Abstract
As opportunistic infections among human T-lymphotrophic virus type 1 (HTLV-1) carriers and patients with adult T-cell leukemia/lymphoma (ATL) pose a serious problem, it is necessary to clarify their clinical characteristics and outcomes in these patients. We retrospectively analyzed the clinical features and outcomes of opportunistic infections in 127 HTLV-1 carriers and 153 ATL patients between 2006 and 2016. The cumulative incidence rates of opportunistic infections among HTLV-1 carriers and ATL patients were 1.5% (2/127) and 6.5% (10/153), respectively. The etiology of opportunistic infections was as follows: fungal infections (3 cases), pneumocystis pneumonia, and cytomegalovirus (CMV) infections. Even after aggressive treatment, the prognosis of opportunistic infections was poor (50% of overall survival at 28 days). Regarding prognostic factors affecting the OS of opportunistic infections, higher SOFA scores (especially the respiratory subscore) and higher LDH values were identified by univariate analysis. Moreover, 3 out of 6 patients achieved spontaneous remission of ATL as the short-term outcome after the development of opportunistic infection. However, 5 out of 6 surviving patients exhibited ATL progression or relapse after a median of 194 days (133–226) after contracting an opportunistic infection as the long-term outcome of ATL. In conclusion, opportunistic infections should be carefully followed among HTLV-1 carriers and ATL patients because of their aggressive clinical course and poor outcomes. Furthermore, early diagnosis and subsequent prompt treatment are necessary in clinical practice.
Keywords: : adult T-cell leukemia/lymphoma, HTLV-1 carrier, opportunistic infection, spontaneous remission of ATL, progression of ATL
INTRODUCTION
It is well known that human T-lymphotrophic virus type 1 (HTLV-1) infection causes T-cell dysfunction based on the study by Mituya et al. in 1984.1 Moreover, Shimoyama reported in 1991 that opportunistic infections, including fungal and viral infections as well as Pneumocystis pneumonia, are a major cause of morbidity and mortality among HTLV-1 carriers and patients with adult T-cell leukemia-lymphoma (ATL).2 Subsequently, Suzumiya et al. published a study on autopsy cases of patients with ATL in Miyazaki prefecture in 1993, demonstrating the high incidence rates, clinical features, and outcomes of opportunistic infection among these patients.3 Although the reasons or mechanisms underlying the high incidence of opportunistic infections in HTLV-1 carriers and patients with ATL are not completely understood at present, one of the mechanisms involved is the reduction of CD4+ T-cell functions due to HTLV-1 infection.4,5 Recently, Yanagihara et al. reported that one of the mechanisms of the susceptibility of HTLV-1–infected patients to opportunistic infection is the expression of PD-1 and PDL-1 on cytotoxic T lymphocytes (CTLs) to suppress host defense against pathogens.6
Although there has been marked progress in the discovery of biomarkers (β-D-glucan, Asp Ag, and CMV C7 HRP) for diagnosis, and in antifungal and antiviral treatment [fluconazole (FLCZ), itraconazole (ITCZ), voriconazole (VRCZ), and liposomal amphotericin B (L-AMPH-B)] of opportunistic infections over the past few decades, opportunistic infections in HTLV-1 carriers and ATL patients still represent a serious problem.6,7 Furthermore, spontaneous remission of ATL after these patients contract an opportunistic infection has been previously documented in several case reports.7-24
However, the incidence, clinical features, and treatment outcomes of opportunistic infections among HTLV-1 carriers and patients with ATL are unclear. Thus, in this study, we elucidated these parameters of opportunistic infections in HTLV-1 carriers and ATL patients. Moreover, we investigated the short- and long-term effects of antimicrobial treatment on opportunistic infection and ATL in clinical practice.
PATIENTS AND METHODS
We retrospectively analyzed 12 patients with opportunistic infections among 153 ATL patients and 127 HTLV-1 carriers during 11 years from January 1, 2006, to December 31, 2016. At present, ATL is diagnosed by Shimoyama’s diagnostic criteria (acute, lymphoma, chronic, and smoldering types).1 Each HTLV-1 carrier in our study was diagnosed by the presence of anti–HTLV-1 antibody and asymptomatic chronic clinical course that did not meet the diagnostic criteria of smoldering ATL type or chronic type according to Shimoyama’s guidelines because of the absence of definite diagnostic criteria of HTLV-1.1 In all patients, HTLV-1 clonality was examined by Southern blot analysis using nonradioactive probes specific for the entire HTLV-I genome.
To clarify the etiology of opportunistic infection among HTLV-1 carriers and ATL patients, we examined the serological markers of β-D glucan, CMV pp65 antigenemia (CMV C7 HRP), Aspergillus antigen, Cryptococcus antigen, and Candida antigen in peripheral blood (PB), computed tomography (CT), cytology, culture, and genetic analysis [polymerase chain reaction (PCR) analysis for Pneumocystis jirovecii] of bronchoalveolar lavage fluid (BALF). Furthermore, TBLB was performed to investigate nodular/mass lesions to exclude lung cancer or fungal infection by CT.
In case 4, to clarify the Aspergillus species, PCR analysis of BALF was performed.
According to the laboratory findings, radiological findings, and BALF or TBLB findings, we diagnosed the following opportunistic infections: Pneumocystis pneumonia, cytomegalovirus (CMV) pneumonia, Aspergillus pneumonia, and cryptococcal pneumonia based on the guidelines of the Infectious Disease Society of America (IDSA), Mycoses Forum, the Japan Society for Hematopoietic Cell Transplantation (JSHCT), and the Japan Society for Medical Mycology.25-28
We retrospectively analyzed the etiology, diagnosis, and treatment outcomes of the 12 cases of opportunistic infection. We compared the parameters related to opportunistic infections (β-D-glucan, CMV C7-HRP, and SOFA score) and the parameters related to ATL (WBC, Ab-ly, LDH, and sIL-2R) after treatment of opportunistic infections. Subsequently, we examined the overall survival (OS) in the 12 cases of opportunistic infection. Thus, to improve the poor outcome of opportunistic infections, we compared the profiles of surviving patients and those that died among the 12 cases of opportunistic infection. Lastly, we examined the impact of opportunistic infections on the disease status of patients with ATL and of HTLV-1 carriers.
In case 6, to examine the presence of HTLV-1–specific CTLs and CMV-specific CTLs, we examined the HLA-A24–restricted HTLV-1–specific CTLs and the HLA-A24–restricted CMV-specific CTLs by HLA-A24 flow cytometric (FCM) tetramer assay.
The changes in the parameters related to opportunistic infections and ATL were examined by the Wilcoxon signed-rank test. The parameters between survivors and non-survivors were compared using the Mann–Whitney U test. Significance was determined using a 2-sided P-value (should be <0.05).
This retrospective study was performed in compliance with good clinical practices and the ethical principles of the Declaration of Helsinki. We received prior approval from the ethics review board at Miyazaki Prefectural Miyazaki Hospital.
RESULTS
The clinical features and treatment outcomes of 12 HTLV-1 carriers and patients with ATL with opportunistic infections are shown in Table 1.
