Abstract
The chemotherapy response score (CRS) is used to score histopathologic response to neoadjuvant chemotherapy (NACT) of patients with extrauterine high grade serous carcinoma. This study was undertaken to determine if the CRS in the omentum, adnexa or when combined correlates with 1) progression free survival (PFS) or overall survival (OS), 2) laparoscopic score of abdominal disease, 3) CA-125 levels, 4) BRCA status and 5) platinum resistant disease.
158 cases were retrospectively collected that received NACT between April 2013 and February 2018 at a single institution. The 3-tier Böhm CRS system was applied to the omentum and adnexa. Survival outcomes between scored subgroups were analyzed using Cox proportional hazards regression. Spearman rank correlation analyses were used to assess CRS and clinical data.
119 cases were treated only with Carboplatinum/Paclitaxel. Omental CRS was: 1 (23 cases, 19.3%), 2 (65 cases, 54.6%), and 3 (31 cases, 26.1%), while adnexal CRS was: 1 (50 cases, 42%), 2 (48 cases, 40.3%) and 3 (21 cases, 17.6%). The omental CRS was significantly associated with PFS as a 2-tier score (HR 0.612, 95% CI 0.378-0.989, p=0.045) but not associated with the PFS using the 3-tier score or with OS using either system. Adnexal CRS was not associated with OS but was significantly associated with PFS using the 3-tier (HR 0.49, 95% CI 0.263-0.914, p=0.025) and 2-tier scores (HR 0.535, 95% CI 0.297-0.963, p=0.037). The combined score was not associated with OS but was significantly associated with PFS using the 3-tier (HR 0.348, 95%CI 0.137-0.88, p=0.026) and 2-tier scores (HR 0.364, 95% CI 0.148-0.896, p=0.028). No CRS system used associated with laparoscopic assessment of disease. CRS in the omentum had no significant association with platinum resistance; however, the adnexal CRS 1/2 were three times as likely to develop platinum resistance compared to CRS 3 (RR 3.94, CI 1.03-15.09, p=0.046).
The CRS, when used on the omentum, adnexa, and as a combined score, was significantly associated with PFS but not with OS. Adnexal CRS 1/2 are more likely to develop platinum resistant disease. Therefore, the use of this pathology parameter may be useful for clinical management.
Keywords: ovary, fallopian tube, peritoneal, extrauterine, high grade serous carcinoma, adjuvant, chemotherapy, chemotherapy response, score, survival, progression-free survival, overall survival
Introduction
Neoadjuvant chemotherapy (NACT) followed by interval debulking has become a viable treatment option for advanced stage extrauterine high-grade serous carcinoma (HGSC).1,2 For those treated with NACT, a 3-tier chemotherapy response score (CRS) based on histologic features has been proposed, with the aim to identify patients at risk for recurrence and those at higher risk for mortality, and has been adapted for use in synoptic reports by the College of American Pathologists and the International Collaboration on Cancer Reporting.3 The CRS, when used on the omentum, has been shown to be reproducible and to correlate with progression free survival (PFS).3-7 However, its correlation with overall survival (OS) has had mixed results.3-5,7-10 Additionally, the CRS has shown some promise in identifying patients at risk for platinum resistant disease (i.e., progression/recurrence within 6 months following platinum-based chemotherapy).3,8 When the CRS is used on the adnexa, there are also mixed results, ranging from no correlation to correlation with only PFS to correlation with PFS and OS. 4,5,11
While the CRS is used in the post-operative setting, other relevant data in the pre- and peri-operative settings have been examined to determine their impact on patient outcomes. Since residual tumor volume at the time of debulking significantly correlates with recurrence and overall survival, a laparoscopic score of disease and resectability, which has been shown to predict optimal cytoreduction, is being used to triage patients into NACT or primary debulking.12-14
The aims of this study were to determine if the CRS obtained either in the omentum, adnexa or combined (i.e., omental and adnexal scores added together) correlates with 1) PFS or OS, 2) laparoscopic score of abdominal disease, 3) CA-125 levels, 4) BRCA status and 5) platinum resistant disease. In addition, we evaluated the correlation of the laparoscopic score independently with PFS and OS.
