Abstract
Background
Non-ST elevation acute coronary syndrome (NSTEACS) occurs more frequently in older patients with an increased occurrence of recurrent cardiac events following the index presentation. Telomeres are structures consisting of repeated DNA sequences as associated shelterin proteins at the ends of chromosomes. We aim to determine whether telomere length (TL) and telomerase activity (TA) predicted poor outcomes in older patients presenting with NSTEACS undergoing invasive care.
Method
Older patients undergoing invasive management for NSTEACS were recruited to the ICON-1 biomarker study (NCT01933581). Peripheral blood mononuclear cells (PBMC) were recovered on 153 patients. DNA was isolated and mean TL was measured by quantitative PCR expressed as relative T (telomere repeat copy number) to S (single copy gene number) ratio (T/S ratio), and a telomere repeat amplification assay was used to assess TA during index presentation with NSTEACS. Primary clinical outcomes consisted of death, myocardial infarction (MI), unplanned revascularisation, stroke and significant bleeding recorded at 1 year. TL and TA were divided into tertile groups for analysis. Cox proportional hazards regression was performed. Ordinal regression was performed to evaluate the relationship between TL and TA and traditional cardiovascular risk factors at baseline.
Results
298 patients were recruited in the ICON-1 study of which 153 had PBMC recovered. The mean age was 81.0 ± 4.0 years (64% male). Mean telomere length T/S ratio was 0.47 ± 0.25 and mean TA was 1.52 ± 0.61 units. The primary composite outcome occurred in 44 (28.8%) patients. There was no association between short TL or low TA and incidence of the primary composite outcome (Hazard Ratio [HR] 1.50, 95% Confidence Interval [CI] 0.68–3.34, p = 0.32 and HR 1.33, 95% CI 0.52–3.36, p = 0.51 respectively).
Conclusion
TL and TA are not found to be associated with the incidence of adverse outcomes in older patients presenting with NSTEACS undergoing invasive care.
Clinical trial registration
URL: https://www.clinicaltrials.gov Unique identifier: NCT01933581
Introduction
Older age is a well-known cardiovascular disease (CVD) risk factor, especially for coronary artery disease (CAD)[1–3]. In a rapidly progressing ageing population, CAD prevalence, and the related detrimental consequences, can only be expected to increase. Non ST-elevation acute coronary syndromes (NSTEACS) are more common within the older population, with the UK’s Myocardial Ischaemia National Audit Project (MINAP) data showing that 46% of all non ST elevation myocardial infarction (NSTEMIs) suffered between 2006 and 2010 occurred in patients aged ≥75 years old[4].
Telomeres are structures of tandemly repeated hexanucleotide TTAGGG sequences associated with specific shelterin proteins at the end of eukaryotic chromosomes. They protect internal chromosomal regions of DNA from degradation during cell division and gradually shorten with each cycle due to the end replication problem as well as the sensitivity to oxidative stress[5]. At a certain point the telomeres become too short to facilitate cell division, resulting in cell senescence or apoptosis. Telomere length (TL) and telomerase activity (TA) have been investigated regarding their possible applicability as biomarkers for age-related chronic diseases, including CVD. Shorter TL has also been linked to an increased risk of adverse events in patients with pre-existing CAD[6]. These studies have mostly been restricted to younger patients, resulting in a paucity of research investigating this relationship in older patients. Therefore we sought to investigate the association of TL and TA with adverse outcomes in older patients presenting with NSTEACS undergoing invasive management.
Methods
Study design
The Improve Cardiovascular Outcomes in high-risk older patients with acute coronary syndrome (ICON1) study is a multicentre prospective cohort study which aimed to develop a risk score for high-risk older adults, the FRAIL-HEART score.[7–9] The study protocol has been published previously[10]. The current study is a planned study as outlined in the the ICON1 study protocol[10]. Older patients (aged ≥ 65 years old) presenting with NSTEACS with planned invasive management were recruited from two tertiary cardiac care hospitals. The study was carried out in accordance with the Declaration of Helsinki, and approved by the regional ethics committee (NRES Committee North East–Sunderland 12/NE/0160). Participants gave written informed consent to take part in the study. The a priori primary outcome was created from data on death, acute coronary syndrome (ACS), unplanned revascularisation, stroke and BARC (Bleeding Academic Research Consortium)-defined bleeding (type 2 or greater) at 1 year[11]. Follow-up was carried out in research clinics, and for patients unable to attend telephone consultations were carried out. In participants where more than one component of the composite outcome occurred, time-to-first-event was used. Detailed information on baseline assessment had been previously published.[10] Frailty was assessed using Fried Frailty Index, derived from the Cardiovascular Health Study with a score of 0 classed as robust, 1–2 as pre-frail and ≥3 as frail.[12]
Telomere and telomerase analysis
Peripheral blood mononuclear cells were isolated using leucosep tubes, and DNA extraction performed using QIAamp® DNA mini kit (Qiagen). DNA concentration and quality was ascertained using 260/280 nm spectrophotometry (NanoDropTM). Samples were diluted to 10 ng/ul prior to TL assay. Analysis of mean TL was performed using a quantitative, real-time polymerase chain reaction-based assay, in quadruplicate using a 7900 HT Fast Real Time PCR system (Applied Biosciences™). Comparison between signals from T (telomere repeat copy number) to S (single copy gene number) were made, with the relative T/S ratio calculated. The intra-assay coefficient of variation was 2.7%, and the inter-assay coefficient of variation was 5.1%. Telomerase activity was assessed using a telomere repeat amplification assay (TeloTAGGG™; Roche, Switzerland)[13] using 500ng lysate from PBMCs isolated in the same way as above.
Statistical analysis
Statistical analyses were conducted with the use of SPSS software version 22 (SPSS Inc., Chicago, IL, USA) and a two-tailed-test p value <0.05 was considered statistically significant. Those variables classed as normally distributed were reported as mean ± standard deviation (SD). Variables that were non-normally distributed were reported as median [interquartile range (IQR)]. For TL analysis, data were split into three equal tertiles with patients categorized into long TL (LTL, ratio ≥0.5), medium TL (MTL, ratio 0.3468–0.5), and short TL (STL, ratio ≤ 0.3467). TA data were also split into three equal tertiles for analysis: high TA (≥ 1.86 units), mid TA (1.32–1.86 units), and low TA (≤ 1.31 units).
