were affected by CLA treatment, with higher values in CLA 0.5% and lower in CLA 1%. Additionally, eicosanoic acid cis 11 (C20:1) was decreased by 17.4% by CLA 1% vs. CON. Linolenic acid (C18:3), arachidonic acid (C20:4), and sum of the PUFA tended to decrease in CLA 1% vs. CON. CLA supplementation decreased epididymis total fatty acids in a dose dependent manner (P < 0.01).
Table 5. Effect of long term dietary CLA supplementation on fatty acid profile and desaturation indices of epididymal fat1.
| Fatty acid, (mg/g tissue) | CON2 | CLA 0.5%3 | CLA 1%4 | SEM | P-value |
|---|---|---|---|---|---|
| C10:0 | 0.60ab | 0.64a | 0.53b | 0.03 | 0.03 |
| C12:0 | 0.22b | 0.27a | 0.22b | 0.01 | 0.02 |
| C14:0 | 33.09b | 36.27a | 30.07c | 0.75 | <0.01 |
| C16:0 | 279.95 | 272.46 | 257.90 | 16.48 | 0.64 |
| C16:1 | 36.35 | 34.87 | 32.37 | 1.75 | 0.31 |
| C18:0 | 40.71 | 39.83 | 33.68 | 2.50 | 0.15 |
| C18:1 | 67.70 | 65.20 | 61.68 | 3.72 | 0.54 |
| C18:3 | 242.11 | 242.71 | 196.22 | 15.22 | 0.10 |
| C20:0 | 1.22 | 1.17 | 1.07 | 0.07 | 0.31 |
| C20:1 | 3.16a | 3.01a | 2.61b | 0.13 | 0.04 |
| C20:2 | 2.47 | 2.29 | 2.24 | 0.13 | 0.45 |
| C20:4 | 2.14 | 2.15 | 1.78 | 0.12 | 0.10 |
| Σ SFA5 | 355.80 | 350.64 | 323.46 | 15.87 | 0.35 |
| Σ MUFA6 | 107.21 | 103.08 | 96.66 | 4.00 | 0.23 |
| Σ PUFA7 | 246.72 | 247.14 | 200.23 | 15.12 | 0.09 |
| Σ Fatty acids | 709.73a | 670.79b | 620.36c | 8.54 | <0.01 |
| Elongase8 | 0.15 | 0.15 | 0.13 | 0.01 | 0.62 |
| Δ 9 desaturase (16)9 | 10.66 | 11.40 | 11.14 | 0.50 | 0.55 |
| Δ 9 desaturase (18)10 | 62.44 | 61.93 | 64.76 | 2.10 | 0.61 |
| Δ 9desaturase (c16+18) 11 | 22.90 | 24.41 | 24.37 | 1.08 | 0.53 |
1Values are least square mean, n = 4 samples/group.
2Control group receiving basal diet supplemented with 1% oleic acid to balance for energy.
3Group receiving diet supplemented with 0.5% CLA and 0.5% Oleic acid.
4Group receiving diet supplemented with 1% CLA.
5 Saturated fatty acids.
6 Monounsaturated fatty acids.
7 Poly unsaturated fatty acids.
8 Elongase = C18:0/C16:0.
9 Δ9 desaturase (16) = [C16:1/(C16:1 + C16:0)]*100.
10 Δ9 desaturase (18) = [C18:1/(C18:1 + C18:0)]*100.
11 Δ9 desaturase (16 18) = [(C16:1 + C18:1)/(C16:1 + C16:0 + C18:1 + C18:0)]*100.
Different superscript letters denote P ≤ 0.05 between treatments.
Histomorphometry findings
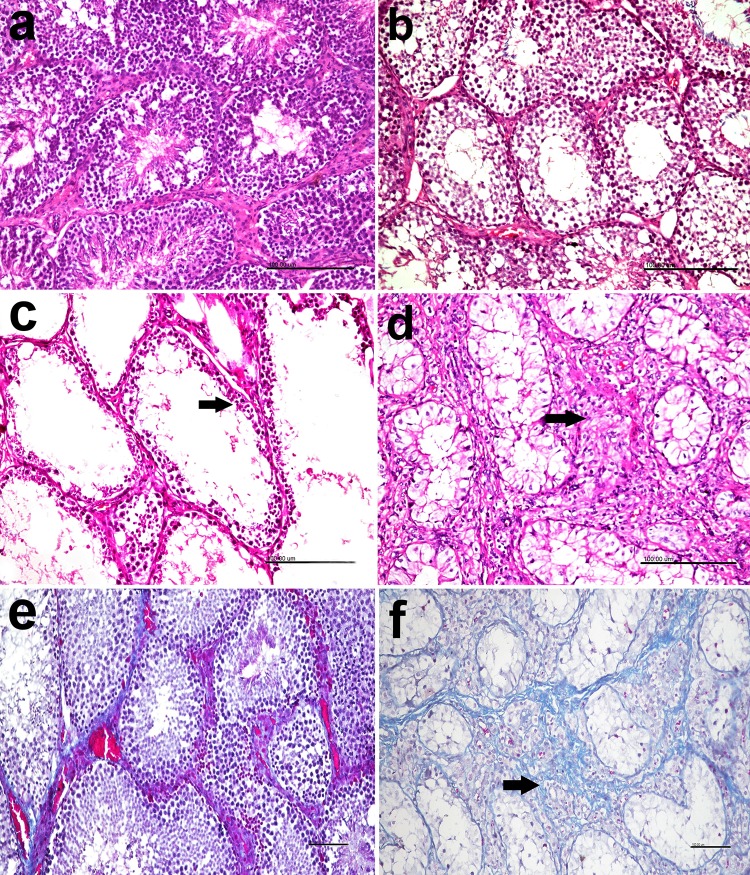
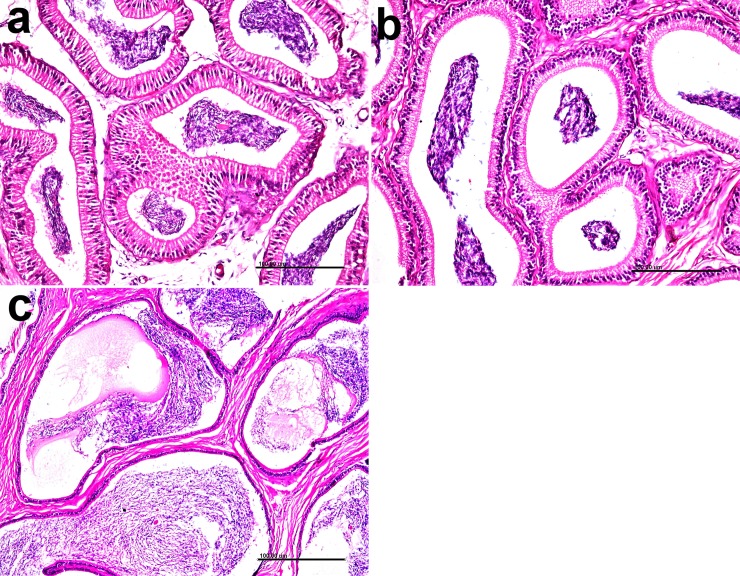
