Abstract
The stimulant, methylphenidate (MPH), is commonly used to treat attention deficit hyperactivity disorder (ADHD) and has been increasingly prescribed for school age children and adolescents. Concerns regarding its long-term effects on later substance use disorders (SUDs) have been raised. Previous animal studies have produced contradictory results regarding whether early exposure to MPH increases or protects against SUD in adulthood. The goal of our study was to determine if clinically relevant doses of MPH during adolescence alter cocaine responsiveness in adulthood in a rat model of ADHD, the spontaneous hypertensive rat (SHR). We pretreated SHRs with saline or MPH (2.5 mg/kg once or twice day) via oral gavage during their dark cycle from postnatal day 35 (p35) to p44. Adult rats (p80) were assessed in an eight-session cocaine-conditioned place preference test (CPP). Four doses of cocaine were administered via intraperitoneal injection (i.p.) during the conditioning sessions: 1, 5, 10 and 20 mg/kg. Once per day MPH treatment had a small sensitizing effect on baseline general locomotor activity in a novel environment at p80 as well as a limited suppressive effect on reward-specific locomotor activity as measured by the decreased preference to enter the cocaine-paired chamber. This treatment did not have any effect on the amount of time that rats chose to spend in the cocaine-paired chamber. Twice per day MPH treatment had no effect on locomotion or drug-preference. Our results suggest that MPH treatment of ADHD rats during adolescence does not alter preference for cocaine in adulthood.
Keywords: methylphenidate, ADHD, cocaine, conditioned place preference, locomotion
INTRODUCTION
The stimulant methylphenidate (MPH) treats attention deficit hyperactivity disorder (ADHD) [1] and is often prescribed for prolonged periods given that the disorder persists into adulthood in two-thirds of cases [2]. MPH increases extracellular dopamine by blocking the dopamine transporter (DAT). This mechanism underlies both its therapeutic and reinforcing effects [3]. MPH is abused by adolescents and adults [4–6] and the diversion and misuse of ADHD medications is a clinical concern [7,8]. Adolescent rats readily self-administer MPH, as they do cocaine [9]. Although a slow uptake of medication in the brain via oral treatment has a lower potential for abuse or diversion [10], it is still not clear whether long-term exposure to therapeutic dose of MPH during youth alters the risk for substance abuse in adulthood.
A meta-analysis suggested a protective effect of early methylphenidate treatment on the development of SUDs in adolescence, but not adulthood [11]. Since then, several studies reported either no association of SUDs with early stimulant treatment [12–15], or a protective effect [16–19]. A randomized, placebo-controlled trial found that stimulant treatment improved SUDs outcomes in adolescents with co-morbid attention and conduct problems [20,21]. This work reported that age at stimulant treatment initiation was positively related to the later development of non-alcohol SUDs. Groenman et al. [22] also reported that children who started stimulant medication at younger ages were better protected against later SUDs. But in that study, the effect of age of first stimulant use on SUD development diminished with age.
Animal studies have produced far more inconsistent results. Some found that MPH treatment increased aversion or decreased liking to cocaine [23–26]. Others found that MPH treatment led to enhanced cocaine self-administration, ethanol intake, behavioral sensitization, or drug-conditioned place preference [27–30]. These studies differed in many methodological features including: dose, route of administration, age, gender, individual genetic predisposition (animal strains) and time of treatment (during dark or light cycles of the rodent circadian rhythm), that contributed to variation in the data [31,32]. A typical treatment dose of MPH for ADHD administered orally (e.g., b.i.d, t.i.d. or slow-release) achieves an 8–40 ng/mL plasma peak level after approximately 2 h in humans [32–34]. This induces a slow-steady increase of brain dopamine [4,32,35]. A 1–3 mg/kg oral dose in rats was estimated to produce a similar plasma peak level but with a much shorter half-life due to the between species differences in drug absorption and metabolism [32,36]. Unfortunately, most rat studies used i.p. or intravenous injections of this or higher dose levels, leading to faster and larger increases of plasma drug levels and brain dopamine levels, which are often associated with locomotor stimulation and sensitization instead of a calming effect for hyperactivity [37–39]. Gaytan et al. [40] have found that the most robust sensitization occurred if treatment was during the light phase, while no sensitization was observed if treatment was during the dark phase. Treatment given during the dark cycle was also associated with smaller effects of high dose MPH on stereotypic behaviors [41].
Rat strains also play important roles in different response to MPH treatment [42,43]. For example, repeated 2.5 mg/kg MPH treatment elicited locomotor sensitization in behaviorally “normal” control strains, such as Sprague Dawley (SD) and Wistar Kyoto (WKY) rats, but not in the SHR rat, a validated rat model for ADHD [43]. Similar to ADHD human patients, SHR rats could benefit from a paradoxical calming effect of stimulant treatment, whereas “normal” individuals and rats experienced locomotor activation [36,44,45]. SHR rats readily self-administered MPH and developed conditioned place preference to MPH confirming a rewarding effect of MPH for SHR rats. However, SHR rats learned MPH self-administration faster and responded more to MPH infusions than did Wistar rats, particularly more so in the adolescent SHR rats [46,47]. This is in concordance with higher rates of SUDs in ADHD than non-ADHD youth [14].
Among 400 articles retrieved from PubMed by searching “methylphenidate AND cocaine” or “methylphenidate AND drug abuse” in non-human species, 238 used rat species. However, only 10 studies used SHR rats. Five of these examined the effects of MPH treatment during adolescence on adult animals’ drug response or use behavior [26,30,46,48,49]. Among all 10 studies, only one specified that the experiments were conducted during the rats’ dark cycle [48]. Although all the treatment studies modeled clinically relevant doses, the majority used i.p. injections; only two administered MPH orally [30,48]. Soeters et al. [48] was the only study that treated SHR rats with 2 mg/kg MPH via oral consumption in their dark cycle. The authors did not find any effect of MPH treatment during adolescence (p21–35) on ethanol consumption later in adulthood, although they found that SHRs consumed less ethanol voluntarily than did the control WKY rats, which is contrary to the clinical observation of a higher risk of alcohol abuse in ADHD individuals [22].
