Abstract
BACKGROUND:
Around 15-25% of diabetes mellitus (DM) patients will develop diabetic foot ulcers (DFUs) with high morbidity, many studies have been proposed to search the most effective healing techniques.
AIM:
This study was conducted to demonstrate the ability of hyperbaric oxygen therapy (HBOT) as a complementary therapy in DFUs healing through raising vascular endothelial growth factor (VEGF) levels and suppressing tumour necrosis factor-alpha (TNF-α).
METHODS:
All patients received the same treatment including wound debridement and wound care, but the patients in the HBOT group, breathed 100% oxygen at 2.4 ATA for 90 minutes in total of 20 sessions (four weeks).
RESULT:
There were 32 diabetic patients with DFUs Wagner 3-4. VEGF levels after four weeks of HBOT was significantly elevated compared to the control group (p = 0.013). The effect size of VEGF levels was p = 0.005. TNF-α levels after four weeks of therapy were decreased (p = 0.01). Faster epithelialization is seen in the HBOT group (p < 0.001). We also performed path analysis, HBOT had a significant effect on the epithelialization (p < 0.001) and VEGF levels affected the epithelialization process (p = 0.042).
CONCLUSION:
HBOT administration leads to increased VEGF levels, decreased TNF-α levels, and accelerated wound healing of DFUs patients. HBOT directly aids epithelialization and indirectly through VEGF upsurge and TNF-α downturn.
Keywords: VEGF, TNF-α, Epithelialization, Diabetic foot ulcers, Hyperbaric oxygen therapy
Introduction
Diabetes mellitus (DM) is a chronic disease caused by inadequate insulin production, or when insulin cannot be used effectively. This results in elevated blood sugar levels causing damage to the heart, blood vessels, eyes, kidneys, and nerves in longstanding uncontrolled DM [1], [2]. In patients with DM, the most common complications in blood vessels are macroangiopathy, neuropathy, immunosuppre-ssion that facilitate inflammation, ischemia, infection, and cell death [2], [3]. These complications may lead to foot abnormalities such as a chronic ulcer, called diabetic foot ulcers (DFUs).
An ulcer occurs because, at tissue damage or death, that associated with the degree of peripheral vascular disease in inferior limb and may be accompanied by infection [4], [5]. It is estimated 15-25% of DM patients will develop DFUs with high morbidity, 40-80% will have high infection risk, and 10-20% will require amputation [6].
The standard therapies of DFUs are to regulate normal blood sugar levels, antibiotics medication to prevent and treat infection, ulcer debridement, wound care, off-loading the affected limb, and to improve blood flow or revascularisation [2], [6], [7], [8], [9]. In addition to these standard therapies, there are many adjuvant therapies, such as hyperbaric oxygen therapy (HBOT), growth factor therapy, stem cells therapy [7], [10], autolytic debridement [4], and percutaneous transluminal angioplasty [11]. These modalities are also performed in the management of DFUs. Because of DFUs high morbidity risk, many studies have been proposed to search the most effective healing techniques.
According to Undersea and Hyperbaric Medical Society (UHMS), HBOT is an intervention in which a person breathes 100% oxygen intermittently inside a hyperbaric chamber at a pressure greater than sea level pressure (1 atmosphere absolute or ATA). Increased 1 ATA pressure is equivalent to a depth of 10 meters underwater. The therapeutic condition is achieved with a minimum pressure of 1.4 ATA and breathes with 100% oxygen [12]. In general, DFUs therapy uses 100% oxygen and pressure 2-3 ATA inside the hyperbaric chamber for 90 minutes per day [10], [13], [14].
The HBOT’s mechanisms increase the tissue oxygen levels, decreased oedema, and kills anaerobic bacteria, resulting in the acceleration of wound healing [14]. Based on these mechanisms, many researchers use HBOT as one of the method therapies to DFUs [2], [6], [9], [13], [15]. HBOT is also able to improve the angiogenesis process, characterised by an increased in vascular endothelial growth factor (VEGF) levels. It can boost epithelial and granulation processes [13], [16], [17].
The wound or ulcer healing theory with angiogenesis mechanisms through the role of platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), and vascular endothelial growth factor (VEGF) have been extensively studied in animals. However, the use of HBOT in DFUs patients, in HBOT’s role in increasing VEGF through the angiogenesis process has not been widely discussed [18]. Tumour necrosis factor-alpha (TNF-α) is certainly elevated in inflammation, but in DFUs patients who get HBOT has not been explained why HBOT can decrease TNF-α levels. Fundamentally, the role of HBOT heals wounds through oxidative stress and can suppress inflammatory reactions with decreased TNF-α [19].
