Abstract
CD19-targeted CAR-T cell therapy has shown excellent anti-tumor activity in patients with relapsed/refractory B cell malignancies, with very encouraging response rates and outcomes. However, the late effects following this therapy are still unknown. Here we report late adverse events, defined as beginning or persisting beyond 90 days after CAR-T cell infusion, in patients who survived at least one year after therapy. Median follow-up was 28.1 months (range, 12.5–62.6). At last follow-up 73% of patients were still alive, and 24% were in ongoing complete remission (1). The most common late adverse event was hypogammaglobulinemia (IgG <400 mg/dL or IVIG replacement), observed in 67% of patients with available data. Infection density was 0.55 infections/100 days at risk (2.08/patient year). 80% of the infections were treated in the outpatient setting, and 5% required admission to the intensive care unit. Subsequent malignancies occurred in 15% of patients, including 5% myelodysplastic syndrome (MDS). Among patients with ongoing CR and with no MDS, 16% experienced prolonged cytopenias, requiring transfusions or growth factor support. Graft versus host disease (GVHD) occurred in three of 15 patients (20%) who had a prior allogeneic hematopoietic cell transplantation. Most of the late events observed in this cohort were not severe and many could be related to prior or subsequent therapies, suggesting a safe long-term profile of CD19-targeted CAR-T cell immunotherapy.
Keywords: CD19-targeted CAR-T cells, late events, hypogammaglobulinemia, cytopenia, subsequent malignancies
Introduction
CD19-targeted chimeric antigen receptor modified T-cell (CD19 CAR-T cell) immunotherapy has shown excellent anti-tumor activity in patients with relapsed/refractory acute lymphoblastic leukemia (ALL) (2) and non-Hodgkin lymphoma (NHL) (3, 4), which led to the approval of tisagenlecleucel (Kymriah®) and axicabtagene ciloleucel (Yescarta®) by the USA Food and Drug Administration (FDA).
At Fred Hutchinson Cancer Research Center (FHCRC), a phase I/II clinical trial using CD19 CAR-T cells demonstrated high response rates in patients with relapsed/refractory ALL, NHL and chronic lymphocytic leukemia (CLL). Patients were treated with lymphodepletion chemotherapy followed by infusion of autologous T cells modified to express a second generation CD19-targeting chimeric antigen receptor incorporating single chain variable fragment (scFv) derived from the murine IgG1 anti-CD19 monoclonal antibody, FMC63, and a 4–1BB costimulatory domain (5, 6). The CD19 CAR-T cells were manufactured from defined T cell subsets and administered in a 1:1 CD4+/CD8+ CAR-T cell ratio. Optimal results were achieved with cyclophosphamide and fludarabine (Cy/Flu) lymphodepletion prior to CAR-T cells infusion (7). The trial evaluated three dose levels of 2 × 10^5, 2 × 10^6 and 2 × 10^7 CAR-T cells/kg as been previously published (8). Across all dose levels, among ALL patients, 85% achieved minimal residual disease (MRD)-negative complete remission determined by high resolution flow cytometry (9). The overall response rate among NHL patients was 51% with 40% CR (10). Among CLL patients, the ORR and CR were 74% and 21%, respectively (11).
CD19 CAR-T cells can cause unique early toxicities, such as cytokine release syndrome (CRS) (12, 13) and acute neurotoxicity (14). However, potential long-term adverse events are still unknown (15–18), thus the objective of the current study was to describe late events after treatment with CD19 CAR-T cells among one-year survivors who achieved or did not achieve CR after treatment.
Patients and Methods
The study cohort included 86 patients with relapsed/refractory ALL, NHL, or CLL treated with CD19 CAR-T cells on a phase I/II clinical trial () between July 2013 and February 2017 who survived at least 12 months after treatment. The study was approved by the FHCRC institutional review board, and all patients provided informed consent for treatment and for long-term follow-up.
We report late events in the entire study cohort, as well as late events in patients with ongoing CR, defined as CR that was achieved at first evaluation (approximately 4 weeks after CAR-T cell administration) and continued until last follow-up with no additional therapy. CR was defined according to the diagnosis: NHL, based on the Lugano criteria (5); CLL, based on International Workshop on Chronic Lymphocytic Leukemia (IWCLL; 2008) criteria (11); ALL, based on disease detection in bone marrow sample by morphology, flow cytometry and molecular testing: CR was defined as <5% blasts by morphology and minimal residual disease (MRD) was defined as <5% blasts by morphology, but evidence of disease by flow cytometry or molecular testing. Patients who achieve CR after CAR-T cells and received consolidation allogeneic transplant were not included in the ongoing CR group.
We retrospectively reviewed patient’s medical records for evaluation of late events after CAR-T cell therapy. Late events were defined as events that presented and/or persisted beyond 90 days after CAR-T cell infusion. The following events categories were identified and are reported: (1) significant cytopenias, (2) hypogammaglobulinemia, (3) infections, (4) subsequent malignancies, (5) immune-related events, (6) Graft Versus Host Disease (GVHD) in previous allogeneic HCT recipients, and (7) neurologic and psychiatric events.