Table 1. The overview of clinical features and treatment outcomes in 12 immunocompromised patients with infections among HTLV-1 carriers and ATL cases.
| Case | Age | Sex | Clinical subtype | Opportunistic infection | Onset of opportunistic infection | βD Glucan (PB) | CM V C7 HRP (PB) | Aspergillus antigen (PB) | Cryptococcus antigen (PB) | The histological Findings (BALF or TBLB) | PS | SOFA | HTLV-1 clonality | Treatment for opportunistic infection | Outcome of opportunistic infection at 28 days | Outcome of ATL at 28 days | Relapse of ATL | Salvage therapy | Final outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 | M | acute type | Pneumocystis pneumonia | During treatment (LSG 15, PD status) | 1278 | Negative | negative | negative | Grocott stain+ Pneumocystis PCR+ | 3 | 13 | Monoclonality | ST, Pentamidine, mPSL | Not improved (4 days) | PD | - | not done | Dead (4 days) |
| 2 | 71 | F | carrier type | Pneumocystis pneumonia | Initial diagnosis | 117 | negative | negative | negative | Grocott stain+ Pneumocystis PCR+ | 3 | 6 | n.e. | ST, Pentamidine, mPSL | Not improved (27 days) | SD | - | not done | Dead (27 days) |
| 3 | 65 | M | chronic type | Cryptococcus pneumonia | Initial diagnosis | negative | negative | negative | positive | Grocott stain+ Cryptococcal organism+ | 3 | 10 | Monoclonality | L-AMPH-B | Improved (55 days needed to remission) | SD | Relapse at 226 days | CHOP | Dead (305 days) |
| 4 | 73 | F | acute type | Aspergillus pneumonia | Initial diagnosis | negative | 4 | 2.2 | negative | Grocott stain+ Aspergillus bundles, PCR+ | 3 | 8 | Monoclonality | L-AMPH-B | Not improved (26 days) | PD | - | not done | Dead (26 days) |
| 5 | 74 | F | smoldering type | Cryptococcus pneumonia | Initial diagnosis | negative | negative | negative | positive | Grocott stain+ Cryptococcal organism+ | 0 | 0 | Clonality (-) | Surgery FLCZ | Improved (16 days needed to remission) | SD | - | not done | Alive (2689 days) |
| 6 | 56 | F | acute type | CMV pneumonia | During treatment (CHOP-VMMV, PD status) | 18 | 1132 | negative | negative | CMV PCR+ | 3 | 6 | Biclonality | DHPG mPSL | Improved (52 days needed to remission) | CR | Relapse at 213 days | RTx | Dead (240 days) |
| 7 | 67 | M | acute type | CMV pneumonia | During treatment (CHOP-VMMV, PD status) | negative | 124 | negative | negative | CMV PCR+ | 1 | 8 | Monoclonality | DHPG mPSL | Improved (51 days needed to remission) | PR | Relapse at 133 days | CHOP | Dead (246 days) |
| 8 | 78 | F | chronic type | CMV pneumonia | Initial diagnosis | negative | 360 | negative | negative | CMV PCR+ | 0 | 6 | Monoclonality | DHPG mPSL | Not improved (14 days) | SD | - | not done | Dead (14 days) |
| 9 | 74 | M | acute type | CMV pneumonia | Initial diagnosis | negative | 121 | negative | negative | CMV PCR+ | 4 | 8 | Monoclonality | DHPG mPSL | Not improved (15 days) | PD | - | not done | Dead (15 days) |
| 10 | 72 | F | carrier | CMV pneumonia | Initial diagnosis | negative | 520 | negative | negative | CMV PCR+ | 4 | 10 | Clonality (-) | DHPG mPSL | Not improved (2 days) | SD | - | not done | Dead (2 days) |
| 11 | 54 | M | chronic type | Pneumocystis pneumonia | Initial diagnosis | 353 | negative | negative | negative | Grocott stain+ Pneumocystis PCR+ | 2 | 2 | Monoclonality | ST, mPSL | Improved (18 days needed to remission) | PR | Relapse at 153 days | mLSG 15 →allo- HSCT | Alive (631 days) |
| 12 | 78 | M | smoldering type | Pneumocystis pneumonia | Initial diagnosis | 374 | negative | negative | negative | Grocott stain+ Pneumocystis PCR+ | 2 | 3 | Monoclonality | ST, mPSL | Improved (29 days needed to remission) | SD | Relapse at 194 days | BSC | Dead (215 days) |
Among the 12 cases of opportunistic infection, the average age was 66.3 years (range 34–78) and the male/female ratio was 1:1. Patients with the following subtypes of ATL developed opportunistic infections: acute type (5 patients), lymphoma type (0 patients), chronic type (3 patients), smoldering type (2 patients), and HTLV-1 carriage (2 patients). The cumulative incidence rates of opportunistic infections among HTLV-1 carriers and ATL patients were 1.5% (2/127) and 6.5% (10/153), respectively. To clarify the etiology of opportunistic infection among HTLV-1 carriers and ATL patients, we measured the serological markers of β-D glucan, CMV C7HRP, Aspergillus antigen, and Cryptococcus antigen, and performed cytology, culture, and genetic analysis (PCR for Pneumocystis jirovecii) of BALF. Furthermore, focal lesions found on CT underwent TBLB to exclude lung cancer. We empirically treated bacterial, viral, or fungal infections using antibiotics, antifungal agents, or antiviral drugs, including cefepime (CFPM) or meropenem (MEPM), caspofungin, liposomal amphotericin B (L-AMPH-B), ganciclovir (DHPG), and trimethoprim-sulfamethoxazole (ST), respectively, until a definite diagnosis of the opportunistic infection was made. The identified etiologies of opportunistic infections were Pneumocystis pneumonia (4 cases), cryptococcal pneumonia (2 cases), Aspergillus pneumonia (1 case), and CMV pneumonia (5 cases).
Pneumocystis pneumonia (cases 1, 2, 11, and 12)
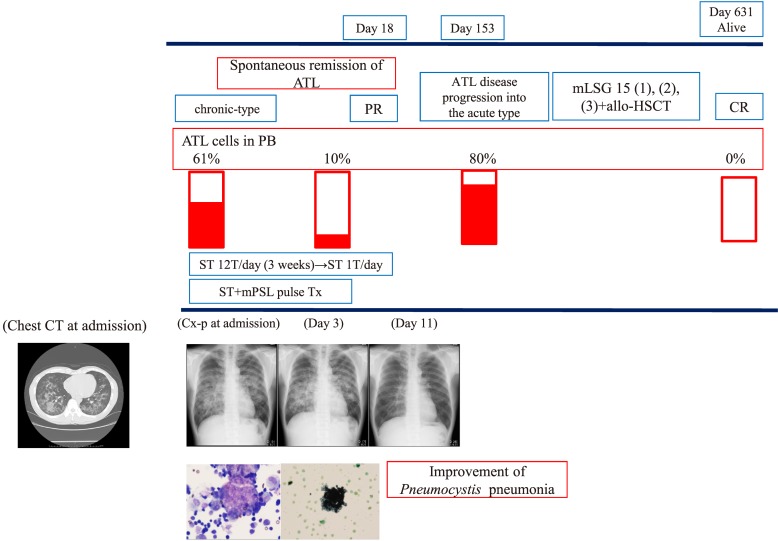
Pneumocystis pneumonia was diagnosed by combination of clinical signs such as progressive respiratory failure, serological findings, CT findings, and cytology and genetic analysis (PCR for Pneumocystis jirovecii) of BALF. Serological findings included high β-D-glucan levels. In addition, CT demonstrated bilateral diffuse ground-glass opacities with peripheral sparing.4 Subsequent cytology analysis of BALF revealed cysts of Pneumocystis jirovecii and PCR analysis of BALF revealed the presence of Pneumocystis jirovecii. Regarding treatment, sulfamethoxazole trimethoprim (ST) or pentamidine was administered. mPSL pulse was performed according to the degree of acute respiratory failure. A representative clinical course (case 11) is shown in Fig. 1A.
