I. Introduction
Drivers are increasingly studied ablation targets for atrial fibrillation (AF), and rotational or focal activation patterns are supported by most translational studies1–3 with steadily increasing evidence in patients4–10. However, results from ablation studies remain controversial. First, despite promising outcomes of AF driver ablation in several meta-analyses11–13, outcomes vary between centers and patients. Second, it is unclear how best to perform driver ablation. Recent data from the randomized REAFFIRM trial showed 77.7% freedom from atrial arrhythmias by driver ablation plus pulmonary vein isolation (PVI) in persistent AF, trending higher than PVI,14 yet success was lower when lines and fractionated electrograms were added which rendered the trial neutral overall. Since prior strategies beyond PVI have all trended to lower results,15 these data provide an important signal that improved driver ablation could improve persistent AF ablation outcomes overall. Third, there is a lack of practical guidance on how to optimally map and ablate AF drivers, particularly to differentiate critical from secondary sites using different AF mapping methods. This chapter addresses each of these issues.
II. Mechanistic targets for Therapy:
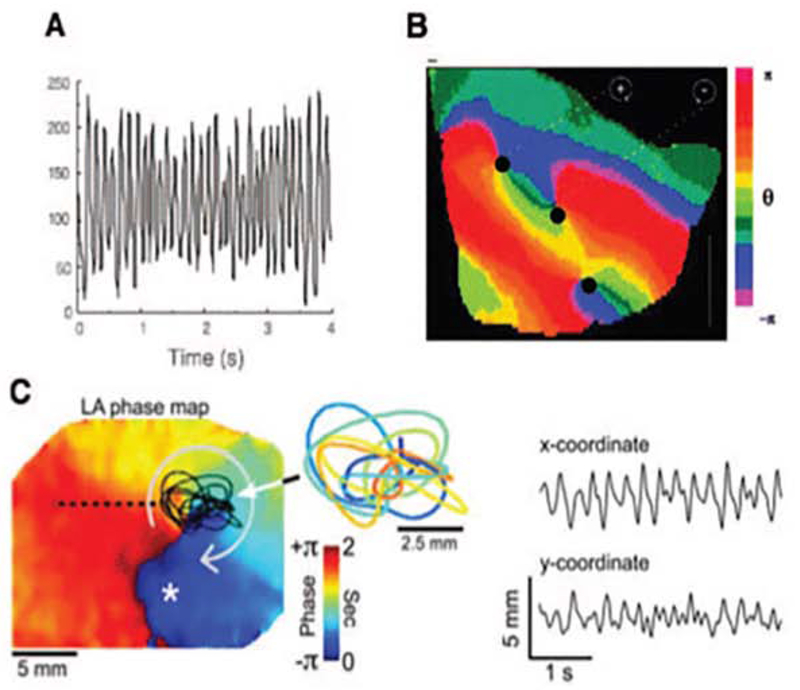
There is now considerable evidence that localized rotational or focal drivers sustain fibrillation using optical mapping in isolated hearts from multiple species including human AF. Optical mapping (figure 1) uses video imaging of voltage-sensitive dye imaging, coupled with activation, phase or other analysis approaches to map AF at high spatial and temporal resolution. Figure 1A illustrates rapid irregular action potentials at one point mapped optically. Figure 1B plots such action potentials across the cardiac surface in fibrillation. Each color represents phases of cardiac activation (from depolarization to repolarization), in which rotations are represented as a full color spectrum from red to blue. Points in the atrium where activation and repolarization meet (figure 1C), i.e. an entire cycle, have undefined phase and are termed phase singularities (PS) which may represent rotor cores in fibrillating myocardium. AF rotational circuits are not fixed like macro-reentry around an obstacle, but precess in small regions (figure 1C). This also distinguishes them from fleeting rotational activity in multi-wavelet (leading circle) reentry. Fibrillation may thus terminate when the rotor collides with a boundary3, does not have enough “elbow room” to spin or by eliminating drivers such that fibrillatory waves are no longer replenished and progressively extinguish.
Figure 1: Optical Mapping of Fibrillatory Conduction from an AF Source.
(A) High Resolution optical action potentials obtained from explanted fibrillating tissue. (B) Snapshot of phase movie of a fibrillating rabbit ventricle, showing rotors as red to blue phase angles, and phase singularities (PS) as dark black dots where all phases (colors) converge. (C) Rotor meandering and fractionation during AF in isolated sheep heart. On the left, a left atrial phase snapshot demonstrates reentry in the left atrium (LA) free wall. The inset shows the time-space trajectory of the tip (PS), while the x and y coordinate signals are shown on the right. (Adapted from Gray R, Pertsov A, Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature [Internet]. 1998 [cited 2013 Mar 13];392(May):75–8. Available from: http://www.nature.com/nature/journal/v392/n6671/abs/392075a0.html; and Zlochiver S, Yamazaki M, Kalifa J, Berenfeld O. Rotor meandering contributes to irregularity in electrograms during atrial fibrillation. Heart Rhythm [Internet]. 2008 Jun [cited 2013 May 14];5(6):846–54. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3079377&tool=pmcentrez&rendertype=abstract; with permission.)
Optical mapping of AF in human hearts shows endocardial micro-reentrant circuits which may be focal on the epicardium16, and hence reconcile the apparent paradox between surgical and clinical mapping studies.1–3 Intriguingly, these optically mapped drivers were correctly detected as rotational circuits by clinical baskets and mapping algorithms (FIRM),17 thus validating these clinical mapping approaches. In these unique translational human studies, rotational circuits localized to regions of micro-fibrosis which may or may not represent those sites identified by clinical atrial MRI.
Clinically, the terms rotational activity, rotational driver or even ‘reentry’ are equally effective to describe the phenomena mapped clinically, and cannot be easily separated by the resolution of clinical tools. Classically, unlike anatomic reentry, entrainment is not possible due to complex or absent excitable gaps.3
III. Comparing Different AF Mapping Methods
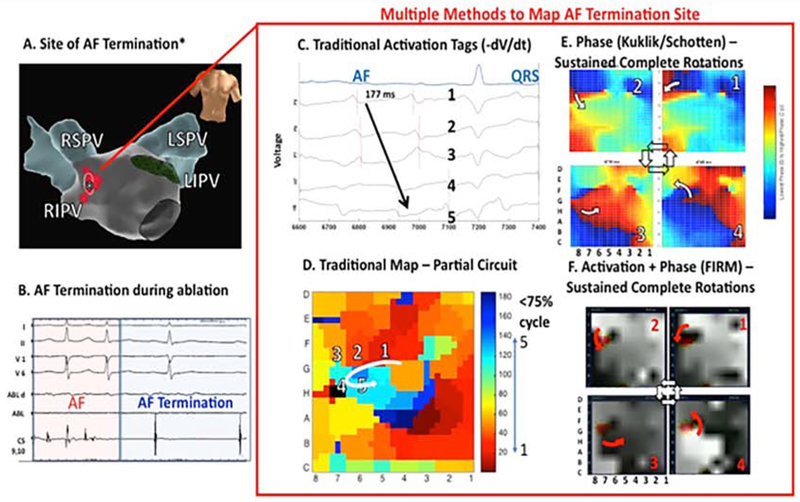
Few studies have compared methods in the same patient data. We assembled an international registry to compare mapping methods in patients in whom ablation terminated persistent AF (), for which we have made data available online (http://narayanlab.stanford.edu). Figure 2 shows termination of persistent AF to sinus rhythm AF in a 65 year old man (panels A, B). In panel C, AF maps using traditional methods showed only partial and transient rotations (shown), which do not explain the site of termination. In panel D, a freely available phase mapping approach18 shows consistent rotations at this site, and were corroborated by FIRM maps (panel E) by which show gray-scale activation maps and phase singularities (in red). Thus, sites of AF termination by ablation may show rotational AF drivers by phase and activation plus phase (FIRM), which can be missed by traditional activation mapping of AF.19
Figure 2. Comparing AF Mapping Methods In The Same Patient, Where Ablation Terminates Persistent AF.
