Highlights
-
•
BMI and genetic risk for AD correlated with medial temporal lobe volume.
-
•
Link between BMI and AD risk on medial temporal volume was strongest in females.
-
•
BMI and AD genetic risk independently correlated with amyloid beta, tau, and p-tau.
Keywords: Atrophy, Alzheimer's disease, Body mass index, Hippocampus, Entorhinal cortex, Polygenic risk
Abbreviations: Aβ, β-amyloid peptide; AD, Alzheimer's disease; ADNI, Alzheimer's Disease Neuroimaging Initiative; BMI, body mass index; GWAS, genome-wide association study; MCI, mild cognitive impairment; p-tau, phosphorylated tau; PHS, polygenic hazard score; SNPs, single nucleotide polymorphisms; CSF, cerebrospinal fluid
Abstract
Body mass index (BMI) has a complex relationship with Alzheimer's disease (AD); in midlife, high BMI is associated with increased risk for AD, whereas the relationship in late-life is still unclear. To clarify the relationship between late-life BMI and risk for AD, this study examined the extent to which genetic predisposition for AD moderates BMI and AD-related biomarker associations. Participants included 126 cognitively normal older adults at baseline from the Alzheimer's Disease Neuroimaging Initiative (ADNI) cohort. Genetic risk for AD was assessed via polygenic hazard score. AD-related biomarkers assessed were medial temporal lobe volume and cerebrospinal fluid (CSF) biomarkers. Hierarchical linear regressions were implemented to examine the effects of BMI and polygenic hazard score on AD-related biomarkers. Results showed that BMI moderated the relationship between genetic risk for AD and medial temporal lobe volume, such that individuals with high BMI and high genetic risk for AD showed lower volume in the entorhinal cortex and hippocampus. In sex-stratified analyses, these results remained significant only in females. Finally, BMI and genetic risk for AD were independently associated with CSF biomarkers of AD. These results provide evidence that high BMI is associated with lower volume in AD-vulnerable brain regions in individuals at genetic risk for AD, particularly females. The genetic pathways of AD may be exacerbated by high BMI. Environmental and genetic risk factors rarely occur in isolation, which underscores the importance of looking at their synergistic effects, as they provide insight into early risk factors for AD that prevention methods could target.
1. Introduction
Alzheimer's disease (AD), the most common form of dementia, is a global health concern that places an epic burden on families, caregivers, healthcare systems, and the economy. An estimated 5.6 million Americans currently live with AD, and this number is expected to increase rapidly as the number of individuals over the age of 65 increases (Hebert et al., 2013). Brain changes, including atrophy and accumulation of β-amyloid peptide (Aβ) and tau, begin years before noticeable clinical and cognitive symptoms develop (Braak and Braak, 1991; Jack et al., 2013; Villemagne et al., 2013), making it imperative to investigate early risk factors that prevention methods could target to delay or prevent progression to AD.
One variable that may play a role in development of AD is obesity. Obesity is a serious and growing health concern that impacts 38.9% of U.S. adults (Hales et al., 2018), and is associated with numerous deleterious health conditions, including diabetes and cardiovascular disease, as well as impaired quality of life (Dixon, 2010). One measure of obesity, body mass index (BMI), has a complex relationship with AD. In midlife, obesity is consistently associated with increased risk for dementia, including AD (Albanese et al., 2017; Anstey et al., 2011). Although the precise pathways linking obesity and neurodegenerative disease are unknown, likely mechanisms include inflammation, insulin resistance, oxidative stress, and blood-brain barrier disturbances (Alford et al., 2018; O'Brien et al., 2017). However, there is an “obesity paradox” when BMI is measured in older adults. There is some evidence that high BMI is associated with lower risk of developing dementia, including AD (Atti et al., 2008; Fitzpatrick et al., 2009; Kivimäki et al., 2018). Additionally, studies have shown that lower BMI is associated with increased AD-pathology including Aβ, total tau, and phosphorylated tau (p-tau) (Ewers et al., 2012; Vidoni et al., 2011), and accelerated cognitive decline (Cronk et al., 2010). Possible explanations include changes in olfaction that alter eating habits, damage to brain regions that are involved in controlling weight and food intake such as the hypothalamus and medial temporal lobe (Buchman et al., 2005; Grundman et al., 1996; Morris et al., 1989), and higher levels of leptin in obesity which may facilitate hippocampal synaptic plasticity and consequently improve learning and memory (Harvey et al., 2006). However, the relationship between late-life BMI and AD remains to be elucidated. Higher BMI in late-life was associated with increased risk for AD (Gustafson et al., 2003), while a meta-analysis showed no relationship between late-life BMI (measured continuously) and dementia risk (Anstey et al., 2011).
Complex phenotypes such as late-onset AD cannot fully be explained by environmental factors alone, but rather a multitude of environmental and genetic factors and their interactions. Examining moderating factors, such as genetic risk for AD, may provide additional insight into the relationship between obesity and AD. It is well-established that genetics contributes to the development of AD, and evidence suggests that late-onset AD is 60–80% heritable (Gatz et al., 2006). Recent work suggests that polygenic risk scores for AD are better predictors of AD than are single candidate variants such as the APOE ε4 allele (Ridge et al., 2013). A polygenic risk score incorporates multiple genetic variants, identified from a genome-wide association study (GWAS) of a particular trait, into a genetic propensity score for that trait. Polygenic risk for AD has also been associated with other markers of AD-related pathology, including neurofibrillary tangles, neuritic plaques, Aβ, tau, and volume loss in the hippocampus and entorhinal cortex (Desikan et al., 2017). These results suggest that polygenic risk scores may serve as predictors of prodromal AD-related brain pathology.
The present study examined the relationship between BMI, polygenic risk for AD, and medial temporal lobe volume (entorhinal cortex and hippocampus) using the Alzheimer's Disease Neuroimaging Initiative (ADNI) database. Our analyses focused on these medial temporal lobe regions as they are known to be particularly vulnerable to AD (Braak and Braak, 1991), and genetic risk for AD is associated with lower volume in these regions (Desikan et al., 2017). Additionally, given data showing that females have a greater risk of developing AD (Gao et al., 1998) and some evidence that the relationship between BMI and dementia risk is stronger in females (Gustafson et al., 2003; Joo et al., 2018), we also examined sex differences in the relationship between BMI, polygenic risk for AD, and medial temporal lobe volume. Finally, we examined the relationship between BMI, polygenic risk for AD, and other known AD-biomarkers, including Aβ, tau, and p-tau. Investigating how environmental and genetic factors interact to influence AD-related pathology has important implications for the development of specific prevention methods aimed at delaying or preventing progression to AD. These factors were examined in cognitively normal individuals in an attempt to identify early risk factors that can be targeted prior to onset of clinical symptoms.
