Conspectus
The lipid bilayer, together with embedded proteins, is the central structure in biomembranes. While artificial lipid bilayers are useful to model natural membranes, they are generally symmetric, with the same membrane lipid composition in each lipid monolayer (leaflet). In contrast, natural membranes are often asymmetric, with different lipids in each leaflet. To prepare asymmetric lipid vesicles, we developed cyclodextrin-catalyzed phospholipid exchange procedures. The basic method is that an excess of vesicles with one set of lipids (the donor vesicles) is mixed with a second set of vesicles (acceptor vesicles) with a different set of lipids. Cyclodextrin is introduced into the external aqueous solution, so that lipids in the outer leaflet of the vesicles bind to it and are shuttled between the vesicles. At equilibrium the lipids in the outer leaflet of the acceptor vesicles are replaced by those from the donor vesicles. The exchanged acceptor vesicles are then isolated. Asymmetric vesicles are versatile in terms of vesicle sizes and lipid compositions that can be prepared. Measuring asymmetry is often difficult. A variety of assays can be used to measure the extent of asymmetry, but most are specific for one particular membrane lipid type or class, and there are none that can be used in all situations. Studies using asymmetric vesicles have begun to explore how asymmetry influences lipid movement across the bilayer, the formation of ordered lipid domains, coupling between the physical properties in each leaflet, and membrane protein conformation. Lipid domain formation stands out as of the most important properties in which asymmetry is likely to be crucial. Lipid bilayers can exist in both liquid-like and solid/ordered-like states depending on lipid structure, and in lipid vesicles with a mixture of lipids highly ordered and disordered domains can co-exist. However, until very recently such studies only had been carried out in symmetric artificial membranes. Whether ordered domains (often called lipid rafts) and disordered lipid domains co-exist in asymmetric cell membranes remains controversial partly because lipids favoring formation of an ordered state are largely restricted in the leaflet facing the external environment. Studies using asymmetric vesicles have recently shown that each leaflet can influence the physical behavior of the other, i.e. that the domain forming properties in each leaflet tend to be coupled, with consequences highly dependent upon the details of lipid structure. Future studies investigating the dependence of coupling and properties upon the details of lipid composition should clarify the potential of natural membranes to form lipid domains. In addition, we recently extended the exchange method to living mammalian cells, using exchange to efficiently replace virtually the entire phospholipid and sphingolipid population of the plasma membrane outer leaflet with exogenous lipids without harming cells. This should allow detailed studies of the functional impact of lipid structure, asymmetry, domain organization, and interactions with membrane proteins in living cells.
Graphical Abstract
1. Introduction:
1.1. Lipid Asymmetry
Artificial lipid bilayers that self-assemble when phospholipids are dispersed in aqueous solution have been invaluable for studying the physical properties and function of lipids within biomembranes. In addition, incorporation (reconstitution) of membrane proteins into artificial bilayers has provided insights into membrane protein function and interaction with lipid. The most widely used artificial membranes are freely floating vesicles, often called liposomes. They contain a lipid bilayer and entrapped aqueous solution. However, a long-standing issue limiting the value of artificial lipid vesicles has been that they generally lack lipid asymmetry. Many biological membranes, including the plasma membrane surrounding eukaryotic cells, are asymmetric, with different lipids in the two lipid monolayers (leaflets) that form the lipid bilayer. Inner and outer leaflet lipids can differ in chemical structure, electrostatic charge and physical properties. How these properties differ in asymmetric and symmetric bilayers is poorly understood.
1.2. Lipid Physical State
One critical property likely to be influenced by asymmetry is lipid physical state. The physical behavior of lipids and lipid mixtures is shown schematically in the phase diagram of Figure 1. At higher temperatures, pure phospholipids and sphingolipids form lipid bilayers in the liquid disordered (Ld) state (formerly called liquid crystalline state). It has loose lateral lipid packing, acyl chains with gauche kinks, and fast lateral diffusion.1
Figure 1:
Schematic illustration of membrane lipid phase behavior on a temperature/composition phase diagram. At low temperature, the solid-like gel state predominates (for example in lipid mixture of about equal amounts of Lipid “a” and Lipid “b”at point 1, lipids shown in black at right). At high temperature the liquid disordered (Ld) state predominates (for example at point 3, lipids shown in gray at right). For pure membrane lipids there is a distinct melting temperature (Tm) in which the lipid bilayer switches between these states. Tm depends on lipid structure. Notice that Lipid “a” has a lower Tm than Lipid “b” in the example shown. In a mixture of lipids there is can be a region of phase co-existence at intermediate temperature (for example at point 2) in which two phases/domains with different properties, and lipid compositions, co-exist.
At low temperature, the solid-like gel state usually predominates. It contains tightly packed lipids with extended and ordered acyl chains and has very slow lateral diffusion of lipids. The gel state, at least at sufficiently low temperatures, is relatively detergent insoluble, i.e. lipid-lipid interactions are stronger than lipid-detergent interactions.2 The thermal transition between gel and Ld states in a bilayer of a pure lipid occurs at a melting/freezing temperature (Tm) specific to each lipid. The presence of cis double bonds (unsaturation) in acyl chains strongly influences Tm. Sphingolipids tend to have acyl chains without double bonds and high Tm, while most phospholipids have at least one unsaturated acyl chain and low Tm. This raises the possibility that biomembranes containing a mixture of both of these lipid classes, such as plasma membranes, could contain co-existing gel and Ld domains, as shown in Figure 1 .3 Complicating this, in the presence of cholesterol an intermediate state can form, the liquid ordered (Lo) state. The Lo state is characterized by tight lipid packing and a high degree of order, but fast lateral diffusion 1. Based on studies showing that sphingolipid and cholesterol rich detergent insoluble membranes can be isolated from eukaryotic plasma membranes4, that the Lo state was detergent-insoluble, and that detergent insolubility is linked with the appearance of Lo domains in artificial lipid vesicles, our group together with that of Dr. Deborah Brown proposed Lo domain/Ld domain co-existence in biological membranes.5,6. This is the working model for the origin of sphingolipid and cholesterol rich rafts.7,8
However, sphingolipids are most highly enriched in the outer leaflet of the plasma membrane, while ordered domains are thought to span the bilayer.1 An obvious possibility is that Lo domains in one leaflet induce them in the opposite leaflet via interactions that couple their physical properties. This could induce transmembrane signal transduction directly via the lipid bilayer, as suggested long ago.9
This raises the question: under what conditions does this interleaflet coupling occur? This cannot be answered using symmetric lipid bilayers. Asymmetric bilayers are needed. This is what triggered the studies our lab has carried out on asymmetric vesicles. However, as described below, the ability to control lipid asymmetry can impact additional aspects of our understanding of lipid structure and function.