It is explained that in the DFUs healing process requires the study of the biomolecular role. Based on these theories, we were challenged to learn, understand, and explore more about the HBOT, not only in accelerating wound healing as a clinical feature, but also the changes in biomolecular that may help DFUs patients.
This study aimed to demonstrate the HBOT role as a complementary therapy to provide more rapid clinical recovery through increased VEGF and decreased TNF-α in DFUs wound healing.
Material and Methods
This research is an experimental study using randomised pre and post-test control groups design, with permuted block to HBOT and control group. All patients received the same wound treatment. In the HBOT group, the patient got HBOT, but the control group did not. This research was conducted at Sanglah General Hospital, Denpasar, Bali, after obtaining the ethical clearance of research 1582/UN.14.2/Litbang/2015 from Research Unit of Medical Faculty of Udayana University and Sanglah General Hospital. All subjects of this research were willing to follow the research by signing the agreement after getting the explanation (informed consent).
Inclusion criteria were DM patients with DFU Wagner grade 3 and 4, aged 40-60 years, and TcPO2 > 40 mmHg. TcPO2 measurement was performed at the proximal of the ulcerated foot. Exclusion criteria were DM patients with DFU Wagner 1, 2, and 5, patients with other organ abnormalities such as heart failure, pulmonary infections, pulmonary emphysema, pneumothorax, chronic obstructive pulmonary disease, liver disease or hepatitis, stroke, kidney failure, and sepsis or multiple organ failure.
Before the debridement, serum sampling was performed to get a baseline value of VEGF and TNF-α biomarkers. Another laboratory test was taken, such as random blood sugar and serum albumin. Blood centrifugation was done at the velocity of 3000 rpm for 10 minutes. Serum was inserted in the safe lock microcentrifuge tube and stored in a freezer at -20°C in the laboratory. Biomarker examination used quantitative sandwich enzyme immunoassay. VEGF test used the catalogue number PDVE00, and TNF-α used the catalogue number DTA00C. After debridement, the wounds were treated with normal saline, sterile gauze, and elastic bandage as standard management.
The total sample was 32 patients, divided into two groups (HBOT and control), with 16 patients, respectively. In HBOT group, patients breathed 100% oxygen at 2.4 ATA in a multiplace hyperbaric chamber for 90 minutes each session per day, and five days in a week until 20th session (four weeks). At the end of therapy after four weeks from the surgical debridement, second serum sampling was performed again to check VEGF and TNF-α biomarkers in the same way as serum sampling at the beginning of the study. Epithelialization was also performed at the end of therapy. We measured the average of epithelial growth of the entire edges of the wound, in a circle, every 1 cm, from healthy skin to the edge of the ulcer.
Data analysis was conducted by the IBM SPSS statistics version 23.0 for Windows (IBM Corporation). Descriptive analysis to described patient characteristics in both groups. We evaluated the normality of numerical data with the Shapiro-Wilk test. If the data were normally distributed, parametric test with paired T-test for pre and post-test data, and evaluation of value between groups with independent T-test. If numerical data were not normally distributed, data transformation with base-e logs was also known as natural logs (Ln), and it tested by the same test. If after transformation, the data was not normally distributed, then used the non-parametric test, such as a paired T-test replaced with Wilcoxon test and independent T-test with Mann-Whitney U test. The further analysis used path analysis with Stata 12.0 (StataCorp). Significant test was p-value < 0.05.
Results
The subjects collected in this study were 32 DM patients with DFUs Wagner 3-4 (Table 1), with average age 52 years old, duration of DM 6 years, and body mass index (BMI) around 23 kg/m2 (normal limit). In the control group, patients had foot ulcers longer, slightly higher TcPO2, higher random blood sugar, and lower albumin serum than HBOT group. Both groups were comparable with p-value > 0.05.
Table 1.
Characteristics of patients before treatment
| HBOT (n = 16) | Control (n = 16) | p value | |
|---|---|---|---|
| Age (years)a | 52.56 ± 5.81 | 52.75 ± 5.17 | 0.924b |
| DFUs duration (weeks)a | 5.75 ± 4.19 | 7.53 ± 12.98 | 0.779c |
| DM duration (years)a | 6.33 ± 6.81 | 6.75 ± 6.16 | 0.319c |
| BMI (kg/m2) | 23.43 ± 3.86 | 23.99 ± 4.23 | 0.687c |
| TcPO2 (mmHg)a | 58.11 ± 4.87 | 59.18 ± 12.94 | 0.759b |
| Random blood sugar (mg/dl)a | 238.00 ± 106.55 | 266.50 ± 122.40 | 0.488b |
| Albumin serum (mg/dl)a | 3.08 ± 0.75 | 2.92 ± 0.55 | 0.692d |
| Sex (%) | |||
| Male | 8 (50) | 5 (31.25) | 0.280e |
| Female | 8 (50) | 11 (68.75) |
Mean ± standard deviation; independent T-test;
Ln Independent T-test;
Mann-Whitney U test;
Chi-square test.
