Abstract
Chronic kidney disease (CKD) is characterized by loss of renal function and a consequent increase of serum uremic toxins, which contribute to inflammation status. Deficiency of 25-vitamin D, often found in patients with CKD, has been included as an inflammatory factor since it might modulate the immune system. The aim of this study was to investigate the role of 25-vitamin D on inflammatory pathways in healthy and uremic environment. Toll-like receptor 4 (TLR4), oxidative stress (ROS), vitamin D receptor (VDR), 1-α hydroxylase (CYP27), 24 hydroxylase, cathelicidin, and MCP-1 were evaluated in monocytes exposed to a uremic serum pool compared with healthy pool. The human monocytes lineage (U937) was incubated with or without 25-vitamin D (50 ng/ml for 24 hours). TRL4, VDR, CYP27, CYP24, and ROS were evaluated by flow cytometry. We used ELISA to measure IL-6, TNF-α, IL-10, cathelicidin, and MCP-1 in the cell culture supernatant. We observed a higher expression of TRL-4, IL-6, TNF-α, IL-10, cathelicidin and MCP-1 in monocytes incubated with uremic serum when compared with serum from healthy individuals. Supplementation of 25-vitamin D was able to reduce the expression of TRL4, cathelicidin, and MCP-1 in the uremic environment. There was no difference in the expression of VDR, CYP27 and CYP24 intracellular enzymes. This in vitro study showed that the uremic pool activates inflammatory response in monocytes, which was reversed by 25-vitamin D supplementation; this finding suggests that 25-vitamin D has an anti-inflammatory role in the uremic environment.
Subject terms: Applied immunology, Haemodialysis
Introduction
Chronic Kidney disease (CKD) is characterized by the loss of glomerular filtration rate resulting in serum accumulation of toxic substances, called uremic toxins1. The accumulation of these compounds is defined as the uremic environment.
It has been shown that uremic toxins are capable to induce an inflammatory response in patients with CKD2–4. Uremic toxins can cause oxidative stress and stimulate the production of proinflammatory cytokines in this population. Several studies have been reported a relationship between uremic toxins (mainly indoxyl sulfate, p-cresyl sulfate, and indole-3-acetic acid) and inflammation3,5,6. The indoxyl sulfate has been described to activate NF-ĸB, NADPH oxidase, upregulated mRNA and expression of intercellular adhesion molecule-1 (ICAM-1)7–9, and is also capable to induce endothelial injury with the formation of microvesicles that contribute to endothelial cell progenitors dysfunction10. The p-cresyl sulfate and indole-3-acetic acid are associated with increased IL-6 and CRP, respectively, in patients with CKD3,11.
When renal function deteriorates to <15 ml/min/1,73 m2, patients usually need dialysis support. However, despite the technological advances in the dialysis procedures, thrice weekly conventional hemodialysis is not able to remove all toxins, particularly those too large and/or protein-bounded12. Therefore, patients on dialysis still have serum circulating uremic toxins. Some studies have demonstrated a direct relationship between uremic toxins and inflammation and cardiovascular disease, which is the main cause of mortality in patients with CKD13,14.
Uremic toxins can cause immune activation with production of proinflammatory cytokines including TNF-α, IL-6 and MCP-115,16 mainly by monocytes17,18. Although these proinflammatory cytokines enhance host defense, their excessive production leads to unresolved inflammation19. The uremic toxins may induce the toll-like receptor (TLR) activation, resulting in the production of several inflammatory mediators20. Our group has recently reported that TLR4 expression is increased in neutrophils and monocytes obtained from hemodialysis patients that correlated with IL-6, reinforcing the role of TLR4 in the mechanism of inflammation21.
Besides cytokines, 25-vitamin D has also been implicated as an inflammatory marker, participating in both innate and adaptive immunity22. Hypovitaminosis D has been often reported in patients with CKD, reaching up to 80% of prevalence23. Although the levels of 1,25-vitamin D are important, it is the circulating concentration of 25-vitamin D that determines the vitamin D status of a given individual. Levels of serum 25-vitamin D from 20 to 60 ng/mL are considered normal and values below 20 ng/mL are considered indicative of vitamin D deficiency24.
