Abstract
The vulnerability of forest species and tree populations to climate change is related to the exposure of the ecosystem to extreme climatic conditions and to the adaptive capacity of the population to cope with those conditions. Adaptive capacity is a relatively under-researched topic within the forest science community and there is an urgent need to understand to what extent particular combinations of traits have been shaped by natural selection under climatic gradients, potentially resulting in adaptive multi-trait associations. Thus, our aim was to quantify genetic variation in several leaf and woody traits that may contribute to multi-trait associations in which intraspecific variation could represent a source for species adaptation to climate change. A multi-trait approach was performed using nine Quercus petraea provenances originating from different locations that cover most of the species’ distribution range over Europe and that were grown in a common garden. Multiple adaptive differences were observed between oak provenances but also some evolutionary stasis. Also, our results revealed higher genetic differentiation in traits related to phenology and growth than in those related to xylem anatomy, physiology and hydraulics for which no genetic differentiation was observed. The multiple associations between those traits and climate variables resulting from multivariate and path analyses suggest a multi-trait association largely involving phenological and growth traits for Quercus petraea.
Keywords: Climate change, adaptive capacity, phenology, embolism resistance, plant ecophysiology, plant functional traits
Introduction
Climate change and the associated increase in mean temperature and reduction in precipitation are expected to induce significant shifts in species’ distributions due to drought-induced population diebacks (Bertin 2008, Allen et al. 2010 201, Delzon et al. 2013, Allen 2014). This has raised important concerns not only about our ability to predict population mortality and its impact on ecosystem function (McDowell et al. 2013, Cailleret et al. 2017) but also about the capacity of species to adapt in a timely manner to the expected warmer and drier climates (Corlett and Westcott 2013, Sáenz-Romero et al. 2017, González-Muñoz et al. 2018). These concerns are especially relevant to forest species given, on the one hand, the rapid rate of environmental change and, on the other, the long life-span of most tree species (Aitken et al. 2008). Therefore, crucial questions for evaluating and predicting the consequences of ongoing climate change are i) which key traits can evolve within a few generations allowing the adaptation of trees to climate change?; and ii) how fast can such adaptation occur in response to a changing environment?
During the last decade, many studies have focused on addressing these questions by monitoring woody plants under controlled or induced environmental changes (Hoffmann and Sgrò 2011, Franks et al. 2014). As a result, different traits have been identified that respond phenotypically to temperature, thus making them potential targets for microevolution (e.g. Amano Tatsuya et al. 2010). Phenology is one of the most well–known sensitive indicators of climate change. In oaks, previous studies have shown genetic differentiation in bud phenology that has resulted in a shift of two to three days in spring and 0.3 to 1.6 days in autumn per decade over the last 50 years, extending the growing season (Vitasse et al. 2009). Although the benefits can vary across species, it has been shown that individuals that flower early produce flowers throughout the entire growing season, thus maximizing fitness compared with late-flowering individuals (Anderson et al. 2012). However, much less is known about the genetic determinism of leaf functional traits in oaks such as stomatal density, leaf size and leaf thickness, that have significant influence on net carbon gain but also on plant water balance. The genetic determinism of xylem and hydraulic traits that are related to the hydraulic failure of the plant water transport system due to embolism formation is also largely unknown in oak despite embolism formation being considered to be one of the main mechanism leading drought-induced plant mortality (Brodribb and Cochard 2009, Urli et al. 2015, Salmon et al. 2015, Choat et al. 2018). Understanding intra-specific variation in these traits would therefore help us to evaluate the capacity of tree species to face and adapt to new environmental conditions induced by ongoing climate change.
In this study, we investigated intraspecific variation of numerous traits related to leaf phenology and physiology with the aim to better understand the potential for adaptation of oak populations. Indeed, genetic variation could help to ensure survival of at least some individuals or populations during extreme events, buffering the population or the species against extinction (Meireles et al. 2017).
Some of these traits have previously been assessed in common garden experiments and exhibited clinal genetic variation along geographic gradients as a result of diversifying selection (Vitasse et al. 2009, Alberto et al. 2011). Thus, divergent intraspecific profiles for “intrinsic” water-use efficiency (Farquhar and Richards 1984) have been linked to the distribution of genotypes across gradients in air humidity and soil water availability for different species (Pennington et al. 1999, Cregg and Zhang 2001, Aletà i Soler et al. 2009). Also, a previous study in oaks carried out in a common garden with populations from different locations along an elevation gradient showed how genetic differentiation accounted for up to 28% of total variation in traits such as leaf mass area and nitrogen content for European oak and beech (Bresson et al. 2011). However, some studies reported no evidence of genetic differentiation for growth traits between populations along an aridity gradient (Deacon and Cavender-Bares 2015, Ramírez-Valiente et al. 2017). Environmental changes can also affect wood and water metabolism-related traits, such as wood density, vulnerability to embolism and water use efficiency (Mencuccini 2003, Schume et al. 2004, Brienen et al. 2011). In fact, genetic differences in both wood density and vulnerability to embolism have been reported for some species (Arnold et al. 2004, Sotelo Montes and Weber 2009, David-Schwartz et al. 2016) but not for others (Lamy et al. 2011).
In this contribution, we purposely implemented a multitrait approach by considering different functional traits related to either growth, phenology, structure (wood density), and physiology. Our aim was to investigate whether diversifying selection along climate gradients triggered specific associations of multiple traits whose genetic variation could represent a source for adaptation. Although previous studies have shown that some traits may follow clinal genetic variation along climatic gradients, their pattern of genetic variation has never been investigated collectively in a single and large common garden. We studied nine Quercus petraea L. provenances diverging from a common source population (from the last glacial period, 15000 years BP) and originating from different locations that cover most of the species’ distribution range over Europe and grown in a common garden. We focused on a paneuropean oak species: Quercus petraea (sessile oak). This species has been extensively monitored at the molecular level to document spatial and temporal differences across Europe, but to a much lesser extent at the phenotypic level, which was our main aim. Also, Quercus petraea is an ideal species for drawing adaptive inferences based on genetic divergence. Indeed, recent microevolutionary patterns of variation were shown to be less blurred by historical or demographic noise in oaks in comparison to other species, due to their very low level of subdivision on the basis of neutral markers (Firmat et al. 2017).
Material and Methods
Common-garden experiment
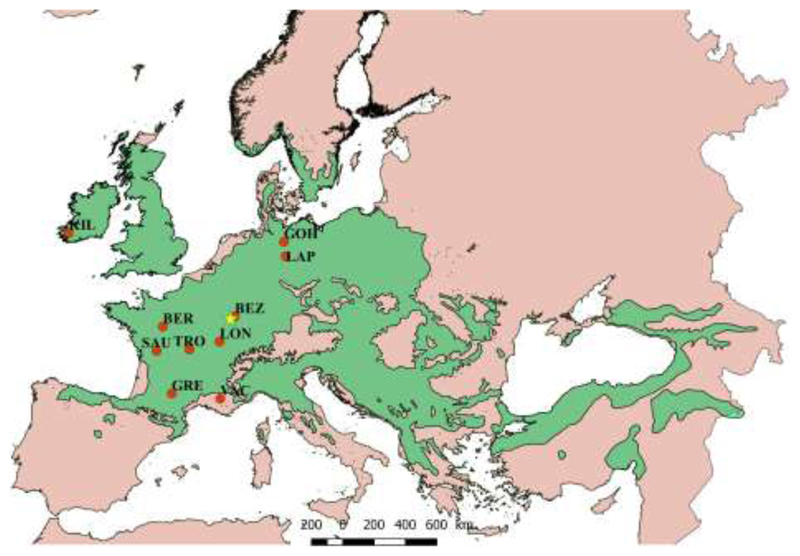
This study utilised a common garden experiment that was planted in 1989 and 1993 in the Forêt Domaniale de Sillégny (France) which contains 107 sessile oak provenances (Ducousso et al. 1996). From these, 9 provenances diverging from a common source population and representing different climatic regions within the distribution range of the species in Europe, from Northern Germany to Southern France, were selected for this study (Fig. 1 and Table 1, climate data source: Worldclim; period: 1960-1990). The initial density of the plantation was 1904 individuals per hectare (spacing 3 m × 1.75 m) with each provenance replicated from ten to fifteen plots with 24 trees per plot. At the time of the study, most of the trees were 25 year-old and 10 m tall on average (see details about plantation years in Table 1). Although sample size differed between the different traits studied because of varying complexity of measurements, all traits were evaluated in the exact same set of trees per provenance.
Fig. 1.
Distribution of Quercus petraea in Europe. Red dots indicate the origin of the provenances grown in the common garden located in Sillegny (yellow star) and used in this study (See Table 1 for climate information and abbreviation of each site).
Table 1.