Due to the different nature of the events, there is variability in the patient population that was included for evaluation of each event. Table 1 describes in detail the late events and the specific patient population that was evaluated for each event. Briefly, Significant cytopenias were defined as cytopenias that required red blood cell or platelet transfusions, or growth factor support. For cytopenia analysis we included only patients with ongoing CR who were not diagnosed with myelodysplastic syndrome (MDS) after CAR-T cell therapy. Late hypogammaglobulinemia was defined as IgG level < 400mg/dL and/or documentation in medical records of at least one-time intravenous immunoglobulin (IVIG) replacement beyond day 90 after CAR-T cell infusion. Evaluation for hypogammaglobulinemia was censored at the time of any systemic therapy for the underlying disease. For analysis of late infections we considered infection events reported in medical records between day 90 after CAR-T cell infusion until death or initiation of any systemic therapy for treatment of the underlying disease. All newly diagnosed malignancies after CD19 CAR-T cell therapy were reported. All possible immune-related events, neurological events and psychiatric conditions that required medical interventions (referral to specialist or pharmacological therapy), which presented or persisted beyond day 90 after CAR-T cells, were reported. All late events observed are reported without taking the level of attribution to CAR-T cell therapy into account.
Table 1:
Definitions of late events and patient population evaluated for each event.
| Event | Definition | Censoring criteria | Denominator | |
|---|---|---|---|---|
| Subsequent systemic therapy for underlying disease | Subsequent transplant | |||
| Late significant cytopenias | Cytopenias that required red blood cell or platelet transfusions, or growth factors support. Occurred and/or persisted beyond 90 days after first CAR-T cell infusion. |
Yes | Yes | N=19 Included patients with ongoing CR without diagnosis of subsequent MDS. |
| Late hypogammaglobulinemia | IgG levels < 400 mg/dL and/or intravenous immunoglobulin (IVIG) replacement. Occurred and/or persisted beyond 90 days after first CAR-T cell infusion. |
Yes | Yes | N=42 Included patients with available IgG data > 90 days after CAR-T cells |
| Late infections | Any infection documented in medical records (with or without microbiologic or radiologic evidence). Occurred and/or persisted beyond 90 days after first CAR-T cell infusion. We considered two different infection events if: (1) occur at different time points (2) different pathogen groups (viral, bacterial, fungal, parasite) were identified (even if documented at the same time point) or (3) different non-adjacent organs were affected (even if documented at the same time point). |
Yes | Yes | N=54 Included patients with available infection data > 90 days after CAR-T cells |
| Subsequent malignancies | All malignancies with pathology confirmation that were diagnosed after CAR-T cell administration. | No | Yes | N=86 Entire cohort |
| Immune-related events | Any possible immune-related condition (except infections), even without a formal diagnostic confirmation. Occurred and/or persisted beyond 90 days after first CAR-T cell infusion. |
No | Yes | N=86 Entire cohort |
| Psychiatric and neurologic disorders | Occurred and/or persisted beyond 90 days after first CAR-T cell infusion. | No | No | N=86 Entire cohort |
| GVHD | GVHD requiring systemic therapy. | No | Yes | N=15 Patients with history of allogeneic HCT prior to first CAR-T cell infusion. |
Statistical Methods
This is a retrospective observational study. To evaluate association between CR status and late events p-values were calculated using Wilcoxon rank-sum test for continuous characteristics, and Fisher’s exact test for categorical characteristics. Infection density was reported as the mean number of infections per 100 days at risk, and was calculated as the total number of infection events in the entire cohort divided by the number of days at risk and multiplied by 100. Due to the limited sample size, multivariate analyses were not performed. P-values reported are two-sided without multiplicity adjustment.
Results
Patients characteristics and outcome
Of 163 patients treated on through the end of February 2017, 86 (53%) patients survived at least one year following CD19 CAR-T cell infusion as of March 2018 and were included in this study: 43 with NHL, 26 with ALL, and 17 with CLL. Median age of the study cohort was 57 years (rage, 23 to 75) and 73% were males. The median number of prior lines of therapy was 4 (range, 1 to 8). Twenty-four patients with NHL had prior autologous hematopoietic cell transplantation (HCT), accounting for 56% of NHL patients and 28% of the entire cohort. Fifteen patients (17%) had prior allogeneic HCT: 23% of ALL, 18% of CLL and 14% of NHL patients. Characteristics of the cohort are shown in table 2.
Table 2:
Patient characteristics
| Patient characteristics | Entire cohort (N=86) | NHL* (N=43) | CLL (N=17) | ALL (N=26) | Ongoing CR (N=21) | Non-ongoing CR (N=65) | p-value** |
|---|---|---|---|---|---|---|---|
| Gender: | |||||||
| Male | 63 (73%) | 32 (74%) | 12 (71%) | 19 (73%) | 12 (57%) | 51 (78%) | 0.09 |
| Female | 23 (27%) | 11 (26%) | 5 (29%) | 7 (27%) | 9 (43%) | 14 (22%) | |
| Median age, in years (range) | 57 (23–75) | 59 (34–70) | 63 (41–73) | 40 (23–75) | 58 (22–73) | 56 (23–74) | 0.92 |
| Median number of prior lines of therapy (range) | 4 (1–8) | 4 (1–7) | 5 (2–8) | 2 (10–7) | 4 (2–8) | 3 (1–7) | 0.51 |
| Prior HCT: | 35 (41%) | 26 (60%) | 3 (18%) | 6 (23%) | 13 (62%) | 22 (34%) | 0.04 |
| Autologous HCT before CAR-T cells | 24 (28%) | 24 (56%) | 0 | 0 | 8 (38%) | 16 (25%) | 0.27 |
| Allogeneic HCT before CAR-T cells | 15 (17%) | 6 (14%) | 3 (18%) | 6 (23%) ⋄ | 8 (38%) | 7 (11%) | 0.008 |
| Median time from prior allogeneic HCT to first CAR-T cell infusion, in months (range) | 23.5 (3.2–143.6) | 32.1 (6.7–55.2) | 88.4 (26.5–143.6) | 16.8 (3.2–76.5) | 18 (3–144) | 24 (16–88) | 0.46 |
| Lymphodepletion chemotherapy | |||||||
| Cy/Flu ♦ | 74 (86%) | 35 (81%) | 16 (94%) | 23 (88%) | 20 (95%) | 54 (83%) | 0.28 |
| Other ♦ | 12 (14%) | 8 (19%) | 1 (6%) | 3 (12%) | 1 (5%) | 11 (17%) | |
| Dose (CAR T-cells/Kg) | |||||||
| Level 1 (2×105) | 20 (23) | 2 (4%) | 4 (23%) | 14 (54%) | 1 (5%) | 19 (29%) | 0.04 |
| Level 2 (2×106) | 60 (70%) | 36 (84%) | 13 (77%) | 11 (42%) | 18 (85%) | 42 (65%) | |
| Level 3 (2×107) | 6 (7%) | 5 (12%) | 0 | 1 (4%) | 2 (10%) | 4 (6%) |
NHL: DLBCL (N=21); FL (N=13); mantle cell lymphoma (N=6); marginal zone lymphoma (N=2), Burkitt lymphoma (N=1).