The vitamin-D receptor (VDR) and the enzyme 1α-hydroxylase (CYP27) are present in cells of the immune system25–27. 25-vitamin D regulates the expression of cathelicidin, an endogenous antimicrobial peptide28. This modulation occurs by activation of TLRs, which sign for increased expression of VDR and CYP27, the enzyme that converts 25-vitamin D to the active form, 1,25-vitamin D. This active form regulates the VDR that sign the hCAP18 encoding gene, which is a pro-protein that upon be cleaved releases cathelicidin29 to act against gram-negative and positive bacteria, viruses and fungi30.
Uremic toxins and hypovitaminosis D are important inflammatory markers in patients with CKD. However, few studies evaluated the effect of supplementation of 25-vitamin D on the cells and the mechanisms involved in the innate immunity response. Therefore, the goal of the current study was to evaluate the in vitro effect of 25-vitamin D supplementation on inflammation through monocytes cells. The expression of the receptors TLR4, VDR, 1-α hydroxylase, cathelicidin, in addition to oxidative stress and inflammatory cytokines were also evaluated.
Matherial and Methods
Culture assay: The U937 lineage31 (human monocytic lineage, ATCC #TIB-202) was differentiated into monocytes using phorbol 12-myristate 13- acetate (PMA; Sigma-Aldrich P1585) to evaluate the modulation of 25-vitamin D on the expression of TLR4, VDR, CYP27, CYP24, cathelicidin, IL-6, IL-10, MCP-1, and oxidative stress, as previously described32. U937 was cultured in RPMI 1640 with (pH 7.4, Sigma Chemical Co., St. Louis, MO, USA), 10 mmol/L HEPES (Sigma Chemical Co., St. Louis, MO, NY, USA), 2 mmol/L L-Glutamine (Merck, Darmstadt, Germany), 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco, BRL, Life Technologies, USA), 10% fetal bovine serum and maintained in culture with 5% CO2 incubator at 37 °C.
The culture medium was changed every 48 hours until the cell concentration reached 1 × 106/mL. Subsequently, the cells were stimulated by 10 nM/mL PMA for 24 hours in a 75 cm3 culture bottle containing 1 × 106 cells/mL. After differentiation, the cells were washed in 10 mL of PBS (Sigma, Cat P3744-1PAK) to remove the PMA and incubated with 5 mL of 0.5% trypsin, in order to detach the cells from the surface of the plaque, obtaining a homogeneous cell suspension. The cells were then washed with culture medium (5 mL) for trypsin neutralization, and cell viability testing was performed using Trypan Blue.
After differentiation, U937 cells were transferred to culture bottles of 25 cm2, preincubated with 25-vitamin D (Sigma-Aldrich CAT: 101443236) at the concentration of 50 ng/mL, for 24 hs. Afterward, the cells were incubated with serum from uremic patients or serum from healthy subjects. Detailed description of Monoclonal antibodies is shown in Supplementary Table 1.
Preparation of human serum pool
Healthy and uremic serum pool were prepared according to our previous study33, as follows:
Healthy Serum Pool: Blood samples (20 mL) were collected from 20 healthy volunteers in tubes with anticoagulants and centrifuged at 500 G for 10 minutes. The supernatant was stored in freezer aliquots −80 °C.
Uremic Serum Pool: blood samples were collected with anticoagulant tubes from 30 patients in hemodialysis treatment imediately before the begining of the second session of the week. The tubes were centrifuged at 500 G for 10 minutes. The supernatant was stored in a freezer −80 °C. Patients that had any type of infection, use of immunosuppressive drugs, vitamin D supplementation, diabetes mellitus or neoplasia disease were excluded from the study.
An aliquot from both sample pools were sent to the laboratory to be sure that uremic pool was representative of uremic environment, detected by the increase of levels of creatinine, urea, parathyroid hormone (PTH), phosphorus and decrease of calcium and 25-vitamin D. Besides, we performed the Limulus amebocyte lysate test (LAL) on both uremic and healthy pool serum to evaluated lipopolysaccharide (LPS) levels, which cause monocyte activation in levels >0.25mlU/mL.
This study was approved by the Institutional Review Board of the Federal University of São Paulo (#0727/10). Healthy and uremic serum samples were obtained after written informed consent of participants or legal representative, according to the Helsinki Declaration and local regulations.