Fig. 1A.
Clinical course of case 11. Age 54 years, chronic-type ATL, pneumocystis pneumonia → improvement → the development of acute-type ATL.
A 54-year-old male was followed for chronic-type ATL with the CD3+CD4+CD25+CD5+CD7+ phenotype on FCM analysis and a monoclonal band on Southern blot analysis. The patient presented with acute progressive respiratory failure. Laboratory findings were as follows: WBC 32550/μL (60%), LDH 500 IU/ml, sIL2R 14500 IU/ml, CRP 2 mg/dl, β-D-glucan: 353.7 pg/mL, Aspergillus Ag(-), and CMV C7 HRP(-). Furthermore, CT showed bilateral interstitial pneumonia sparing the normal lung field under the pleural membrane. BAL analysis revealed a foamy population after May–Giemsa staining, Papanicolaou staining, and Grocott staining. Furthermore, Pneumocystis jiroveci was confirmed by PCR analysis. These findings led to the final diagnosis of Pneumocystis pneumonia. Subsequent ST treatment and mPSL pulse therapy led to the cure of Pneumocystis pneumonia. During the treatment of Pneumocystis pneumonia, the status of ATL was PR. However, chronic-type ATL progressed to acute-type ATL on day 153. Subsequent chemotherapy including mLSG 15 and allo-HSCT led to CR of ATL on day 631.
Aspergillus pneumonia (case 4)
Aspergillus pneumonia was diagnosed by the combination of aggressive clinical symptoms, CT findings, Aspergillus antigen-positive status, the culture findings of BALF and the histological findings of TBLB. Chest CT demonstrated right upper lobe cavitary lesions consistent with pulmonary aspergillosis. Moreover, histopathological findings included marked inflammation with neutrophilic infiltration, coagulation necrosis, and fungal elements. PCR analysis of BALF revealed the etiology of fungal infection to be Aspergillus fumigatus. L-AMPH-B was administered for treatment.
Cryptococcus pneumonia (cases 3 and 5)
Cryptococcus pneumonia was diagnosed by the subacute clinical course, serum cryptococcus antigen-positive status, CT findings, histological findings of TBLB, and culture of BALF. CT demonstrated solitary1 or multiple nodules2 requiring differential diagnosis from lung cancer. Moreover, histological findings of the TBLB specimen included histiocytic aggregation with multinuclear giant cells and cryptococcal organisms. Furthermore, to exclude cryptococcus meningitis, lumbar puncture was performed. L-AMPH-B was administered for treatment.
CMV pneumonia (cases 6, 7, 8, 9, and 10)
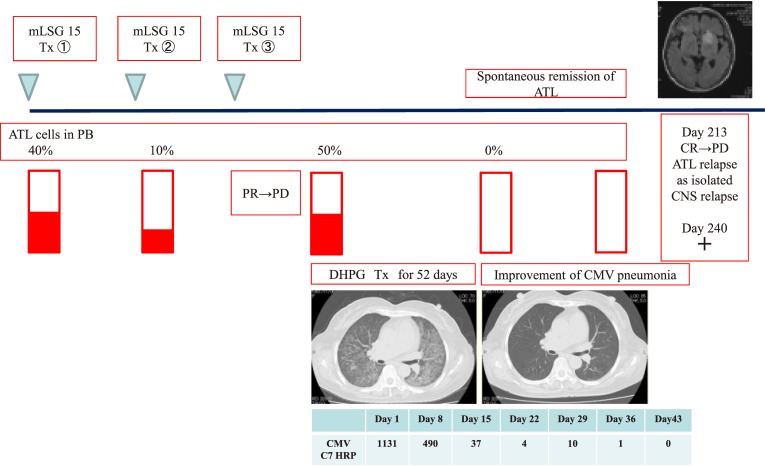
CMV pneumonia was diagnosed by the combination of clinical symptoms, CT findings, CMV pp65 antigenemia, CMV PCR, and cytology of BALF. CT demonstrated ground-glass opacities in all patients, with bilateral patchy (n = 1) and bilateral diffuse (n = 4) distribution, respectively. Serological CMV C7 HRP in PB was positive in all patients. In addition, PCR analysis of BALF for CMV was positive in all patients. Furthermore, cytology analysis of BALF for CMV also revealed the presence of inclusion bodies, suggesting the reactivation of CMV in all patients. Regarding treatment, ganciclovir (DHPG) was administered. For patients with bilateral diffuse distribution in CT (n = 4), mPSL pulse therapy was performed because of the aggressive respiratory failure mimicking acute interstitial lung disease on CT. A representative clinical course (case 6) is shown in Fig. 1B.
Fig. 1B.
Clinical course of case 6. Age 56 years, acute-type ATL, CMV pneumonia → improvement → ATL relapse.
A 56-year-old female was diagnosed with acute-type ATL and treated using the mLSG 15 regimen. However, the disease status of ATL was PD after mLSG 15 therapy. During chemotherapy, the patient developed acute progressive respiratory failure due to opportunistic infection. Subsequent CT revealed bilateral interstitial pneumonia. Based on the positive findings of CMV C7-HRP in PB and CMV PCR in BALF, CMV pneumonia was diagnosed. Subsequent DHPG therapy and mPSL pulse therapy cured CMV pneumonia within 52 days. During the treatment of CMV pneumonia, the disease status of ATL was CR, but she developed isolated CNS relapse on day 213. Subsequent RTx did not affect the CNS lesions of ATL at 240 days.
The onset of opportunistic infection was observed at initial diagnosis (9/12) or during the treatment of ATL (3/12). Prophylactic administration of antimicrobial drugs for opportunistic infections was not carried out in the group that developed opportunistic infections at initial diagnosis. For the group that contracted opportunistic infections during the treatment of ATL (cases 1, 6, and 7), ST and ITCZ were administered. Regarding the prophylactic treatment, e.g., ST and FLCZ/ITCZ for opportunistic infections, it was not administered to HTLV-1 carriers. Although prophylactic treatment, such as the administration of ST and ITCZ, was performed for patients with ATL during chemotherapy, opportunistic infections developed in cases 1, 6, and 7.