In a 65 year old man, localized ablation (A) near the right superior pulmonary vein carina prior to PVI (B) terminates persistent AF to sinus rhythm. (C,D) Traditional activation maps show only a partial rotation (75% of cycle, orange to light blue) which may not explain AF termination by ablation in this region. Notably, (E) phase maps by an independent method and (F) activation plus phase maps (FIRM; activation in gray scale, phase in red) each revealed sustained rotations at the site where ablation terminates AF. Ablation site was identified prospectively by map (F). (Adapted from Kowalewski CAB, Shenasa F, Rodrigo M, Clopton P, Meckler G, Alhusseini MI, et al. Interaction of Localized Drivers and Disorganized Activation in Persistent Atrial Fibrillation. Circ Arrhythmia Electrophysiol [Internet]. 2018 Jun 8 [cited 2018 Jun 11];11(6):e005846. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29884620; with permission.)
While termination of persistent AF by ablation is not equivalent to elimination, it is rare to terminate persistent AF by limited ablation of a of atrial tissue before PVI. Moreover, termination provides one of the few accepted acute endpoints for ablation. Long-term outcomes are clearly the ultimate goal, yet may not be ideal for assessing the accuracy of AF mapping, since they include long-term confounding issues unrelated to mapping such as the effects of additional lesion sets including PVI, lesion recovery, or disease progression. Thus, the approach of comparing maps and acute ablation response eliminates ‘apples to oranges’ comparisons of patient populations, and may continue to be a useful strategy to compare mapping method/algorithm moving forwards.
AF drivers have now been identified by multiple mapping systems, with many similarities 7,20. We recently summarized these similarities in meta-analyses of mapping11,12. Table 1 summaries some of these details. Studies of FIRM typically show 2-4 biatrial AF drivers, ECGI5 showed 3-5 and other techniques such as that by Lin et al. showed 2-3.21 When bi-atrial mapping is performed, about two thirds of AF drivers lie in left atrium and one third in right atrium, as also reported by other mapping modalities. This distribution may explain ablation outcomes, where lesion sets may coincidentally hit AF drivers22.
Table 1.
Summary of AF Driver Mapping and Ablation Strategies
| Mapping Technique | AF type mapped | Number of ablation targets | Atrial Location | Source Characterisation | Acute termination % | Freedom from AF at 12 months, with PVI |
|---|---|---|---|---|---|---|
| Focal Impulse and Rotor Mapping (FIRM)4,23,39 | Paroxysmal, persistent and long standing persistent | 3-5 | LA 70% RA 30% PV 24% |
Stable rotations 76% Focal sources 24%4 |
56% (60% to sinus)4 RA in 22%39 |
Meta-analysis: 72.5%12 Persistent AF RCT: 77.7% (FIRM+PVI subgroup)14 |
| Endocardial phase19 | Paroxysmal, persistent and long standing persistent | 3-5 | LA 66% RA 34% PV 40% |
Stable rotations 100% | 100% (83% to sinus) | Similar to FIRM |
| Body Surface, ECGI5,40,41 | Persistent and long standing persistent | 3-6 | LA 70% RA 30% LPV/LAA 82%5 LA 53% RA 27% Septum 20%40 |
Re-entries 80% Focal breakthrough 20%5 |
80% (66% to AT)5 64% (79% to AT) (PVs 37%, LA 35%, RA in 28%)40 |
85%5 78%40 |
| CartoFinder6,42–44 | Persistent and long standing persistent | 1-3 | LA 63% RA 27% Non-PV 79%8 |
Rotational activity 70% Focal activations 30%42 Focal activations 100%44 |
63% (58% to AT)42 15% (all sinus)8 |
71%44 70%8 |
| Spatiotemporal Dispersion10 | Persistent AF | 4-6 | LA 80% RA 20% PV/LAA 80% |
Regions of microreentry | 95% (85% to AT) | 85% without PVI (1.4 procedures, at 18 months) |
| Dominant Frequency45 | Paroxysmal and persistent | 3-4 | LA 80% RA 20% PV>70% |
High frequency sites | 80-90% in HFSA arm | 70% persistent |
| Charge/Dipole Density7,46 | Persistent AF | 2-3 | RA not mapped LA anterior 70% | Localized irregular activity Localized rotational activity Focal activity |
50-60% | 73%46 |
| Electrographic flow mapping47,48 | Persistent AF | 4-6 | LA 70% RA 30% PV 40% |
Rotational 51% Focal 49% |
100% RA in 10% |
Pending |
| Stochastic Trajectory Analysis of Ranked Signals (STAR)9 | Persistent AF | 2-3 (post PVI) | LA 95% RA 5% |
Early sites of activation | 29% (75% to AT) | 80% (AT/AF at 18 months) |
Further comparative mapping studies are needed for new techniques, particularly those that compare methods in the same patient datasets, ideally compared to a references such as optical mapping as has been conducted for some methods17.
IV. Clinical Outcomes of FIRM-guided Ablation
Multiple studies now exist on the outcomes from AF driver ablation. As with any new approach, initial reports were promising and some subsequent reports were disappointing. In recent meta-analyses, adjunctive driver-guided ablation was associated with higher rates of acute AF termination, lower recurrence of any atrial arrhythmia and comparable complication incidence. 11
Multicenter randomized data of AF driver ablation was recently presented. The REAFFIRM trial enrolled 375 patients and randomized them 1:1 to conventional ablation, the intention-to-treat arms being PVI versus PVI + rotor ablation (FIRM). By intention-to-treat analysis, freedom from atrial arrhythmias after a single procedure was 67.5% in the PVI and 69.3% in the FIRM arms at 1 year. However, nearly half of all patients in both limbs had additional ablation not prescribed by protocol. Examining on-treatment subgroups,14 FIRM +PVI (77.7%) achieved higher success than PVI (65.5%) with a trend (p=0.09) as PVI success was higher than anticipated and the trial was not powered for these subgroups. Non-prescribed groups were PVI+complex fractionated and linear ablation (69.6%), and all strategies combined with the lowest success (57.7%). Notably, the FIRM+PVI result in REAFFIRM was similar to early studies (82.4% success in CONFIRM4). These results appear to re-iterate the signal for lower success by ablation of complex fractionated atrial electrograms or lines observed in STAR-AF2 and other recent randomized trials. The final publication of REAFFIRM is awaited and may address some unanswered questions, including why ablation times in the PVI-only arm were longer than the FIRM+PVI arm and what additional ablation was performed.