The testis of the CON group had multiple layers of spermatogonia lining the seminiferous tubules (Fig 4A). However, in CLA 0.5% group, there was testicular degeneration, Sertoli cell vacuolations, fewer spermatogonial (germ) cells in seminiferous tubules, and few sperms in the lumen (Fig 4B). The same findings were in the CLA 1% group with increase severity of the lesions with areas of seminiferous tubules that lost germ cells (Fig 4C). In two rabbits of the CLA 1% group, there was a noticeable increase in the interstitial tissue and interstitial (Leydig) cells hyperplasia (Fig 4D) which was confirmed by Masson’s trichrome stain (Fig 4E and 4F).
Fig 4. Effect of long term CLA supplementation on testicular tissue of rabbit.
Normal histological structure in CON group (4a) Vacuolation of Sertoli cells and decreased spermatogenesis in CLA 0.5% group (4b). Absence of spermatogenesis with few spermatogonial cells lining seminiferous tubules (4c). Testicular degeneration and thickening of interstitial tissue (4d) in CLA 1% group. Hematoxylin and eosin stain X 200. Thickening of interstitial tissue due to Leydig cell hyperplasia in the group treated with 1% CLA (4e) and (4f). Masson’s Trichrome X 200.
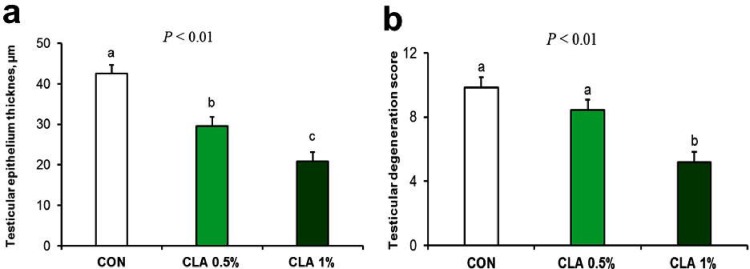
Lesion scoring results of testicular tissue are presented in Fig 5. Testicular epithelial thickness was decreased when CLA dose increased (P < 0.01); however, testicular degeneration lesion scoring was significantly affected (i.e., degeneration was increased) by CLA 1% (P < 0.01).
Fig 5. Effect of long-term CLA supplementation on testicular epithelium thickness and lesion scoring of rabbit testicle “Johnsen's scoring system”.
Long term of dietary CLA supplementation decreased testicular epithelium thickness at a dose dependent manner (5a) but induced testicular degeneration (5b) in CLA 1% group only.
Histopathological examination of the epididymis is shown in Fig 6. The epididymis of the CON (Fig 6A) and CLA 0.5% (Fig 6B) groups had normal histological structure, whereas in CLA 1% group there was cystic dilatation of epididymal tubules and sperm stasis in cauda epididymis (Fig 6C).
Fig 6. Effect of long-term CLA supplementation on rabbit epididymis.
Normal histological structure in CON (6a) and CLA 0.5% (6b) groups, dilation of the epididymal duct with sperm stasis, in the group treated with 1% CLA (6c). Hematoxylin and eosin stain X 200.
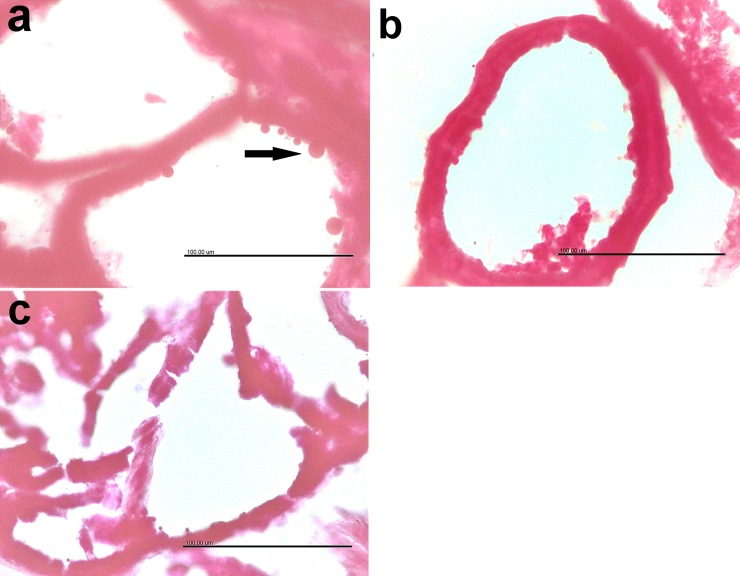
The staining of the epididymis with Sudan black stain is shown in Fig 7. Fat globules were present on the surface of the epithelium of epididymis in the CON group (Fig 7A), whereas they were minute in the CLA 0.5% group (Fig 7B), and almost absent in the CLA 1% group (Fig 7C).
Fig 7. Effect of long-term CLA supplementation on epididymal fat globules “stained with Sudan black”.
