Abstract
Objectives:
The objective of our study was to evaluate the association between occupational exposure to trichloroethylene (TCE), a suspected lymphomagen, and serum levels of miRNAs in a cross-sectional molecular epidemiology study of TCE exposed workers and comparable unexposed controls in China.
Methods:
Serum levels of 40 miRNAs were compared in 74 workers exposed to TCE (median: 12 ppm) and 90 unexposed control workers. Linear regression models were used to test for differences in serum miRNA levels between exposed and unexposed workers and to evaluate exposure-response relationships across TCE exposure categories using a three-level ordinal variable (i.e., unexposed, <12 ppm, the median value among workers exposed to TCE) and ≥12 ppm)). Models were adjusted for sex, age, current smoking, current alcohol use, and recent infection.
Results:
Seven miRNAs showed significant differences between exposed and unexposed workers at FDR (false discovery rate) < 0.20. miR-150–5p and let-7b-5p also showed significant inverse exposure-response associations with TCE exposure (Ptrend=0.002 and 0.03, respectively). The % differences in serum levels of miR-150–5p relative to unexposed controls were −13% and −20% among workers exposed to <12 ppm and ≥12 ppm TCE, respectively.
Conclusions:
miR-150–5p is involved in B-cell receptor pathways and let-7b-5p plays a role in the innate immune response processes that are potentially important in the etiology of non-Hodgkin lymphoma (NHL). Further studies are needed to replicate these findings and to directly test the association between serum levels of these miRNAs and risk of NHL in prospective studies.
Keywords: microRNAs, trichloroethylene, epigenetics
Introduction
Trichloroethylene (TCE) is widely used as an industrial organic solvent primarily for degreasing and remains a ubiquitous environmental contaminant found in air, soil, ground water, and food. In 2012, the International Agency for Research on Cancer (IARC) classified TCE as a known human carcinogen (Group 1) based on associations with kidney cancer along with limited evidence for non-Hodgkin lymphoma (NHL) risk (IARC, 2014).
MicroRNAs (miRNAs) are a group of regulatory RNAs consisting of 19–22 nucleotides that exert regulatory functions through base-pairing with complementary sequences within target messenger RNA (mRNA) molecules, resulting in gene silencing via translational repression or target degradation of their target mRNAs (Hudder and Novak, 2008). Numerous studies have demonstrated that miRNAs can participate in the regulation of crucial biological processes such as cell growth, differentiation, development, and apoptosis (Salimienejad et al., 2018). Aberrant miRNA expression profiles are known to play a critical role in tumorigenesis and tumor development and may serve as biomarkers for diagnosis and therapies (Hayes et al., 2014). Dysregulation of miRNAs have been observed to play crucial roles in the pathogenesis of lymphomas through altered regulation of target oncogenes or tumor suppressors (Tagawa et al., 2013; Fernadez-Mercado et al., 2015).
Environmental exposures including metals (e.g. arsenic, cadmium, and aluminum), cigarette smoke, ambient air pollutants (e.g. particulate matter and diesel exhaust particles), and persistent organic pollutants have been observed to alter expression levels of miRNAs in various biological specimens (Hou et al., 2011; Krauskopf et al., 2017). Only a few studies to date have investigated whether TCE exposure is associated with altered expression of miRNAs. A recent study of miRNA expression profiles in mouse embryonic liver cell lines (Jiang et al., 2017) reported that TCE up-regulates miR-182–5p expression by DNA hypomethylation, which could suppress the tumor suppressor gene Cited2 and improve cell proliferation rates resulting in liver tumors. Another experimental study (Cui et al., 2016) reported that TCE-induced global hypomethylation could be partly attributed to the disrupted DNA methyltransferase 3a (Dnmt3a) and that a group of cancer-related miRNAs (i.e., miR-1–1, miR-32, let-7g, miR-210, miR-30, miR-126) experienced promoter demethylation following TCE exposure. In the only human study to evaluate associations between TCE exposure and miRNA levels, Varshney et al. (2015) found no differences in plasma levels of one candidate miRNA (let-7c) between 49 workers exposed to TCE during metal degreasing and 19 unexposed controls in India.
We previously observed a decrease in lymphocyte subsets and a substantial decline in markers of immune regulation among TCE-exposed workers compared with controls and suggested those as potential intermediate markers that may be involved in lymphomagenesis (Lan et al., 2010; Hosgood et al., 2012; Zhang et al., 2013; Bassig et al., 2013). In the current study, we evaluated the association between TCE exposure and serum levels of miRNAs with the objective of providing additional data on biologic plausibility and the potential mechanistic processes that may support reported epidemiologic associations between occupational TCE exposure and risk of NHL.