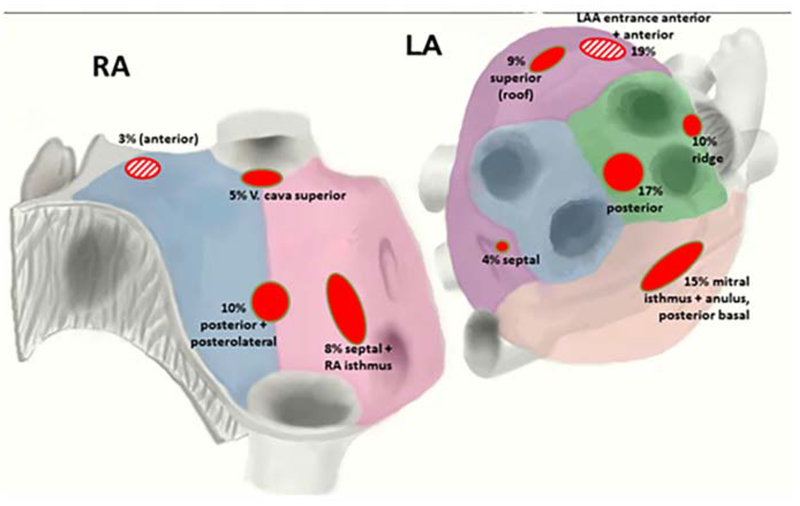
In FIRM studies from multiple groups, AF sources arise in diverse locations, overall with 25-40% near pulmonary veins, 25-40% elsewhere in the left atrium, and 25-40% in right atrium (figure 3 and table 2).23 Body surface mapping and ECG imaging show similar AF driver distributions but in larger regions,5 which may represent greater ‘meander’ in projecting from the heart to the torso.24 In multiple studies, right atrial drivers are mostly in the free wall, posterolateral to the right atrial appendage, and rarely near the superior vena cava or cavotricuspid isthmus (figure 3). AF drivers are present in higher numbers and more widely distributed in patients with persistent than paroxysmal AF.25
Figure 3:
Location of focal sources and rotors in patients using FIRM mapping. (From Spitzer SG, Károlyi L, Rämmler C, Scharfe F, Weinmann T, Zieschank M, et al. Treatment of Recurrent Non-Paroxysmal Atrial Fibrillation Using Focal Impulse and Rotor Mapping (FIRM) -Guided Rotor Ablation : Early Recurrence and Long-Term Outcomes Short title : FIRM ablation outcomes for recurrent non-paroxysmal AF. J Cardiovasc Electrophysiol. 2017;1–30; with permission.)
Table 2:
Considerations for successful FIRM-guided ablation
| - General | - Basket coverage (multiple positions ideally used) and careful map interpretation are the cornerstones of AF driver ablation. |
| - Rotational and Focal Driver regions fluctuate but remain in spatially stable regions which are ablation targets. | |
| - Sources lie in 2-3 cm2 areas (1.5 X 1.5 cm). | |
| - FIRM sites lie between, i.e. are typically bounded by physical electrodes. | |
| - Given the size of each ablation lesion (>7 mm), one should bracket the driver between electrodes rather than define a precise ‘core coordinate’ | |
| - Right Atrial Sources | - Observed in one third of patients, paroxysmal as well as persistent AF (see figure 3) |
| - Typically fewer in number than in left atrium | |
| - No stereotypical locations, but more likely in the free wall (where phrenic capture should be tested), septum and posterior wall. | |
| - Rarely at the superior vena cava or cavotricuspid isthmus. | |
| - Left Atrial Sources | - Observed in nearly all patients. |
| - Typically multiple (average of 2-3), with higher numbers in patients with more advanced disease (persistent-long-standing AF) | |
| - No stereotypical location, but 40-50% at sites covered by a typical wide area PV antral ablation |
The emergence of randomized data of driver ablation with a favorable safety and procedure time profile, showing a trend to improve results from PVI, differs from all other additive strategies which trended to worsen PVI.15 This signal potentially improving outcomes from persistent AF ablation should prompt investigation into the most suitable patients in whom rotor mapping and ablation is performed. To address some of the variety in this technique, we include a practical ‘how-to’ for using FIRM mapping in the following sections.
V. How To Create Basket-Derived Driver Maps (FIRM)
The general considerations for FIRM mapping and ablation are summarized in table 2. The procedure requires skills that include assessing adequate basket coverage of the atrium, interpretation of complex AF maps, and appropriate ablation guidance. We estimate that approximately 20 cases are adequate for an operator to achieve proficiency.
The basket catheter should be sized to left atrial dimensions, then deployed sequentially in right then left atria via standard sheaths. New baskets cover >80% of the atria (figure 4) and may improve over prior designs.26 Suboptimal basket deployment (noncontact) may cause AF sources to be missed. Importantly, comprehensive atrial coverage may not be possible with a single basket position, and so repeat maps after re-positioning may reveal all AF sources (figure 5). Video 1 shows preparation of the compliant basket, whose splines have rectangular cross section designed to not separate or spread significantly despite radial pressure. The basket is then collapsed for insertion into the femoral vein.
Figure 4: Positioning Basket Catheters.
(A) In right atrium, the sheath and catheter are advanced to the SVC where retracting the sheath causes the basket to self-expand. Slight torque may be required to maximize expansion and apposition. Optimally only 0-1 splines should traverse the tricuspid valve orifice. (B) In left atrium the sheath and catheter are advanced into the left superior pulmonary vein and the sheath is retracted. Slight manipulation may be needed to expand the basket outside of the vein. Again, only 0–1 spline should traverse the mitral valve orifice. (C) An undersized basket catheter will assume its natural spherical shape without any deformation suggesting limited endocardial contact. Intracardiac echocardiography (ICE) is often helpful in assessing endocardial contact. (D) An oversized basket catheter is most commonly recognized by an inability to expand the basket secondary to distal electrode restriction in a PV antrum. Further basket withdrawal is limited by the proximal electrodes encountering the trans-septal access site. (E) Withdrawal of basket out of the LUPV orifice results in slight spline prolapse into the left ventricle, although > 56 electrodes are in contact with the left atrial walls. (F) Regional Oversampling using the basket catheter. Here, the basket is manipulated to ensure higher sampling density at the LA roof, where a rotor was observed, at the cost of lower density sampling in the posterior and anterior walls where rotors were previously not seen. If no AF sources are identified, repositioning to systematically sample regions of the atria is suggested. (From Narayan SM, Krummen DE, Rappel W-J. Clinical mapping approach to diagnose electrical rotors and focal impulse sources for human atrial fibrillation. J Cardiovasc Electrophysiol [Internet]. 2012 May [cited 2012 Aug 6];23(5):447–54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22537106; with permission.)