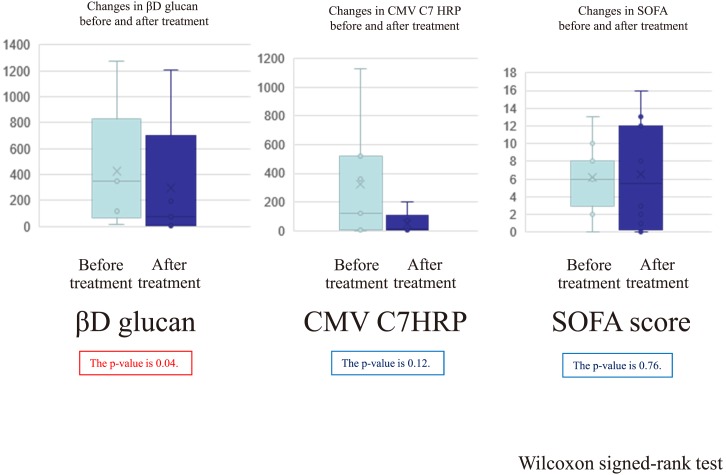
Next, we compared the changes in parameters related to infection among immunocompromised patients after treatment in the 12 cases of opportunistic infection (Fig. 2). The values of β-D-glucan and CMV C7-HRP decreased after treatment of opportunistic infections (Pneumocystis pneumonia and CMV pneumonia, respectively). The values of SOFA did not significantly improve and varied from progression to improvement after treatment (Fig. 2). In case 11 (survivor), the β-D-glucan level slowly declined over 2 months despite the improvement of bilateral interstitial pneumonia after 1 month. In case 6 (survivor), the CMV C7-HRP level slowly decreased over 2 months despite the improvement of bilateral interstitial pneumonia after 1 month.
Fig. 2.
The changes in parameters related to opportunistic infection after treatment.
We compared the changes of parameters related to infection in immunocompromised patients after treatment in 12 cases of opportunistic infection. The values of β-D-glucan and CMV C7-HRP decreased after treatment of opportunistic infections (Pneumocystis pneumonia and CMV pneumonia, respectively). The SOFA scores decreased in survivors and deteriorated in non-survivors.
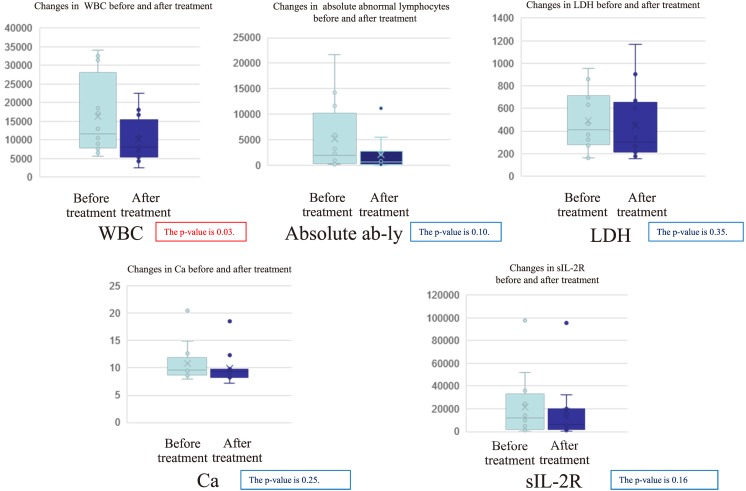
We also examined the changes in parameters related to ATL after treatment (Fig. 3). The values of WBC, absolute numbers of abnormal-lymphocytes, LDH, Ca, and sIL-2R decreased after treatment of opportunistic infections (Fig. 3).
Fig. 3.
The changes in parameters related to ATL after treatment.
We examined the changes in parameters related to ATL after treatment. The values of WBC, Ab-ly, LDH, Ca, and sIL-2R decreased after treatment of opportunistic infection.
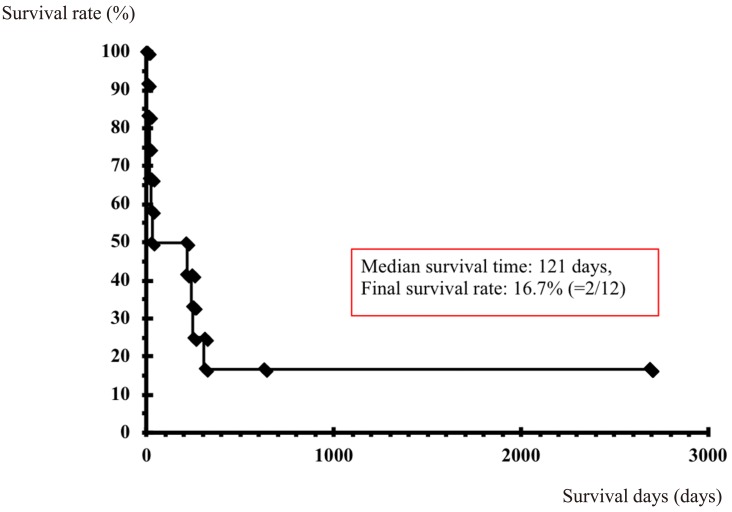
Consequently, we investigated treatment outcomes of the 12 cases of opportunistic infection among ATL patients and HTLV-1 carriers (Fig. 4). The OS of 28 days indicated a poor prognosis (OS rate of 50%; 6/12). Among the 6 surviving patients, 5 developed PD (3 patients) or relapse of ATL (2 patients) at a median of 194 days (range 133–226). Thus, the median OS in this group was 121 days, and OS rate was 16.7% (2/12).
Fig. 4.
Treatment outcomes of opportunistic infection among HTLV-1 carriers and ATL patients.
We investigated treatment outcomes of 12 cases of opportunistic infection among ATL patients and HTLV-1 carriers. The prognosis was poor: OS of 28 days and OS rate of 50% (6/12). Among 6 survivors, 5 presented with ATL PD (3 patients) and relapse of ATL (2 patients) at a median of 194 days (range 133–226). Thus, the median OS was 121 days and OS rate was 16.7% (2/12).
We next compared the physiological, laboratory, and immunological findings of ATL patients who achieved spontaneous remission after the treatment of opportunistic infections (remission group: cases 6, 7, and 11) with those whose disease progressed after the treatment of opportunistic infections (non-remission group; cases 1, 2, 3, 4, 5, 8, 9, 10, and 12; Table 2). Our analysis revealed that the absolute numbers of abnormal lymphocytes in the peripheral blood (PB) of the remission group were slightly higher than those of the non-remission group (Table 2). Moreover, based on our analysis, the non-remission group had hyperbilirubinemia (Table 2).
Table 2. The differences in laboratory markers between responders (n = 3) and non-responders (n = 9).
