Abstract
Chronic pain is a complex neuropsychiatric disorder, characterized by sensory, cognitive, and affective symptoms. Over the last two decades, researchers have made significant progress towards understanding the impact of mesolimbic dopamine circuitry in acute and chronic pain. These efforts have provided insights into the circuits and intracellular pathways in the brain reward center that are implicated in sensory and affective manifestations of chronic pain. Studies have also identified novel therapeutic targets as well as factors that impact treatment responsiveness. Dysregulation of dopamine function in the brain reward center may further promote comorbid mood disorders and vulnerability to addiction. This review discusses recent clinical and preclinical findings on the neuroanatomical and neurochemical adaptations triggered by prolonged pain states in the brain reward pathway. Furthermore, this discussion highlights evidence of mechanisms underlying comorbidities between pain, depression, and addiction.
Keywords: Pain circuitry, fMRI, prefrontal cortex, nucleus accumbens, depression, opioid addiction
Introduction
The mesolimbic system is a central nervous system circuit in which dopaminergic inputs from the ventral tegmental area (VTA) innervate brain regions involved in executive, affective, and motivational functions, including the prefrontal cortex (PFC), amygdala, and nucleus accumbens (NAc). Dysfunctions within this system can contribute to neuropsychiatric diseases, including major depression disorder (MDD) and addiction (1–3). Emerging evidence points to a critical role of the mesolimbic system in the perception and modulation of chronic pain symptoms (4–9), highlighting the importance of this pathway in pain therapeutics.
Understanding the function of the brain reward pathway under chronic pain states can also provide insight into clinically detrimental comorbidities, such as depression and addiction vulnerability. Indeed, clinical research has demonstrated substantial comorbidity between pain and depression, with one study estimating a prevalence of 30% comorbidity (10). Considering that approximately 9% and 20% of the U.S. population suffers from depressive or chronic pain disorders, respectively, this increased potential for comorbidity is not trivial (11, 12). Notably, patients with debilitating mental conditions, such as MDD, have a higher comorbid presence of chronic pain, with each of these conditions positively reinforcing the other (13–18). Managing chronic pain is challenging because symptoms often vary between patients, while the currently available medications target only a subset of symptoms. Furthermore, we have limited knowledge of the gender- and age-related mechanisms of pain modulation.
The misuse of opioid analgesics has also been a major concern in pain management. Morphine, oxycodone, and other synthetic opioids show limited efficacy in the treatment of chronic pain, they promote hyperalgesia, and they contain the risk of physical dependence and addiction (19–24). The 300% increase in opioid analgesic prescriptions for chronic non-cancer pain patients between 2000 and 2010 has contributed to the substantial rise in the incidence of physical dependence and substance use disorders (19–25). Based on the existence of affective and nociceptive comorbidities related to the mesolimbic system, it falls within reason that this network could underlie vulnerabilities to addiction and depression in chronic pain patients (26).
Mesolimbic Functional Magnetic Resonance Imaging (fMRI) in Chronic Pain Patients Compared to Depression and Addiction Populations
Functional imaging studies have been fundamental in documenting the impact of pain conditions on the brain reward circuitry (see Figure 1). By reviewing the literature on depression and addiction, below we summarize structural and functional commonalities between these conditions that may underlie comorbidities in chronic pain populations. Specifically, we focus on the ventral tegmental area (VTA), nucleus accumbens (NAc), prefrontal cortex (PFC), anterior cingulate cortex (ACC), and the amygdala, which play major roles in managing executive decision-making, emotional perception, and motivational drive.
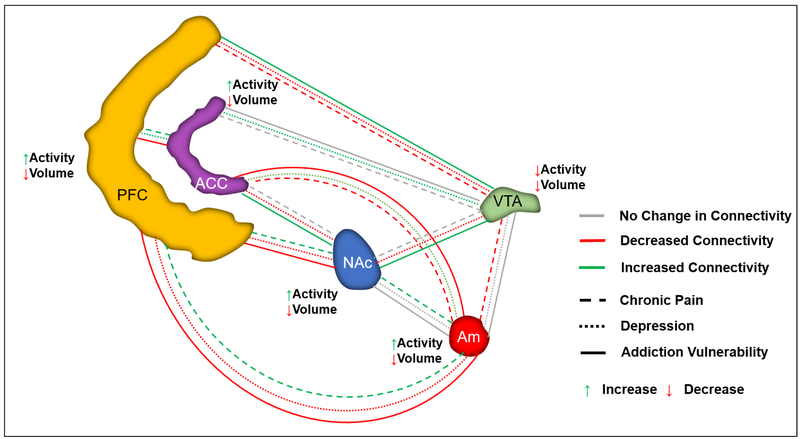
Figure 1:
fMRI studies have provided data on structural and functional changes within the mesolimbic circuitry underlying chronic pain and depression, independently and within comorbid states. This schematic highlights intra-regional changes in collective baseline activity and gray matter volume within chronic pain populations, as well as changes in functional connectivity within chronic pain, depression, and addiction populations. PFC=prefrontal cortex; ACC=anterior cingulate cortex; NAc=nucleus accumbens; VTA=ventral tegmental area; Am=amygdala.
Ventral Tegmental Area.
Normal mesolimbic function and affective processes, such as reward- mediated drive, are highly dependent on dopaminergic neurotransmission emanating from the VTA (27–29). Fibromyalgia and trigeminal neuralgia patient populations demonstrate a reduction in gray matter volume and decreased VTA activity (30, 31). Researchers have found that chronic migraine is associated with decreased connectivity between the extended amygdala and the VTA, which are both areas significantly involved in the management of executive function and emotional responses (32). Interestingly, studies on MDD populations have reported increased VTA connectivity to the ACC, a structure involved in pain processing (33). Decreased VTA activity also has been observed in MDD patients (33). The contribution of reduced VTA activity to mesolimbic affective disorders is supported by the finding that deep brain stimulation (DBS) of the VTA is effective in treating chronic, refractory cluster headaches and treatment-resistant depression (TRD) (34–36). Alterations in VTA-NAc connectivity have also been observed in individuals suffering from substance abuse disorders, during active use as well as during abstinence phases; such changes may further impact executive or affective pain-processing centers (26, 37, 38). Specifically, changes in VTA-NAc connectivity in individuals suffering from substance abuse disorders occur in an opposite direction to those observed in individuals suffering from depression, as 3-day abstinent cocaine users show increased effective connectivity from the VTA to the NAc and medial PFC (mPFC) (37). However, another study found decreased functional connectivity between the VTA and NAc of active cocaine users (38). Differences between these studies may be related to the abstinent versus active status of the patients, or to the form of connectivity analyzed (effective connectivity analyzes a unidirectional influence of one brain area over another, while functional connectivity provides a broader bidirectional relationship between two regions).
Nucleus Accumbens.
The NAc projects to various executive, emotional, and motor regions and is considered a master regulator of motivational drive (39, 40). Although a decrease in NAc gray matter volume occurs in states of chronic pain (as with heroin addiction), the activity in this region collectively increases (31, 41–43). Human imaging studies also have helped elucidate the neurobiological mechanisms contributing to the transition from acute to chronic pain, which is fundamental for the development of efficient treatment tools. As shown by a longitudinal brain imaging study that followed subacute back pain patients for one year, increased functional connectivity of the NAc with the PFC predicts pain persistence, suggesting that this circuit contributes to the transition to chronic pain (44). A different study on chronic back pain patients indicated that the higher incidence of white matter and functional connections within the dorsal medial PFC (dmPFC)-amygdala-NAc circuit, and the observed reductions in amygdala volume, represent risk factors for pain persistence. This work demonstrated that the opioid receptor delta 1 (OPRD1) rs678849 single nucleotide polymorphism (SNP) is associated with amygdala volume, and the opioid receptor mu 1 (OPRM1) rs1799971 SNP is associated with incidence of connections within the dmPFC-amygdala-NAc white matter network (45). Interestingly, the status of NAc-PFC connectivity also contributes to depression and addiction, although these conditions arise from a net decrease in functional connectivity (33, 46, 47). Small-scale clinical studies have demonstrated promising efficacy of NAc DBS for depression and sustained heroin abstinence (48–51), suggesting that such approaches may be used for the treatment of comorbid conditions.