Presence of fat globules on the surface epithelium of epididymis in the control group (7a), minute fat globules in CLA 0.5% group (7b), and almost diminished in CLA 1% group (7c). Sudan Black X 400.
Immunohistochemistry findings
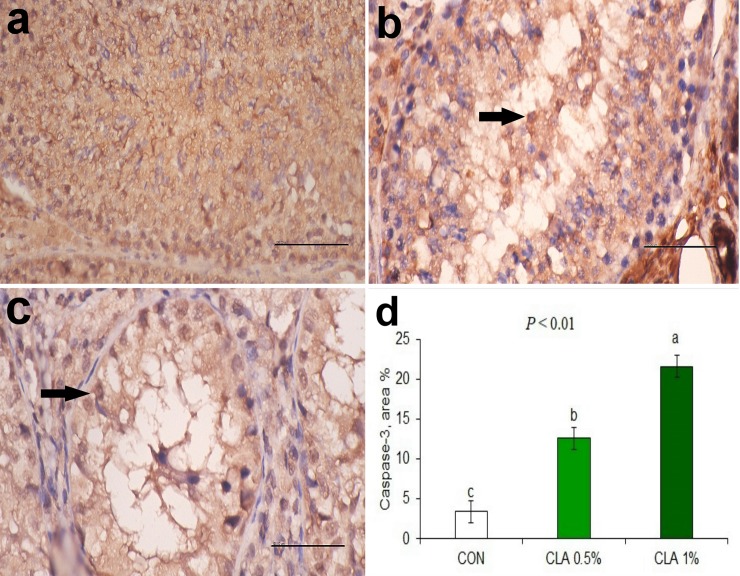
The results of caspase-3 staining of testicular tissue are shown in Fig 8. The testis of the CON group presented a minimal (< 5%) staining for caspase-3 (Fig 8A). On the other hand, brown positively stained spermatogonial cells were observed in the lumen and in the lining epithelium of seminiferous tubules in both CLA 0.5% (Fig 8B) and CLA 1% (Fig 8C) groups in a dose dependent manner. Area of positive caspase-3 cells was significantly increased when CLA dose increased (Fig 8D, P < 0.01).
Fig 8. Effect of long-term CLA supplementation on immune histochemistry of Caspase-3 rabbit testicular tissue.
Negative staining for caspase-3 in the control group (8a). Brown positively stained spermatogonial cells were observed in the lumen and in the lining epithelium of seminiferous tubules in CLA 0.5% (8b), and CLA 1% (8c) groups. Immuno-peroxidase and hematoxylin counterstain X 400. Area percent of Caspase-3 in different groups, long term dietary supplementation of CLA increased area percentage of Caspase-3 in a dose dependent manner (8d).
Transcription of lipid synthesis and apoptosis related genes in testicular tissue and epididymal fat
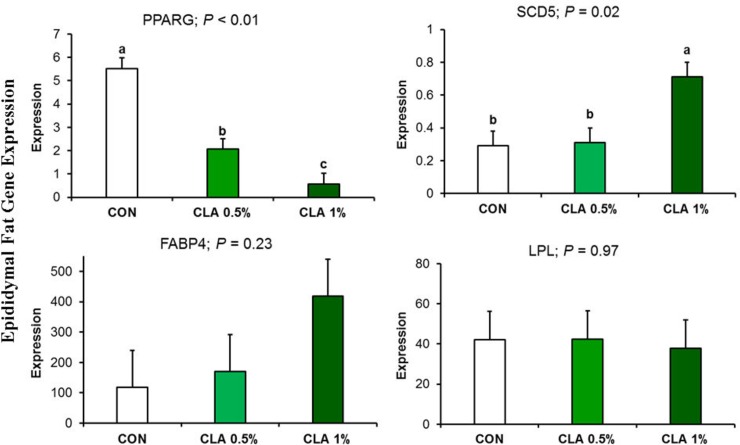
The expression of key lipogenic genes in epididymal fat is shown in Fig 9. The expression of PPARG was downregulated by CLA in a dose dependent manner (P < 0.01). The expression of SCD5 gene was upregulated in CLA 1% but not in CLA 0.5% (P = 0.02).
Fig 9. Epididymal fat gene expression in male rabbits receiving long-term CLA supplementation.
SCD5 gene was upregulated in CLA1% group only, while PPARG was downregulated in a dose dependent manner. FABP and LPL did not significantly different.
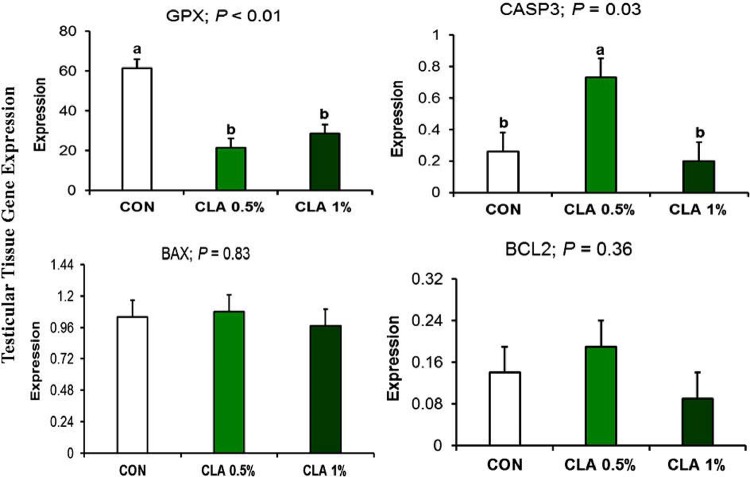
The expression of key apoptosis regulatory genes (CASP3, BAX, and BCL2) and the antioxidant-related gene GPX in the testicular tissue is shown in Fig 10. The expression of the pro-apoptotic CASP3 gene was increased by CLA 0.5% group but not CLA 1% group (P = 0.03). The expression of GPX was downregulated in both CLA 0.5% and CLA 1% fed groups (P < 0.01).