Table 2 showed average VEGF levels at baseline was similar 151 pg/ml in both groups, but after four weeks therapy there was significant escalation of VEGF in HBOT group than control group (277.42 ± 171.75 pg/ml, 95% CI 185.90-368.94 pg/ml vs 169.21 ± 78.92 pg/ml, 95% CI 127.16-211.26 pg/ml, respectively, p = 0.013). We showed pre and post-test VEGF levels in HBOT was different significant p < 0.001 with escalation value of 125.75 ± 116.76 pg/ml, but in control group was a slight escalation of 17.94 ± 81.26 pg/ml, p = 0.439. The escalation value (effect size) between HBOT and control groups was a significant difference, p = 0.005.
Table 2.
VEGF levels between groups
| VEGF (pg/ml) | HBOT | Control | p-value |
|---|---|---|---|
| Baseline | 151.67 ± 96.46 | 151.27 ± 51.98 | 0.501b |
| After therapy | 277.42 ± 171.75 | 169.21 ± 78.92 | 0.013b |
| p value | < 0.001a | 0.439a |
All value in mean ± standard deviation;
Ln Dependent T test;
Ln Independent T test.
We analysed TNF-α levels between groups in Table 3. It showed similar value at baseline (p = 0.91), but TNF-α levels at four weeks therapy was significantly different between groups (p = 0.01), in HBOT group was 28.51 ± 4.25 pg/ml, 95%CI 26.24-30.77 pg/ml, and in control group was 35.33 ± 13.82 pg/ml, 95%CI 27.96-42.69 pg/ml. TNF-α levels in HBOT group was reduction 4.43 ± 5.03 pg/ml, but in control group was escalation 1.94 ± 10.72 pg/ml (p = 0.02). There was a significant reduction of TNF-α levels in the HBOT group (p = 0.005).
Table 3.
TNF-α levels between groups
| TNF-α (pg/ml) | HBOT | Control | p-value |
|---|---|---|---|
| Baseline | 32.93 ± 4.43 | 33.38 ± 5.29 | 0.91b |
| After therapy | 28.51 ± 4.25 | 35.33 ± 13.82 | 0.01b |
| p value | 0.005a | 0.814a |
All value in mean ± standard deviation;
Wilcoxon test;
Mann-Whitney U test.
Besides the biomolecular analysis, we measured the epithelialization of ulcers at the end of therapy. There was faster epithelialization in HBOT group than the control group (3.81 ± 1.38 mm, 95%CL 3.07-4.54 mm vs 1.27 ± 0.61 mm, 95%CI 0.95-1.60 mm, respectively, p < 0.001). We also performed path analysis to evaluate the relationship between HBOT, VEGF, and TNF-α to epithelialization (Figure 1). The relationship that occurs as a direct and indirect effect on epithelialization. Directly, the HBOT had a significant effect on the epithelialization (p < 0.001). But indirectly, HBOT affected epithelialization through the escalation of VEGF levels and reduction of TNF-α levels. However, the most important role of epithelialization was VEGF levels (p = 0.042).
Figure 1.
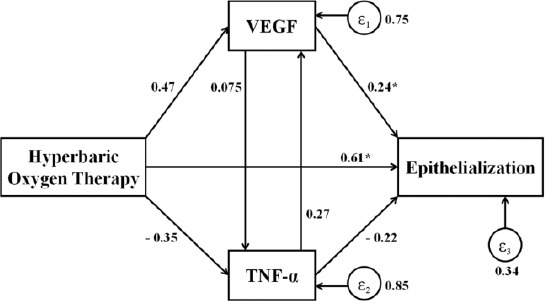
Diagram of path analysis of HBOT to VEGF, TNF-α, and epithelialisation. Note: *p <0.05. - HBOT did not significantly escalate VEGF by 47.35%, p = 1.00; - HBOT did not significantly reduce TNF-α by 35.46%, p = 1.00; - HBOT significantly escalated epithelialization by 61.02%, p <0.001; - VEGF did not significantly escalate TNF-α by 7.52%, p = 1.00; - VEGF significantly escalated epithelialization by 24.13%, p = 0.042; - TNF-α did not significantly escalate VEGF by 26.96%, p = 1.00; - TNF-α did not significantly reduce epithelialization by 22.14%, p = 0.054
Discussion
Hyperbaric oxygen therapy is tailored made for each disease. The frequency of HBOT varies from three to five sessions for acute cases [13], [20]. Ten to twenty HBOT sessions for DFU [2], [6], [9], [10], even fifty to sixty times for chronic cases and slow healing wound [13], [20]. The 5-10 HBOT sessions or less than 20 sessions on DFUs gives no clinical results maximum. The 30 HBOT sessions or more 20 sessions gives the same result with 20 HBOT sessions. Therefore, we use 20 sessions of 100% oxygen and 2.4 ATA in a multiplaced hyperbaric room, one session for 90 minutes intermittent (every 30 minutes, patients resting 5 minutes), one session per day, and five sessions per week [2], [9], [10], [13], [14]. For this study indicated for type 2 DM with DFU Wagner 3-4.