2. Materials and methods
2.1. Participants
Data used in the preparation of this article were obtained from the ADNI database (adni.loni.usc.edu). ADNI was launched in 2004 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial MRI, PET, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early AD. Additional information can be found at www.adni-info.org.
The final sample included cognitively normal subjects at screening/baseline with available demographic, height and weight, polygenic hazard score (PHS), and 3T structural MRI data. Cognitively normal was defined according to ADNI's cognitively normal inclusion criteria, such that participants cannot have cognitive/functional impairments or memory complaints beyond what would be expected for age-related changes in memory. Additionally, participants must have normal memory function, as indicated by education-adjusted scores of at least 9 for 16 or more years of education, at least 5 for 8–15 years of education, or at least 3 for 0–7 years of education on the Logical Memory II subscale (Delayed Paragraph Recall, Paragraph A) of the Wechsler Memory Scale – Revised. Participants must also have a Mini-Mental State Examination score between 24 and 30, as well as Clinical Dementia Rating global and memory box scores of zero. Furthermore, individuals in the ADNI dataset are excluded if they have any significant neurological disease or mental health diagnoses including depression/bipolar disorder, schizophrenia, or substance abuse.
For the current study, 180 white, non-Hispanic/Latino subjects were identified to avoid population stratification effects. This sample included both accelerated (n = 30) and non-accelerated (n = 150) T1-weighted MRI scans. We restricted our analysis to the 150 subjects with non-accelerated scans, which typically have fewer image artifacts and better signal to noise ratio than accelerated scans (Jack et al., 2010). Of these, 20 scans failed one or more regions of the visual quality check performed by the UCSF processing team and were excluded. Three participants had two baseline scans and, in these cases, only one scan was included in our analyses. One participant had a BMI more than three standard deviations above the mean and was excluded from analyses. The final sample included 126 subjects. Four participants had diabetes. Demographics of the entire sample and as a function of BMI based on a median split are shown in Table 1. Demographics as a function of sex are shown in Inline Supplementary Table 1. Nine of these subjects were excluded from hippocampal analyses for missing (n = 1) or failing (n = 8) the left and/or right hippocampal quality check. Sixteen of these subjects were missing tau, p-tau, and Aβ data and therefore analyses with these cerebrospinal fluid (CSF) biomarkers had a total of n = 110. Study procedures were approved by site-specific Institutional Review Boards and all participants and/or authorized representatives provided written informed consent consistent with the Declaration of Helsinki.
Table 1.
Demographic and clinical characteristics.
| Variable | Total (n = 126) | Low BMI (n = 63) | High BMI (n = 63) | P-Value | Effect Sizea |
|---|---|---|---|---|---|
| Age in years, mean ± SD (range) | 74.0 ± 5.9 (63.5 – 89.0) | 74.9 ± 5.9 (65.0 – 89.0) | 73.0 ± 5.7 (63.5 – 84.7) | 0.071 | 0.32 |
| Males, n (%) | 60 (47.6) | 27 (42.9) | 33 (52.4) | 0.285 | 0.095+ |
| Years of education, mean ± SD (range) | 16.5 ± 2.5 (12.0 – 20.0) | 16.7 ± 2.6 (12.0 – 20.0) | 16.3 ± 2.4 (12.0 – 20.0) | 0.394 | 0.15 |
| Intracranial volume, mean ± SD | 1,483,619 ± 150,782 | 1,473,510 ± 145,261 | 1,493,729 ± 156,616 | 0.454 | −0.13 |
| Total geriatric depression, mean ± SD (range) | 0.72 ± 1.1 (0 – 6) | 0.71 ± 1.0 (0 – 5) | 0.73 ± 1.2 (0 – 6) | 0.937 | −0.01 |
| Polygenic hazard score, mean ± SD (range) | 0.01 ± 0.7 (−1.5 – 2.0) | 0.09 ± 0.7 (−1.5 – 2.0) | −0.07 ± 0.6 (−1.2 – 1.8) | 0.173 | 0.24 |
| Body mass index, mean ± SD (range) | 26.9 ± 3.8 (20.0 – 36.8) | 23.9 ± 1.8 (20.0 – 26.3) | 29.9 ± 2.7 (26.4 – 36.8) | < 0.001* | −2.61 |
| Entorhinal cortex volume, mean ± SD | 3850 ± 609 | 3766 ± 581 | 3935 ± 628 | 0.118 | −0.28 |
| Hippocampal volume, mean ± SD | 7429 ± 906 | 7329 ± 833 | 7529 ± 971 | 0.218 | −0.22 |
Reported effect sizes are Cohen's d unless otherwise indicated. +Reported effect size is phi correlation coefficient because variable is binary.
P < 0.05.
2.2. Body mass index
Height (inches or centimeters) and weight (pounds or kilograms) were measured at baseline for all participants. All height values were converted to meters and all weight values were converted to kilograms. BMI was calculated using the following formula: weight (in kilograms) divided by height (in meters) squared. The sample consisted of 42 normal weight (18.5 ≤ BMI < 25), 58 overweight (25 ≤ BMI < 30), and 26 obese (BMI ≥ 30) participants. There were no underweight individuals (BMI < 18.5).
2.3. MRI analysis
Participants were scanned on ADNI-approved 3T MRI scanners. T1-weighted images were acquired for each subject and pre-processed by Mayo Clinic. Automated cortical reconstruction and volumetric segmentation were performed by the University of California San Francisco with FreeSurfer image analysis suite (version 5.1), which is available for download online (http://surfer.nmr.mgh.harvard.edu/). The scans were processed cross-sectionally using the 2010 Desikan-Killany atlas. See supplementary material for details regarding imaging analysis with FreeSurfer.