Cox proportional hazard models were used to model the risk of incidence of the primary composite end-point by tertile of TL and TA. Differences in baseline characteristics were assessed between tertiles with one-way ANOVA for normally distributed continuous data, Kruskal-Wallis testing for non-normally distributed continuous data, and chi-squared test (χ2) or Fisher exact test (as appropriate with expected cell counts of <5) for categorical variables. Baseline characteristics did not vary between tertiles of TL or TA (see Table 1) therefore univariate cox proportional hazard models were used. In addition, adjusted Cox proportional hazards regression models were also used, adjusting for age, gender and frailty. Kaplan-Meier survival analysis was performed to compare event-free survival from incidence of the primary composite outcome between tertiles of TL and TA, probed with the Log-rank test. TL and TA was also assessed as continuous variables (S1–S3 Figs). The mean TL and TA subjects with composite events were compared to subjects without composite events with Wilcoxon rank sum test. In addition TL and TA was also classified into two groups based on receiver operating characteristic curve to determine the best cut-off. Details can be found in S1 Table.
Table 1. Baseline characteristics.
| Total (N = 135) | LTL (N = 43) |
MTL (N = 47) |
STL (N = 45) |
p-value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years (SD) | 80.8 (4.1) | 80.2 (3.4) | 80.7 (4.4) | 81.6 (3.9) | 0.39 |
| Female, n (%) | 47 (34.8) | 20 (46.5) | 12 (25.5) | 15 (33.3) | 0.49 |
| NSTEMI, n (%) | 246 (82.0) | 31 (72.1) | 39 (83.0) | 36 (80.0) | 0.43 |
| UA, n (%) | 54 (18.0) | 12 (27.9) | 8 (17.0) | 9 (20.0) | 0.44 |
| Clinical Measures | |||||
| BMI, kg m-2 (SD) | 26.7 (4.2) | 26.7 (4.7) | 26.7 (3.9) | 26.7 (4.5) | 0.95 |
| Systolic BP, mmHg (SD) | 145.2 (26.5) | 147.1 (19.9) | 145.5 (26.5) | 142.8 (32.3) | 0.80 |
| NYHA functional class, n (%): | |||||
| 1—no limitation of activity | 51 (38.1) | 16 (38.1) | 21 (44.7) | 14 (31.1) | 0.18 |
| 2—slight limitation of activity | 50 (37.3) | 18 (42.9) | 15 (31.9) | 17 (37.8) | |
| 3—marked limitation of activity | 32 (23.9) | 3 (9.1) | 21(31.3) | 8 (23.5) | |
| 4—unable to carry out activity | 1 (0.7) | 1 (2.4) | 0 (0.0) | 0 (0.0) | |
| GRACE Score, points (SD) | 130.2 (18.5) | 130.2 (17.1) | 129.8 (18.5) | 130.7 (20.2) | 0.97 |
| Medical History | |||||
| Diabetes, n (%) | 30 (22.2) | 13 (30.2) | 7 (14.9) | 10 (22.2) | 0.64 |
| Hypertension, n (%) | 92 (68.1) | 25 (58.1) | 35 (74.5) | 32 (71.1) | 0.30 |
| Hyperlipidaemia, n (%) | 69 (51.1) | 23 (53.5) | 25 (53.2) | 21 (46.7) | 0.53 |
| Renal impairment, n (%) | 33 (24.4) | 9 (20.9) | 15 (31.9) | 9 (20.0) | 0.39 |
| Previous MI, n (%) | 37 (27.4) | 12 (27.9) | 11 (23.4) | 14 (31.1) | 0.47 |
| Previous angina, n (%) | 57 (42.2) | 18 (41.9) | 21 (44.7) | 18 (40.0) | 0.85 |
| Previous PCI, n (%) | 24 (17.8) | 7 (16.3) | 11 (23.4) | 6 (13.3) | 0.86 |
| Previous CABG, n (%) | 8 (5.9) | 4 (9.3) | 1 (2.1) | 3 (6.7) | 0.07 |
| HF, n (%) | 10 (7.4) | 3 (7.0) | 4 (8.5) | 3 (6.7) | 0.82 |
| AF, n (%) | 19 (14.1) | 4 (9.3) | 8 (17.0) | 7 (15.6) | 0.36 |
| PVD, n (%) | 13 (9.6) | 4 (9.3) | 4 (8.5) | 5 (11.1) | 0.86 |
| Previous TIA/Stroke, n (%) | 19 (14.1) | 3 (7.0) | 9 (19.1) | 7 (15.6) | 0.63 |
| Osteoarthritis, n (%) | 4 (3.0) | 1 (2.3) | 1 (2.1) | 2 (4.4) | 0.46 |
| Peptic ulcer disease, n (%) | 6 (4.4) | 2 (4.7) | 1 (2.1) | 3 (6.7) | 0.85 |
| COPD, n (%) | 23 (17.0) | 6 (14.0) | 11 (23.4) | 6 (13.3) | 0.51 |
| Malignancy, n (%) | 11 (8.1) | 3 (7.0) | 6 (12.8) | 2 (4.4) | 0.43 |
| Bleeding problems, n (%) | 4 (3.0) | 0 (0.0) | 2 (4.3) | 2 (4.4) | 0.47 |
| Anaemia, n (%) | 11 (8.1) | 2 (4.7) | 5 (10.6) | 4 (8.9) | 0.45 |
| Smoking Status | |||||
| Current smoker, n (%) | 9 (6.8) | 1 (2.4) | 4 (8.5) | 4 (8.9) | 0.36 |