1 patient with 2 allogeneic HCT before CAR-T cells.
Patients received one of several lymphodepleting regimens: cyclophosphamide (Cy), 2 to 4g/m2 iv (intravenously) on day 1; Cy, 2 to 4 g/m2 iv on day 1 plus Etoposide (E), 100 to 200 mg/m2/day iv on days 1 to 3 (Cy/E); Cy, 60 mg/kg iv on day 1 plus fludarabine (Flu) 25 mg/m2/day iv on either days 2 to 4 or days 2 to 6, Cy 300 mg/m2/day × 3 days plus Flu 30 mg/m2/day × 3 days, or Cy 500 mg/m2/day × 3 days plus Flu 30 mg/m2/day × 3days (Cy/Flu); Bendamustine 90 mg/m2/day (one patient).
Ongoing CR versus non-ongoing CR. P-values calculated using Wilcoxon Rank sum test for continuous characteristics, and Fisher’s Exact test for categorical characteristics.
Patients were followed from first CAR-T cell infusion until last contact or death. The median duration of follow-up was 28.1 months (range, 12.5 to 62.6). Fifteen patients (17%) received a second CAR-T cell infusion and two patients (2%) received a third infusion. Thirty-five patients (41%) received allogeneic HCT post CAR-T cells, including twelve patients (14%) who received allogeneic HCT as consolidation after achieving CR with CAR-T cell therapy (11 ALL patients with MRD-neg CR and one NHL patient).
Seventeen patients (20%) died of disease progression, at a median of 19 months (range, 12.5 to 39) after first CAR-T cell infusion. Six patients died of non-relapse causes (7%): five patients died of complications after allogeneic HCT and one patient died of complications of multiple myeloma. Sixty-three patients (73%) were alive at last follow-up. Twenty-one patients (24%) were in ongoing CR (NHL=14, CLL=4, ALL=3) at a median follow-up of 34 months (range, 18–44).
Late Events after CAR-T Cell Therapy
1. Significant prolonged cytopenias
Three of 19 patients (16%) with ongoing CR and no diagnosis of MDS experienced prolonged significant cytopenias that lasted for 15.2 to 21.7 months after CAR-T cell infusion. Supplemental table 1 describes the cytopenia events in detail.
2. Hypogammaglobulinemia
Thirty-four patients (40%) had hypogammaglobulinemia (documented IgG levels < 400 mg/dL and/or IVIG replacement) prior to lymphodepletion. Data on IgG levels or IVIG replacement beyond day 90 after CAR-T cell infusion and before subsequent systemic therapy for underlying disease were available for 42 patients (19 ongoing CR; 23 non-ongoing CR). Twenty-eight patients (67%) had evidence of hypogammaglobulinemia beyond day 90 and prior to initiation of subsequent therapy, of whom 18 already had hypogammaglobulinemia before CAR-T cells. Among the ongoing CR group, 14 patients (74%) had documented hypogammaglobulinemia beyond day 90 after CAR-T cell infusion (table 3).
Table 3:
Hypogammaglobulinemia
| NHL (N=43) | CLL (N=17) | ALL (N=26) | Entire cohort (N=86) | Ongoing CR (N=21) | Non-ongoing CR (N=65) | P value# | |
|---|---|---|---|---|---|---|---|
| Hypogammaglobulinemia (IgG < 400 mg/dL or IVIG) pre-lymphodepletion ^ | 13 (30%) | 13 (76%) | 8 (31%) | 34 (40%) | 9 (43%) | 25 (38%) | 0.80 |
| Late hypogammaglobulinemia (% of patients with available data; N=42) * | 13/23 (56%) | 10/13 (77%) | 5/6 (83%) | 28/42 (67%) | 14/19 (74%) | 14/23 (61%) | 0.51 |
Last IgG level/IVIG data before lymphodepletion (within 60 days)
Documentations in medical records of IgG level <400 mg/dL and/or IVIG replacement at any time beyond 90 days after CAR-T cell infusion and before subsequent therapy for the underling disease HCT.