Cortical volume values were extracted from the entorhinal cortex and hippocampus. These regions of interest were selected because they are particularly vulnerable to the early effects of AD (Braak and Braak, 1991) and are associated with genetic risk for AD (Desikan et al., 2017). Regions of interest were registered to each individual subject's cortical representation via surface-based registration and cortical volume values were extracted for each subject. Values were summed across left and right hemispheres to create a bilateral volume value. Two additional FreeSurfer regions of interest, the precentral gyrus and postcentral gyrus, were selected to serve as control regions for the putative AD-vulnerable regions of interest. Intracranial volume was also extracted from the FreeSurfer analysis to serve as a covariate in analyses.
2.4. Polygenic hazard score
Genetic risk for AD was assessed via PHS. Methods used to calculate PHS in the present study have been previously published (Desikan et al., 2017). Briefly, 1854 AD-associated single nucleotide polymorphisms (SNPs) (at p < 10−5) were identified using GWAS data from 17,008 AD patients and 37,154 controls in the International Genomics of Alzheimer's Project. Next, in a step-wise procedure, genotype data from 6409 AD patients and 9386 controls in Phase 1 of the Alzheimer's Disease Genetics Consortium was used to identify the top AD-associated SNPs and develop a survival model for PHS, while controlling for the effects of gender, APOE, and population stratification. In each step, the SNP that most improved model prediction was added, and this process continued until residuals did not improve with the addition of another SNP. In the final model, two APOE variants, the ε2 and ε4 alleles, and 31 AD-associated SNPs were integrated into a Cox proportional hazard model to generate a PHS that reflects an individual's risk for developing AD based their age and genotype. This PHS has been replicated in numerous independent samples, including Phase 2 of the Alzheimer's Disease Genetics Consortium, the National Institute on Aging Alzheimer's Disease Centers, and ADNI.
2.5. CSF biomarkers
The analyses of CSF Aβ1-42, tau, and p-tau181 were performed at the UPenn/ADNI Biomarker laboratory using the fully automated Roche Elecsys immunoassay and following a Roche Study protocol. The Elecsys Aβ CSF immunoassay is not a commercially available in vitro diagnostic assay. It is an assay that is currently under development and for investigational use only. The measuring range of the assay is 200 (lower technical limit) – 1700 pg/mL (upper technical limit). The performance of the assay beyond the upper technical limit has not been formally established. Therefore, use of values above the upper technical limit, which are provided based on an extrapolation of the calibration curve, is restricted to exploratory research purposes and is excluded for clinical decision making or for the derivation of medical decision points. In the present study, 36 subjects had Aβ values greater than the upper technical limit, which were truncated to 1700. There were no values below the lower technical limit for Aβ or outside of the technical limits for tau (80–1300 pg/mL) or p-tau (8–120 pg/mL).
2.6. Statistical approach
Statistical analyses were performed using IBM SPSS Statistics for Macintosh, version 25.0. Linear regression models were implemented to parse the relative effects of BMI, PHS, and covariates on AD-vulnerable regions and biomarkers. In the first model, age (years), sex, education (years), and total geriatric depression score were entered as predictors for the dependent variable of interest. For analyses examining brain volumes (entorhinal cortex, hippocampus, precentral gyrus, and postcentral gyrus), intracranial volume was also entered into the first model. In the second model, the main effects of BMI and PHS were entered. Finally, the BMI x PHS interaction term was entered into the third model. To examine sex differences in the relationship between BMI, PHS, and medial temporal lobe volume, the hippocampal and entorhinal cortex regressions were also run separately for males and females. All models also examined the contribution of cerebrovascular risk factors including hypertension, smoking history, and hypercholesterolemia/hyperlipidemia. However, these factors were not significant predictors in the model and are not reported in the final results.
3. Results
3.1. Entorhinal cortex
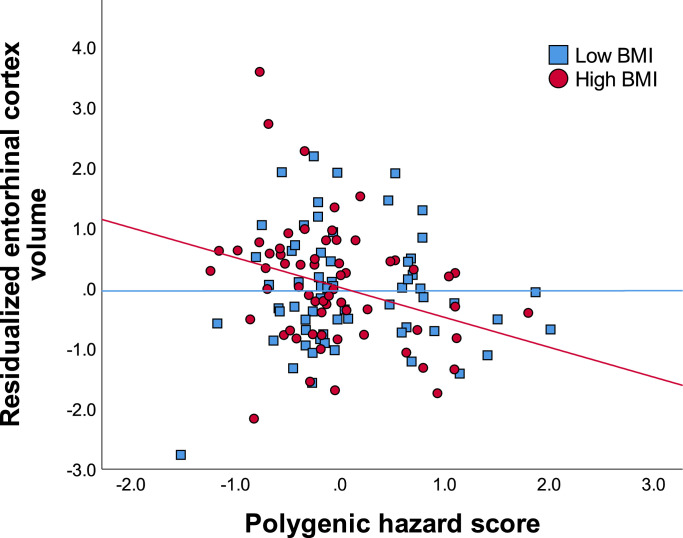
Intracranial volume, age, education, sex, and depression (model 1) accounted for 34.7% of the variance in entorhinal cortex volume. Adding PHS and BMI accounted for an additional 3.7% of the variance. Adding the BMI x PHS interaction term accounted for an additional 3.2% of the variance, which was a significant change to the model. The results suggest that the combination of greater genetic risk for AD and high BMI was associated with lower volume in the entorhinal cortex [ΔF(1,117) = 6.411, P = 0.013, R2 = 0.416] (See Fig. 1 and Table 2).
Fig. 1.
Reduced entorhinal cortex volume among subjects with high BMI and high genetic risk for AD. Values on the x-axis represent polygenic hazard scores for AD, with higher scores indicating increased risk for AD. Values on the y-axis represent standardized residuals of entorhinal cortex volume (accounting for age, sex, education, intracranial volume, and depression).
Table 2.