Materials and Methods
Study population and exposure assessment
The study design, participants, environmental exposure assessment, and biological sample collection in this study have been described (Lan et al., 2010). Briefly, we conducted a cross-sectional molecular epidemiological study of 80 workers who were currently exposed to TCE in six study factories with TCE cleaning operations, and 96 unexposed controls in Guangdong, China. Controls were frequency-matched to exposed workers by sex and age (±5 years) and were enrolled from two clothing manufacturing factories, one food production factory, and a hospital that did not use TCE. Workers with a history of cancer, chemotherapy, radiotherapy or a previous occupation with notable exposure to benzene, butadiene, styrene and/or ionizing radiation were excluded from the study. A questionnaire-based interview that assessed demographic and lifestyle characteristics as well as an occupational history was administered to all subjects. Blood samples were delivered on ice to the laboratory within 6 hours of being collected and centrifuged, and serum samples were preserved in −80 °C. All samples collected from workers exposed to TCE and from the unexposed control workers were processed and stored using the same biological sample protocols. The study was approved by Institutional Review Boards at the US National Cancer Institute and the Guangdong National Poison Control Center, China.
Full-shift personal air exposure measurements were taken in the factories using 3M organic vapor monitoring (OVM) badges before blood collection. All samples were analyzed for TCE and a subset (48 from TCE-exposed workers) was analyzed for a panel of organic hydrocarbons including benzene, methylene chloride, perchloroethylene, and epichlorohydrin. OVM samples were also obtained on a subgroup of control workers. TCE air levels in parts per million (ppm) were based on the arithmetic mean of an average of two to three measurements per subject. TCE air levels were highly correlated with urinary concentrations of trichloroacetic acid (TCA) (r=0.44, p=0.0001) and total trichloroethanol (TCEOH) (r=0.66, p=0.0001) (Supplementary Table 1). Ninety-six percent of workers were exposed to TCE below the current US Occupational Safety and Health Administration Permissible Exposure Limit (100 ppm 8h time-weighted average), with a mean (SD) of 22.2 (36.0) ppm.
Assays for micro RNAs
miRNAs were quantified using the Firefly Circulating miRNA Assay (Abcam, Inc., Cambridge, MA) providing direct detection of microRNAs from serum. This approach does not require a separate RNA isolation and purification step, which is advantageous for high-throughput applications and reducing pre-analytical variability, as described elsewhere (Abcam Inc., 2018). Data from serum generated from the assay have been demonstrated to have high correlation (>90%) with results from purified RNA and from qRT-PCR and sequencing based approaches (Abcam Inc., 2017 and 2018). Initially, we conducted a small pilot study including 6 workers exposed to TCE and 6 unexposed controls and measured 407 miRNAs using untargeted discovery panels. These discovery panels included miRNAs with known expression in plasma/serum and reflected diverse biologic pathways including regulation of immune/inflammatory processes. Detectable miRNAs were assessed in the pilot study for evidence of initial differences in serum levels between exposed and control workers based on non-parametric tests and expression fold changes. We further conducted a comprehensive literature review to identify miRNAs with biologic functions potentially relevant for suspected TCE-associated diseases (e.g. immune regulation and liver toxicity). A total of 40 miRNAs from this initial screening were selected for further evaluation in the primary study of 74 workers exposed to TCE and 90 unexposed controls.
A standard laboratory protocol provided by the manufacturer was followed for the miRNA analyses (Abcam, 2018). Briefly, miRNAs from up to 40 μL serum were hybridized to complementary oligonucleotides covalently attached to encoded hydrogel microparticles. The bound target was ligated to oligonucleotide adapter sequences that serve as universal PCR priming sites. The miR-adapter hybrid molecules were then denatured from the particles at 95°C and reverse transcription polymerase chain reaction (RT-PCR) was performed using a fluorescent forward primer. Once amplified, the fluorescent target was rehybridized to the original capture particles and scanned on a EMD Millipore Guava 8HT Flow Cytometer (Merck KGaA Darmstadt, Germany). Signals (expressed in arbitrary units, A.U.) were normalized based on the geNorm algorithm that identifies the evaluated probes with the most stable expression levels across samples (has-let-7d-3p, has-miR-27a-3p, and has-miR-24–3p in our study) and uses the geometric mean expression of these miRNAs to normalize each sample for each miRNA target (Mestdagh et al., 2009).
Assay quality control (QC) was conducted by including 5–7 blind replicate samples from 3 different QC subjects in the assay batches. Of the 40 evaluated miRNAs, 7 were found to have coefficients of variation (CV) ≥ 40% and were excluded from the statistical analyses, leaving 33 miRNAs in the final study. Of these 33 mRNAs, 27 are involved in immunological processes. The list of these 33 miRNAs are shown in Supplementary Table 2.