1.3. Preparing Asymmetric Membranes Via Monolayer Assembly or Bilayer Modification
Progress preparing asymmetric lipid bilayers has been slow. One approach has been to bring two lipid monolayers of different lipid compositions together. For supported planar bilayers prepared this way, a cushion between the bilayer and the substrate can minimize perturbation by the support, and some studies of interleaflet interaction have used this approach.10 Methods to prepare asymmetric planar bilayers and vesicles involving use of lipids dispersed in oils have also been described.11–19 Perturbed membrane properties due to residual oil is a concern, although there have been attempts to minimize this.20 Methods avoiding solvent include use of pH gradients, which achieve some asymmetry due to translocation of anionic lipids to the side of the membrane exposed to high pH21. Outer leaflet headgroup exchange via phospholipase D 22, or decarboxylation of phosphatidylserine (PS) to produce phosphatidylethanolamine (PE)23 are also promising and have important advantages, but are limited in what combinations of acyl chains and headgroups can be studied.
2. Preparation, Analysis and Properties of Vesicles Prepared by Lipid Exchange
2.1. Lipid Exchange
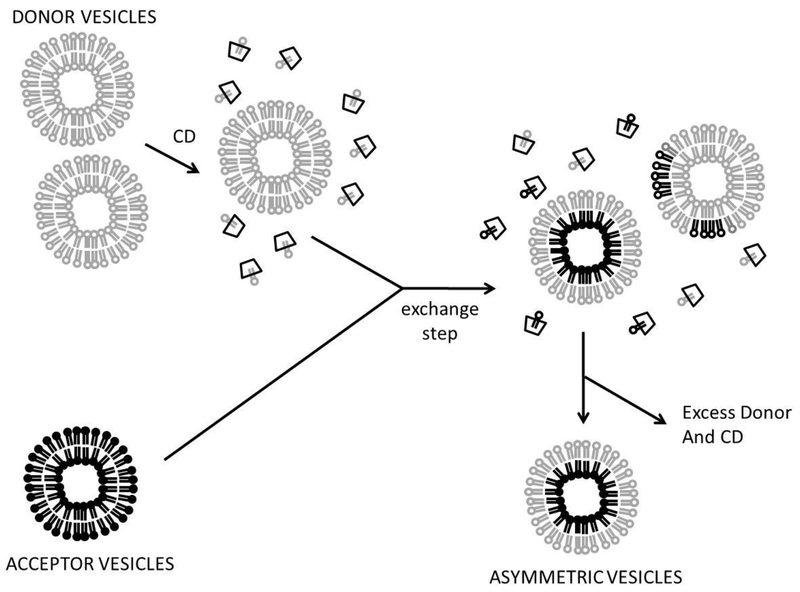
Lipid exchange is probably the most versatile method to prepare asymmetric lipid vesicles. In this approach a population of acceptor vesicles composed of the desired inner leaflet lipids, and a donor vesicle population (in excess), which donates what becomes the outer leaflet of the asymmetric vesicles are mixed. In the presence of a water-soluble, membrane-impermeable, lipid-carrying agent outer leaflet lipids between the two populations are exchanged and equilibrate (Figure 1). The exchanged acceptor vesicles are then isolated. In some cases, exchange agent preloaded with lipid is added to the acceptor vesicles. In early studies phospholipid exchange proteins were used.24–26 However, lipid specificity was an obstacle to wide use, and high extents of exchange were generally not the goal, and so not achieved.
2.2. Preparing Asymmetric Vesicles With Cyclodextrin-Catalyzed Lipid Exchange
Cyclodextrins (CDs) have turned out to be the most versatile agents for lipid exchange. CDs are cyclic oligomers of glucose, and have a hydrophobic cavity that binds diverse hydrophobic molecules. CDs are often highly water soluble. The three commonly used classes of CD have six (alpha), seven (beta), or eight (gamma) glucose. Methyl beta CD (MβCD) is widely used to exchange sterols in and out of cells or artificial lipid membranes.27 Studies showed that MβCD and other CDs could also bind to phospholipids, presumably by binding their acyl chains, and introduce them into membranes, or exchange them between membranes.28–30
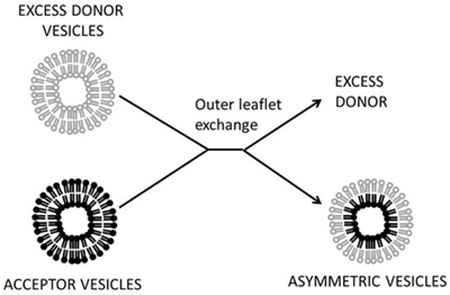
We first exploited exchange with MβCD to prepare highly asymmetric vesicles, with efficient (75-100%) replacement of acceptor vesicle outer leaflet lipid with donor lipid (Figure 2).31 This requires very high CD concentrations, and excess donor lipid. Since high concentrations of MβCD can dissolve vesicles, the protocol involves premixing MβCD with excess donor vesicles, to saturate the MβCD with lipid and prevent any further vesicle solubilization. Then acceptor vesicles are added. In the first study using this approach, we produced asymmetric small unilamellar vesicles (SUV). These were separated from larger vesicles and small soluble molecules by size.
Figure 2:
General protocol for preparing asymmetric vesicles by exchange. Exchange agent, here CDs, shown as trapezoids. Excess donor lipid and CD are removed by size chromatography, centrifugation, or filtration. Note: a CD molecule likely accommodates a single acyl chain in its cavity.
It is desirable to prepare asymmetric vesicles with less curvature than SUV because most natural membrane are not as highly curved as SUV. To do this, acceptor large unilamellar vesicles (LUV) were prepared containing entrapped sucrose to increase their density. After exchange, asymmetric vesicles could be isolated by centrifugation.32 A variant avoiding entrapping sucrose in acceptor vesicles is to entrap sucrose within the donor vesicles.33,34 After exchange, donor lipid vesicles are removed by pelleting, and soluble components are removed by filtration. Exchange has also been extended by us to giant unilamellar vesicles (GUV)35, and by others to planar bilayers.36 In these cases the exchange solution with donor is removed by washing. Removal of excess donor vesicles from the donor vesicle-MβCD mixture prior to donor addition to acceptor prevents artifacts arising from sticking of donor vesicles to acceptor, but decreases exchange efficiency. It should be noted that GUV are sensitive to osmotic pressure. Even a small osmotic gradient can lead to transient pore formation37, which might degrade the lipid asymmetry.
Exchange can be carried out with various lipids. We found phosphatidylcholine (PC ) or sphingomyelin (SM) containing donor lipid vesicles can be used to efficiently exchange many types of PC and SM in the outer leaflet of acceptor lipid vesicles composed of almost any type of phospholipid.31,38 Exchange efficiency tends to increase as acyl chain length decreases.39,40 Few studies have looked at exchanging donor lipids with other headgroups41–43, and do so with high efficiency remains a challenge.
Controlling membrane cholesterol content is often desired. We first introduced cholesterol after phospholipid exchange by adding cholesterol-loaded MβCD to vesicles at an MβCD concentration too low to bind phospholipids. We subsequently switched to using αCDs, which have a cavity large enough to bind an acyl chain but which does not bind cholesterol.44–46 We found they catalyze efficient phospholipid exchange43,47, and devised a method using hydroxypropyl alpha CD (HPαCD), and later using methyl alpha cyclodextrin (MαCD) to exchange lipids using cholesterol-containing acceptor lipid vesicles.43,44 In these protocols cholesterol concentration in the asymmetric vesicles is virtually the same as in the acceptor vesicles from which they are prepared. This allows preparation of asymmetric vesicles with any cholesterol concentration up to its solubility limit in the lipid bilayer.48
Cholesterol flips across the bilayer rapidly in the Ld state, and these protocols do not achieve cholesterol asymmetry.28 At present, this is not a crucial issue because the extent of cholesterol asymmetry in cell membranes is highly controversial.49–52 Thus, it is not clear what cholesterol asymmetry would mimic natural membranes.