P values are for ongoing CR versus non-ongoing CR
3. Late infections
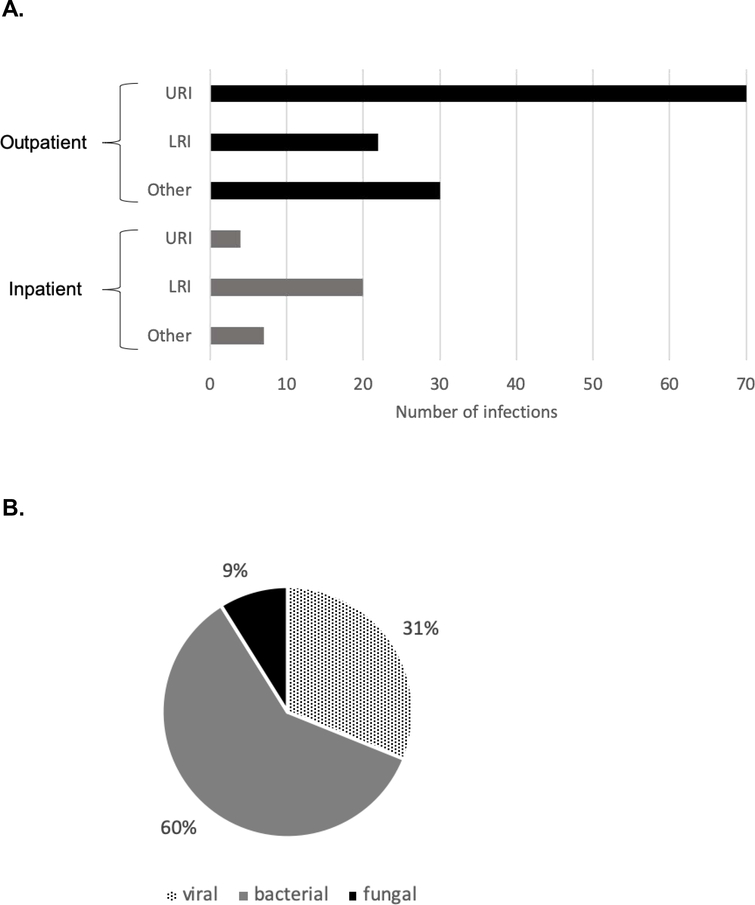
Data on late infections were available for 54 patients, among them 33 patients (61%) had at least one infection for a total of 153 infection events. The infection density was 0.55 infections/100 days at risk (2.08/patient year). The most common infections were upper respiratory tract infections (48%), followed by lower respiratory infections (23%). Eighty percent of the infections were treated in the outpatient setting, and 20% required admission, including 5% requiring intensive care (Figure 1A, B). Thirty-seven of 153 (24%) recorded infection events had a microbiological etiology, including 60% bacterial infections, 31% viral infections (mostly respiratory viruses) and 9% fungal infections (aspergillus (N=2), candida (N=1) and coccidioidomycoses (N=1)) (Figure 1C). Supplemental Table 2 describes the infection events in detail, and the identified organism are shown in supplemental Table 3.
Figure 1. Late infections.
A. Late infections managed outpatient (N=122) and inpatient (N=31), including infections requiring intensive care (N=7, all LRI). URI – upper respiratory infection, LRI – lower respiratory infection, Other – other infections (N=37): bacteremia (N=1), febrile neutropenia (N=1), conjunctivitis (N=2), oral infections (including HSV and candida) (N=4), genitourinary tract infections (N=4), gastrointestinal infections (N=5), osteomyelitis (N=1), skin including cellulitis, HPV, HSV, zoster, tinea (N=19).
B. Infections with microbiologic evidence: 37 infections.
4. Subsequent malignancies
Thirteen patients (15%) in the entire cohort developed subsequent malignancies: Six (7%) non-melanoma skin cancer, four (5%) MDS, one (1%) melanoma, one (1%) non-invasive bladder cancer, and one (1%) multiple myeloma (MM). Among the 21 patients with ongoing CR, six (29%) developed subsequent malignancies, including MDS (N=2), non-melanoma skin cancer (N=2), melanoma (N=1), MM (N=1).
Among the 13 patients diagnosed with subsequent malignancies, the median number of prior lines of therapy was four (range, 1–7), and eight patients (62%) had prior autologous or allogeneic HCT. Importantly, two of the four patients with subsequent diagnosis of MDS had cytogenetics abnormalities prior to CAR-T cell therapy, and the patient who developed subsequent MM had diagnosis of MGUS prior to CAR-T cell therapy.
The median time from first CAR-T cell infusion to diagnosis of non-melanoma skin cancer was 16 months (range, 1–35) and to diagnosis of MDS was 6 months (range, 4–17). The cases of bladder cancer, MM and melanoma were diagnosed 2, 6 and 8 months after CAR-T cell infusion, respectively. No replication-competent lentivirus (RCL) was detected in any of the CAR-T cell products prior to infusion and in all blood samples tested after CAR-T cell infusion.
All patients with skin cancer and the patient with bladder cancer were treated with resection, with or without topical treatment. Among the patients with MDS one patient died from MDS and active NHL, one patient received allogeneic HCT for MDS and CLL, and two patients with ongoing CR for NHL received hypomethylation treatment for MDS. The patient with MM died as consequence of that disease. Supplemental table 4 describes the subsequent malignancies in detail.
5. Immune-related events
Among the 86 patients in the cohort we identified 7 patients (8%) with new possible immune-related events, including: lymphocytic alveolitis (associated with elevated ferritin level); persistent skin rash (with biopsy consistent with spongiosis and psoriaform dermatitis, and with CAR-T cells detected in skin biopsy tissue by qPCR); eosinophilic pneumonia; pneumonitis not otherwise specified (NOS); granulomatous disease NOS; persistent flu-like syndrome (malaise, fatigue, arthralgia, myalgia) for several months with negative infectious workup; and collagenous colitis. Median time of symptoms onset was 234 days after CAR-T cell infusion (range, 67–1099). The eosinophilic pneumonia occurred in the setting of relapsed NHL, and the pneumonitis NOS occurred in the setting of ibrutinib therapy. Two events occurred in patients with ongoing CR (lymphocytic alveolitis and flu like syndrome). No re-expansion of CAR-T cell counts in blood was seen around the time of clinical events, and no association could be made between CAR-T cells and the presumed immune-related events. Supplemental table 5 describes the immune-related events in detail.