Materials and Methods
Following Institutional Review Board (IRB) approval, a retrospective search for in-house surgical resection cases of extrauterine HGSC was done by searching for “high grade serous”, “carcinoma”, “ovary”, “fallopian tube”, and “peritoneum” within the database of the Department of Pathology at The University of Texas M.D. Anderson Cancer Center. This search was restricted to a period of time, April 2013 and February 2018, which corresponded to the use of the laparoscopic assessment to score the extent of abdominal disease in cases of extrauterine high grade serous carcinoma. 460 cases were identified, with 304 cases determined to have NACT following review of surgical pathology reports and clinical records. The inclusion criteria in this study were: 1) high grade serous carcinoma histotype exclusively (mixed carcinomas were excluded), 2) extrauterine origin only (tumors associated with a uterine neoplasm that could be interpreted as synchronous primaries were excluded), 3) FIGO stage III or stage IV disease, 4) the adnexa and omentum were removed during the same surgical procedure, 5) chemotherapy regimen based on Carboplatinum and Paclitaxel, with or without additional agents used (i.e., bevacizumab), 6) a follow-up of at least 6 months after completion of the post-operative chemotherapy, and 7) slides from the adnexa and omentum available for review. Six cases were excluded based upon no tumor seen on microscopic examination of the omentum and upon chart review no evidence of omental disease was detected by pre-NACT radiologic imaging. The following clinical parameters were recorded: patients’ age at time of diagnosis, date of diagnosis, FIGO stage, pre-chemotherapy CA-125, pre-interval debulking CA-125, post-interval debulking CA-125, date of first chemotherapy treatment, date of last chemotherapy treatment after interval debulking, date of recurrence, date of surgery, laparoscopic score (if performed), BRCA status, date of last follow-up and vital status. Follow-up time was calculated from the date of first neoadjuvant chemotherapy to date of last follow-up, with a cut-off date in December 2018. In the end, 158 cases were reviewed, of which 151 had sufficient slides available for evaluation. The remaining 7 cases were retained in the study to assess the relationship of laparoscopic score with PFS and OS.
Before scoring slides for chemotherapy response, the reviewing pathologists (A.M., B.L.) independently reviewed the original publication by Böhm, et al., and independently underwent the online training for the CRS system as provided by the aforementioned publication (http://www.gpecimage.ubc.ca/aperio/images/crs).3 Each pathologist completed the test section of the training with a kappa agreement of 1.0 to the test scores. Omental and adnexal slides were scored by pathologists jointly, with each site given a single score of 1 to 3. A full description of the CRS is provided in Table 1, as originally described by Böhm, et al.3 Briefly, the CRS is defined as follows: CRS 1, there is no or minimal tumor response, CRS 2, there is appreciable tumor response amidst viable tumor and CRS 3, there is complete or near complete response with no residual tumor or minimal irregularly scattered tumor foci seen as individual cells, cell groups or nodules up to 2 mm. For the purposes of analysis and ability to compare data to previous studies, CRS scores 1 and 2 were merged for comparison versus CRS score 3, referred to as modified 2-tier CRS. A combined score was also calculated by adding the adnexal and omental scores together, for a range of 2 to 6. The number of slides examined for each site (i.e., adnexa and omentum) was recorded.
Table 1.
Chemotherapy Response Score Criteria
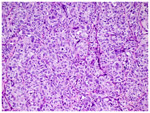 |
CRS 1 No or minimal tumor response. Mainly viable tumor with no or minimal regression-associated fibroinflammatory changes, limited to a few foci; cases in which it is difficult to decide between regression and tumor-associated desmoplasia or inflammatory cell infiltration. |
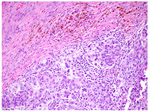 |
CRS 2 Appreciable tumor response amid viable tumor that is readily identifiable. Tumor is regularly distributed, ranging from multifocal or diffuse regression-associated fibroinflammatory changes with viable tumor in sheets, streaks, or nodules to extensive regression-associated fibroinflammatory changes with multifocal residual tumor, which is easily identifiable. |
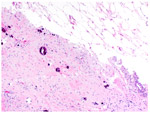 |
CRS 3 Complete or near-complete response with no residual tumor or minimal, irregularly scattered tumor foci seen as individual cells, cell groups or nodules up to 2 mm maximum size. Mainly regression-associated fibroinflammatory changes or very little residual tumor in the complete absence of any inflammatory response. |
At our institution, some patients are assessed laparoscopically to determine resectability of disease and to triage a patient to either upfront debulking or NACT. This is done by a laparoscopic score, which is based on peritoneal carcinomatosis, omental caking, diaphragmatic involvement, mesenteric retraction, bowel infiltration, stomach infiltration and superficial liver disease, with each site positive given a score of 2, with a possible score of 0 (negative) to 14 (each site scoring positive) and is graded by two gynecologic oncologic surgeons.12,13 As previously mentioned, the laparoscopic assessment has been able to predict optimal tumor debulking, with cases scoring 8 or greater having low probability for receiving optimal tumor debulking prior to chemotherapy.12,13 If the scores assigned by both surgeons were the same, the score was recorded. For the purpose of this study, if the scores assigned by the surgeons differed the scores were averaged together, with the averaged score recorded (e.g., if scores assigned were 0 and 2, the score recorded was 1).