Fig 10. Testicular gene expression in male rabbits fed receiving long-term CLA supplementation.
GPX gene was down regulated in CLA fed male rabbits, however, CASP3 gene coding for protein regulating apoptosis was upregulated in CLA 1% group.
Discussion
Several studies were conducted to investigate the growth promoting [36], immune stimulant [37], anti-inflammatory, and anti-carcinogenic effects of CLA [38]. However, studies investigating the long-term effects of CLA on body weight and other body organs and systems like male reproductive system are very limited [39]. In the current study dietary supplementation of CLA for 26 weeks (from weaning till puberty) slightly increased feed intake and body weight similar to previous finding [40]. However, other studies detected opposite effect of CLA on body weight and feed intake [41] or no effect [36,42]. Additionally, longitudinal studies of CLA supplementation revealed also different effects of CLA on body weight and body weight gain, some researchers detected body weight re-gain after supplementation of CLA [43,44], and other researchers observed body weight loss [45,46]. To the authors knowledge there are no longitudinal studies that investigated the effect of CLA on growth rate of male rabbits; however, CLA supplementation for short period (49 d) did not affect body weight and feed intake in growing rabbits [27,47]. The contrasting effects of CLA on body weight and feed intake in the above studies could be explained by the difference in species and the period of CLA supplementation. Our study, which is the first one where CLA was fed for a long period, indicated a significant positive effect of CLA on both feed intake and body weight; however, the increase, especially of body weight, can be considered minimal and probably not important in front of its negative impact on fertility. Moreover, it was previously reported an association between decreased fertility and increased body weight in male rabbits [48].
The effect of long-term dietary CLA supplementation on male rabbit reproduction was not formerly investigated. Prior studies investigating the effect of CLA on semen parameters either through dietary supplementation [12] or direct application on semen [8] were of short term and, for the most part, conducted after puberty [7]. In the current study, long-term dietary supplementation of CLA did not significantly affect sperm kinetics or morphology. However, CLA at a dose of 1% reduced sperm concentration. The negative effect of CLA 1% on sperm progressive motility as well other motility parameters measured, might be due to changes in epididymal histological structure, fatty acid profile, and gene expression. PUFA are a special fatty acid components of sperm plasma membrane [49], they easily undergo lipoperoxidation; thus, presence of powerful antioxidant system to protect them from lipoperoxidative damage is crucial.
Our data suggest that feeding 1% CLA negatively impacted sperm nutrition by reducing epididymal fat PUFA portion and disappearance of fat globules in the epididymis. The reduced PUFA might be consequence also of a higher oxidative status with lower antioxidant capacity, as indicated by the lower vitamin E, L-carnitine, and GPX gene expression and by the higher accumulation of MDA in testicular tissue [49–51]. The increased MDA and downregulation of testicular GPX transcript in our study was similar to prior findings [52]. The effect of CLA on regulation of tocopherol is not clear. In short term studies CLA increased liver and adipose tissue tocopherol [52]. However, longer supplementation of CLA during pregnancy and lactation reduced liver tocopherol by 24% in rat dams and increased it in their adipose tissue [53].
The upregulation of SCD5 in the current study was associated with a decreased PUFA. This finding appears contradictory; however, it has been reported that PUFA inhibit SCD activity and transcription [54,55] The downregulation of PPARG in this study is similar to prior studies [56,57] and might explain the overall decrease of total epididymal fatty acid, similar to Hue et al., [58].
Supplementation of 1% CLA for 26 weeks tended to reduce the level of arachidonic acid in epididymal fat pad, similar to a prior study [59]. Arachidonic acid is essential for the synthesis of prostaglandin D2, known to inhibit cyclooxygenase-2-induced apoptosis [49].
Leydig cells hyperplasia was also observed in the testicular tissue of group treated with 1% CLA which could be due to hormonal imbalance [60]. A study that was conducted to investigate the effect of CLA on the pituitary-testes axis in rams showed an increased concentration of luteinizing hormone (LH) by CLA [61]; the depletion of LH was associated with Leydig cell atrophy in adult rats [62].
In the present study, the caspase-3 was positively stained in spermatogonial cells of the seminiferous tubules in the groups treated with CLA which demonstrated a dose-related increase in apoptosis [63]. However, on the transcriptional level the transcription of CASP3 was upregulated only in CLA 0.5% but not in CLA 1%. L-carnitine is known to inhibit the activity of caspases [64]. Thus, the higher level of L-carnitine in CLA 0.5% compared to CLA 1% might explain the higher apoptosis in CLA 1% despite higher CASP3 expression in CLA 0.5%.
In former reports applying different doses of CLA isomers mixture exerted different effects on several body metabolites [65–67], that could be explained by tissue affinity of CLA, whereas, low dose of CLA affected only retroperitoneal fat depot, while the higher dose affected both retroperitoneal and epididymal fat in mice model [67].
To our knowledge, there are no former studies that showed a positive effect of CLA on apoptosis in the testis although it has been recorded in adipose tissue [68]. Whether the CLA exerts its effect on the testes directly or indirectly is unknown. However, it could be suggested that CLA mediate theirs effect on the testis by affecting the fatty acid composition of epididymal fat with consequent reduction of spermatogenesis [69] and affecting the synthesis of hormones involved in reproduction by disturbing the pituitary-testis axis [70].
Study limitation
The rabbit number (n = 4/group) used in this study was similar to prior studies conducted on rabbits [71–77]. Despite the proper selection of the statistical model, proper sampling and technical replicates analyzed, the low number of animals per group is considered a limitation of the present study.
Conclusion
Our data suggest that long-term supplementation of CLA is not beneficial to male rabbits, especially if fed with a dose larger than 0.5%. The growth promoting effect might be of interest, but the magnitude does not seem to balance the negative effects on fertility.