Prefrontal Cortex.
The PFC is a major component of the mesolimbic circuit, which affects several structures involved in pain perception, motivational drive, substance seeking, and anxiodepressive states (52–58). A resting-state fMRI study with rheumatoid arthritis patients revealed that prolonged pain states are associated with increased connectivity between the insula and the PFC (59). Using fMRI, Baliki et al. demonstrated that chronic back pain results in increased PFC activity, and this activity is strongly related to pain intensity (60). The degree of change in BOLD (blood-oxygen-level-dependent) signal in the mPFC and the ACC were highly correlated with the severity of spontaneous pain in chronic back patients. In contrast, the level of insular activation was significantly associated with duration (years) of pain (60). A reduction in PFC thickness has also been reported in chronic pain populations (61, 62). Interestingly, effective treatment of low back pain (spinal surgery or facet joint injections) reverses the anatomical and functional maladaptations in ACC circuits (62). Since studies using repetitive transcranial magnetic stimulation (rTMS) and DBS of the PFC demonstrate significant and rapid improvement of fibromyalgia-induced chronic pain and TRD, along with a reduction of cue-evoked heroin cravings, it is possible that such therapeutic interventions may successfully manage comorbidities between these disorders (63–65).
Anterior Cingulate Cortex:
Evidence suggests that the ACC and the periaqueductal gray (PAG) form a central network that is primarily involved in spontaneous pain, and that the connectivity between these regions is increased in chronic pain patients (66, 67). This hypothesis is substantiated by the practice of treating patients exhibiting comorbid neuropathic pain and MDD with bilateral anterior cingulotomy, which effectively relieves symptoms associated with both disorders (68). Indeed, studies have shown that the serotonin-norepinephrine reuptake inhibitor (SNRI) Milnacipran is effective for treating fibromyalgia patients, likely because of its ability to decrease functional connectivity within the ACC-insular cortex-PAG network (69). Enhanced ACC activity has been associated with chronic pain and depression, whereas decreased ACC gray matter volume coincides with these two conditions as well as with heroin dependence (70–75). Notably, clinical trials suggest that interrupting abnormal ACC signaling by DBS or transcranial direct current stimulation has promise for the management of neuropathic pain, TRD, and cue-evoked cocaine cravings (76–78).
Amygdala:
Nuclei of the amygdala help regulate emotional stress and pain perception (79–81). Elevated baseline activity in the amygdala is associated with fibromyalgia- and inflammatory bowel syndrome-induced chronic pain and depression. By contrast, reductions in amygdala gray matter volume have been noted across the comorbidities discussed above (82–84). The amygdala also appears to modulate affective abnormalities associated with chronic pain, such as catastrophizing, through exaggerated and abnormal functional connectivity with the central executive network (85). This is important because catastrophizing can further exacerbate chronic pain conditions and increase the likelihood of poor clinical outcomes (86, 87). Similar to the observations in chronic pain patients, studies have found that using oral morphine for one month leads to persistent decreases in amygdala gray matter volume (88). Other reports show decreased functional connectivity between the amygdala and the ACC in heroin-addicted individuals (89, 90). We expect that future work on the impact of pain-addiction comorbidities on the function of this circuitry will fill a major gap in our understanding of addiction mechanisms.
Common Clinical Alterations in Mesolimbic Dopamine Neurotransmission Among Chronic Pain-related Comorbidities
Both pre- and post-synaptic stages of dopaminergic neurotransmission are compromised during chronic pain (91, 92), as well as in other affective disorders (see Figure 2). In accordance with reduced VTA activity in chronic pain patients, a pilot study found that chronic pain states diminish the pre-synaptic metabolism of dopamine in the VTA, as well as in the anterior cingulate gyrus and the insular cortex (91). Furthermore, chronic back pain patients demonstrate blunted dopamine release in response to noxious challenges (92). Positron emission tomography (PET) imaging studies in chronic back pain and fibromyalgia patients have demonstrated a reduction in ventral striatal dopamine receptor subtype 2 and subtype 3 (D2/D3R) binding (92, 93). These studies also found that the reduction in dopamine receptor availability correlates with lower thermal pain thresholds. By contrast, a Serine-9-Glycine mutation in the DRD3 gene, which is known to increase D3R activity, restores thermal pain thresholds to healthy control levels (94). Activity-lowering polymorphisms in dopamine metabolism genes, such as DAT-1 (dopamine transporter-1) and MAO-A (monoamine oxidase-A), have been associated with increased sensitivity to noxious cold (95). Crucially, activity-reducing mutations in dopamine-clearing genes, such as COMT (catechol-O-methyltransferase), also appear to modify pain sensitivity by changing functional responses of μ-opioid receptors in the NAc and other pain-modulating circuits. Such maladaptations may ultimately impact pain tolerance and affective states (96).
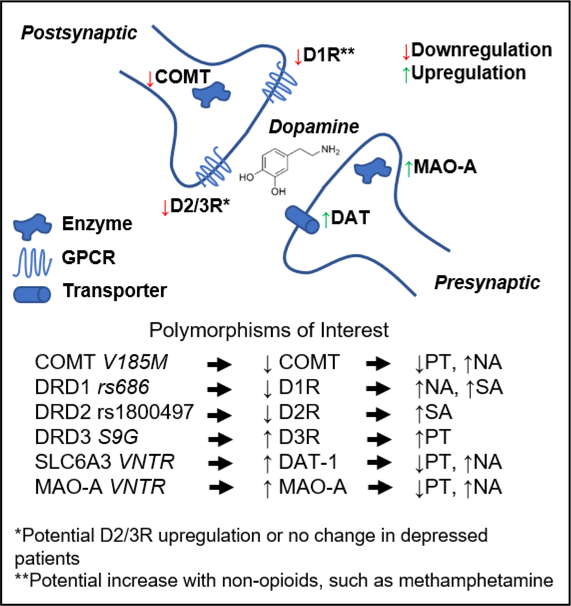
Figure 2:
Gene mutations underlying alterations in mesolimbic dopamine neurotransmission commonly encode proteins involved in dopamine synthesis, clearance, and release, as well as pre-synaptic reuptake and post-synaptic receptor binding. This representation shows examples of mutations altering the regulation of these genes, along with the respective abnormal affective phenotypes. COMT=catechol-O-methyltransferase; MAO-A=monoamine oxidase A; DAT=dopamine active transporter; D1/2/3R=dopamine receptor D1/2/3; PT=pain tolerance; NA=negative affect; SA=substance abuse risk
As opposed to the downregulation of inhibitory postsynaptic mechanisms in the chronic pain population, MDD patients can display an upregulation of D2/D3R availability, although evidence varies between studies. This conclusion would potentially support the finding that fibromyalgia patients with comorbid depression have higher ventral striatum D2/D3R binding potential than a non-depressed comparison group (93, 97, 98). Furthermore, Cannon et al. noted a depression-related reduction of excitatory D1R expression in the caudate, which strongly innervates the ACC, highlighting the importance of balanced dopamine activity in regulating affective processing (99). Similar to observations from chronic pain patients, the dopamine metabolism genes DAT-1 and COMT play a critical role in mediating susceptibility to depression. One study found that activity-enhancing polymorphisms in these genes protect against negative emotionality, further supporting the role of dopamine in resilience to affective disorders (100). Overall, evidence suggests that polymorphisms leading to a reduction in dopamine neurotransmission promote both chronic pain and depression. Notably, tricyclic antidepressants and SNRIs promote the activity of dopamine and effectively ameliorate sensory and affective symptoms of neuropathic pain (15, 101). Given the slow onset and several side effects of antidepressants, there is a pressing need for novel fast-acting therapeutic interventions that promote dopaminergic activity in the brain reward pathway.