2.3. Exchange Efficiency and the Extent of Asymmetry
The level of exchange partly depends on the binding of CD to different lipids, which is highly cooperative in CD concentration.30 Even when lipid is in excess, only a small fraction of total CD is bound to lipid.30,41 In only few cases have any binding parameters been measured.30,41,53,54 Binding to CD, and thus exchange, should also be a function of the lipid composition of the donor and acceptor vesicles, and lipid packing density within the bilayer. Some information about lipid-CD interactions comes from studies of what concentrations of CDs dissolve lipid vesicles. We found methyl CDs can dissolve vesicles, while hydroxypropyl CDs do not47, perhaps indicative of stronger lipid binding by methyl CDs. There are differences in the concentration of CDs that dissolve vesicles as a function of lipid type, generally within a factor of two when headgroup is varied.43,47 Lipids with relatively short acyl chains dissolve at lower concentrations than lipid with longer ones.43,47 Variability in lipid interactions may arise from batch-to-batch differences in CDs which are randomly modified with either methyl or hydroxypropyl groups.
Exchange appears to involve nearly a 1:1 replacement of acceptor with donor lipid molecules, as suggested by unaltered acceptor vesicle size after exchange.31,44 A possible explanation for this balance is if the relative affinity of lipids for the bilayer vs. for CD increases when the outer leaflet has a lipid deficit (exchange is less than 1:1) relative to the inner leaflet, and decreases if there is a lipid excess (exchange is more than 1:1). This would set up a feedback loop maintaining lipid balance. (If the lipid exchanged into the outer leaflet occupies a different cross-sectional area than that being removed, then to balance inner and outer leaflet surface area exchange should not be exactly 1:1.) Changes in vesicle shape or flow of cholesterol between leaflets could also help maintain lipid balance.
The level of lipid asymmetry achieved after lipid exchange reflects two distinct parameters. The first is partial vs. full replacement of outer leaflet lipids, i.e. exchange efficiency. The second is scrambling of inner and outer leaflet lipids. In many studies, partial replacement is the goal, so a moderate exchange efficiency is desired. Scrambling is often more problematic, but a difference between the behavior of a partly scrambled and symmetric (fully scrambled) vesicle can still be used to evaluate effects of asymmetry.
When the level of exchange is that expected for replacement of the entire outer leaflet of the acceptor vesicles with donor lipid, it likely reflects efficient, complete exchange of just the outer leaflet. Even so proving such vesicles are asymmetric is difficult. Different asymmetry assays are often needed for different lipids. When one lipid is anionic, one approach is to assay electric charge on the vesicle outer surface. We determined this from the extent of binding of cationic species31,32,44, and others by measuring vesicle outer surface zeta potential.41 In both cases, a standard curve derived from symmetric vesicles of mixed lipid composition, at the appropriate lipid concentration, is used to calibrate response vs. outer leaflet composition.
In SUV with SM or PC we used the difference in NMR chemical shift of choline methyl protons in the outer and inner leaflets to assay asymmetry.38 This can also be done in LUV, but shift reagents must be added to the external solution to resolve outer and inner leaflet choline protons chemical shifts.33 The presence of multiple choline-containing species with overlapping signals can be alleviated by use of one species with a deuterated choline. Incompatibility of cationic shift reagents with anionic vesicles due to vesicle aggregation and fusion is a complication.
Chemical labeling, such as of outer leaflet PE with TNBS can be used to define PE asymmetry.44 However, incomplete labeling or permeability resulting in labeling of inner leaflet PE can complicate this approach. Careful control experiments are needed. Similarly, although not widely used for asymmetric vesicles to date, digestion of outer leaflet lipids with water soluble phospholipases could be used to define asymmetry. The degree to which reaction goes to completion and digestion-induced perturbation of asymmetry would be concerns.
Empirical differences in asymmetric and symmetric vesicle properties are also signatures of asymmetry. In vesicles with ordered membrane domains we found Tm differs in asymmetric and symmetric vesicles of identical overall lipid composition.31,32,38,55 This may not reveal the absolute level of asymmetry, but the rate at which Tm decays to the symmetric vesicle value can reveal the rate at which asymmetry decays.38 Others have shown measuring time-dependent changes in domain size/number can be used for this purpose.36 The level of asymmetry can also be estimated by comparing outer leaflet order in asymmetric and symmetric vesicles in cases in which the outer leaflet forms ordered domains independently of inner leaflet lipid composition. To do so we added an order-sensitive probe that binds only to the outer leaflet, and the level of membrane order in asymmetric vesicles was compared to that in symmetric vesicles with different amounts of the order-favoring lipid.31,55 This method cannot be used when the inner leaflet affects ordered domain formation in the outer leaflet.40
2.4. Asymmetry and Transverse Diffusion
Because asymmetry is degraded, i.e. lipids are scrambled, when lipids undergo transverse diffusion across the bilayer (flip-flop, see Figure 3), understanding spontaneous lipid flip is important. It is also of interest to know how asymmetry influences flip. Lipid flip is controlled by both the chemical structure of a lipid and the physical properties of the hydrophobic core and polar regions of the vesicle across which it flips. For any pure lipid vesicle, lipid chemical structure controls both properties. However, with a mixture of lipids, the lipid that flips does not by itself determine bilayer properties. The situation is even more complex in an asymmetric bilayer, in which each leaflet can have different properties, and in membranes with lipid domains, as flip across Ld and Lo domains will have different rates. By lateral diffusion lipids could gain access to the domain with the fastest flip rate.
Figure 3:
Section of lipid bilayer showing the distinction between lateral diffusion and transverse diffusion (flip-flop). The diffusion events shown by solid arrows. Notice that the latter degrades lipid asymmetry in the absence of other processes.
Studies in symmetric vesicles composed of commonly used PCs revealed that phospholipid flip in the Ld state is usually extremely slow, many hours to days.24,25,56,57 This is likely to reflect the low ability of the charged lipid headgroups to dissolve in the hydrophobic core of the bilayer.38,58 Interruption of van der Waals interactions between acyl chains as a lipid crosses the bilayer might also slow flip. Acyl unsaturation can accelerate flip in Ld state PC vesicles.56 In the case of a PC having two polyunsaturated chains (which are rare in nature), lipid flip was as fast as minutes. This might result from loose packing in the hydrophobic core, or increased hydrophilicity of the bilayer core due to the presence of double bonds.55 Another study suggested lipid flip in symmetric PC vesicles is faster in thinner bilayers.59 In symmetric vesicles with both ordered and Ld domains we found flip consistent with the rate limiting step being flip in the Ld domains.55
In asymmetric vesicles we found asymmetry is stable for days, i.e. flip is slow, in SUV and LUV containing SM outer leaflets and PC, PE/PS or PS inner leaflets, both in the absence and presence of cholesterol.31,32,44 We carried out more detailed studies in asymmetric SUV with SM in the outer leaflet and various PCs in the inner leaflet.55 (Controls in symmetric vesicles indicated flip in SUV and LUV do not differ greatly.) Results were largely consistent with observations in symmetric vesicles. As measured by the loss of asymmetry, lipid flip rate increased with increasing PC unsaturation, and little or no asymmetry was present if PC had two polyunsaturated chains. Lipid flip was also faster with shorter acyl chain PCs, likely reflecting faster flip in thinner bilayers.