6. GVHD
Fifteen patients had prior allogeneic HCT at a median of 37 months (range, 3.2–143.6) before CAR-T cell infusion and were at risk of developing GVHD. None had GVHD at the time of CAR-T cell infusion. We identified three patients (20%) who developed GVHD requiring systemic therapy after CAR-T cells; all were in ongoing CR for their baseline disease. One patient with no prior history of GVHD developed late acute GVHD after CAR-T cell affecting gastrointestinal tract, liver and skin, and had complete resolution after treatment with prednisone. Two patients, one of whom with prior history of acute GVHD after transplant, developed chronic GVHD after CAR-T cells, requiring multiple lines of therapy. GVHD developed 1.9 to 3.2 months after CAR-T cell infusion, and all patients had low level CAR-T cell persistence at the time of initial GVHD manifestation. Supplemental table 6 describes the GVHD events in detail.
7. Neurologic and psychiatric events
Among the 86 patients in the cohort nine patients (10%) were found to have 11 new neurologic findings, including three cerebrovascular accident (CVA) events and one transient ischemic attack (TIA). Two events occurred in two patients (13%) with ongoing CR: Alzheimer dementia and peripheral neuropathy.
We identified eight patients (9%) with psychiatric events requiring intervention: four with newly diagnosed and four with exacerbation of previous mood disorders (depression and anxiety). Two events occurred in patients with ongoing CR: one patient had overlap depression and dementia, and the other had exacerbation of depression, including a suicide attempt. Supplemental table 7 describes the neuropsychiatric events in details.
Late events in patients with ongoing CR
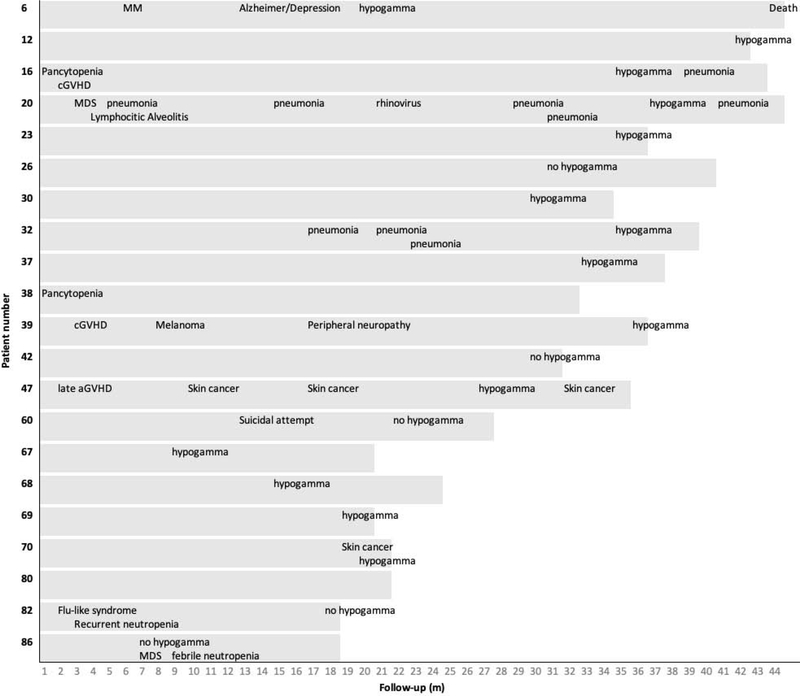
Three of 19 patients (16%) with ongoing CR and without subsequent diagnosis of MDS were found to have prolonged cytopenia lasting for 15.2 to 21.7 months after CAR-T cell infusion. Seventy-four percent of patients with available data had prolonged hypogammaglobulinemia, and the infection density in this group was 0.56/100 days at risk. Six patients (29%) developed subsequent malignancies (MDS (N=2), non-melanoma skin cancer (N=2), melanoma (N=1), MM (N=1)), and two patients (10%) experienced possible immune-related events. Figure 2 shows the late events in this group and Table 4 summarizes the late events in this group and in the entire cohort. Although small numbers, our data suggest no significant differences in late events between patients with ongoing CR to patients who did not achieve ongoing CR (Table 4).
Figure 2. Late events in patients with ongoing CR.
MM = multiple myeloma, hypogamma = hypogammaglobulinemia (IgG <400 and/or IVIG replacement), MDS = myelodysplastic syndrome, cGVHD = chronic graft-versus-host disease, aGVHD = acute graft-versus-host disease. Hypogammaglobulinemia data were not available for patients 38 and 80.
