Abstract
Cyp27b1 and Cyp24a1 are reciprocally regulated in the kidney by the key hormones PTH, FGF23, and 1,25(OH)2D3. Our recent genomic studies in mice identified a complex kidney-specific enhancer module located within the introns of adjacent Mettl1 (M1) and Mettl21b (M21) genes that mediate basal and PTH induction of Cyp27b1 as well as suppression by FGF23 and 1,25(OH)2D3. Gross deletion of these segments in mice has severe consequences on skeletal health, and directly affects Cyp27b1 expression in the kidney. Deletion of both M1 and M21 submodules together fully eliminates basal Cyp27b1 expression in the kidney, leading to a systemic and skeletal phenotype similar to that of the Cyp27b1-KO mouse due to depletion of 1,25(OH)2D3 and high PTH. Cyp24a1 levels in the double KO mouse were low due to compensatory regulation by elevated PTH and reduced FGF23. However, expression of Cyp27b1 and retention of its regulation by inflammation (LPS) in the NRTCs remained unperturbed. Dietary normalization of calcium, phosphate, PTH, and FGF23 rescues this aberrant phenotype and normalizes the skeletal issues. Cyp24a1 is controlled by its own unique enhancers for 1,25(OH)2D3, FGF23, and PTH. We were also able to eliminate these activities in mice. Collectively, the hormone-mediated enhancer regulation of both Cyp27b1 and Cyp24a1 in the kidney is responsible for the circulating levels of 1,25(OH)2D3 in the blood which in turn primarily affects calcium and phosphate regulation. Importantly, we can now manipulate this system with our enhancer deletion animal models to study 1,25(OH)2D3 production in non-renal target cells and tissues not only in disease, where it is known to affect the immune system, but also in healthy individuals. Here we will review our studies that have defined a finely balanced homeostatic control mechanism employed by PTH and FGF23 with catastrophic toxicity protection from 1,25(OH)2D3 in the genomic regulation of vitamin D metabolism and its accompanied control of mineral maintenance.
Keywords: Cytochrome P450; CRISPR/Cas9; ChIP-seq; vitamin D; gene regulation; 1,25(OH)2D3; Cyp27b1-KO; Cyp24a1; fibroblast growth factor 23 (FGF23); parathyroid hormone (PTH)
1. Introduction
The path to bioactivation of vitamin D traverses several tissues on its way to the final step in the kidney. The liver enzyme CYP2R1 converts vitamin D3 to 25(OH)D3, which is then further hydroxylated by the CYP27B1 enzyme to become the most active vitamin D metabolite, 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3) [1, 2]. The main function of 1,25(OH)2D3 in the circulation is to bind to and activate the vitamin D receptor (VDR) to control genes that help maintain the delicate balance of calcium and phosphate at specific target tissues, however, 1,25(OH)2D3 can also have a wide range of effects on the cells of the immune system and cell proliferation. The excess of 1,25(OH)2D3 is neutralized by the CYP24A1 enzyme to create 1,24,25(OH)2D3, a first step in the catabolism of 1,25(OH)2D3 [2]. Importantly, CYP24A1 also converts the 25(OH)D3 as well as numerous catabolic intermediates that eventually find their way to calcitroic or calcioic acid that is then excreted from the body. Therefore, both CYP27B1 and CYP24A1 share a common substrate in 25(OH)D3 and while the generation of 25(OH)D3 by CYP2R1 is generally unregulated, the Cyp27b1 and Cyp24a1 genes are tightly regulated by a triumvirate of phosphate (P) and calcium (Ca) sensing endocrine hormones, fibroblast growth factor 23 (FGF23), parathyroid hormone (PTH), and 1,25(OH)2D3 itself [3-7]. These hormones regulate Cyp27b1 and Cyp24a1 in the kidney in a reciprocal manner. 1,25(OH)2D3 and FGF23 suppress Cyp27b1 and induce Cyp24a1, whereas PTH is a potent inducer of Cyp27b1, it suppresses Cyp24a1. The relationship of both Cyp27b1 and Cyp24a1 expression allows the body to maintain the appropriate levels 1,25(OH)2D3 and therefore extracellular calcium and phosphate levels.