Expression of CD14, TLR4, VDR, CYP27 and CYP24 by flow cytometry
3 × 105 cells from healthy and uremic serum (pre-incubated or not with 25 vitamin D) were used to label monoclonal antibodies to CD14-FITC and TLR4 PE and VDR-APC, CYP27-Alexa Fluor 647 and CYP24-Alexa Fluor 488 (Supplementary Table 2) according to manufactures instructions, and was added to healthy and uremic serum pool.
The expression of these monoclonal antibodies was checked by the flow cytometer (FacsCanto I, BD, USA) immediately.
Flow cytometry analysis
A forward scatter plot versus side scatter plot was used to make a gate for the U937 cells and to exclude debris. DOT-PLOT graphs were used to analyze the CD14, TLR-4 and VDR (Supplementary Fig. 1) and histograms were used to analyze the CYP27 and CYP24 (Supplementary Fig. 2) and ROS (Supplementary Fig. 3. The results were described in mean fluorescence intensity (MFI), such that the higher the MFI the greater the expression of these receptors.
Detection of reactive oxygen species (ROS) by Flow Cytometry
The 3 × 105 cells each condition were transferred into the 12 × 75 mm tube and incubated with 100 uL of 2′-7′-dichlorofluorescein diacetate (DCFH-DA)(FITC) (Sigma, St. Louis, MO, USA) to evaluated ROS, according to previously described34. The analyses were performed by the flow cytometer (FacsCanto I, BD).
Detection of TNF-α, IL-6, IL-10, cathelicidin, MCP-1 by ELISA technique
The culture supernatants were stored at −80 °C freezer to detection TNF-α, IL-6, IL-10, cathelicidin and MCP-1 by enzyme-linked immunosorbent assay (ELISA) assays. ELISA tests were performed using commercial Kits according to manufacturers’ instructions (Supplementary Table 1).
Detection of NF-κB
The monocytes were evaluated for NF-κB activity, according to the manufacturer’s instructions (NF-κB p50/p65 Transcription Factor Assay Kit, eBioscience, San Diego, USA). The respective inter- and intra-assay coefficient of variation was 4.1% and 5.9%.
Statistical analysis
The results according to each group (healthy, uremic, with 25-vitamin D supplementation and without 25-vitamin D supplementation) were expressed as median (minimum and maximum), and the differences tested by Kruskal Wallis. Relationships between independent variables were performed in the entire group. Kolmogorov-Smirnov test was used to test normality of data. We used Pearson or Spearman correlation coefficient, as appropriate. The value of p < 0.05 was considered for statistical significance. The analyses were performed using SPSS (Statistical Package Social Sciences) software version 22.0 for Windows.
Results
Comparison of characteristics between healthy and uremic pool:
Biochemical characteristics: As expected, uremic pool had higher creatinine, urea, PTH, and phosphorus, whereas serum calcium and 25-vitamin D were lower (Table 1).
Table 1.
Biochemical data from healthy and uremic serum pool.
| Healthy Pool | Uremic Pool | |
|---|---|---|
| Creatinine (mg/dL) | 0.82 | 8.92 |
| Urea (mg/dL) | 33 | 135 |
| PTH (pg/mL) | 23 | 392 |
| Calcium (mg/dL) | 9.7 | 7.4 |
| Phosphorus (mg/dL) | 3.4 | 4.3 |
| 25-vitamin D (ng/mL) | 19.2 | 10.5 |
| LPS levels (mlU/mL) | <0.25 | <0.25 |
PTH, parathyroid hormone; LPS, lipopolisaccharide.
Inflammatory markers: We observed a higher expression of TRL4, ROS, IL-6, TNF-α, IL-10, cathelicidin, and MCP-1 in monocytes incubated with uremic serum when compared to healthy serum (Table 2). When monocytes were incubated with uremic serum previously treated with 25-vitamin D, there was a reduction in the expression of TLR4, MCP-1, and cathelicidin. There were no differences regarding the expression of VDR, CYP27, CYP24, and NF-κB between groups (Table 2).
Table 2.