Cox proportional hazards regression was used to assess the association between various covariates of interest. Spearman rank correlation analysis was used to assess the association between chemotherapy response scores and laparoscopic assessment of disease, number of slides examined, pre-interval debulking CA-125, and change in CA-125 between pre-NACT CA-125 and pre-interval debulking CA-125. Wilcoxon rank-sum tests were used to compare the chemotherapy response scores between BRCA positive and BRCA negative patients and between patients with and without additional chemotherapy agents used prior to interval debulking. All statistical analyses were performed using R version 3.6.0. All statistical tests used a significance level of 5%. No adjustments for multiple testing were made.
Results
Clinical findings are summarized in Table 2. Patients age ranged from 36 to 86 years (median 64 years). FIGO stage distribution was as follows: 78 (49.4%) stage III and 80 (50.6%) stage IV. The median follow up was 2.1 years (min 0.97 years, max 5.5 years). All 158 cases were treated with a base of Carboplatinum/Paclitaxel; however, additional agents, such as bevacizumab, were given neoadjuvantly in 2 cases, adjuvantly in 18 cases and both neoadjuvantly and adjuvantly in 12 cases. One hundred and twenty-six cases were treated with only Carboplatinum/Paclitaxel neoadjuvantly and adjuvantly, of which 119 had sufficient slides for review; as noted previously, the remaining 7 cases were retained in the study for assessment of the laparoscopic score. Results given are for the group of 119 cases with sufficient slides for review and only Carboplatinum/Paclitaxel, unless otherwise noted.
Table 2.
Baseline Characteristics of All Cases
| Characteristic | No. (%) |
|---|---|
| Median age, years (range) | 64 (36-86) |
| High-grade serous histology | 158 (100) |
| FIGO stage | |
| III | 78 (49.4) |
| IV | 80 (50.6) |
| Outcomes of surgical debulking | |
| Optimal | 143 (90.5) |
| Suboptimal | 15 (9.5) |
| Cycles of neoadjuvant chemotherapy | |
| Three or four | 122 (77.2) |
| > Four | 36 (22.8) |
| Total of six or more cycles of chemotherapy (neoadjuvant + adjuvant) | 153 (96.8) |
| Regiment of chemotherapy | |
| Carboplatinum/paclitaxel only | 126 (79.7) |
| Carboplatinum/paclitaxel and additional agent(s) given neoadjuvantly | 2 (1.3) |
| Carboplatinum/paclitaxel and additional agent(s) given adjuvantly | 18 (11.4) |
| Carboplatinum/paclitaxel and additional agent(s) given neoadjuvantly and adjuvantly | 12 (7.6) |
| BRCA status | |
| BRCA + | 20 (12.7) |
| BRCA − | 103(65.2) |
| Cases scored for laparoscopic bulk of disease | 41 (25.9) |
| Cases with sufficient slides for scoring CRS | 151 (95.6) |
| Median follow up, years (range) | 2.1 (0.97-5.5) |
There was a significant association between age at diagnosis and overall survival (HR=1.308, 1.11 to 1.542 95% CI, p=0.0013); however, there was no evidence of association between age at diagnosis and progression free survival (HR=1.064, 0.963 to 1.175 95% CI, p=0.23)
Omental Score
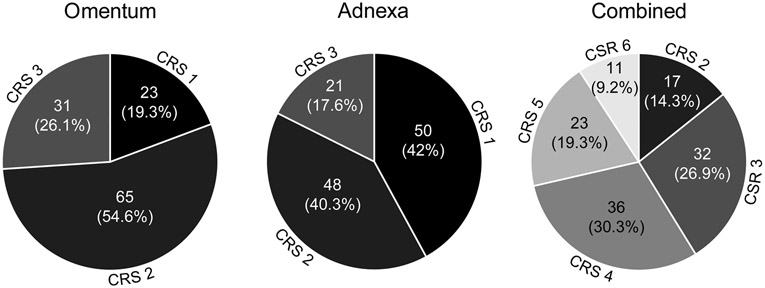
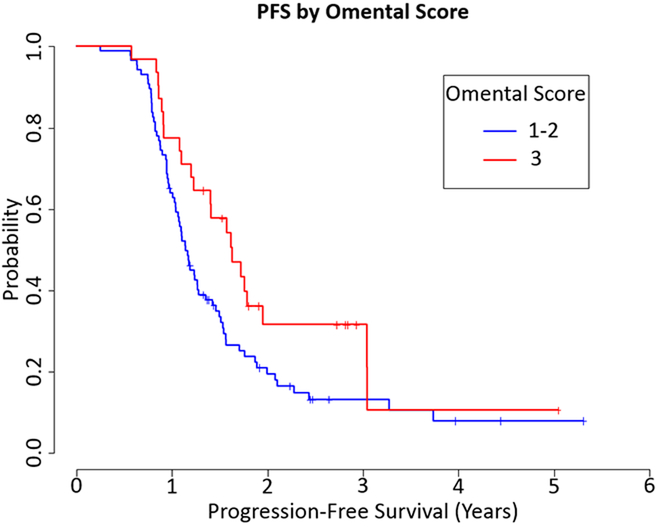
The omental CRS was as follows: CRS 1, 23 (19.3%) cases, CRS 2, 65 (54.6%) cases and CRS 3, 31 (26.1%) cases (Fig. 1). There was no evidence of an association between OS and the 3-tier omental score (HR=1.024, 0.418 to 2.508 95% CI, p=0.96) or the modified 2-tier omental score (HR=0.96, 0.495 to 1.865 95% CI, p=0.91). There was no significant association between the 3-tier omental score and PFS (HR=0.652, 0.349 to 1.219 95% CI, p=0.18). However, there was a significant association between the modified 2-tier omental score and PFS (HR=0.612, 0.378 to 0.989 95% CI, p=0.045, Fig. 2).