Outside of the kidney, the expression of these enzymes in non-renal target cells or tissues (NRTCs) is extremely low in healthy tissue [8, 9]. Cyp27b1 is not responsive to FGF23, PTH, or 1,25(OH)2D3 as it is in the kidney, and Cyp24a1 is only, and strikingly, up-regulated by 1,25(OH)2D3, undoubtedly as a protective mechanism in the rare case of toxic circulating 1,25(OH)2D3 levels. There are a few cell types and tissues in which Cyp27b1 expression may be somewhat regulated by 1,25(OH)2D3 such as the skin or parathyroid glands, but overall this expression is far more modest than the effects observed in the kidney [9]. Cyp27b1 and Cyp24a1 are regulated in cells of the immune system (macrophages and T cells) by inflammation [8, 10]. The expression of non-renal Cyp27b1 has the potential to create non-renal 1,25(OH)2D3, or “local” 1,25(OH)2D3 production. Apart from the most striking example of a diseased sarcoidosis patient [11, 12], it is not believed that 1,25(OH)2D3 from NRTCs makes its way back into the circulation in any meaningful amounts and if produced, may be retained for local actions within those tissues in vivo. Cell culture and ex vivo models of Cyp27b1 enzymatic activity have been shown in keratinocytes, macrophages, and T cells [10]. While nearly all cells may contain these enzymes, many of these NRTCs lack the transport mechanisms of megalin receptor and accessory protein cubilin to deliver the physiologic concentrations of 25(OH)D3 substrate necessary as they do in the kidney [13]. So despite the potential for NRTC-produced 1,25(OH)2D3 and expression of Cyp27b1, the enzyme may not see any of the required substrate in vivo that may otherwise present itself in artificial or cancerous cell culture models.
Even though the Cyp27b1 gene was discovered many decades ago, its genomic regulation has remained poorly understood until recent [14, 15]. The endocrine regulation of kidney Cyp27b1 that exists in vivo is difficult to recapitulate in cultured cells for reasons that remain unknown. Cyp24a1 regulation by 1,25(OH)2D3 in cells was believed to be only a promoter driven event [16, 17], until we discovered a downstream enhancer cluster [18] that parts of which, we have learned more recently, were kidney specific [19]. Therefore, the only model in which to faithfully study the regulation of Cyp27b1, as well as Cyp24a1, is within a whole organism model system like the mouse. Unlike cell culture, animal models have the ability to sense and respond to changing calcium or phosphate concentrations in the circulation and release endocrine hormones like PTH (from the parathyroid) and FGF23 (from skeletal cells). These changes then may have systemic consequences such as hormonal imbalance, stunted growth, and deterioration of skeletal integrity. Recently, we turned to the mouse model and identified the responsible genomic enhancers via in vivo kidney ChIP-seq analysis of the transcription factors VDR and CREB as well as histone modification marks that indicate enhancer position and activity [14, 15, 19]. These studies provided the first road map with which we could start to manipulate the genome via CRISPR/Cas genomic editing, eliminating enhancers that appear to be kidney specific and may disturb the regulation Cyp27b1 or Cyp24a1. While we manipulated each gene enhancer separately, there were consequences on the regulation of both genes, for example deletion of the M1 Cyp27b1 enhancer resulted in an extremely low expression of Cyp24a1 and thus, very low vitamin D metabolic products. This highlights the exquisite sensitivity of the reciprocal regulation between Cyp27b1 and Cyp24a1. Here, we will review this process, what we discovered about the genetic landscape around these genes, and lessons we have learned about vitamin D metabolism as a result.