Expression of TLR-4, ROS, VDR, CYP27, CYP24, IL-6, TNF-α, IL-10, cathelicidin, MCP-1 and NF- κB.
| Healthy pool (HS) | Uremic pool (US) | |||
|---|---|---|---|---|
| HS | HS + 25 vitamin D | US | US + 25 vitamin D | |
| Expression (MIF) | ||||
| TLR4 |
716 (654–936) |
644 (644–870) |
2653* (1853–3372) |
1692† (1022–2292) |
| ROS |
343 (313–498) |
378 (320–563) |
550* (394–607) |
544 (435–599) |
| VDR |
7882 (6574–9687) |
8394 (6475–10708) |
7657 (6670–9420) |
7556 (5699–9420) |
| CYP27 |
1201 (692–1461) |
1207 (747–1521) |
1396 (931–1532) |
1170 (955–1521) |
| CYP24 |
439 (319–563) |
363 (329–501) |
342 (331–676) |
375 (333–485) |
| Cytokines (pg/mL) | ||||
| IL-6 |
3.8 (3.3–4.9) |
3.6 (3.1–5.3) |
39.3* (28.3–45.3) |
37.3 (28.8–42.5) |
| TNF-α |
2.2 (1.6–2.7) |
2.3 (2.1–3.2) |
3.9* (3.2–4.7) |
3.4 (2.9–4.8) |
| IL-10 |
45 (41–62) |
47 (45–62) |
61* (53–64) |
60 (56–73) |
| CATHELICIDIN |
0.44 (0.43–0.46) |
0.43 (0.4–0.49) |
0.58* (0.51–0.65) |
0.52† (0.48–0.59) |
| MCP-1 |
11076 (7899–11948) |
7336 (4820–8905) |
14370* (9117–16936) |
7697† (5940–10310) |
| NF- κB (%) |
4.8 (1.6–1.2) |
3.1 (1.7–3.4) |
3.4 (2.1–5.5) |
4.1 (2.3–6.4) |
*US ≠ HS: p < 0.04.
†US + 25 vitamin D ≠ US: p < 0.03.
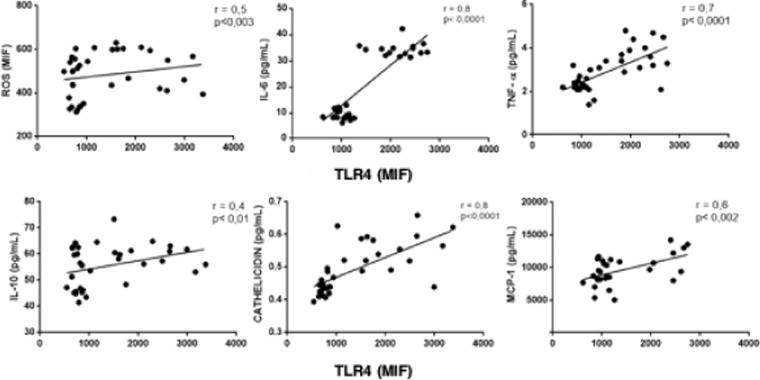
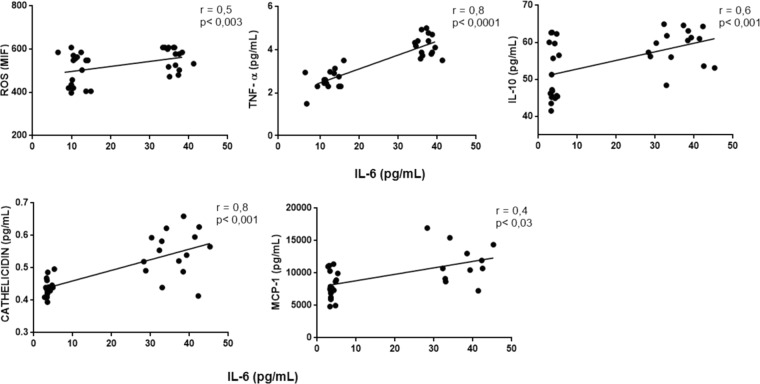
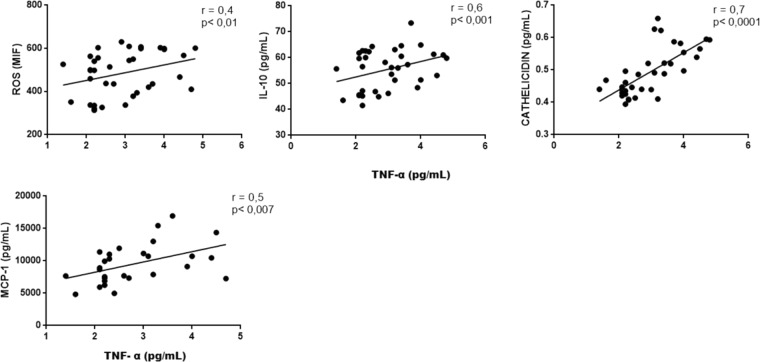
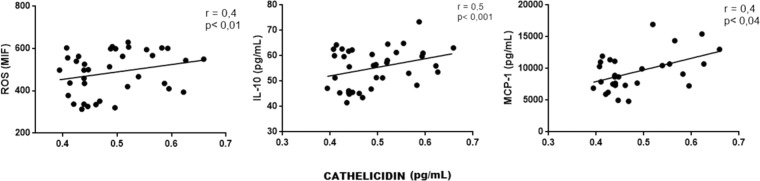
Correlations among inflammatory markers: We performed correlation between TLR4 and other variables to address the interplay among this receptor and inflammatory markers. In fact, there was a positive correlation between the expression of TLR4 and ROS, IL-6, TNF-α and IL-10, MCP-1 and cathelicidin (all p values < 0.05). There was also a positive correlation between IL-6 with ROS, IL-10, cathelicidin and MCP-1, a positive correlation between TNF-α and ROS, IL-10, cathelicidin and MCP-1, and between cathelicidin and ROS, IL-10 and MCP-1 (all p values < 0.05). These correlations are illustrated in Figs. 1, 2, 3 and 4).
Figure 1.
Correlation between TLR-4 and ROS, IL-6, TNF-α, IL-10, cathelicidin and MCP-1.
Figure 2.
Correlation between expression of IL-6 and ROS, TNF-α, IL-10, Cathelicidin and MCP-1.
Figure 3.
Correlation between expression of TNF-α and ROS, IL-10, cathelicidin and MCP-1.
Figure 4.
Correlation between expression of Cathelicidin and ROS, IL-10 and MCP-1.