Statistical analysis
Linear regression models using the natural logarithm (ln) of each miRNA was used to test for differences between control and exposed workers and to evaluate exposure-response relationships across TCE exposure categories using a three-level ordinal variable (i.e., controls, low exposure group (<12 ppm, the median value among workers exposed to TCE) and high exposure group (≥12 ppm)). Models were adjusted for sex, age, current smoking, current alcohol use, and recent infection. To account for multiple comparisons, we evaluated our findings in relation to the false discovery rate (FDR) and considered FDR values less than 0.20 as noteworthy. As sensitivity analyses, we conducted multiple linear regression analyses with additional adjustment for selected immune regulation markers that were evaluated for an association with TCE exposure in our previous studies. For the miRNAs that showed a significant exposure-response association with TCE exposure, we also conducted analyses using different cut points for low and high exposure groups (i.e., <5, <10, <20, <30, and <40) to assess the robustness of these associations at different levels of exposure. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Demographic characteristics including age, sex, BMI, current smoking, alcohol status, and recent infection were comparable between the controls (n=90) and exposed subjects (n=74) (Table 1). The mean TCE exposure among the exposed group was 18.70 ppm (SD 27.95) and exposure levels were negligible in the control group.
Table 1.
Demographic characteristics of the study population in a cross-sectional molecular epidemiology study of TCE exposure in China
| Controls (n=90) | TCE-exposed workers | |||
|---|---|---|---|---|
| All (n=74) | <12 ppm (n=40) | ≥12 ppm (n=34) | ||
| Age, mean (SD) | 27 (7) | 25 (7) | 23 (5) | 27 (8) |
| BMI, mean (SD) | 22 (3) | 21 (3) | 21 (2) | 22 (3) |
| Sex, n (%) | ||||
| Female | 21 (23) | 21 (28) | 16 (40) | 5 (15) |
| Male | 69 (77) | 53 (72) | 24 (60) | 29 (85) |
| Current smoking, n (%) | ||||
| No | 54 (60) | 42 (57) | 23 (58) | 19 (56) |
| Yes | 36 (40) | 32 (43) | 17 (42) | 15 (44) |
| Current alcohol use, n (%) | ||||
| No | 53 (59) | 50 (68) | 27 (67) | 23 (68) |
| Yes | 37 (41) | 24 (32) | 13 (33) | 11 (32) |
| Recent infection, n (%) | ||||
| No | 69 (77) | 59 (80) | 32 (80) | 27 (79) |
| Yes | 21 (23) | 15 (20) | 8 (20) | 7 (21) |
| TCE exposure | ||||
| TCE air level (ppm), mean (SD) | <0.03 | 18.70 (27.95) | 5.36 (3.59) | 34.39 (35.27) |
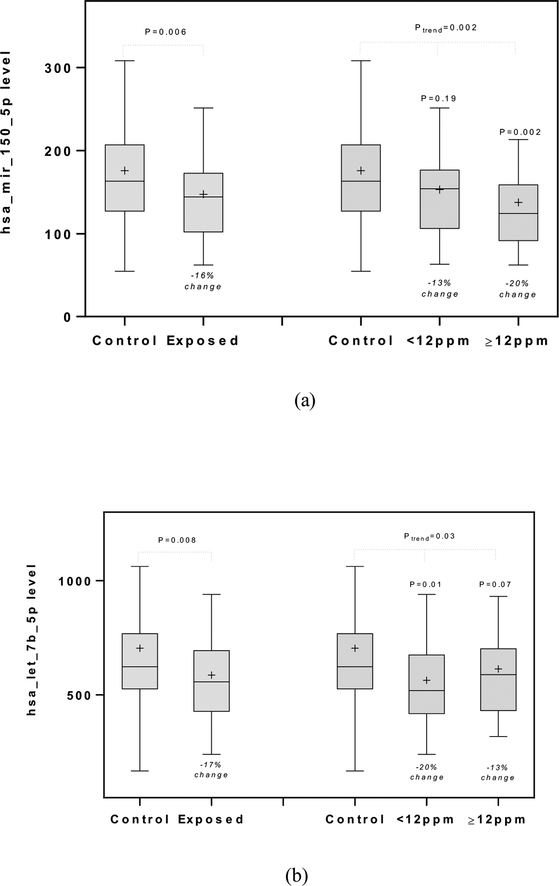
Of the 33 miRNAs that were evaluated (Supplementary Table 2), serum levels of 7 miRNAs (i.e., miR-150–5p, let-7b-5p, miR-92a-3p, miR-122–5p, let-7g-5p, miR-20a-5p, and miR-140–5p) were significantly different between TCE-exposed and control workers (P<0.05) and remained noteworthy after considering the FDR (<0.20). However, only two miRNAs (i.e., miR-150–5p and let-7b-5p) were observed to have a significant exposure-response relationship with the level of TCE exposure (i.e., controls, <12 ppm, and ≥12 ppm) (P<0.05) (Table 2). Mean serum levels of miR-150–5p were 176.0 for controls, 153 (% difference: −13%) for low-exposed, and 140.5 (% difference: −20%) for high-exposed workers (Ptrend=0.002). Mean serum levels of let-7b-5p were 705.0 for controls, 563.8 (% difference: −20%) for low-exposed, and 613.6 (% difference: −13%) for high-exposed workers (Ptrend=0.003). A suggestive inverse exposure-response relationship between let-7g-5p levels and increasing TCE exposure was also observed (P=0.05).