Climatic data, location, altitude and aridity index of the studied Quercus petraea provenances
| Provenance | Country | Latitude(DD) | Longitude(DD) | Altitude(m) | Mean Temperature(C) | Precipitation(mm) | Aridity index | Common garde n plantation year |
|---|---|---|---|---|---|---|---|---|
| St Sauvant (SAU) | France | 46.38 | 0.12 | 155 | 11.35 | 838 | 0.91 | 1989 |
| Killarney (KIL) | Ireland | 52.01 | -9.50 | 50 | 10.14 | 1374 | 2.09 | 1990 |
| Grésigne (GRE) | France | 44.04 | 1.75 | 310 | 11.97 | 806 | 0.81 | 1990 |
| Bézange (BEZ) | France | 48.76 | 6.49 | 275 | 9.54 | 761 | 0.99 | 1993 |
| Bercé (BER) | France | 47.81 | 0.39 | 165 | 10.71 | 709 | 0.85 | 1993 |
| Longchamp (LON) | France | 47.26 | 5.31 | 235 | 10.85 | 793 | 0.96 | 1993 |
| Tronçais (TRO) | France | 46.68 | 2.83 | 245 | 10.55 | 752 | 0.86 | 1993 |
| Vachères (VAC) | France | 43.98 | 5.63 | 650 | 10.77 | 779 | 0.92 | 1993 |
| Göhrde (GOH) | Germany | 53.10 | 10.85 | 85 | 8.49 | 629 | 0.90 | 1993 |
| Lappwald (LAP) | Germany | 52.26 | 10.99 | 180 | 8.64 | 598 | 0.83 | 1993 |
Phenology and tree height
We monitored spring and fall phenology in the nine selected provenances in 2014. Leaf unfolding (LU) in spring was monitored every ten days in 25 individuals per provenance distributed in 7 to 12 replicated plots. Leaf senescence (LS) was monitored two times in late September and mid-October 2014 in the same individuals monitored for LU. Phenological observations were made using binoculars (magnifying power: 109) at a distance of approximately 10 m from each tree, by the same observer. In spring, we recorded the development stages from bud dormancy to leaf unfolding, using a scale with five intermediate stages according to Vitasse et al. (2009). We considered that a bud had reached leaf unfolding (LU) stage when at least one of its leaves was fully unfolded. At the tree level, LU date was determined when 50% of the buds had reached this threshold. In fall, due to the low number of field campaigns, we were not able to estimate a date of LS and therefore used the score of LS obtained during the October campaign (the September campaign was not discriminative enough). The senescence score corresponds in percentage to the amount of non-functional leaves, either coloured or fallen according to Vitasse et al. (2009). Height of all individuals was measured in January 2015.
Mean leaf area, specific leaf area and wood density
Mean leaf area (MLA, mm2) and specific leaf area (SLA, m2 kg-1) was determined for 22 to 28 trees per provenance randomly selected in 7 to 12 blocks per provenance and with at least 1 tree per block. Between 10 to 15 fully expanded and non-damaged leaves were collected per tree. To avoid any possible effects of different light exposure within the crown on leaf traits, all samples were collected from the upper and outer part of the crown to ensure a similar light exposure among them. All leaves were collected within 48 hours starting on June 14th, 2014. They were collected from a single branch per tree using a pole pruner or by shooting them down. Immediately after being collected, leaves were placed in sealed plastic bags to avoid desiccation, stored in cooling boxes and transported to the lab. Once in the lab, 6 to 8 leaves per tree were scanned to measure the area of each individual leaf using a desktop scanner (Expression 10000 XL, Epson, Japan) and WinFolia software (Regent Instruments Inc., Quebec, Canada). For determining the SLA, the dry mass of leaves was measured after drying them in an oven at 65°C until a constant mass was reached. The SLA was assessed as the ratio of the leaf area to its mass. Individual leaf data were later averaged over all leaves per tree.
Wood density was estimated using X-ray imagery (Polge 1966) on a section of dry branch. One ca. 10-cm long branch segment per individual was collected from 4 to 16 individuals per provenance. As for leaves, to avoid any possible effects of different light exposure within the crown on wood density, all branches were collected from the upper and outer part of the crown to ensure similar light exposure conditions among them. All branches had the same age (two years-old) within and between provenances. For each sample, we used a double-bladed saw to cut a transverse section with a constant thickness of 2 mm. Wood density was measured on the transverse section by using an x-ray image calibration procedure. Sections were exposed to X rays and were then scanned with a microdensitometer. Images were analysed using Windendro (Guay et al. 1992) to obtain two radial density profiles per section. Ring limits were determined automatically, checked manually, and then corrected with this software. We then calculated mean wood density (D, g cm-3).
Stable C and N isotopes analysis
After MLA and SLA measurements, the same leaves were used for determining the carbon and nitrogen content (C and N g/kg, respectively) and isotopic discrimination (Δ13C and Δ15N for C and N, respectively). The dry samples were ground to powder using a wood grinding sample system (Labman, Stokesley, North Yorkshire, UK) and put into a tin capsule for mass spectrometry. The C and N isotope ratios as well as C and N contents were measured on 3 mg samples at the Microbiology & Agronomics Platform at INRA Reims (France) by using an isotope ratio mass spectrometer (Delta Advantage, Thermo Scientific, Bremen Germany). The carbon isotopic composition expressed as δ13C in ‰ (Craig 1957) was then converted into carbon discrimination Δ13C in ‰ (Farquhar and Richards 1984). The Δ13C values were corrected for the Suess Effect (decrease in δ13C of atmospheric CO2 since the beginning of industrialization) resulting from the emission of fossil carbon dioxide, which is depleted in 13C (Francey et al. 1999, McCarroll and Loader 2004) even if the sampling has been done at the same date. Δ13C can be related to the ratio of CO2 assimilation (A) to stomatal conductance (gs), also named the intrinsic water-use efficiency.
Leaf vein and stomatal density
Vein density (total vein length per mm2 of leaf area) was determined from paradermal sections of five fresh leaves (one leaf per tree, five trees per provenance) similar to those used for MLA and SLA. Sections were prepared and measured following the protocols described by Carins Murphy et al. (2012). In brief, this involved removing the adaxial epidermis and palisade tissue, clearing all pigment with bleach, and measuring VD from slide mounts of the sections using image analysis of digital photomicrographs (5 fields of view per section). Stomatal density (total stomata per mm2 of leaf area) was also determined from cuticles (1 per leaf and 5 fields of view per cuticle) prepared and measured following the protocols of Carins Murphy et al. (2012).
Vulnerability to embolism
Due to the complexity of these measurements, vulnerability to xylem embolism was determined in three out of the 9 selected provenances and one extra provenance (originally from Ireland) for which the aridity of the provenance origin was much lower (Table 1). For each provenance, 15 individuals randomly selected in 7 to 9 blocks per provenance and with at least 1 tree per block were evaluated in June/July 2015. These four provenances were selected according to their aridity index (AI) which was calculated as:
where MAP and MAE represent the mean annual precipitation and mean annual potential evapotranspiration, respectively. The four provenances selected were Grésigne (Southeastern France), Killarney (Southern Ireland), Vachères (Southwestern France) and Göhrde (Northern Germany), and represent different climatic regions, ranging from a dry Mediterranean region in France to a continental temperate climate in Germany (plus the additional provenance from a cool and humid oceanic climate in Ireland).
Xylem vulnerability to embolism was measured using the Cavitron technique (Cochard, 2002; Cochard, 2005) at the Caviplace laboratory (GENOBOIS platform, INRA-University of Bordeaux, France). To prevent artefactual losses in hydraulic conductance due to the induction of embolism during the sample preparation (Torres-Ruiz et al. 2015) or the presence of open vessels in the samples (Torres-Ruiz, Cochard, Choat, et al. 2017), >2 m-long branches were collected from the trees (one branch per tree), wrapped in moist paper and plastic bags to kill transpiration and transported to the laboratory. Once in the lab, branches were progressively recut under water to release the xylem tension according to Torres-Ruiz et al. (2015) and to adjust them to a 1 m-long length. Branches were debarked at both ends and installed in a large cavitron equipped with a 1 m-diameter custom-built honeycomb rotor (DGMeca, Gradignan, France; (Lobo et al. 2018). Several branches were used to test the presence of open vessels by air injection at 2 bars and none of them presented open vessels in 1 m long branches. Samples were spun for three minutes at a given speed to decrease the xylem pressure progressively at its centre from -0.8 MPa to -10.5 MPa (those pressures correspond to centrifugation rotation from 764 rpm to 2768 rpm respectively). Vulnerability curves to embolism were generated by plotting the percentage loss of hydraulic conductivity (PLC) at the different target pressures applied and fitting a sigmoidal equation (Pammenter and Van der Willigen 1998). Mean P50 values, i.e. the xylem pressure inducing 50% of PLC, were obtained by averaging the values of 13 to 15 samples per provenance. The P50 value is commonly used as a proxy for tree drought resistance: the lower the P50 value, the more drought tolerant the species (Delzon 2015, Torres-Ruiz, Cochard, Fonseca, et al. 2017).
Statistical analyses
Differences in phenological, physiological, anatomical and hydraulic traits among the nine provenances of Q. petraea were tested with a generalized linear mixed model (MIXED procedure, restricted maximum likelihood (REML) method in SAS, version 9.4, SAS Institute, NC, USA) where plots and provenances were respectively treated as fixed and random factors. The provenance effect was further assessed using a log likelihood ratio test from the full and reduced models (Littell et al. 2007). The data were checked to satisfy the assumptions of normality and homogeneous variance prior to analyses. The ratio of the provenance variance component to total variance was estimated according to Vitasse et al. (2009) using the VARCOMP procedure with the restricted maximum likelihood (REML). These analyses used the following statistical model: Yijk = μ + Pi + bj + (Pb)ij + εijk, with Yijk being the observed trait of the seedling k from the provenance i and block j, μ the overall mean of the analysed characters, Pi the random effect of provenance i, bj the fixed effect of block (here replicated plot) j, (Pb)ij the interaction between provenance i and block j, and εijk the residual variation including the effect of tree k belonging to combination ijk. Variances of random effects (provenance σP2, interaction σbP2, residual σε2) were also computed. The overall differentiation among provenances (D) was calculated as the ratio (%) of the variance component of provenance to total variance estimated by analysis of variance, i.e as σP2 / (σP2 + σbP2 + σε2), and varied from 0 to 100. D is an analog of Qst (Spitze 1993), which is the genetic differentiation of quantitative traits (Qst = σP2 / (σP2 + 2σA2), where σA2 is the within provenance additive variance. In our study based on provenance and not descendant test, σA2 could not be estimated and we thus used the overall within population phenotypic variance (i.e. D) rather than the overall genetic variance as it is for Qst (Vitasse et al. 2009, Bresson et al. 2011).