Summary of regression analysis for association with entorhinal cortex volume.
| Variable | Model 1 |
Model 2 |
Model 3 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE (B) | β | P | B | SE (B) | β | P | B | SE (B) | β | P | |
| Age | −30.035 | 7.852 | −0.289 | < 0.001⁎⁎ | −26.026 | 7.979 | −0.251 | 0.001⁎⁎ | −24.656 | 7.821 | −0.238 | 0.002⁎⁎ |
| Sex | 305.340 | 109.937 | 0.252 | 0.006⁎⁎ | 266.592 | 109.092 | 0.220 | 0.016* | 259.644 | 106.709 | 0.214 | 0.016* |
| Education | −15.951 | 18.923 | −0.065 | 0.401 | −14.795 | 18.927 | −0.061 | 0.436 | −18.424 | 18.563 | −0.076 | 0.323 |
| Intracranial volume | 0.002 | 0.000 | 0.405 | < 0.001⁎⁎ | 0.002 | 0.000 | 0.411 | < 0.001⁎⁎ | 0.002 | 0.000 | 0.428 | < 0.001⁎⁎ |
| Depression | −15.247 | 40.145 | −0.028 | 0.705 | −21.858 | 39.413 | −0.041 | 0.580 | −53.249 | 40.485 | −0.099 | 0.191 |
| PHS | −96.502 | 67.976 | −0.105 | 0.158 | 1207.461 | 519.279 | 1.312 | 0.022* | ||||
| BMI | 24.989 | 12.376 | 0.154 | 0.046* | 15.887 | 12.624 | 0.098 | 0.211 | ||||
| BMI x PHS | −49.883 | 19.701 | −1.442 | 0.013* | ||||||||
| R2 | 0.347 | 0.384 | 0.416 | |||||||||
| Model F | 12.756⁎⁎ | 10.494⁎⁎ | 10.404⁎⁎ | |||||||||
PHS = polygenic hazard score; BMI = body mass index.
P < 0.05.
P < 0.01.
To further explore this pattern of results, partial correlations were used to examine the relationship between PHS and entorhinal cortex volume for low/high BMI groups (based on median split; Mdn = 26.33). Correcting for all covariates, results revealed that among individuals with high BMI, PHS was negatively correlated with entorhinal cortex volume (pr = −0.328, P = 0.012). Among individuals with low BMI, PHS was not significantly correlated with entorhinal cortex volume (pr = 0.018, P = 0.892).
Next, sex differences in the relationship between BMI, PHS, and entorhinal cortex volume were examined. The sex-stratified linear regressions revealed that the BMI x PHS interaction remained significant in females [ΔF(1,58) = 5.495, P = 0.023, R2 = 0.320] (See Inline Supplementary Table 2). In males, the BMI x PHS interaction was not significant [ΔF(1,52) = 0.059, P = 0.809, R2 = 0.337], but there was a main effect of PHS on entorhinal cortex volume (P = 0.039) (See Inline Supplementary Table 3).
3.2. Hippocampus
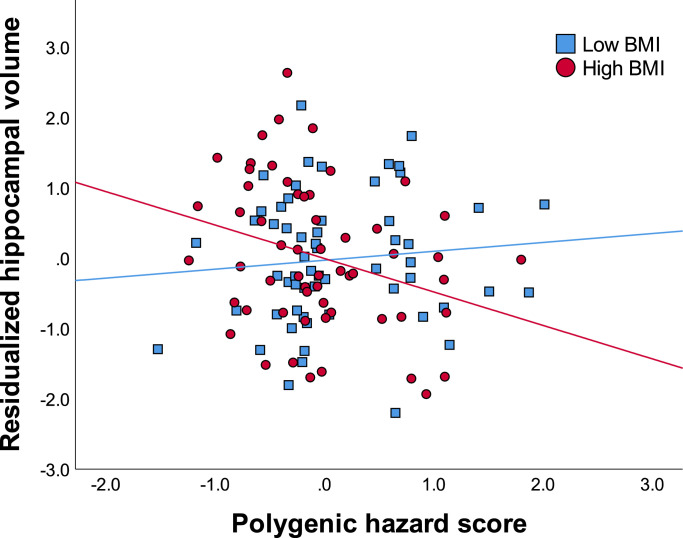
Intracranial volume, age, education, sex, and depression (model 1) accounted for 40.3% of the variance in hippocampal volume. Adding PHS and BMI (model 2) accounted for an additional 1.2% of the variance, which was not significant. Adding BMI x PHS (model 3) accounted for an additional 3.5% of the variance. The BMI by PHS interaction term was significant, whereby individuals with high BMI and high PHS had reduced volume in the hippocampus [ΔF(1,108) = 6.855, P = 0.010, R2 = 0.450] (See Fig. 2 and Table 3).
Fig. 2.
Reduced hippocampal volume among subjects with high BMI and high genetic risk for AD. Values on the x-axis represent polygenic hazard scores for AD, with higher scores indicating increased risk for AD. Values on the y-axis represent standardized residuals of hippocampal volume (accounting for age, sex, education, intracranial volume, and depression).
Table 3.
Summary of regression analysis for association with hippocampal volume.
| Variable | Model 1 |
Model 2 |
Model 3 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE (B) | β | P | B | SE (B) | β | P | B | SE (B) | β | P | |
| Age | −61.564 | 11.332 | −0.408 | < 0.001⁎⁎ | −58.399 | 11.758 | −0.387 | < 0.001⁎⁎ | −56.070 | 11.489 | −0.372 | < 0.001⁎⁎ |
| Sex | 51.758 | 159.993 | 0.029 | 0.747 | 15.997 | 162.196 | 0.009 | 0.922 | 10.418 | 158.022 | 0.006 | 0.948 |
| Education | −24.915 | 27.709 | −0.070 | 0.371 | −23.674 | 28.262 | −0.067 | 0.404 | −30.852 | 27.668 | −0.087 | 0.267 |
| Intracranial volume | 0.003 | 0.001 | 0.538 | < 0.001⁎⁎ | 0.003 | 0.001 | 0.545 | < 0.001⁎⁎ | 0.003 | 0.001 | 0.563 | < 0.001⁎⁎ |
| Depression | 5.165 | 60.136 | 0.006 | 0.932 | −2.048 | 60.260 | −0.003 | 0.973 | −55.058 | 62.098 | −0.068 | 0.377 |
| PHS | −92.951 | 99.233 | −0.070 | 0.351 | 1852.735 | 749.380 | 1.398 | 0.015* | ||||
| BMI | 19.003 | 18.233 | 0.081 | 0.300 | 5.879 | 18.455 | 0.025 | 0.751 | ||||
| BMI x PHS | −74.388 | 28.411 | −1.496 | 0.010* | ||||||||
| R2 | 0.403 | 0.415 | 0.450 | |||||||||
| Model F | 14.984⁎⁎ | 11.059⁎⁎ | 11.053⁎⁎ | |||||||||
PHS = polygenic hazard score; BMI = body mass index.