To address the limitations of prior animal studies of the link between MPH treatment and subsequent substance use behaviors, our study treated adolescent SHR rats orally during the dark cycle and examined their cocaine-conditioned place preference behavior during adulthood. To compensate for the shorter half-life of MPH in rats, we also compared the effects of once per day and twice per day treatments.
METHODS
Animals
108 male SHR rats (4 weeks old) obtained from Charles River Laboratories were housed in pairs in plastic cages (24 cm × 46 cm × 20 cm) with ad libitum access to food and water and kept on a 12 h reverse light cycle (lights on from 6 pm to 6 am). All drug treatment and behavioral test procedures were approved by the Animal Care and Use Committee of the State University of New York at Buffalo.
Conditioning Apparatus
Eight in-house constructed conditioning apparatuses were used. Each apparatus had three compartments separated by removable dividers. The left and right compartments were 23 cm × 35 cm and featured differential tactile and visual cues. Tactile cues consisted of stainless-steel square wire-mesh grid flooring with either 0.8 cm or 1.5 cm grid-spacing. Visual cues consisted of two-colored walls which were either black or white. The tactile and visual cues were uncorrelated and evenly distributed between left and right compartments. The middle compartment was 16.5 cm × 15 cm and when the compartment dividers were removed had two openings measuring 15.9 cm × 11.7 cm that allowed subjects to freely travel between compartments. All compartments were 29 cm tall and were covered using a removable Plexiglas top.
Drugs
Animals were pretreated with (±)-methylphenidate hydrochloride (MPH) by oral gavage on days p35–44 twice a day at 9 am and 1 pm. MPH was dissolved in saline (SAL) at a concentration of 2.5 mg/mL and administered to animals at 2.5 mg/kg so that rats received equal gavage volumes by weight. Animals were randomly divided into three pretreatment groups. The control group received SAL twice a day. MPH was given either once per day (MPH1 group, MPH gavage at 9 am and SAL at 1 pm) or twice per day (MPH2 group, MPH gavage at both times).
(−)-Cocaine hydrochloride (gifted by NIDA-RIT Log no: 13070–12C, ref. #013277) was dissolved in saline and injected (intraperitoneal) to rats from p82 to p86 during CPP conditioning. Only rats that were weighted between 220 g and 335 g (M = 282.0 g, SD = 19.7 g) at the time of testing (total = 89) were used for conditioned place preference (CPP) testing. Animals from each pretreatment group were randomly assigned to four different cocaine dose groups (1, 5, 10 and 20 mg/kg) and received injections of equal volumes by weight.
Test for Cocaine-Induced Conditioned Preference
CPP testing used an unbiased procedure and consisted of three phases: pre-conditioning, conditioning and post-conditioning. Sessions were 30 min in duration. During the pre-conditioning session (p81), all dividers were removed, and animals were placed in the center compartment and allowed to freely travel between compartments. The conditioning phase consisted of test sessions 2–7. Immediately prior to conditioning sessions 2, 4, and 6, half the rats were injected with one of four doses of cocaine (COC) and confined in the paired cocaine compartments while the other half were injected with saline and placed in the paired saline compartments. During the conditioning phase the dividers were in place and animals could not travel between compartments. Immediately prior to conditioning sessions 3, 5, and 7, rats that were previously injected with cocaine were injected with saline and placed in the paired saline compartment and those rats that were previously injected with saline were injected with cocaine and placed in the paired cocaine compartment. Animals received a total of 3 saline and 3 cocaine conditioning tests. The scores from the pre-conditioning phase were used to determine the unconditioned compartment preference. Because a biased design, i.e., paring cocaine with the non-preferred compartment, is susceptible for false positive findings [50], we randomly assigned half of the animals to receive drug in their preferred compartment and the other half to receive drug in their non-preferred compartment during the conditioning phase. The post-conditioning phase consisted of one session. Animals were placed in the center compartment, with all dividers removed so that they were able to freely travel between compartments. Both pre- and post-conditioning phases were video-taped for analysis.
Data Analysis
The videos were analyzed using ANY-maze software by an experimenter blinded to the treatment conditions. The software tracks animals’ heads and defines the rats to be in a given compartment if ≥85% of its body was in that compartment. Three additional animals were excluded from the analysis because software failed to correctly track the animals. The final number of animals used for the analysis was 86 with group sizes ranging from 6 to 9. The primary measurements were the number of entries, the distance traveled, and amount of time spent in each compartment. Previous studies of cocaine CPP have primarily examined the time spent in the drug-paired chambers. We sought to examine a full spectrum of behavioral measurements that are available in our data in order to disentangle the reinforcing effect of the drug from the animals’ locomotor sensitization and hyperactivity. A total recording time of 30 min for each rat was divided into six 5-minute segments. 5-Minute segments were chosen based on others and our previous studies to examine locomotor activity and habituation in experimental chambers [51,52]. Only the first 5 segments were used because some of the videos were truncated during the last segment. For the pre-conditioning and post-conditioning phases, general locomotion was represented by the sum of the number of entries or distance traveled in all three compartments. The preference to cocaine was represented by subtracting the number of entries, distance traveled, and time spent in the cocaine-paired compartment in the pre-conditioning session from those of the post-conditioning session. We also calculated the relative preference for cocaine vs saline by subtracting the above measurements of the saline-paired compartment from those of the cocaine-paired compartment during only the post-conditioning sessions. We examined all the measurements in a longitudinal data format consisting of five 5-minute segments and used a random effect general linear regression model to evaluate the effects of MPH pretreatment and cocaine dose in STATA 12.0.