Acknowledgments
The authors would like to acknowledge Dr. Mohammed Said Amer for helping with anesthesia and providing the anesthetic medications.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This study was partially funded by Faculty of Veterinary Medicine, Cairo University, Research band #3 to AMA, http://vet.cu.edu.eg/Home. No other external or internal organization have funded this work. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chen PB and Park Y. Conjugated Linoleic Acid in Human Health: Effects on Weight Control In Nutrition in the Prevention and Treatment of Abdominal Obesity. Academic Press; 2019; 355–382. [Google Scholar]
- 2.Mitchell PL and McLeod RS. Conjugated linoleic acid and atherosclerosis: studies in animal models. Biochemistry and Cell Biology. 2008; 86 (4), pp. 293–301. 10.1139/o08-070 [DOI] [PubMed] [Google Scholar]
- 3.Viladomiu M, Hontecillas R, and Bassaganya-Riera, J. Modulation of inflammation and immunity by dietary conjugated linoleic acid. European Journal of Pharmacology. 2016; 785, pp. 87–95. 10.1016/j.ejphar.2015.03.095 [DOI] [PubMed] [Google Scholar]
- 4.Wood JD. Meat composition and nutritional value In Lawrie´s Meat Science. 2017; January 1 (pp. 635–659). Woodhead Publishing. [Google Scholar]
- 5.Matin S, Nemati A, Ghobadi H, Alipanah-Moghadam R, and Rezagholizadeh L. The effect of conjugated linoleic acid on oxidative stress and matrix metalloproteinases 2 and 9 in patients with COPD. International Journal of Chronic Obstructive Pulmonary Disease. 2018; 13: p. 1449–1459. 10.2147/COPD.S155985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onakpoya IJ, Posadzki PP, Watson LK, Davies LA, and Ernst E. The efficacy of long-term conjugated linoleic acid (CLA) supplementation on body composition in overweight and obese individuals: a systematic review and meta-analysis of randomized clinical trials. European Journal of Nutrition. 2012; 51(2), pp. 127–134. 10.1007/s00394-011-0253-9 [DOI] [PubMed] [Google Scholar]
- 7.Karimi R, Towhidi A, Zeinoaldini S, Rezayazdi K, Mousavi M, Safari H et al. Effects of supplemental conjugated linoleic acids (CLA) on fresh and post‐thaw sperm quality of Holstein bulls. Reproduction in Domestic Animals. 2017; 52(3), pp. 459–467. 10.1111/rda.12932 [DOI] [PubMed] [Google Scholar]
- 8.Teixeira SMP, Chaveiro AEN, and da Silva JFM. Effect of trans-10, cis-12 isomer of conjugated linoleic acid on boar semen quality after cryopreservation. Animal Reproduction. 2017; 14(2), pp. 400–405. [Google Scholar]
- 9.De Veth MJ, Bauman DE, Koch W, Mann GE, Pfeiffer AM and Butler WR. Efficacy of conjugated linoleic acid for improving reproduction: A multi-study analysis in early-lactation dairy cows. Journal of Dairy Science. 2009; 92(6), pp. 2662–2669. 10.3168/jds.2008-1845 [DOI] [PubMed] [Google Scholar]
- 10.Zamora-Zamora V, Figueroa-Velasco JL, Cordero-Mora JL, Nieto-Aquino R, García-Contreras ADC, Sánchez-Torres MT. Conjugated linoleic acid supplementation does not improve boar semen quality and does not change its fatty acid profile. Veterinaria México. 2017; 4(3), pp. 1–15. [Google Scholar]
- 11.Khodaei H, Chamani M, Sadeghi A, and Hejazi H. Effects of Conjugated Linoleic Acid (CLA) on hormones and factors involved in murine ovulation. Journal of Reproduction & Infertility. 2009; 10(2), pp. 101–108. [Google Scholar]
- 12.Aydin R, Karaman M, Toprak HHC, Ozugur AK, Aydin D, and Cicek T. The effect of long-term feeding of conjugated linoleic acid on fertility in Japanese quail. South African Journal of Animal Science. 2006; 36(2), pp. 99–104. [Google Scholar]
- 13.Ali TM. A manual for the primary animal health care worker. Food and Agriculture Organization (FAO) 1994. [Google Scholar]
- 14.Gliozzi TM, Zaniboni L, Maldjian A, Luzi F, Maertens L, and Cerolini S. Quality and lipid composition of spermatozoa in rabbits fed DHA and vitamin E rich diets. Theriogenology. 2009; 71(6), pp. 910–919. 10.1016/j.theriogenology.2008.10.022 [DOI] [PubMed] [Google Scholar]
- 15.National Research Council. Nutrient Requirements of Rabbits: 1977. National Academies Press; 1977. February 1. [Google Scholar]
- 16.Garcia MR, Amstalden M, Morrison CD, Keisler, DH, and Williams GL. Age at puberty, total fat and conjugated linoleic acid content of carcass, and circulating metabolic hormones in beef heifers fed a diet high in linoleic acid beginning at four months of age. J. Anim. Sci. 2003; 81(1), 261–268. 10.2527/2003.811261x [DOI] [PubMed] [Google Scholar]
- 17.AOAC. Official methods of analysis. 17th ed, Vols. 1 and 2. Gaithersburg, MD: Association of the Official Analytical Chemists, 2000. [Google Scholar]
- 18.Macari M, and Machado CR. Sexual maturity in rabbits defined by the physical and chemical characteristics of the semen. Laboratory Animals. 1978; 12: 37–39. 10.1258/002367778780953305 [DOI] [PubMed] [Google Scholar]
- 19.Boulbina I, Ain-Baziz H, Ilès I, Zenia S, Belabbas R, Temim S. Effect of birth season on onset of puberty and semen characteristics in male rabbit of Algerian population (Oryctolagus cuniculus). World Rabbit Science Association Proceedings 10 th World Rabbit Congress. 2012; 335–339.