Table 2.
Exposure-response relationships with TCE exposure for miRNAs that showed significant differences between exposed workers and unexposed controls
| No. | miRNA | Controls (n=90) | TCE-exposed | Low-exposed (<12 ppm) (n= 40) | High-exposed (≥12 ppm) (n=34) | Ptrendb | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meana | SDa | Meana | SDa | % difference | P | FDR | Meana | SDa | % change | Pb | Meana | SDa | % difference | Pb | ||||
| 1 | miR-150–5p | 176.0 | 75.9 | 147.3 | 63.1 | −16% | 0.006 | 0.13 | 153.0 | 56.5 | −13% | 0.19 | 140.5 | 70.3 | −20% | 0.002 | 0.002 | |
| 2 | let-7b-5p | 705.0 | 325.2 | 586.6 | 239.3 | −17% | 0.008 | 0.13 | 563.8 | 226.2 | −20% | 0.01 | 613.6 | 254.5 | −13% | 0.07 | 0.03 | |
| 3 | miR-92a-3p | 2478.1 | 798.7 | 2166.8 | 792.2 | −13% | 0.02 | 0.17 | 2054.6 | 861.7 | −17% | 0.02 | 2298.7 | 690.9 | −7% | 0.22 | 0.09 | |
| 4 | miR-122–5p | 1037.7 | 1177.2 | 576.5 | 610.3 | −44% | 0.03 | 0.17 | 364.9 | 280.4 | −65% | 0.00 | 825.4 | 782.8 | −20% | 0.69 | 0.28 | |
| 5 | let-7g-5p | 67.5 | 36.1 | 59.2 | 26.8 | −12% | 0.03 | 0.17 | 59.8 | 26.5 | −11% | 0.09 | 58.6 | 27.5 | −13% | 0.09 | 0.05 | |
| 6 | miR-20a-5p | 649.8 | 249.9 | 573.4 | 222.1 | −12% | 0.03 | 0.17 | 565.1 | 239.6 | −13% | 0.06 | 583.1 | 202.7 | −10% | 0.14 | 0.07 | |
| 7 | miR-140–5p | 8.7 | 4.5 | 7.6 | 4.7 | −13% | 0.04 | 0.19 | 7.8 | 5.1 | −11% | 0.11 | 7.4 | 4.3 | −15% | 0.11 | 0.06 | |
Signals in arbitrary unit (A.U.);
Estimated by multiple linear regression model adjusted for age, sex, smoking, alcohol use, and recent infection with each log-transformed miRNA level as dependent variable;
false discovery rate;
values <0.20 were considered noteworthy.
Although serum levels of miR-150–5p and let-7b-5p showed significant exposure-response associations with increasing TCE exposure levels, the patterns of these relationships differed for these miRNAs in relation to the TCE exposure level. Specifically, whereas serum levels of miR-150–5p were significantly different among high-exposed (≥12 ppm) workers compared to control workers, for let-7b-5p serum levels were significantly different only among low-exposed (<12 ppm) workers compared to controls (Figure 1). Weak correlations were observed between levels of miR-150–5p and let-7b-5p (rsp=0.24 among workers exposed to TCE), as well as between levels of let-7b-5p and let-7g-5p, which belong to the same let-7 family of miRNAs (rsp=0.35 among workers exposed to TCE) (Supplementary Table 3).
Figure 1.
Box plots for the levels of miR-150–5p (a) and let-7b-5p (b) for controls, all exposed, low-exposed (<12 ppm), and high-exposed (≥12 ppm) (Signals in arbitrary unit (A.U.)). Percentage changes of the mean values and p-values are added.
In addition, only weak to moderate correlations were found between several immune markers and the two miRNA that showed a significant exposure-response with TCE (i.e., miR-150–5p and let-7b-5p) (Supplementary Table 4). The associations between TCE exposure and serum levels of these two miRNAs remained statistically significant after additional adjustment for these immune regulation markers in the regression models (data not shown). The miRNAs associated with TCE exposure were weakly correlated with age (Supplemental Table 5). Levels of some miRNAs were significantly different in men and women and in relation to current smoking status and alcohol consumption among exposed workers (Supplemental Table 5), but adjustment for these variables did not alter the significance of the associations with TCE exposure.