| Ex-smoker, n (%) | 66 (49.6) | 20 (48.8) | 25 (53.2) | 21 (46.7) | 0.93 |
| Never-smoker, n (%) | 58 (43.6) | 20 (48.8) | 18 (38.3) | 20 (44.4) | 0.94 |
| Frailty Indices | |||||
| Fried index components | |||||
| Shrinking criterion, n (%) | 24 (17.8) | 4 (9.5) | 10 (21.3) | 10 (22.2) | 0.36 |
| Low physical endurance, n (%) | 33 (24.6) | 6 (14.3) | 15 (31.9) | 12 (26.7) | 0.14 |
| Low physical activity, n (%) | 35 (25.9) | 6 (14.3) | 15 (31.9) | 14 (31.1) | 0.34 |
| Weakness, n (%) | 83 (62.4) | 25 (59.5) | 28 (60.9) | 30 (66.7) | 0.80 |
| Slow walking speed, n (%) | 23 (17.0) | 7 (16.7) | 7 (15.6) | 9 (20.0) | 0.85 |
| Rockwood score, n (%) | |||||
| 1 − 2 | 46 (34.1) | 18 (41.9) | 13 (27.7) | 15 (33.3) | 0.28 |
| 3 − 4 | 72 (53.3) | 22 (51.2) | 27 (57.4) | 23 (51.1) | |
| 5 − 7 | 17 (12.6) | 3 (7.0) | 7 (14.9) | 7 (15.6) | |
| Quality of Life and Co-morbidity | |||||
| MoCA, points (SD) | 25.1 (3.4) | 25.5 (2.5) | 25.4 (3.6) | 24.2 (3.8) | 0.29 |
| SF-36 PCS, points (SD) | 36.6 (11.3) | 35.4 (8.9) | 37.6 (12.2) | 35.8 (11.7) | 0.98 |
| SF-36 MCS, points (SD) | 49.6 (10.2) | 47.6 (10.7) | 50.0 (10.2) | 50.9 (9.8) | 0.91 |
| Health state, % (SD) | 63.7 (19.4) | 59.8 (19.8) | 66.2 (19.7) | 62.3 (18.0) | 0.88 |
| Charlson index, points (SD) | 5.3 (1.7) | 5.4 (1.5) | 5.1 (1.8) | 5.6 (1.9) | 0.63 |
| Blood results | |||||
| Haemoglobin, g L-1 (SD) | 12.9 (1.9) | 13.0 (1.6) | 13.2 (1.7) | 12.4 (2.5) | 0.88 |
| Creatinine, μmol L-1 (SD) | 103.5 (32.7) | 106.7 (39.0) | 99.5 (28.4) | 108.3 (34.1) | 0.60 |
| Total cholesterol, mmol L-1 (SD) | 4.3 (1.3) | 4.5 (1.5) | 4.3 (1.3) | 3.9 (0.8) | 0.71 |
| hsCRP, mg L-1 (SD) | 12.3 (37.6) | 7.5 (6.7) | 15.8 (50.9) | 9.5 (16.1) | 0.29 |
| Troponin T, ng L-1 (SD) | 511.8 (983.7) | 606.0 (1159.1) | 403.6 (860.9) | 636.8 (1035.8) | 0.55 |
| eGFR, mL min-1 1.73 m-2 (SD) | 54.5 (19.5) | 54.3 (21.2) | 56.2 (18.1) | 51.2 (20.5) | 0.71 |
Abbreviations: AF—atrial fibrillation, BMI—body mass index, BP—blood pressure, CABG—coronary artery bypass graft, COPD—chronic obstructive pulmonary disease, EQ—EuroQol form, GRACE—Global Registry of Acute Coronary Events, HF—heart failure, IDAOPI—Income Deprivation Affecting Older People Index, IMD—Index of Multiple Deprivation, IQR—interquartile range, LTL- long telomere length, MTL- medium telomere length, STL-short telomere length, MCS—mental component summary, MI—myocardial infarction, MoCA—Montreal Cognitive Assessment, NYHA—New York Heart Association class, PCI—percutaneous coronary intervention, PCS—physical component summary, PVD—peripheral vascular disease, SD—standard deviation, SF-36—short form 36, TIA—transient ischaemic attack, eGFR—estimated glomerular filtration rate, hsCRP—C-reactive protein
Results
Baseline characteristics
A total of 153 patients had PBMC samples recovered as part of the ICON1 biomarker sub-study and of these samples, 135 patients had appropriate samples for TL analysis and 67 samples were analysed for TA. Patient baseline characteristics are illustrated in Table 1. There was no significant difference in baseline characteristics between tertiles of TL.
The mean age of participants was 81.0 ± 4.0 years and 64.0% were male, with 87.5% of participants classed as pre-frail or frail. The mean cohort telomere length was 0.47 ± 0.25 and was split into tertiles for analysis: LTL (n = 43, mean 0.74 ± 0.27), MTL (n = 47, mean 0.42 ± 0.05) and STL (n = 45, mean 0.25 ± 0.06). The mean cohort TA was 1.52 ± 0.61 units and split into tertiles for analysis: high TA (n = 23, 2.09 ± 0.24), mid TA (n = 22, 1.61 ± 0.15) and low TA (n = 22, 0.83 ± 0.48).
Of the 153 patients in the study, 111 patients (82.2%) underwent PCI. As demonstrated in Table 2, there were no significant differences in the coronary arteries affected, number of stents used or medical therapy on discharge between tertiles of TL.
Table 2. Procedural charactersitics.