| All patients (n=12) | Responders (n=3) | Non-responders (n=9) | Statistical analysis | |
|---|---|---|---|---|
| Age | 71.5 (34-78) | 56 (54-67) | 73 (34-78) | 0.07 |
| PS | 3(0-4) | 2 (1-3) | 3 (0-4) | 0.44 |
| WBC | 11695 (5630-34000) | 9070 (6440-32500) | 12900 (5630-34000) | 0.78 |
| Absolute numbers of abnormal lymphocytes | 875 (56-19855) | 5895 (3220-19855) | 979 (56-14280) | 1.75 |
| Hb | 11.9 (4-15) | 7.8 (7.5-15) | 12.1 (4-13.9) | 0.64 |
| Plt | 21.9 (0.6-31.8) | 5.7 (0.6-31.8) | 22.7 (2-30.6) | 0.51 |
| PT-INR | 1.3 (1-1.4) | 1.1 (1-1.4) | 1.3 (1-1.3) | 0.60 |
| FIB | 257 (77.1-663) | 250 (192-663) | 262 (77.1-325) | 0.92 |
| FDP | 8.3 (1.5-14.1) | 8.2 (1.5-8.6) | 8.4 (3.2-14.1) | 0.51 |
| T.Bil | 1.15 (0.3-13.1) | 0.4 (0.3-0.7) | 1.2 (0.5-13.1) | 0.03 * |
| GOT | 37 (13-355) | 28 (14-58) | 38 (13-355) | 0.57 |
| GPT | 20.5 (8-286) | 24 (10-28) | 19 (8-286) | 0.85 |
| LDH | 414.5 (163-956) | 368 (285-461) | 634 (163-956) | 0.64 |
| BUN | 18.2 (7.6-97.7) | 14.1 (7.6-23.2) | 19.1 (9.7-97.7) | 0.31 |
| Cr | 0.8 (0.6-5.6) | 0.8 (0.7-0.8) | 0.8 (0.6-5.6) | 0.70 |
| CRP | 3.3 (0.24-29.2) | 5.2 (1.5-6.4) | 2.4 (0.24-29.2) | 0.51 |
| Ca | 9.55 (8-20.5) | 8.9 (8-9.5) | 9.6 (8.5-20.5) | 0.11 |
| sIL-2R | 12145 (481-97900) | 14500 (9790-36200) | 4520 (481-97900) | 0.51 |
| SOFA score | 7 (0-13) | 6 (2-8) | 8 (0-13) | 0.39 |
| DIC score | 2 (1-5) | 2 (2-2) | 2 (1-5) | 0.71 |
*, Mann-Whitney U test
Furthermore, we carefully analyzed the non-survivors with opportunistic infections (Table 1). All six non-survivors presented with aggressive acute respiratory failure requiring intensive treatment with intubation and ventilation in an intensive care unit. The patients with Pneumocystis pneumonia (cases 1 and 2) died on days 18 and 27, respectively. The patient with Aspergillus pneumonia (case 4) died on day 26. The patients with CMV pneumonia (cases 8, 9, and 10) died on days 14, 15, and 2, respectively. Thus, all six non-survivors exhibited aggressive progressive disease within 4 weeks.
To elucidate the factors affecting the poor prognosis, we analyzed the survivors and non-survivors among the 12 cases of opportunistic infection (patients with ATL and HTLV-1 carriers; Table 3). Non-survivors had a shorter OS and higher SOFA score and LDH than survivors. Moreover, all 6 non-survivors presented with aggressive acute respiratory failure requiring intensive treatment with intubation and ventilation in an intensive care unit. Thus, we evaluated each factor composing the SOFA score such as the cardiovascular subscore, renal subscore, respiratory subscore, coagulation subscore, hepatic subscore, and neurological subscore. Among these six subscores, the respiratory subscore was found to be a significant factor affecting OS by univariate analysis, as shown in Table 3. There was no difference in platelet number, serum T-bilirubin, or creatinine levels between the two groups, as shown in Table 3.
Table 3. The analysis of surviving patients and those that died among 12 immunocompromised patients with infection among HTLV-1 carriers and ATL cases.
| Improved cases of opportunistic infection |
Not improved cases of opportunistic infection |
P-value | |
|---|---|---|---|
| Age | 66 (51-77) | 72.5 (34-78) | 0.47 |
| WBC | 8985 (5630-32550) | 17790 (7590-34000) | 0.14 |
| Absolute numbers of AB-Ly | 2385 (56-19855) | 1285 (171-14280) | 0.32 |
| Hb | 12.5 (7.5-15) | 11.6 (4-12.8) | 0.33 |
| Plt | 24.6 (0.6-31.8) | 17.35 (2-30.6) | 0.52 |
| PT-INR | 1.1 (1.0-1.4) | 1.3 (1.3-1.4) | 0.06 |
| FIB | 271 (192-663) | 257 (77.1-325) | 1.0 |
| FDP | 7.5 (1.5-8.6) | 8.8 (5-14.1) | 0.05 |
| T.BIL | 0.6 (0.3-1.3) | 1.2 (0.5-13.1) | 0.12 |
| GOT | 21 (13-58) | 57 (17-355) | 0.05 |
| GPT | 23 (8-28) | 16 (9-286) | 0.87 |
| LDH | 303.5 (163-461) | 707 (275-956) | 0.02 * |
| BUN | 12.3 (7.6-28.1) | 30.45 (13.3-97.7) | 0.07 |
| Cr | 0.8 (0.7-0.9) | 0.75 (0.6-5.6) | 0.86 |
| CRP | 2.85 (0.24-6.4) | 4 (0.47-29.2) | 0.33 |
| Ca | 9.25 (8-9.6) | 11.15 (8.5-20.5) | 0.19 |
| sIL-2R | 7155 (481-36200) | 20100 (1420-97900) | 0.33 |
| SOFA score | 4 (0-8) | 8 (6-13) | 0.03 * |
| Hepatic subscore | 0 (0-1) | 0 (0-4) | 0.40 |
| Renal subscore | 0 (0-0) | 0 (0-3) | 0.139 |
| Coagulation subscore | 0 (0-4) | 0.5 (0-3) | 0.788 |
| Respiratory subscore | 2 (0-3) | 3 (2-3) | 0.0259* |
| Cardiovascular subscore | 1.5 (0-3) | 2.5 (2-3) | 0.0749 |
| Neurological subscore | 0 (0-4) | 0 (0-1) | 0.673 |
| DIC score | 2 (1-2) | 2 (2-5) | 0.09 |
| βD glucan | 9 (0-374) | 0 (0-1278) | 0.72 |
| CMV C7 HRP | 0 (0-1132) | 62.5 (0-520) | 0.49 |
| PS | 2 (0-3) | 3 (0-4) | 0.11 |
| OS | 275.5 (215-2689) | 14.5 (2-27) | 0.003 ** |
*, Mann-Whitney U test **, Log-rank test
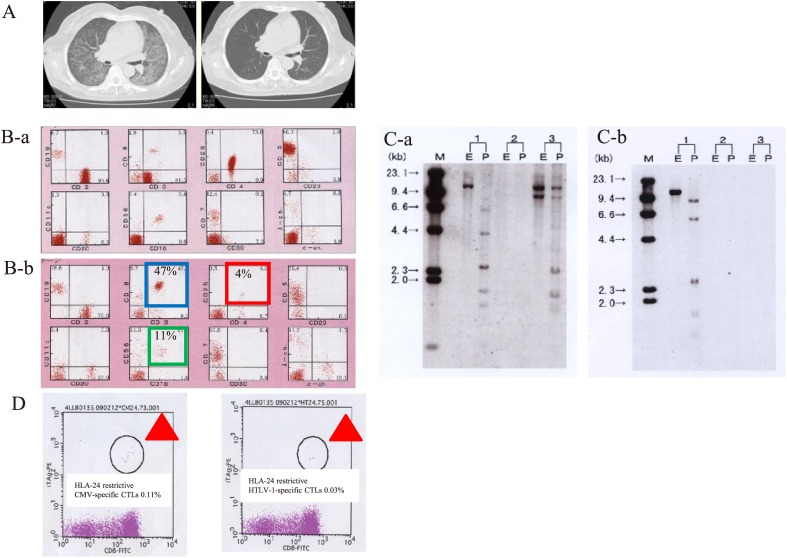
In addition, we examined the short-term outcome of ATL patients who achieved spontaneous remission after the development of opportunistic infection (spontaneous remission; Table 4). In this regard (spontaneous remission), 3 out of 6 survivors (cases 6, 7, and 11) developed spontaneous remission after opportunistic infection (complete response [CR]: 1 patient and partial response [PR]: 2 patients). Of note, 2 of these 3 patients (cases 6 and 7) achieved remission despite PD status during systemic chemotherapy. The etiologies of the opportunistic infections leading to spontaneous remission were CMV pneumonia (2 cases) and Pneumocystis pneumonia (1 case). Presented are the CT findings, FCM analysis, southern blot analysis and CTL analysis by a tetramer assay in a representative case of spontaneous remission of ATL (case 6) after treatment of opportunistic infection (Fig. 5A-D). CT findings after treatment of CMV pneumonia demonstrated the improvement of CMV pneumonia (Fig. 5A-a and 5A-b). FCM findings after treatment of opportunistic infection confirmed the improvement of CMV pneumonia (Fig. 5B-a and 5B-b). Southern blot analysis after treatment of opportunistic infection revealed the absence of HTLV-1 clonality in PB (Fig. 5C-a and 5C-b). Thus, CR was achieved in case 6 because of the disappearance of ATL cells on smears of PB, FCM analysis, and Southern blot analysis after CMV pneumonia (Fig. 5A–D). Furthermore, case 6 had 47% CD8+ T cells and 11% NK cells according to FCM analysis of PB. Furthermore, based on the HLA-A24 FCM tetramer assay, there were 0.03% HLA-24–restricted HTLV-1–specific CTLs and 0.1% HLA-24–restricted CMV-specific CTLs (Fig. 5D-a and 5D-b). Thus, CTL analysis by HLA 24 restrictive FCM analysis after treatment of opportunistic infection demonstrated the presence of CMV-specific CTLs and HTLV-1-specific CTLs.