The variability of each trait was evaluated by estimating both the intra- and inter-provenance coefficient of variation. The CVintra was calculated for each provenance and then averaged at the species level, while the CVinter was obtained from the between-provenance standard deviation and the overall mean value.
Weighted linear regression analyses were used to assess (i) relationships between the functional traits that showed significant differences among provenances, and (ii) whether genetic variation was explained by the local environmental conditions (i.e. latitude, temperature and precipitation) of the provenances’ origin. Also, relationships between phenological and functional traits with climate variables were investigated using principal component analysis (PCA) in order to identify patterns of interrelationships. Finally, a path analysis was carried out to test different conceptual models that could explain how the climate at the original location of each provenance determines the variance of the different functional traits. Those models were based on different hypotheses considering only the traits that were the most influenced by temperature and precipitation according to the PCA analyses (Supplementary information Fig. S3). The best model based on the Akaike information criterion (AIC) would therefore provide valuable information about the associations of climate and multitrait values.
Results
Genetic differentiation
Significant genetic differentiation between provenances was observed in four out of the 13 studied traits (Table 2). The provenances differ in both the timing of leaf spring and autumn phenology (leaf unfolding and senescence, respectively). Tree height (H) and SLA were also significantly different between provenances, whereas a lack of genetic differentiation was found for MLA, Δ 13C, Δ 15N, leaf C and N content, stomatal density, P50, vein density and wood density. Overall genetic differentiation for the different traits evaluated ranged from 0 up to 79. Thus, SLA and leaf unfolding amounted the highest values with 79 and 62, respectively, whereas it was weaker for, leaf senescence (32) and MLA (5). A null (0.0) overall differentiation was observed for all the other traits (Table 2). Most traits showed, to a greater or a lesser extent, higher coefficient of variation across provenances than within provenances (Table 2). Only leaf vein density showed a slightly higher variation within (9) than across provenances (8).
Table 2.
Genetic differentiation (D) among provenances, coefficient of variation (%) intra- and inter-provenances and log likelihood ratios (LLR) for the different phenological and functional traits. LLR are given as provenance was treated in the GLMM as a random factor. LU = leaf unfolding; P50 = xylem pressure inducing 50% of loss in stem conductivity. LS = leaf senescence; SLA = Specific leaf area; MLA = Mean leaf area; D (%) is the ratio of the variance component of provenance to total variance estimated by analysis of variance (σP2 / (σP2 + σbP2 + σε2)). * P < 0.05, ** P < 0.01, *** P < 0.001
| Trait | df | LLR | D | CVintra | CVinter |
|---|---|---|---|---|---|
| Phenology | |||||
| LU | 8 | 45.30*** | 62 | 4 | 6 |
| LS | 8 | 18.90*** | 32 | 57 | 64 |
| Growth | |||||
| Height | 8 | 7.20** | 0 | 9 | 10 |
| Physiology | |||||
| SLA | 8 | 5.80* | 79 | 15 | 16 |
| MLA | 8 | 0.60 | 5 | 22 | 23 |
| Δ13C | 8 | 0.00 | 0 | 4 | 4 |
| Δ15N | 8 | 0.00 | 0 | 11 | 13 |
| C content | 8 | 0.00 | 0 | 5 | 5 |
| N content | 8 | 0.00 | 0 | 10 | 11 |
| Stomatal density | 8 | 0.00 | 0 | 16 | 16 |
| Hydraulics | |||||
| P50 | 3 | 0.00 | 0 | 9 | 10 |
| Anatomy | |||||
| Vein density | 8 | 0.00 | 0 | 9 | 8 |
| Wood density | 7 | 0.70 | 0 | 14 | 14 |
Genetic clines
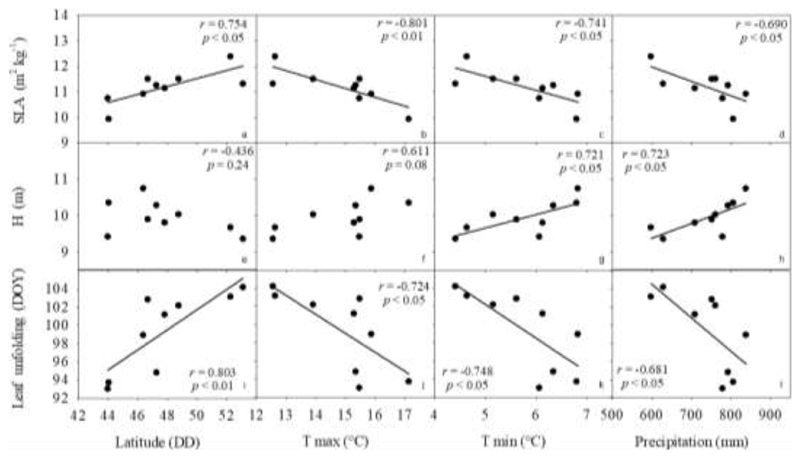
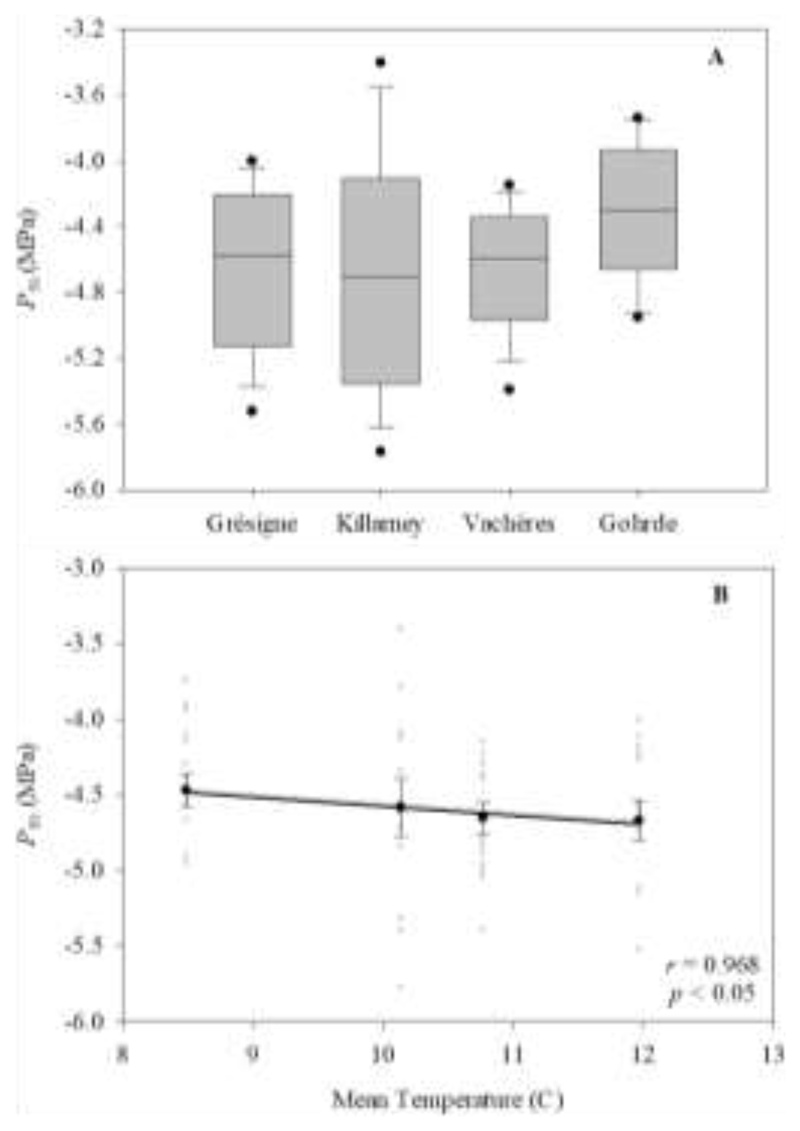
Correlations between traits and the climate and latitude of provenance origin reveal some relevant trends (Fig 2). Thus, positive and negative trends were observed between leaf unfolding and both latitude and temperature of the provenance origin, respectively. Thus, the earliest provenance to begin leaf flushing was the one originating from the southern margin (Latitude 43.98°) whereas the latest was one from northern Germany (Latitude 53.18°). Both the mean annual maximum and minimum temperatures correlate with the date of leaf unfolding, with earlier budburst in provenances from warmer areas. Also, late leaf unfolding was observed in areas with lower precipitation. Thus, for the Grésigne provenance with a mean annual precipitation of 806 mm leaf unfolding occurred in DOY (day of year) 93.7, whereas for Göhrde with a precipitation of 629 mm/year it occurred in DOY 104.2, i.e 10.5 days later than for Grésigne. Concerning leaf shedding, while significant genetic differentiation was observed between provenances (Table 2) no significant cline with the climate of provenances was detected (Supplementary information Table S1). A similar pattern as in LU was observed for SLA, with higher values in provenances originating from higher latitudes and colder and drier areas. Thus, mean SLA ranged from 9.95 to 12.39 m2 kg-1 within a range in temperature of 17.14 to 4.42°C and in total annual precipitation of 838 to 598mm. Tree height, however, was not significantly affected by latitude or maximum temperature, but significantly increased with increasing minimum temperature and precipitation. Thus, trees are taller (up to 10.74 m) in provenances with higher minimum temperatures (6.8°C) and annual precipitation (838 mm) (Fig. 2). Interestingly, xylem resistance to embolism showed similar P50 values across the four provenances evaluated (i.e. no genetic differentiation, Fig. 3A), but a significant correlation with mean annual temperature (MAT), showing increased resistance to embolism with higher MAT (Fig. 3B). Surprisingly, aridity index did not correlate with any of the evaluated traits (Supplementary information Table S1).
Fig. 2.