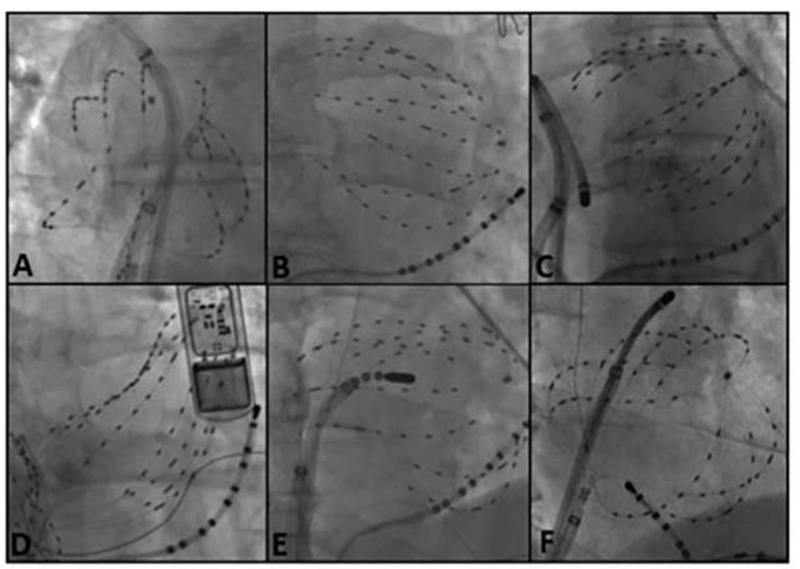
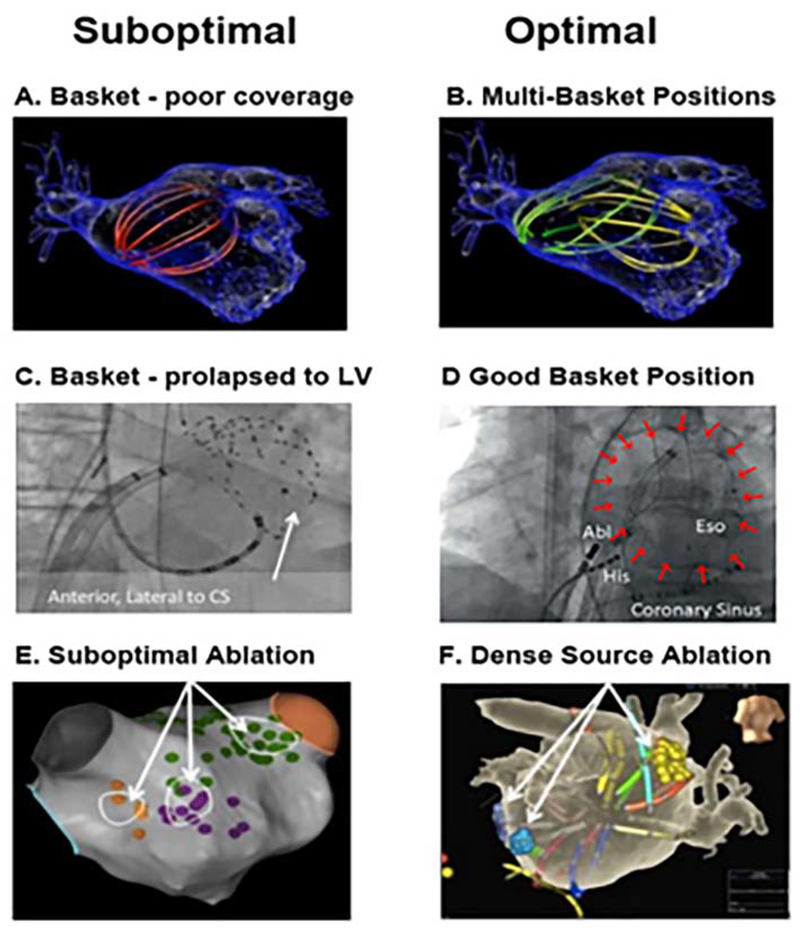
Figure 5. Pearls and Pitfalls in AF Basket Placement.
A) Suboptimal basket position in left atrium. B) Multi-Position Basket Mapping, the method of choice in large atria. C) Suboptimal basket in a disappointing report,52 consistent with inadvertent LV prolapse – conical shape, anterolateral to coronary sinus, ventricular electrograms; (D) Good basket position covering atrium well; (E) Sparse lesions over source area in a disappointing report53; (F) Dense source area lesions in a successful series. ([C] from Buch E, Benharash P, Frank P, Share M, Tung R, Shivkumar K, et al. Quantitative Analysis of Localized Sources Identified by Focal Impulse and Roter Modulation Mapping in Atrial Fibrillation. Circ Arrhythmia Electrophysiol [Internet]. 2015;554–62. Available from: http://circep.ahajournals.org/cgi/doi/10.1161/CIRCEP.115.002721; [D,E] from Gianni C, Mohanty S, Di Biase L, Metz T, Trivedi C, Gökoğlan Y, et al. Acute and early outcomes of FIRM-guided rotors-only ablation in patients with non-paroxysmal atrial fibrillation. Hear Rhythm [Internet]. 2015 Dec 16 [cited 2016 Jan 10]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/26706193; [F] from Sommer P, Kircher S, Rolf S, John S, Arya A. Successful Repeat Catheter Ablation of Recurrent Longstanding Persistent Atrial Fibrillation with Rotor Elimination as the Procedural Endpoint: A Case Series. J Cardiovasc Electrophysiol 27. 2015;27(3):274–80. 27; with permission.
Right atrial contact is more forgiving of basket size. For the left atrium, a more anterior trans-septal puncture orients the basket posteriorly, which is ideal, while a posterior puncture encourages partial prolapse across the mitral annulus which is suboptimal. Basket size is selected by measuring distance from the inter-atrial septum to the Coumadin ridge via catheter spacing on fluoroscopy, intra-cardiac echocardiography or pre-procedural imaging to avoid the need to upsize or downsize.
Figure 4 outlines deployment techniques for the basket catheter. In the right atrium the basket catheter is unsheathed in the superior vena cava (SVC) and slowly retracted into the right atrial body. Slight clockwise or counterclockwise torque is applied for optimal deployment. Particular care is required if a right atrial pacing lead is present, rotating the basket so that splines span the lead rather than displace it27 and typically advancing from the inferior vena cava rather than pulling down from the SVC. Video 2 shows rapid creation of a right atrial geometry by insertion into the superior vena cava, pull down to the right atrium, then to the inferior vena cava. This process takes less than a minute, created several hundred geometric points with voltage data, and the basket is then used to map AF in 2-3 right atrial positions.
In left atrium, the basket is unsheathed in the left superior pulmonary vein and slowly retracted into the atrial body. Slight clockwise or counterclockwise torque may help achieve optimal basket deployment, maximizing endocardial contact with the fewest splines over the mitral valve orifice. Alternatively, the basket catheter can be carefully ‘reflected’ off the carina of the left pulmonary veins. Video 3 shows rapid creation of left atrial geometry, by insertion to the left superior pulmonary vein, the posterior wall, the right pulmonary veins then the anterior left atrium. This process takes less than a minute, created many hundred geometric points with voltage data, and the basket is then used to map AF in 2-3 left atrial positions.
Once the basket catheter is in place, AF electrograms are recorded in several 1 minute epochs, and exported for near-real time analysis. Localization signals from electroanatomic mapping systems are best switched off while recording. Signals are typically recorded in unfiltered (0.05-250 Hz or 0.05-500 Hz) unipolar configuration, referenced to the Wilson Central Terminal, a body patch or a catheter in the SVC. This also serves to maximize the functional resolution of the basket by preserving spatial relations between 64 unipoles rather than 32 pairs of bipoles and use of direction sensitive bipolar data may hamper detection of rotational activity.
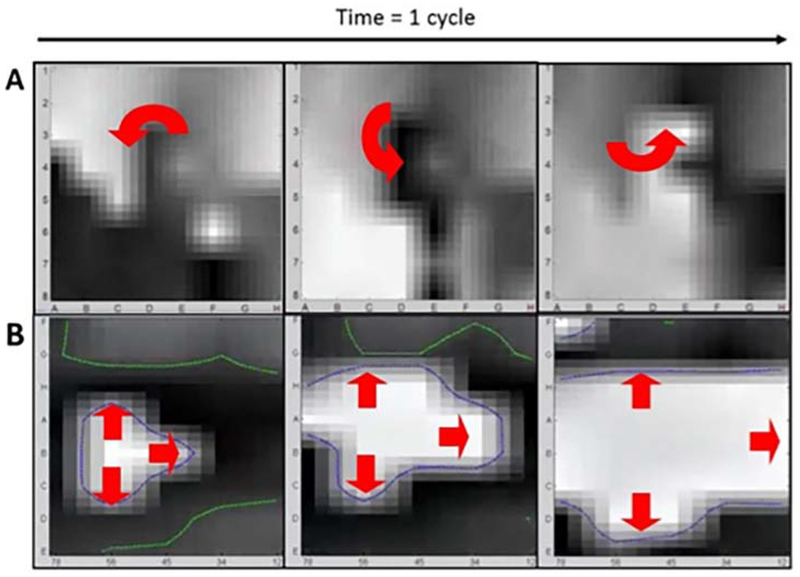
Computational analysis uses described algorithms28 based on tissue physiology (repolarization and conduction dynamics in patients with persistent and paroxysmal AF29–33 to create a propagation movie of the arrhythmia (AF). Three dimensional activation on the basket catheter is translated to 2 dimensions to display a FIRM movie. Figure 6 A and B depict 3 successive frames of a FIRM movie of AF spanning 100-160ms showing a spiral wave and focal impulse, respectively. During a case, ~25 consecutive cycles (4 seconds) are typically viewed and analyzed per “epoch”. Figures 7A, B show isochronal snapshots that summarize these movies. Rotational drivers (spiral waves) or focal sources are diagnosed using criteria in table 3, and targeted only if they remain in stable regions over 1 minute or more, with precession that has been quantified in <2 cm2 regions.34 This criterion eliminates transient, partial or migratory rotations.