| Total (N = 135) | LTL (N = 43) |
MTL (N = 47) |
STL (N = 45) |
p-value | |
|---|---|---|---|---|---|
| Killip on admission | |||||
| 1, n (%) | 115 (81.2) | 35 (81.4) | 41 (87.2) | 39 (86.7) | 0.79 |
| 2, n (%) | 16 (11.8) | 7 (16.3) | 4 (8.5) | 5 (11.1) | |
| 3, n (%) | 4 (0.03) | 1 (2.3) | 2 (4.3) | 1 (2.2) | |
| PCI, n (%) | 111 (82.2) | 34 (79.1) | 40 (85.1) | 37 (82.2) | 0.76 |
| Coronary arteries affected | |||||
| One vessel disease, n (%) | 85 (63.0) | 26 (60.5) | 29 (61.7) | 30 (66.7) | 0.81 |
| Multi-vessel disease, n (%) | 27 (20.0) | 9 (20.9) | 11 (23.4) | 7 (15.6) | 0.63 |
| LMS, n (%) | 4 (3.0) | 0 (0.0) | 2 (4.3) | 2 (4.4) | 0.38 |
| LAD, n (%) | 64 (47.4) | 19 (44.2) | 23 (48.9) | 22 (48.9) | 0.88 |
| LCx, n (%) | 34 (25.2) | 9 (20.9) | 12 (25.5) | 13 (28.9) | 0.69 |
| RCA, n (%) | 39 (28.9) | 14 (32.6) | 15 (31.9) | 10 (22.2) | 0.48 |
| Number of stents used | |||||
| 0, n (%) | 27 (20.0) | 11 (25.6) | 7 (14.9) | 9 (20.0) | 0.78 |
| 1, n (%) | 55 (40.7) | 14 (32.6) | 22 (46.8) | 19 (42.2) | |
| 2, n (%) | 30 (22.2) | 11 (25.6) | 10 (21.3) | 9 (20.0) | |
| 3, n (%) | 13 (9.6) | 6 (14.0) | 4 (8.5) | 3 (6.7) | |
| 4, n (%) | 7 (5.2) | 1 (2.3) | 3 (6.4) | 3 (6.7) | |
| 5, n (%) | 2 (1.5) | 0 (0.0) | 1 (2.1) | 1 (2.2) | |
| 6, n (%) | 1 (0.7) | 0 (0.0) | 0 (0.0) | 1 (2.2) | |
| Medical therapy on discharge | |||||
| Aspirin, n (%) | 132 (97.8) | 41 (95.3) | 46 (97.9) | 45 (100.0) | 0.33 |
| Clopidogrel, n (%) | 78 (57.8) | 25 (58.1) | 26 (55.3) | 27 (60.0) | 0.90 |
| Prasugrel, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 | |
| Ticagrelor, n (%) | 52 (38.5) | 16 (37.2) | 19 (40.4) | 17 (37.8) | 0.94 |
| Statin, n (%) | 126 (93.3) | 42 (97.7) | 42 (89.4) | 42 (93.3) | 0.98 |
| ACEi/A2RB, n (%) | 117 (86.7) | 36 (83.7) | 42 (89.4) | 39 (86.7) | 0.73 |
| BB, n (%) | 113 (83.7) | 37 (86.0) | 42 (89.4) | 34 (75.6) | 0.18 |
| CCB, n (%) | 42 (31.1) | 12 (27.9) | 15 (31.9) | 15 (33.3) | 0.85 |
| ISMN, n (%) | 42 (31.1) | 15 (34.9) | 16 (34.0) | 11 (24.4) | 0.49 |
| Nicorandil, n (%) | 20 (14.8) | 8 (18.6) | 7 (14.9) | 5 (11.1) | 0.61 |
| PPI, n (%) | 63 (46.7) | 20 (46.5) | 16 (34.0) | 27 (60.0) | 0.055 |
| Warfarin, n (%) | 10 (7.4) | 2 (4.7) | 4 (8.5) | 4 (8.9) | 0.70 |
| DOAC, n (%) | 5 (3.7) | 2 (4.7) | 2 (4.3) | 1 (2.2) | 0.80 |
Abbreviations: ACEi/A2RB–angiotensin converter inhibitor/angiotensin 2 receptor blocker, BB–beta blocker, CCB–calcium channel blocker, DOAC—direct acting oral anticoagulants, ISMN–isosorbide mononitrate, LAD–left anterior desceding coronary artery disease, LCx–left coronary artery disease, LMS–left main stem coronary artery disease, LTL- long telomere length, MTL- medium telomere length, STL-short telomere length, PPI–proton pump inhibitor, RCA–right coronary artery disease
Telomere length, telomerase activity and adverse outcomes
Incidence of the primary composite outcome at 1 year is detailed in Table 3. The primary composite outcome occurred in 44 patients (28.8%). There was no significant difference in the incidence of the primary composite outcome between tertiles of TL (p = 0.57) or TA (p = 0.62).
Table 3. Outcome measures.
| 1-year Outcomes | Total (N = 135) | LTL (N = 43) |
MTL (N = 47) |
STL (N = 45) |
p-value |
|---|---|---|---|---|---|
| Composite, n (%) | 38 (28.1) | 10 (23.3) | 13 (27.7) | 15 (33.3) | 0.57 |
| Death, n (%) | 6 (4.4) | 1 (2.3) | 2 (4.2) | 3 (6.7) | 0.61 |
| Myocardial infarction, n (%) | 12 (8.9) | 5 (11.6) | 4 (8.4) | 3 (6.7) | 0.71 |
| Unplanned revascularisation, n (%) | 6 (4.4) | 4 (9.3) | 0 | 2 (4.4) | 0.10 |
| Stroke, n (%) | 2 (1.5) | 1 (2.3) | 0 | 1 (2.2) | 0.58 |
| Significant bleeding, n (%) | 21 (15.6) | 4 (9.3) | 9 (19.1) | 8 (17.8) | 0.54 |
| All-cause re-hospitalisation, n (%) | 69 (51.1) | 21 (48.8) | 25 (53.2) | 23 (51.1) | 0.92 |
LTL long telomere length, MTL medium telomere length, STL short telomere length.
Cox proportional hazard models between tertiles of TL and TA and the incidence of the primary composite outcome are detailed in Table 4. There was an increased hazard of the primary composite outcome in patients with a MTL (hazard ratio HR 1.28, 95% Confidence Interval CI 0.56–2.91, p = 0.56) and STL (HR 1.50, 95% CI 0.68–3.34, p = 0.32) when compared to the reference patients with a LTL, however this was not statistically significant. There was no significant difference between TA and the incidence of the primary composite outcome in patients with a mid TA (HR 0.90, 95% CI 0.94–2.60, p = 0.89) and low TA (HR 1.33, 95% CI 0.52–3.36, p = 0.51). The results are consistent after adjusting for age, gender and frailty in the analysis model. Kaplan-Meier survival curves for TL (Fig 1) and TA (Fig 2) show no difference between groups for both TL (p = 0.61) and TA (p = 0.74). These results are consistent when primary outcome were analysed without BARC. See S2 Table.