Table 4:
Summary of Late Events after CD19 targeted CAR-T cells
| Late adverse events* | Entire cohort (N=86) | Ongoing CR (N=21) | Non-ongoing CR (N=65) | p-value** |
|---|---|---|---|---|
| Cytopenias (evaluated only in patients with ongoing CR and no diagnosis of subsequent MDS) | - | 3/ 19 (16%) | - | - |
| Hypogammaglobulinemia (% of patients with available data) | 28/42 (67%) | 14/19 (74%) | 14/ 23 (61%) | 0.51 |
| Infection density; mean number of infections/100 days at risk (Number of patients evaluated) | 0.57 (53) | 0.57 (20) | 0.58 (33) | 0.58† |
| Subsequent malignancies (%) | 0.08 | |||
| - All subsequent malignancies | 13 (15%) | 6 (29%) | 7 (11%) | |
| - MDS | 4 (5%) | 2 (10%) | 2 (3%) | 0.25 |
| - non-melanoma skin cancer | 6 (7%) | 2 (10%) | 4 (6%) | 0.63 |
| Immune-related events (%) | 7 (8%) | 2 (10%) | 5 (8%) | 1.00 |
| Neurologic events (%) | 9 (10%) | 2 (10%) | 7 (11%) | 1.00 |
| Patients with CVA/TIA | 4 (5%) | 0 (0%) | 4 (5%) | 0.57 |
| Psychiatric events (%) | 8 (9%) | 2 (10%) | 6 (9%) | 1.00 |
| GVHD (% of patients with prior allogeneic HCT) | 3/15 (20%) | 3/8 (38%) | 0/7 (0%) | 0.20 |
Number of patients with late adverse evens
Ongoing CR versus non-ongoing CR. P-values calculated using Fisher’s exact test.
P-value calculated using t-test from 100 bootstrap samples
Discussion
CD19-targeted CAR-T cell therapy has revolutionized the treatment of relapsed/refractory B-cell malignancies, with unprecedented response rates in heavily pre-treated patient population. However, data regarding long-term events after this novel therapy are still scarce. In this study we described late events in 86 patients with NHL, CLL and ALL, who had at least one-year follow-up after treatment with CD19-targeted CAR-T cells on a dose finding phase I/II clinical trial.
Overall, our data suggest that CD19 CAR-T cell therapy has acceptable long-term safety. Only six patients in our cohort (7%) died of non-relapse causes, with five deaths due to HCT-related complications.
The most common late event identified was hypogammaglobulinemia, an expected “on-target off-tumor” effect of CD19 CAR-T cells. Among patients with available data, 67% of the patients had documented hypogammaglobulinemia/IVIG replacement. Locke et al. reported 44% IVIG replacement among DLBCL patients with ongoing remission in the ZUMA1 study (18), and Park et al. demonstrated low IgG levels in 83% of ALL patients at least one month after achieving CR following treatment with CD19 CAR-T cells on a phase 1 study. Maude et al. demonstrated B-cell aplasia at 6 months after treatment with tisagenlecleucel in 83% of ALL patients (2). Taking together, our data support prior findings demonstrating hypogammaglobulinemia as the most common late event after treatment with CD19 CAR-T cells.
Hill et al. studied early infections (up to 90 days after CAR-T cell infusion) in patients treated on the clinical trial reported here, and found that the incidence and severity of infections were comparable to those seen after other chemo-immunotherapies. The majority of the late infections identified in our study were mild and managed in the outpatient setting. In most cases that required hospital or ICU admission, there were other factors that contributed to an increased risk of infection. Locke et al. reported that 28% of patients on the ZUMA1 trial developed grade ≥ 3 infections, including early infections; however, only one serious lung infection was reported beyond 12 months post treatment (18). Maude et al. reported grade ≥ 3 infections in 36% of patients during a median follow-up of 13.1 months, and 3 deaths associated with infections more than 30 days after CAR-T cell infusion (2). Park et al. showed that 10 of 32 patients (31%) had 15 infections between day 31 and day 180 after CAR-T cells, 6 (40%) with documented hypogammaglobulinemia. Similar to our findings, Kochenderfer et al. reported mild viral infections and one pneumonia required hospitalization among four patients with DLBCL with ongoing (> 3) CR after CD19 CAR-T cell therapy (15). Porter et al. reported one death in a patient in CR, 21 months after CAR-T cell infusion from complications of a pseudomonas wound infection after basal cell carcinoma removal, despite IVIG replacement (19). Comparing our results with other CD19 CAR-T cell studies is challenging as we only included infections occurring at least 90 days after CAR-T cell infusion in patients who survived at least one year after CAR-T cell infusion, while others included early infections as well. However, in line with others, our findings support low rate of severe late infections after CD19 CAR-T cell therapy.
Cytopenias are expected early after CAR-T cell therapy due to lymphodepletion chemotherapy. Some patients on our study received high intensity lymphodepletion regimens, which may increase the risk of developing prolonged cytopenia. Three of 19 patients (16%) with ongoing CR and with no MDS diagnosis had persistent significant cytopenias (requiring RBC or platelet transfusion, or growth factor support) more than 3 months after treatment (two of whom received high intensity lymphodepletion regimen). Similarly, 17% of patients in the ZUMA1 study had grade ≥ 3 cytopenia three months after CAR-T cell infusion or later (18), and 12% and 11% of patients in the ELIANA study had grade ≥ 3 thrombocytopenia and neutropenia, respectively, at median follow-up of 13.1 months after CAR-T cells (2). The mechanism of prolonged cytopenia after CD19 CAR-T cell therapy is unclear, and more work is needed to evaluate its etiology. Additionally, as all patients are heavily pre-treated it is important to rule out pre-existing treatment-related marrow hypoplasia or MDS as cause of cytopenia after CD19 CAR-T cell therapy.