Figure 6: AF Sources On FIRM Movies. Three successive FIRM movie snapshots demonstrating.
(A) A counterclockwise AF rotor centered at electrode D3–4. (B) A focal impulse source for AF originating near electrode B5-6. Each AF source precessed within their spatially reproducible region for tens of minutes, until eliminated by brief FIRM targeted ablation.
Figure 7. Snapshots Illustrate FIRM-based AF movies.
(A) Right atrial AF rotor lies at D3-4 (precesses between cycles), and was eliminated by ablation. Same patient as figure 6A (B) Left Atrial AF Focal Source lies at B56 (precesses between cycles), and was eliminated by ablation. Same patient as figure 6B. (From Narayan SM, Krummen DE, Rappel W-J. Clinical mapping approach to diagnose electrical rotors and focal impulse sources for human atrial fibrillation. J Cardiovasc Electrophysiol [Internet]. 2012 May [cited 2012 Aug 6];23(5):447–54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22537106; with permission.)
Table 3:
Diagnostic Criteria
| - Rotational AF Driver | - A rotational site that emanates waves to cause disorganized activity within the chamber. - Driver may precess (meander) but should remain within a region of 1-2 cm2 for ~ 1 minute or more, with temporal fluctuations. |
| - Focal AF Driver | - A focal site with rapid activation which emanates waves to cause disorganized activation within the chamber - Driver may precess (meander) but should remain within a region of 1-2 cm2 for ~ 1 minute or more, with temporal fluctuations. |
| - Disorganized (fibrillatory) activity | - Absence of organized rotational or focal activation. |
VI. How to Ablate FIRM-Identified AF Drivers
The basic approach to ablating AF drivers is to eliminate viable tissue in the region of precession. If right and left atria are mapped in that sequence then ablation typically follows that sequence, but this order can be varied.
The rotational core or focal impulse origin of each driver is tagged relative to its closest electrode on the movie and on the multipolar catheter. Figures 2 and 5 show how drivers identified on AF maps are translated to physical electrode projections on electroanatomic shells. This is straightforward in principle, but should avoid errors of projecting basket electrodes to the wrong regions of the shell. We ensure that the shell is viewed with the basket spline en face, to avoid parallax errors which would lead to the wrong regions marked on the shell. The ablation target area is an area of 1-2 cm diameter bounded by the electrodes which bracket it.
The ablation endpoint is elimination of the driver on a post-ablation map. Irrigated and non-irrigated radiofrequency and cryoablation energy have all demonstrated success in FIRM-guided ablation.4,35 The typical approach using RF is to apply successive lesions, moving the catheter within the precession area. Local electrogram abatement and failure to capture with high output pacing within the ablation target region can help ensure complete ablation of target area. If AF terminates during ablation, attempts are made to reinitiate AF for remapping. If AF does not terminate during ablation, repeat FIRM mapping sometimes identifies slight shift of the driver. Additional AF drivers are sometimes clearer on a remap after competing sources are eliminated. Table 4 summarizes how to approach common problems encountered in FIRM cases.
Table 4:
Troubleshooting The Difficult Case
| - Basket Catheter Sizing and Positioning | - Select basket based on left atrial rather than right atrial size. |
| - Select basket based on measuring the distance from trans-septal crossing to Coumadin ridge | |
| - Select a more anterior trans-septal puncture, to advance the basket posteriorly into the left atrium | |
| - If in doubt, undersize rather than oversize the basket | |
| - Unable to identify focal sources on a FIRM map. | - Assess for proper basket catheter size and position. See figure 5 |
| - Maximize signal fidelity by catheter opposition; ensure adequate filtering and unipolar reference. | |
| - Choose another time slice within the epoch, since AF drivers may fluctuate then re-appear in the same location. Alternatively, collect another epoch. | |
| - Play movie initially fast, to localize general region of rotation or focal activity, then slow movie down. For focal source, play movie in reverse to show activation ‘collapse’ to an origin. | |
| - Consider sources outside the mapping area. | |
| - Evaluate regions of under sampling, adjust basket positioning, and re-sample. | |
| - Choose an alternative spline to “cut and open”. | |
| - Inability to eliminate Rotor or Focal Source Despite FIRM ablation | - Ensure adequate ablation at targeted sites by lack of capture with high output pacing. |
| - Utilize additional ablation if needed, taking into account standard safety considerations e.g. near the esophagus or phrenic nerves. | |
| - Repeat FIRM map. Sources may become clearer with elimination of other sources. | |
| - Evaluate regions of under sampling, adjust basket positioning, and re-sample. | |
| - Consider sources outside the mapping area. |
If AF driver ablation results in an atrial tachycardia, that mechanism is typically ablated as part of the procedure. Preliminary studies show that ablation of an AF driver may anchor activation to a localized micro-reentrant atrial tachycardia,36 which may arise near the original driver, as well as other tachycardias.37
The safety profile of basket mapping and FIRM-guided ablation appears excellent38 with no documented cases of thromboembolism or perforation using basket catheters. This was supported by the safety profile in the REAFFIRM trial. Ablation safety appears similar to traditional AF ablation, and sensitive sites, such as near the esophagus or phrenic nerves, should be screened in routine fashion.
XII. Conclusions
There is mounting evidence that human AF is maintained by rotational and focal drivers, analogous to those long described in optical mapping of AF in animal models. Single center studies and now multicenter randomized ablation trials show that driver ablation has the potential to improve outcomes for persistent AF, with a favorable safety profile and ablation time. Notably, additional ablation of complex electrograms and lines may reduce the success of AF driver ablation. Translational and clinical studies are needed to explain these clinical results, and personalize mapping in individual patients and reconcile technical differences between approaches. It is important for next generation approaches to simplify the technique, including multipolar mapping and basket placement, AF map interpretation and ablation guidance.
Supplementary Material
Video 1. Preparation of the compliant basket, whose splines have rectangular cross section designed to not separate or spread significantly despite radial pressure. The basket is collapsed for insertion into the femoral vein.
Video 2. Rapid creation of right atrial geometry by insertion into the superior vena cava, pull down to the right atrium, then to the inferior vena cava. This process takes 1-2 minutes, and creates several hundred geometric points with voltage data. The basket is then used to map AF in 2-3 right atrial positions (64-pole basket, FIRMap, Abbott). (Courtesy of Abbott, Abbott Park, IL.)