Table 4. Telomere length and telomerase activity as predictors of the primary composite outcome.
| Hazard ratio | 95% confidence interval | p-value | |
|---|---|---|---|
| Model 1 | |||
| TL* | |||
| MTL | 1.28 | 0.56–2.91 | 0.56 |
| STL | 1.50 | 0.68–3.34 | 0.32 |
| TA† | |||
| Mid | 0.90 | 0.94–2.60 | 0.89 |
| Low | 1.33 | 0.52–3.36 | 0.51 |
| Model 2 | |||
| TL* | |||
| MTL | 1.49 | 0.64–3.45 | 0.35 |
| STL | 1.58 | 0.71–3.55 | 0.26 |
| TA† | |||
| Mid | 1.04 | 0.37–2.92 | 0.94 |
| Low | 1.18 | 0.46–3.06 | 0.73 |
Univariate (model 1) and multivariate (model 2; adjusted for age, gender and frailty) Cox regression analysis preformed for combined and primary outcomes alone using telomere length and telomerase activity as predictors. Both predictors were divided into tertiles for analysis.
* LTL used as reference.
† High used as reference. MTL- medium telomere length, STL-short telomere length and TA-telomerase activity.
Fig 1. Telomere length and cumulative event rates.
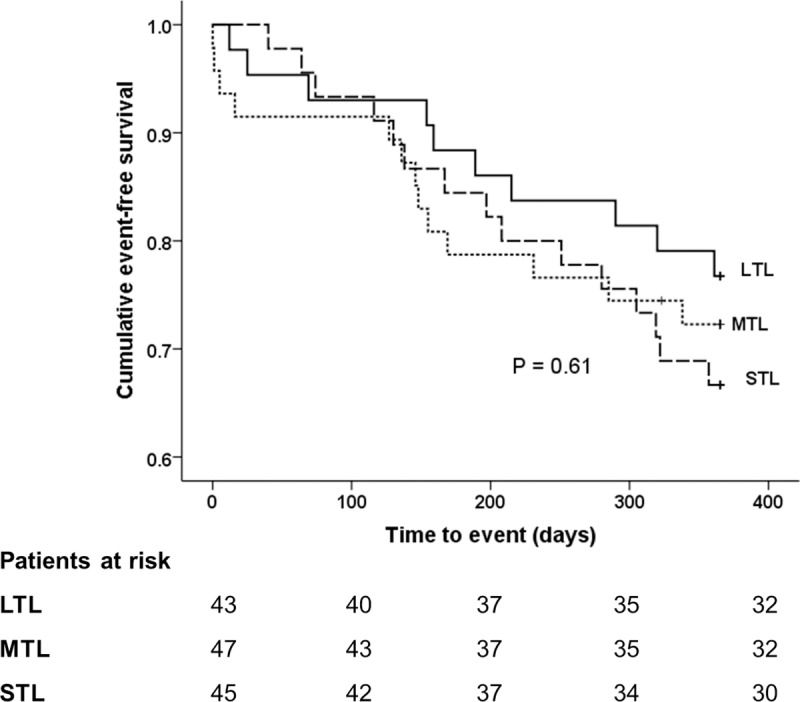
Cumulative event-free survival from the composite primary end-point by tertile of telomere length. LTL ≥ 0.5 T/S ratio, MTL 0.3468 to 0.5 T/S ratio and STL ≤ 0.3467 T/S ratio. P value from the Log-rank test. LTL, long telomere length; MTL; medium telomere length and STL; short telomere length.
Fig 2. Telomerase activity and cumulative event rates.
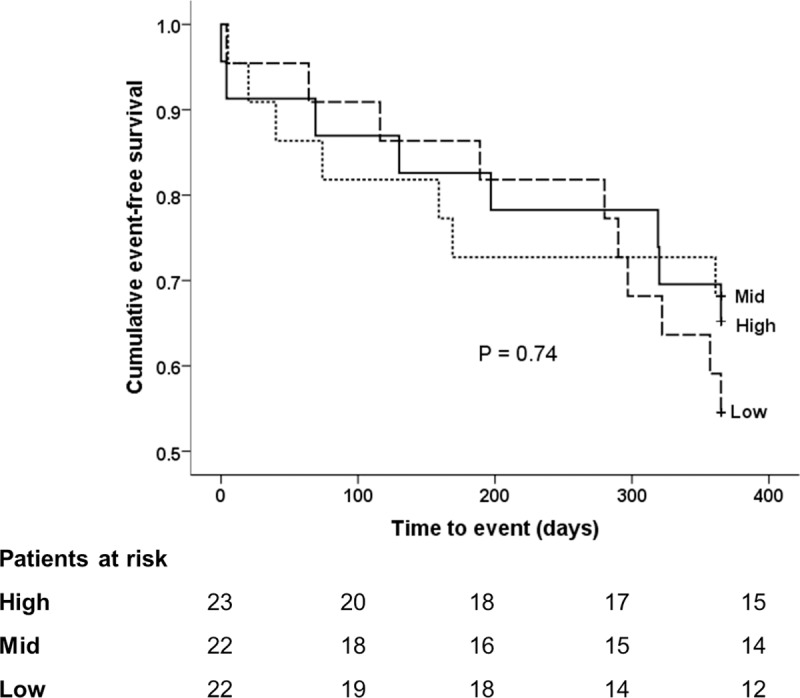
Cumulative event-free survival from the composite primary end-point by tertile of telomerase activity. High telomerase activity denotes ≥1.86 units, mid telomerase activity 1.32 to 1.86 units, and low telomerase activity ≤1.31 units. P value from the Log-rank test.
Discussion
This is the first study to examine the association of TL and TA with adverse clinical outcomes in older patients with NSTEACS undergoing an invasive treatment strategy. Neither TL nor TA were found to be associated with adverse outcomes in older patients with NSTEACS.
Ischaemic heart disease (IHD) is the leading cause of death worldwide[14] and importantly, the demographic of the condition is changing, with increasing frequency of older patients presenting with IHD. Studies have also shown that advancing age is associated with increased risk of mortality following cardiovascular events[15]. The increase of IHD burden in older patients, together with the increased risk of poorer outcome, drives a need for markers which could guide the management of ACS in this group of patients.
Telomeres are protective structures at the end of chromosomes which act to stabilise genome integrity, and have been implicated as a marker of biological age and age-related diseases. The length of telomere shortens with subsequent cell division, and this shortening is accelerated by inflammation and oxidative stress, processes implicated in the pathophysiology of IHD. To prevent telomere shortening to a critical length, in cells such as germ cells and stem cells, telomerase activity is high to maintain and elongate telomeres. In most somatic cells, telomerase activity is absent or low, but a mouse study has suggested that telomerase might play a role in regulating tissue repair in cardiomyocytes and endothelial progenitor cells[16].