RESULTS
General Locomotor Activity
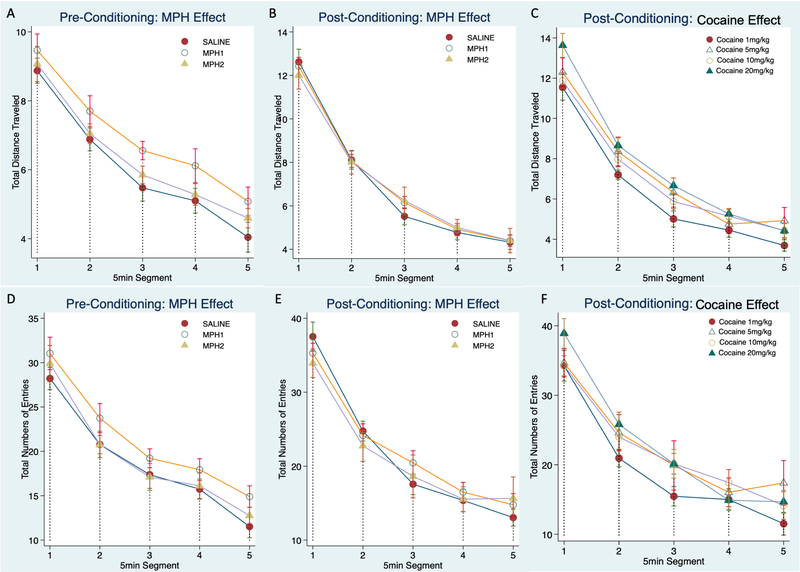
We assessed the general locomotor activity during the pre- and post-conditioning sessions. Figure 1A,D plots total number of entries and distance traveled over five 5-minute segments to show the main effect of MPH pre-treatment during the pre-conditioning phase, which assessed the baseline activity of the pre-treated animals in adulthood. Locomotor activity during the post-conditioning phase were plotted for the main effects of MPH-pretreatment (Figure 1B,E) and the main effects of cocaine doses (Figure 1C,F). The main effects of time segments were significant for both the total number of entries during pre-conditioning (χ2(4) = 400.75, p < 0.0001) and post-conditioning sessions (χ2(4) = 324.89, p < 0.0001), and the total distance traveled during pre-conditioning (χ2(4) = 520.63, p < 0.0001) and post-conditioning sessions (χ2(4) =1650.08, p < 0.0001).
Figure 1.
General locomotor activity during the pre- and post-conditioning tests. The total distance traveled (A–C) and the total numbers of entries (D–F) to all three compartments were plotted over five segments to show the general locomotor and habituation. Group mean and standard errors were shown for each time segment. Animals were grouped in individual figures to show the main effect of MPH pretreatment for the pre-conditioning test (A,D) and post-conditioning test(B,E), and the main effect of the cocaine doses (C,F) at the post-conditioning test. MPH1, 2.5 mg/kg MPH once per day treatment; MPH2, 2.5 mg/kg MPH twice per day treatment.
MPH pretreatment had no effect on the locomotor activity. The small increase of total distance traveled observed for the MPH1 group compared with the SAL group during the pre-conditioning phase was non-significant (χ2(2) = 4.84, p = 0.09, Figure 1A). The MPH2 group did not differ from the SAL group. A similar pattern was observed for the total entries but was also not significant (Figure 1D). The randomly assigned cocaine treatment groups were not different during the pre-conditioning phase (not shown).
During the post-conditioning phase, we also found no effects of MPH pretreatment, nor the interaction of MPH and time-segment (Figure 1B,E). There was a significant effect of interaction between cocaine dose and time-segment (χ2(15) = 39.21, p = 0.0006) for the total entries, primarily due to the lower number of entries of the 1 mg/kg cocaine dose group during time segments 2, 3 and 5 (Figure 1F). The overall effect of cocaine dose on the total distance traveled was modest, and there was no effect of interaction between cocaine dose and time-segment.
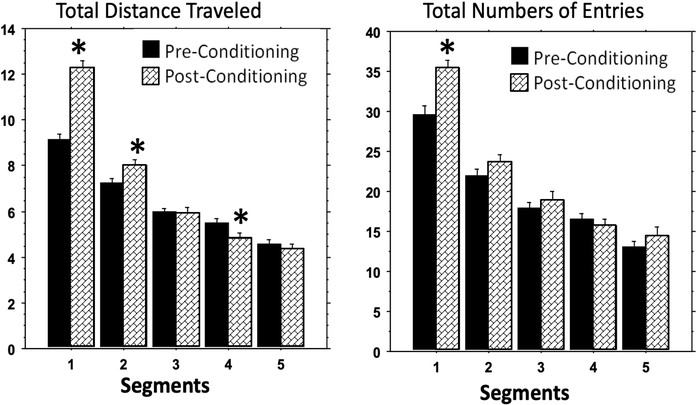
Cocaine conditioning significantly increased the spontaneous locomotor activity in the test apparatus, particularly during the first 10 min as measured by total distance traveled (first 5-minute segment: F(1,173) = 64.42, p < 0.0001; second 5-minute segment: F(1,171) = 6.01, p = 0.015) and during the first 5minutes as measured by total number of entries (F(1,176) = 16.74, p = 0.0001) (Figures 1 and 2). The effect of conditioning-induced locomotor stimulation disappeared after the first 10 min as measured by distance traveled and after 5 minutes as measured by total number of entries. There was a moderate effect of locomotor depression during the fourth 5 minute segment as measured by total distance traveled (F(1,173) = 4.45, p = 0.034), but that effect disappeared during the final segment (Figure 2 Left). Such time-dependent change of activity pattern was similarly observed for all cocaine and MPH treatment groups, and there was no effect of MPH or cocaine dose on the conditioning-induced locomotor increase.
Figure 2.
Cocaine conditioning induced locomotion stimulation. The total distance traveled and the total numbers of entries to all three compartments were plotted for all groups combined to show the main effect of the cocaine conditioning induced locomotion stimulation over time. * indicated significant difference (p < 0.05) between pre- and post-conditioning tests.