2. Enhancer-mediated regulation of Cyp27b1 and Cyp24a1 expression
Predicting regulatory enhancers based on genomic sequence alone is a difficult task. Often we can learn much about the genomic environment from the sequence surrounding a gene of interest as well as the density of genes found within that genomic locus. That information coupled with histone modification markers like histone H3 monomethylated lysine 4 (H3K4me1), which has been attributed to enhancers, or H3 acetylated lysine 9 (H3K9ac) or lysine 27 (H3K27ac, active regions/enhancer), will quickly detail regions of the genome that are likely being actively utilized for transcription in one form or another [20-22]. Of course other techniques like DNase-seq or ATAC-seq may indicate which of the genomic regions are “open” to allow transcription factors and other complexes bind to DNA, however may not be as dynamic of markers as the histone modifications [23, 24]. Genes that reside in a “gene desert”, meaning very few genes either upstream or downstream (by 10s if not 100s of kb), that are surrounded by nearby peak(s) of H3K4me1 or peak(s) that indicate these regions to be open by ATAC- or DNase-seq may mean that these genomic regions (peaks) may in fact regulate the nearby gene. Unfortunately though, many genes reside in very gene dense locations, like Cyp27b1 does on chromosome 10 in the mouse (Fig. 1A) [14, 15]. In these gene dense areas, assignment of peaks to their associated genes is almost impossible without either a chromosomal conformation assay (3C, 4C, HiC) or genetic editing using CRISPR/Cas, TALENs, or other approach [25-28]. The former approach (HiC application) is also difficult to evaluate in gene dense regions as closer structures associate more frequently in this assay than distant connections giving false positive correlations. The ideal assay for causative evidence of an enhancer regulating a gene of interest is with genetic modification, either deletion or mutation, of the sequences involved. We have successfully employed genetic modification through CRISPR/Cas methods to demonstrate a hierarchy of multiple enhancer control of the Mmp13 gene in cultured cells [29] and also here to explore this complicated relationship between Cyp27b1 and Cyp24a1 [14, 15].
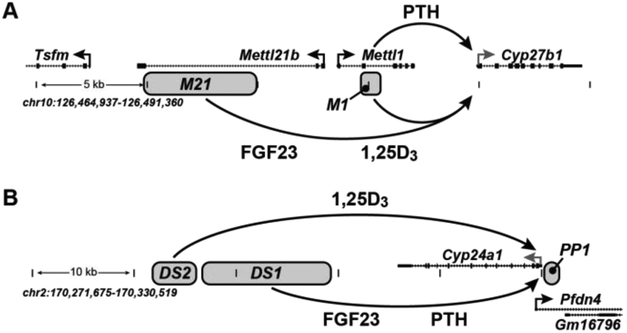
Figure 1:
Schematic representation of the genomic enhancers for either Cyp27b1 (A) or Cyp24a1 (B). Enhancers are shown as grey boxes, which are deleted in the mouse with the same name. Genomic location and scale are given for both loci (mouse, mm9). Gene transcriptional direction is indicated by an arrow and exons by boxes.
For our investigations surrounding Cyp27b1, we used a variety of histone modification markers as well as VDR and pCREB in the mouse kidney [15]. We also compared these tracks to data we had generated in other cell lines, tissues, as well as the publicly available ENCODE consortium data tracks [30]. From these data, we identified a series of 4 peaks that appeared to be kidney specific which will be summarized succinctly here, (for primary genomic data please refer to our recent publications [14, 15]). The first of these peaks (closest to Cyp27b1) was found in an intronic region of the Mettl1 gene, an enhancer we called “M1”, and the following 3 peaks were found in an intronic region of the Mettl21b gene (termed M21). This is shown schematically in Fig. 1A. Notably, the M1 region is approximately 5 kb away from the Cyp27b1 TSS and the M21 region is between 10-15 kb. From our sequence information, we learned that nearly 75% of the M21 region contained repetitive genomic elements that were not conserved in the human sequence [14, 15].