Discussion
In this study, we confirmed that the uremic environment is associated with an inflammatory status, which was depicted by elevated levels of cytokines and ROS. In addition, uremic serum, similarly to what happens in patients with CKD presented low levels of 25-vitamin D. The novelty of our study, however, was demonstrating that 25-vitamin D was capable to reduce TLR-4, MCP-1, and cathelicidin in a uremic environment. Taken together, our findings provide evidence that vitamin D has a role in protecting against inflammation, at least mediated by monocytes.
The high TLR4 expression is important to improve the infection response, however the continuous expression leads to a release of pro-inflammatory cytokines. We observed a high expression of TLR4 in monocytes incubated with uremic serum compared with healthy serum and a positive association with cytokines and ROS. The TLR4 expression may activate the intracellular nuclear factor-κB (NF-κB) pathway and enhances the expression of NF-κB-controlled genes such as for inflammatory cytokines and adhesion molecules not only in monocytes but also in endothelial cells35,36. In our in vitro study, we did not observe a difference in the percentage of NF-κB, despite the overexpression of TLR4 in monocytes incubated with uremic serum.
The short-time incubation could lead to this contradictory result, although our results are similar to a previous study that did not show an effect of a 16-week supplementation of vitamin D on NF-κB activity37. TLR4, however, associated with cytokines, confirming previous studies21,38 in patients on hemodialysis.
We observed an increased production and secretion of ROS and MCP-1 in monocytes incubated with uremic serum compared to healthy serum. The Monocyte chemoattractant peptide protein 1 (MCP-1) has been described to attract circulating cells from blood vessels to the local injury sites and together with inflammatory mediators and ROS may contribute to the increased vascular injury39 and also cardiovascular disease in patients with CKD40. The relationship between uremia and the vascular lesion has been demonstrated by Stinghen A et al.41, describing in an in vitro study that indoxyl sulfate, a uremic toxin, causes endothelial lesion that may contribute to cardiovascular disease in patients with CKD.