We also studied the effect of headgroup structure on flip in asymmetric SUV composed of SM in their outer leaflet and phospholipids with various headgroups in their inner leaflet.38 Asymmetry decayed somewhat over hours for anionic inner leaflets composed of phosphatidylglycerol (PG), phosphatidylinositol (PI) or cardiolipin (CL), Headgroup-dependent lipid packing differences may control flip in these vesicles. Alternately, repulsions between anionic groups could raise the ground state free energy, and so may decrease the difference between energy of the transition state (a lipid with its headgroup buried in the hydrophobic core of the bilayer) vs. the unflipped state. In contrast, asymmetry was stable for days in vesicles in which the inner leaflet was formed from PS or formed from PE mixed with PG, PI, CL or PS.
While cholesterol flips quickly in the Ld state at room temperature or 37°C, cholesterol slows both spontaneous and peptide-enhanced phospholipid flip.59,60 This presumably reflects tighter packing of the hydrophobic core of the bilayer when cholesterol is present. It should be noted that at very low temperatures in the gel or Lo state cholesterol flip can be very slow.61
Phospholipid flip in PC vesicles labeled asymmetrically by lipid exchange was found by NMR to be much slower in the gel state than in the Ld state, and accelerated when temperature was increased in the Ld state.57 Nevertheless, at the gel to Ld transition temperature, at which packing defects exist at Ld/gel boundaries62, flip was somewhat accelerated relative to higher temperatures. In supported planar bilayer studies flip rates much faster than in vesicles have been reported.63–65 It was proposed that this might be explained by bilayer defects in planar bilayers.57 It should also be noted that lipid flip might be accelerated by CDs59, especially if they bind to vesicles, or perturb lipid balance by net extraction or delivery of outer leaflet lipid. For some lipid compositions some asymmetry might be lost during the lipid exchange step.
Hydrophobic peptides are known to accelerate lipid flip between leaflets.59,60,66–69 This property of the peptide alamethicin was used to destroy lipid asymmetry.32 Recent studies have found that the peptide gramicidin can also accelerate lipid flip in asymmetric vesicles.70 In other studies, the loss of asymmetry due to lipid flip was used to create asymmetric vesicles that gradually expose PS on their outer leaflet, and become susceptible to engulfment by macrophages.71 Such vesicles would be targeted to first bind virus, and then allow the virus to be engulfed by a macrophage
2.5. Asymmetry and Interleaflet Coupling
An important question is: to what degree are physical properties in one leaflet of the bilayer coupled to those in the opposite leaflet? As noted above, if ordered domains in one leaflet induce them in the opposite leaflet, they could trigger lipid-induced signal transduction across the bilayer, a very different process than the familiar signal transduction mediated by transmembrane proteins. There are many ways in which interleaflet coupling might be manifest in a bilayer in which one leaflet contains lipid preferring to form an ordered state.32,40 As shown in Figure 4, the bilayer could have uncoupled leaflets, with each leaflet in the same physical state as in a symmetric bilayer formed by pure outer or inner leaflet lipids. At the other extreme is strong coupling in which an ordered leaflet could induce order in the opposite leaflet (outer leaflet dominates), or in which a disordered leaflet could induce disorder in the opposite leaflet (inner leaflet dominates). There could also be strong coupling in which neither leaflet dominates and the bilayer forms a uniform intermediate physical state, or in which an order gradient forms such that intermediate properties exist near the bilayer midplane. Not illustrated, is partial coupling in which an ordered state or disordered state leaflet imposes a partly ordered or disordered state upon the opposite leaflet.
Figure 4:
Schematic illustration of different degrees of physical state coupling. Top: Behavior of symmetric lipid vesicles composed of single lipids. Middle: weak coupling (left), strong coupling with ordered leaflet dominant (middle) and strong coupling with disordered leaflet dominant (right). Bottom: strong coupling without a dominant leaflet. Not illustrated, an intermediate degree of coupling, and scenarios for temperature dependence of coupling. For the latter see 40.
Coupling can also be temperature dependent. Consider asymmetric vesicles at a temperature at which the two leaflets would have domains in different physical states in symmetric vesicles. At lower temperatures, the leaflet with ordered lipids might dominate bilayer properties while at higher temperatures the disordered lipid leaflet might dominate. Alternately, there could be strong coupling as some temperatures and a lack of coupling at other temperatures.
Measuring interleaflet coupling is difficult. Partly this reflects experimental limitations. Disappearance of domains at the level of light microscopy might only represent their conversion to a submicroscopic size. If the asymmetric bilayer preparation has residual oil between leaflets, interleaflet coupling could be disrupted. Finally, if domains are detected by a fluorescent probe concentrating in Lo or Ld domains, a change in bilayer properties such that a probe partitions close to equally between domains would lead to a loss of contrast which could be mistaken for loss of domains.
The amount of the bilayer in the form of different domains might also alter coupling. For example, when the outer leaflet has a mixture of Lo and Ld domains, and coupling induces the formation of an Lo domain in the inner leaflet, inner leaflet lipids would rearrange laterally, and lipid leaflet lipids in contact with the outer leaflet Lo domain will not be identical to the overall inner leaflet composition.72 The physical properties can then depend on the extent of lipid rearrangement. This lateral rearrangement cannot occur in the inner leaflet when the entire outer leaflet is composed of a single physical state, e.g. all Lo. Another complication is that different physical parameters (domain formation, diffusion, membrane order) may be coupled to different extents, which can lead to different conclusions depending on the parameter being measured.