Supplementary Table 6 shows the results from comparison of the levels of miR-150–5p and let-7b-5p between controls and low exposure group with different cut points used. The p value for the difference between the low exposure group and controls became significant (i.e., <0.05) when 30 ppm was used as a cut point for miR-150–5p and 10 ppm was used as a cut point for let-7b-5p.
Discussion
We evaluated the association between TCE exposure and serum levels of miRNAs in a cross-sectional molecular epidemiological study in China. We found evidence of a significant exposure-response association with serum levels of two miRNAs (i.e., miR-150–5p and let-7b-5p) in workers occupationally exposed to TCE compared to unexposed controls. Our study is the first to evaluate effects of TCE on circulating levels of multiple miRNAs in humans and provides additional data on the underlying biologic perturbations of this exposure.
miR-150, which showed the strongest association with TCE exposure in our study, is mainly expressed in the lymph nodes and spleen and is highly up-regulated during the development of mature B and T cells (Zhou et al., 2007; Monticelli et al., 2005). MiR-150 has a critical role in regulating both lymphopoiesis and myelopoiesis (He et al., 2014). Dysregulation of miR-150 has been frequently observed in hematopoietic malignancies including mantle cell lymphoma (Zhao et al., 2010), mucosa-associated lymphoid tissue lymphoma (Cai et al., 2012), and NK/T-cell lymphoma (Watanabe et al., 2011). Although the underlying molecular mechanism of these associations has not been fully elucidated, direct targets of miR-150 include genes that have been directly implicated in carcinogenesis (e.g. MYB, FLT3, CBL, EGR2, AKT2 and DKC1), and experimental evidence suggests a tumor-suppressor role of miR-150 in leukemogenesis or lymphomagenesis (He et al., 2014). Specifically, Watanabe et al. (2011) reported that the aberrant downregulation of miR-150 induces continuous activation of the PI3K-AKT pathway, leading to telomerase activation and immortalization of NK/T-cell lymphoma cells. Recently, miR-150 knock-out B cells were shown to have altered B cell receptor pathways and significantly enhanced antigen presentation upon stimulation, ultimately leading to elevated inflammation compared to wild type B cells (Ying et al., 2016).
let-7b, a member of the let-7 family of miRNAs, is involved in inflammation and immune responses. The let-7 family includes 13 miRNAs that play an important role in tumor suppression and regulation of the innate immune response (Sathe et al., 2014). In an experimental study of gastric mucosa specimens and gastric epithelial cell lines infected with H.pylori (Teng et al., 2013), let-7b was shown to target toll-like receptor 4 (TLR4) mRNA, an important initiator in innate immune responses to microbial infections. TLR4 also regulates the activation of NF-kB and the expression of the downstream genes related to the inflammation and immune responses (Teng et al., 2013). Li et al. (2012) reported that TLR4 signaling induced the release of microparticles by hepatocarcinoma tumor cells that regulate levels of the inflammatory cytokine IL-6 of macrophages via miRNA let-7b. Therefore, decreased levels of let-7b could potentially contribute to increased inflammation that may have implications for lymphomagenesis. Consistent with this hypothesis, over-expression of let-7b-5p significantly suppressed cell proliferation in multiple myeloma (MM) tissues and cell lines (Xu et al., 2014) and mimics of let-7b-5p reduced the expression of IL6 and TNF in monocytes, and SERPINE1 expression in LPS-activated macrophages (Marques-Rocha et al., 2018). Given the broader role of let-7 in innate immunity, the suggestive exposure-response relationship also observed between levels of let-7g-5p and TCE exposure may possibly reflect the same underlying biologic response.
There is growing evidence that environmental or occupational exposure to chemicals may cause changes in miRNA profiles (Hou et al., 2011; Krauskopf et al., 2017). Our study adds to this evidence as we found two miRNAs that showed a significant exposure-response association with TCE exposure. In addition to considerable experimental evidence, recent results from molecular epidemiology studies suggest that miRNAs may potentially be intermediate markers linking environmental exposures and their associated health effects. Chen et al. (2018) suggested that effects of PM2.5 on cardiovascular diseases may be related to acute effects on cytokine expression, which may be partly mediated through effects of PM2.5 on miRNAs that regulate cytokine expression. Yokoyama et al. (2018) suggested that miRNAs may act as potential mediators of inflammation and atherosclerosis induced by cigarette smoking. Rahman et al. (2018) suggested that some placenta-derived miRNAs were significantly associated with birth weight, and the effects of the miRNAs were modified by in utero arsenic exposure. It is noteworthy that both miRNAs (i.e., miR-150–5p and let-7b) that were identified to be associated with TCE exposure in our study have been implicated in the tumorigenesis of non-Hodgkin lymphoma (NHL), as discussed above.