Specific leaf area (SLA), mean tree height (H) and leaf unfolding of the nine provenances studied in the common-garden at Sillegny versus latitude, mean annual maximum and minimum temperature (Tmax and Tmin, respectively) and precipitation. r indicates Pearson product-moment correlation; p indicates significance levels of the p values of the slope.
Fig. 3.
A) Box plot of P50 (i.e. pressure at which 50% loss of stem conductivity occurs) for each of the four provenances in which resistance to embolism was evaluated. From 13 to 15 different branches from different trees were used for each provenance. No significant differences between provenances were observed in P50 (p = 0.21). B) Significant correlation between P50 and mean temperature. A linear regression was fitted to the whole dataset (r, Pearson product-moment correlation; p, significance levels of the p values of the slope). The error bars represent ± the standard error.
Relationships between phenological and functional traits, and climate variables
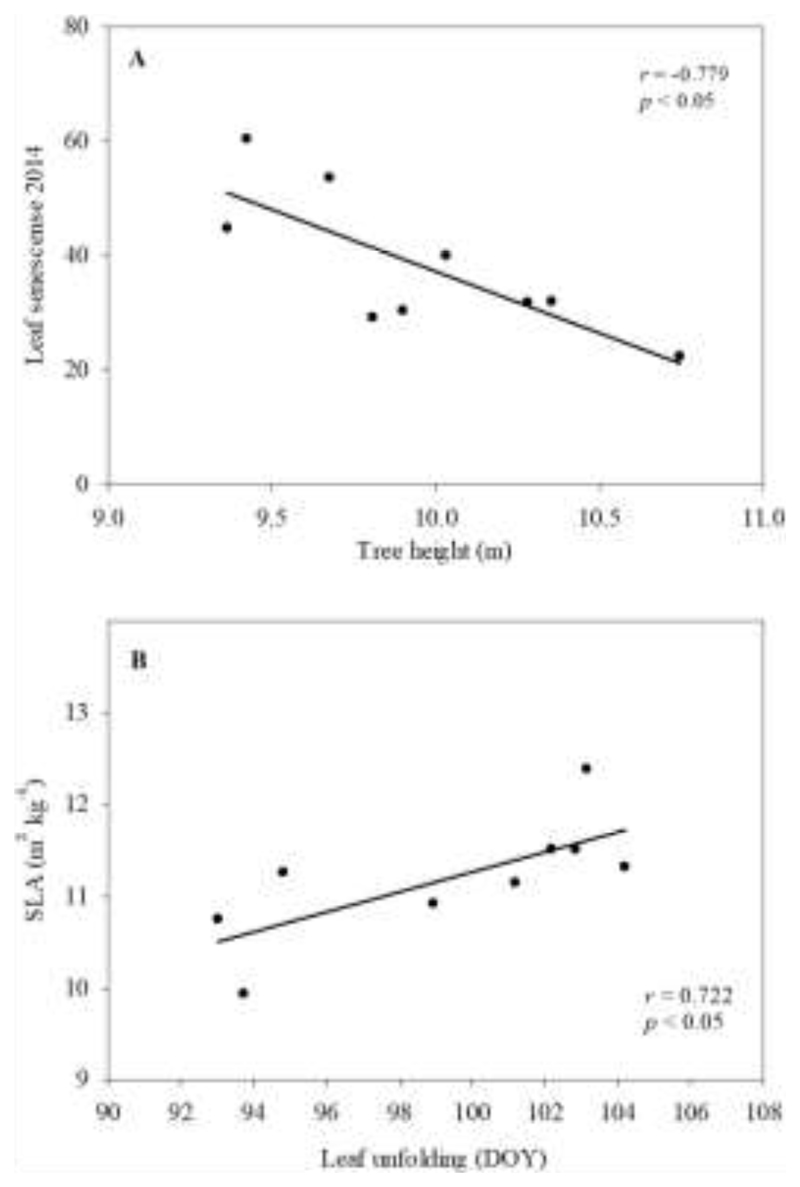
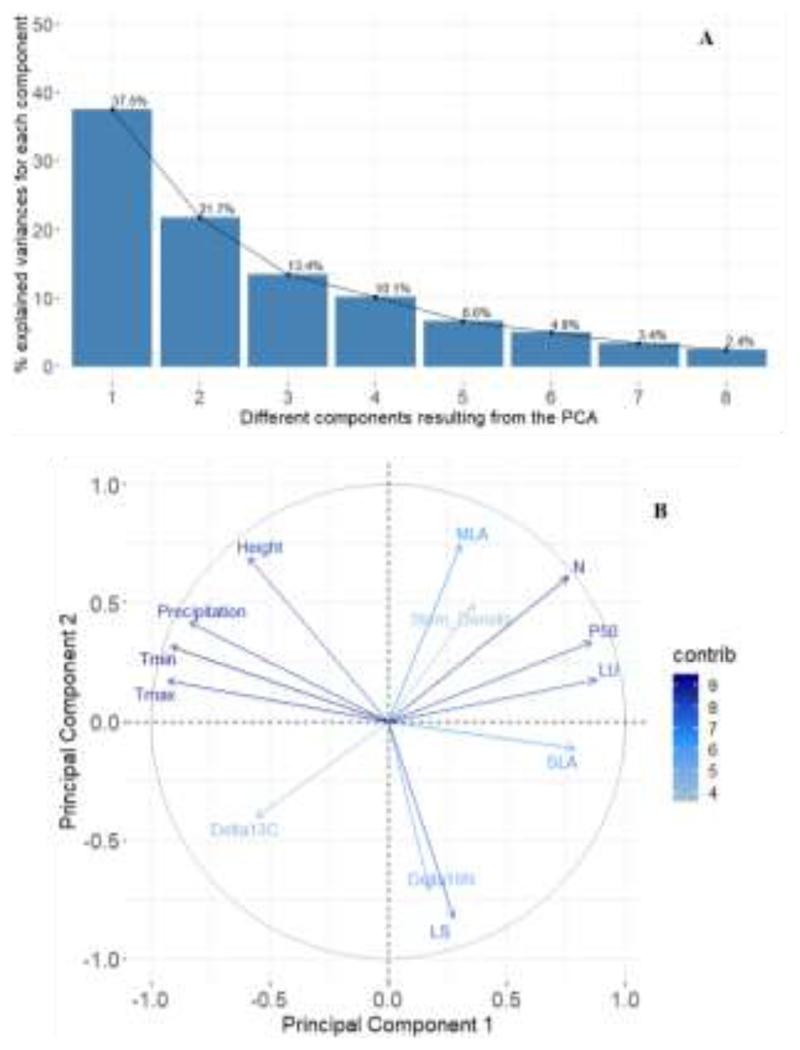
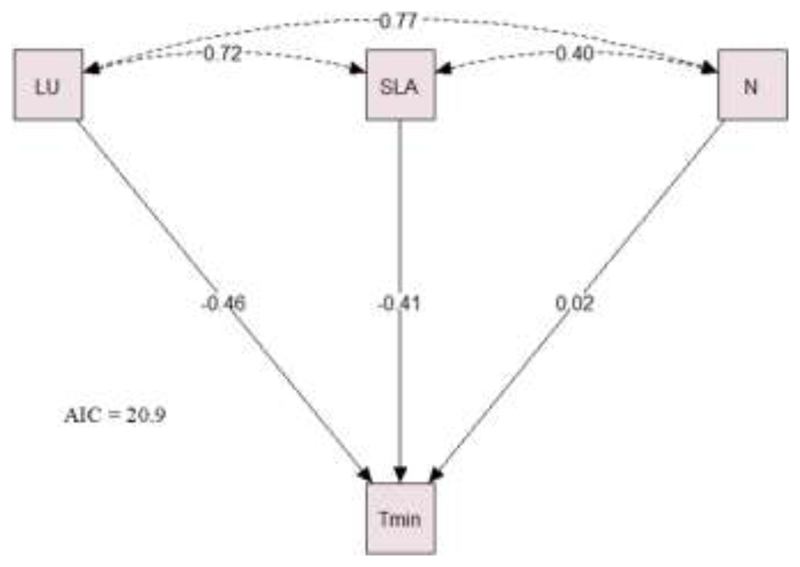
Only a few significant correlations were observed between those traits exhibiting a provenance effect (Table 3). Thus, tight correlations were observed between LS and H (Fig. 4A) and between SLA and leaf unfolding (Fig. 4B). Thus, lower SLA was observed in those provenances with an earlier flushing, with Grésigne having the lowest SLA value (9.9 m2 kg-1) and flushing in DOY= 94. On the contrary, the highest SLA values was observed for Lappwald (12.4 m2 kg-1) that flushed in DOY= 103. The correlation between LS and H was observed (Fig.4A) reporting a lower percentage of coloured leaves on in taller trees. The PCA showed how the first two principal components together explained 59.2% of the variance (Fig. 5A). Thus, Axis 1 (variance 37.5%) was strongly associated with the main climate variables, i.e. maximum and minimum temperature and precipitation, as well as with functional leaf traits leaf unfolding, P50, SLA and N content (Fig. 5B). Axis 2 (variance 21.7%), on the contrary, was more defined by the variance in leaf senescence, MLA, Δ15N and tree height than by climate variables (Supplementary information Fig. S2). When running the path analyses to detect associations between the main climate variables and the functional traits we found that the minimum temperature at the provenance origin seemed to be the most relevant trait determining their leaf unfolding, SLA and N content values (Fig. 6).
Table 3.
Statistics for the Pearson correlation at the individual level to each pair of traits exhibiting a provenance effect. Values in each cell indicate the Pearson correlation coefficient. Asterisk (*) indicates a significant correlation between two traits (p<0.01).
| Tree height | Leaf unfolding | Leaf senescence | |
|---|---|---|---|
| SLA | -0.079 | 0.226(*) | 0.079 |
| Tree height | -0.094 | -0.448(*) | |
| Leaf unfolding | 0.001 | ||
Fig. 4.