Effects of MPH Pretreatment on Cocaine-Conditioned Place Preference
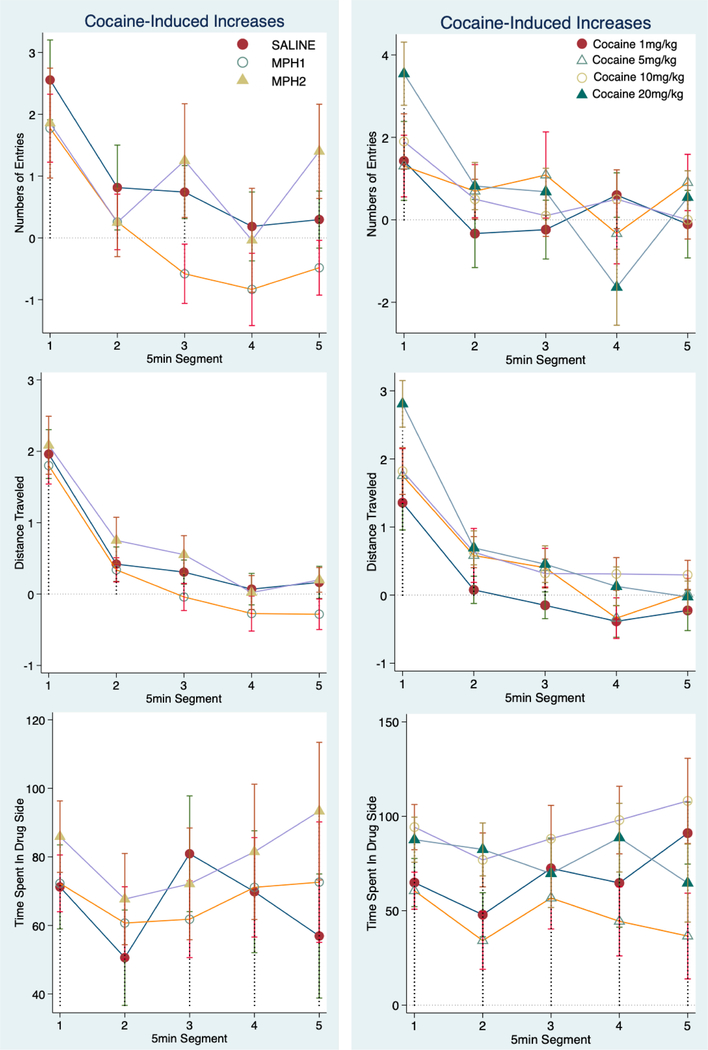
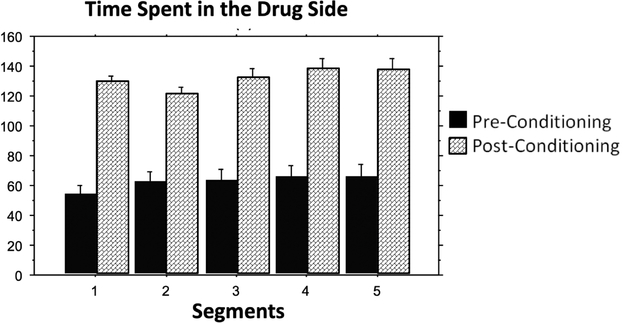
Preference for cocaine was calculated as the post- and pre-conditioning difference (cocaine-conditioning induced increase) for all three measurements: number of entries to the cocaine-paired compartment, distance traveled, and time spent in the cocaine-paired compartment. Figure 3 plots these measurements to show the main effect of MPH pretreatment (Left) and cocaine dose (Right) over time. There was a significant effect of time segment on the numbers of entries (χ2(4) = 34.29, p < 0.0001) and distance traveled (F(4,427) = 32.63, p < 0.0001), but not for time spent in the drug-paired compartment. Cocaine-induced increases of locomotor activity (entries and distance traveled) within the cocaine-paired compartment were the most robust in the first 5-minute segment and subsequently decreased drastically afterwards to the levels of the pre-conditioning phase (Figure 3 Top two rows).
Figure 3.
Cocaine-conditioned place preference measures. Cocaine preference was calculated by the post-conditioning increase of all three measures to the drug-paired compartment from those of the pre-conditioning baselines: the number of entries (Top Row), the distance traveled (Middle Row) and the time spent in the cocaine-paired compartment (Bottom Row). The group means and standard errors were plotted over time segments to show the effects of MPH pretreatment groups (Left Panel) and cocaine doses (Right Panel). MPH1, 2.5 mg/kg MPH once per day treatment; MPH2, 2.5 mg/kg MPH twice per day treatment.
We found a modest but overall non-significant effect of MPH pretreatment on cocaine–induced increase in number of entries to the drug-paired compartment (χ2(4) = 4.88, p = 0.087; Figure 3 Top Left). The effect was due to reduced increase of entries to the drug-paired compartment for the MPH1 treatment group in comparison with the saline treatment (χ2(4) = 5.87, p = 0.015). This suppression was observed only during the last three 5-minute segments, after cocaine-induced locomotion stimulation had waned. MPH2 treatment had no effect on this measure. There was no effect of cocaine dose or interaction of cocaine dose, MPH pretreatment and time on the increased entries (Figure 3 Top row). We also found a modest but significant effect of cocaine dose effect on the increase of distance traveled in the cocaine-paired compartment (Figure 3 Middle Right, χ2(3) = 8.4, p = 0.039), due to lower increase for the 1 mg/kg cocaine group. There was no effect of either MPH pretreatment, time segments or cocaine dose on the increase of time spent in the cocaine-paired compartment (Figure 3 Bottom row).