Although miRNAs can be downregulated or upregulated in accordance with genomic or epigenomic events, the exact mechanisms by which TCE exposure may result in alterations of miRNA expression remain to be elucidated. One possible explanation for increased miRNA expression is that severe oxidative stress caused by TCE exposure could lead to compromising the reservoir of the methyl donor (i.e., S-adenosylmethioinine (SAM)), which subsequently may contribute to DNA hypomethylation including miRNAs coding genes (Cui et al., 2016). However, this mechanism would not explain the significantly decreased levels of miR-150–5p or let-7b observed among the workers exposed to TCE in our study. We note that decreased numbers of lymphocytes and other immune markers observed among TCE exposed workers in our previous studies (Lan et al., 2010; Hosgood et al., 2012; Zhang et al., 2013; Bassig et al., 2013) also seemingly do not entirely explain the decreased levels of miR-150–5p and let-7b-5p considering that only weak to moderate correlations were found between these various markers (Supplementary Table 3).
This is the first cross-sectional molecular epidemiology study to evaluate associations between occupational TCE exposure and serum levels of multiple miRNAs. A key strength of the study was the range in exposure levels, including a subgroup of workers that were highly exposed to TCE, that enabled exposure-response analyses. The study also featured a well-characterized exposure assessment that was conducted over a year to identify factories with minimal co-exposures in the workplace. As such, the associations reported in our study are unlikely to be attributed to another occupational co-exposure. While our study has identified two miRNAs that showed an exposure-response relationship with TCE exposure, further studies are needed to confirm these results and to conduct agnostic “omic” approaches such as RNA sequencing that identify a more comprehensive suite of markers including other types of extracellular RNAs that may be involved in disease processes.
Limitations of our study include a relatively long storage (>10 years) time for the serum samples that were used in the miRNA analysis. Although miRNAs appear to be stable in frozen plasma or serum samples over months to years (Grasedieck et al., 2012), expression levels may be impacted by a decade or more of storage. However, from an epidemiologic perspective it is important to note that we used a standard protocol for biologic specimen collection that ensured that all samples were collected, processed, and stored using the same procedures among all subjects in our study. All samples for this study were also collected during the same time period and have been stored for the same amount of time. Thus, any potential measurement error in miRNA levels that may result from storage conditions is expected to be non-differential with respect to exposure status (i.e. storage related effects on miRNA levels should be relatively consistent in samples collected from exposed and unexposed workers) and would not be a likely explanation for the differences in serum miRNA levels observed between exposure groups. Second, we adjusted for several potential confounders that may influence serum miRNA levels but it is possible that other unmeasured lifestyle differences between the exposed and unexposed groups could have impacted our findings to some degree. Finally, the external generalizability of our study with respect to other ethnic groups is not clear given that miRNA levels in general may vary according to ethnicity (Huang et al. 2011). Potential ethnic-related differences in expression levels for miRNAs that showed significant associations with TCE exposure in our study (i.e., miR-150–5p, let-7b-5p, or let-7g-5p) have not been previously investigated to our knowledge.
In conclusion, our results suggest that exposure to TCE may be associated with decreased serum levels of miR-150–5p and let-7b-5p, which are involved in B-cell receptor pathways and the innate immune response. However, further studies in other populations occupationally exposed to TCE would be useful to replicate and expand upon our findings. In addition, the etiologic relevance of these miRNAs for risk of NHL would need to be evaluated in prospective cohorts with availability of pre-diagnostic blood specimens.
Supplementary Material
Acknowledgements
Funding Support:
Intramural funds from National Institutes of Health and National Cancer Institute; National Institute of Environmental Health Sciences (P42ES04705 and P30ES01896 to M.T.S.); Northern California Center for Occupational and Environmental Health and Department of Science and Technology of Guangdong Province, China (2007A050100004 to X.T.).
Funding: This work was supported by intramural funds from the National Cancer Institute and the National Institutes of Health and Guangdong Provincial Key Laboratory of Occupational Disease Prevention and Treatment (grant no. 2012A061400007), National Natural Science Foundation of China (grant no. 81502769), and International Research Fund (2018) from Korea National Open University.
Footnotes
Conflicts of Interest:
The authors have no conflicts of interest to declare.