In this study VEGF levels can be seen in Table 2, that the control group VEGF levels increased, but not statistically significant (p = 0.439), while the HBOT group experienced a very significant increase (p < 0.001). The increase in VEGF in the HBOT group was higher than the control group with significant difference (p = 0.005).
In the Asano et al., Study [21] using mice, ligated left femoral artery and given HBOT found VEGF mRNA increased, at 24 hours and 72 hours, but returned to normal after day 14. Increased VEGF mRNA is due to ischemia process. In contrast to bFGF (basic fibroblast growth factor) and HGF (hepatocyte growth factor) which at 24 and 72 hours also increased, but greatly improved on day 14 after administration of HBOT. This indicates that VEGF mRNA is elevated because it is induced by ischemia alone. Yuan et al., Study [22] obtaining VEGF did not increase in the administration of HBOT. The early increasing of VEGF, occurs due to lactate and increased NO (nitric oxide) and not due to hyperoxia in HBOT.
In the Al Waili et al., Study [23] found that VEGF levels increased significantly after HBOT administration, while PGE2 (prostaglandin E2) and Cox-2 (cyclooxygenase-2) mRNA values decreased. It was concluded that cytokines, prostaglandins, and NO probably induced by increased VEGF levels. VEGF plays an important role in liver regeneration, and the effects of VEGF are mediated through two tyrosine kinase receptors [24]. Different oxygen pressures will lead to activation of signalling pathway differences that stimulate VEGF expression to angiogenesis. Hyperoxia causes ROS (reactive oxygen species) production that affects HIF-1 (hypoxia-inducible factor-1) and causes VEGF expression [25].
The increase of VEGF levels in the HBOT group was greater and significantly higher than the control group; this represents that HBOT may activate angiogenesis through increasing VEGF levels. According to Thom [19], HBOT can increase the reactive oxygen compound, will synthesise more growth factors through increased SDF-1 (stromal-derived factor-1) ingredients, angiopoietin, FGF, TGF-β1, and VEGF through HIF-1. From these components, it is the improvement of neovascularisation. The ROS or RNS (reactive nitrogen species) compound affects the cell, in which the PKC (protein kinase C) is activated and various gene expression occurs such as endothelin-1, VEGF, TGF-β, PAI-1 (plasminogen activator inhibitor-1), NF-κB, NAD(P)H oxidase, and decreased eNOS (endothelial nitric oxide synthase) [26].
Wound healing factors in DFUs depend on growth factor, angiogenic response, macrophage function, collagen accumulation, the barrier function of the epidermal cell, keratocyte granulation quality, fibroblast migration, epidermal nerve proliferation, bone healing, ECM (extracellular matrix) accumulation, and remodelling of MMPs (matrix metalloproteinases) [27].
The most important growth factor in the angiogenesis process is VEGF. VEGF of 17-23 kDa can stimulate proliferation and endothelial cell migration. VEGF-A is believed to be responsible for fatty tissue angiogenesis [28]. VEGF-B (21 kDa) of 43% is identical to VEGF-A 165. It also stimulates angiogenesis and has implications of ECM degradation through plasminogen activation regulation [29]. VEGF-C (23 kDa) showed 35% homologous with VEGF-A 165, which play an important role in angiogenesis and lymphangiogenesis [30], [31]. VEGF-D (22 kDa) of 48% identical to VEGF-C also promotes the growth of lymphatic channels [32].
The TNF-α levels changes in our study, shown in Table 3. There was no significant increase in TNF-α levels (p = 0.814). In the HBOT group, there was a significant decrease in TNF-α levels (p = 0.005) after treatment. TNF-α levels after four weeks were significantly different between the control group and the HBOT group (p = 0.01). The difference of TNF-α levels between the initial treatment and after four weeks was significantly different (p = 0.02).