Similar to chronic pain and depression, D2/D3R function plays a critical role in addiction vulnerability. [11C]raclopride D2/D3R binding potential in heroin-dependent subjects both at baseline and after methylphenidate treatment is significantly lower than that of control subjects, particularly in the limbic striatum, caudate, and putamen (102). Researchers have also observed a decrease in striatal D2R in opiate-dependent individuals upon naloxone-precipitated withdrawal (103). The DRD2 rs1800497 polymorphism, which attenuates D2R activity, also has been linked to vulnerability to heroin addiction (104). In accordance with reported changes in dopamine transport in MDD and chronic pain, patients on methadone maintenance or prolonged heroin abstinence also demonstrate lower presynaptic DAT activity in the striatum (105). In the next few years, the use of advanced PET imaging tools is expected to enhance our knowledge on the genetic and neurochemical substrates of chronic pain and its affective comorbidities.
Chronic Pain-induced Alterations in the Mesolimbic Pathway: Insights from Preclinical Studies
Studies on Neuronal Circuits
Several rodent studies have demonstrated that noxious stimuli affect the release of dopamine in the brain reward center. Consistent with observations in human studies, chronic pain states in rodents lead to a reduction in NAc dopamine release and pain relief is associated with increased dopamine levels in the NAc shell (106). In preclinical settings, compounds with pain-alleviating properties promote place preference when mice are conditioned to environmental cues. Pain relief promotes place preference in studies using the spared nerve injury (SNI) model of neuropathic pain, the complete Freund’s adjuvant model of inflammatory pain, as well as models of post-surgical, cancer, and osteoarthritic pain (107). Thus, place preference to drugs with analgesic (but not rewarding) properties, such as lidocaine or the alpha-2 adrenoreceptor agonist clonidine, provides a valid model for evaluating spontaneous pain in rodents (108).
As mentioned earlier, there is major neurophysiological overlap between pain and depression (10, 17). Consistent with evidence from human studies, hypodopaminergic states in rodent models of neuropathic pain are associated with depression-like behaviors and reduced motivation (109–114). These affective deficits are linked to long-term depression of excitatory synaptic transmission in the medium spiny neurons of the indirect pathway (114).
Opto- and chemogenetic approaches have provided information on the functional role of brain reward circuits in affective and sensory pain symptoms. Ren and colleagues utilized fluorescent retrograde vectors and patch clamp electrophysiology to demonstrate that prolonged peripheral nerve injury using the SNI model leads to elevated intrinsic excitability of NAc shell medium spiny neurons of the indirect pathway (115). Long-term neuropathic pain states were also associated with a reduction in dendritic number and size, as well as with a decrease in extracellular dopamine levels. Furthermore, peripheral nerve injury caused a reduction in the spontaneous spiking of VTA dopamine cells projecting to NAc medium spiny neurons. Chemogenetic excitation of medium spiny neurons of the indirect pathway worsened SNI-induced mechanical allodynia, whereas inhibition alleviated allodynia (115). These results further support a role of the indirect dopamine pathway in modulating sensory hypersensitivity symptoms of peripheral nerve injury. Pharmacologic blockade of calcium-permeable AMPA receptors in the NAc core increased depression-like behaviors in models of neuropathic pain (116). The same study demonstrated that delivery of an AMPA receptor potentiator into the NAc ameliorates behavioral manifestations of depressive states. Thus, calcium-permeable AMPA receptors and their downstream pathways may provide new avenues for the management of pain-induced depression.
In a rat model of peripheral neuropathy, activation of PFC neurons projecting to the NAc core increased excitatory postsynaptic potentials and alleviated sensory hypersensitivity symptoms (117). In these experiments, light activation of neurons that were infected with adeno-associated viruses encoding channelrhodopsin-2 induced action potential spikes within the prelimbic PFC of rats. This optogenetic activation alleviated both mechanical and cold allodynia. Using the place-conditioning paradigm, the investigators demonstrated that optical activation of these neurons relieved pain and promoted a preference for the associated compartment. Optical stimulation of the prelimbic PFC also reversed depression-like behaviors that were observed several weeks after nerve injury. Subsequent experiments utilized photoactivation of channelrhodopsin-2-expressing NAc medium spiny neurons to confirm that the activation of projections from the prelimbic PFC to the NAc was responsible for these effects, pointing to this corticostriatal circuit as an important target of neuromodulation therapy. On the contrary, inhibiting rat pyramidal mPFC neurons projecting to the NAc core by halorhodopsin exacerbated acute pain symptoms, suggesting a role of this circuitry in the endogenous antinociceptive pathway (118). This intervention also heightened affective and sensory symptoms of peripheral nerve injury, suggesting that therapeutic interventions targeting the activity of this pathway may efficiently alleviate chronic pain symptoms.
Rodent models have also demonstrated a role of the ACC in mood disorders triggered by chronic pain states. Lesions of the ACC prevent the development of anxiodepressive behaviors without impacting hypersensitivity symptoms, whereas optogenetic activation of the ACC promotes depressive states (119). A study by Sellmeijer and colleagues applied the cuff model of peripheral nerve injury to demonstrate a potent role of the ACC in modulating anxiodepressive behaviors (120). Using in vivo electrophysiological recordings, the investigators observed an increased firing rate and bursting activity within the ACC at time points at which anxiety and depression-like behaviors had developed (120).
Using a rat neuropathic pain model, a recent study demonstrated that inactivation of amygdala nuclei alleviates hyperalgesia/allodynia and depression-like behaviors (121). More recently, Corder and colleagues combined circuit optogenetics with in vivo calcium imaging in mice to investigate the function of an amygdala-NAc circuit in pain-free and neuropathic pain states (122). These studies showed that while this amygdala-NAc circuit did not have a prominent role in nociceptive thresholds in pain-naïve mice, it played a major role in modulating affective pain symptoms in mice suffering from prolonged peripheral nerve injury. Inhibiting this pathway with opsins alleviated the aversive components of pain without impacting mechanical allodynia (122). Table 1 summarizes key preclinical findings on mesolimbic circuits in the modulation of chronic pain symptoms.
Table 1:
Summary of preclinical findings highlighting the role of different mesolimbic brain regions in the modulation of affective and sensory symptoms of chronic pain.