P < 0.05.
P < 0.01.
To further explore this pattern of results, partial correlations were conducted based on median split (Mdn = 26.45), as described above for the entorhinal cortex. Correcting for all covariates, results revealed that among individuals with high BMI, PHS was negatively correlated with hippocampal volume (pr = −0.289, P = 0.036). Among individuals with low BMI, PHS was not significantly correlated with hippocampal volume (pr = 0.125, P = 0.367).
Next, sex differences in the relationship between BMI, PHS, and hippocampal volume were examined. The sex-stratified linear regressions revealed that the BMI x PHS interaction remained significant in females [ΔF(1,52) = 4.073, P = 0.049, R2 = 0.560] (See Inline Supplementary Table 4). However, in males, age (P = 0.009) and intracranial volume (P = 0.001) were the only predictors of hippocampal volume (See Inline Supplementary Table 5).
3.3. Control regions
To investigate the specificity of BMI and PHS findings on AD-vulnerable brain regions, we examined associations in two control regions of interest. Intracranial volume, age, education, sex, and depression (model 1) accounted for 34.5% of the variance in the precentral gyrus and 40.1% of the variance in the postcentral gyrus. Adding PHS and BMI (model 2) and BMI x PHS (model 3) did not account for additional significant variance in the precentral gyrus or postcentral gyrus. Age (P = 0.011) and intracranial volume (P < 0.001) were the only predictors of precentral gyrus volume. Intracranial volume (P < 0.001) was the only predictor of postcentral gyrus volume.
3.4. CSF biomarkers
The relationship between BMI, PHS, and CSF biomarkers of AD was also explored. Results of the linear regressions revealed that the BMI x PHS interaction term did not significantly predict levels of Aβ (P = 0.116), tau (P = 0.221), or p-tau (P = 0.443). There was, however, a main effect of PHS on levels of Aβ (P < 0.001), whereby higher PHS was associated with lower CSF levels of Aβ. This is consistent with the notion that lower CSF levels of Aβ are related to greater Aβ burden in the brain. Additionally, there was a main effect of BMI (P = 0.022), such that higher BMI was associated with higher tau levels. There was a marginally significant main effect of higher PHS associated with higher tau (P = 0.080). There were main effects of BMI (P = 0.028) and PHS (P = 0.029) on levels of p-tau, such that higher BMI and higher PHS were independently associated with higher p-tau levels.
4. Discussion
This study examined the associations of BMI, polygenic risk for AD, and their interaction on medial temporal lobe volume and CSF biomarkers. There were three main findings. First, results revealed that the combination of two risk factors, being overweight/obese and genetic risk for AD, was associated with lower volume in AD-vulnerable brain regions, and in particular, the entorhinal cortex and hippocampus, in cognitively normal older adults. Second, the effect was observed primarily in female participants. Finally, polygenic risk for AD and BMI independently influence levels of CSF biomarkers.
Previous work has shown inconsistent relationships between obesity and risk for AD in older adults. The findings reported here may help clarify the relationship between obesity and risk for AD by demonstrating the moderating influence of genetic risk on this relationship. In particular, high BMI may confer greater risk for neurodegenerative processes in the context of predisposing genetic risk for AD. Although the precise mechanisms that underlie the synergistic effects are unknown, it is important to note that there is substantial overlap between the metabolic consequences of obesity and the genetic pathways associated with AD. Genetic pathways of AD include immune response, lipid metabolism, cholesterol, endocytosis, cell adhesion molecules, and inflammation (Jones et al., 2010; Verheijen and Sleegers, 2018). Similarly, obesity is associated with inflammation, altered lipid metabolism, cholesterol, insulin resistance, and immune response (Jones and Rebeck, 2019; Martí et al., 2001; O'Brien et al., 2017). One interpretation of the findings is the deleterious metabolic cascades associated with genetic risk for AD are exacerbated with high BMI.
Results of this study also showed that the relationship between BMI and genetics on medial temporal lobe volume was pronounced in female participants. Females are more likely to develop AD than males (Andersen et al., 1999; Farrer et al., 1997; Gao et al., 1998), which cannot simply be explained by increased longevity among females (Lautenschlager et al., 1996). The relationship between BMI and dementia risk appears to be stronger in females than males both in mid-life and late-life, although not all studies have found sex differences (Hassing et al., 2009; Kivipelto et al., 2005). Gustafson et al. (2003) showed that females with higher BMI in older adulthood had a greater risk of developing dementia. Previous research has also shown differential genetic influences in women vs. men, whereby healthy older female APOE ε4 carriers had the greatest risk for conversion to MCI or AD (Altmann et al., 2014). In sub-analyses, Altmann et al. (2014) also showed that among healthy older adults, a single copy of the APOE ε4 allele increases risk of conversion to MCI or AD in females, but not males. A recent study also found associations between genetic risk for AD, measured both by APOE ε4 status and a polygenic risk score that excluded APOE and TREM2, and lower hippocampal volume in older adults, and these associations were more pronounced in females (Lupton et al., 2016). A potential mechanism contributing to sex differences may be the role that estrogen plays in AD risk (for review, see Merlo et al., 2017; Pike, 2017), and future studies should investigate how estrogen may interact with genetics to confer risk for AD.
The current study also examined the relationship between BMI and genetic risk for AD in two control regions of interest: the precentral gyrus and postcentral gyrus. BMI, genetic risk, and their interaction did not influence volume in the precentral gyrus or postcentral gyrus. These results suggest that the influence of BMI and genetic risk on brain volume may be specific to AD-vulnerable regions. Although atrophy is observed in normal aging, its neural signature is distinct from AD-related atrophy. The medial temporal lobe regions, including the entorhinal cortex and hippocampus, are typically the first to be impacted by AD-related cortical atrophy (Pini et al., 2016), however these regions are relatively spared in regards to age-related atrophy (Bakkour et al., 2013; Ohnishi et al., 2001). This further supports the interpretation that the lower volume observed specifically in the medial temporal lobe regions in the present study may reflect some of the earliest AD-related pathology. As AD progresses, atrophy extends throughout the cortex, following a neuropathological trajectory similar to Braak neurofibrillary tangle staging. Cortical atrophy spreads through the temporal, parietal, and frontal regions, while sensory and motor regions are minimally impacted until late stages of the disease (Frisoni et al., 2009; Pini et al., 2016).