This condition was described by Thom [19], that hyperbaric oxygen therapy increases oxygen levels in the cell, resulting in the formation of reactive oxygen compounds or reactive nitrogen compounds (ROS or RNS). These reactive compounds will increase at hyperoxia state. Reactive oxygen compounds will suppress monocyte cells and reduce the synthesis of chemokines. The small number of monocytes and low level of chemokines will decrease the amount of cytokine production such as TNF-α. Thus, HBOT decreases the overall inflammatory response. In vivo, TNF-α is a major regulator of inflammatory immune responses, both locally and systemically. There are homologous genes from TNF, such as TNF-α and lymphotoxin (TNF-ß). These genes are present on the short arm of chromosome 6 [33]. A systemic inflammatory response will decrease the synthesis of various cytokines, including a decrease in TNF-α levels. Besides, the decrease of TNF-α may be induced by the HIF-1 effect mechanism [19]. TNF-α is a molecule formed by activated mononuclear phagocytes, including endothelial cells and fibroblast cells. In hyperglycaemia, increased ROS level may induce the release of TNF-α, IL-1, and IL-6 through the NF-κB pathway [34]. While on the administration of HBOT, this condition will not happen [19].
Excessive formation of ROS or RNS will be followed by the formation of an oxidant as a scavenger, that will counter the overproduction of ROS. There are two kinds of antioxidants, such as enzymatic (superoxide dismutase, catalase, thioredoxin-glutathione dependent peroxidase, and reductase) and non-enzymatic (vitamin C, vitamin E, thioredoxin, glutathione, uric acid, β carotene, and carotene). These antioxidants are adequate to fight against oxidants. The decrease of TNF-α in the HBOT adjuvant therapy, depending on duration, frequency is given HBOT, and HBOT dose per session. Administration of HBOT 3 ATA for one-hour at massive bleeding decreased TNF-α compared to control. One-hour 100% oxygen delivery with 2.8 ATA also inhibits elevated TNF-α in ischemia-reperfusion injury of intestinal [35], [36]. Clinical judgement of DFUs was done by observation and measurement of epithelial width growth, from the margin of healthy skin into the wound, measured after four weeks of treatment. The control group, the epithelial growth of DFUs, was 1.27 ± 0.61 mm. The HBOT group, the epithelial growth of the patient, was 3.81 ± 1.38 mm. There was a significant difference in both groups’ epithelial growth. Epithelial growth in the HBOT group was much better than the control group (p < 0.001).
In acute lesions, the epithelial growth of normal skin cells is 0.1 mm per day or 0.75 mm per week and 3 cm during the proliferative phase [37], whereas in chronic wounds, the epithelial growth slows down. In a chronic condition, the network healing process fails to achieve functional and anatomy integrity as in normal condition. Various factors that cause chronic wounds are still not fully understood, but one of the most important factors is the occurrence of oxygen deficiency resulting in prolonged tissue hypoxia. The ECM deposit also becomes less due to the production of fibroblasts and collagen remodelling highly dependent on the adequacy of tissue oxygen. In the development of chronic wounds, the healing process can be stopped at every phase, especially in the inflammatory or proliferative stage. Inhibition of the proliferative phase causes the build-up of neutrophil production in tissues that would otherwise destroy growth factors and degrade ECM components. This causes the tissue to become fragile [38]. In diabetes, the persistent inflammatory phase leads to prolonged maturation time of the granulation tissue and the parallel reduction of tensile strength [39]. In acute healing, there will be a gradual wound healing process based on the hemostasis phase, the inflammatory phase, the proliferation phase, and the remodelling phase. While in chronic wounds, the inflammatory phase persistently occurs [40].
Based on the description above, we can assemble the role of each molecule or compound in the cell or body to physiologically wound healing process and progression [41]. HBOT role as adjuvant therapy may activate the system to accelerate the DFUs healing process. According to Mendes et al., [41] several molecules and compounds take a big role in DFUs healing process, including genetic changes. It is an endless orchestra of harmony; there are many things that have not been revealed and fully understood. HBOT offers a variety of beneficial effects. In this study that HBOT can be used for the treatment of DFUs because HBOT causes a change in pathobiology, such as increased VEGF in the plasma will stimulate angiogenesis and neovascularisation. A decrease of TNF-α as a sign of reduced inflammatory reactions and improvement of immunity in plasma, followed by increased granulation as fibroblast work undergoes proliferation and migration. The clinical improvement of epithelial acceleration in adjuvant HBOT proved that HBOT improves ulcer healing through various growth factors, cell proliferation and migration, increased vascular permeability through vasoconstriction mechanisms, and improves vascular endothelial function.
The role of HBOT to VEGF, TNF-α and epithelial growth can be seen in the path analysis (Figure 1). By path analysis, the relationship was HBOT directly affecting epithelialization significantly (p < 0.001). HBOT indirectly affects epithelialization via VEGF and TNF-α. However, the most important role in the indirect relationship between HBOT and epithelialisation was VEGF (p = 0.042). According to Mendes et al., [41] in the wound healing process, many things are involved and influential. Molecules and compounds take their roles, genetic change of chain, interrelated, and dependent. But Yuan et al., Study [22] obtaining VEGF did not increase in the administration of HBOT.