| Brain Region | Pain Model | Intervention | Outcome | Reference |
|---|---|---|---|---|
| Nucleus Accumbens | Spared Nerve Injury | Optogenetic Activation (PFC-NAc core circuit) | Alleviated both mechanical and cold allodynia, reversed depression-like behaviors | 115 |
| Chemogenetic Activation | Increased mechanical allodynia | 115 | ||
| Chemogenetic Inhibition | Decreased mechanical allodynia | 115 | ||
| Pharmacological blockade of calcium-permeable AMPA receptors | Increased depression-like behaviors | 116 | ||
| Pharmacological activation of calcium-permeable AMPA receptors | Decreased depression-like behaviors | 116 | ||
| Prefrontal Cortex | Spared Nerve Injury | Electrophysiology (activation of mPFC-NAc circuit) | Increased excitatory postsynaptic potential, alleviated sensory hypersensitivity | 117 |
| Optogenetic Activation | Alleviation of both mechanical and cold allodynia | 117 | ||
| Optogenetic Inhibition | Increased nociceptive sensitivity and aversive responsiveness | 118 | ||
| Anterior Cingulate Cortex | Sciatic Nerve Cuff | Optogenetic Activation | Promoted depressive states | 120 |
| In vivo electrophysiology | Increased firing rates and bursting activity coinciding with timepoints of development of anxiodepressive-like behaviors | 120 | ||
| Amygdala | Chronic Constriction Injury | Pharmacological inactivation of BLA and CeA Nuclei | Reversed hyperalgesia, allodynia and depressive-like behaviors | 121 |
| Spared Nerve Injury | In vivo calcium imaging and optogenetics (Amygdala-NAc circuits) | No effect on nociceptive threshold, decreased affective pain symptoms | 122 |
Studies on Transcriptional and Epigenetic Adaptations in the Brain Reward Center
Prolonged neuropathic pain states also promote adaptations in gene expression in the mPFC and the NAc. Evidence from next-generation RNA sequencing (RNA-Seq) and subsequent bioinformatic analyses suggest that prolonged pain states affect several intracellular pathways within the mPFC and the NAc, including G protein, cAMP, and nitric oxide signaling, as well as immune, glutamatergic and glucocorticoid pathways (123). Several of the identified genes and pathways have documented roles in neuropsychiatric disorders. For example, recent studies pointed to the role of brain-derived neurotrophic factor and tumor necrosis factor-alpha (TNF-α) in regulating symptoms of chronic pain, depression, and addiction (124–127). RNA-Seq studies by Mitsi et al., which used the SNI model to monitor gene expression adaptations in response to desipramine treatment, showed that recovery from chronic pain states coincides with an upregulation of genes and intracellular cascades in neurons of the indirect pathway. In particular, recovery from chronic pain was associated with decreased phosphorylation of the transcription factor CREB (c-AMP response element binding) and the AMPA receptor subunit GluR1 (128) in the NAc. These studies also highlight a negative modulatory role of histone deacetylase 5 on the onset of action and efficacy of desipramine, suggesting that targeting histone function in the NAc may efficiently accelerate the expression of genes necessary for recovery from sensory and affective pain symptoms. Changes in the epigenetic landscape may also play critical roles in the transition from acute to chronic pain, the maintenance of pain, or the development of co-morbid depression. For example, there is a decrease in global gene methylation in the PFC six months after peripheral nerve injury, which is a time point at which both sensory deficits (mechanical and cold allodynia) and anxio-depressive behaviors are typically observed (129). This adaptation is particularly important as DNA methylation and other histone modifications may lead to the suppression of genes necessary for synaptic remodeling and recovery from chronic pain states (130). In this study, partial recovery from sensory hypersensitivity coincided with a return of global DNA methylation to normal levels. Thus, interventions in transcriptional or epigenetic processes may provide a powerful avenue for pharmacological management of chronic pain and comorbidities. Given the wide presence of gene expression modulators, a detailed understanding of the transcriptional and epigenetic adaptations underlying chronic pain and comorbidities is crucial for the development of medications with limited off-target effects.
Brain Reward Center Modulation of Opiate Actions Under Chronic Pain States
Recent studies assessed the impact of pain on opiate addiction vulnerability using a rat model of inflammatory pain in combination with heroin self-administration (131). In this study, rats showed decreased sensitivity to self-administration of low heroin doses, but increased intake with higher amounts of available heroin (131). It will be important to utilize animal models of chronic pain to determine if prior exposure to opioids or prolonged treatment regimens that cause physical dependence affect addiction-related behaviors. Various laboratories have observed a reduction in morphine reward sensitivity under long-term pain states. For instance, using the conditioned place preference test researchers have shown that the loss of morphine reward sensitivity is accompanied by cellular adaptations in the VTA, including adaptations in G protein-coupled extracellular kinase-2 (109, 110). Nerve injury in mice is also accompanied by upregulation of TNF-α in the NAc; genetic or pharmacologic inactivation of TNF-α restores sensitivity to morphine place preference (125). Work from our group demonstrated that three weeks after SNI, mice show a small but significant reduction in oxycodone reward sensitivity in the conditioned place preference assay (132). Importantly, blocking intracellular modulators of mu opioid receptor function, such as regulator of G protein signaling-9–2, attenuated the rewarding effects of oxycodone and prevented the reinstatement of oxycodone place preference (132). Using a peripheral nerve injury paradigm, Taylor and colleagues demonstrated a potent role of VTA microglia in the rewarding effects of opioids and other drugs that increase dopamine levels in the NAc (133). These studies highlight various mesolimbic-related mechanisms that may affect sensitivity to the behavioral effects of opioids, and show that chronic pain states do not afford protection from physical dependence or addiction.
Future Directions
Substance abuse-related deaths have reached an alarming level, with the National Institutes of Health reporting over 70,000 U.S. total deaths in 2017, ~68% of which were attributed to opioids (134). With the rising prevalence of chronic pain and depression, this death toll is likely to be exacerbated by prescription or comorbidity-related substance abuse (135, 136). The current situation necessitates a better understanding of structural changes within brain networks that manage executive/cognitive function, emotion, and perception. Various studies have linked the mesolimbic pathway to chronic pain, depressive, and addictive disorders. Given the limited comorbidity-specific preclinical models, human imaging studies provide a means to highlight and compare adaptations in the mesolimbic system that are associated with the symptomatology of specific diseases. These studies, in turn, can guide the development of new models that better reflect the human condition. Comorbidity analyses are also crucial for directing therapeutic approaches, such as TMS and DBS (76, 137–139). Information from clinical imaging analyses could also provide insight for translational research directed towards synaptic and cell type-specific mechanisms of chronic pain and comorbid disorders. The identification of circuits and cellular populations of the brain reward pathway may guide drug development efforts towards a range of new targets, from neuropeptide receptors to epigenetic modifiers. Collectively, these research and technological directions will help address a pressing challenge in therapeutics: the development of tailored, effective, and safe treatment approaches that reverse hypodopaminergic states and other pathological adaptations to chronic pain and related comorbidities.