The development of a new malignancy is a potential risk of genetically modified cellular therapies. However, to date, there have been no reports of T-cell malignancy following CAR-T cell therapy nor reports of replication-competent retro/lenti-virus in T-cell products (20). One case of transduction of leukemic cells associated with resistant ALL has been reported (21). We identified subsequent malignancies in 13 (15%) patients; however, most (7%) were non-melanoma skin cancer. Four patients (5%) were diagnosed with MDS, three of whom had prior autologous HCT and two received high/intermediate intensity lymphodeplytion regimen, which may increase the risk of subsequent MDS. A rate of 12.5% (3/24) treatment related MDS is within the range of the expected risk in a post-autologous transplant population (22). Other groups also reported cases of MDS after CD19 CAR-T cell therapy likely related to prior therapies (15) (18). The rate of 11% secondary malignancies among NHL patients in our cohort is similar to the expected incidence of secondary malignancies after conventional treatment of NHL (4–10%) (23–26). At this time we have no data regarding the effect of CAR-T cells on development or aggressiveness of subsequent malignancies. Additional studies are needed to further assess whether or not CAR-T cells increase risk for subsequent malignancies.
Autoimmune reactions are another potential concern regarding CAR-T cell therapy. In this study, we identified seven patients (8%) with possible immune-related events. Most of the events were idiopathic and did not meet diagnostic criteria for any autoimmune disease. Although one event was associated with elevated ferritin level, and in one event CAR-T cells were detected in the skin biopsy by qPCR, there was no good evidence to indicate that the subsequent events were due to CAR-T cell therapy. Hu et al. reported that 0.2% of patients with NHL treated with conventional treatment in a large registry study developed autoimmune diseases over a median time of 2.6 years after the lymphoma diagnosis (27). An important difference between the report by Hu et al. and our study is that we report any possible immune-related event while Hu el al. reported only diseases that met diagnostic criteria of defined autoimmune diseases.
Acute neurotoxicity is a well documented adverse event after CAR-T cells (14, 28). We identified late neurologic events in 10% of the patients. Although none of the events could be directly attributed to the CAR-T cell therapy, the presence of vascular phenomena may be related to endothelial activation reported during acute CAR-T cell-mediated neurotoxicity (14). Neuropsychiatric events are very hard to analyze, due to subjectivity and confounding factors in this heavily pre-treated population, and ongoing efforts are in progress to determine if there are lasting neuropsychiatric sequelae after CAR-T cell therapy.
Patient who receive CAR-T cell therapy after prior allogeneic HCT could be at risk for GVHD exacerbation associated with alloreactive T cell infusion and systemic inflammation during CRS. Fifteen patients in our cohort had prior allogeneic HCT, among them three patients (20%) developed GVHD requiring systemic immunosuppressive therapy after CAR-T cells. Similarly, Hu et al. reported acute GVHD in 2 out of 11 patients (18%) who received CAR-T cells after prior allogeneic HCT (29). This is not an unexpected rate of GVHD at this time period after transplant (30), suggesting the risk of exacerbation of GVHD is not markedly increased after CAR-T cell therapy. Although the three patients in our cohort who developed GVHD had CAR-T cell persistence at time of GVHD diagnosis, at this time there is no data to support association between CAR-T cell persistence and development of GVHD. Larger studies are needed to further assess the potential association between CAR-T cell persistence and development of GVHD after CAR-T cell therapy.
This study has several limitations, the most significant being the retrospective data collection and missing data, the small sample size and the progressively smaller number of subjects in remission on whom results are reported, as well as the phase I/II dose finding nature of the clinical trial patients were treated on. Several patients in the cohort returned to the care of their primary oncologists outside of our center, and thus data collection was not consistent for all patients. Despite the limitations this study provides useful approximations of the incidences of late adverse events that will be helpful in counselling patients undergoing CD19 CAR-T cell therapy.
Conclusion
Our results suggest that CD19 CAR-T cell therapy has acceptable safety, as most of late events seen in our cohort were mild and likely related to the underlining disease and/or prior or subsequent therapies. Continuing prospective systematic follow-up is needed for better understanding of late effects of CAR-T cell therapy and for establishing evidence-based long-term follow-up and treatment guidelines.
Supplementary Material
Highlights.
Hypogammaglobulinemia was the most common late event.
Most infections were mild and treated in the outpatient setting.
Subsequent malignancies occurred in 15% of patients.
16% of patients with ongoing CR and no MDS, experienced prolonged cytopenias.
GVHD occurred in 20% of patients who had a prior allogeneic HCT.
Acknowlegemnet
The authors thank the patients who participated in the clinical trial, the members of the research and clinical staff at the Fred Hutchinson Cancer Research Center (FHCRC)/Seattle Cancer Care Alliance (SCCA), and referring physicians for their care of our patients after CAR-T cell therapy.
This study was supported by the National Institutes of Health (NIH) National Cancer Institute grants R01 CA136551 and P30 CA15704, NIH National Institute of Diabetes and Digestive and Kidney Diseases grant P30 DK56465, NIH National Heart, Lung, and Blood Institute funded National Gene Vector Biorepository at Indiana University (Contract # 75N92019D00018), Life Science Discovery Fund, Bezos Family, FHCRC Immunotherapy Integrated Research Cente, and Juno Therapeutics/Celgene, Inc.
Financial Disclosure:
A.C., E.B., A.V.H., M.S., M.B. declare no competing financial interests.
J.A.H. has served as a consultant for Nohla Therapeutics, Inc. and Amplyx. and has received research support from Nohla Therapeutics, Karius, and Shire.
D.G.M. has received research funding from Kite Pharma, a Gilead Company, Juno Therapeutics, a Celgene/Bristol-Myers Squibb company, and Celgene; has served on advisory boards for Kite Pharma, Gilead, Genentech, Novartis and Eureka Therapeutics.