Previous studies evaluating the association of telomere lenth and telomerase activity on clinical outcomes in different populations have shown varied results[17–25]. In a prospective WOSCOPS study of 484 participants with a mean age of 55 years, participants in the middle and the lowest tertiles of telomere length were more at risk of developing a coronary heart disease event than were individuals in the highest tertile (odds ratio [OR] for coronary heart disease: 1.51, 95% CI 1.15–1.98; p = 0.0029 in the middle tertile; 1.44, 1.10–1.90, p = 0.0090 in the lowest)[26]. In a meta-analysis of prospective and retrospective studies with 5566 participants with CHD, the pooled relative risk for CHD of shortest versus longest tertile telomere length was 1.54 (95% CI 1.30 to 1.83)[27]. Moreover, in a previous study of 170 patients with stable angina or acute coronary syndrome and using virtual histology intravascular ultrasound, it was shown that shorter TL was associated with a high risk of unstable plaques (calcified thin-capped fibroatheroma), with an OR of 1.24 (95% CI 1.01 to 1.53)[28]. Our results contradict the previous literature, a finding which may be partly explained by differences in the study design, methodology and patient cohort. Our study population is generally older, underwent invasive management, and we specifically examined NSTEACS as this is the predominant ACS phenotype in this age group. This differs to previous research, which was in a majority performed in a younger cohort and included all ACS types.
Epel and colleagues[25] found that the rate of telomere shortening was predictive of mortality from cardiovascular disease in elderly men, suggesting that low telomerase activity contribute to cardiovascular risk. However, our study found that telomerase activity was not associated with adverse outcomes following invasive management of NSTEACS. This may partly be explained by the difference of study populations. Epel et al. recruited healthy older volunteers prior to cardiovascular event, compared to the patients presenting with NSTEACS in the current study. Hence, this study focused on telomere length in those whom already developed coronary artery disease. The results together would suggest that dependent upon the cumulative oxidative stress and inflammatory burden, telomere attrition may play a different role in the process of atherosclerosis in older patients. Consistent with this hypothesis, Perez-Rivera et al[29]. found worse prognosis with short telomere length in middle aged men with ACS but not in older patients.
It has been speculated that telomere attrition and the resulting cell senescence may act as a mechanism for restricting atheromas[30]. Indeed, this is consistent with our study result showing low telomerase activity was not associated with adverse outcomes in older patients. Furthermore, telomerase-deficient mice TERC-/- ApoE-/- had fewer atherosclerotic lesions compared to TERC+/+ ApoE-/-[31], and Willeit et al[32] showed that TL was a risk predictor for myocardial infarction, but not for de novo stable angina and intermittent claudication. Therefore, telomere attrition causes senescence and apoptosis, but may have process-specific effect on atherosclerosis[30].
Recently Werner and colleagues found that specific exercise modalities were associated with increased telomere length and telomerase activity[33].This raises the interesting possibility that the benefits of exercise in reducing and preventing frailty in the older population may act through regulating cellular senescence. The current study found no association between telomere length and frailty, which is consistent with the results demonstrated by Brault et al. in older adults with cardiac disease[34]. These results suggest that frailty may be the consequence of repeated physiological stress induced premature cellular senescence in addition to progressive telomere shortening. It would be interesting to investigate the effect of exercise on telomere length and frailty in older adults with established cardiac disease.
Limitations
The limitation of this study is the relatively small number of adverse outcomes observed in the 1 year follow-up, therefore these results should be interpreted with caution. Recruiting older patients to clinical research is very challenging[35, 36]. Very few studies have evaluated older patients with NSTEACS. A lot of factors and therapy that show positive correlation in younger patients, don’t quite show this in older patients. Given the growth of older patients with coronary disease, it is important the cardiovascular community are aware of such differences in the best care of older patients. Our study with the sample size included with detailed statistical analysis provides important information regarding these patients.
Larger follow-up studies would be required to evaluate the true value of TL and TA as predictors in this setting. A further limitation is that we have not considered leukocyte subpopulations distributions, a factor that can determine the measurements of both TL and TA TL[37] as well as the association of both parametres to cardiac dysfunction[38].
Conclusion
The present study found TL and TA were not associated with adverse outcomes in older patients presenting with NSTEACS.
Supporting information
Scatter plot analysis of TL with age yields a non statstiacally significant increasing regression line in aged 75–80 (Spearman's correlation coefficient, rs = 0.052, p = 0.66) and a decreasing regression line in aged > 80 (rs = -0.11, p = 0.34).
(TIF)
Scatter plot analysis of TA with age yields a non-statistically significant decreasing regression line (Spearman's correlation coefficient, rs = -0.22, p = 0.071).
(TIF)
A Wilcoxon signed–rank test showed that telomere lengths did not elicit a significant change for subjects with primary outcomes compared to subjects without composite events (Z = -0.655, p = 0.071).
(TIF)
A Wilcoxon signed–rank test showed that telomerase activity did not elicit a significant change for subjects with primary outcomes compared to subjects without composite events (Z = -1.274, p = 0.20).
(TIF)
Cox regression analysis preformed for combined and primary outcomes alone using telomere length and telomerase activity as predictors. Both predictors were divided into two groups for analysis based upon area under curve (ROC). For TL, area under curve was 0.57 (p = 0.17). The best cutoff was 0.61 with sensitivity of 87% and specificity of 80%. With this cut-off, 111 participants had STL (82.2%) and 24 had LTL (17.8%). The area under curve was also measured for TA at 0.54 (p = 0.57), with cut-off of 1.88 (sensitivity of 80% and specificity of 64%). 47 (70.1%) participants classified as low TA and 20 (29.9) as high TA. * LTL used as reference. † High used as reference. TL- telomere length and TA-telomerase activity.
(DOCX)
Cox regression analysis preformed for combined outcomes without major bleeding using telomere length and telomerase activity as predictors. Both predictors were divided into tertiles for analysis. * LTL used as reference. † High used as reference. MTL- medium telomere length, STL-short telomere length and TA-telomerase activity.