The lack of interaction between BMI and polygenic risk for AD on CSF biomarkers was surprising, as there is evidence that atrophy reflects the accumulation of Aβ, tau, and p-tau in the brain (Fagan et al., 2009; Jack et al., 2013). However, there is additional evidence that MRI and CSF biomarkers may independently relate to and reflect unique parts of AD (Schoonenboom et al., 2008; Vemuri et al., 2009). Thus, the pathways implicated by high BMI and genetic risk may differentially impact medial temporal lobe volume and CSF biomarkers. Despite the lack of interaction, there were still independent effects of BMI and PHS on CSF biomarkers. In the present study, individuals with high genetic risk for AD had lower CSF Aβ (reflecting greater intracranial Aβ burden), higher p-tau, and marginally higher tau, which is consistent with previous research showing that higher PHS is associated with greater AD-related pathology (Desikan et al., 2017). Furthermore, higher BMI was associated with greater levels of tau and p-tau, suggesting that high BMI in late-life may be a risk factor for AD.
This study has several limitations that should be considered. First, although this study observed AD-related vulnerabilities using MRI biomarkers, it cannot be determined whether any of the individuals in this study will develop AD in the future. Additionally, ADNI does not collect data on BMI prior to the baseline assessment, and thus the influence of lifetime BMI on the relationship between late-life BMI, genetic risk for AD, and AD-related biomarkers could not be examined. Investigating the relationship between AD-related pathology, BMI, and polygenic risk for AD longitudinally can further elucidate how these variables interact to influence the progression of AD. Furthermore, the sample did not include any individuals with a BMI below 20 and thus the relationship between underweight BMI, genetics, and AD-related biomarkers could not be examined. However, this relationship should be investigated, as previous research has shown that having a BMI below 20 in older adulthood may increase risk for dementia (Fitzpatrick et al., 2009). Another limitation is the selectivity of the sample. The findings of this study may not generalize to ethnic groups other than white, non-Hispanic/Latino older adults. Additional work is necessary to determine how BMI and genetic risk for AD interact in other samples.
5. Conclusions
This study reports that the combination of high BMI and high genetic risk for AD is associated with lower volume in the medial temporal lobe, and this relationship is more pronounced in females. Additionally, high BMI and high genetic risk are independently associated with increased CSF biomarker burden. These results underscore the importance of examining the synergistic effects of genetic and environmental risk factors on markers of AD, with the overarching goal of developing methods aimed at delaying or preventing progression to AD.
Funding
This work was supported by the National Institute on Aging (NIA) of the National Institutes of Health (NIH) R21AG056921 (awarded to SMH), NIA R01AG058822 (awarded to JPH), and The Ohio State University Discovery Themes Chronic Brain Injury Initiative (SMH and JPH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (NIH Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the NIA, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.;Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the NIH (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Appendix A. supplementary data
Supplementary material contains additional details regarding MRI analysis with FreeSurfer.
CRediT authorship contribution statement
Jasmeet P. Hayes: Conceptualization, Writing - original draft, Writing - review & editing, Supervision, Funding acquisition. Jena N. Moody: Data curation, Formal analysis, Validation, Writing - original draft, Writing - review & editing, Visualization. Juan Guzmán Roca: Data curation, Software, Writing - review & editing, Visualization. Scott M. Hayes: Conceptualization, Writing - review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2019.102156.
Contributor Information
Jasmeet P. Hayes, Email: hayes.1075@osu.edu.
Jena N. Moody, Email: moody.279@buckeyemail.osu.edu.
Juan Guzmán Roca, Email: juan.guzman@upr.edu.
Scott M. Hayes, Email: hayes.1074@osu.edu.
Appendix. Supplementary materials
References
- Albanese E., Launer L.J., Egger M., Prince M.J., Giannakopoulos P., Wolters F.J., Egan K. Body mass index in midlife and dementia: systematic review and meta-regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimers Dement (Amst) 2017;8:165–178. doi: 10.1016/j.dadm.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford S., Patel D., Perakakis N., Mantzoros C.S. Obesity as a risk factor for Alzheimer's disease: weighing the evidence. Obes. Rev. 2018;19:269–280. doi: 10.1111/obr.12629. [DOI] [PubMed] [Google Scholar]
- Altmann A., Tian L., Henderson V.W., Greicius M.D., Alzheimer's Disease Neuroimaging Initiative Sex modifies the APOE-related risk of developing Alzheimer disease. Ann. Neurol. 2014;75:563–573. doi: 10.1002/ana.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K., Launer L.J., Dewey M.E., Letenneur L., Ott A., Copeland J.R., Dartigues J.F., Kragh-Sorensen P., Baldereschi M., Brayne C., Lobo A., Martinez-Lage J.M., Stijnen T., Hofman A., Incidence Research Group E.U.R.O.D.E.M. Gender differences in the incidence of AD and vascular dementia: the Eurodem Studies. Neurology. 1999;53:1992–1997. doi: 10.1212/wnl.53.9.1992. [DOI] [PubMed] [Google Scholar]
- Anstey K.J., Cherbuin N., Budge M., Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes. Rev. 2011;12:e426–e437. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- Atti A.R., Palmer K., Volpato S., Winblad B., Ronchi D.D., Fratiglioni L. Late-life body mass index and dementia incidence: nine-year follow-up data from the Kungsholmen Project. J. Am. Geriatr. Soc. 2008;56:111–116. doi: 10.1111/j.1532-5415.2007.01458.x. [DOI] [PubMed] [Google Scholar]