Figure 1.
Chemotherapy response scores by site
Figure 2.
Progression Free Survival (PFS) by Omental CRS 2-tier system (HR=0.612, 0.378 to 0.989 95% CI, p=0.045)
Adnexal Score
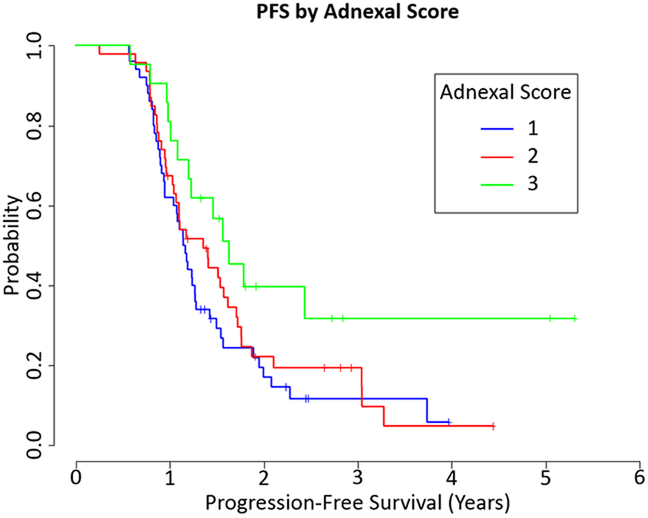
The adnexal CRS was: CRS 1, 50 (42%) cases, CRS 2, 48 (40.3%) cases, and CRS 3, 21 (17.6%) cases (Fig. 1). There was no significant association between OS and the 3-tier adnexal score (HR=0.71, 0.301 to 1.673 95% CI, p=0.43) or the modified 2-tier adnexal score (HR=0.734, 0.327 to 1.645 95% CI, p=0.45). However, there was a significant association between PFS and the 3-tier score (HR=0.49, 0.263 to 0.914 95% CI, p=0.025, Fig. 3), as well as the modified 2-tier score (HR=0.535, 0.297 to 0.963 95% CI, p=0.037, Fig. 3).
Figure 3.
Progression Free Survival (PFS) by Adnexal CRS 3-tier system (HR=0.49, 0.263 to 0.914 95% CI, p=0.025) and 2-tier system (HR=0.535, 0.297 to 0.963 95% CI, p=0.037)
Score comparison
Comparing the scores between the adnexa and omentum, the omentum scored higher in 47 (39.5%) cases, the adnexa scored higher in 15 (12.6%) cases and the score was equal in the omentum and adnexa in 57 (47.9%) cases.
Combined Score
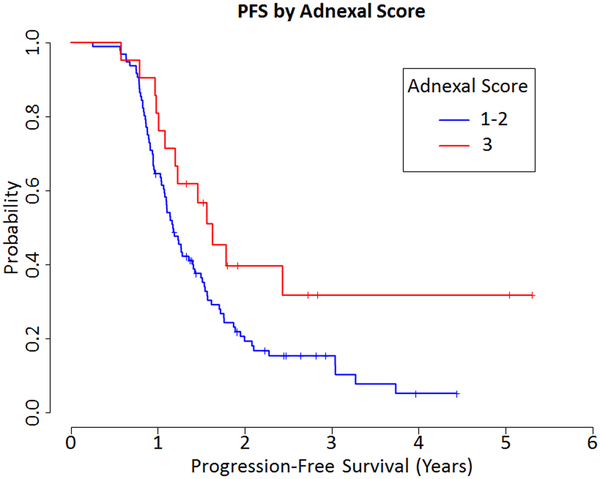
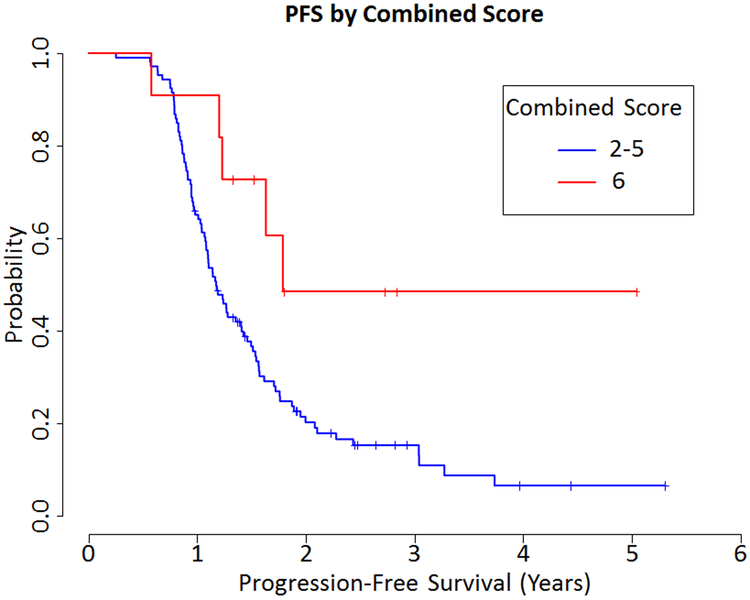
Combining the adnexal and omental scores, for a range of 2 to 6, resulted in 17 (14.3%) cases with a score of 2, 32 (26.9%) cases with a score of 3, 36 (30.3%) cases with a score of 4, 23 (19.3%) cases with a score of 5, and 11 (9.2%) cases with a score of 6 (Fig. 1). In addition to a 5-tier score system, analysis included a modified 3-tier system (scores 2 and 3 together versus scores 4 and 5 together versus 6), as well as a modified 2-tier system (combining scores 2 through 5 together versus 6). There was no evidence of an association between OS and the combined score using the 5-tier score system (HR=0.592, 0.147 to 2.382 95% CI, p=0.46), the 3-tier system (HR=0.747, 0.22 to 2.537 95% CI, p=0.64) or 2-tier system (HR=0.66, 0.205 to 2.131 95% CI, p=0.49). There was marginal evidence of an association between PFS and the combined score using the 5-tier score (HR 0.392, 0.138 to 1.117 95% CI, p=0.080). There was a significant association between PFS with both the combined 3-tier score (HR=0.348, 0.137 to 0.88 95% CI, p=0.026) and the 2-tier score (HR=0.364, 0.148 to 0.896 95% CI, p=0.028, Fig. 4)
Figure 4.
Progression Free Survival (PFS) by Combined Score 2-tier system (HR=0.364, 0.148 to 0.896 95% CI, p=0.028)
Laparoscopic Score