Ethical approval: “All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Institutional Review Boards at the US National Cancer Institute and the Guangdong National Poison Control Center, China) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- Abcam. Benchmarking the FirePlex miRNA Assay. A comparison with other platforms. 2017. (https://docs.abcam.com/pdf/general/JP_miRNA_Benchmarking_Technote_2017.pdf) [accessed 28 Nov. 2018]
- Abcam. MicroRNA profiling directly from low amounts of plasma or serum using the Multiplex Circulating miRNA Assay with Firefly™ particle technology. Technical Note. 2018. (https://docs.abcam.com/pdf/kits/circulating-mirna-assay-technology-note.pdf) [accessed 28 Nov. 2018]
- Bao MH, Feng X, Zhang YW, Lou XY, Cheng Y, Zhou HH. Let-7 in cardiovascular diseases, heart development and cardiovascular differentiation from stem cells. Int J Mol Sci. 2013. November 21;14(11):23086–102. doi: 10.3390/ijms141123086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassig BA, Zhang L, Tang X, et al. Occupational exposure to trichloroethylene and serum concentrations of IL-6, IL-10, and TNF-alpha. Environ Mol Mutagen 2013;54(6):450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Liu X, Cheng J, Li Y, Huang X, Li Y, Ma X, Yu H, Liu H, Wei R. MicroRNA-200 is commonly repressed in conjunctival MALT lymphoma, and targets cyclin E2. Graefes Arch Clin Exp Ophthalmol. 2012. April;250(4):523–31. [DOI] [PubMed] [Google Scholar]
- Chen R, Li H, Cai J, Wang C, Lin Z, Liu C, Niu Y, Zhao Z, Li W, Kan H. Fine Particulate Air Pollution and the Expression of microRNAs and Circulating Cytokines Relevant to Inflammation, Coagulation, and Vasoconstriction. Environ Health Perspect. 2018. January 17; 126(1):017007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Choudhury SR, Irudayaraj J. Epigenetic Toxicity of Trichloroethylene: A Single-Molecule Perspective. Toxicol Res. 2016;5(2):641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mercado M, Manterola L, Lawrie CH. MicroRNAs in Lymphoma: Regulatory Role and Biomarker Potential. Curr Genomics. 2015. October;16(5):349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasedieck S, Schöler N, Bommer M, Niess JH, Tumani H, Rouhi A, Bloehdorn J, Liebisch P, Mertens D, Döhner H, Buske C, Langer C, Kuchenbauer F. Impact of serum storage conditions on microRNA stability. Leukemia. 2012;26(11):2414–6. [DOI] [PubMed] [Google Scholar]
- Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014. August;20(8):460–9. [DOI] [PubMed] [Google Scholar]
- He Y, Jiang X, Chen J. The role of miR-150 in normal and malignant hematopoiesis. Oncogene. 2014. July 24;33(30):3887–93. [DOI] [PubMed] [Google Scholar]
- Hosgood HD 3rd, Zhang L, Tang X, et al. Decreased Numbers of CD4(+) Naive and Effector Memory T Cells, and CD8(+) Naive T Cells, are Associated with Trichloroethylene Exposure. Front Oncol 2012; 10;1:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Wang D, Baccarelli A. Environmental chemicals and microRNAs. Mutat Res. 2011. September 1;714(1–2):105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RS, Gamazon ER, Ziliak D, Wen Y, Im HK, Zhang W, Wing C, Duan S, Bleibel WK, Cox NJ, Dolan ME. Population differences in microRNA expression and biological implications. RNA Biol. 2011;8(4):692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudder A, Novak RF. miRNA: Effectors of environmental influences on gene expression and disease. Toxicol Sci. 2008; 103(2):228–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. IARC Monographs 106: Tricholoetheylene, tetrachloroethylene, and some other chlorinated agents. 2014.