Table 4. The short-term outcomes of ATL patients who developed opportunistic infections (spontaneous remission).
| No. | Age/Sex | Subtype | CR/PR | Remission duration | Clonality | Author (year) |
|---|---|---|---|---|---|---|
| 1 | 36/M | ND | CR | 4 years | ND | Kimura (1983) |
| 2 | 54/M | chronic | PR | ND | ND | Kawano (1983) |
| 3 | 73/M | acute | CR | several weeks | ND | Schnizer (1983) |
| 4 | 74/M | ND | PR | ND | ND | Tagawa (1984) |
| 5 | 50/F | ND | PR | ND | ND | Tagawa (1984) |
| 6 | 41/M | ND | CR | 5 years | ND | Mattock (1986) |
| 7 | 57/F | chronic | CR | 1 year | ND | Murakawa (1990) |
| 8 | 55/F | acute | CR | 10 months | (+) | Taniguchi (1993) |
| 9 | 50/F | acute | CR | 11 months | (+) | Shimamoto (1993) |
| 10 | 64/M | lymphoma | CR | 3 months | (+) | Shimamoto (1993) |
| 11 | 49/F | acute | CR | 6 years | (+) | Shimamoto (1993) |
| 12 | 42/M | acute | CR | 2 months | (+) | Suzuki (1995) |
| 13 | 62/F | lymphoma | CR | 2 months | (+) | Jinnohara (1997) |
| 14 | 70/M | chronic | CR | ND | (+) | Mizumoto (1997) |
| 15 | 42/M | acute | CR | 2 years | (+) | Kawada (1998) |
| 16 | 76/M | acute | PR | ND | (+) | Matsushita (1999) |
| 17 | 79/M | acute | CR | 1 year | (+) | Takezako (2000) |
| Present case 6 | 56/F | acute | CR | 213 days | biclonality | The present study |
| Present case 7 | 67/M | acute | PR | 133 days | monoclonality | The present study |
| Present case 11 | 54/M | chronic | PR | 194 days | monoclonality | The present study |
Abbreviations: Abbreviations: CR, complete remission; PR, partial remission; ND, not described or not done.
Fig. 5.
Clinical presentation of a spontaneous-remission case before and after treatment according to FCM analysis, Southern blotting, and CTL analysis.
Presented are the CT findings, FCM analysis, southern blot analysis, and CTL analysis by a tetramer assay in a representative case of spontaneous remission of ATL (case 6) after treatment of opportunistic infection (A-D). CT findings after treatment of CMV pneumonia showed the improvement of CMV pneumonia (A-a and A-b). FCM findings after treatment of opportunistic infection demonstrated the improvement of CMV pneumonia (B-a and B-b). Southern blot analysis after treatment of opportunistic infection showed the absence of HTLV-1 clonality in PB (C-a and C-b). Thus, CR was achieved because of the disappearance of ATL cells on smears of PB, FCM analysis, and Southern blot analysis after CMV pneumonia (A–D). Furthermore, case 6 had 47% CD8+ T cells and 11% NK cells according to FCM analysis of PB. In the HLA-A24 FCM tetramer assay, there were 0.03% HLA-24–restricted HTLV-1–specific CTLs and 0.1% HLA-24–restricted CMV-specific CTLs (D). Thus, CTL analysis by HLA 24 restrictive FCM analysis after treatment of opportunistic infection confirmed the presence of CMV-specific CTLs and HTLV-1-specific CTLs.
We next examined the long-term outcomes of ATL patients who achieved spontaneous remission after the development of opportunistic infection (ATL progression or ATL relapse; Table 5). Five out of 6 survivors after opportunistic infection presented with ATL progression (3 patients) or relapse (2 patients). The 3 patients with ATL progression after opportunistic infection had monoclonal ATL clones and high levels of sIL-2R at the initial diagnosis of opportunistic infection.
Table 5. The long-term outcomes of ATL patients who developed opportunistic infections (ATL progression or ATL relapse).
| Age | Sex | Subtype | Infection | Onset | Outcome of opportunistic infection | Response of ATL | Duration of ATL disease progression | ATL progression | Clonality | sIL- 2R | Final outcome after ATL development | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Previous report’s case 1 Tashiro T et al. 1992 | 66 | F | smoldering | Cryptococcus pneumonia | Initial diagnosis | Alive | SD | 16 months | Aggressive Type | n.e. | n.e. | dead (4 months) |
| Previous report’s case 2 Tashiro T et al. 1992 | 46 | M | smoldering | Pneumocystis pneumonia | Initial diagnosis | Alive | SD | 16 months | Aggressive Type | n.e. | n.e. | dead (3 months) |
| Previous report’s case 3 Tashiro T et al. 1992 | 55 | F | carrier | Cryptococcus pneumonia | Initial diagnosis | Alive | SD | 14 months | Aggressive type | n.e. | n.e. | alive (12 months) |
| Previous report’s case 4 Tanaka T et al. 2015 | 67 | M | carrier | CMV enteritis | Initial diagnosis | Alive | n.d. | n.d. | Smoldering type | n.e. | 4304 | n.d. |
| Previous report’s case 5 Tanaka T et al. 2015 |
78 | F | carrier | Pneumocystis pneumonia, herpes infection | Initial diagnosis | Dead | n.d. | n.d. | Smoldering type | n.e. | 14058 | dead (4 months) |
| Present case 3 | 65 | M | Chronic | Cryptococcus pneumonia, meningitis | Initial diagnosis | Alive | SD | 226 days | Acute type | monoclonality | 2280 | dead (79 days) |
| Present case 6 | 56 | F | acute | CMV pneumonia | During treatment | Alive | CR | 213 days | Relapse after self remission | biclonality | 9790 | dead (27 days) |
| Present case 7 | 67 | M | acute | CMV pneumonia | During treatment | Alive | PR | 133 days | Relapse after self remission | monoclonality | 36200 | dead (133 days) |
| Present case 11 | 54 | M | chronic | Pneumocystis pneumonia | Initial diagnosis | Alive | PR | 194 days | Acute type | monoclonality | 14500 | alive (194 days) |
| Present case 12 | 78 | M | smoldering | Pneumocystis pneumonia | Initial diagnosis | Alive | SD | 153 days | Acute type | monoclonality | 4520 | dead (153 days) |
DISCUSSION
This study demonstrated that opportunistic infections should be carefully followed among HTLV-1 carriers and ATL patients because of the aggressive clinical course exhibited by the non-survivors; they had a median OS of 14.5 days (range 2–27) and poor outcome–50% OS rate and OS of 28 days–despite the low cumulative incidence rates of opportunistic infections among HTLV-1 carriers (1.5% = 2/127) and ATL patients (6.5% = 10/153). As for the prognostic factors affecting opportunistic infections, our study revealed that higher SOFA scores (especially the respiratory subscore) and higher LDH values are closely related to the poor outcome of opportunistic infections. Thus, early diagnosis and subsequent prompt treatment are necessary for opportunistic infections among HTLV-1 carriers and ATL patients. Moreover, the detection of opportunistic infections may be predictive of the progression of ATL because of the high incidence rates of ATL progression and relapse in our study (83.3% = 5 out of 6 patients) at a median of 194 days (range 133–226 days). Based on our present study, further studies are needed to clarify the characteristics of opportunistic infections and the relationship between opportunistic infections and ATL, as well as to identify predictive factors for ATL progression in the future.