A similar methodology was employed for the investigation of Cyp24a1 as well [19]. The same kidney ChIP-seq data were analyzed in the chromosome 2 region that contains the Cyp24a1 gene and its surrounding genes Prefoldin 4 (Pfdn4) and breast carcinoma amplified sequence 1 (Bcas1). Unlike the Cyp27b1 locus, this region contained a gene desert region (~55 kb) between Cyp24a1 and the downstream Bcas1 gene [18]. In 2010, we discovered that part of this intergenic region helped contribute to the 1,25(OH)2D3 mediated regulation of Cyp24a1 in NRTCs like human colonic and mouse osteoblastic cells [18] along with the known promoter proximal region [16, 17]. While illuminating for the activities of 1,25(OH)2D3, Cyp24a1 in the NRTCs is not regulated by PTH or FGF23, therefore ChIP-seq analysis from the mouse kidney was key to understanding these latter regulatory components. From these studies, we determined there were a series of peaks of histone modification marks that were differentially changed after PTH and FGF23 treatments. We named this collection of peaks “DS1” (downstream 1) which is shown schematically in Fig. 1B. The aforementioned 1,25(OH)2D3 responsive enhancer discovered in the NRTCs was also present in the kidney and was named “DS2” (downstream 2); finally, the previously discovered promoter proximal set of VDREs was named PP1 [19]. After the identification of these kidney specific peaks near both Cyp27b1 and Cyp24a1, we then sought to understand if and how these regions were able to control Cyp27b1 and Cyp24a1 through deletions in the mouse.
3. Animal knockouts and enhancer anomalies
Since the re-purposing of the CRISPR/Cas bacterial defense system to mammalian systems in 2012, it has been used in various ways to create gene deletions, frame mismatch mutations, genomic insertions, reporter gene introduction, and even disease correcting SNPs [26, 31-38]. Our application of CRISPR/Cas genomic editing has been directed towards understanding enhancer-specific genomic control [29]. As such, we focus our efforts in two ways: first, the elimination of enhancers (200 – 2000+ bp) largely by non-homologous end joining (NHEJ) and second, sequence-specific mutation utilizing homology directed repair (HDR) after Cas9 nuclease digestion of detailed segments we discover in our enhancers. While this can be accomplished in cell lines with some effort, the ideal system for our Cyp27b1 and Cyp24a1 investigations has been in the mouse model system to fully appreciate the homeostatic regulation of these genes. The CRISPR/Cas machinery (Cas9 protein and RNA guides) is directly injected into the pronucleus of a fertilized, single-cell zygote which is then implanted to a foster mother [15]. In this way, the CRISPR machinery is able to mutate the genome as directed and then is rapidly degraded, with little chance of any materials being integrated into the host cell. Even in the case of HDR, we utilize single stranded donor oligos (ssODNs) which are degraded more rapidly than double stranded DNA. The resulting founder pups may contain a multitude of differential mutations or deletions that must be carefully monitored and investigated for fidelity of the designed deletion.
3.1. M1 and M21 enhancer control of Cyp27b1 in the mouse kidney
For the Cyp27b1 deletions depicted in Fig. 1A, we used a pair of RNA guides to direct the Cas9 digestions [15]. The M1 deletion created a mouse missing < 400 bp of intronic DNA from the Mettl1 gene (M1 intronic knock out, M1-IKO). The M21 deletion removed ~ 5 kb of intronic DNA from the Mettl21b gene (M21 intronic knock out, M21-IKO). These animals and their phenotypes are summarized in Table 1. Both of these mice were indistinguishable from WT littermates at weaning, however shortly thereafter, the M1-IKO animals’ growth appeared stunted resulting in mice of lower weight and smaller stature compared to their WT littermates. The M21-IKO animals on the other hand continued to be unremarkable in gross physical phenotype compared to their WT littermates. By 8 weeks of age, the M1-IKO animals resembled the physical characteristics of a Cyp27b1-null mouse [15, 39]. The M1-IKO animals also had nearly identical serum calcium, phosphate, PTH, and intact FGF23 (iFGF23) as the Cyp27b1-null animals in that Ca (7-8 mg/dL, WT 10-11 mg/dL) and P (1.8-2.0 mM, WT 2.4 mM) were low, iFGF23 extremely low (~6-10 pg/mL, WT 190-200 pg/mL), and PTH values were excessively high ( > 3,500 pg/mL, WT is 40-60 pg/mL). By comparison, the M21-IKO animals had normal Ca, P, PTH, and about half the levels of iFGF23 (90 pg/mL, WT 190-200 pg/mL). The skeletal morphology of the M1-IKO mouse also mimicked the Cyp27b1-null mouse with low BMD, low body weight, and severe loss of cortical and trabecular bone, whereas the M21-IKO mice were not different than WT littermates in any parameter [15].