Thirty-seven cases were scored by our gynecologic oncologic surgeons for laparoscopic assessment of abdominal disease in which patients only received Carboplatinum/Paclitaxel. Scoring of cases ranged from 2 to 14, with a mean of 9 and median of 10. This assessment was significantly associated with PFS, with the cases that scored higher (i.e., more abdominal disease) having worse PFS (HR=1.154, 1.016-1.312 95% CI, p=0.028); however, there was no evidence of an association with OS (HR=1.132, 0.934-1.374 95%CI, p=0.21). Using Spearman rank correlation, there was no correlation between the laparoscopic score and the omental CRS (rank correlation 0.12, p=0.4922), adnexal CRS (rank correlation 0.20, p=0.2753) or the combined CRS (rank correlation 0.19, p=0.2802).
BRCA
BRCA status was known in 99 cases, with 83 cases BRCA negative and 16 BRCA positive. There was no correlation with BRCA and OS (HR=0.36, 0.11 to 1.165 95% CI, p=0.088) or PFS (HR=0.71, 0.384 to 1.312 95% CI, p=0.27).
In the subset of 144 cases with only Carboplatinum/Paclitaxel administered (126 cases) or who received an additional chemotherapy agent(s) adjuvantly (18 cases), BRCA was known in 106 cases, with 89 BRCA negative and 17 BRCA positive. There was no evidence of an association between BRCA status and the omental CRS (median=2 for both BRCA+ and BRCA−, p=0.38), adnexal CRS (median=2 for both BRCA+ and BRCA−, p=0.41), or combined CRS (median=3 for both BRCA+ and BRCA−, p=0.82).
CA-125
Of the one hundred and nineteen cases with sufficient slides for review and only Carboplatinum/Paclitaxel, all had pre-interval debulking CA-125 for review. The pre-interval debulking CA-125 had no significant association with OS (HR=0.999, 0.992 to 1.006 95% CI, p=0.73) or PFS (HR=1.001, 0.998 to 1.004 95% CI, p=0.35). There was a significant association between the pre-interval debulking CA-125 and the adnexal CRS (rank correlation −0.22, p=0.017) and the combined CRS (rank correlation −0.23, p=0.014), while there was a non-significant trend between the pre-interval debulking CA-125 levels and the omental CRS (rank correlation −0.16, p=0.079). Of these one hundred and nineteen cases, one hundred and twelve cases had both pre-chemotherapy CA-125 and pre-interval debulking CA-125 levels available. There was no correlation between the change in pre-chemotherapy CA-125 and pre-interval debulking CA125 and the omental CRS (rank correlation −0.01, p=0.90), adnexal CRS (rank correlation −0.08, p=0.41), or the combined CRS (rank correlation −0.06, p=0.50).