- Bakkour A., Morris J.C., Wolk D.A., Dickerson B.C. The effects of aging and Alzheimer’s disease on cerebral cortical anatomy: specificity and differential relationships with cognition. Neuroimage. 2013;76:332–344. doi: 10.1016/j.neuroimage.2013.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Buchman A.S., Wilson R.S., Bienias J.L., Shah R.C., Evans D.A., Bennett D.A. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65:892–897. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- Cronk B.B., Johnson D.K., Burns J.M., Initiative A.D.N. Body mass index and cognitive decline in mild cognitive impairment. Alzheimer Dis. Assoc. Disord. 2010;24:126–130. doi: 10.1097/WAD.0b013e3181a6bf3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Fan C.C., Wang Y., Schork A.J., Cabral H.J., Cupples L.A., Thompson W.K., Besser L., Kukull W.A., Holland D., Chen C.H., Brewer J.B., Karow D.S., Kauppi K., Witoelar A., Karch C.M., Bonham L.W., Yokoyama J.S., Rosen H.J., Miller B.L., Dillon W.P., Wilson D.M., Hess C.P., Pericak-Vance M., Haines J.L., Farrer L.A., Mayeux R., Hardy J., Goate A.M., Hyman B.T., Schellenberg G.D., McEvoy L.K., Andreassen O.A., Dale A.M. Genetic assessment of age-associated Alzheimer disease risk: development and validation of a polygenic hazard score. PLoS Med. 2017;14 doi: 10.1371/journal.pmed.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J.B. The effect of obesity on health outcomes. Mol. Cell. Endocrinol. 2010;316:104–108. doi: 10.1016/j.mce.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Ewers M., Schmitz S., Hansson O., Walsh C., Fitzpatrick A., Bennett D., Minthon L., Trojanowski J.Q., Shaw L.M., Faluyi Y.O., Vellas B., Dubois B., Blennow K., Buerger K., Teipel S.J., Weiner M., Hampel H. Body mass index is associated with biological CSF markers of core brain pathology of Alzheimer's disease. Neurobiol. Aging. 2012;33:1599–1608. doi: 10.1016/j.neurobiolaging.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan A.M., Head D., Shah A.R., Marcus D., Mintun M., Morris J.C., Holtzman D.M. Decreased cerebrospinal fluid Aβ42 correlates with brain atrophy in cognitively normal elderly. Ann. Neurol. 2009;65:176–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer L.A., Cupples L.A., Haines J.L., Hyman B., Kukull W.A., Mayeux R., Myers R.H., Pericak-Vance M.A., Risch N., van Duijn C.M. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Fitzpatrick A.L., Kuller L.H., Lopez O.L., Diehr P., O’Meara E.S., Longstreth W.T., Luchsinger J.A. Midlife and late-life obesity and the risk of dementia: cardiovascular Health Study. Arch. Neurol. 2009;66:336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisoni G.B., Prestia A., Rasser P.E., Bonetti M., Thompson P.M. In vivo mapping of incremental cortical atrophy from incipient to overt Alzheimer's disease. J. Neurol. 2009;256:916–924. doi: 10.1007/s00415-009-5040-7. [DOI] [PubMed] [Google Scholar]
- Gao S., Hendrie H.C., Hall K.S., Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch. Gen. Psychiatry. 1998;55:809–815. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- Gatz M., Reynolds C.A., Fratiglioni L., Johansson B., Mortimer J.A., Berg S., Fiske A., Pedersen N.L. Role of genes and environments for explaining Alzheimer disease. Arch. Gen. Psychiatry. 2006;63:168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- Grundman M., Corey-Bloom J., Jernigan T., Archibald S., Thal L.J. Low body weight in Alzheimer’s disease is associated with mesial temporal cortex atrophy. Neurology. 1996;46:1585–1591. doi: 10.1212/wnl.46.6.1585. [DOI] [PubMed] [Google Scholar]
- Gustafson D., Rothenberg E., Blennow K., Steen B., Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch. Intern. Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- Hales C.M., Fryar C.D., Carroll M.D., Freedman D.S., Aoki Y., Ogden C.L. Differences in obesity prevalence by demographic characteristics and urbanization level among adults in the United States, 2013-2016. JAMA. 2018;319:2419–2429. doi: 10.1001/jama.2018.7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J., Solovyova N., Irving A. Leptin and its role in hippocampal synaptic plasticity. Prog. Lipid Res. 2006;45:369–378. doi: 10.1016/j.plipres.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassing L.B., Dahl A.K., Thorvaldsson V., Berg S., Gatz M., Pedersen N.L., Johansson B. Overweight in midlife and risk of dementia: a 40-year follow-up study. Int. J. Obes. (Lond) 2009;33:893–898. doi: 10.1038/ijo.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert L.E., Weuve J., Scherr P.A., Evans D.A. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Bernstein M.A., Borowski B.J., Gunter J.L., Fox N.C., Thompson P.M., Schuff N., Krueger G., Killiany R.J., DeCarli C.S., Dale A.M., Carmichael O.W., Tosun D., Weiner M.W. Update on the magnetic resonance imaging core of the Alzheimer’s disease neuroimaging initiative. Alzheimers Dement. 2010;6:212–220. doi: 10.1016/j.jalz.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Knopman D.S., Jagust W.J., Petersen R.C., Weiner M.W., Aisen P.S., Shaw L.M., Vemuri P., Wiste H.J., Weigand S.D., Lesnick T.G., Pankratz V.S., Donohue M.C., Trojanowski J.Q. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L., Holmans P.A., Hamshere M.L., Harold D., Moskvina V., Ivanov D., Pocklington A., Abraham R., Hollingworth P., Sims R., Gerrish A., Pahwa J.S., Jones N., Stretton A., Morgan A.R., Lovestone S., Powell J., Proitsi P., Lupton M.K., Brayne C., Rubinsztein D.C., Gill M., Lawlor B., Lynch A., Morgan K., Brown K.S., Passmore P.A., Craig D., McGuinness B., Todd S., Holmes C., Mann D., Smith A.D., Love S., Kehoe P.G., Mead S., Fox N., Rossor M., Collinge J., Maier W., Jessen F., Schürmann B., van den Bussche H., Heuser I., Peters O., Kornhuber J., Wiltfang J., Dichgans M., Frölich L., Hampel H., Hüll M., Rujescu D., Goate A.M., Kauwe J.S.K., Cruchaga C., Nowotny P., Morris J.C., Mayo K., Livingston G., Bass N.J., Gurling H., McQuillin A., Gwilliam R., Deloukas P., Al-Chalabi A., Shaw C.E., Singleton A.B., Guerreiro R., Mühleisen T.W., Nöthen M.M., Moebus S., Jöckel K.