The principal focus of this manuscript was the (i) clinical characteristics of opportunistic infections (aggressive clinical course and poor outcome) among HTLV-1 carriers and ATL patients, (ii) short-term outcomes of ATL patients who achieved spontaneous remission after the development of opportunistic infection, and (iii) and long-term outcomes of ATL patients who achieved spontaneous remission after the development of opportunistic infection (ATL progression and ATL relapse).
First, regarding the clinical characteristics of opportunistic infections (aggressive clinical course and poor outcome) among HTLV-1 carriers and ATL patients, our present study revealed the cumulative incidence rates of opportunistic infections among HTLV-1 carriers and ATL patients [1.5% (2/127) and 6.5% (10/153), respectively] to be consistent with those reported by Shimoyama in 1991, with an incidence rate of opportunistic infections including bacterial, viral, and Pneumocystis pneumonia of 11.9% (98/818) in ATL patients.2 Opportunistic infections also developed in HTLV-1 carriers. Furthermore, consistent with the report by Suzumiya on the etiology of opportunistic infections among autopsies of ATL patients in 1993,3 the etiologies of opportunistic infections were diverse, such as Cryptococcus, Aspergillus, Pneumocystis, or CMV, with the main target organ being the lungs (12/12). Consequently, our present study demonstrated that the clinical course and prognosis of opportunistic infections are aggressive and poor, respectively; 50% OS rate, OS of 28 days, and median OS of 121 days. Of note, the 6 non-survivors had an aggressive clinical course in terms of OS; median OS of 14.5 days (range 2–27 days). Regarding the prognostic factors affecting opportunistic infections, a higher SOFA score and higher LDH values were closely related to the poor outcome of opportunistic infections. Among the factors composing the SOFA score, such as cardiovascular subscore, renal subscore, respiratory subscore, coagulation subscore, hepatic subscore, and neurological subscore, the respiratory subscore was identified as a significant factor affecting OS by univariate analysis. This is consistent with all 6 non-survivors presenting with aggressive acute respiratory failure requiring intensive treatment with intubation and ventilation in an intensive care unit. Regarding LDH values as a prognostic factor in opportunistic infections, further accumulation of cases is essential because higher LDH values may affect lung failure due to opportunistic infection and ATL. As such, early diagnosis and subsequent prompt treatment are necessary for opportunistic infections among HTLV-1 carriers and ATL patients.
Second, we focused on the short-term outcomes of ATL patients who developed opportunistic infection (spontaneous remission) and the long-term outcomes of ATL patients who developed opportunistic infection (ATL progression and ATL relapse).
Regarding the short-term outcomes of ATL patients who developed opportunistic infection, half of the survivors (3/6) with opportunistic infections presented with spontaneous remission (1 CR and 2 PR) during relatively short periods (213, 133, and 194 days). Previously, 17 ATL patients who achieved spontaneous remission after the development of opportunistic infection, including fungal infections and CMV infections, were reported (Table 4).7-24
Previous reports and our study suggest that the onset of opportunistic infections induces an unknown immune system attack on ATL. In one CR case after opportunistic infection in our study, the patient attained CR according FCM and Southern blot analyses with the presence of HLA-24–restricted CTLs for CMV infection and ATL. Moreover, our analysis revealed more abnormal lymphocytes in the PB of the remission group than in the non-remission group (Table 2). Furthermore, the non-remission group had hyperbilirubinemia (Table 2). Further accumulation of data is needed to identify factors predicting remission, e.g., by multivariate analysis.
As for the long-term outcomes of ATL patients who achieved spontaneous remission after the development of opportunistic infection, there were high incidence rates of ATL progression (3 patients) and relapse (2 patients) after opportunistic infection in our study (83.3% = 5 out of 6 patients) at median 194 days (133–226 days). Previously, 2 ATL patients and 3 HTLV-1 carriers exhibited disease progression after opportunistic infection (Table 5).23,24 In our study, 3 (cases 3, 6, and 11) out of 5 ATL patients were treated by chemotherapy, radiation therapy, and allogeneic hematopoietic stem cell transplantation (allo-HSCT), respectively. Only one patient (case 11) was successfully treated by allo-HSCT because of good PS and ADL after the treatment of opportunistic infection.
Of note, the 3 patients with ATL PD after opportunistic infections had monoclonality of ATL clones and high levels of sIL-2R. Thus, the detection of opportunistic infections may be predictive of the progression of ATL in clinical practice. In our study, only 1 patient was successfully treated by mLSG 15 and subsequent allo-HSCT among the 6 survivors. The other 5 patients were contraindicated for allo-HSCT because of poor PS and ADL after the treatment of opportunistic infections. For further elucidation, maintenance of the PS and ADL is essential during treatment of opportunistic infections. Allo-HSCT may be necessary to overcome the poor outcome of ATL progression after opportunistic infection.
In conclusion, opportunistic infections should be carefully followed among HTLV-1 carriers and ATL patients because of their aggressive clinical course and poor outcomes. Thus, early diagnosis and subsequent prompt treatment are important. Our present study demonstrated that further studies are needed to clarify the characteristics of opportunistic infections and the relationship between opportunistic infections and ATL, as well as to identify predictive factors of ATL progression in the future.
Footnotes
CONFLICTS OF INTEREST: The authors state that they have no Conflict of Interest (COI).