Pioneering studies with asymmetric planar bilayers revealed that coupling can induce ordered domains large enough to detect by light microscopy in a manner dependent on lipid composition, but the issues noted above limit more detailed interpretation.10,11 A number of studies have now been carried out using asymmetric vesicles prepared by CD exchange. In vesicles lacking cholesterol, we found that in asymmetric SUV and LUV having fully ordered outer leaflets composed of SM, inner leaflets composed of unsaturated PC were only weakly coupled to the outer leaflet in terms of membrane order. This was true both in the absence and presence of cholesterol.31,32 However, our study of lipid diffusion in asymmetric GUV with a mixed SM and unsaturated PC outer leaflet that should be partly in a gel state detected coupling slowing inner leaflet lipid diffusion, for some, but not all, unsaturated PC species.73 Interestingly, overall inner leaflet order was not increased by substitution of SM into the outer leaflet. However, this does not rule out more local changes in the inner leaflet. In terms of the inner leaflet influence on the outer leaflet, small angle neutron scattering and X-ray studies in asymmetric LUV prepared by MβCD-induced exchange have indicated that the level of order of ordered domains in a leaflet containing saturated DPPC and unsaturated PC was decreased by contact with an inner leaflet composed of unsaturated PC.33,74
We carried out confocal light microscopy studies in cholesterol-containing asymmetric GUV having a SM and an unsaturated PC outer leaflet forming co-existing Lo and Ld domains and an inner leaflet composed of the same unsaturated PC. SM rich outer leaflet Lo domains induced Lo domain formation in the inner leaflet.72 Under some conditions, there was a difference in fluorescent probe partition between ordered and disordered domains in the inner and outer leaflet that suggested inner leaflet ordered domains were less ordered than those in the outer leaflet. The low concentration of the unsaturated lipid fluorescent probe in inner leaflet ordered domains (relative to that in disordered domains) suggested ordered domains would also be relatively depleted in unsaturated PC, as thus would have to be enriched in cholesterol.
Our recent studies in cholesterol-containing asymmetric LUV having an outer leaflet composition favoring formation of co-existing Lo and Ld domains and an inner leaflet composed of unsaturated PC have examined domains at the nanodomain size scale using FRET.40 We found that the thermal stability of outer leaflet ordered domains was strongly dependent on the structure of the order-forming outer leaflet lipid, saturated PC. As saturated PC acyl chain length was decreased, there was an increasing loss of outer leaflet ordered domain formation relative to that in symmetric vesicles with the composition of the corresponding outer leaflet. This implies that as saturated PC acyl chain length decreased the disordered inner leaflet increasingly dominated physical properties of the outer leaflet.
Membrane curvature can influence coupling. With asymmetric vesicles with SM-rich outer leaflets and unsaturated lipid rich inner leaflets we found little difference in the coupling of membrane order in asymmetric LUV and SUV, which have different degrees of curvature.32 This was true both with and without cholesterol. However, studies by others in LUV with mixtures of PE and PC found that coupling is affected by the difference in inner and outer leaflet curvature. Pure PE vesicles formed gel state domains at higher temperatures than the PC used.42 In asymmetric vesicles, when the outer leaflet had a PE and PC mixture and the inner leaflet enriched in PC, two melting events were observed, as if each leaflet melted independently, indicating weak coupling. However, when the compositions of the leaflets were reversed, only a single melting event at an intermediate temperature was observed, indicating strong coupling. Clearly, interleaflet coupling is a complex phenomenon.
2.6. Asymmetry Alters the Effects of Sterols Upon Membrane Domain Formation
We found that the influence of sterol structure on Lo domain formation differs in symmetric and asymmetric LUV.75 In addition to sterol, the latter had a SM and DOPC containing outer leaflet, and a DOPC containing inner leaflet. 7-dehydrocholesterol most strongly stabilized ordered domain formation, cholesterol was the next most stabilizing, and 4-cholesten-3-one the least ordered domain stabilizing in both symmetric and asymmetric LUV. However, in asymmetric vesicles desmosterol stabilized ordered domains nearly as well as cholesterol, while epicholesterol stabilized ordered domain much more weakly. This has biological implications, because the correlation between the ability to support ordered domain formation and the ability of cells to carry out endocytosis (transport of molecules from the plasma membrane to internal membranes)76 is stronger when assessed via the ability of sterols to support ordered domain formation in asymmetric vesicles.
2.7. Deriving Other Insights into Membrane Structure and Function Via CD-Catalyzed Lipid Exchange
In addition to membrane domain formation, lipid asymmetry can influence membrane proteins. We found asymmetry of charged lipid increased the fraction of a membrane-bound peptide in a transmembrane orientation as opposed to a non-transmembrane “surface” state.31 Others found asymmetry of PS influences configuration of other transmembrane helices.77 We also studied the response of the protein perfringolysin O (PFO) to asymmetry.78 PFO binding to, and insertion into, membranes involve a series of events: cholesterol binding, oligomerization, insertion of transmembrane beta strands, and formation of a transmembrane beta barrel with a large internal pore. These processes occur if membranes contain sufficient cholesterol. We found lipid asymmetry in cholesterol-containing LUV that mimics plasma membrane asymmetry had a marked effect on these processes. In symmetric vesicles the extent to which each step was triggered had a similar dependence on cholesterol concentration, as if the insertion process was largely all or none. In the asymmetric vesicles, there were two distinct steps. At intermediate cholesterol concentrations PFO bound, oligomerized and inserted, but did not form pores. It should be noted that these studies were aided by the ability of PFO to insert in membranes from the outside in a unidirectional orientation.
In studies of others using CD-induced lipid exchange it was found that lipid asymmetry alters partitioning of a receptor protein between Lo and Ld domains.79 Also, CD-induced lipid exchange was used to demonstrate the ability of in situ changes in lipid composition to alter membrane protein topography.80,81
An exciting application for CD-induced lipid exchange is to replace the endogenous lipids in the outer leaflet of the plasma membranes of cultured cells with exogenous lipids. Prior efforts introduced exogenous lipids into cell membranes using MβCD39,82 or carboxyethyl gamma CD.83 However, the degree to which endogenous lipids were replaced was not measured. Removal of cholesterol by MβCD was circumvented by mixing exogenous cholesterol with MβCD prior to add MβCD to cells.39
We reinvestigated lipid exchange using cells with MαCD, which has favorable properties for phospholipid (and sphingolipid)-specific exchange: strong binding to many lipids and a lack of interaction with cholesterol. Complete or nearly complete replacement of endogenous plasma membrane outer leaflet lipids (other than cholesterol) with exogenous lipids (SM or PC) is achieved without harming cells.43 Our published data and unpublished work shows alteration of cell lipid composition with SM and PC persists for hours after exchange, and the exogenous lipids largely reside in the plasma membrane outer leaflet (plus membrane compartments rapidly cycling to the plasma membrane). The composition of the endogenous lipids removed by exchange, PC and SM with a little PE, was consistent with the asymmetry of mammalian plasma membranes.84
3. Conclusions
The ability to prepare asymmetric vesicles with CD-catalyzed lipid exchange has opened up important areas of membrane structure and function to detailed studies. It seems likely that they will allow derivation of a detailed and realistic understanding of coupling between the leaflets of a lipid bilayer. How lipid asymmetry affects membrane proteins is another area that has been widely ignored, but can now be studied. Note that this will require improved methods to control the orientation of proteins incorporated into artificial membranes.
Potential applications of the ability to change lipid composition in cells are particularly exciting. The ability to introduce unnatural lipids, or lipids with defined combinations of lipid acyl chains and polar head groups could allow studies at an unprecedented level of detail. How membrane organization and lipid composition controls protein function can be investigated. By analyzing endogenous lipid removed from the plasma membrane, plasma membrane asymmetry under different conditions could be evaluated.
Acknowledgement
This work was supported by N.I.H. Grant GM 122493 and N.S.F. grant DMR1709035.