- Jiang Y, Chen J, Yue C, Zhang H, Tong J, Li J, Chen T. The Role of miR-182–5p in Hepatocarcinogenesis of Trichloroethylene in Mice. Toxicol Sci. 2017. March 1;156(1):208–216. [DOI] [PubMed] [Google Scholar]
- Krauskopf J, de Kok TM, Hebels DG, Bergdahl IA, Johansson A, Spaeth F, Kiviranta H, Rantakokko P, Kyrtopoulos SA, Kleinjans JC. MicroRNA profile for health risk assessment: Environmental exposure to persistent organic pollutants strongly affects the human blood microRNA machinery. Sci Rep. 2017. August 23;7(1):9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Q, Zhang L, Tang X, et al. Occupational exposure to trichloroethylene is associated with a decline in lymphocyte subsets and soluble CD27 and CD30 markers. Carcinogenesis 2010;31(9):1592–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Jia H, Zhang H, Lv M, Liu J, Zhang Y, Huang T, Huang B. TLR4 signaling induces the release of microparticles by tumor cells that regulate inflammatory cytokine IL-6 of macrophages via microRNA let-7b. Oncoimmunology. 2012. August 1;1(5):687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Rocha JL, Garcia-Lacarte M, Samblas M, Bressan J, Martínez JA, Milagro FI. Regulatory roles of miR-155 and let-7b on the expression of inflammation-related genes in THP-1 cells: effects of fatty acids. J Physiol Biochem. 2018. November;74(4):579–589. doi: 10.1007/s13105-018-0629-x. Epub 2018 May 22. [DOI] [PubMed] [Google Scholar]
- Mestdagh P, Van Vlierberghe P, De Weer A, Muth D, Westermann F, Speleman F, Vandesompele J. A novel and universal method for microRNA RT-qPCR data normalization. Genome Biol. 2009;10(6):R64. doi: 10.1186/gb-2009-10-6-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli S, Ansel KM, Xiao C, Socci ND, Krichevsky AM, Thai TH, Rajewsky N, Marks DS, Sander C, Rajewsky K, Rao A, Kosik KS. MicroRNA profiling of the murine hematopoietic system. Genome Biol. 2005;6(8):R71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman ML, Liang L, Valeri L, Su L, Zhu Z, Gao S, Mostofa G, Qamruzzaman Q, Hauser R, Baccarelli A, Christiani DC. Regulation of birthweight by placenta-derived miRNAs: evidence from an arsenic-exposed birth cohort in Bangladesh. Epigenetics. 2018;13(6):573–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2018. November 23. doi: 10.1002/jcp.27486. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Sathe A, Ayyar K, Reddy KVR. MicroRNA let-7 in the spotlight: Role in innate immunity. Inflamm Cell Signal 2014;1:66–75. [Google Scholar]
- Tagawa H, Ikeda S, Sawada K. Role of microRNA in the pathogenesis of malignant lymphoma. Cancer Sci. 2013. July;104(7):801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng GG, Wang WH, Dai Y, Wang SJ, Chu YX, Li J. Let-7b is involved in the inflammation and immune responses associated with Helicobacter pylori infection by targeting Toll-like receptor 4. PLoS One. 2013;8(2):e56709. doi: 10.1371/journal.pone.0056709. Epub 2013 Feb 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney M, Chandra A, Jain R, Ahmad R, Bihari V, Chandran CK, Mudiam MKR, Patnaik S, Goel SK. Occupational health hazards of trichloroethylene among workers in relation to altered mRNA expression of cell cycle regulating genes (p53, p21, bax and bcl-2) and PPARA. Toxicol Rep. 2015. May 15;2:748–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Tagawa H, Yamashita J, Teshima K, Nara M, Iwamoto K, Kume M, Kameoka Y, Takahashi N, Nakagawa T, Shimizu N, Sawada K. The role of microRNA-150 as a tumor suppressor in malignant lymphoma. Leukemia. 2011. August;25(8):1324–34. [DOI] [PubMed] [Google Scholar]
- Xu H, Liu C, Zhang Y, Guo X, Liu Z, Luo Z, Chang Y, Liu S, Sun Z, Wang X. Let-7b-5p regulates proliferation and apoptosis in multiple myeloma by targeting IGF1R. Acta Biochim Biophys Sin (Shanghai). 2014. November;46(11):965–72. doi: 10.1093/abbs/gmu089. Epub 2014 Sep 30. [DOI] [PubMed] [Google Scholar]
- Ying W, Tseng A, Chang RC, Wang H, Lin YL, Kanameni S, Brehm T, Morin A, Jones B, Splawn T, Criscitiello M, Golding MC, Bazer FW, Safe S, Zhou B. miR-150 regulates obesity-associated insulin resistance by controlling B cell functions. Sci Rep. 2016. February 1;6:20176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama Y, Mise N, Suzuki Y, Tada-Oikawa S, Izuoka K, Zhang L, Zong C, Takai A, Yamada Y, Ichihara S. MicroRNAs as Potential Mediators for Cigarette Smoking Induced Atherosclerosis. Int J Mol Sci. 2018. April 6;19(4). pii: E1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Bassig BA, Mora JL, Vermeulen R, Ge Y, Curry JD, Hu W, Shen M, Qiu C, Ji Z, Reiss B, McHale CM, Liu S, Guo W, Purdue MP, Yue F, Li L, Smith MT, Huang H, Tang X, Rothman N, Lan Q. Alterations in serum immunoglobulin levels in workers occupationally exposed to trichloroethylene. Carcinogenesis. 2013;34(4):799–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JJ, Lin J, Lwin T, Yang H, Guo J, Kong W, Dessureault S, Moscinski LC, Rezania D, Dalton WS, Sotomayor E, Tao J, Cheng JQ. microRNA expression profile and identification of miR-29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphoma. Blood. 2010. April 1;115(13):2630–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B1, Wang S, Mayr C, Bartel DP, Lodish HF. miR-150, a microRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurely. Proc Natl Acad Sci U S A. 2007. April 24;104(17):7080–5. Epub 2007 Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.