Acknowledgements:
Supported by National Institute of Neurological Disorders and Stroke NS086444 (V.Z.), NS093537 (V.Z.), and National Institute on Drug Abuse PPG-POIDAO8227 (V.Z.), T32 5T32DA007135-34 (K.P.). The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Russo SJ, Nestler EJ (2013): The Brain Reward Circuitry in Mood Disorders. Nat Rev Neurosci. 14: 609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper S, Robison AJ, Mazei-Robison MS (2017): Reward Circuitry in Addiction. Neurotherapeutics. . doi: 10.1007/s13311-017-0525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomasi D, Volkow ND (2013): Striatocortical pathway dysfunction in addiction and obesity: Differences and similarities. Crit Rev Biochem Mol Biol. . doi: 10.3109/10409238.2012.735642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, et al. (2012): Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 15: 1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitsi V, Zachariou V (2016): Modulation of pain, nociception, and analgesia by the brain reward center. Neuroscience. 338: 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashmi JA, Baliki MN, Huang L, Baria AT, Torbey S, Hermann KM, et al. (2013): Shape shifting pain: Chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain. 136: 2751–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz N, Miller C, Fields HL (2017): Cortico-Accumbens Regulation of Approach-Avoidance Behavior Is Modified by Experience and Chronic Pain. Cell Rep. 19: 1522–1531. [DOI] [PubMed] [Google Scholar]
- 8.Navratilova E, Xie JY, Meske D, Qu C, Morimura K, Okun A, et al. (2015): Endogenous Opioid Activity in the Anterior Cingulate Cortex Is Required for Relief of Pain. J Neurosci. 35: 7264–7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S, et al. (2012): Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc Natl Acad Sci. 109: 20709–20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chopra K, Arora V (2014): An intricate relationship between pain and depression: clinical correlates, coactivation factors and therapeutic targets. Expert Opin Ther Targets. 18: 159–176. [DOI] [PubMed] [Google Scholar]
- 11.Gonzales O, Berry J, McKnight-Eily L, Strine T, Edwards V, Lu H, Croft J (2010): Current Depression Among Adults — United States , 2006 and 2008. Morb Mortal Wkly Rep. (Vol. 59). [PubMed] [Google Scholar]
- 12.Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, et al. (2018): Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults — United States , 2016. Morb Mortal Wkly Rep. (Vol. 67). doi: 10.15585/mmwr.mm6736a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnow BA, Hunkeler EM, Blasey CM, Lee J, Constantino MJ, Fireman B, et al. (2006): Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 68: 262–268. [DOI] [PubMed] [Google Scholar]
- 14.Kroenke K, Wu J, Bair MJ, Krebs EE, Damush TM, Tu W (2011): Reciprocal relationship between pain and depression: A 12-month longitudinal analysis in primary care. J Pain. 12: 964–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blair M, Robinson R, Katon W, Kroenke K (2003): Depression and Pain Comorbidity. Arch Intern Med. 163: 2433–2445. [DOI] [PubMed] [Google Scholar]
- 16.Miller LR, Cano A (2009): Comorbid Chronic Pain and Depression: Who Is at Risk? J Pain. 10: 619–627. [DOI] [PubMed] [Google Scholar]
- 17.Bair MJ, Robinson RL, Katon W, Kroenke K (2003): Depression and Pain Comorbidity. Arch Intern Med. 163: 2433. [DOI] [PubMed] [Google Scholar]
- 18.Bair M, Wu J, Damush T, Sutherland J, Kroenke K (2008): Association of Depression and Anxiety Alone and in Combination with Chronic Musculoskeletal Pain in Primary Care Patients. 70: 890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elman I, Borsook D (2016): Common Brain Mechanisms of Chronic Pain and Addiction. Neuron. 89: 11–36. [DOI] [PubMed] [Google Scholar]
- 20.Pohl M, Smith L (2012): Chronic pain and addiction: Challenging co-occurring disorders. J Psychoactive Drugs. 44: 119–124. [DOI] [PubMed] [Google Scholar]
- 21.Fishbain DA, Cole B, Lewis J, Rosomoff HL, Rosomoff RS (2008): What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? a structured evidence-based review. Pain Med. 9: 444–459. [DOI] [PubMed] [Google Scholar]
- 22.Pitcher MH, Von Korff M, Bushnell MC, Porter L (2018): Prevalence and Profile of High-Impact Chronic Pain in the United States. J Pain. 00: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manchikanti L, Damron KS, McManus CD, Barnhill RC (2004): Patterns of illicit drug use and opioid abuse in patients with chronic pain at initial evaluation: a prospective, observational study. Pain Physician. 7: 431–437. [PubMed] [Google Scholar]
- 24.Dersh J, Polatin PB, Gatchel RJ (2002): Chronic pain and psychopathology: Research findings and theoretical considerations. Psychosom Med. 64: 773–786. [DOI] [PubMed] [Google Scholar]
- 25.Volkow N, Benveniste H, McLellan AT (2017): Use and Misuse of Opioids in Chronic Pain. Annu Rev Med. 69: 451–465. [DOI] [PubMed] [Google Scholar]
- 26.Wachholtz A, Gonzalez G (2014): Co-morbid pain and opioid addiction: Long term effect of opioid maintenance on acute pain. Drug Alcohol Depend. 1: 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han MH, Nestler EJ (2017): Neural Substrates of Depression and Resilience. Neurotherapeutics. 14: 677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esposito E (2006): Serotonin-Dopamine Interaction as a Focus of Novel Antidepressant Drugs. Curr Drug Targets. 7: 177–185. [DOI] [PubMed] [Google Scholar]
- 29.Holly EN, Miczek KA (2016): Ventral tegmental area dopamine revisited: Effects of acute and repeated stress. Psychopharmacology (Berl). 233: 163–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loggia ML, Berna C, Kim J, Cahalan CM, Gollub RL, Wasan AD, et al. (2014): Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia. Arthritis Rheumatol. 66: 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayes DJ, Chen DQ, Zhong J, Lin A, Behan B, Walker M, Hodaie M (2017): Affective Circuitry Alterations in Patients with Trigeminal Neuralgia. Front Neuroanat. 11: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Androulakis XM, Rorden C, Peterlin BL, Krebs K (2018): Modulation of salience network intranetwork resting state functional connectivity in women with chronic migraine. Cephalalgia. 38: 1731–1741. [DOI] [PubMed] [Google Scholar]
- 33.Gong L, Yin Y, He C, Ye Q, Bai F, Yuan Y, et al. (2017): Disrupted reward circuits is associated with cognitive deficits and depression severity in major depressive disorder. J Psychiatr Res. 84: 9–17. [DOI] [PubMed] [Google Scholar]
- 34.Akram H, Miller S, Lagrata S, Hyam J, Hariz M, Matharu M, Zrinzo L (2014): Deep brain stimulation for refractory chronic cluster headache. Stereotact Funct Neurosurg. 92: s39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlaepfer TE, Bewernick BH, Kayser S, Mädler B, Coenen VA (2013): Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 73: 1204–1212. [DOI] [PubMed] [Google Scholar]
- 36.Grubert C, Hurlemann R, Bewernick B, Kayser S, Hadrysiewicz B, Axmacher N, et al. (2011): Neuropsychological safety of nucleus accumbens deep brain stimulation for major depression: Effects of 12-month stimulation. World J Biol Psychiatry. 12: 516–527. [DOI] [PubMed] [Google Scholar]
- 37.Ray S, Di X, Biswal BB (2016): Effective Connectivity within the Mesocorticolimbic System during Resting-State in Cocaine Users. Front Hum Neurosci. 10: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA (2010): Mesocorticolimbic Circuits are Impaired in Chronic Cocaine Users as Demonstrated by Resting State Functional Connectivity. Neuroimage. 53: 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salamone JD, Correa M, Mingote SM, Weber SM (2005): Beyond the reward hypothesis: Alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol. 5: 34–41. [DOI] [PubMed] [Google Scholar]