Biography
Erwin London is a Distinguished Professor in the Department of Biochemistry and Cell Biology, and a joint appointment in the Dept. of Chemistry, at Stony Brook University. He received his Ph.D. from Cornell University in 1980. His research has focused using fluorescence spectroscopy and biochemical methods to define the principles of membrane organization, membrane protein-lipid interaction, and membrane protein structure.
References
- (1).London E How principles of domain formation in model membranes may explain ambiguities concerning lipid raft formation in cells. Biochimica et biophysica acta 2005, 1746, 203–220. [DOI] [PubMed] [Google Scholar]
- (2).London E; Brown DA Insolubility of lipids in triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts). Biochimica et biophysica acta 2000, 1508, 182–195. [DOI] [PubMed] [Google Scholar]
- (3).Thompson TE; Tillack TW Organization of glycosphingolipids in bilayers and plasma membranes of mammalian cells. Annual review of biophysics and biophysical chemistry 1985, 14, 361–386. [DOI] [PubMed] [Google Scholar]
- (4).Brown DA; Rose JK Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 1992, 68, 533–544. [DOI] [PubMed] [Google Scholar]
- (5).Ahmed SN; Brown DA; London E On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry 1997, 36, 10944–10953. [DOI] [PubMed] [Google Scholar]
- (6).Schroeder R; London E; Brown D Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proceedings of the National Academy of Sciences of the United States of America 1994, 91, 12130–12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Simons K; Ikonen E Functional rafts in cell membranes. Nature 1997, 387, 569–572. [DOI] [PubMed] [Google Scholar]
- (8).Brown DA; London E Structure of detergent-resistant membrane domains: does phase separation occur in biological membranes? Biochemical and biophysical research communications 1997, 240, 1–7. [DOI] [PubMed] [Google Scholar]
- (9).Schmidt C; Barenholz Y; Huang C; Thompson T Monolayer coupling in sphingomyelin bilayer systems. Nature 1978, 271, 775–777. [DOI] [PubMed] [Google Scholar]
- (10).Wan C; Kiessling V; Tamm LK Coupling of cholesterol-rich lipid phases in asymmetric bilayers. Biochemistry 2008, 47, 2190–2198. [DOI] [PubMed] [Google Scholar]
- (11).Collins MD; Keller SL Tuning lipid mixtures to induce or suppress domain formation across leaflets of unsupported asymmetric bilayers. Proceedings of the National Academy of Sciences of the United States of America 2008, 105, 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Pautot S; Frisken BJ; Weitz DA Engineering asymmetric vesicles. Proc Natl Acad Sci U S A 2003, 100, 10718–10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Hamada T; Miura Y; Komatsu Y; Kishimoto Y; Vestergaard M. d.; Takagi M. Construction of asymmetric cell-sized lipid vesicles from lipid-coated water-in-oil microdroplets. The journal of physical chemistry B 2008, 112, 14678–14681. [DOI] [PubMed] [Google Scholar]
- (14).Hwang WL; Chen M; Cronin B; Holden MA; Bayley H Asymmetric droplet interface bilayers. Journal of the American Chemical Society 2008, 130, 5878–5879. [DOI] [PubMed] [Google Scholar]
- (15).Hu PC; Li S; Malmstadt N Microfluidic fabrication of asymmetric giant lipid vesicles. ACS applied materials & interfaces 2011, 3, 1434–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Arriaga LR; Huang Y; Kim SH; Aragones JL; Ziblat R; Koehler SA; Weitz DA Single-step assembly of asymmetric vesicles. Lab on a chip 2019, 19, 749–756. [DOI] [PubMed] [Google Scholar]
- (17).Arriaga LR; Datta SS; Kim SH; Amstad E; Kodger TE; Monroy F; Weitz DA Ultrathin shell double emulsion templated giant unilamellar lipid vesicles with controlled microdomain formation. Small 2014, 10, 950–956. [DOI] [PubMed] [Google Scholar]
- (18).Richmond DL; Schmid EM; Martens S; Stachowiak JC; Liska N; Fletcher DA Forming giant vesicles with controlled membrane composition, asymmetry, and contents. Proceedings of the National Academy of Sciences 2011, 108, 9431–9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Lu L; Schertzer JW; Chiarot PR Continuous microfluidic fabrication of synthetic asymmetric vesicles. Lab on a chip 2015, 15, 3591–3599. [DOI] [PubMed] [Google Scholar]
- (20).Kamiya K; Kawano R; Osaki T; Akiyoshi K; Takeuchi S Cell-sized asymmetric lipid vesicles facilitate the investigation of asymmetric membranes. Nature chemistry 2016, 8, 881–889. [DOI] [PubMed] [Google Scholar]
- (21).Redelmeier TE; Hope MJ; Cullis PR On the mechanism of transbilayer transport of phosphatidylglycerol in response to transmembrane pH gradients. Biochemistry 1990, 29, 3046–3053. [DOI] [PubMed] [Google Scholar]
- (22).Takaoka R; Kurosaki H; Nakao H; Ikeda K; Nakano M Formation of asymmetric vesicles via phospholipase D-mediated transphosphatidylation. Biochimica et biophysica acta. Biomembranes 2018, 1860, 245–249. [DOI] [PubMed] [Google Scholar]
- (23).Drechsler C; Markones M; Choi JY; Frieling N; Fiedler S; Voelker DR; Schubert R; Heerklotz H Preparation of Asymmetric Liposomes Using a Phosphatidylserine Decarboxylase. Biophysical journal 2018, 115, 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Rothman JE; Dawidowicz EA Asymmetric exchange of vesicle phospholipids catalyzed by the phosphatidylcholine exhange protein. Measurement of inside--outside transitions. Biochemistry 1975, 14, 2809–2816. [DOI] [PubMed] [Google Scholar]
- (25).Bloj B; Zilversmit D Asymmetry and transposition rate of phosphatidylcholine in rat erythrocyte ghosts. Biochemistry 1976, 15, 1277–1283. [DOI] [PubMed] [Google Scholar]
- (26).Everett J; Zlotnick A; Tennyson J; Holloway PW Fluorescence quenching of cytochrome b5 in vesicles with an asymmetric transbilayer distribution of brominated phosphatidylcholine. The Journal of biological chemistry 1986, 261, 6725–6729. [PubMed] [Google Scholar]
- (27).Kim J; London E Using Sterol Substitution to Probe the Role of Membrane Domains in Membrane Functions. Lipids 2015, 50, 721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Leventis R; Silvius JR Use of cyclodextrins to monitor transbilayer movement and differential lipid affinities of cholesterol. Biophysical journal 2001, 81, 2257–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Niu SL; Litman BJ Determination of membrane cholesterol partition coefficient using a lipid vesicle-cyclodextrin binary system: effect of phospholipid acyl chain unsaturation and headgroup composition. Biophysical journal 2002, 83, 3408–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Anderson TG; Tan A; Ganz P; Seelig J Calorimetric measurement of phospholipid interaction with methyl-beta-cyclodextrin. Biochemistry 2004, 43, 2251–2261. [DOI] [PubMed] [Google Scholar]