In conclusion, HBOT administration leads to increased VEGF, decreased TNF-α, and accelerated wound healing of DFUs patients. Clinical efficacy in humans reported by previous investigators can be demonstrated through this biomolecular study. The biomolecular findings reinforce the theoretical basis, helping to explain human clinical findings, and adding biomolecular research to animals thus adding to the validity of previous studies. HBOT directly affects epithelialization, but also induces indirectly through VEGF enhancement mechanism and decreases TNF-α. However, the most important role in indirect epithelialization process is VEGF.
Data Availability
The data of this study is available by request.
Author contributions
I Nyoman Semadi contributed to study design, conduct research, surgical debridement, data and statistical analysis, interpretation of findings, and drafting of the manuscript.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Global Report on Diabetes. Switzerland: WHO Press; 2016. World Health Organization. [Google Scholar]
- 2.Irawan H, Semadi IN, Devi A. Effect of Hyperbaric Oxygen Therapy to Improve Serum Albumin for Patients with Diabetic Foot Ulcer. Biomed Pharmacol J. 2018;11(1):569–75. https://doi.org/10.13005/bpj/1409. [Google Scholar]
- 3.Masharani U, Karam JH, German MS. Pancreatic Hormones &Diabetes Mellitus. In: Greenspan FS, Gardner DG, editors. Basic and Clinical Endocrinology. 7thEd. Philadelphia London: 2004. pp. 3–32S. [Google Scholar]
- 4.Irawan H, Yasa KP. A case report of diabetic foot ulcer underwent an autolytic debridement using hydrogel and hydrocellular foam combination. Bali Med J. 2017;6(3):S93–6. https://doi.org/10.15562/bmj.v6i3.411. [Google Scholar]
- 5.Payne C. Health sciences:Introduction to the diabetic foot. New York: La Trobe University; 2002. pp. 35–48. [Google Scholar]
- 6.Irawan H, Semadi IN, Widiana IGR. A Pilot Study of Short Duration Hyperbaric Oxygen Therapy to Improve HbA1c, Leukocyte, and Serum Creatinine in Patient with Diabetic Foot Ulcer Wagner 3-4. Scientific World Journal. 2018;2018 doi: 10.1155/2018/6425857. https://doi.org/10.1155/2018/6425857 PMid:30158840 PMCid:PMC6109474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frykberg RG, Zgonis T, Armstrong DG, Driver VR, Giurini JM, et al. Diabetic Foot Disorders:A Clinical Practice Guideline (2006 revision) J Foot Ankle Surg. 2006;45(5):S1–66. doi: 10.1016/S1067-2516(07)60001-5. https://doi.org/10.1016/S1067-2516(07)60001-5. [DOI] [PubMed] [Google Scholar]
- 8.Wounds International. International Best Practice Guidelines:Wound Management in Diabetic Foot Ulcers. London: Wounds International; 2013. [Google Scholar]
- 9.Semadi IN, Irawan H. Blood Glucose and Lipid Profile in Patients with Diabetic Foot Ulcer that Underwent Hyperbaric Oxygen Therapy. Bali Med J. 2017;6(2):405–8. https://doi.org/10.15562/bmj.v6i2.606. [Google Scholar]
- 10.Kessler L, Bilbault P, Ortéga F. Hyperbaric oxygenation accelerates the healing rate of nonischemic chronic diabetic foot ulcers. Diabetes Care. 2003;26(8):2378–82. doi: 10.2337/diacare.26.8.2378. https://doi.org/10.2337/diacare.26.8.2378 PMid:12882865. [DOI] [PubMed] [Google Scholar]
- 11.Irawan H, Mooy DZ, Yasa KP. Short Time Result of Percutaneous Transluminal Angioplasty on Ischemia Diabetic Foot Ulcer. Asian J Pharm Clin Res. 2018;11(5):4–6. https://doi.org/10.22159/ajpcr.2018.v11i5.24759. [Google Scholar]
- 12.Weaver LK. Hyperbaric Oxygen Therapy Indications. 13th Ed. USA: Undersea and Hyperbaric Medical Society; 2014. pp. ix–xi. [PubMed] [Google Scholar]
- 13.Bhutani S, Vishwanath G. Hyperbaric oxygen and wound healing. Indian J Plast Surg. 2012;45(2):316–24. doi: 10.4103/0970-0358.101309. https://doi.org/10.4103/0970-0358.101309 PMid:23162231 PMCid:PMC3495382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irawan H. Kartika. Terapi Oksigen Hiperbarik sebagai Terapi Adjuvan Kaki Diabetik. Cermin Dunia Kedokteran-245. 2016;43(10):782–5. [Google Scholar]