- 40.Soares-Cunha C, Coimbra B, David-Pereira A, Borges S, Pinto L, Costa P, et al. (2016): Activation of D2 dopamine receptor-expressing neurons in the nucleus accumbens increases motivation. Nat Commun. 7: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geha PY, Baliki MN, Chialvo DR, Harden RN, Paice JA, Apkarian AV. (2007): Brain activity for spontaneous pain of postherpetic neuralgia and its modulation by lidocaine patch therapy. Pain. 128: 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai Y-H, Yuan R, Patel D, Chandrasekaran S, Weng H, Yang J-T, et al. (2017): Altered structure and functional connection in patients with classical trigeminal neuralgia. Hum Brain Mapp. 39: 609–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seifert CL, Magon S, Sprenger T, Lang UE, Huber CG, Denier N, et al. (2015): Reduced volume of the nucleus accumbens in heroin addiction. Eur Arch Psychiatry Clin Neurosci. 265: 637–645. [DOI] [PubMed] [Google Scholar]
- 44.Baliki M, Petre B, Torbey S, Herrmann K, Huang L, Schnitzer T, et al. (2013): Corticostriatal functional connectivity predicts transition to chronic pain. Nat Neurosci. 15: 1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vachon-Presseau E, Tétreault P, Petre B, Huang L, Berger SE, Torbey S, et al. (2016): Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain. 139: 1958–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Froeliger B, McConnell PA, Stankeviciute N, McClure EA, Kalivas PW, Gray KM (2015): The effects of N-Acetylcysteine on frontostriatal resting-state functional connectivity, withdrawal symptoms and smoking abstinence: A double-blind, placebo-controlled fMRI pilot study. Drug Alcohol Depend. 156: 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang S, Li CSR (2018): Ventral striatal dysfunction in cocaine dependence - difference mapping for subregional resting state functional connectivity. Transl Psychiatry. 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, et al. (2010): Nucleus Accumbens Deep Brain Stimulation Decreases Ratings of Depression and Anxiety in Treatment-Resistant Depression. Biol Psychiatry. 67: 110–116. [DOI] [PubMed] [Google Scholar]
- 49.Kuhn J, Möller M, Treppmann JF, Bartsch C, Lenartz D, Gruendler TOJ, et al. (2014): Deep brain stimulation of the nucleus accumbens and its usefulness in severe opioid addiction. Mol Psychiatry. 19: 145–146. [DOI] [PubMed] [Google Scholar]
- 50.Chen L, Li N, Ge S, Lozano AM, Lee DJ, Yang C, et al. (2019): Long-term results after deep brain stimulation of nucleus accumbens and the anterior limb of the internal capsule for preventing heroin relapse: An open-label pilot study. Brain Stimul. 12: 175–183. [DOI] [PubMed] [Google Scholar]
- 51.Zhou H, Xu J, Jiang J (2011): Deep brain stimulation of nucleus accumbens on heroin-seeking behaviors: A case report. Biol Psychiatry. 69: 69–70. [DOI] [PubMed] [Google Scholar]
- 52.Arnsten AFT (2015): Stress weakens prefrontal networks: Molecular insults to higher cognition. Nat Neurosci. 18: 1376–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koob GF, Volkow ND (2016): Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry. 3: 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krishnan V, Nestler EJ (2010): Linking Molecules To Mood: New Insight Into the Biology of Depression. Am J Psychiatry. 167: 1305–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murray EA, Wise SP, Drevets WC (2011): Localization of dysfunction in major depressive disorder: Prefrontal cortex and amygdala. Biol Psychiatry. 69: e43–e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abdallah CG, Sanacora G, Duman RS, Krystal JH (2018): The neurobiology of depression, ketamine and rapid-acting antidepressants: Is it glutamate inhibition or activation? Pharmacol Ther. 190: 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giesecke T, Gracely RH, Williams DA, Geisser ME, Petzke FW, Clauw DJ (2005): The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 52: 1577–1584. [DOI] [PubMed] [Google Scholar]
- 58.Vania Apkarian A, Baliki MN, Farmer MA (2013): Predicting transition to chronic pain. Curr Opin Neurol. 26: 360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flodin P, Martinsen S, Altawil R, Waldheim E, Lampa J, Kosek E, Fransson P (2016): Intrinsic Brain Connectivity in Chronic Pain: A Resting-State fMRI Study in Patients with Rheumatoid Arthritis. Front Hum Neurosci. 10: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. (2006): Chronic Pain and the Emotional Brain: Specific Brain Activity Associated with Spontaneous Fluctuations of Intensity of Chronic Back Pain. J Neurosci. 26: 12165–12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Polli A, Weis L, Biundo R, Thacker M, Turolla A, Koutsikos K, et al. (2016): Anatomical and functional correlates of persistent pain in Parkinson’s disease. Mov Disord. 31: 1854–1864. [DOI] [PubMed] [Google Scholar]
- 62.Jarzem P, Seminowicz DA, Fallatah S, Ware MA, Stone LS, Ouellet JA, et al. (2011): Effective Treatment of Chronic Low Back Pain in Humans Reverses Abnormal Brain Anatomy and Function. J Neurosci. 31: 7540–7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sampson SM, Rome JD, Rummans TA (2006): Slow-Frequency rTMS Reduces Fibromyalgia Pain. Pain Med. 7: 115–118. [DOI] [PubMed] [Google Scholar]
- 64.Pascual-Leone A, Rubio B, Pallardo F, Catala M (1996): Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 348: 233–237. [DOI] [PubMed] [Google Scholar]
- 65.Shen Y, Cao X, Tan T, Shan C, Wang Y, Pan J, et al. (2016): 10-Hz Repetitive Transcranial Magnetic Stimulation of the Left Dorsolateral Prefrontal Cortex Reduces Heroin Cue Craving in Long-Term Addicts. Biol Psychiatry. 80: e13–e14. [DOI] [PubMed] [Google Scholar]
- 66.Kong J, Tu P, Zyloney C, Su T (2010): Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behav Brain Res. 211: 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Truini A, Tinelli E, Gerardi MC, Calistri V, Iannuccelli C, Cesa S La, et al. (2016): Abnormal resting state functional connectivity of the periaqueductal grey in patients with fibromyalgia. Clin Exp Rheumatol. 34: S129–S133. [PubMed] [Google Scholar]
- 68.Deng Z, Pan Y, Li D, Zhang C, Jin H, Wang T, et al. (2018): Effect of Bilateral Anterior Cingulotomy on Chronic Neuropathic Pain with Severe Depression. World Neurosurg. 121: 196–200. [DOI] [PubMed] [Google Scholar]
- 69.Schmidt-Wilcke T, Ichesco E, Hampson JP, Kairys A, Peltier S, Harte S, et al. (2014): Resting state connectivity correlates with drug and placebo response in fibromyalgia patients. Neuroimage Clin. 6: 252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kregel J, Meeus M, Malfliet A, Dolphens M, Danneels L, Nijs J, Cagnie B (2015): Structural and functional brain abnormalities in chronic low back pain: A systematic review. Semin Arthritis Rheum. 45: 229–237. [DOI] [PubMed] [Google Scholar]
- 71.Obermann M, Rodriguez-Raecke R, Naegel S, Holle D, Mueller D, Yoon MS, et al. (2013): Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. Neuroimage. 74: 352–358. [DOI] [PubMed] [Google Scholar]
- 72.Malinen S, Vartiainen N, Hlushchuk Y, Koskinen M, Ramkumar P, Forss N, et al. (2010): Aberrant temporal and spatial brain activity during rest in patients with chronic pain. Proc Natl Acad Sci. 107: 6493–6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu J, Ren L, Womer FY, Wang J, Fan G, Jiang W, et al. (2014): Alterations in Amplitude of Low Frequency Fluctuation in Treatment-Naive Major Depressive Disorder Measured With Resting-State fMRI. 4988: 4979–4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Tol M, van der Wee N, van den Heuvel O, Nielen M, Demenescu L, Aleman A, et al. (2010): Regional Brain Volume in Depression and Anxiety Disorders. JAMA Psychiatry. 67: 1002–1011. [DOI] [PubMed] [Google Scholar]