Table 1:
Animal models and phenotypes
| Strain/type | Phenotype | Reference |
|---|---|---|
| Wildtype (WT) | none | |
| Cyp27b1-null | Cyp27b1 null, low Cyp24a1 basal | [14,15] |
| M1-IKO | Low Cyp27b1 basal, Cyp27b1 PTH insensitive | [14,15] |
| M1-IKOP | Low Cyp27b1 basal, Cyp27b1 small PTH sensitivity | [14] |
| M1-IKOD | Elevated Cyp27b1 basal, Cyp27b1 PTH response normal | [14] |
| M21-IKO | Low Cyp27b1 basal, Cyp27b1 FGF23 insensitive | [14,15] |
| Cyp24a1-null | Cyp24a1 null | [19] |
| C24-DS1KO | Low Cyp24a1 basal, Reciprocally low Cyp27b1 basal |
[19] |
| C24-DS2KO | Cyp24a1 1,25(OH)2D3 sensitive in NRTC only | [19] |
| M1/M21-DIKO | Low Cyp27b1 basal, Cyp27b1 PTH/FGF23/1,25(OH)2D3 insensitive |
[14] |
The reasons for these phenotypic differences revealed themselves in the gene expression pattern for Cyp27b1 from the mouse kidney. Typically, Cyp27b1 is strongly induced by PTH and potently inhibited by FGF23 and 1,25(OH)2D3 in wildtype animals [14, 15]. Both the M1-IKO and M21-IKO mice not only had regulatory defects in the expression of Cyp27b1, but also severe decreases in basal expression. M1-IKO animals were unable to respond to increasing levels of PTH, however Cyp27b1 was still inhibited by FGF23 and 1,25(OH)2D3. M21-IKO animals were unable to be inhibited by FGF23, but were still inhibited by 1,25(OH)2D3 and induced by PTH. The insensitivity of Cyp27b1 to the increasing levels of PTH prevents 1,25(OH)2D3 production to stave off the losses in Ca which further drives the release of PTH. This vicious cycle drives the skeletal losses as the mouse attempts to correct the Ca depletion by resorbing bone. For this reason, the M21-IKO animals have a normal skeleton as Cyp27b1 is still sensitive to PTH, enough 1,25(OH)2D3 can be made to interrupt the cycle of skeletal depletion. It is important to note that only the kidney Cyp27b1 (and Cyp24a1) expression was affected by these deletions. The non-renal target cells (NRTCs) like macrophages, T cells, skin, L5 vertebrae, calvaria, intestine, and others all had WT levels of basal Cyp27b1 expression [15]. It would appear that during normal mouse development (at least through 8-10 weeks), the FGF23 suppressive effect has less of a consequence on Cyp27b1 expression and production of 1,25(OH)2D3 than does sensitivity to Ca and the resulting elevation in PTH since the M21-IKO mice are normal in their skeletal and systemic phenotypes.
The basal decrease of the Cyp27b1 expression in the M1-IKO and M21-IKO mice lead to overall lower levels of 1,25(OH)2D3 being produced compared to WT littermates. As a reminder, 25(OH)D3 is the substrate for both the CYP27B1 and CYP24A1 enzymes, the former leading to 1,25(OH)2D3 and the latter leading to a catabolic product 24,25(OH)2D3 [2]. CYP24A1 also catabolizes 1,25(OH)2D3 to 1,24,25(OH)3D3 which is then further degraded and excreted. Importantly, since the expression of Cyp27b1 and Cyp24a1 are so tightly linked, we found an accommodation of Cyp24a1 basal expression to the levels of 1,25(OH)2D3 being produced [15]. Therefore, the low basal levels of Cyp27b1 (~85 - 95% reduction from WT) resulted in an equivalently lowered Cyp24a1 expression in the kidney. This led to less catabolism of 1,25(OH)2D3 to 1,24,25(OH)3D3 in an attempt to salvage all possible 1,25(OH)2D3 for the biological functions necessary to keep the animal healthy. In our M1- and M21-IKO mice, the lowered production of Cyp27b1 and Cyp24a1 resulted in a high level of substrate 25(OH)D3 (80 and 60 ng/mL, ~15 ng/mL WT) and low levels of 1,25(OH)2D3 (13 and 20 pg/mL, ~25-30 pg/mL WT) product. Interestingly, M1-IKO mice made very little 24,25(OH)2D3 (2 ng/mL, ~10 ng/mL WT) and undetectable amounts of 1,24,25(OH)3D3 (< limit of detection (LOD), ~65 pg/mL WT) whereas the M21-IKO animals were able to make both 24,25(OH)2D3 (18 ng/mL) and 1,24,25(OH)3D3 (25 pg/mL). This indicates that the reduced concentration of Cyp24a1 expression leads to an enzymatic level that is inadequate for conversion of either 25(OH)D3 or 1,25(OH)2D3 in the M1-IKO, yet is adequate for conversion in the M21-IKO animals. Cyp24a1 expression is suppressed by PTH in the kidney and therefore the M1-IKO animals and the high circulating PTH levels continually suppress the Cyp24a1 levels below what is required for enzymatic conversion. It is also possible the CYP24A1 enzyme is being inhibited by an unknown mechanism related to PTH, perhaps though enzymatic inhibition [40]. In total, the M1-IKO mice represent a very similar phenotype to the Cyp27b1-null animal and the levels of Cyp24a1 expression and its relationship to Cyp27b1 are vital to the ability of the mouse to maintain a healthy skeleton.
3.2. Hormonal control of renal Cyp24a1 expression
The genomic mechanism of the reciprocal regulation of Cyp24a1 in the kidney has also been poorly understood beyond the actions of 1,25(OH)2D3. Therefore, we applied our observations in the mouse kidney ChIP-seq and eliminated the DS1 and DS2 genomic regions just as we did for the Cyp27b1 M1 and M21 investigations resulting in C24-DS1KO (17.5 kb deletion) and C24-DS2KO (2.4 kb deletion) mice (Fig. 1B) [19]. The phenotypes of these animals are summarized in Table 1. The DS1KO and DS2KO mice had very little systemic or overt physical phenotypes unlike the M1-IKO and M21-IKO animals. The skeletal quality and BMD was unchanged from WT as were the levels of Ca and P. The DS1KO had slightly lower levels of PTH and higher levels of iFGF23 from WT, whereas the DS2KO animals were not statistically different than WT in any parameter. These results for the DS1KO indicated that there may be some mineral homeostatic compensation that keep the animals healthy [19].
Interestingly, the gene expression demonstrated a kidney-specific defect in Cyp24a1 regulation in the DS1KO animals, whereas the DS2KO only affected expression in NRTCs [19]. Cyp24a1 expression was unaffected by DS1KO deletion in the NRTCs we tested (intestine, skin, bone, thymus, spleen, and the thyroparathyroid), however in the kidney there was a drop in the basal activity (~90%). The DS1KO animals also demonstrated a resistance to PTH and FGF23 regulation of Cyp24a1, however, 1,25(OH)2D3 fully induced Cyp24a1. In the NRTCs, PTH and FGF23 do not regulate Cyp24a1 so those levels were unchanged, and the induction of 1,25(OH)2D3 was also the same. DS2KO mice however, were not different from WT mice in Cyp24a1 kidney basal expression or the response to PTH, FGF23, or 1,25(OH)2D3. These DS2KO animals only showed a loss of Cyp24a1 activity in the NRTCs. These results indicated that the DS1 region was in fact a kidney specific region that not only controls FGF23 and PTH regulation, but also controls the basal activity of Cyp24a1. The basal expression of Cyp24a1 in NRTCs is very low, nearly undetectable in most cases even by qPCR analysis. In contrast, the Cyp24a1 expression in the kidney is easily and reliably detectable tens of fold higher than observed in any NRTC. We believe that the DS1 enhancer is responsible for the higher basal activity in the kidney as the chromatin structure is unique to the kidney. The promoter proximal region (PP1) and the DS2 regions clearly mediate the 1,25(OH)2D3 response otherwise and while the PP1 carries the majority of the activation burden, the DS2 contributes as well. The Cyp24a1 regulation requires cooperation of the promoter proximal and downstream enhancers for both its kidney-specific and more ubiquitous activation roles in the body.