Significant correlations between pairs of phenological and ecophysiological traits. Each point represents the mean value of 22 to 28 trees per provenance. Linear regressions were fitted for the whole dataset (r, Pearson product-moment correlation; p, significance levels of the p values of the slope).
Fig. 5.
A) Percentage of variances explained by the different axes (components) resulting from the principal component analysis (PCA). B) Variables factor map on the first two axes of the PCA performed on the different climate variables and functional traits. For facilitating its evaluation, only those traits with a higher contribution to component 1 and 2 (cos2>0.25) are shown. The variables factor map including all traits is included in the supplementary information of the article (Fig. S1).
Fig. 6.
Path diagram of the model that best describes (lower Akaike information criterion (AIC)) the relationship between climate variables and functional traits. Solid and dashed lines indicate direct or indirect effects, respectively, between variables and traits. Values in the arrows indicate the standardized coefficients (std.all), and refer to standardized estimates of the variances of both continuous observed and latent variables. LU: Leaf unfolding; SLA: Specific Leaf Area; N: Leaf Nitrogen content; Tmin: minimum temperature.
Discussion
Nine provenances of Q. petraea originating from an extensive portion of the species’ overall distribution in Europe showed significant genetic differentiation in traits related to leaf phenology, morphology and growth but not in traits related to xylem anatomy and hydraulics when grown in a common-garden. Phenology and some functional traits such as P50 also showed significant clines with the latitude and climate of provenance origin. Results from PCA and path analyses reported multiple associations between climate variables and both phenology and functional traits that would be driven by a diversifying selection along the studied climatic gradient.
Multi-trait associations
The effect of climate change on phenological traits has been extensively studied (Peñuelas et al. 2002, Gordo and Sanz 2005), especially the effect of temperature on the timing of leaf unfolding and flowering, which both occur earlier as temperature rises (Doi and Katano 2008, Chung et al. 2013). However, unlike this study, many previous studies have been carried out ‘in situ’, and thus do not allow for any attribution of trait variation to either plasticity or genetic differentiation. Our results from trees grown in a common garden showed a significant cline between leaf unfolding and the climate of provenance origin, with earlier bud burst in provenances from the warm margin. Therefore, local adaptation of phenology to the environmental conditions is observed for Q. petraea. Similar results have been already reported for different tree species including oaks (Menzel and Fabian 1999, Matsumoto et al. 2003, Vitasse et al. 2009), showing the important role of local temperature in driving adaptation of the growing season length. This may partially explain the significant differences in growth (i.e. tree height) observed between provenances although a more complete understanding of the role of the vegetative growth period length and assimilation rates on the final growth of the populations would require a more focused study. In fact, our results also agree with a previous study by Kuster et al. (2014) in which three different oak species were grown on two different soils and exposed to air warming and drought. Their results showed earlier leaf unfolding for trees exposed to higher air temperatures, leading to an earlier start of shoot growth, in that they estimated an advance in leaf unfolding of 1-3 days °C−1. Our results also agree with those reported by (Vitasse et al. 2009) who observed negative genetic clines in leaf unfolding with increasing temperature at the origin of the provenance for Quercus petraea. More recent studies (Alberto et al. 2011, Firmat et al. 2017) reported a similar genetic cline for both germination and bud burst timing with provenance elevation in Q. petraea, showing that spring leaf phenological traits are critical for local adaptation in oaks. Those genetic variations in leaf phenology observed in oaks are probably due to differences in heat requirement for bud burst rather than differences in chilling-associated with leaf shedding (Dantec et al. 2015).
In contrast with other studies (Vitasse et al. 2009), a clinal trend with temperature was not observed for leaf senescence in our study despite the significant variation in this trait among provenances and despite showing the highest between-provenance variation value. This is confirmed by the results from the PCA showing that, contrary to leaf unfolding, the variation in leaf senescence is barely determined by the temperature and precipitation regimes of the provenances’ origin.
Significant genetic differentiation in SLA, a trait directly related to light conditions and nutrient availability (Milla et al. 2008), was observed between provenances, indicating genetic variation among provenances induced by the climate at their original location. Similar to “in situ” observations from Bresson et al. (2011) for Quercus petraea, we observed a negative correlation between SLA and both temperature and precipitation. In fact, results from the PCA show exactly this: a high influence of the minimum and maximum temperatures and precipitation not only on SLA, but also on other traits such as P50 and leaf unfolding. Intraspecific variation in SLA with air temperature and precipitation have been already reported for other species including maize and wheat for which, under N fertilization and irrigation controlled conditions, responses to both climate variables explained 43.7 % of the variation in SLA (Martin et al. 2018). Earlier leaf unfolding would increase leaf lifespan which has been reported to be correlated with SLA. Thus, across species, Reich et al. (1991) showed that species with short leaf lifespan generally have thinner leaves (high SLA) than those with longer leaf lifespan, which agrees with our results within species. As the authors suggested, the correlation between leaf lifespan, and therefore leaf unfolding, and SLA would result from different allocation trade-offs between species to enhance productivity, nutrient conservation or defence. This could also be the case at the intraspecific level and may explain observed differences between provenances. The relevance of the adaptation of SLA to climate resides in the fact that it may contribute to a species’ ability to adjust to different air temperatures and precipitation regimes (Albert et al. 2010, Long et al. 2011), because SLA is a key plant functional trait reflecting the trade-off between resource capture and conservation (Wright et al. 2004). Interestingly, no genetic differences were found in leaf size between provenances. This finding links with a recent study evaluating the bivariate leaf size-climate relationships for 7670 plant species from 682 sites worldwide that shows how day and night time leaf-to-air temperature differences are key to explaining the latitudinal gradient in leaf size (Wright et al. 2017). So, considering the results from Wright et al. (2017) and our results, latitudinal changes in leaf size would be likely due to phenotypic plasticity rather than to genetic variation.
Lack of evidence for local adaptation
Leaf vein and stomatal density are directly linked with plant transpiration (Brodribb et al. 2007, Franks and Beerling 2009) and also highly influenced by environmental factors (Woodward and Bazzaz 1988, Uhl and Mosbrugger 1999), with higher stomatal and venation densities in drier areas (Herbig and Kull 1992, Carlson et al. 2016). Furthermore, both traits, tend to remain proportional during leaf acclimation to light intensity and VPD in woody angiosperm species (Brodribb and Jordan 2011, Carins Murphy et al. 2012, 2014, 2016). However, the determinism of these traits has received limited attention so far (Zhu et al. 2012). Assuming these traits varied between provenances in situ, which is likely given the capacity for acclimation in vein density to variation in evaporative demand among the upper and lower canopy in the closely related Quercus rubra (Zwieniecki et al. 2004), our results suggest that leaf capacity to acclimate to different conditions via changes to these anatomical traits is not translated into genetic differences. In fact, there is no evidence for genetically driven effects on stomatal density in Quercus petraea L. and Fagus sylvatica, although the phenotypic effect of temperature is large (Bresson et al. 2011). However, contrary to this, vein density was found to be genotypically fixed in Quercus variabilis (Zhu et al. 2012). A possible explanation for our observation that these traits that tend to show large phenotypic variation appear to have not responded to divergent selection here is that the populations sampled could have not spanned all the variation within the distribution area of this species. However, an efficient phenotypic plasticity response could also have had an important role in the lack of variation observed for these traits since it would lead to convergence among populations in trait expression when grown in a common garden. An alternative explanation is that other traits not measured in this study but that also contribute to leaf water supply and demand varied among provenances. For example, stomatal size, along with stomatal density, determines stomatal conductance to water vapour. Likewise, the distance between vein tips and stomata, along with the horizontal spacing of veins, determines the length of the post-vein pathway from the end of the vascular system to the sites of evaporation in the leaf which itself is correlated with leaf hydraulic conductance (Brodribb et al. 2007). In this study differences were found in SLA but not in leaf size implying that leaf thickness varied among provenances. This may have altered the post-vein pathlength for water. However, the distance between veins tends to equal the distance between veins and the evaporative surface in derived angiosperms (Zwieniecki and Boyce 2014). More detailed study is therefore required to determine whether the lack of variation in vein and stomatal density among provenances is due to strong plasticity or genetic changes in other leaf traits.
In many cases, traits exhibiting plasticity also show genetic differentiation (Kremer et al. 2013), unless their heritability is extremely low. Therefore, an interesting follow up of this study would be to explore whether there is enough genetic diversity within populations on which diversifying selection may act since it may be very important for the species potential to adapt to future changes in climate.
Increases in shoot growth and leaf area allocation due to higher temperatures result in decreases in plant N concentration and leaf N content (Weih and Karlsson 2002). This explains the negative correlation reported by the PCA between leaf N content and temperature and precipitation for the different provenances. In fact, from all the models tested to identify which climate variable determines the variance in the functional traits most influenced by climate, the one that fits best with our results suggests that minimum temperature is the main climate variable determining leaf N content, SLA and leaf unfolding for each provenance.
Water Use Efficiency (WUE), which corresponds to the ratio of biomass produced to the rate of transpiration can be estimated by measuring the carbon isotope discrimination. While previous studies in oak showed intraspecific genetic variations in Δ13C in Quercus (Q. robur and Q. petraea, Ponton et al. 2002) (Q. robur and Q. pyrenaica,Granda et al. 2018) in Pinus pinaster (Lamy et al. 2014) and in Populus nigra (Guet et al. 2015), our results showed no significant genetic differentiation between provenances in Quercus petraea. The lack of genetic differentiation between populations might be explained by the advantage of increased efficiency of water use in conserving soil moisture, which could be more predominant in Mediterranean and semiarid climates. Our results however agree with those reported by Chamaillard et al. (2011) showing no differences in leaf C content among three populations of Populus nigra grown in a common garden, even when they were subjected to different water treatments. Whether our target species, Quercus petraea, is less prone to the occurrence of genetic differences for given physiological traits than other species is an emerging question that needs to be addressed in future studies.