Additional Chemotherapy Given and Correlation with Chemotherapy Response Score
Of the cases in which any additional chemotherapeutic agents other than Carboplatinum/Paclitaxel were given neo-adjuvantly (14 cases total) versus those who received no additional chemotherapeutic agents or additional agents only adjuvantly (137 cases total), there was no association with the use of additional agents neo-adjuvantly and the CRS in the omentum (median 2 for both groups, p=0.47) or adnexa (median 2 for both groups, p=0.99).
Number of slides examined
Of all cases, 151 cases had sufficient slides available for review, with 977 slides evaluated on the omentum (median 5 per case, ranging from 1 to 26) and 1566 slides evaluated on the adnexa (median 9 per case, ranging from 1 to 33). Of the subset of 119 cases with no additional chemotherapy agents and with sufficient slides for examination, 803 slides were evaluated on the omentum (median 6, ranging from 1 to 26) and 1208 slides were evaluated on the adnexa (median 9, ranging from 2 to 33).
Using Spearman rank correlation, there was a non-significant trend between the omental CRS and the number of slides examined (rank correlation 0.17, p=0.061) while there was no correlation between the adnexal CRS and the number of slides examined (rank correlation 0.04, p=0.66).
Platinum resistant disease
One hundred and seventeen cases that received only Carboplatinum/Paclitaxel had specific data for post-chemotherapy progression/recurrence. In regards to omental CRS, progression/recurrence occurred within 6 months in 8 of 23 (34.8%) cases that scored a 1, 23 of 63 (36.5%) of those that scored a 2, and 7 of 31 (22.6%) of those that scored a 3. Overall, omental CRS was not statistically significant in predicting platinum resistant disease when comparing CRS 1+2 vs. 3 (RR=1.596, 0.785-3.247 95% CI, p=0.20). In regards to adnexal CRS, progression/recurrence occurred within 6 months in 22 of 50 (44%) cases that scored a 1,14 of 46 (30.4%) of those that scored a 2, and 2 of 21 (9.5%) that scored a 3. Overall, adnexal CRS was statistically significant in predicting platinum resistant disease when comparing CRS 1+2 vs. 3 (RR=3.938, 1.029-15.09 95% CI, p=0.046).
Discussion
Our study showed that the modified omental 2-tier CRS (i.e., CRS 1 and 2 versus CRS 3), not the 3-tier system, correlated with PFS, while neither the modified 2-tier nor the 3-tier system correlated with OS. This finding is in concordance with the Coghlan, et al, and Ditzel, et al, studies, totaling 139 cases.4,10 Singh, et al, additionally showed correlation with the omental 2-tier CRS and PFS while showing no correlation with OS in 103 cases.5 The first derivation of the current CRS was tested on the omentum and adnexa using a 6-tier system, with favorable results seen in the omentum.3 The current 3-tier CRS was further developed and validated on the omentum, with correlation with PFS repeatedly shown predominantly with the modified 2-tier system, combining scores 1 and 2 versus 3.3,4,6,8 While the original publication by Böhm, et al., showed the omental 3-tier system to have a non-significant trend with OS, a study with 36 months of extended follow up does show significant correlation with OS.3,9 Additional studies have also shown this correlation between omental CRS and OS; however, others have shown no correlation, including this study.7,8 Noting the difference between the follow-up time of our study (median 2.1 years) to that of the extended follow-up time of Böhm, et al., (median 4.3 years) may explain why we did not show significant correlation with the omental CRS and OS.9 Additional studies will be needed in order to determine if this association is seen in our cohort after extended follow-up.
The experience with the use of CRS on the adnexa is limited. Our results showed that the adnexal CRS 3-tier and modified 2-tier systems correlate with PFS; however, both systems showed no correlation with OS. Our findings are in keeping with the results of Michaan, et al., which showed that the CRS when applied to the adnexa had significant association with PFS but not OS and partially contrasts with Singh, et al, which showed that the CRS when applied to the adnexa had no significant association with either PFS or OS.5,11 Böhm, et al., when first developing the CRS extended scoring criteria to the adnexa and found it to not correlate with PFS or OS.3 Ditzel, et al., showed that the adnexal CRS had statistically significant correlation with PFS and OS; however, statistical significance was lost after further training on the CRS.4 Ditzel, et al., suggested that their change in statistical significance with the adnexal score after training was due to the fact that the online training and the original CRS were focused on the omentum only, not the adnexa, and that there may be slight variations to chemotherapy response by anatomic site.4 The authors also noted the small percentage of adnexal CRS 3 cases, 11.9%, may play into the loss of statistical significance, as compared to our study with more cases and a higher percentage of adnexal CRS 3 cases (17.6%).4
Our study examined a combined scoring system, by adding the omental and adnexal chemotherapy response scores together, and showed no correlation with OS. However, there was significant correlation with PFS when using a 3-tier system (scores 2+3 versus 4+5 versus 6) as well as a 2-tier system (scores 2 through 5 versus 6). This result is similar to Ditzel, et al., and Michaan, et al., which showed that a combined score, derived from the combination of the omental and adnexal CRS, has significant correlation with PFS but not OS.4,11 These findings, along with our own, suggest that the use of the CRS may not be limited to the omentum and that grading more diffuse disease response is possible.