- 20.Viudes-de-Castro MP, Lavara R, Safaa HM, Marco-Jiménez F, Mehaisen GMK, and Vicente JS. Effect of freezing extender composition and male line on semen traits and reproductive performance in rabbits. Animal. 2014; 8(5), pp. 765–770. 10.1017/S1751731114000135 [DOI] [PubMed] [Google Scholar]
- 21.Perumal P. Effect of superoxide dismutase on semen parameters and antioxidant enzyme activities of liquid stored (5 C) Mithun (Bos frontalis) semen. Journal of Animals. 2014; 1–9. 10.3390/ani5010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simón L, Funes AK, Yapur MA, Cabrillana ME, Monclus MA, Boarelli PV, et al. Manchette-acrosome disorders during spermiogenesis and low efficiency of seminiferous tubules in hypercholesterolemic rabbit model. PloS one. 2017; 12(2):e0172994 10.1371/journal.pone.0172994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ijaz A, Javed I, Aslam B, Khan JA, Khaliq T, Khan MZ, et al. Nephroprotective and antioxidant effects of Moringa Oleifera (Sohanjna) in paracetamol induced nephrotoxic albino rabbits. Pak. Vet. J. 2016; 36(3): 292–296. [Google Scholar]
- 24.Ahmed-Farid OA, Nasr M, Ahmed RF, and Bakeer RM. Beneficial effects of curcumin nano-emulsion on spermatogenesis and reproductive performance in male rats under protein deficient diet model: enhancement of sperm motility, conservancy of testicular tissue integrity, cell energy and seminal plasma amino acids content. Journal of Biomedical Science. 2017; 24(1), p. 66 10.1186/s12929-017-0373-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ling B, Aziz C, and Alcorn J. Systematic evaluation of key L-Carnitine homeostasis mechanisms during postnatal development in rat. Nutrition & Metabolism. 2012; 1:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMurray CH, Blanchflower WJ, and Rice DA. Influence of extraction techniques on determination of alpha tocopherol in animal feedstuffs. Journal-Association of Official Analytical Chemists. 1980; 63(6), pp. 1258–1261. [PubMed] [Google Scholar]
- 27.Abdelatty AM, Mohamed SA, Moustafa MMA, Al-Mokaddem AK, Baker MR, Elolimy AA, et al. Nutrigenomic effect of conjugated linoleic acid on growth and meat quality indices of growing rabbit. PLoS ONE. 2019; 14(10): e0222404 10.1371/journal.pone.0222404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'fallon JV, Busboom JR, Nelson ML, and Gaskins CT. A direct method for fatty acid methyl ester synthesis: application to wet meat tissues, oils, and feedstuffs. J. Anim. Sci. 2007; 85(6), pp. 1511–1521. 10.2527/jas.2006-491 [DOI] [PubMed] [Google Scholar]
- 29.Sirri F, Castellini C, Roncarati A, Franchini A, and Meluzzi A. Effect of feeding and genotype on the lipid profile of organic chicken meat. European Journal of Lipid Science and Technology. 2010; 112(9), pp. 994–1002. [Google Scholar]
- 30.Aslan Koşar P, Tuncer H, Cihangir Uğuz A, Espino Palma J, Darıcı H, Onaran İ, Çiğ B, et al. The efficiency of Poly (ADP‐Ribose) Polymerase (PARP) cleavage on detection of apoptosis in an experimental model of testicular torsion. International Journal of Experimental Pathology. 2015. 96(5), pp. 294–300. 10.1111/iep.12137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Y, Wolcott RD, and Dowd SE. Tag-encoded FLX amplicon pyrosequencing for the elucidation of microbial and functional gene diversity in any environment In High-Throughput Next Generation Sequencing. 2011. pp. 129–141. Humana Press, Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- 32.Norollahi SA, Kokhaee P, Rashidy-Pour A, Hojati V, Norollahi SE, Larijani LV, and Samadan AA. Comparison of Methods of RNA Extraction From Breast and Gastric Cancer Tissues. Crescent J. Med. Biol. Sci. 2018; 5(1), pp. 25–28. [Google Scholar]
- 33.Bionaz M and Loor JJ. Identification of reference genes for quantitative real-time PCR in the bovine mammary gland during the lactation cycle. Physiological Genomics. 2007; 29(3), 312–319. 10.1152/physiolgenomics.00223.2006 [DOI] [PubMed] [Google Scholar]
- 34.Andersen CL, Jensen JL, and Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Research. 2004; 64(15), pp. 5245–5250. 10.1158/0008-5472.CAN-04-0496 [DOI] [PubMed] [Google Scholar]
- 35.Wiegand BR, Sparks JC, Parrish FC, and Zimmerman, DR. Duration of feeding conjugated linoleic acid influences growth performance, carcass traits, and meat quality of finishing barrows. Journal of Animal Science. 2002; 80 (3), pp. 637–643. 10.2527/2002.803637x [DOI] [PubMed] [Google Scholar]
- 36.Moraes ML, Ribeiro AML, Santin E, and Klasing KC. Effects of conjugated linoleic acid and lutein on the growth performance and immune response of broiler chickens. Poultry Science. 2015; 95(2), pp. 237–246. 10.3382/ps/pev325 [DOI] [PubMed] [Google Scholar]
- 37.Koronowicz AA, and Banks P. Antitumor properties of CLA-enriched food products. Nutrition and Cancer. 2018; 70(4), pp. 529–545. 10.1080/01635581.2018.1460684 [DOI] [PubMed] [Google Scholar]
- 38.Onankpoya I, Posadzki P, Watson L, Davies L, and Ernst E. Efficacy of long-term conjugated linoleic acid (CLA) supplementation in the management of overweight and obesity: a systematic review and meta-analysis of randomized clinical trials. European Journal of Nutrition. 2011; 51(2), 127–134. 10.1007/s00394-011-0253-9 [DOI] [PubMed] [Google Scholar]