Video 3. Rapid creation of left atrial geometry. The basket is first advanced to the left superior pulmonary vein, then the posterior wall, the right pulmonary veins then the anterior left atrium. This process takes 1-2 minutes, and creates several hundred geometric points with voltage data. The basket is then used to map AF in 2-3 left atrial positions (64-pole basket, FIRMap, Abbott). (Courtesy of Abbott., Abbott Park, IL.)
SYNOPSIS.
Drivers are increasingly studied ablation targets for atrial fibrillation (AF). However, results from ablation remain controversial. First, outcomes vary between centers and patients. Second, it is unclear how best to perform driver ablation. Third, there is a lack of practical guidance on how to optimally map and ablate AF drivers, particularly to identify critical from secondary sites using different AF mapping methods. This chapter addresses each of these issues.
KEY POINTS.
Rotational and focal sources are increasingly identified in persistent atrial fibrillation using a variety of mapping methods.
Ablation at these sites has both short term and long term efficacy in treating atrial fibrillation in meta-analyses and subgroups of recent randomized clinical trials.
Most of these sites lay remote to the pulmonary veins, even in the right atrium and may explain the limited success of pulmonary vein based ablation for treatment of persistent atrial fibrillation.
There are technical, logistical and theoretical considerations for successful mapping of rotational and focal drivers which are not part of traditional electrophysiology training.
Acknowledgments
DISCLOSURE STATEMENT
Dr. Narayan reports funding by NIH (R01 HL83359, K24 HL103800); intellectual property owned by the University of California Regents and Stanford University; consulting income from Abbott, the American College of Cardiology, Beyond Limits.ai, TDK Inc and UpToDate. Dr. Baykaner reports funding by American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Junaid Zaman, Stanford University, Stanford, California, USA, Imperial College London, UK.
Tina Baykaner, Department of Medicine/Cardiovascular Medicine, Stanford University, Stanford, California, USA.
Sanjiv Narayan, Department of Medicine/Cardiovascular Medicine, Stanford University, Stanford, California, USA.
XIII. References
- 1.Nattel S, Xiong F, Aguilar M. Demystifying rotors and their place in clinical translation of atrial fibrillation mechanisms. Nat Rev Cardiol 14. 2017;14:509–20. 14 [DOI] [PubMed] [Google Scholar]
- 2.Nattel S, Dobrev D. Controversies About Atrial Fibrillation Mechanisms: Aiming for Order in Chaos and Whether it Matters. Circ Res [Internet]. 2017. April 28 [cited 2019 Jun 9];120(9):1396–8. Available from: https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.116.310489 [DOI] [PubMed] [Google Scholar]
- 3.Pandit S V, Jalife J Rotors and the Dynamics of Cardiac Fibrillation. Circ Res [Internet]. 2013. February 28 [cited 2013 Mar 1];112(5):849–62. Available from: http://circres.ahajournals.org/cgi/doi/10.1161/CIRCRESAHA.111.300158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel W-J, Miller JM. Treatment of Atrial Fibrillation by the Ablation of Localized Sources. J Am Coll Cardiol [Internet]. American College of Cardiology Foundation; 2012. July [cited 2012 Jul 20];60(x):628–36. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0735109712021377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haissaguerre M, Hocini M, Denis A, Shah AJ, Komatsu Y, Yamashita S, et al. Driver domains in persistent atrial fibrillation. Circulation [Internet]. 2014. August 12 [cited 2014 Sep 26];130(7):530–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25028391 [DOI] [PubMed] [Google Scholar]
- 6.Daoud EG, Zeidan Z, Hummel JD, Weiss R, Houmsse M, Augostini R, et al. Identification of Repetitive Activation Patterns Using Novel Computational Analysis of Multielectrode Recordings During Atrial Fibrillation and Flutter in Humans. JACC Clin Electrophysiol [Internet]. JACC: Clinical Electrophysiology; 2017. March 1 [cited 2017 Nov 17];3(3):207–16. Available from: http://linkinghub.elsevier.com/retrieve/pii/S2405500X16302766 [DOI] [PubMed] [Google Scholar]
- 7.Grace A, Willems S, Meyer C, Verma A, Heck P, Zhu M, et al. High-resolution noncontact charge-density mapping of endocardial activation. JCI Insight [Internet]. 2019. March 21 [cited 2019 Jun 9];4(6). Available from: http://www.ncbi.nlm.nih.gov/pubmed/30895945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calvo D, Rubin J, Perez D, Moris C. Ablation of rotor domains effectively modulates dynamics of human long-standing persistent atrial fibrillation. Circ Arrhythmia Electrophysiol. 2017; [DOI] [PubMed] [Google Scholar]
- 9.Honarbakhsh S, Hunter RJ, Ullah W, Keating E, Finlay M, Schilling RJ. Ablation in Persistent Atrial Fibrillation Using Stochastic Trajectory Analysis of Ranked Signals (STAR) Mapping Method. JACC Clin Electrophysiol [Internet]. JACC: Clinical Electrophysiology; 2019. May 7 [cited 2019 Jun 9];910 Available from: https://linkinghub.elsevier.com/retrieve/pii/S2405500X19302968 [DOI] [PubMed] [Google Scholar]
- 10.Seitz J, Bars C, Théodore G, Beurtheret S, Lellouche N, Bremondy M, et al. AF Ablation Guided by Spatiotemporal Electrogram Dispersion Without Pulmonary Vein Isolation: A Wholly Patient-Tailored Approach. J Am Coll Cardiol [Internet]. 2017;69(3):303–21. Available from: http://myaccess.library.utoronto.ca/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=rzh&AN=120635135&site=ehost-live [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin CY, Lin YJ, Narayan SM, Baykaner T, Lo MT, Chung FP, et al. Comparison of phase mapping and electrogram-based driver mapping for catheter ablation in atrial fibrillation. PACE - Pacing Clin Electrophysiol [Internet]. 2019. December 10 [cited 2019 Jun 9];42(2):216–23. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/pace.13573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baykaner T, Rogers AJ, Meckler GL, Zaman J, Navara R, Rodrigo M, et al. Clinical Implications of Ablation of Drivers for Atrial Fibrillation. Circ Arrhythmia Electrophysiol [Internet]. Lippincott Williams & Wilkins Hagerstown, MD; 2018. May [cited 2019 Feb 12];11(5). Available from: https://www.ahajournals.org/doi/10.1161/CIRCEP.117.006119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramirez FD, Birnie DH, Nair GM, Szczotka A, Redpath CJ, Sadek MM, et al. Efficacy and safety of driver-guided catheter ablation for atrial fibrillation: A systematic review and meta-analysis. J Cardiovasc Electrophysiol [Internet]. 2017;(May):1–8. Available from: http://doi.wiley.com/10.1111/jce.13313 [DOI] [PubMed] [Google Scholar]
- 14.Brachmann Johannes, Hummel John D., Wilber David J., Sarver Anne E., Rapkin Joshua, Shlomo Shpun P, Szili-Torok T. S-LBCT01-02 Prospective Randomized Comparison Of Rotor Ablation Vs Conventional Ablation For Treatment Of Persistent Atrial Fibrillation - The REAFFIRM Trial [Internet]. Heart Rhythm. 2019. [cited 2019 Jun 9]. Available from: https://www.abstractsonline.com/pp8/#!/5753/presentation/31210 [Google Scholar]
- 15.Clarnette JA, Brooks AG, Mahajan R, Elliott AD, Twomey DJ, Pathak RK, et al. Outcomes of persistent and long-standing persistent atrial fibrillation ablation: a systematic review and meta-analysis. Europace [Internet]. 2018. November 1 [cited 2019 Jun 9];20(FI_3):f366–76. Available from: https://academic.oup.com/europace/article/20/FI_3/f366/4753706 [DOI] [PubMed] [Google Scholar]