Of interest, when comparing the CRS scores in the omentum and adnexa in our study, the CRS was similar in 47.9% of cases while the omental score was greater 39.5% of cases. When originally applying the CRS to the adnexa, Böhm, et al. had 43% of cases having equivalent CRS in the omentum and adnexa and 41% of cases having a CRS higher in the omentum.3
As the chemotherapy response score is determined on the slide with the least amount of response and there can be heterogeneity in the response seen from slide to slide, our study attempted to determine if the number of slides examined correlated with the CRS. However, there was no significant correlation with the number of slides examined per site with either the omental or adnexal CRS, though a non-significant trend was noted between the omental CRS and number of slides examined. Cases with complete response may show fibro-inflammatory regression associated changes, though this is not always the case and tissue sections may appear completely unremarkable. Our current practice for HGSC is to submit 1 section per 2 cm of grossly unremarkable omentum and complete submission of grossly unremarkable adnexa in an attempt to detect disease. This practice is likely why our study showed a non-significant trend between the omental CRS and the number of slides examined for the omentum, as more slides were submitted for grossly negative omentum that would be more likely to be CRS 3 than a grossly positive omentum. Following slide review, we excluded cases that had no tumor or regression associated changes on submitted sections and were negative for involvement by carcinoma on pre-NACT imaging. The exclusion of such cases has not been explicitly stated in prior studies of the CRS and may explain differences in results of correlations with omental CRS between this and other studies.
We found no correlation between the laparoscopic score and the omental, adnexal or combined CRS, though the laparoscopic score did independently correlate with PFS. While the CRS does not take into account pre-NACT bulk of disease, the laparoscopic score can aid in prognosis in the pre-NACT setting. The pre-interval debulking CA-125 did correlate with the adnexal and combined CRS and trended with the omental CRS, suggesting the CRS may correlate with the amount of disease at the time of resection. When examining NACT response with CA-125 levels, our study found no correlation with the change between the pre-NACT and the pre-interval debulking CA-125 levels and the CRS in the omentum, adnexa or combined. This is similar to what Böhm, et al., described in validation of the omental CRS in which the change in CA-125 had no correlation with the CRS.3 However, the CRS does appear to correlate with other measurements of response, such as in the case of McNulty, et al, which showed that the CRS and radiological imaging response are significantly associated.15 However, that study also showed that cases described as complete radiologic response could show no histologic response (CRS 1) and that cases with radiologically stable disease after NACT can show complete or near complete histologic response (CRS 3).15
Potentially the most clinically useful aspect of the CRS is the association with platinum resistant disease, allowing for prediction of cases at risk for recurrence for possible therapeutic action. In this study, of the subset with only Carboplatinum/Paclitaxel given and with sufficient data for progression/recurrence, 38 of 117 (32.5%) patients recurred within 6 months of post-operative chemotherapy and thus were classified as having platinum resistant disease. Böhm, et al., in the original development and validation of the CRS first noted an association with the omental CRS and platinum resistant disease, which has been seen in other studies when examining the omental CRS.3,8 However, other studies have not seen a statistically significant association with platinum resistant disease and the omental CRS.7 Our study also does not show significant association with platinum resistant disease with the omental CRS but does show significant association with the adnexal CRS.
Overall, this study validated what previous studies have shown, that the CRS when used on the omentum only correlates with PFS when used as a modified 2-tier system. We also showed that the CRS when used on the adnexa correlates with PFS when graded on the 3-tier system and the modified 2-tier system. The CRS when used as a combined score from the omentum and adnexa also correlated with PFS. However, no scoring system in this study correlated with OS. The use of the CRS then is limited only to that of disease progression. While other studies have shown a correlation with the omental CRS and prediction of platinum resistant disease, we did not find this in our study; however, we did note that the adnexal CRS was significant in predicting platinum resistant disease. Further studies are warranted to explore the usefulness of the CRS, especially with association with platinum resistant disease and exploration of treatment options in the immediate post-operative window when CRS is applied, to determine if the CRS is a clinically useful tool for patient management. Additionally, cancer reporting protocols (such as those by the College of American Pathologists and the International Collaboration on Cancer Reporting) should consider the use of the 2-tier omental CRS and possibly expanding the CRS use to the adnexa.