- 75.Liu H, Hao Y, Kaneko Y, Ouyang X, Zhang Y, Xu L, et al. (2009): Frontal and cingulate gray matter volume reduction in heroin dependence: Optimized voxel-based morphometry. Psychiatry Clin Neurosci. 63: 563–568. [DOI] [PubMed] [Google Scholar]
- 76.Boccard SGJ, Fitzgerald JJ, Pereira EAC, Moir L, Van Hartevelt TJ, Kringelbach ML, et al. (2014): Targeting the Affective Component of Chronic Pain. Neurosurgery. 74: 628–637. [DOI] [PubMed] [Google Scholar]
- 77.Riva-Posse P, Choi KS, Holtzheimer PE, McIntyre CC, Gross RE, Chaturvedi A, et al. (2014): Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 76: 963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Conti Catarine Lima, Nakamura-Palacios Ester Miyuki (2014): Bilateral Transcranial Direct Current Stimulation Over Dorsolateral Prefrontal Cortex Changes the Drug-cued Reactivity in the Anterior Cingulate Cortex of Crack-cocaine Addicts. Brain Stimul. 7: 130–132. [DOI] [PubMed] [Google Scholar]
- 79.Daviu N, Bruchas MR, Moghaddam B, Sandi C, Beyeler A (2019): Neurobiological links between stress and anxiety. Neurobiol Stress. 11: 100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koob GF (2019): Neurobiology of Opioid Addiction: Opponent Process, Hyperkatifeia, and Negative Reinforcement. Biol Psychiatry. 5: 1–10. [DOI] [PubMed] [Google Scholar]
- 81.Veinante P, Yalcin I, Barrot M (2013): The amygdala between sensation and affect: a role in pain. J Mol Psychiatry. 1: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burgmer M, Gaubitz M, Konrad C, Wrenger M, Hilgart S, Heuft G, Pfleiderer B (2009): Decreased gray matter volumes in the cingulo-frontal cortex and the amygdala in patients with fibromyalgia. Psychosom Med. 71: 566–573. [DOI] [PubMed] [Google Scholar]
- 83.Drevets WC, Bogers W, Raichle M (2002): Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neuropsychopharmacol. 12: 527–544. [DOI] [PubMed] [Google Scholar]
- 84.Hong J, Kilpatrick LA, Labus J, Gupta A, Jiang Z, Ashe-mcnalley C, et al. (2013): Patients with Chronic Visceral Pain Show Sex-Related Alterations in Intrinsic Oscillations of the Resting Brain. 33: 11994–12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiang Y, Oathes D, Hush J, Darnall B, Charvat M, Mackey S, Etkin A (2016): Perturbed connectivity of the amygdala and its subregions with the central executive and default mode networks in chronic pain. 157: 1970–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Edwards RR, Iii COB, Bathon J, Haythornthwaite JA (2006): Catastrophizing and Pain in Arthritis, Fibromyalgia, and Other Rheumatic Diseases. Arthritis Rheum. 55: 325–332. [DOI] [PubMed] [Google Scholar]
- 87.Yakobov E, Stanish W, Tanzer M, Dunbar M, Richardson G, Sullivan MJL (2018): The prognostic value of pain catastrophizing in health-related quality of life judgments after Total knee arthroplasty. Health Qual Life Outcomes. 16: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Younger JW, Chu LF, DArcy NT, Trott KE, Jastrzab LE, MacKey SC (2011): Prescription opioid analgesics rapidly change the human brain. Pain. 152: 1803–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Y, Gong J, Xie C, Ye EM, Jin X, Song H, et al. (2015): Alterations in brain connectivity in three sub-regions of the anterior cingulate cortex in heroin-dependent individuals: Evidence from resting state fMRI. Neuroscience. 284: 998–1010. [DOI] [PubMed] [Google Scholar]
- 90.Wang W, Wang YR, Qin W, Yuan K, Tian J, Li Q, et al. (2010): Changes in functional connectivity of ventral anterior cingulate cortex in heroin abusers. Chin Med J (Engl). 123: 1582–1588. [PubMed] [Google Scholar]
- 91.Wood PB, Patterson JC, Sunderland JJ, Tainter KH, Glabus MF, Lilien DL (2007): Reduced Presynaptic Dopamine Activity in Fibromyalgia Syndrome Demonstrated With Positron Emission Tomography: A Pilot Study. J Pain. 8: 51–58. [DOI] [PubMed] [Google Scholar]
- 92.Martikainen IK, Nuechterlein EB, Pecina M, Love TM, Cummiford CM, Green CR, et al. (2015): Chronic Back Pain Is Associated with Alterations in Dopamine Neurotransmission in the Ventral Striatum. J Neurosci. 35: 9957–9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ledermann K, Jenewein J, Sprott H, Hasler G, Schnyder U, Warnock G, et al. (2016): Relation of dopamine receptor 2 binding to pain perception in female fibromyalgia patients with and without depression - A [11C] raclopride PET-study. Eur Neuropsychopharmacol. 26: 320–330. [DOI] [PubMed] [Google Scholar]
- 94.Potvin S, Larouche A, Normand E, de Souza JB, Gaumond I, Grignon S, Marchand S (2009): DRD3 Ser9Gly Polymorphism Is Related to Thermal Pain Perception and Modulation in Chronic Widespread Pain Patients and Healthy Controls. J Pain. 10: 969–975. [DOI] [PubMed] [Google Scholar]
- 95.Treister R, Pud D, Ebstein RP, Laiba E, Gershon E, Haddad M, Eisenberg E (2009): Associations between polymorphisms in dopamine neurotransmitter pathway genes and pain response in healthy humans. Pain. 147: 187–193. [DOI] [PubMed] [Google Scholar]
- 96.Zubieta J-K, Heitzeg M, YR S, JA B, Xu K, Xu Y, et al. (2003): COMT val 158 met Genotype Affects μ-Opioid Neurotransmitter Responses to a Pain Stressor. Science (80-). 299: 1240–1244. [DOI] [PubMed] [Google Scholar]
- 97.Peciña M, Sikora M, Avery ET, Heffernan J, Peciña S, Mickey BJ, Zubieta JK (2017): Striatal dopamine D2/3 receptor-mediated neurotransmission in major depression: Implications for anhedonia, anxiety and treatment response. Eur Neuropsychopharmacol. 27: 977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schneier FR, Slifstein M, Whitton AE, Pizzagalli DA, Reinen J, McGrath PJ, et al. (2018): Dopamine Release in Antidepressant-Naive Major Depressive Disorder: A Multimodal [11C]-(+)-PHNO Positron Emission Tomography and Functional Magnetic Resonance Imaging Study. Biol Psychiatry. 84: 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cannon DM, Klaver JM, Peck SA, Rallis-Voak D, Erickson K, Drevets WC (2009): Dopamine type-1 receptor binding in major depressive disorder assessed using positron emission tomography and [11C]NNC-112. Neuropsychopharmacology. 34: 1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Felten A, Montag C, Markett S, Walter NT, Reuter M (2011): Genetically determined dopamine availability predicts disposition for depression. Brain Behav. 1: 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cruccu G (2007): Treatment of painful neuropathy. Curr Opin Neurol. 20: 531–535. [DOI] [PubMed] [Google Scholar]
- 102.Martinez D, Saccone PA, Liu F, Slifstein M, Orlowska D, Grassetti A, et al. (2012): Deficits in dopamine D 2 receptors and presynaptic dopamine in heroin dependence: Commonalities and differences with other types of addiction. Biol Psychiatry. 71: 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang G, Volkow ND, Fowler JS, Ph D, Logan J, Ph D, et al. (1997): Dopamine D2 Receptor Availability in Subjects before and after Naloxone-Precipitated Withdrawal. Neuropsychopharmacology. 16: 174–182. [DOI] [PubMed] [Google Scholar]
- 104.Hou QF, Li S Bin (2009): Potential association of DRD2 and DAT1 genetic variation with heroin dependence. Neurosci Lett. 464: 127–130. [DOI] [PubMed] [Google Scholar]
- 105.Shi J, Zhao LY, Copersino ML, Fang YX, Chen Y, Tian J, et al. (2008): PET imaging of dopamine transporter and drug craving during methadone maintenance treatment and after prolonged abstinence in heroin users. Eur J Pharmacol. 579: 160–166. [DOI] [PubMed] [Google Scholar]