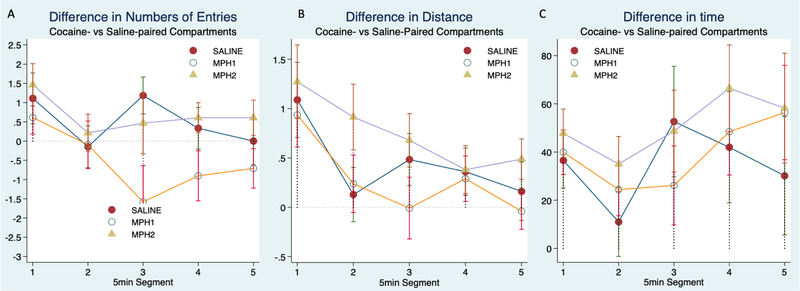
We also calculated a relative preference to the cocaine-paired compartment by subtracting the measurements to the saline-paired compartment from that of the cocaine-paired compartment during only the post-conditioning session (Figure 4). Because half of the animals were paired with cocaine in their preferred compartments and half in their non-preferred compartments, we included it as a covariate in our model. Overall, we found that the cocaine induced changes in either preferred or non-preferred compartments were not significantly different and that the effect of cocaine dose was also not significant. Consistent with what we have seen, time segment effect was significant for both the numbers of entries (Figure 4A, χ2(4) = 10.45, p = 0.033), and distance traveled (Figure 4B, χ2(4) = 30.52, p < 0.0001), but not for the time (Figure 4C). The lack of time segment effect on the increased time spent in the cocaine-paired compartment was further shown in Figure 5. Figure 4 showed the effect of MPH pretreatment on these measurements. The significant effect of MPH treatment on the differences in numbers of entries remained (χ2(2) = 6.22, p = 0.04, Figure 4A), with MPH1 pretreatment showing fewer entries to the cocaine-paired compartments than to the saline-paired compartments. Because an increase of entries to the drug-paired chambers could simply be due to enhanced locomotor stimulation, we included the total number of entries to all chambers at the post-conditioning phase as a covariate in the analysis. We found that the total number of entries had no effect on this measure of relative preference to cocaine. The addition of this covariate completely eliminated the effect of time segments, confirming that the total number of entries represents motor habituation. The effect of MPH pretreatment, namely the suppressive effect of MPH1 treatment on the difference in entries, however, remained significant (χ2(2) = 6.34, p = 0.04).
Figure 4.
A relative preference to the cocaine- vs saline-paired compartments was calculated by subtracting the number of entries (A), distance traveled (B), or time spent (C) in the saline compartment from that of the cocaine-paired compartment during the post-conditioning phase. This relative preference was plotted over time segment to show the main effect of MPH treatment groups.
Figure 5.
The time animals spent in the cocaine-paired compartment was plotted for pre- and post-conditioning phases to show the effectiveness of cocaine conditioning and the lack of change over time.
DISCUSSION
Animal models are widely used in studies of childhood and adolescent MPH treatment effects on adult substance abuse behaviors. However, the majority of these studies have significant methodological problems that have led to contradictory results and further obfuscated the issue. Addressing this issue in a manner that is relevant to the clinical treatment of ADHD requires selecting the proper MPH dose and the route and timing of drug administration. We used SHR rats, the most widely used and validated genetic rat model for ADHD. Our goal was to address the issues that limited the interpretation of previous studies and to determine if the clinically relevant use of MPH in an adolescent ADHD rat model would lead to changes in their cocaine-conditioned place preference behavior in adulthood.
All four doses of cocaine induced significant locomotion stimulation and increased preference for the cocaine-paired compartment. The observation that cocaine is rewarding for the SHR is consistent with previous work [26,30]. However, one study did not observe a rewarding effect at the 1 mg/kg dose [26].This discrepancy could be because that our experiment conducted three trials of cocaine and saline paring (6 days total), while Augusyniak et al. only conducted two trials of cocaine and saline pairing (4 days total) [26]. The differences could also be due to the treatment regimen, (light vs. dark cycle) and genetic variations in the SHR strains from different vendors [40,53]. It is not surprising to see the lack of dose-response curves in the CPP results. Augusyniak et al. also showed similar levels of preference to the cocaine chambers for all effective doses (5, 10 and 20 mg/kg) in SHR rats [26]. We did observe a small but significant cocaine dose-dependent locomotor stimulation (Figure 1C). Self-administration paradigm maybe better suited than the CPP paradigm for study of dose-dependent rewarding effects of cocaine.
One strength of our current study is detailed analysis of the CPP behavior during a prolonged period of time (25 min total), which revealed two separate behavioral dimensions in SHR rats: locomotor sensitization and conditioned preference to the cocaine. Locomotor sensitization was only present during the first 10 min of post-conditioning testing. Context alone was enough to elicit the behavioral sensitization for all the effective doses of cocaine used, and it did not require a challenge injection. In contrast, conditioned preference to cocaine, measured by the increased time that animals spent in the cocaine-paired compartment, was not time dependent. Once conditioning was well established, animals continued to spend more time in the drug-paired compartment, regardless of the decreasing number of entries into that chamber due to locomotion habituation. It is important to note that animal entries to the drug-specific compartment may reflect both the general locomotor stimulation and reward-invoked drug seeking. In our analysis of the number of entries to the drug vs. saline paired compartments, we statistically controlled the effects of general locomotor activation on the reward-specific increase of entries to the cocaine compartment. Interestingly, when general locomotion was taken into account, the specific change of entries to the cocaine compartment was no longer dependent on time. This observation was consistent with the lack of time segment effect on the cocaine preference measured by the time. Both of which support the conclusion that established cocaine conditioned preference does not change over time during the course of our CPP testing, although locomotion habituates over time.
Our main finding is the lack of effect of MPH pretreatments on the increased preference to cocaine-paired chambers as measured by the time. Five prior studies used SHR rats to examine the effect of MPH treatment in adolescence on drug response in adulthood. Three studies administered MPH once per day. Augustyniak et al. [26] found a reduced CPP to cocaine and De la Pena et al. [46] found a reduced CPP to methylphenidate. Both studies administered MPH via i.p. during the rats’ light cycles. Harvey et al. [30] used oral administration during the light cycle and found that it enhanced the SHR’s speed to acquire cocaine self-administration and exerted the opposite effect for Wistar rats. Two studies used a twice per day treatment regimen, one via i.p during the light cycle [29] and one via oral consumption during the dark cycle [48]. Both studies found no effect on adult male SHR rats’ voluntary alcohol consumption. Our study is the only one that examined both once and twice per day regimens via oral administration during rats’ dark cycles. Our results showed that neither treatment regimens had significant impact on animals’ preference to cocaine-paired chambers as measured by the time.