(DOCX)
Acknowledgments
The authors would like to thank:
Dr. J Ahmed, Dr. A Bagnall, Dr. R Das, Dr. R Edwards, Dr. M Egred, Dr. I Purcell, Professor. I Spyridopoulos and Professor. A Zaman of Freeman Hospital, Newcastle upon Tyne for their help with data collection.
Cardiology CRN research team at Freeman Hospital: Mrs. Kathryn Proctor and Mrs. Jennifer Adams-Hall for their support with follow-up of study patients.
Dr. Mark de Belder and Mrs. Bev Atkinson, the James Cook University Hospital, South Tees Hospitals NHS Foundation Trust, Middlesbrough, United Kingdom for their help with data collection.
The authors would like to thank Clinical research fellows, Dr H. Sinclair, Dr M. Veerasamy, Dr. J Batty, Dr. B Beska of Freeman Hospital, Newcastle upon Tyne for their hard work in patient recruitment and data collection
Data Availability
There are ethical restrictions on sharing a de-identified data set, as data contain potentially sensitive information as imposed by study sponsor and consent for data sharing not obtained from patients. Data are available upon request to the study Sponsor tnu-tr.sponsormanagement@nhs.net.
Funding Statement
The research is supported by the National Institute for Health Research (NIHR) Newcastle Biomedical Research Centre based at Newcastle-upon-Tyne Hospitals NHS Foundation Trust and Newcastle University. VK has received research funding from the British Heart Foundation (CS/15/7/31679). WQ is salaried by Sanofi Genzyme. The funder provided support in the form of salaries for authors VK and WQ but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation. 1999;99(9):1165–72. Epub 1999/03/09. 10.1161/01.cir.99.9.1165 . [DOI] [PubMed] [Google Scholar]
- 2.Castelli WP. Epidemiology of coronary heart disease: the Framingham study. Am J Med. 1984;76(2A):4–12. Epub 1984/02/27. 10.1016/0002-9343(84)90952-5 . [DOI] [PubMed] [Google Scholar]
- 3.Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90(1):583–612. Epub 1994/07/01. 10.1161/01.cir.90.1.583 . [DOI] [PubMed] [Google Scholar]
- 4.Zaman MJ, Stirling S, Shepstone L, Ryding A, Flather M, Bachmann M, et al. The association between older age and receipt of care and outcomes in patients with acute coronary syndromes: a cohort study of the Myocardial Ischaemia National Audit Project (MINAP). Eur Heart J. 2014;35(23):1551–8. Epub 2014/03/20. 10.1093/eurheartj/ehu039 . [DOI] [PubMed] [Google Scholar]
- 5.von Zglinicki T. Role of oxidative stress in telomere length regulation and replicative senescence. Ann N Y Acad Sci. 2000;908:99–110. Epub 2000/07/27. 10.1111/j.1749-6632.2000.tb06639.x . [DOI] [PubMed] [Google Scholar]
- 6.Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Abdelhadi N, Alkhoder A, et al. Telomere Shortening, Regenerative Capacity, and Cardiovascular Outcomes. Circ Res. 2017;120(7):1130–8. Epub 2016/12/14. 10.1161/CIRCRESAHA.116.309421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batty J, Qiu W, Gu S, Sinclair H, Veerasamy M, Beska B, et al. One-year clinical outcomes in older patients with non-ST elevation acute coronary syndrome undergoing coronary angiography: An analysis of the ICON1 study. International journal of cardiology. 2018. Epub 2018/10/06. 10.1016/j.ijcard.2018.09.086 . [DOI] [PubMed] [Google Scholar]
- 8.Gu SZ, Qiu W, Batty JA, Sinclair H, Veerasamy M, Brugaletta S, et al. Coronary artery lesion phenotype in frail older patients with non-ST elevation acute coronary syndrome undergoing invasive care. EuroIntervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2019. Epub 2019/02/20. 10.4244/eij-d-18-00848 . [DOI] [PubMed] [Google Scholar]
- 9.Gu SZ, Beska B, Chan D, Neely D, Batty JA, Adams-Hall J, et al. Cognitive Decline in Older Patients With Non- ST Elevation Acute Coronary Syndrome. J Am Heart Assoc. 2019;8(4):e011218 Epub 2019/02/19. 10.1161/JAHA.118.011218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunadian V, Neely RD, Sinclair H, Batty JA, Veerasamy M, Ford GA, et al. Study to Improve Cardiovascular Outcomes in high-risk older patieNts (ICON1) with acute coronary syndrome: study design and protocol of a prospective observational study. BMJ Open. 2016;6(8):e012091 Epub 2016/08/25. 10.1136/bmjopen-2016-012091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736–47. Epub 2011/06/15. 10.1161/CIRCULATIONAHA.110.009449 . [DOI] [PubMed] [Google Scholar]
- 12.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. Epub 2001/03/17. 10.1093/gerona/56.3.m146 . [DOI] [PubMed] [Google Scholar]
- 13.Kim NW, Wu F. Advances in quantification and characterization of telomerase activity by the telomeric repeat amplification protocol (TRAP). Nucleic Acids Res. 1997;25(13):2595–7. Epub 1997/07/01. 10.1093/nar/25.13.2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349(9061):1269–76. Epub 1997/05/03. 10.1016/S0140-6736(96)07493-4 . [DOI] [PubMed] [Google Scholar]
- 15.Veerasamy M, Edwards R, Ford G, Kirkwood T, Newton J, Jones D, et al. Acute coronary syndrome among older patients: a review. Cardiol Rev. 2015;23(1):26–32. Epub 2014/01/11. 10.1097/CRD.0000000000000016 . [DOI] [PubMed] [Google Scholar]