- 16.Hansen BJ, Zhao J, Csepe T a., Moore BT, Li N, Jayne L a., et al. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur Heart J [Internet]. 2015;36(35):2390–401. Available from: http://eurheartj.oxfordjournals.org/cgi/doi/10.1093/eurheartj/ehv233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen BJ, Zhao J, Li N, Zolotarev A, Zakharkin S, Wang Y, et al. Human Atrial Fibrillation Drivers Resolved With Integrated Functional and Structural Imaging to Benefit Clinical Mapping. JACC Clin Electrophysiol [Internet]. JACC: Clinical Electrophysiology; 2018. December 1 [cited 2019 Jun 9];4(12):1501–15. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2405500X1830793X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuklik P, Zeemering S, Maesen B, Maessen J, Crijns HJ, Verheule S, et al. Reconstruction of Instantaneous Phase of Unipolar Atrial Contact Electrogram Using a Concept of Sinusoidal Recomposition and Hilbert Transform. IEEE Trans Biomed Eng 62. 2015;62(1):296–302. 62 [DOI] [PubMed] [Google Scholar]
- 19.Alhusseini M, Vidmar D, Meckler GL, Kowalewski CA, Shenasa F, Wang PJ, et al. Two Independent Mapping Techniques Identify Rotational Activity Patterns at Sites of Local Termination During Persistent Atrial Fibrillation. J Cardiovasc Electrophysiol 28. 2017;28(6):615–22. 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaman JAB, Rogers AJ, Narayan SM. Rotational Drivers in Atrial Fibrillation. Circ Arrhythmia Electrophysiol [Internet]. 2017. December [cited 2017 Dec 21];10(12):e006022 Available from: http://www.ncbi.nlm.nih.gov/pubmed/29254949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Y-J, Lo M-T, Chang S-L, Lo L, Hu Y, Chao T, et al. Benefits of Atrial Substrate Modification Guided by Electrogram Similarity and Phase Mapping Techniques to Eliminate Rotors and Focal Sources Versus Conventional Defragmentation in Persistent Atrial Fibrillation. JACC Clin Electrophysiol [Internet]. 2016;2(6):667–78. Available from: http://linkinghub.elsevier.com/retrieve/pii/S2405500X16302833 [DOI] [PubMed] [Google Scholar]
- 22.Narayan SM, Krummen DE, Clopton P, Shivkumar K, Miller JM. Direct Or Coincidental Elimination of Stable Rotors or Focal Sources May Explain Successful Atrial Fibrillation Ablation: On-Treatment Analysis of the CONFIRM (CONventional ablation for AF with or without Focal Impulse and Rotor Modulation) Trial. J Am Coll Cardiol [Internet]. 2013. April 3 [cited 2013 Jun 1];62(2):138–47. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23563126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spitzer SG, Károlyi L, Rämmler C, Scharfe F, Weinmann T, Zieschank M, et al. Treatment of Recurrent Non-Paroxysmal Atrial Fibrillation Using Focal Impulse and Rotor Mapping (FIRM) -Guided Rotor Ablation : Early Recurrence and Long-Term Outcomes Short title : FIRM ablation outcomes for recurrent non-paroxysmal AF. J Cardiovasc Electrophysiol. 2017;1–30. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigo M, Guillem MS, Climent AM, Pedrón-Torrecilla J, Liberos A, Millet J, et al. Body surface localization of left and right atrial high-frequency rotors in atrial fibrillation patients: A clinical-computational study. Heart Rhythm [Internet]. Elsevier; 2014. May 17 [cited 2014 Jul 15];11:1584–91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24846374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krummen DE, Bayer JD, Ho J, Ho G, Smetak MR, Clopton P, et al. Mechanisms for Human Atrial Fibrillation Initiation: Clinical and Computational Studies of Repolarization Restitution and Activation Latency. Circ Arrhythm Electrophysiol [Internet]. 2012. October 1 [cited 2012 Nov 12]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/23027797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Honarbakhsh S, Schilling RJ, Providência R, Dhillon G, Sawhney V, Martin CA, et al. Panoramic atrial mapping with basket catheters: A quantitative analysis to optimize practice, patient selection, and catheter choice. J Cardiovasc Electrophysiol [Internet]. 2017. September 26 [cited 2017 Nov 19]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/28862787 [DOI] [PubMed] [Google Scholar]
- 27.Narayan SM, Krummen DE, Rappel W-J. Clinical mapping approach to diagnose electrical rotors and focal impulse sources for human atrial fibrillation. J Cardiovasc Electrophysiol [Internet]. 2012. May [cited 2012 Aug 6];23(5):447–54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22537106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narayan SM, Krummen DE, Enyeart MW, Rappel W-J. Computational mapping identifies localized mechanisms for ablation of atrial fibrillation. PLoS One [Internet]. 2012. January [cited 2013 Jan 31];7(9):e46034 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3458823&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narayan SM, Bode F, Karasik P, Franz MR. Alternans of Atrial Action Potentials During Atrial Flutter as a Precursor to Atrial Fibrillation. Circulation [Internet]. 2002. September 23 [cited 2013 Mar 11];106(15):1968–73. Available from: http://circ.ahajournals.org/cgi/doi/10.1161/01.CIR.0000037062.35762.B4 [DOI] [PubMed] [Google Scholar]
- 30.Narayan SM, Franz MR, Clopton P, Pruvot EJ, Krummen DE. Repolarization alternans reveals vulnerability to human atrial fibrillation. Circulation [Internet]. 2011. June 28 [cited 2012 Aug 6];123(25):2922–30. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3135656&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lalani GG, Schricker A, Gibson M, Rostamian A, Krummen DE, Narayan SM. Atrial conduction slows immediately before the onset of human atrial fibrillation: a bi-atrial contact mapping study of transitions to atrial fibrillation. J Am Coll Cardiol [Internet]. Elsevier Inc.; 2012. February 7 [cited 2012 Aug 6];59(6):595–606. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3390156&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narayan SM, Krummen DE, Kahn AM, Karasik PL, Franz MR. Evaluating fluctuations in human atrial fibrillatory cycle length using monophasic action potentials. Pacing Clin Electrophysiol [Internet]. 2006. November [cited 2015 Oct 11];29(11):1209–18. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17100673 [DOI] [PubMed] [Google Scholar]
- 33.Narayan SM, Kazi D, Krummen DE, Rappel W-J. Repolarization and activation restitution near human pulmonary veins and atrial fibrillation initiation: a mechanism for the initiation of atrial fibrillation by premature beats. J Am Coll Cardiol [Internet]. 2008. October 7 [cited 2012 Jul 14];52(15):1222–30. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2604131&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narayan SM, Shivkumar K, Krummen DE, Miller JM, Rappel W-J. Panoramic Electrophysiological Mapping but not Electrogram Morphology Identifies Stable Sources for Human Atrial Fibrillation: Stable Atrial Fibrillation Rotors and Focal Sources Relate Poorly to Fractionated Electrograms. Circ Arrhythm Electrophysiol [Internet]. 2013. February 1 [cited 2013 Feb 28];6(1):58–67. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23392583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller JM, Kowal RC, Swarup V, Daubert JP, Daoud EG, Day JD, et al. Initial Independent Outcomes from Focal Impulse and Rotor Modulation Ablation for Atrial Fibrillation: Multicenter FIRM Registry. J Cardiovasc Electrophysiol [Internet]. 2014. June 19 [cited 2014 Jun 23];25:921–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24948520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baykaner T, Zografos TA, Zaman JAB, Pantos I, Alhusseini M, Navara R, et al. Spatial relationship of organized rotational and focal sources in human atrial fibrillation to autonomic ganglionated plexi. Int J Cardiol 240. 