However, we did find a suppressive effect of MPH1 treatment on the number of entries to the cocaine-paired compartment. Interestingly this suppressive effect was only observed after the cocaine-associated locomotion stimulation had waned and the effect remained significant after removing the influence of general locomotion on the entries. The decrease of entries to drug side in the MPH1 treatment group may be due to reduced unnecessary and impulsive entries that rats made, considering that the total increased time that rats stayed in the drug side remained same for all pretreatment groups. A recent meta-analysis of SHR behavior did find a significant effect of MPH treatment on reducing impulsivity [54]. It is not clear why the effect on the entries was limited to the once per day treatment group. Considering that MPH has a much shorter half-life in rodents than in humans, a twice per day treatment paradigm likely offers a more consistent therapeutic plasma drug level over a longer duration. This treatment paradigm is more similar to human clinical use.
The main limitations of the study are small sample sizes and a balanced design of biased apparatus. Our final sample sizes were 6–9 animals per treatment group, which may have limited our ability to detect small changes. The sample size also prevented us from adopting a truly unbiased design. Future studies with more animals per group in unbiased apparatus will be needed to replicate our findings. Limitations in translational utility of inferring SUDs in human ADHD patients should be considered for several reasons. First, the negative findings in CPP test can only suggest a lack of change in sensitivity to the conditioned rewarding effect of cocaine [50]. Self-administration paradigm will be needed to directly address the link with addiction. Secondly, there are limitations associated with using SHR rats as the ADHD model. The adolescent SHR rats are the most widely used and validated animal model for ADHD. However, adult rats develop hypertension and it is not clear how hypertension affects the cocaine CPP behavior in adult rats. Furthermore, future studies with inclusion of and comparison with appropriate control strains are warranted to demonstrate the therapeutic effects of MPH for ADHD without any abusive liabilities of cocaine. Finally, ADHD is known to have high co-morbidity with mood disorders such as major depression and anxiety. Treatment with MPH and other stimulants has been shown to decrease the incidence and improve the symptoms of these disorders, both of which are known to independently contribute as risk factors to SUDs. Furthermore, improvement in key symptom areas such as impulsivity and subsequent improvements in social and occupational performance may profoundly influence outcomes for developing SUDs. The connection between impulsivity and drug seeking and using behaviors are well established and poor social and occupational outcomes contribute to low self-esteem, which itself is linked to SUDs. From the social, occupation and emotional perspective, rat studies of ADHD models have limited utility in explaining the potential protective effect of MPH treatment on subsequent SUDs observed in some clinical studies.
ACKNOWLEDGEMENTS
We thank Dr. Jerry Rechards, Marita Paredez, Leah Millitello and Brandon Kuehlewind for their assistance with data collection and Ms. Gail DePalma for administrative support.
FUNDING
The study was supported by SUNY REACH Award to Drs. Faraone and Richards, and NARSAD Young Investigator Award to Dr. Yanli ZhangJames. Dr. Faraone is supported by the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no 602805, the European Union’s Horizon 2020 research and innovation programme under grant agreements No 667302 & 728018 and NIMH grants 5R01MH101519 and U01 MH109536-01.
CONFLICTS OF INTEREST
In the past year, SVF received income, potential income, travel expenses continuing education support and/or research support from Tris, Otsuka, Arbor, Ironshore, Shire, Akili, Enzymotec, Sunovion, Supernus and Genomind. With his institution, he has US patent US20130217707 A1 for the use of sodium-hydrogen exchange inhibitors in the treatment of ADHD. In previous years, he received support from: Shire, Ironshore, Neurovance, Alcobra, Rhodes, CogCubed, KemPharm, Enzymotec, Akili, Neurolifesciences, Lundbeck/Takeda, Otsuka, McNeil, Janssen, Novartis, Pfizer and Eli Lilly. SVF receives royalties from books published by Guilford Press: Straight Talk about Your Child’s Mental Health, Oxford University Press: Schizophrenia: The Facts and Elsevier: ADHD: Non-Pharmacologic Interventions. He is principal investigator of https://adhdinadults.com/. YZJ, DRL, MLJ, JBR, and LY, do not have any conflict of interest.