- 39.Lauridsen C, Mu H, and Henckel P. Influence of dietary conjugated linoleic acid (CLA) and age at slaughtering on performance, slaughter-and meat quality, lipoproteins, and tissue deposition of CLA in barrows. Meat Science. 2005; 69(3), pp. 393–399. 10.1016/j.meatsci.2004.08.009 [DOI] [PubMed] [Google Scholar]
- 40.Szymczyk B, Pisulewski P, Szczurek W, and Hanczakowski P, The effects of feeding conjugated linoleic acid (CLA) on rat growth performance, serum lipoproteins and subsequent lipid composition of selected rat tissues. Journal of the Science of Food and Agriculture. 2000; 80(10), pp. 1553–1558. [Google Scholar]
- 41.Lindsey CE. Influence of conjugated linoleic acid supplementation on body composition of weaned pigs. Ph.D. thesis, Sam Houston State University. 2017. Available from: https://shsu-ir.tdl.org/handle/20.500.11875/2188
- 42.Liu H, Wu F, Bai LL, Chen YF, Lai CH, Ren LQ et al. Effect of dietary conjugated linoleic acid supplementation during late gestation on colostrum yield, fatty acid composition, and IgG concentrations in primiparous sows. Canadian Journal of Animal Science. 2018; 98(4), pp. 732–740. [Google Scholar]
- 43.Larsen TM, Toubro S, Gudmundsen O, and Astrup A. Conjugated linoleic acid supplementation for 1 y does not prevent weight or body fat regain. The American Journal of Clinical Nutrition. 2006; 83(3), pp. 606–612. 10.1093/ajcn.83.3.606 [DOI] [PubMed] [Google Scholar]
- 44.Racine NM, Watras AC, Carrel AL, Allen DB, McVean JJ, Clark RR. Effect of conjugated linoleic acid on body fat accretion in overweight or obese children. The American Journal of Clinical Nutrition. 2010; 91(5), pp. 1157–1164. 10.3945/ajcn.2009.28404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaullier JM, Halse J, Høye K, Kristiansen K, Fagertun H, Vik H, et al. Conjugated linoleic acid supplementation for 1 y reduces body fat mass in healthy overweight humans. The American Journal of Clinical Nutrition. 2004; 79(6), pp. 1118–1125. 10.1093/ajcn/79.6.1118 [DOI] [PubMed] [Google Scholar]
- 46.Gaullier JM, Halse J, Høivik HO, Høye K, Syvertsen C, Nurminiemi Met al. Six months supplementation with conjugated linoleic acid induces regional-specific fat mass decreases in overweight and obese. British Journal of Nutrition. 2007; 97(3), pp. 550–560. 10.1017/S0007114507381324 [DOI] [PubMed] [Google Scholar]
- 47.Corino C, Mourot J, Magni S, Pastorelli G, and Rosi F. Influence of dietary conjugated linoleic acid on growth, meat quality, lipogenesis, plasma leptin and physiological variables of lipid metabolism in rabbits. Journal of Animal Science. 2002; 80(4), pp. 1020–1028. 10.2527/2002.8041020x [DOI] [PubMed] [Google Scholar]
- 48.Vernet P, Aitken RJ, and Drevet JR. Antioxidant strategies in the epididymis. Molecular and Cellular Endocrinology. 2004; 216(1–2), pp. 31–39. 10.1016/j.mce.2003.10.069 [DOI] [PubMed] [Google Scholar]
- 49.Marco-Jiménez F, Vicente JS. Overweight in young males reduce fertility in rabbit model. PLoS ONE. 2017; 12(7): e0180679 10.1371/journal.pone.0180679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abd-Allah AR, Helal GK, Al-Yahya AA, Aleisa AM, Al-Rejaie SS, et al. Pro-inflammatory and oxidative stress pathways which compromise sperm motility and survival may be altered by L-carnitine. Oxidative Medicine and Cellular Longevity. 2009; 2(2), pp. 73–81. 10.4161/oxim.2.2.8177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castellini C, Lattaioli P, Dal Bosco A, Minelli A, and Mugnai C. Oxidative status and semen characteristics of rabbit buck as affected by dietary. Reproduction Nutrition Development. 2003; 43(1), pp. 91–103. [DOI] [PubMed] [Google Scholar]
- 52.Chen WH, Li YJ, Wang MS, Kang ZC, Huang HL, and Shaw HM. Elevation of tissue α-tocopherol levels by conjugated linoleic acid in C57BL/6J mice is not associated with changes in vitamin E absorption or α-carboxyethyl hydroxychroman production. Nutrition. 2012; 28(1), pp. 59–66. 10.1016/j.nut.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 53.Zeitz JO, Most E, and Eder K. Conjugated linoleic acid influences the metabolism of tocopherol in lactating rats but has little effect on tissue tocopherol concentrations in pups. Lipids in Health and Disease. 2016; 15(1), p. 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sessler AM, Kaur N, Palta JP, and Ntambi JM. Regulation of stearoyl-CoA desaturase 1 mRNA stability by polyunsaturated fatty acids in 3T3-L1 adipocytes. Journal of Biological Chemistry. 1996; 271(47), pp. 29854–29858. 10.1074/jbc.271.47.29854 [DOI] [PubMed] [Google Scholar]
- 55.Ntambi JM. Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. Journal of Lipid Research. 1999; 40(9), pp. 1549–1558. [PubMed] [Google Scholar]
- 56.Brown JM, Boysen MS, Jensen SS, Morrison RF, Storkson J, Lea Currie R, et al. Isomer-specific regulation of metabolism and PPARγ signaling by CLA in human preadipocytes. Journal of Lipid Research. 2003; 44(7), pp. 1287–1300. 10.1194/jlr.M300001-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramiah SK, Meng GY, Sheau Wei T, Swee Keong Y, and Ebrahimi M. Dietary conjugated linoleic acid supplementation leads to downregulation of PPAR transcription in broiler chickens and reduction of adipocyte cellularity. PPAR Research, 2014; 1–10. 10.1155/2014/137652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hue JJ, Lee KN, Jeong JH, Lee SH, Lee YH, Jeong SW, Nam SY, Yun YW and Lee BJ. Anti-obesity activity of diglyceride containing conjugated linoleic acid in C57BL/6J ob/ob mice. Journal of Veterinary Science. 2009; 10(3), pp. 189–195. 10.4142/jvs.2009.10.3.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu KL and Belury MA. Conjugated linoleic acid reduces arachidonic acid content and PGE2 synthesis in murine keratinocytes. Cancer Letters.1998; 127(1–2), pp. 15–22. 10.1016/s0304-3835(97)00479-5 [DOI] [PubMed] [Google Scholar]