2017;240 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rappel W-J, Zaman JAB, Narayan SM. Mechanisms For the Termination of Atrial Fibrillation by Localized Ablation: Computational and Clinical Studies. Circ Arrhythmia Electrophysiol 8. 2015;8(6):1325–33. 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krummen DE, Baykaner T, Schricker AA, Kowalewski CAB, Swarup V, Miller JM, et al. Multicentre safety of adding Focal Impulse and Rotor Modulation (FIRM) to conventional ablation for atrial fibrillation. EP Eur [Internet]. 2017. May 1 [cited 2019 Jun 9];19(5):769–74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28339546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller JM, Kalra V, Das MK, Jain R, Garlie JB, Brewster JA, et al. Clinical Benefit of Ablating Localized Sources for Human Atrial Fibrillation. J Am Coll Cardiol [Internet]. Elsevier; 2017;69(10):1247–56. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0735109717301213 [DOI] [PubMed] [Google Scholar]
- 40.Knecht S, Sohal M, Deisenhofer I, Albenque JP, Arentz T, Neumann T, et al. Multicentre evaluation of non-invasive biatrial mapping for persistent atrial fibrillation ablation: The AFACART study. Europace [Internet]. 2017. August 1 [cited 2017 Dec 13];19(8):1302–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28204452 [DOI] [PubMed] [Google Scholar]
- 41.Rodrigo M, Climent AM, Liberos A, Fernandez-Aviles F, Berenfeld O, Atienza F, et al. Technical Considerations on Phase Mapping for Identification of Atrial Reentrant Activity in Direct- and Inverse-Computed Electrograms. Circ Arrhythm Electrophysiol [Internet]. 2017. September 8 [cited 2017 Nov 17];10(9):e005008 Available from: http://www.ncbi.nlm.nih.gov/pubmed/28887361 [DOI] [PubMed] [Google Scholar]
- 42.Honarbakhsh S, Schilling RJ, Dhillon G, Ullah W, Keating E, Providencia R, et al. A Novel Mapping System for Panoramic Mapping of the Left Atrium. JACC Clin Electrophysiol [Internet]. 2018. January [cited 2019 Jun 10];4(1):124–34. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2405500X17309490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Honarbakhsh S, Schilling RJ, Dhillon G, Ullah W, Keating E, Providencia R, et al. A Novel Mapping System for Panoramic Mapping of the Left Atrium. JACC Clin Electrophysiol [Internet]. JACC: Clinical Electrophysiology; 2017. November 15 [cited 2017 Dec 13];546 Available from: http://linkinghub.elsevier.com/retrieve/pii/S2405500X17309490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Honarbakhsh S, Schilling RJ, Providencia R, Keating E, Sporton S, Lowe M, et al. Automated detection of repetitive focal activations in persistent atrial fibrillation: Validation of a novel detection algorithm and application through panoramic and sequential mapping. J Cardiovasc Electrophysiol [Internet]. 2019. January [cited 2019 Jun 9];30(1):58–66. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30255666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atienza F, Almendral J, Ormaetxe JM, Moya Á, Mart½nez-Alday JD, Hernández-Madrid A, et al. Comparison of Radiofrequency Catheter Ablation of Drivers and Circumferential Pulmonary Vein Isolation in Atrial Fibrillation. J Am Coll Cardiol [Internet]. 2014. December [cited 2014 Dec 10];64(23):2455–67. Available from: http://www.sciencedirect.com/science/article/pii/S073510971406584X [DOI] [PubMed] [Google Scholar]
- 46.Verma A Utilizing Novel Dipole Density Capabilities to Objectively Visualize the Etiology of Rhythms in Atrial Fibrillation - UNCOVER-AF [Internet]. [cited 2019 Jun 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT02825992
- 47.Swerdlow M, Tamboli M, Alhusseini MI, Moosvi N, Rogers AJ, Leef G, et al. Comparing phase and electrographic flow mapping for persistent atrial fibrillation. Pacing Clin Electrophysiol [Internet]. 2019. May 24 [cited 2019 Jun 9];42(5):499–507. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30882924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bellmann B, Lin T, Ruppersberg P, Zettwitz M, Guttmann S, Tscholl V, et al. Identification of active atrial fibrillation sources and their discrimination from passive rotors using electrographical flow mapping. Clin Res Cardiol [Internet]. 2018. November 9 [cited 2019 Jun 9];107(11):1021–32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29744616 [DOI] [PubMed] [Google Scholar]
- 49.Gray R, Pertsov A, Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature [Internet]. 1998. [cited 2013 Mar 13];392(May):75–8. Available from: http://www.nature.com/nature/journal/v392/n6671/abs/392075a0.html [DOI] [PubMed] [Google Scholar]
- 50.Zlochiver S, Yamazaki M, Kalifa J, Berenfeld O. Rotor meandering contributes to irregularity in electrograms during atrial fibrillation. Heart Rhythm [Internet]. 2008. June [cited 2013 May 14];5(6):846–54. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3079377&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kowalewski CAB, Shenasa F, Rodrigo M, Clopton P, Meckler G, Alhusseini MI, et al. Interaction of Localized Drivers and Disorganized Activation in Persistent Atrial Fibrillation. Circ Arrhythmia Electrophysiol [Internet]. 2018. June 8 [cited 2018 Jun 11];11(6):e005846 Available from: http://www.ncbi.nlm.nih.gov/pubmed/29884620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buch E, Benharash P, Frank P, Share M, Tung R, Shivkumar K, et al. Quantitative Analysis of Localized Sources Identified by Focal Impulse and Roter Modulation Mapping in Atrial Fibrillation. Circ Arrhythmia Electrophysiol [Internet]. 2015;554–62. Available from: http://circep.ahajournals.org/cgi/doi/10.1161/CIRCEP.115.002721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gianni C, Mohanty S, Di Biase L, Metz T, Trivedi C, Gokoglan Y, et al. Acute and early outcomes of FIRM-guided rotors-only ablation in patients with non-paroxysmal atrial fibrillation. Hear Rhythm [Internet]. 2015. December 16 [cited 2016 Jan 10]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/26706193 [DOI] [PubMed] [Google Scholar]
- 54.Sommer P, Kircher S, Rolf S, John S, Arya A. Successful Repeat Catheter Ablation of Recurrent Longstanding Persistent Atrial Fibrillation with Rotor Elimination as the Procedural Endpoint: A Case Series. J Cardiovasc Electrophysiol 27. 2015;27(3):274–80. 27 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Preparation of the compliant basket, whose splines have rectangular cross section designed to not separate or spread significantly despite radial pressure. The basket is collapsed for insertion into the femoral vein.
Video 2. Rapid creation of right atrial geometry by insertion into the superior vena cava, pull down to the right atrium, then to the inferior vena cava. This process takes 1-2 minutes, and creates several hundred geometric points with voltage data. The basket is then used to map AF in 2-3 right atrial positions (64-pole basket, FIRMap, Abbott). (Courtesy of Abbott, Abbott Park, IL.)
Video 3. Rapid creation of left atrial geometry. The basket is first advanced to the left superior pulmonary vein, then the posterior wall, the right pulmonary veins then the anterior left atrium. This process takes 1-2 minutes, and creates several hundred geometric points with voltage data. The basket is then used to map AF in 2-3 left atrial positions (64-pole basket, FIRMap, Abbott). (Courtesy of Abbott., Abbott Park, IL.)