Resistance to embolism varies across plants species and helps to explain species distributions across climatic gradients (i.e. water scarcity; Choat et al. 2012). However, low or no variability in resistance to embolism are usually reported within species (Lamy et al. 2014, González-Muñoz et al. 2018). Lamy et al. (2011) showed a strong uniform selection or canalisation for this trait shaped the lack of genetic variation. There are some studies showing a significant correlation between P50 and climate variables (Brodribb et al. 2014, Larter et al. 2017), especially temperature (Kavanagh et al. 1999), although this is not a common pattern in either ‘in situ’ studies or common garden experiments (Martínez-Vilalta et al. 2009, Lamy et al. 2014). Recently, Stojnić et al. (2018) attributed lack of variability to an ascertainment bias, as most populations in reported studies originate from the core of the species distribution. Hence, significant differences are observed when marginal populations growing in areas subject to unsuitable conditions for the species are considered. In our study, despite the contrasting climate at the original location of the four provenances that would induce differences in potential evapotranspiration (PET, no data available), xylem resistance to embolism showed similar P50 values. Therefore, contrary to Cavender-Bares (2018) our results do not evidence a role of evolvability for hydraulic traits that could have allowed adaptation to changing environments. The minor differences observed however, correlate with mean temperature, with more resistant individuals originating from the warm margin. This could affect the performance of individuals from the different populations although only slightly. In fact, as for SLA, leaf unfolding and leaf N content, P50 seems to be altered by the main climate variables evaluated (i.e. precipitation and maximum and minimum temperatures) as the PCA shows. This result is similar to what has been observed for Quercus oleoides in which variation among populations associated with climates of origin have been found despite a lack of local adaptation (Cavender-Bares 2018). Blackman et al. (2017) also showed how P50 was related to mean annual temperature across eight populations of Corymbia calophylla grown in a common garden experiment. Wood density has been theoretically linked with resistance to embolism based on the avoidance of implosion/collapse events in the xylem vessels (Hacke et al. 2001), because it is closely related to climate, and in particular precipitation and aridity, across species (Martínez-Cabrera et al. 2009). The lack of significant correlation between wood density and the climate variables analysed and the low contribution to the principal component 1 of the PCA (i.e. the one more influenced by climate), suggests that this anatomical trait is differently affected by climate than resistance to embolism and that, therefore, it could not work as an accurate proxy for P50 at an intra-specific level. Despite this, more studies including more provenances from the marginal distribution area of the species and more species with different xylem anatomical characteristics are required to definitely reject wood density as proxy for embolism resistance within species.
Conclusions
Our results evidenced that different Quercus petraea provenances originating from an extensive portion of the species’ overall distribution in Europe showed important genetic variation for traits related to phenology and growth. In fact, genetic differentiation is much higher for leaf phenological traits than for other functional traits directly related with plant tolerance to drought such as hydraulic traits. Most anatomical, physiological and hydraulics traits evaluated, except SLA, did not show any significant variation between provenances, or very minor genetic differentiation, although large between trees (within populations) variations were observed. This raises the question of whether the multiple-trait associations are more driven by very integrated traits, i.e. by traits that can give synthetic information about interactions between the plant and their environment such as growth, phenology and SLA. Despite this, there are some less integrated traits, e.g. leaf N content, highly influenced by the climate at the origin of the provenances that, although they did not show genetic divergence, they could be a good target of selection for individuals from marginal populations, i.e. with highly contrasted climate conditions. Whether the lack of variation in certain traits in this species is due to phenotypic plasticity remains to be investigated. In any case, we suspect that there is enough genetic variation within populations to trigger genetic divergence among populations. While experimental evolution cannot be implemented to check our hypothesis, future investigations will explore whether anatomical, physiological and hydraulics traits do exhibit significant selection gradients.
Supplementary Material
Acknowledgements
We thank the Experimental Unit of INRA Nancy (UEFL, Unité Expérimentale Forestière de Lorraine) for their contribution during field assessments and field collection. TREEPEACE team for field sampling and trait measurements in the lab.
Funding Information.
This study was supported by the ERC project TREEPEACE (FP7-339728) and the ‘Investments for the Future’ (ANR-10-EQPX-16, XYLOFOREST) programme of the French National Agency for Research and from the Labex COTE program. JMTR was supported by the Visiting Fellows & Visiting Scholars Program of the University of Tasmania to carry out part of the measurements at the University of Tasmania.
Footnotes
Authors’ Contributions
AK and SD conceived the ideas and, together with JMT-R, designed methodology. JMT-R, LT, FB, AD and SD collected the samples and carried out most of the measurements related with phenology and anatomy. JMT-R, MRC-M and TB collected and analysed the vein and stomatal density data. LJL and JMT-R ran the statistical analyses. All authors contributed critically to the drafts and gave final approval for publication.
References
- Aitken SN, Yeaman S, Holliday JA, Wang T, Curtis-McLane S. Adaptation, migration or extirpation: climate change outcomes for tree populations. Evol Appl. 2008;1:95–111. doi: 10.1111/j.1752-4571.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert C, Thuiller W, Yoccoz N, Soudant A, Boucher F, Saccone P, Lavorel S. Intraspecific functional variability: Extent, structure and sources of variation. J Ecol. 2010;98:604–613. [Google Scholar]
- Alberto F, Bouffier L, Louvet J-M, Lamy J-B, Delzon S, Kremer A. Adaptive responses for seed and leaf phenology in natural populations of sessile oak along an altitudinal gradient. J Evol Biol. 2011;24:1442–1454. doi: 10.1111/j.1420-9101.2011.02277.x. [DOI] [PubMed] [Google Scholar]
- Aletà i Soler N, Vilanova A, Díaz R, Voltas Velasco J. Genetic variation for carbon isotope composition in Juglans regia L.: relationships with growth, phenology and climate of origin. [17 June 2019, date last accessed];2009 https://repositori.udl.cat/handle/10459.1/30357.
- Allen CD. Forest ecosystem reorganization underway in the Southwestern US: A preview of widespread forest changes in the Anthropocene. U.S. Department of Agriculture: Rocky Mountain Research Station; 2014. [13 June 2019, date last accessed]. http://pubs.er.usgs.gov/publication/70156788. [Google Scholar]
- Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, Kitzberger T, Rigling A, Breshears DD, Hogg EH (Ted), Gonzalez P, et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manag. 2010;259:660–684. [Google Scholar]
- Amano Tatsuya, Smithers Richard J, Sparks Tim H, Sutherland William J. A 250-year index of first flowering dates and its response to temperature changes. Proc R Soc B Biol Sci. 2010;277:2451–2457. doi: 10.1098/rspb.2010.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JT, Inouye DW, McKinney AM, Colautti RI, Mitchell-Olds T. Phenotypic plasticity and adaptive evolution contribute to advancing flowering phenology in response to climate change. Proc Biol Sci. 2012;279:3843–3852. doi: 10.1098/rspb.2012.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold R, Johnson IG, Owen JV. Genetic variation in growth, stem straightness and wood properties in Eucalyptus dunnii trials in northern New South Wales. For Genet. 2004;11:1–12. [Google Scholar]
- Bertin RI. Plant Phenology And Distribution In Relation To Recent Climate Change. J Torrey Bot Soc. 2008;135:126–146. [Google Scholar]
- Blackman CJ, Aspinwall MJ, Tissue DT, Rymer PD. Genetic adaptation and phenotypic plasticity contribute to greater leaf hydraulic tolerance in response to drought in warmer climates. Tree Physiol. 2017;37:583–592. doi: 10.1093/treephys/tpx005. [DOI] [PubMed] [Google Scholar]