REFERENCES
- 1.Faraone SV, Buitelaar J. Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. Eur Child Adolesc Psychiatry. 2010;19(4):353–64. [DOI] [PubMed] [Google Scholar]
- 2.Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36(2):159–65. [DOI] [PubMed] [Google Scholar]
- 3.Faraone SV. The pharmacology of amphetamine and methylphenidate: Relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018;87:255–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry. 2003;160(11):1909–18. [DOI] [PubMed] [Google Scholar]
- 5.Kollins SH, MacDonald EK, Rush CR. Assessing the abuse potential of methylphenidate in nonhuman and human subjects: a review. Pharmacol Biochem Behav. 2001;68(3):611–27. [DOI] [PubMed] [Google Scholar]
- 6.Mannuzza S, Klein RG, Truong NL, Moulton JL 3rd, Roizen ER, Howell KH, et al. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: prospective follow-up into adulthood. Am J Psychiatry. 2008;165(5):604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faraone SV, Upadhyaya HP. The effect of stimulant treatment for ADHD on later substance abuse and the potential for medication misuse, abuse, and diversion. J Clin Psychiatry. 2007;68(11):e28. [DOI] [PubMed] [Google Scholar]
- 8.Faraone SV, Rostain AL, Montano CB, Mason O, Antshel KM, Newcorn JH. Systematic Review: Nonmedical Use of Prescription Stimulants: Risk Factors, Outcomes, and Risk Reduction Strategies. J Am Acad Child Adolesc Psychiatry. 2020;59(1):100–12. [DOI] [PubMed] [Google Scholar]
- 9.Burton CL, Nobrega JN, Fletcher PJ. The effects of adolescent methylphenidate self-administration on responding for a conditioned reward, amphetamine-induced locomotor activity, and neuronal activation. Psychopharmacology. 2010;208(3):455–68. [DOI] [PubMed] [Google Scholar]
- 10.Faraone SV, Wilens TE. Effect of stimulant medications for attention-deficit/hyperactivity disorder on later substance use and the potential for stimulant misuse, abuse, and diversion. J Clin Psychiatry. 2007;68(Suppl 11):15–22. [PubMed] [Google Scholar]
- 11.Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111(1):179–85. [DOI] [PubMed] [Google Scholar]
- 12.Winters KC, Lee S, Botzet A, Fahnhorst T, Realmuto GM, August GJ. A Prospective Examination of the Association of Stimulant Medication History and Drug Use Outcomes among Community Samples of ADHD Youths. J Child Adolesc Subst Abuse. 2011;20(4):314–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biederman J, Monuteaux MC, Spencer T, Wilens TE, Macpherson HA, Faraone SV. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: a naturalistic controlled 10-year follow-up study. Am J Psychiatry. 2008;165(5):597–603. [DOI] [PubMed] [Google Scholar]
- 14.Molina BS, Hinshaw SP, Eugene Arnold L, Swanson JM, Pelham WE, Hechtman L, et al. Adolescent substance use in the multimodal treatment study of attention-deficit/hyperactivity disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. J Am Acad Child Adolesc Psychiatry. 2013;52(3):250–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humphreys KL, Eng T, Lee SS. Stimulant medication and substance use outcomes: a meta-analysis. JAMA Psychiatry. 2013;70(7):740–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammerness P, Joshi G, Doyle R, Georgiopoulos A, Geller D, Spencer T, et al. Do stimulants reduce the risk for cigarette smoking in youth with attention-deficit hyperactivity disorder? A prospective, long-term, open-label study of extended-release methylphenidate. J Pediatr. 2013;162(1):22–7.e2. [DOI] [PubMed] [Google Scholar]
- 17.Hammerness P, Petty C, Faraone SV, Biederman J. Do Stimulants Reduce the Risk for Alcohol and Substance Use in Youth With ADHD? A Secondary Analysis of a Prospective, 24-Month Open-Label Study of Osmotic-Release Methylphenidate. J Atten Disord. 2017;21(1):71–7. doi: 10.1177/1087054712468051 [DOI] [PubMed] [Google Scholar]
- 18.Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104(2):e20. [DOI] [PubMed] [Google Scholar]
- 19.Schoenfelder EN, Faraone SV, Kollins SH. Stimulant treatment of ADHD and cigarette smoking: a meta-analysis. Pediatrics. 2014;133(6):1070–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamm L, Trello-Rishel K, Riggs P, Nakonezny PA, Acosta M, Bailey G, et al. Predictors of treatment response in adolescents with comorbid substance use disorder and attention-deficit/hyperactivity disorder. J Subst Abuse Treat. 2013;44(2):224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riggs PD, Winhusen T, Davies RD, Leimberger JD, Mikulich-Gilbertson S, Klein C, et al. Randomized controlled trial of osmotic-release methylphenidate with cognitive-behavioral therapy in adolescents with attention-deficit/hyperactivity disorder and substance use disorders. J Am Acad Child Adolesc Psychiatry. 2011;50(9):903–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groenman AP, Oosterlaan J, Rommelse N, Franke B, Roeyers H, Oades RD, et al. Substance use disorders in adolescents with attention deficit hyperactivity disorder: a 4-year follow-up study. Addiction. 2013;108(8):1503–11. [DOI] [PubMed] [Google Scholar]
- 23.Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA Jr. Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci. 2002;5(1):13–4. [DOI] [PubMed] [Google Scholar]
- 24.Carlezon WA Jr, Mague SD, Andersen SL. Enduring behavioral effects of early exposure to methylphenidate in rats. Biol Psychiatry. 2003;54(12):1330–7. [DOI] [PubMed] [Google Scholar]
- 25.Mague SD, Andersen SL, Carlezon WA Jr. Early developmental exposure to methylphenidate reduces cocaine-induced potentiation of brain stimulation reward in rats. Biol Psychiatry. 2005;57(2):120–5. [DOI] [PubMed] [Google Scholar]
- 26.Augustyniak PN, Kourrich S, Rezazadeh SM, Stewart J, Arvanitogiannis A. Differential behavioral and neurochemical effects of cocaine after early exposure to methylphenidate in an animal model of attention deficit hyperactivity disorder. Behav Brain Res. 2006;167(2):379–82. [DOI] [PubMed] [Google Scholar]
- 27.Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology. 2001;25(5):651–61. [DOI] [PubMed] [Google Scholar]