- 16.Richardson GD, Breault D, Horrocks G, Cormack S, Hole N, Owens WA. Telomerase expression in the mammalian heart. FASEB J. 2012;26(12):4832–40. Epub 2012/08/25. 10.1096/fj.12-208843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bischoff C, Petersen HC, Graakjaer J, Andersen-Ranberg K, Vaupel JW, Bohr VA, et al. No Association Between Telomere Length and Survival Among the Elderly and Oldest Old. Epidemiology. 2006;17(2):190–4. 10.1097/01.ede.0000199436.55248.10 00001648-200603000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Kimura M, Hjelmborg JV, Gardner JP, Bathum L, Brimacombe M, Lu X, et al. Telomere length and mortality: a study of leukocytes in elderly Danish twins. Am J Epidemiol. 2008;167(7):799–806. Epub 2008/02/14. 10.1093/aje/kwm380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terry DF, Nolan VG, Andersen SL, Perls TT, Cawthon R. Association of longer telomeres with better health in centenarians. J Gerontol A Biol Sci Med Sci. 2008;63(8):809–12. Epub 2008/09/06. 10.1093/gerona/63.8.809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin-Ruiz CM, Gussekloo J, van Heemst D, von Zglinicki T, Westendorp RG. Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: a population-based study. Aging Cell. 2005;4(6):287–90. Epub 2005/11/23. 10.1111/j.1474-9726.2005.00171.x . [DOI] [PubMed] [Google Scholar]
- 21.Gardner MP, Martin-Ruiz C, Cooper R, Hardy R, Sayer AA, Cooper C, et al. Telomere length and physical performance at older ages: an individual participant meta-analysis. PLoS One. 2013;8(7):e69526 Epub 2013/08/08. 10.1371/journal.pone.0069526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishikawa N, Nakamura K, Izumiyama N, Aida J, Sawabe M, Arai T, et al. Telomere length dynamics in the human pituitary gland: robust preservation throughout adult life to centenarian age. Age (Dordr). 2012;34(4):795–804. Epub 2011/07/08. 10.1007/s11357-011-9280-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houben JM, Giltay EJ, Rius-Ottenheim N, Hageman GJ, Kromhout D. Telomere length and mortality in elderly men: the Zutphen Elderly Study. J Gerontol A Biol Sci Med Sci. 2011;66(1):38–44. Epub 2010/10/05. 10.1093/gerona/glq164 . [DOI] [PubMed] [Google Scholar]
- 24.Halaschek-Wiener J, Vulto I, Fornika D, Collins J, Connors JM, Le ND, et al. Reduced telomere length variation in healthy oldest old. Mech Ageing Dev. 2008;129(11):638–41. Epub 2008/09/04. 10.1016/j.mad.2008.07.004 . [DOI] [PubMed] [Google Scholar]
- 25.Epel ES, Merkin SS, Cawthon R, Blackburn EH, Adler NE, Pletcher MJ, et al. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging (Albany NY). 2008;1(1):81–8. Epub 2009/01/01. 10.18632/aging.100007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369(9556):107–14. Epub 2007/01/16. 10.1016/S0140-6736(07)60071-3 . [DOI] [PubMed] [Google Scholar]
- 27.Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227 Epub 2014/07/10. 10.1136/bmj.g4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calvert PA, Liew TV, Gorenne I, Clarke M, Costopoulos C, Obaid DR, et al. Leukocyte telomere length is associated with high-risk plaques on virtual histology intravascular ultrasound and increased proinflammatory activity. Arterioscler Thromb Vasc Biol. 2011;31(9):2157–64. Epub 2011/06/18. 10.1161/ATVBAHA.111.229237 . [DOI] [PubMed] [Google Scholar]
- 29.Perez-Rivera JA, Pabon-Osuna P, Cieza-Borrella C, Duran-Bobin O, Martin-Herrero F, Gonzalez-Porras JR, et al. Effect of telomere length on prognosis in men with acute coronary syndrome. Am J Cardiol. 2014;113(3):418–21. Epub 2013/12/03. 10.1016/j.amjcard.2013.10.009 . [DOI] [PubMed] [Google Scholar]
- 30.Yeh JK, Wang CY. Telomeres and Telomerase in Cardiovascular Diseases. Genes (Basel). 2016;7(9). Epub 2016/09/07. 10.3390/genes7090058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong LS, Oeseburg H, de Boer RA, van Gilst WH, van Veldhuisen DJ, van der Harst P. Telomere biology in cardiovascular disease: the TERC-/- mouse as a model for heart failure and ageing. Cardiovasc Res. 2009;81(2):244–52. Epub 2008/12/03. 10.1093/cvr/cvn337 . [DOI] [PubMed] [Google Scholar]
- 32.Willeit P, Willeit J, Brandstatter A, Ehrlenbach S, Mayr A, Gasperi A, et al. Cellular aging reflected by leukocyte telomere length predicts advanced atherosclerosis and cardiovascular disease risk. Arterioscler Thromb Vasc Biol. 2010;30(8):1649–56. Epub 2010/05/29. 10.1161/ATVBAHA.110.205492 . [DOI] [PubMed] [Google Scholar]
- 33.Werner CM, Hecksteden A, Morsch A, Zundler J, Wegmann M, Kratzsch J, et al. Differential effects of endurance, interval, and resistance training on telomerase activity and telomere length in a randomized, controlled study. Eur Heart J. 2019;40(1):34–46. Epub 2018/11/30. 10.1093/eurheartj/ehy585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brault ME, Ohayon SM, Kwan R, Bergman H, Eisenberg MJ, Boivin JF, et al. Telomere length and the clinical phenotype of frailty in older adults undergoing cardiac surgery. J Am Geriatr Soc. 2014;62(11):2205–7. Epub 2014/11/22. 10.1111/jgs.13076 . [DOI] [PubMed] [Google Scholar]
- 35.Sinclair H, Batty JA, Qiu W, Kunadian V. Engaging older patients in cardiovascular research: observational analysis of the ICON-1 study. Open Heart. 2016;3(2):e000436 Epub 2016/08/23. 10.1136/openhrt-2016-000436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinclair H, Kunadian V. Coronary revascularisation in older patients with non-ST elevation acute coronary syndromes. Heart. 2016;102(6):416–24. Epub 2016/01/08. 10.1136/heartjnl-2015-307859 . [DOI] [PubMed] [Google Scholar]
- 37.Blackburn EH, Epel ES, Lin J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350(6265):1193–8. Epub 2016/01/20. 10.1126/science.aab3389 . [DOI] [PubMed] [Google Scholar]
- 38.Yu HT, Youn JC, Kim JH, Seong YJ, Park SH, Kim HC, et al. Arterial Stiffness Is Associated With Cytomegalovirus-Specific Senescent CD8(+) T Cells. J Am Heart Assoc. 2017;6(9). Epub 2017/08/30. 10.1161/jaha.117.006535 [DOI] [PMC free article] [PubMed] [Google Scholar]