- 15.Flood MS. Hyperbaric Oxygen Therapy for diabetic Foot Ulcers. The Journal of Lancaster General Hospital. 2007;2(4):140–5. [Google Scholar]
- 16.Abadia A, Laden G, Kuhan G. The role of hyperbaric oxygen therapy in ischaemic diabetic lower extremity ulcers:a double blind randomised-controlled trial. Eur J Vasc Endovasc Surg. 2003;25(6):513–8. doi: 10.1053/ejvs.2002.1911. https://doi.org/10.1053/ejvs.2002.1911 PMid:127≁2. [DOI] [PubMed] [Google Scholar]
- 17.Kalani M, Jörneskog G, Naderi N, Lind F, Brismar K. Hyperbaric oxygen (HBO) therapy in treatment of diabetic foot ulcers. Long term follow-up. J Diabetes Complications. 2002;16(2):153–8. doi: 10.1016/s1056-8727(01)00182-9. https://doi.org/10.1016/S1056-∗(01)00182-9. [DOI] [PubMed] [Google Scholar]
- 18.Kang TS, Gorti GK, Quan SY, Ho M, Koch RJ. Effect of Hyperbaric Oxygen on the Growth Factor Profile of Fibroblasts. Arch Facial Plast Surg. 2004;6(1):31–5. doi: 10.1001/archfaci.6.1.31. https://doi.org/10.1001/archfaci.6.1.31 PMid:14732642. [DOI] [PubMed] [Google Scholar]
- 19.Thom SR. Hyperbaric Oxygen:Its Mechanisms and Efficacy. Plast Reconstr Surg. 2011;127(1):131S–41. doi: 10.1097/PRS.0b013e3181fbe2bf. https://doi.org/10.1097/PRS.0b013e3181fb.e2bf PMid:21200283 PMCid:PMC3058327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wibowo A. Oksigen Hiperbarik:Terapi Percepatan Penyembuhan Luka. JuKe Unila. 2015;5(9):124–8. [Google Scholar]
- 21.Asano A, Kaneko E, Shinozaki S, Imai Y, Shibayama M, Chiba T, et al. Hyperbaric Oxygen Induces Basic Fibroblast Growth Factor and Hepatocyte Growth Factor Expression, and Enhances Blood Perfusion and Muscle Regeneration in Mouse Ischemic Hind Limbs. Circ J. 2007;71(3):405–11. doi: 10.1253/circj.71.405. https://doi.org/10.1253/circj.71.405 PMid:17322643. [DOI] [PubMed] [Google Scholar]
- 22.Yuan J, Handy RD, Moody AJ, Bryson P. Response of blood vessels in vitro to hyperbaric oxygen (HBO):Modulation of VEGF and NO release by external lactate or arginine. Biochimica et Biophysica Acta. 2009;1787(7):828–34. doi: 10.1016/j.bbabio.2009.03.009. https://doi.org/10.1016/j.bbabio.2009.03.009 PMid:19298791. [DOI] [PubMed] [Google Scholar]
- 23.Al-Waili NS, Butler J. Effects of Hyperbaric Oxygen on Inflammatory Response to Wound and Trauma:Possible mechanism of Action. ScientificWorldJournal. 2006;6:425–41. doi: 10.1100/tsw.2006.78. https://doi.org/10.1100/tsw.2006.78 PMid:16604253 PMCid:PMC5917171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu H, Miyazaki M, Wakabayashi Y, Mitsuhashi N, Kato A, Ito H, et al. Vascular endothelial growth factor secreted by replicating hepatocytes induces sinusoidal endothelial cell proliferation during regeneration after partial hepatectomy in rats. J Hepatol. 2002;34(5):683–9. doi: 10.1016/s0168-8278(00)00055-6. https://doi.org/10.1016/S0168-8278(00)00055-6. [DOI] [PubMed] [Google Scholar]
- 25.Ijichi H, Taketomi A, Yoshizumi T, Uchiyama H, Yonemura Y, Soejima Y, et al. Hyperbaric oxygen induces vascular endothelial growth factor and reduces liver injury in regenerating rat liver after partial hepatectomy. J Hepatol. 2006;45(1):28–34. doi: 10.1016/j.jhep.2005.12.021. https://doi.org/10.1016/j.jhep.2005.12.021 PMid:16513203. [DOI] [PubMed] [Google Scholar]
- 26.Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, Sonoda N, et al. Protein Kinase Dependent Increase in Reactive Oxygen Species (ROS) Production in Vascular Tissue of Diabetes:Role of Vascular NAD(P)H Oxidase. J Am Soc Nephrol. 2003;14(3):S227–32. doi: 10.1097/01.asn.0000077407.90309.65. https://doi.org/10.1097/01.ASN.0000077407.90309.65 PMid:12874436. [DOI] [PubMed] [Google Scholar]