The supplementation of 25-vitamin D, tested in the current study as an anti-inflammatory agent, seems to have an effect on monocytes. The lower TLR4 expression suggests a downregulation effect of 25-vitamin D on this receptor. Recently, Zhang Y et al.42 described a beneficial effect of 25-vitamin D supplementation on the decrease of IL-6 and TNF-α, though this study was conducted in patients without renal disease. We found no effect of the 25-vitamin D supplementation downregulating cytokines that might be related to the short period of treatment (24-hour).
The 25-vitamin D decreased MCP-1 in monocytes incubated with uremic serum. We expected that low levels of MCP-1 would result in fewer leukocytes recruitment and endothelial injury. Cathelicidin is an endogenous antimicrobial peptide23 that also works as an inflammatory marker43. In the current study, we showed a positive correlation of cathelicidin with IL-6 and TNF-α, reinforcing this hypothesis.
Giffoni et al.33 demonstrated that lymphocytes obtained from uremic patients supplemented with 25-vitamin D exhibited an increase of VDR and CYP27 expression, resulting in reduced production of pro-inflammatory cytokines. Contrary to expectations, we did not observe an increased intracellular expression of VDR and CYP27 when the monocytes were treated with 25-vitamin D. It supposed that the effect of 25-vitamin D might be dependent on the cell type and exposure time to regulate inflammatory mechanisms.
Our study has some limitations such as the short time of follow-up and the lack of gene expression evaluation, which could help to elucidate the mechanism of anti-inflammatory effect of vitamin D. The strength of our study was to demonstrate the effect of 25-vitamin D in TLR4, cathelicidin and MCP-1 expressions suggesting that this pre-activated form of vitamin D may minimize inflammation. Whether the supplementation of 25-vitamin D in patients with CKD is capable to reduce inflammation needs to be confirmed in further studies.
Supplementary information
Acknowledgements
The authors thank all the professionals who provided essential technical support during the conduction of this work. This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo, FAPESP (Grant Number 2014-14192-1).
Author contributions
Study design, data collection, flow cytometry data acquisition, analysis, interpretation, and approval of the final version of the article: Brito, R.; Rebello J.F.; ELISA data acquisition, analysis and interpretation, signifcant intellectual insights, approval of the final version of the article: Grabulosa, C.C.; Bioinformatics analysis, interpretation and significant intellectual insights, approval of the final version of the article: Pinto, W. and Morales, A. Bioinformatics analysis, interpretation and significant intellectual insights and review and approval of the final version of the article, approval of the final version of the article: Elias, R.M. and Moysés, R.M.; Study design, data analysis, interpretation, intellectual insights, manuscript writing, review, and approval of the final version of the article, approval of the final version of the article: Dalboni, M.A.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-56874-1.
References
- 1.Vanholder RC, Glorieux GL. An overview of uremic toxicity. Hemodialysis international. International Symposium on Home Hemodialysis. 2003;7:156–161. doi: 10.1046/j.1492-7535.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- 2.Borges NA, et al. Protein-Bound Uremic Toxins from Gut Microbiota and Inflammatory Markers in Chronic Kidney Disease. Journal of renal nutrition: the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2016;26:396–400. doi: 10.1053/j.jrn.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Rossi M, et al. Protein-bound uremic toxins, inflammation and oxidative stress: a cross-sectional study in stage 3-4 chronic kidney disease. Archives of medical research. 2014;45:309–317. doi: 10.1016/j.arcmed.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Kaminski TW, Pawlak K, Karbowska M, Mysliwiec M, Pawlak D. Indoxyl sulfate - the uremic toxin linking hemostatic system disturbances with the prevalence of cardiovascular disease in patients with chronic kidney disease. BMC nephrology. 2017;18:35. doi: 10.1186/s12882-017-0457-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onal EM, Afsar B, Covic A, Vaziri ND, Kanbay M. Gut microbiota and inflammation in chronic kidney disease and their roles in the development of cardiovascular disease. Hypertension research: official journal of the Japanese Society of Hypertension. 2019;42:123–140. doi: 10.1038/s41440-018-0144-z. [DOI] [PubMed] [Google Scholar]