C.J.T. receives research funding from and has patents licensed or pending with Juno Therapeutics, a Celgene company, and Nektar Therapeutics; has served on advisory boards and has equity in Caribou Biosciences, Eureka Therapeutics, and Precision Biosciences; and has served on advisory boards for Aptevo, Bluebird Bio, Adaptive Biotechnologies, Juno Therapeutics, a Celgene company, Kite, a Gilead Company, Humanigen, Nektar Therapeutics, Novartis, T-CURX, and Allogene.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377(26):2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019;380(1):45–56. [DOI] [PubMed] [Google Scholar]
- 5.Turtle CJ, Hanafi LA, Berger C, Hudecek M, Pender B, Robinson E, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8(355):355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sommermeyer D, Hudecek M, Kosasih PL, Gogishvili T, Maloney DG, Turtle CJ, et al. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30(2):492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turtle CJ, Berger C, Sommermeyer D, Hanafi L-A, Pender B, Robinson EM, et al. Anti-CD19 Chimeric Antigen Receptor-Modified T Cell Therapy for B Cell Non-Hodgkin Lymphoma and Chronic Lymphocytic Leukemia: Fludarabine and Cyclophosphamide Lymphodepletion Improves In Vivo Expansion and Persistence of CAR-T Cells and Clinical Outcomes. Blood. 2015;126:184. [Google Scholar]
- 8.Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hay KA, Gauthier J, Hirayama AV, Voutsinas JM, Wu Q, Li D, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood. 2019;133(15):1652–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirayama AV, Gauthier J, Hay KA, Voutsinas JM, Wu Q, Gooley T, et al. The response to lymphodepletion impacts PFS in patients with aggressive non-Hodgkin lymphoma treated with CD19 CAR T cells. Blood. 2019;133(17):1876–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turtle CJ, Hay KA, Hanafi LA, Li D, Cherian S, Chen X, et al. Durable Molecular Remissions in Chronic Lymphocytic Leukemia Treated With CD19-Specific Chimeric Antigen Receptor-Modified T Cells After Failure of Ibrutinib. J Clin Oncol. 2017;35(26):3010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hay KA, Hanafi LA, Li D, Gust J, Liles WC, Wurfel MM, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130(21):2295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, et al. Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017;7(12):1404–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kochenderfer JN, Somerville RPT, Lu T, Yang JC, Sherry RM, Feldman SA, et al. Long-Duration Complete Remissions of Diffuse Large B Cell Lymphoma after Anti-CD19 Chimeric Antigen Receptor T Cell Therapy. Mol Ther. 2017;25(10):2245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill JA, Li D, Hay KA, Green ML, Cherian S, Chen X, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood. 2018;131(1):121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park JH, Romero FA, Taur Y, Sadelain M, Brentjens RJ, Hohl TM, et al. Cytokine Release Syndrome Grade as a Predictive Marker for Infections in Patients With Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia Treated With Chimeric Antigen Receptor T Cells. Clin Infect Dis. 2018;67(4):533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20(1):31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornetta K, Duffy L, Turtle CJ, Jensen M, Forman S, Binder-Scholl G, et al. Absence of Replication-Competent Lentivirus in the Clinic: Analysis of Infused T Cell Products. Mol Ther. 2018;26(1):280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruella M, Xu J, Barrett DM, Fraietta JA, Reich TJ, Ambrose DE, et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat Med. 2018;24(10):1499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pedersen-Bjergaard J, Andersen MK, Christiansen DH. Therapy-related acute myeloid leukemia and myelodysplasia after high-dose chemotherapy and autologous stem cell transplantation. Blood. 2000;95(11):3273–9. [PubMed] [Google Scholar]
- 23.Giri S, Bhatt VR, Verma V, Pathak R, Bociek RG, Vose JM, et al. Risk of Second Primary Malignancies in Patients With Follicular Lymphoma: A United States Population-based Study. Clin Lymphoma Myeloma Leuk. 2017;17(9):569–74. [DOI] [PubMed] [Google Scholar]
- 24.Damlaj M, El Fakih R, Hashmi SK. Evolution of survivorship in lymphoma, myeloma and leukemia: Metamorphosis of the field into long term follow-up care. Blood Rev. 2019;33:63–73. [DOI] [PubMed] [Google Scholar]
- 25.Penne M, Sarraf Yazdy M, Nair KS, Cheson BD. Extended Follow-up of Patients Treated With Bendamustine for Lymphoid Malignancies. Clin Lymphoma Myeloma Leuk. 2017;17(10):637–44. [DOI] [PubMed] [Google Scholar]
- 26.Olszewski AJ, Reagan JL, Castillo JJ. Late infections and secondary malignancies after bendamustine/rituximab or RCHOP/RCVP chemotherapy for B-cell lymphomas. Am J Hematol. 2018;93(1):E1–E3. [DOI] [PubMed] [Google Scholar]
- 27.Hu S, Zhou D, Wu Y, Zhao Y, Wang S, Han B, et al. Autoimmune disease-associated non-Hodgkin’s lymphoma-a large retrospective study from China. Ann Hematol. 2019;98(2):445–55. [DOI] [PubMed] [Google Scholar]
- 28.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu Y, Wang J, Wei G, Yu J, Luo Y, Shi J, et al. A retrospective comparison of allogenic and autologous chimeric antigen receptor T cell therapy targeting CD19 in patients with relapsed/refractory acute lymphoblastic leukemia. Bone Marrow Transplant. 2018. [DOI] [PubMed] [Google Scholar]
- 30.Arora M, Cutler CS, Jagasia MH, Pidala J, Chai X, Martin PJ, et al. Late Acute and Chronic Graft-versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2016;22(3):449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.