- 60.Creasy D, Bube A, Rijk ED, Kandori H, Kuwahara M, Masson R. Proliferative and non-proliferative lesions of the rat and mouse male reproductive system. Toxicol. Pathol. 2012; 40:40S–121S; 10.1177/0192623312454337 [DOI] [PubMed] [Google Scholar]
- 61.Mahdavi R, Faramarzi E, Mohammad-Zadeh M, Nasirimotlagh B, and Ghaemmaghami SJ. Effects of conjugated linoleic acid supplementation on nutritional status, symptoms of eating problems and dietary intake in rectal cancer patients undergoing chemoradiotherapy. Current Topics in Nutraceutical Research. 2013; 11(1–2), pp. 15–21. [Google Scholar]
- 62.Dym M and Madhwa Raj HG. Response of adult rat sertoli cells and leydig cells to depletion of luteinizing hormone and testosterone. Biology of Reproduction. 1977; 17(5), pp. 676–696. 10.1095/biolreprod17.5.676 [DOI] [PubMed] [Google Scholar]
- 63.Li J and Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008; 27(48), p. 6194 10.1038/onc.2008.297 [DOI] [PubMed] [Google Scholar]
- 64.Mutomba MC, Yuan H, Konyavko M, Adachi S, Yokoyama CB, Esser V, et al. Regulation of the activity of caspases by L‐carnitine and palmitoyl carnitine. FEBS Letters. 2000; 478(1–2), pp. 19–25. 10.1016/s0014-5793(00)01817-2 [DOI] [PubMed] [Google Scholar]
- 65.Miner JL, Cederberg CA, Nielsen MK, Chen X, and Baile CA. Conjugated linoleic acid (CLA), body fat, and apoptosis. Obesity Research. 2001; 9(2), pp. 129–134. 10.1038/oby.2001.16 [DOI] [PubMed] [Google Scholar]
- 66.Nazari M, Karandish M, Jalali MT, and Saberi AH. Weight management effects of a mixture of conjugated linoleic acid and L-carnitine in diet induced obese rats. British Journal of Medicine and Medical Research. 2016; 12(1): 1–8. 10.9734/BJMMR/2016/21609 [DOI] [Google Scholar]
- 67.Bezan P, Holland H, de Castro G, Cardoso J, Ovidio P, Calder P, et al. High dose of a conjugated linoleic acid mixture increases insulin resistance in rats fed either a low fat or a high fat diet. Exp. Clin. Endocrinol. Diabetes. 2018; 126, 379–386. 10.1055/s-0043-118348 [DOI] [PubMed] [Google Scholar]
- 68.Parra P, Palou A, and Serra F. Moderate doses of conjugated linoleic acid reduce fat gain, maintain insulin sensitivity without impairing inflammatory adipose tissue status in mice fed a high-fat diet. Nutrition & Metabolism. 2010; 7(1), 5 10.1186/1743-7075-7-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Srinivasan V, Thombre DP, Lakshmanan S, and Chakrabarty AS. Effect of removal of epididymal fat on spermatogenesis in albino rats. Indian Journal of Experimental Biology.1986; 24(8), pp. 487–488. [PubMed] [Google Scholar]
- 70.Modaresi M, Mansouri A, and Khodaei HR. The effect of conjugated linoleic acid on male reproductive hormones in mice. Advance Journal of Food Science & Technology. 2013; 5(12), pp. 1618–1620. [Google Scholar]
- 71.Baartscheer A, Schumacher CA, Wüst RCI, Fiolet JWT, Stienen GJM, Coronel R, et al. Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia. 2016; 60(3), 568–573. 10.1007/s00125-016-4134-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blay RM, Adjenti SK, Adutwum-Ofosu KK, Hottor BA, Ahenkorah J, Arko-Boham B, et al. Natural cocoa inhibits maternal hypercholesterolaemia-induced atherogenesis in rabbit pups. Cardiovascular Journal of Africa. 2019; 30(4): 208–215. 10.5830/CVJA-2019-019 [DOI] [PubMed] [Google Scholar]
- 73.Sun X, Shen J, Liu C, Li S, Peng Y, Chen C et al. L-arginine and N-carbamoyl glutamic acid supplementation enhance young rabbit growth and immunity by regulating intestinal microbial community. Asian-Australas J. Anim. Sci. 2019. Available from: https://www.ajas.info/journal/view.php? 10.5713/ajas.18.0984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Werner RA, Kobayashi R, Javadi MS, Köck Z, Wakabayashi H, Unterecker S. et al. Impact of novel antidepressants on cardiac 123i-metaiodobenzylguanidine uptake: experimental studies on SK-N-SH cells and healthy rabbits. Journal of Nuclear Medicine, 59(7), 1099–1103. 10.2967/jnumed.117.206045 [DOI] [PubMed] [Google Scholar]
- 75.Levingstone TJ, Thompson E, Matsiko A, Schepens A, Gleeson JP, O’Brien FJ. Multi-layered collagen-based scaffolds for osteochondral defect repair in rabbits. Acta Biomaterialia. 2016; 32, 149–160. 10.1016/j.actbio.2015.12.034 [DOI] [PubMed] [Google Scholar]
- 76.Torrado J, Cain C, Mauro AG, Romeo F, Ockaili R, Chau VQ, et al. Sacubitril/valsartan averts adverse post-infarction ventricular remodeling and preserves systolic function in rabbits. Journal of the American College of Cardiology. 2018; 72(19), 2342–2356. 10.1016/j.jacc.2018.07.102 [DOI] [PubMed] [Google Scholar]
- 77.Ma H, Zheng L, Liu Y, Zhao C, Harrison TJ, Ma Y, et al. Experimental infection of rabbits with rabbit and genotypes 1 and 4 hepatitis E viruses. PLoS ONE.2010; 5(2): e9160 10.1371/journal.pone.0009160 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.