REFERENCES
- 1.Mitsuya H, Guo H, Cossman J, et al. Functional properties of antigen-specific T cells infected by human T-cell leukemia-lymphoma virus (HTLV-I). Science. 1984; 225: 1484-1486. 10.1126/science.6206569 [DOI] [PubMed] [Google Scholar]
- 2.Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. Br J Haematol. 1991; 79: 428-437. 10.1111/j.1365-2141.1991.tb08051.x [DOI] [PubMed] [Google Scholar]
- 3.Suzumiya J, Marutsuka K, Nabeshima K, et al. Autopsy findings in 47 cases of adult T-cell leukemia/lymphoma in Miyazaki prefecture, Japan. Leuk Lymphoma. 1993; 11: 281-286. 10.3109/10428199309087005 [DOI] [PubMed] [Google Scholar]
- 4.Yasunaga J, Sakai T, Nosaka K, et al. Impaired production of naive T lymphocytes in human T-cell leukemia virus type I–infected individuals: its implications in the immunodeficient state. Blood. 2001; 97: 3177-3183. 10.1182/blood.V97.10.3177 [DOI] [PubMed] [Google Scholar]
- 5.Yamano Y, Takenouchi N, Li HC, et al. Virus-induced dysfunction of CD4+CD25+ T cells in patients with HTLV-I–associated neuroimmunological disease. J Clin Invest. 2005; 115: 1361-1368. 10.1172/JCI23913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yanagihara T, Ikematsu Y, Kato K, et al. Expression of PD-1 and PD-L1 on cytotoxic T lymphocytes and immune deficiency in a patient with adult T cell leukemia/lymphoma. Ann Hematol. 2018; 97: 359-360. 10.1007/s00277-017-3146-z [DOI] [PubMed] [Google Scholar]
- 7.Kawano N, Yoshida S, Kuriyama T, et al. Clinical features and treatment outcomes of 81 patients with aggressive type adult T-cell leukemia-lymphoma at a single institution over a 7-year period (2006-2012). Intern Med. 2015; 54: 1489-1498. 10.2169/internalmedicine.54.1953 [DOI] [PubMed] [Google Scholar]
- 8.Kimura I, Tsubota T, Hayashi K, Ohnoshi T. Spontaneous, complete remission in adult T-cell leukemia: a case report. Jpn J Clin Oncol. 1983; 13(suppl 2): 231-236. [PubMed] [Google Scholar]
- 9.Kawano F, Tsuda H, Yamaguchi K, et al. [Adult T-cell leukemia found in siblings]. Rinsho Ketsueki. 1983; 24: 663-667 [Article in Japanese].. [PubMed] [Google Scholar]
- 10.Schnitzer B, Lovett E, III, Kahn L. Adult T-cell leukaemia with spontaneous remission. Lancet. 1983; 322: 1030. 10.1016/S0140-6736(83)91017-6 [DOI] [PubMed] [Google Scholar]
- 11.Murakawa M, Shibuya T, Teshima T, et al. Spontaneous remission from acute exacerbation of chronic adult T-cell leukemia. Blut. 1990; 61: 346-349. 10.1007/BF01738547 [DOI] [PubMed] [Google Scholar]
- 12.Mattock C, Anderson NA, Sheldon CD, Rustin MH, Hoffbrand BI. Spontaneous remission and relapse in adult T cell lymphoma/leukaemia associated with HTLV-I. BMJ. 1986; 292: 1171-1172. 10.1136/bmj.292.6529.1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimamoto Y, Kikuchi M, Funai N, et al. Spontaneous regression in adult T-cell leukemia/lymphoma. Cancer. 1993; 72: 735-740. [DOI] [PubMed] [Google Scholar]
- 14.Tagawa S, Konishi I, Tokumine Y, et al. Phenotypical heterogeneity of Japanese adult T-cell leukaemia. Scand J Haematol. 1984; 32: 306-312. 10.1111/j.1600-0609.1984.tb01696.x [DOI] [PubMed] [Google Scholar]
- 15.Taniguchi S, Yamasaki K, Shibuya T, Asayama R. Spontaneous remission of acute adult T-cell leukaemia with chromosomal abnormality infiltrating to skin and liver. Br J Haematol. 1993; 85: 413-414. 10.1111/j.1365-2141.1993.tb03189.x [DOI] [PubMed] [Google Scholar]
- 16.Jinnohara T, Tsujisaki M, Sasaki S, Hinoda Y, Imai K. Cytotoxic activity in a case of adult T-cell leukemia/lymphoma with spontaneous regression. Int J Hematol. 1997; 65: 293-298. 10.1016/S0925-5710(96)00564-6 [DOI] [PubMed] [Google Scholar]
- 17.Mizumoto K, Suehara N, Ohuchida J, et al. Pancreatic tumor formed by infiltration of adult T-cell leukemia cells. Int J Gastrointest Cancer. 1997; 21: 253-257. 10.1007/BF02821612 [DOI] [PubMed] [Google Scholar]
- 18.Kawada H, Fukuda R, Suzuki M, et al. Unusual relapse of adult T-cell leukemia/lymphoma after spontaneous remission. Leuk Res. 1998; 22: 197-199. 10.1016/S0145-2126(97)00150-1 [DOI] [PubMed] [Google Scholar]
- 19.Matsushita K, Arima N, Fujiwara H, et al. Spontaneous regression associated with apoptosis in a patient with acute-type adult T-cell leukemia. Am J Hematol. 1999; 61: 144-148. [DOI] [PubMed] [Google Scholar]
- 20.Iroi A, Miyashita N, Nakamura S, Ohizumi H, Mizuno Y. [A patient with marked immunodeficiency in an HTLV-I carrier: a case report]. Rinsho Shinkeigaku. 2000; 40: 135-139 [Article in Japanese].. [PubMed] [Google Scholar]
- 21.Kawahigashi N, Furukawa Y, Tara M, Niina K. [Pneumocystis carinii pneumonia in a HTLV-I carrier with monoclonal proliferation of HTLV-I infected lymphocyte]. Rinsho Ketsueki. 1996; 37: 317-322 [Article in Japanese].. [PubMed] [Google Scholar]
- 22.Ogata M, Satou T, Kawano R, et al. High incidence of cytomegalovirus, human herpesvirus-6, and Epstein-Barr virus reactivation in patients receiving cytotoxic chemotherapy for Adult T cell leukemia. J Med Virol. 2011; 83: 702-709. 10.1002/jmv.22013 [DOI] [PubMed] [Google Scholar]
- 23.Tashiro T, Yamasaki T, Nagai H, Kikuchi H, Nasu M. Immunological studies on opportunistic infection and the development of adult T-cell leukemia. Intern Med. 1992; 31: 1132-1136. 10.2169/internalmedicine.31.1132 [DOI] [PubMed] [Google Scholar]
- 24.Tanaka T, Sekioka T, Usui M, Imashuku S. Opportunistic infections in patients with HTLV-1 infection. Case Rep Hematol. 2015; 2015: 1-5. 10.1155/2015/943867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents. 2015; recommendations from the Centers for Disease Control and Prevention, the National Institutesof Health, and the HIV Medicine Association of the Infectious Diseases Society of America.Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf.
- 26.The guideline of diagnosis and treatment for deep-seated fungal infection from the Mycoses Forum in Japan, 2014.
- 27.Japan Society for Hematopoietic Cell Transplantation (JSHCT) guideline for prophylaxis and treatment of fungal infection. JSHCT monograph Vol 46. September, 2017.
- 28.Japan Society for Hematopoietic Cell Transplantation (JSHCT) guideline for prophylaxis and treatment of cytomegalovirus infection. Vo1 1. May, 2014.