- Bresson CC, Vitasse Y, Kremer A, Delzon S. To what extent is altitudinal variation of functional traits driven by genetic adaptation in European oak and beech? Tree Physiol. 2011;31:1164–1174. doi: 10.1093/treephys/tpr084. [DOI] [PubMed] [Google Scholar]
- Brienen RJW, Wanek W, Hietz P. Stable carbon isotopes in tree rings indicate improved water use efficiency and drought responses of a tropical dry forest tree species. Trees. 2011;25:103–113. [Google Scholar]
- Brodribb TJ, Cochard H. Hydraulic Failure Defines the Recovery and Point of Death in Water-Stressed Conifers. PLANT Physiol. 2009;149:575–584. doi: 10.1104/pp.108.129783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Feild TS, Jordan GJ. Leaf Maximum Photosynthetic Rate and Venation Are Linked by Hydraulics. Plant Physiol. 2007;144:1890–1898. doi: 10.1104/pp.107.101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Jordan GJ. Water supply and demand remain balanced during leaf acclimation of Nothofagus cunninghamii trees. New Phytol. 2011;192:437–448. doi: 10.1111/j.1469-8137.2011.03795.x. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM, Jordan GJ, Martins SCV. Conifer species adapt to low-rainfall climates by following one of two divergent pathways. Proc Natl Acad Sci. 2014;111:14489–14493. doi: 10.1073/pnas.1407930111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailleret M, Jansen S, Robert EMR, Desoto L, Aakala T, Antos JA, Beikircher B, Bigler C, Bugmann H, Caccianiga M, Čada V, et al. A synthesis of radial growth patterns preceding tree mortality. Glob Change Biol. 2017;23:1675–1690. doi: 10.1111/gcb.13535. [DOI] [PubMed] [Google Scholar]
- Carins Murphy MR, Jordan GJ, Brodribb TJ. Differential leaf expansion can enable hydraulic acclimation to sun and shade. Plant Cell Environ. 2012;35:1407–1418. doi: 10.1111/j.1365-3040.2012.02498.x. [DOI] [PubMed] [Google Scholar]
- Carins Murphy MR, Jordan GJ, Brodribb TJ. Cell expansion not cell differentiation predominantly co-ordinates veins and stomata within and among herbs and woody angiosperms grown under sun and shade. Ann Bot. 2016;118:1127–1138. doi: 10.1093/aob/mcw167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JE, Adams CA, Holsinger KE. Intraspecific variation in stomatal traits, leaf traits and physiology reflects adaptation along aridity gradients in a South African shrub. Ann Bot. 2016;117:195–207. doi: 10.1093/aob/mcv146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavender-Bares J. Diversification, adaptation, and community assembly of the American oaks (Quercus), a model clade for integrating ecology and evolution. New Phytol. 2018;221 doi: 10.1111/nph.15450. [DOI] [PubMed] [Google Scholar]
- Chamaillard S, Fichot R, Vincent-Barbaroux C, Bastien C, Depierreux C, Dreyer E, Villar M, Brignolas F. Variations in bulk leaf carbon isotope discrimination, growth and related leaf traits among three Populus nigra L. populations. Tree Physiol. 2011;31:1076–1087. doi: 10.1093/treephys/tpr089. [DOI] [PubMed] [Google Scholar]
- Choat B, Brodribb TJ, Brodersen CR, Duursma RA, López R, Medlyn BE. Triggers of tree mortality under drought. Nature. 2018;558:531–539. doi: 10.1038/s41586-018-0240-x. [DOI] [PubMed] [Google Scholar]
- Choat B, Jansen S, Brodribb TJ, Cochard H, Delzon S, Bhaskar R, Bucci SJ, Feild TS, Gleason SM, Hacke UG, Jacobsen AL, et al. Global convergence in the vulnerability of forests to drought. Nature. 2012;491:752–755. doi: 10.1038/nature11688. [DOI] [PubMed] [Google Scholar]
- Chung H, Muraoka H, Nakamura M, Han S, Muller O, Son Y. Experimental warming studies on tree species and forest ecosystems: a literature review. J Plant Res. 2013;126:447–460. doi: 10.1007/s10265-013-0565-3. [DOI] [PubMed] [Google Scholar]
- Corlett RT, Westcott DA. Will plant movements keep up with climate change? Trends Ecol Evol. 2013;28:482–488. doi: 10.1016/j.tree.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Craig H. Isotopic standards for carbon and oxygen and correction factors for mass-spectrometric analysis of carbon dioxide. Geochim Cosmochim Acta. 1957;12:133–149. [Google Scholar]
- Cregg BM, Zhang JW. Physiology and morphology of Pinus sylvestris seedlings from diverse sources under cyclic drought stress. For Ecol Manag. 2001;154:131–139. [Google Scholar]
- Dantec CF, Ducasse H, Capdevielle X, Fabreguettes O, Delzon S, Desprez-Loustau M-L. Escape of spring frost and disease through phenological variations in oak populations along elevation gradients. Thrall P, editor. J Ecol. 2015;103:1044–1056. [Google Scholar]
- David-Schwartz R, Paudel I, Mizrachi M, Delzon S, Cochard H, Lukyanov V, Badel E, Capdeville G, Shklar G, Cohen S. Indirect Evidence for Genetic Differentiation in Vulnerability to Embolism in Pinus halepensis. Front Plant Sci. 2016;7:768. doi: 10.3389/fpls.2016.00768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon NJ, Cavender-Bares J. Limited Pollen Dispersal Contributes to Population Genetic Structure but Not Local Adaptation in Quercus oleoides Forests of Costa Rica. PLOS ONE. 2015;10:e0138783. doi: 10.1371/journal.pone.0138783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delzon S. New insight into leaf drought tolerance. Funct Ecol. 2015;29:1247–1249. [Google Scholar]
- Delzon S, Urli M, Samalens J-C, Lamy J-B, Lischke H, Sin F, Zimmermann NE, Porté AJ. Field Evidence of Colonisation by Holm Oak, at the Northern Margin of Its Distribution Range, during the Anthropocene Period. PLOS ONE. 2013;8:e80443. doi: 10.1371/journal.pone.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi H, Katano I. Phenological timings of leaf budburst with climate change in Japan. Agric For Meteorol. 2008;148:512–516. [Google Scholar]
- Ducousso A, Guyon JP, Krémer A. Latitudinal and altitudinal variation of bud burst in western populations of sessile oak (Quercus petraea (Matt) Liebl) Ann Sci For. 1996;53:775–782. [Google Scholar]
- Farquhar GD, Richards RA. Isotopic Composition of Plant Carbon Correlates With Water-Use Efficiency of Wheat Genotypes. Funct Plant Biol. 1984;11:539–552. [Google Scholar]
- Firmat C, Delzon S, Louvet J-M, Parmentier J, Kremer A. Evolutionary dynamics of the leaf phenological cycle in an oak metapopulation along an elevation gradient. J Evol Biol. 2017;30:2116–2131. doi: 10.1111/jeb.13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francey RJ, Allison CE, Etheridge DM, Trudinger CM, Enting IG, Leuenberger M, Langenfelds RL, Michel E, Steele LP. A 1000-year high precision record of δ13C in atmospheric CO2. Tellus B Chem Phys Meteorol. 1999;51:170–193. [Google Scholar]
- Franks PJ, Beerling DJ. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc Natl Acad Sci. 2009;106:10343–10347. doi: 10.1073/pnas.0904209106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks SJ, Weber JJ, Aitken SN. Evolutionary and plastic responses to climate change in terrestrial plant populations. Evol Appl. 2014;7:123–139. doi: 10.1111/eva.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Muñoz N, Sterck F, Torres-Ruiz JM, Petit G, Cochard H, von Arx G, Lintunen A, Caldeira MC, Capdeville G, Copini P, Gebauer R, et al. Quantifying in situ phenotypic variability in the hydraulic properties of four tree species across their distribution range in Europe. Heinze B, editor. PLOS ONE. 2018;13:e0196075. doi: 10.1371/journal.pone.0196075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordo O, Sanz JJ. Phenology and climate change: a long-term study in a Mediterranean locality. Oecologia. 2005;146:484–495. doi: 10.1007/s00442-005-0240-z. [DOI] [PubMed] [Google Scholar]
- Granda E, Alla AQ, Laskurain NA, Loidi J, Sánchez-Lorenzo A, Camarero JJ. Coexisting oak species, including rear-edge populations, buffer climate stress through xylem adjustments. Tree Physiol. 2018;38:159–172. doi: 10.1093/treephys/tpx157. [DOI] [PubMed] [Google Scholar]
- Guay R, Gagnon R, Morin H. A new automatic and interactive tree-ring measurement system based on a line scan camera. For Chron. 1992:68. [Google Scholar]
- Guet J, Fabbrini F, Fichot R, Sabatti M, Bastien C, Brignolas F. Genetic variation for leaf morphology, leaf structure and leaf carbon isotope discrimination in European populations of black poplar (Populus nigra L.) Tree Physiol. 2015;35:850–863. doi: 10.1093/treephys/tpv056. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloh KA. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia. 2001;126:457–461. doi: 10.1007/s004420100628. [DOI] [PubMed] [Google Scholar]
- Herbig A, Kull U. Leaves and ramification. [19 June 2019, date last accessed];1992 http://elib.uni-stuttgart.de/handle/11682/2315.