Acknowledgment
Statistical analyses of this work were supported in part by the Cancer Center Support Grant (NCI Grant P30 CA016672).
Footnotes
Presented in part at the 108th annual meeting of the United States and Canadian Academy of Pathology, National Harbor, Maryland, March 16-21, 2019
References
- 1.Vergote I, Tropé CG, Amant F, et al. Neoadjuvant Chemotherapy or Primary Surgery in Stage IIIC or IV Ovarian Cancer. N Engl J Med 2010;363:943–53. [DOI] [PubMed] [Google Scholar]
- 2.Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomized, controlled, non-inferiority trial. Lancet 2015;386:249–57. [DOI] [PubMed] [Google Scholar]
- 3.Böhm S, Faruqi A, Said I, et al. Chemotherapy Response Score: Development and Validation of a System to Quantify Histopathologic Response to Neoadjuvant Chemotherapy in Tubo-Ovarain High-Grade Serous Carcinoma. J Clin Oncol 2015;33:2457–63. [DOI] [PubMed] [Google Scholar]
- 4.Ditzel HM, Strickland KC, Meserve EE, et al. Assessment of a Chemotherapy Response Score (CRS) System for Tubo-Ovarian High-Grade Serous Carcinoma (HGSC). Int J Gynecol Pathol 2018;00:1–11. [DOI] [PubMed] [Google Scholar]
- 5.Singh P, Kaushal V, Rai B, et al. The chemotherapy response score is a useful histological predictor of prognosis in high-grade serous carcinoma. Histopathology 2018;72:619–625. [DOI] [PubMed] [Google Scholar]
- 6.Said I, Böhm S, Beasley J, et al. The Chemotherapy Response Score (CRS): Interobserver Reproducibility in a Simple and Prognostically Relevant System for Reporting the Histologic Response to Neoadjuvant Chemotherapy in Tuboovarian High-grade Serous Carcinoma. Int J Gynecol Pathol 2016;36:172–179. [DOI] [PubMed] [Google Scholar]
- 7.Lee JY, Chung YS, Na K, et al. External validation of chemotherapy response score system for histopathological assessment of tumor regression after neoadjuvant chemotherapy in tubo-ovarian high-grade serous carcinoma. J Gynecol Oncol 2017; 10.3802/jgo.2017.28.e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajkumar S, Polson A, Nath R, et al. Prognostic implications of histological tumor regression (Böhm’s score) in patients receiving neoadjuvant chemotherapy for high grade serous tubal and ovarian carcinoma. Gynecol Oncol 2018;151:264–268. [DOI] [PubMed] [Google Scholar]
- 9.Böhm S, Le N, Lockley M, et al. Histopathologic response to neoadjuvant chemotherapy as a prognostic biomarker in tubo-ovarian high-grade serous carcinoma: updated Chemotherapy Response Score (CRS) results. Int J Gynecol Cancer 2019;29:353–356. [DOI] [PubMed] [Google Scholar]
- 10.Coghlan E, Meniawy TM, Munro A, et al. Prognostic Role of Histological Tumor Regression in Patients Receiving Neoadjuvant Chemotherapy for High-Grade Serous Tubo-ovarian Carcinoma. Int J Gynecol Cancer 2017;27:708–713. [DOI] [PubMed] [Google Scholar]
- 11.Michaan N, Chong WY, Han NY, et al. Prognostic Value of Pathologic Chemotherapy Response Score in Patients with Ovarian Cancer After Neoadjuvant Chemotherapy. Int J Gynecol Cancer 2018;28:1676–1682. [DOI] [PubMed] [Google Scholar]
- 12.Fagotti A, Ferrandina G, Fanfani F, et al. Prospective validation of a laparoscopic predictive model for optimal cytoreduction in advanced ovarian carcinoma. Am J Obstet Gynecol 2008;199:642.e1–642.e6. [DOI] [PubMed] [Google Scholar]
- 13.Fleming ND, Nick AM, Coleman RL, et al. Laparoscopic Surgical Algorithm to Triage the Timing of Tumor Reductive Surgery in Advanced Ovarian Cancer. Obstet Gynecol 2018;132:545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winter WE, Maxwell GL, Tian C, et al. Prognostic Factors for Stage III Epithelial Ovarian Cancer: A Gynecologic Oncology Group Study. J Clin Oncol 2007;25:3621–7. [DOI] [PubMed] [Google Scholar]
- 15.McNulty M, Das A, Cohen PA, et al. Measuring response to neoadjuvant chemotherapy in high-grade serous tubo-ovarian carcinoma: an analysis of the correlation between CT imaging and chemotherapy response score. Int J Gynecol Cancer 2019;29:929–934. [DOI] [PubMed] [Google Scholar]