- (31).Cheng HT; Megha London, E. Preparation and properties of asymmetric vesicles that mimic cell membranes: effect upon lipid raft formation and transmembrane helix orientation. The Journal of biological chemistry 2009, 284, 6079–6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Cheng HT; London E Preparation and properties of asymmetric large unilamellar vesicles: interleaflet coupling in asymmetric vesicles is dependent on temperature but not curvature. Biophysical journal 2011, 100, 2671–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Heberle FA; Marquardt D; Doktorova M; Geier B; Standaert RF; Heftberger P; Kollmitzer B; Nickels JD; Dick RA; Feigenson GW; Katsaras J; London E; Pabst G Subnanometer Structure of an Asymmetric Model Membrane: Interleaflet Coupling Influences Domain Properties. Langmuir 2016, 32, 5195–5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Doktorova M; Heberle FA; Eicher B; Standaert RF; Katsaras J; London E; Pabst G; Marquardt D Preparation of asymmetric phospholipid vesicles for use as cell membrane models. Nature protocols 2018, 13, 2086–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Chiantia S; Schwille P; Klymchenko AS; London E Asymmetric GUVs prepared by MbetaCD-mediated lipid exchange: an FCS study. Biophysical journal 2011, 100, L1–L3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Visco I; Chiantia S; Schwille P Asymmetric supported lipid bilayer formation via methyl-beta-cyclodextrin mediated lipid exchange: influence of asymmetry on lipid dynamics and phase behavior. Langmuir : the ACS journal of surfaces and colloids 2014, 30, 7475–7484. [DOI] [PubMed] [Google Scholar]
- (37).Oglecka K; Rangamani P; Liedberg B; Kraut RS; Parikh AN Oscillatory phase separation in giant lipid vesicles induced by transmembrane osmotic differentials. eLife 2014, 3, e03695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Son M; London E The dependence of lipid asymmetry upon polar headgroup structure. Journal of lipid research 2013, 54, 3385–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Kainu V; Hermansson M; Somerharju P Introduction of phospholipids to cultured cells with cyclodextrin. Journal of lipid research 2010, 51, 3533–3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Wang Q; London E Lipid Structure and Composition Control Consequences of Interleaflet Coupling in Asymmetric Vesicles. Biophysical journal 2018, 115, 664–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Markones M; Drechsler C; Kaiser M; Kalie L; Heerklotz H; Fiedler S Engineering Asymmetric Lipid Vesicles: Accurate and Convenient Control of the Outer Leaflet Lipid Composition. Langmuir : the ACS journal of surfaces and colloids 2018, 34, 1999–2005. [DOI] [PubMed] [Google Scholar]
- (42).Eicher B; Marquardt D; Heberle FA; Letofsky-Papst I; Rechberger GN; Appavou MS; Katsaras J; Pabst G Intrinsic Curvature-Mediated Transbilayer Coupling in Asymmetric Lipid Vesicles. Biophysical journal 2018, 114, 146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Li G; Kim J; Huang Z; St Clair JR; Brown DA; London E Efficient replacement of plasma membrane outer leaflet phospholipids and sphingolipids in cells with exogenous lipids. Proceedings of the National Academy of Sciences of the United States of America 2016, 113, 14025–14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Lin Q; London E Preparation of artificial plasma membrane mimicking vesicles with lipid asymmetry. PloS one 2014, 9, e87903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Monnaert V; Tilloy S; Bricout H; Fenart L; Cecchelli R; Monflier E Behavior of alpha-, beta-, and gamma-cyclodextrins and their derivatives on an in vitro model of blood-brain barrier. The Journal of pharmacology and experimental therapeutics 2004, 310, 745–751. [DOI] [PubMed] [Google Scholar]
- (46).Ohtani Y; Irie T; Uekama K; Fukunaga K; Pitha J Differential effects of alpha-, beta- and gamma-cyclodextrins on human erythrocytes. European journal of biochemistry 1989, 186, 17–22. [DOI] [PubMed] [Google Scholar]
- (47).Huang Z; London E Effect of cyclodextrin and membrane lipid structure upon cyclodextrin-lipid interaction. Langmuir : the ACS journal of surfaces and colloids 2013, 29, 14631–14638. [DOI] [PubMed] [Google Scholar]
- (48).Huang J; Buboltz JT; Feigenson GW Maximum solubility of cholesterol in phosphatidylcholine and phosphatidylethanolamine bilayers. Biochimica et biophysica acta 1999, 1417, 89–100. [DOI] [PubMed] [Google Scholar]
- (49).Courtney KC; Fung KY; Maxfield FR; Fairn GD; Zha X Comment on ‘Orthogonal lipid sensors identify transbilayer asymmetry of plasma membrane cholesterol’. eLife 2018, 7, e38493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Mondal M; Mesmin B; Mukherjee S; Maxfield FR Sterols are mainly in the cytoplasmic leaflet of the plasma membrane and the endocytic recycling compartment in CHO cells. Molecular biology of the cell 2009, 20, 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Liu SL; Sheng R; Jung JH; Wang L; Stec E; O’Connor MJ; Song S; Bikkavilli RK; Winn RA; Lee D; Baek K; Ueda K; Levitan I; Kim KP; Cho W Orthogonal lipid sensors identify transbilayer asymmetry of plasma membrane cholesterol. Nature chemical biology 2017, 13, 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Solanko LM; Sullivan DP; Sere YY; Szomek M; Lunding A; Solanko KA; Pizovic A; Stanchev LD; Pomorski TG; Menon AK; Wustner D Ergosterol is mainly located in the cytoplasmic leaflet of the yeast plasma membrane. Traffic 2018, 19, 198–214. [DOI] [PubMed] [Google Scholar]
- (53).Denz M; Haralampiev I; Schiller S; Szente L; Herrmann A; Huster D; Muller P Interaction of fluorescent phospholipids with cyclodextrins. Chemistry and physics of lipids 2016, 194, 37–48. [DOI] [PubMed] [Google Scholar]
- (54).Bozelli JC Jr.; Hou YH; Epand RM Thermodynamics of Methyl-beta-cyclodextrin-Induced Lipid Vesicle Solubilization: Effect of Lipid Headgroup and Backbone. Langmuir 2017, 33, 13882–13891. [DOI] [PubMed] [Google Scholar]
- (55).Son M; London E The dependence of lipid asymmetry upon phosphatidylcholine acyl chain structure. Journal of lipid research 2013, 54, 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Armstrong VT; Brzustowicz MR; Wassall SR; Jenski LJ; Stillwell W Rapid flip-flop in polyunsaturated (docosahexaenoate) phospholipid membranes. Arch Biochem Biophys 2003, 414, 74–82. [DOI] [PubMed] [Google Scholar]
- (57).Marquardt D; Heberle FA; Miti T; Eicher B; London E; Katsaras J; Pabst G (1)H NMR Shows Slow Phospholipid Flip-Flop in Gel and Fluid Bilayers. Langmuir : the ACS journal of surfaces and colloids 2017, 33, 3731–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Homan R; Pownall HJ Transbilayer diffusion of phospholipids: dependence on headgroup structure and acyl chain length. Biochimica et biophysica acta 1988, 938, 155–166. [DOI] [PubMed] [Google Scholar]