- Hoffmann AA, Sgrò CM. Climate change and evolutionary adaptation. Nature. 2011;470:479–485. doi: 10.1038/nature09670. [DOI] [PubMed] [Google Scholar]
- Kavanagh KL, Bond BJ, Aitken SN, Gartner BL, Knowe S. Shoot and root vulnerability to xylem cavitation in four populations of Douglas-fir seedlings. Tree Physiol. 1999;19:31–37. doi: 10.1093/treephys/19.1.31. [DOI] [PubMed] [Google Scholar]
- Kremer A, Potts B, Delzon S. Genetic divergence in forest trees: Understanding the consequences of climate change. Funct Ecol. 2013;28 [Google Scholar]
- Kuster TM, Dobbertin M, Günthardt-Goerg MS, Schaub M, Arend M. A Phenological Timetable of Oak Growth under Experimental Drought and Air Warming. PLOS ONE. 2014;9:e89724. doi: 10.1371/journal.pone.0089724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy J-B, Bouffier L, Burlett R, Plomion C, Cochard H, Delzon S. Uniform Selection as a Primary Force Reducing Population Genetic Differentiation of Cavitation Resistance across a Species Range. PLOS ONE. 2011;6:e23476. doi: 10.1371/journal.pone.0023476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy J-B, Delzon S, Bouche PS, Alia R, Vendramin GG, Cochard H, Plomion C. Limited genetic variability and phenotypic plasticity detected for cavitation resistance in a Mediterranean pine. New Phytol. 2014;201:874–886. doi: 10.1111/nph.12556. [DOI] [PubMed] [Google Scholar]
- Larter M, Pfautsch S, Domec J-C, Trueba S, Nagalingum N, Delzon S. Aridity drove the evolution of extreme embolism resistance and the radiation of conifer genus Callitris. New Phytol. 2017;215:97–112. doi: 10.1111/nph.14545. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken G, Stroup WW, Wolfinger RE, Schabenberger O. SAS for mixed models. American Statistician - AMER STATIST. 2007 [Google Scholar]
- Lobo A, Torres-Ruiz JM, Burlett R, Lemaire C, Parise C, Francioni C, Truffaut L, Tomášková I, Hansen JK, Kjær ED, Kremer A, et al. Assessing inter- and intraspecific variability of xylem vulnerability to embolism in oaks. For Ecol Manag. 2018;424:53–61. doi: 10.1016/j.foreco.2018.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long W, Zang R, Schamp BS, Ding Y. Within- and among-species variation in specific leaf area drive community assembly in a tropical cloud forest. Oecologia. 2011;167:1103–1113. doi: 10.1007/s00442-011-2050-9. [DOI] [PubMed] [Google Scholar]
- Martin AR, Hale CE, Cerabolini BEL, Cornelissen JHC, Craine J, Gough WA, Kattge J, Tirona CKF. Inter- and intraspecific variation in leaf economic traits in wheat and maize. AoB PLANTS. 2018;10 doi: 10.1093/aobpla/ply006. ply006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Cabrera HI, Jones CS, Espino S, Schenk HJ. Wood anatomy and wood density in shrubs: Responses to varying aridity along transcontinental transects. Am J Bot. 2009;96:1388–1398. doi: 10.3732/ajb.0800237. [DOI] [PubMed] [Google Scholar]
- Martínez-Vilalta J, Cochard H, Mencuccini M, Sterck F, Herrero A, Korhonen JFJ, Llorens P, Nikinmaa E, Nolè A, Poyatos R, Ripullone F, et al. Hydraulic adjustment of Scots pine across Europe. New Phytol. 2009;184:353–364. doi: 10.1111/j.1469-8137.2009.02954.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Ohta T, Irasawa M, Nakamura T. Climate change and extension of the Ginkgo biloba L. growing season in Japan. Glob Change Biol. 2003;9:1634–1642. [Google Scholar]
- McCarroll D, Loader NJ. Stable isotopes in tree rings. Quat Sci Rev. 2004;23:771–801. [Google Scholar]
- McDowell NG, Ryan MG, Zeppel MJB, Tissue DT. Feature: Improving our knowledge of drought-induced forest mortality through experiments, observations, and modeling. New Phytol. 2013;200:289–293. doi: 10.1111/nph.12502. [DOI] [PubMed] [Google Scholar]
- Meireles JE, Beulke A, Borkowski DS, Romero-Severson J, Cavender-Bares J. Balancing selection maintains diversity in a cold tolerance gene in broadly distributed live oaks. Genome. 2017;60:762–769. doi: 10.1139/gen-2016-0208. [DOI] [PubMed] [Google Scholar]
- Mencuccini M. The ecological significance of long-distance water transport: short-term regulation, long-term acclimation and the hydraulic costs of stature across plant life forms. Plant Cell Environ. 2003;26:163–182. [Google Scholar]
- Menzel A, Fabian P. Growing season extended in Europe. Nature. 1999;397:659. [Google Scholar]
- Milla R, Reich P, Niinemets Ü, Castro-Díez P. Environmental and developmental controls on specific leaf area are little modified by leaf allometry. Funct Ecol. 2008;22:565–576. [Google Scholar]
- Murphy MRC, Jordan GJ, Brodribb TJ. Acclimation to humidity modifies the link between leaf size and the density of veins and stomata. Plant Cell Environ. 2014;37:124–131. doi: 10.1111/pce.12136. [DOI] [PubMed] [Google Scholar]
- Pammenter NW, Van der Willigen C. A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiol. 1998;18:589–593. doi: 10.1093/treephys/18.8-9.589. [DOI] [PubMed] [Google Scholar]
- Pennington RE, Tischler CR, Johnson HB, Polley HW. Genetic variation for carbon isotope composition in honey mesquite (Prosopis glandulosa) Tree Physiol. 1999;19:583–589. doi: 10.1093/treephys/19.9.583. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Filella I, Comas P. Changed plant and animal life cycles from 1952 to 2000 in the Mediterranean region. Glob Change Biol. 2002;8:531–544. [Google Scholar]
- Polge H. Établissement des courbes de variation de la densité du bois par exploration densitométrique de radiographies d’échantillons prélevés à la tarière sur des arbres vivants : applications dans les domaines Technologique et Physiologique. Ann Sci For. 1966;23:I–206. [Google Scholar]
- Ponton S, Dupouey J-L, Nathalie B, Dreyer E. Comparison of water-use efficiency of seedlings from two sympatric oak species: Genotype x environment interactions. Tree Physiol. 2002;22:413–22. doi: 10.1093/treephys/22.6.413. [DOI] [PubMed] [Google Scholar]
- Ramírez-Valiente JA, Center A, Sparks JP, Sparks KL, Etterson JR, Longwell T, Pilz G, Cavender-Bares J. Population-Level Differentiation in Growth Rates and Leaf Traits in Seedlings of the Neotropical Live Oak Quercus oleoides Grown under Natural and Manipulated Precipitation Regimes. Front Plant Sci. 2017;8:585. doi: 10.3389/fpls.2017.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich PB, Uhl C, Walters MB, Ellsworth DS. Leaf lifespan as a determinant of leaf structure and function among 23 amazonian tree species. Oecologia. 1991;86:16–24. doi: 10.1007/BF00317383. [DOI] [PubMed] [Google Scholar]
- Sáenz-Romero C, Lamy J-B, Ducousso A, Musch B, Ehrenmann F, Delzon S, Cavers S, Chałupka W, Dağdaş S, Hansen JK, Lee SJ, et al. Adaptive and plastic responses of Quercus petraea populations to climate across Europe. Glob Change Biol. 2017;23:2831–2847. doi: 10.1111/gcb.13576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon Y, Torres-Ruiz JM, Poyatos R, Martinez-Vilalta J, Meir P, Cochard H, Mencuccini M. Balancing the risks of hydraulic failure and carbon starvation: a twig scale analysis in declining Scots pine: Twig role in Scots pine drought-induced mortality. Plant Cell Environ. 2015;38:2575–2588. doi: 10.1111/pce.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schume H, Grabner M, Eckmüllner O. The influence of an altered groundwater regime on vessel properties of hybrid poplar. Trees. 2004;18:184–194. [Google Scholar]
- Sotelo Montes C, Weber JC. Genetic variation in wood density and correlations with tree growth in Prosopis africana from Burkina Faso and Niger. Ann For Sci. 2009;66:713–713. [Google Scholar]
- Spitze K. Population structure in Daphnia obtusa: quantitative genetic and allozymic variation. Genetics. 1993;135:367–374. doi: 10.1093/genetics/135.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojnić S, Suchocka M, Benito-Garzón M, Torres-Ruiz JM, Cochard H, Bolte A, Cocozza C, Cvjetković B, de Luis M, Martinez-Vilalta J, Ræbild A, et al. Variation in xylem vulnerability to embolism in European beech from geographically marginal populations. Tree Physiol. 2018;38:173–185. doi: 10.1093/treephys/tpx128. [DOI] [PubMed] [Google Scholar]
- Torres-Ruiz JM, Cochard H, Choat B, Jansen S, López R, Tomášková I, Padilla-Díaz CM, Badel E, Burlett R, King A, Lenoir N, et al. Xylem resistance to embolism: presenting a simple diagnostic test for the open vessel artefact. New Phytol. 2017;215:489–499. doi: 10.1111/nph.14589. [DOI] [PubMed] [Google Scholar]
- Torres-Ruiz JM, Cochard H, Fonseca E, Badel E, Gazarini L, Vaz M. Differences in functional and xylem anatomical features allow Cistus species to co-occur and cope differently with drought in the Mediterranean region. Tree Physiol. 2017;37:755–766. doi: 10.1093/treephys/tpx013. [DOI] [PubMed] [Google Scholar]
- Torres-Ruiz JM, Jansen S, Choat B, McElrone AJ, Cochard H, Brodribb TJ, Badel E, Burlett R, Bouche PS, Brodersen CR, Li S, et al. Direct X-Ray Microtomography Observation Confirms the Induction of Embolism upon Xylem Cutting under Tension. Plant Physiol. 2015;167:40–43. doi: 10.1104/pp.114.249706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl D, Mosbrugger V. Leaf venation density as a climate and environmental proxy: a critical review and new data. Palaeogeogr Palaeoclimatol Palaeoecol. 1999;149:15–26. [Google Scholar]
- Urli M, Lamy J-B, Sin F, Burlett R, Delzon S, Porté AJ. The high vulnerability of Quercus robur to drought at its southern margin paves the way for Quercus ilex. Plant Ecol. 2015;216:177–187. [Google Scholar]
- Vitasse Y, Delzon S, Bresson CC, Michalet R, Kremer A. Altitudinal differentiation in growth and phenology among populations of temperate-zone tree species growing in a common garden. Can J For Res. 2009;39:1259–1269. [Google Scholar]
- Weih M, Karlsson S. Growth response of Mountain birch to air and soil temperature: Is increasing leaf-nitrogen content an acclimation to lower air temperature? New Phytol. 2002;150:147–155. [Google Scholar]
- Woodward FI, Bazzaz FA. The Responses of Stomatal Density to CO2 Partial Pressure. J Exp Bot. 1988;39:1771–1781. [Google Scholar]
- Wright IJ, Dong N, Maire V, Prentice IC, Westoby M, Díaz S, Gallagher RV, Jacobs BF, Kooyman R, Law EA, Leishman MR, et al. Global climatic drivers of leaf size. Science. 2017;357:917–921. doi: 10.1126/science.aal4760. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, et al. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Kang H, Xie Q, Wang Z, Yin S, Liu C. Pattern of leaf vein density and climate relationship of Quercus variabilis populations remains unchanged with environmental changes. Trees. 2012;26:597–607. [Google Scholar]
- Zwieniecki MA, Boyce CK. Evolution of a unique anatomical precision in angiosperm leaf venation lifts constraints on vascular plant ecology. Proc R Soc B Biol Sci. 2014;281 doi: 10.1098/rspb.2013.2829. 20132829–20132829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieniecki MA, Boyce CK, Holbrook NM. Hydraulic limitations imposed by crown placement determine final size and shape of Quercus rubra L. leaves. Plant Cell Environ. 2004;27:357–365. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.