- 28.Crawford CA, Villafranca SW, Cyr MC, Farley CM, Reichel CM, Gheorghe SL, et al. Effects of early methylphenidate exposure on morphine- and sucrose-reinforced behaviors in adult rats: relationship to dopamine D2 receptors. Brain Res. 2007;1139:245–53. [DOI] [PubMed] [Google Scholar]
- 29.Vendruscolo LF, Izidio GS, Takahashi RN, Ramos A. Chronic methylphenidate treatment during adolescence increases anxiety-related behaviors and ethanol drinking in adult spontaneously hypertensive rats. Behav Pharmacol. 2008;19(1):21–7. [DOI] [PubMed] [Google Scholar]
- 30.Harvey RC, Sen S, Deaciuc A, Dwoskin LP, Kantak KM. Methylphenidate treatment in adolescent rats with an attention deficit/hyperactivity disorder phenotype: cocaine addiction vulnerability and dopamine transporter function. Neuropsychopharmacology. 2011;36(4):837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dafny N, Yang PB. The role of age, genotype, sex, and route of acute and chronic administration of methylphenidate: a review of its locomotor effects. Brain Res Bull. 2006;68(6):393–405. [DOI] [PubMed] [Google Scholar]
- 32.Kuczenski R, Segal DS. Stimulant actions in rodents: implications for attention-deficit/hyperactivity disorder treatment and potential substance abuse. Biol Psychiatry. 2005;57(11):1391–6. [DOI] [PubMed] [Google Scholar]
- 33.Swanson J, Gupta S, Guinta D, Flynn D, Agler D, Lerner M, et al. Acute tolerance to methylphenidate in the treatment of attention deficit hyperactivity disorder in children. Clin Pharmacol Ther. 1999;66(3):295–305. [DOI] [PubMed] [Google Scholar]
- 34.Patrick KS, Mueller RA, Gualtieri CT, Breese GR. Pharmacokinetics and actions of methylphenidate In: Meltzer HY, editor. Psychopharmacology: The third generation of progress. New York (US): Raven Press; 1987. p. 1387–95. [Google Scholar]
- 35.Patrick KS, Ellington KR, Breese GR. Distribution of methylphenidate and p-hydroxymethylphenidate in rats. J Pharmacol Exp Ther. 1984;231(1):61–5. [PubMed] [Google Scholar]
- 36.Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22(16):7264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wargin W, Patrick K, Kilts C, Gualtieri CT, Ellington K, Mueller RA, et al. Pharmacokinetics of methylphenidate in man, rat and monkey. J Pharmacol Exp Ther. 1983;226(2):382–6. [PubMed] [Google Scholar]
- 38.Aoyama T, Kotaki H, Sawada Y, Iga T. Pharmacokinetics and pharmacodynamics of methylphenidate enantiomers in rats. Psychopharmacology. 1996;127(2):117–22. [DOI] [PubMed] [Google Scholar]
- 39.Gerasimov MR, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marsteller D, et al. Comparison between intraperitoneal and oral methylphenidate administration: A microdialysis and locomotor activity study. J Pharmacol Exp Ther. 2000;295(1):51–7. [PubMed] [Google Scholar]
- 40.Gaytan O, Yang P, Swann A, Dafny N. Diurnal differences in sensitization to methylphenidate. Brain Res. 2000;864(1):24–39. [DOI] [PubMed] [Google Scholar]
- 41.Gaytan O, Ghelani D, Martin S, Swann A, Dafny N. Methylphenidate: diurnal effects on locomotor and stereotypic behavior in the rat. Brain Res. 1997;777(1–2):1–12. [DOI] [PubMed] [Google Scholar]
- 42.Amini B, Yang PB, Swann AC, Dafny N. Differential locomotor responses in male rats from three strains to acute methylphenidate. Int J Neurosci. 2004;114(9):1063–84. [DOI] [PubMed] [Google Scholar]
- 43.Yang PB, Amini B, Swann AC, Dafny N. Strain differences in the behavioral responses of male rats to chronically administered methylphenidate. Brain Res. 2003;971(2):139–52. [DOI] [PubMed] [Google Scholar]
- 44.Myers MM, Musty RE, Hendley ED. Attenuation of hyperactivity in the spontaneously hypertensive rat by amphetamine. Behav Neural Biol. 1982;34(1):42–54. [DOI] [PubMed] [Google Scholar]
- 45.Arnsten AF. Stimulants: Therapeutic Actions in ADHD. Neuropsychopharmacology. 2006;31(11):2376–83. [DOI] [PubMed] [Google Scholar]
- 46.Dela Pena I, Lee JC, Lee HL, Woo TS, Lee HC, Sohn AR, et al. Differential behavioral responses of the spontaneously hypertensive rat to methylphenidate and methamphetamine: lack of a rewarding effect of repeated methylphenidate treatment. Neurosci Lett. 2012;514(2):189–93. [DOI] [PubMed] [Google Scholar]
- 47.Dela Pena I, Kim BN, Han DH, Kim Y, Cheong JH. Abuse and dependence liability analysis of methylphenidate in the spontaneously hypertensive rat model of attention-deficit/hyperactivity disorder (ADHD): what have we learned? Arch Pharm Res. 2013;36(4):400–10. [DOI] [PubMed] [Google Scholar]
- 48.Soeters HS, Howells FM, Russell VA. Methylphenidate does not increase ethanol consumption in a rat model for attention-deficit hyperactivity disorder-the spontaneously hypertensive rat. Metab Brain Dis. 2008;23(3):303–14. [DOI] [PubMed] [Google Scholar]
- 49.Vendruscolo LF, Izidio GS, Takahashi RN. Drug reinforcement in a rat model of attention deficit/hyperactivity disorder--the Spontaneously Hypertensive Rat (SHR). Curr Drug Abuse Rev. 2009;2(2):177–83. [DOI] [PubMed] [Google Scholar]
- 50.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12(3–4):227–462. [DOI] [PubMed] [Google Scholar]
- 51.Yang L, Faraone SV, Zhang-James Y. Autism spectrum disorder traits in Slc9a9 knock-out mice. Am J Med Genet B. 2016;171B(3):363–76. [DOI] [PubMed] [Google Scholar]
- 52.Zhang-James Y, Yang L, Middleton FA, Yang L, Patak J, Faraone SV. Autism-related behavioral phenotypes in an inbred rat substrain. Behav Brain Res. 2014;269:103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang-James Y, Middleton FA, Faraone SV. Genetic architecture of Wistar-Kyoto rat and spontaneously hypertensive rat substrains from different sources. Physiol Genom. 2013;45(13):528–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leffa DT, Panzenhagen AC, Salvi AA, Bau CHD, Pires GN, Torres ILS, et al. Systematic review and meta-analysis of the behavioral effects of methylphenidate in the spontaneously hypertensive rat model of attention-deficit/hyperactivity disorder. Neurosci Biobehav Rev. 2019;100:166–79. [DOI] [PubMed] [Google Scholar]