- 6.Lau WL, Savoj J, Nakata MB, Vaziri ND. Altered microbiome in chronic kidney disease: systemic effects of gut-derived uremic toxins. Clinical science. 2018;132:509–522. doi: 10.1042/CS20171107. [DOI] [PubMed] [Google Scholar]
- 7.Bolati D, Shimizu H, Yisireyili M, Nishijima F, Niwa T. Indoxyl sulfate, a uremic toxin, downregulates renal expression of Nrf2 through activation of NF-kappaB. BMC nephrology. 2013;14:56. doi: 10.1186/1471-2369-14-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stockler-Pinto MB, et al. From bench to the hemodialysis clinic: protein-bound uremic toxins modulate NF-kappaB/Nrf2 expression. International urology and nephrology. 2018;50:347–354. doi: 10.1007/s11255-017-1748-y. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu H, Yisireyili M, Higashiyama Y, Nishijima F, Niwa T. Indoxyl sulfate upregulates renal expression of ICAM-1 via production of ROS and activation of NF-kappaB and p53 in proximal tubular cells. Life sciences. 2013;92:143–148. doi: 10.1016/j.lfs.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Carmona A, et al. Microvesicles Derived from Indoxyl Sulfate Treated Endothelial Cells Induce Endothelial Progenitor Cells Dysfunction. Frontiers in physiology. 2017;8:666. doi: 10.3389/fphys.2017.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azevedo ML, et al. p-Cresyl sulfate affects the oxidative burst, phagocytosis process, and antigen presentation of monocyte-derived macrophages. Toxicology letters. 2016;263:1–5. doi: 10.1016/j.toxlet.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Claro Ligia, Moreno-Amaral Andrea, Gadotti Ana, Dolenga Carla, Nakao Lia, Azevedo Marina, de Noronha Lucia, Olandoski Marcia, de Moraes Thyago, Stinghen Andréa, Pécoits-Filho Roberto. The Impact of Uremic Toxicity Induced Inflammatory Response on the Cardiovascular Burden in Chronic Kidney Disease. Toxins. 2018;10(10):384. doi: 10.3390/toxins10100384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England journal of medicine. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 14.Lekawanvijit S, Krum H. Cardiorenal syndrome: acute kidney injury secondary to cardiovascular disease and role of protein-bound uraemic toxins. The Journal of physiology. 2014;592:3969–3983. doi: 10.1113/jphysiol.2014.273078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kracht M, Saklatvala J. Transcriptional and post-transcriptional control of gene expression in inflammation. Cytokine. 2002;20:91–106. doi: 10.1006/cyto.2002.0895. [DOI] [PubMed] [Google Scholar]
- 16.Bhavsar P, et al. Relative corticosteroid insensitivity of alveolar macrophages in severe asthma compared with non-severe asthma. Thorax. 2008;63:784–790. doi: 10.1136/thx.2007.090027. [DOI] [PubMed] [Google Scholar]
- 17.Kim HY, et al. Indoxyl sulfate (IS)-mediated immune dysfunction provokes endothelial damage in patients with end-stage renal disease (ESRD) Scientific reports. 2017;7:3057. doi: 10.1038/s41598-017-03130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trojanowicz B, Ulrich C, Seibert E, Fiedler R, Girndt M. Uremic conditions drive human monocytes to pro-atherogenic differentiation via an angiotensin-dependent mechanism. PloS one. 2014;9:e102137. doi: 10.1371/journal.pone.0102137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parrillo JE. Pathogenetic mechanisms of septic shock. The New England journal of medicine. 1993;328:1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- 20.Charytan DM, Fishbane S, Malyszko J, McCullough PA, Goldsmith D. Cardiorenal Syndrome and the Role of the Bone-Mineral Axis and Anemia. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2015;66:196–205. doi: 10.1053/j.ajkd.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grabulosa CC, et al. Chronic kidney disease induces inflammation by increasing Toll-like receptor-4, cytokine and cathelicidin expression in neutrophils and monocytes. Experimental cell research. 2018;365:157–162. doi: 10.1016/j.yexcr.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Holick MF. Vitamin D deficiency. The New England journal of medicine. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 23.Franca Gois Pedro, Wolley Martin, Ranganathan Dwarakanathan, Seguro Antonio. Vitamin D Deficiency in Chronic Kidney Disease: Recent Evidence and Controversies. International Journal of Environmental Research and Public Health. 2018;15(8):1773. doi: 10.3390/ijerph15081773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holick MF. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Reviews in endocrine & metabolic disorders. 2017;18:153–165. doi: 10.1007/s11154-017-9424-1. [DOI] [PubMed] [Google Scholar]