- 27.Galkowska H, Wojewodzka U, Olszewski WL. Chemokines, cytokines and growth factors in keratinocytes and dermal endothelial cells in the margin if chronic diabetic foot ulcers. Wound Repair Regen. 2006;14(5):558–65. doi: 10.1111/j.1743-6109.2006.00155.x. https://doi.org/10.1111/j.1743-6109.2006.00155.x PMid:17014667. [DOI] [PubMed] [Google Scholar]
- 28.Zhang QX, Magovern CJ, Mack CA, Budenbender KT, Ko W, Rosengart TK. Vascular endothelial growth factor is the major angiogenic factor in omentum:mechanism of the omentum-mediated angiogenesis. J. Surg. Res. 1997;67(2):147–54. doi: 10.1006/jsre.1996.4983. https://doi.org/10.1006/jsre.1996.4983 PMid:9073561. [DOI] [PubMed] [Google Scholar]
- 29.Olofsson B, Korpelainen E, Pepper MS, Mandriota SJ, Aase K, Kumar V, et al. Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc Natl Acad Sci USA. 1998;95(20):11709–14. doi: 10.1073/pnas.95.20.11709. https://doi.org/10.1073/pnas.95.20.11709 PMid:9751730 PMCid:PMC21705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, et al. Anovel vascular endothelial growth factor, VEGFC, is a ligand for the Flt4 (VEGFR-3) and KDR(VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15(2):290–8. https://doi.org/10.1002/j.1460-2075.1996.tb00359.x PMid:8617204 PMCid:PMC449944. [PMC free article] [PubMed] [Google Scholar]
- 31.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 2004;5(1):74–80. doi: 10.1038/ni1013. https://doi.org/10.1038/ni1013 PMid:14634646. [DOI] [PubMed] [Google Scholar]
- 32.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7(2):186–9. doi: 10.1038/84635. https://doi.org/10.1038/84635 PMid:11175849. [DOI] [PubMed] [Google Scholar]
- 33.Durum SK, Muegge K, Rich RR, Fleisher TA, Schwartz BD, Shearer WT, Strober W. Clinical immunology, principles and practice. St. Louis, MO: Mosby; 1996. Cytokines linking the immune and inflammatory systems:IL-1, TNF, IL-6, IFN-ab, and TGF-b; pp. 350–62. [Google Scholar]
- 34.Taylor AA. Pathophysiology of Hypertension and endothelial dysfunction in patients with Diabetes Mellitus. Endocrenol Metab Clin North Am. 2001;30(4):983–97. doi: 10.1016/s0889-8529(05)70223-1. https://doi.org/10.1016/S0889-8529(05)70223-1. [DOI] [PubMed] [Google Scholar]
- 35.Yamashita M, Yamashita M. Hyperbaric oxygen treatment attenuates cytokine induction after massive hemorrhage. Am J Physiol Endocrinol Metab. 2000;278(5):E811–6. doi: 10.1152/ajpendo.2000.278.5.E811. https://doi.org/10.1152/ajpendo.2000.278.5.E811 PMid:10780936. [DOI] [PubMed] [Google Scholar]
- 36.Yang ZJ, Bosco G, Montante A, Ou XI, Camporesi EM. Hyperbaric O2 reduces intestinal ischemia-reperfusion-induced TNF-αproductionand lung neutrophil sequestration. Eur J Appl Physiol. 2001;85(1-2):96–103. doi: 10.1007/s004210100391. https://doi.org/10.1007/s004210100391 PMid:11513327. [DOI] [PubMed] [Google Scholar]
- 37.Fishman DT. Wound Care Information Network. Phase of Wound Healing. 2005. [[2010 Jan. 14]]. Available from: http://www.medicaledu.com/phases.htm .
- 38.Thackham JA, McElwain S, Long RJ. The Use of Hyperbaric Oxygen Therapy to Treat Chronic Wounds:A review. Wound Repair Regen. 2008;16(3):321–30. doi: 10.1111/j.1524-475X.2008.00372.x. https://doi.org/10.1111/j.1524-475X.2008.00372.x PMid:18471250. [DOI] [PubMed] [Google Scholar]
- 39.McLennan S, Yue DK, Twigg SM. Molecular aspects of wound healing in diabetes. Primary Intention. 2006;14(1):10–13. [Google Scholar]
- 40.Flanagan M. The Physiology of Wound Healing. J Wound Care. 2000;9(6):299–300. doi: 10.12968/jowc.2000.9.6.25994. https://doi.org/10.12968/jowc.2000.9.6.25994 PMid:11933346. [DOI] [PubMed] [Google Scholar]
- 41.Mendes JJ, Neves J. Diabetic Foot Infection:Current Diagnosis and Treatment. The Journal of Diabetic Foot Complications. 2012;4(2):26–45. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this study is available by request.