- (59).Nakano M; Fukuda M; Kudo T; Matsuzaki N; Azuma T; Sekine K; Endo H; Handa T Flip-flop of phospholipids in vesicles: kinetic analysis with time-resolved small-angle neutron scattering. The journal of physical chemistry. B 2009, 113, 6745–6748. [DOI] [PubMed] [Google Scholar]
- (60).LeBarron J; London E Effect of lipid composition and amino acid sequence upon transmembrane peptide-accelerated lipid transleaflet diffusion (flip-flop). Biochimica et biophysica acta 2016, 1858, 1812–1820. [DOI] [PubMed] [Google Scholar]
- (61).Courtney KC; Pezeshkian W; Raghupathy R; Zhang C; Darbyson A; Ipsen JH; Ford DA; Khandelia H; Presley JF; Zha X C24 Sphingolipids Govern the Transbilayer Asymmetry of Cholesterol and Lateral Organization of Model and Live-Cell Plasma Membranes. Cell reports 2018, 24, 1037–1049. [DOI] [PubMed] [Google Scholar]
- (62).John K; Schreiber S; Kubelt J; Herrmann A; Muller P Transbilayer movement of phospholipids at the main phase transition of lipid membranes: implications for rapid flip-flop in biological membranes. Biophysical journal 2002, 83, 3315–3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Anglin TC; Conboy JC Kinetics and thermodynamics of flip-flop in binary phospholipid membranes measured by sum-frequency vibrational spectroscopy. Biochemistry 2009, 48, 10220–10234. [DOI] [PubMed] [Google Scholar]
- (64).Anglin TC; Cooper MP; Li H; Chandler K; Conboy JC Free energy and entropy of activation for phospholipid flip-flop in planar supported lipid bilayers. The journal of physical chemistry B 2010, 114, 1903–1914. [DOI] [PubMed] [Google Scholar]
- (65).Brown KL; Conboy JC Lipid flip-flop in binary membranes composed of phosphatidylserine and phosphatidylcholine. The journal of physical chemistry B 2013, 117, 15041–15050. [DOI] [PubMed] [Google Scholar]
- (66).Nakao H; Hayashi C; Ikeda K; Saito H; Nagao H; Nakano M Effects of Hydrophilic Residues and Hydrophobic Length on Flip-Flop Promotion by Transmembrane Peptides. The journal of physical chemistry. B 2018, 122, 4318–4324. [DOI] [PubMed] [Google Scholar]
- (67).Nakao H; Ikeda K; Ishihama Y; Nakano M Membrane-Spanning Sequences in Endoplasmic Reticulum Proteins Promote Phospholipid Flip-Flop. Biophysical journal 2016, 110, 2689–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Kaihara M; Nakao H; Yokoyama H; Endo H; Ishihama Y; Handa T; Nakano M Control of phospholipid flip-flop by transmembrane peptides. Chemical Physics 2013, 419, 78–83. [Google Scholar]
- (69).Wimley WC; White SH Determining the membrane topology of peptides by fluorescence quenching. Biochemistry 2000, 39, 161–170. [DOI] [PubMed] [Google Scholar]
- (70).Doktorova M; Heberle FA; Marquardt D; Rusinova R; Sanford RL; Peyear TA; Katsaras J; Feigenson GW; Weinstein H; Andersen OS Gramicidin Increases Lipid Flip-Flop in Symmetric and Asymmetric Lipid Vesicles. Biophysical journal 2019, 116, 860–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Petazzi RA; Gramatica A; Herrmann A; Chiantia S Time-controlled phagocytosis of asymmetric liposomes: Application to phosphatidylserine immunoliposomes binding HIV-1 virus-like particles. Nanomedicine: Nanotechnology, Biology and Medicine 2015, 11, 1985–1992. [DOI] [PubMed] [Google Scholar]
- (72).Lin Q; London E Ordered raft domains induced by outer leaflet sphingomyelin in cholesterol-rich asymmetric vesicles. Biophysical journal 2015, 108, 2212–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Chiantia S; London E Acyl chain length and saturation modulate interleaflet coupling in asymmetric bilayers: effects on dynamics and structural order. Biophysical journal 2012, 103, 2311–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Eicher B; Heberle FA; Marquardt D; Rechberger GN; Katsaras J; Pabst G Joint small-angle X-ray and neutron scattering data analysis of asymmetric lipid vesicles. Journal of applied crystallography 2017, 50, 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).St Clair JW; London E Effect of sterol structure on ordered membrane domain (raft) stability in symmetric and asymmetric vesicles. Biochimica et biophysica acta. Biomembranes 2019, 1861, 1112–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Kim JH; Singh A; Del Poeta M; Brown DA; London E The effect of sterol structure upon clathrin-mediated and clathrin-independent endocytosis. J Cell Sci 2017, 130, 2682–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Scott HL; Heberle FA; Katsaras J; Barrera FN Phosphatidylserine Asymmetry Promotes the Membrane Insertion of a Transmembrane Helix. Biophysical journal 2019, 116, 1495–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Lin QQ; London E The Influence of Natural Lipid Asymmetry upon the Conformation of a Membrane-inserted Protein (Perfringolysin O). Journal of Biological Chemistry 2014, 289, 5467–5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Perillo VL; Peñalva DA; Vitale AJ; Barrantes FJ; Antollini SS Transbilayer asymmetry and sphingomyelin composition modulate the preferential membrane partitioning of the nicotinic acetylcholine receptor in Lo domains. Archives of biochemistry and biophysics 2016, 591, 76–86. [DOI] [PubMed] [Google Scholar]
- (80).Vitrac H; Bogdanov M; Dowhan W In vitro reconstitution of lipid-dependent dual topology and postassembly topological switching of a membrane protein. Proceedings of the National Academy of Sciences 2013, 110, 9338–9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Vitrac H; MacLean DM; Jayaraman V; Bogdanov M; Dowhan W Dynamic membrane protein topological switching upon changes in phospholipid environment. Proceedings of the National Academy of Sciences of the United States of America 2015, 112, 13874–13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Kainu V; Hermansson M; Somerharju P Electrospray ionization mass spectrometry and exogenous heavy isotope-labeled lipid species provide detailed information on aminophospholipid acyl chain remodeling. The Journal of biological chemistry 2008, 283, 3676–3687. [DOI] [PubMed] [Google Scholar]
- (83).Tanhuanpää K; Somerharju P γ-Cyclodextrins Greatly Enhance Translocation of Hydrophobic Fluorescent Phospholipids from Vesicles to Cells in Culture IMPORTANCE OF MOLECULAR HYDROPHOBICITY IN PHOSPHOLIPID TRAFFICKING STUDIES. Journal of Biological Chemistry 1999, 274, 35359–35366. [DOI] [PubMed] [Google Scholar]
- (84).Verkleij AJ; Zwaal RF; Roelofsen B; Comfurius P; Kastelijn D; van Deenen LL The asymmetric distribution of phospholipids in the human red cell membrane. A combined study using phospholipases and freeze-etch electron microscopy. Biochimica et biophysica acta 1973, 323, 178–193. [DOI] [PubMed] [Google Scholar]