- 25.Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. The Journal of clinical endocrinology and metabolism. 1983;57:1308–1310. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 26.Stumpf WE, Sar M, Reid FA, Tanaka Y, DeLuca HF. Target cells for 1,25-dihydroxyvitamin D3 in intestinal tract, stomach, kidney, skin, pituitary, and parathyroid. Science. 1979;206:1188–1190. doi: 10.1126/science.505004. [DOI] [PubMed] [Google Scholar]
- 27.Gombart AF, O’Kelly J, Saito T, Koeffler HP. Regulation of the CAMP gene by 1,25(OH)2D3 in various tissues. The Journal of steroid biochemistry and molecular biology. 2007;103:552–557. doi: 10.1016/j.jsbmb.2006.12.095. [DOI] [PubMed] [Google Scholar]
- 28.Liu PT, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 29.Sorensen OE, et al. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–3959. doi: 10.1182/blood.V97.12.3951. [DOI] [PubMed] [Google Scholar]
- 30.Ramanathan B, Davis EG, Ross CR, Blecha F. Cathelicidins: microbicidal activity, mechanisms of action, and roles in innate immunity. Microbes and infection. 2002;4:361–372. doi: 10.1016/S1286-4579(02)01549-6. [DOI] [PubMed] [Google Scholar]
- 31.Jain SK, Micinski D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochemical and biophysical research communications. 2013;437:7–11. doi: 10.1016/j.bbrc.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L, Dai F, Tang L, Le Y, Yao W. Macrophage differentiation induced by PMA is mediated by activation of RhoA/ROCK signaling. The Journal of toxicological sciences. 2017;42:763–771. doi: 10.2131/jts.42.763. [DOI] [PubMed] [Google Scholar]
- 33.Carvalho JTG, et al. Cholecalciferol decreases inflammation and improves vitamin D regulatory enzymes in lymphocytes in the uremic environment: A randomized controlled pilot trial. PloS one. 2017;12:e0179540. doi: 10.1371/journal.pone.0179540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasui M, Hirabayashi Y, Kobayashi Y. Simultaneous measurement by flow cytometry of phagocytosis and hydrogen peroxide production of neutrophils in whole blood. Journal of immunological methods. 1989;117:53–58. doi: 10.1016/0022-1759(89)90118-X. [DOI] [PubMed] [Google Scholar]
- 35.Zhang G, Ghosh S. Toll-like receptor-mediated NF-kappaB activation: a phylogenetically conserved paradigm in innate immunity. The Journal of clinical investigation. 2001;107:13–19. doi: 10.1172/JCI11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akira S, Takeda K. Toll-like receptor signalling. Nature reviews. Immunology. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 37.Mousa A, et al. Effect of vitamin D supplementation on inflammation and nuclear factor kappa-B activity in overweight/obese adults: a randomized placebo-controlled trial. Scientific reports. 2017;7:15154. doi: 10.1038/s41598-017-15264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meireles MS, et al. Effect of cholecalciferol on vitamin D-regulatory proteins in monocytes and on inflammatory markers in dialysis patients: A randomized controlled trial. Clinical nutrition. 2016;35:1251–1258. doi: 10.1016/j.clnu.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 39.Outtz HH, Wu JK, Wang X, Kitajewski J. Notch1 deficiency results in decreased inflammation during wound healing and regulates vascular endothelial growth factor receptor-1 and inflammatory cytokine expression in macrophages. Journal of immunology. 2010;185:4363–4373. doi: 10.4049/jimmunol.1000720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukami A, et al. High white blood cell count and low estimated glomerular filtration rate are independently associated with serum level of monocyte chemoattractant protein-1 in a general population. Clinical cardiology. 2011;34:189–194. doi: 10.1002/clc.20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stinghen AE, Pecoits-Filho R. Vascular damage in kidney disease: beyond hypertension. International journal of hypertension. 2011;2011:232683. doi: 10.4061/2011/232683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. Journal of immunology. 2012;188:2127–2135. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinheiro da Silva F, Machado MC. The dual role of cathelicidins in systemic inflammation. Immunology letters. 2017;182:57–60. doi: 10.1016/j.imlet.2017.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.