- 106.Navratilova E, Atcherley CW, Porreca F (2015): Brain Circuits Encoding Reward from Pain Relief. Trends Neurosci. 38: 741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Navratilova E, Porreca F (2014): Reward and motivation in pain and pain relief. Nat Neurosci. 17: 1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, et al. (2009): Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 12: 1364–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ozaki S, Narita M, Narita M, Iino M, Sugita J, Matsumura Y, Suzuki T (2002): Suppression of the morphine-induced rewarding effect in the rat with neuropathic pain: Implication of the reduction in μ-opioid receptor functions in the ventral tegmental area. J Neurochem. 82: 1192–1198. [DOI] [PubMed] [Google Scholar]
- 110.Narita M, Suzuki M, Imai S, Narita M, Ozaki S, Kishimoto Y, et al. (2004): Molecular mechanism of changes in the morphine-induced pharmacological actions under chronic pain-like state: Suppression of dopaminergic transmission in the brain. Life Sci. 74: 2655–2673. [DOI] [PubMed] [Google Scholar]
- 111.Terzi D, Gaspari S, Manouras L, Descalzi G, Mitsi V, Zachariou V (2014): RGS9–2 modulates sensory and mood related symptoms of neuropathic pain. Neurobiol Learn Mem. 115: 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xie JY, Qu C, Patwardhan A, Ossipov MH, Navratilova E, Becerra L, et al. (2014): Activation of mesocorticolimbic reward circuits for assessment of relief of ongoing pain: A potential biomarker of efficacy. Pain. 155: 1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sagheddu C, Aroni S, De Felice M, Lecca S, Luchicchi A, Melis M, et al. (2015): Enhanced serotonin and mesolimbic dopamine transmissions in a rat model of neuropathic pain. Neuropharmacology. 97: 383–393. [DOI] [PubMed] [Google Scholar]
- 114.Schwartz N, Temkin P, Jurado S, Lim B, Heifets B, Polepalli J, Malenka R (2014): Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Sci Mag. 345: 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ren W, Centeno MV, Berger S, Wu Y, Na X, Liu X, Kondapalli J (2016): The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain. Nat Neurosci. 19: 220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goffer Y, Xu D, Eberle SE, D’amour J, Lee M, Tukey D, et al. (2013): Calcium-Permeable AMPA Receptors in the Nucleus Accumbens Regulate Depression-Like Behaviors in the Chronic Neuropathic Pain State. J Neurosci. 33: 19034–19044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee M, Manders TR, Eberle SE, Su C, D’amour J, Yang R, et al. (2015): Activation of Corticostriatal Circuitry Relieves Chronic Neuropathic Pain. J Neurosci. 35: 5247–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhou H, Martinez E, Lin HH, Yang R, Dale JA, Liu K, et al. (2018): Inhibition of the Prefrontal Projection to the Nucleus Accumbens Enhances Pain Sensitivity and Affect. Front Cell Neurosci. 12: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barthas F, Sellmeijer J, Hugel S, Waltisperger E, Barrot M, Yalcin I (2015): The anterior cingulate cortex is a critical hub for pain-induced depression. Biol Psychiatry. 77: 236–245. [DOI] [PubMed] [Google Scholar]
- 120.Sellmeijer J, Mathis V, Hugel S, Li X-H, Song Q, Chen Q-Y, et al. (2018): Hyperactivity of Anterior Cingulate Cortex Areas 24a/24b Drives Chronic Pain-Induced Anxiodepressive-like Consequences. J Neurosci. 38: 3102–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Seno MDJ, Assis DV., Gouveia F, Antunes GF, Kuroki M, Oliveira CC, et al. (2018): The critical role of amygdala subnuclei in nociceptive and depressive-like behaviors in peripheral neuropathy. Sci Rep. 8: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Corder G, Ahanonu B, Grewe B, Wang D, Schnitzer M, Scherrer G (2019): An amygdalar neural ensemble that encodes the unpleasantness of pain. Scierc j nce. 363: 276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Descalzi G, Mitsi V, Purushothaman I, Gaspari S, Avrampou K, Eddie Loh Y-H, et al. (2017): Neuropathic pain promotes gene expression adaptations in brain networks involved in stress and depression. Sci Signal. 10: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Taylor AMW, Mehrabani S, Liu S, Taylor AJ, Cahill CM (2017): Topography of microglial activation in sensory- and affect-related brain regions in chronic pain. J Neurosci Res. 95: 1330–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu Y, Na X, Zang Y, Yu C, Xin W, Pang R, et al. (2014): Upregulation of tumor necrosis factor-alpha in nucleus accumbens attenuates morphine-induced rewarding in a neuropathic pain model. Biochem Biophys Res Commun. 449: 502–507. [DOI] [PubMed] [Google Scholar]
- 126.Zhang H, Qian YL, Li C, Liu D, Wang L, Wang XY, et al. (2017): Brain-Derived Neurotrophic Factor in the Mesolimbic Reward Circuitry Mediates Nociception in Chronic Neuropathic Pain. Biol Psychiatry. 82: 608–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu D, Tang Q-Q, Yin C, Song YY, Liu Y, Yang J-X, et al. (2017): BDNF-mediated projection-specific regulation of depressive-like and nociceptive behaviors in mesolimbic reward circuitry. Pain. 159: 175–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mitsi V, Terzi D, Purushothaman I, Manouras L, Gaspari S, Neve RL, et al. (2015): RGS9– 2–controlled adaptations in the striatum determine the onset of action and efficacy of antidepressants in neuropathic pain states. Proc Natl Acad Sci. 112: E5088–E5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tajerian M, Alvarado S, Millecamps M, Vachon P, Crosby C, Bushnell MC, et al. (2013): Peripheral Nerve Injury Is Associated with Chronic, Reversible Changes in Global DNA Methylation in the Mouse Prefrontal Cortex. PLoS One. 8: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Descalzi G, Ikegami D, Ushijima T, Nestler EJ, Zachariou V, Narita M (2015): Epigenetic mechanisms of chronic pain. Trends Neurosci. 38: 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hipolito L, Wilson-Poe A, Campos-Jurado Y, Zhong E, Gonzalez-Romero J, Virag L, et al. (2015): Inflammatory Pain Promotes Increased Opioid Self-Administration: Role of Dysregulated Ventral Tegmental Area Opioid Receptors. J Neurosci. 35: 12217–12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gaspari S, Papachatzaki MM, Koo JW, Carr FB, Tsimpanouli ME, Stergiou E, et al. (2014): Nucleus accumbens-specific interventions in RGS9–2 activity modulate responses to morphine. Neuropsychopharmacology. 39: 1968–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Taylor AMW, Castonguay A, Taylor AJ, Murphy NP, Ghogha A, Cook C, et al. (2015): Microglia Disrupt Mesolimbic Reward Circuitry in Chronic Pain. J Neurosci. 35: 8442–8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G (2019): Drug and Opioid-Involved Overdose Deaths — United States, 2013–2017. MMWR Morb Mortal Wkly Rep. 67: 1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Freburger JK, Holmes M, Agans RP, Jackman AM, Darter J, Wallace AS, et al. (2009): The rising prevalence of chronic disabling low back pain. Arch Intern Med. 169: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Weinberger AH, Gbedemah M, Martinez AM, Nash D, Galea S, Goodwin RD (2017): Trends in depression prevalence in the USA from 2005 to 2015: widening disparities in vulnerable groups. Psychol Med. 48: 1308–1315. [DOI] [PubMed] [Google Scholar]
- 137.Hamilton J, Lee J, Canales JJ (2015): Chronic unilateral stimulation of the nucleus accumbens at high or low frequencies attenuates relapse to cocaine seeking in an animal model. Brain Stimul. 8: 57–63. [DOI] [PubMed] [Google Scholar]
- 138.Martínez-Rivera FJ, Rodriguez-Romaguera J, Lloret-Torres ME, Do Monte FH, Quirk GJ, Barreto-Estrada JL (2016): Bidirectional Modulation of Extinction of Drug Seeking by Deep Brain Stimulation of the Ventral Striatum. Biol Psychiatry. 80: 682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bruchim-Samuel M, Lax E, Gazit T, Friedman A, Ahdoot H, Bairachnaya M, et al. (2016): Electrical stimulation of the vmPFC serves as a remote control to affect VTA activity and improve depressive-like behavior. Exp Neurol. 283: 255–263. [DOI] [PubMed] [Google Scholar]