-H., Klopp N., Wichmann H.-E., Rüther E., Carrasquillo M.M., Pankratz V.S., Younkin S.G., Hardy J., O’Donovan M.C., Owen M.J., Williams J. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer’s disease. PLoS ONE. 2010;5:e13950. doi: 10.1371/journal.pone.0013950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N.S., Rebeck G.W. The synergistic effects of APOE genotype and obesity on Alzheimer’s disease risk. Int. J. Mol. Sci. 2019;20:63. doi: 10.3390/ijms20010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo S.H., Yun S.H., Kang D.W., Hahn C.T., Lim H.K., Lee C.U. Body mass index in mild cognitive impairment according to age, sex, cognitive intervention, and hypertension and risk of progression to Alzheimer’s disease. Front. Psychiatry. 2018;9:142. doi: 10.3389/fpsyt.2018.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivimäki M., Luukkonen R., Batty G.D., Ferrie J.E., Pentti J., Nyberg S.T., Shipley M.J., Alfredsson L., Fransson E.I., Goldberg M., Knutsson A., Koskenvuo M., Kuosma E., Nordin M., Suominen S.B., Theorell T., Vuoksimaa E., Westerholm P., Westerlund H., Zins M., Kivipelto M., Vahtera J., Kaprio J., Singh-Manoux A., Jokela M. Body mass index and risk of dementia: analysis of individual-level data from 1.3 million individuals. Alzheimers Dement. 2018;14:601–609. doi: 10.1016/j.jalz.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M., Ngandu T., Fratiglioni L., Viitanen M., Kåreholt I., Winblad B., Helkala E.L., Tuomilehto J., Soininen H., Nissinen A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch. Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- Lautenschlager N.T., Cupples L.A., Rao V.S., Auerbach S.A., Becker R., Burke J., Chui H., Duara R., Foley E.J., Glatt S.L., Green R.C., Jones R., Karlinsky H., Kukull W.A., Kurz A., Larson E.B., Martelli K., Sadovnick A.D., Volicer L., Waring S.C., Growdon J.H., Farrer L.A. Risk of dementia among relatives of Alzheimer’s disease patients in the Mirage study: what is in store for the oldest old? Neurology. 1996;46:641–650. doi: 10.1212/wnl.46.3.641. [DOI] [PubMed] [Google Scholar]
- Lupton M.K., Strike L., Hansell N.K., Wen W., Mather K.A., Armstrong N.J., Thalamuthu A., McMahon K.L., de Zubicaray G.I., Assareh A.A., Simmons A., Proitsi P., Powell J.F., Montgomery G.W., Hibar D.P., Westman E., Tsolaki M., Kloszewska I., Soininen H., Mecocci P., Velas B., Lovestone S., Brodaty H., Ames D., Trollor J.N., Martin N.G., Thompson P.M., Sachdev P.S., Wright M.J. The effect of increased genetic risk for Alzheimer's disease on hippocampal and amygdala volume. Neurobiol. Aging. 2016;40:68–77. doi: 10.1016/j.neurobiolaging.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí A., Marcos A., Martínez J.A. Obesity and immune function relationships. Obes. Rev. 2001;2:131–140. doi: 10.1046/j.1467-789x.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- Merlo S., Spampinato S.F., Sortino M.A. Estrogen and Alzheimer's disease: still an attractive topic despite disappointment from early clinical results. Eur. J. Pharmacol. 2017;817:51–58. doi: 10.1016/j.ejphar.2017.05.059. [DOI] [PubMed] [Google Scholar]
- Morris C.H., Hope R.A., Fairburn C.G. Eating habits in dementia: a descriptive study. Br. J. Psychiatry. 1989;154:801–806. doi: 10.1192/bjp.154.6.801. [DOI] [PubMed] [Google Scholar]
- O’Brien P.D., Hinder L.M., Callaghan B.C., Feldman E.L. Neurological consequences of obesity. Lancet Neurol. 2017;16:465–477. doi: 10.1016/S1474-4422(17)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T., Matsuda H., Tabira T., Asada T., Uno M. Changes in brain morphology in Alzheimer disease and normal aging: is Alzheimer disease an exaggerated aging process? AJNR Am. J. Neuroradiol. 2001;22:1680–1685. [PMC free article] [PubMed] [Google Scholar]
- Pike C.J. Sex and the development of alzheimer's disease: sex differences in AD. J. Neurosci. Res. 2017;95:671–680. doi: 10.1002/jnr.23827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pini L., Pievani M., Bocchetta M., Altomare D., Bosco P., Cavedo E., Galluzzi S., Marizzoni M., Frisoni G.B. Brain atrophy in Alzheimer's disease and aging. Ageing Res. Rev. 2016;30:25–48. doi: 10.1016/j.arr.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Ridge P.G., Mukherjee S., Crane P.K., Kauwe J.S.K., Alzheimer’s Disease Genetics Consortium Alzheimer’s disease: analyzing the missing heritability. PLoS ONE. 2013;8:e79771. doi: 10.1371/journal.pone.0079771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonenboom N.S.M., van der Flier W.M., Blankenstein M.A., Bouwman F.H., Van Kamp G.J., Barkhof F., Scheltens P. CSF and mri markers independently contribute to the diagnosis of Alzheimer's disease. Neurobiol. Aging. 2008;29:669–675. doi: 10.1016/j.neurobiolaging.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Vemuri P., Wiste H.J., Weigand S.D., Shaw L.M., Trojanowski J.Q., Weiner M.W., Knopman D.S., Petersen R.C., Jack C.R. MRI and CSF biomarkers in normal, MCI, and AD subjects: diagnostic discrimination and cognitive correlations. Neurology. 2009;73:287–293. doi: 10.1212/WNL.0b013e3181af79e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheijen J., Sleegers K. Understanding Alzheimer disease at the interface between genetics and transcriptomics. Trends Genet. 2018;34:434–447. doi: 10.1016/j.tig.2018.02.007. [DOI] [PubMed] [Google Scholar]
- Vidoni E.D., Townley R.A., Honea R.A., Burns J.M., Alzheimer’s Disease Neuroimaging Initiative Alzheimer disease biomarkers are associated with body mass index. Neurology. 2011;77:1913–1920. doi: 10.1212/WNL.0b013e318238eec1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne V.L., Burnham S., Bourgeat P., Brown B., Ellis K.A., Salvado O., Szoeke C., Macaulay S.L., Martins R., Maruff P., Ames D., Rowe C.C., Masters C.L. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12:357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.