4. The kidney-specific, endocrine deficient Cyp27b1 pseudo-null mouse
Establishing the M1 and M21 regions as bone-a-fide enhancers for Cyp27b1 answered many questions about the mechanism of regulation that has remained elusive for many years [14, 15]. While the PTH and FGF23 regulation appear to be segmented into the M1 and M21 regions, respectively, there was only a partial response for 1,25(OH)2D3 regulation of Cyp27b1 from deleting both regions. In fact, we learned that the M1-IKO region could impart both a positive (PTH) and negative (1,25(OH)2D3) control on Cyp27b1 through our M1-IKOP (proximal) and M1-IKOD (distal) mice in which the M1-IKO region was sub-dissected into 2 mutations. When the region containing a putative VDRE was removed (M1-IKOD), the basal expression of Cyp27b1 increased indicating that the deleted region may have been exerting a suppressive effect on Cyp27b1. These observations led us to ask questions beyond the mechanism of Cyp27b1 regulation, focusing on how we might exploit these mechanisms to learn more about the homeostatic implications of 1,25(OH)2D3 production in not only the kidney, but importantly in NRTCs as well. We had the unique opportunity to create an animal whose vitamin D metabolism is resistant to endocrine signaling and decoupled from mineral metabolism, however could still respond to inflammatory induction of Cyp27b1 in cells outside of the kidney. Ideally, we could eliminate the Cyp27b1 expression altogether from the kidney with a kidney-specific Cre-Lox knockout, however the lack of Cre drivers for the complexity of cell types in the kidney has made this difficult to achieve. By combining the M1 and M21 intronic enhancer deletions into a single mouse (M1/M21-DIKO, double intronic knock out), we in effect created an endocrine-deficient, kidney-specific Cyp27b1 pseudo-null mouse. This animal has very low kidney Cyp27b1 expression levels, on par with non-renal cell Cyp27b1 expression, very low levels of circulating 1,25(OH)2D3, and exhibits the phenotype of the Cyp27b1-null animal.
To accomplish this, we performed CRISPR in the M21-IKO line for the M1 enhancer guide RNAs [14]. We ensured that the founder mice deletions were identical to the M1-IKO animals and outbred the animals several generations to remove any undetected off-target or mosaic deletions. The M1/M21-DIKO animals shared the same characteristics of the M1-IKO and Cyp27b1-null animals in serum Ca, P, PTH, and iFGF23 [14]. The BMD and skeletal quality were also equivalent to the Cyp27b1-null mouse. We confirmed by gene expression analysis that the kidney Cyp27b1 was unresponsive to time course administration of PTH or FGF23, as expected. There was also no inhibition of Cyp27b1 throughout a time course of 1,25(OH)2D3 injections. The levels of circulating 1,25(OH)2D3 in the M1/M21-DIKO mice were reduced further from the M1-IKO animals to 10 pg/mL (LOD of assay is 5 pg/mL), and the 25(OH)D3 levels were elevated as they were with the M1-IKO mouse. Similar to the M1-IKO and Cyp27b1-null mice, the CYP24A1 enzyme appeared inactive in the M1/M21-DIKO animals as well, since there was very low 24,25(OH)2D3 and no detectable 1,24,25(OH)3D3. Much like the M1-IKO animals, the CYP24A1 substrate 1,25(OH)2D3 was accumulating and no 1,24,25(OH)3D3 product was being made, therefore the 1,25(OH)2D3 levels in the M1/M21-DIKO animals appeared low yet still detectable. This level of 1,25(OH)2D3 however, was unable to save these mice from skeletal destruction.
Fortunately, the M1/M21-DIKO animals were able to be rescued from their skeletal maladies through a diet high in Ca and P containing lactose (2% Ca, 1.25% P, 20% lactose) [14, 41]. If the animals were fed this “rescue diet” from weaning, the serum Ca and P were rapidly corrected prior to 8 weeks. The serum PTH and iFGF23 values, however, take several additional months to correct. For example, by 3 months of rescue diet, the PTH values decreased from ~3500 pg/mL to 100-300 pg/mL. It takes a full 4 months to fully correct these values to the WT levels in the 40-60 pg/mL range. After this time period, we found these animals normalized their levels of Cyp24a1 expression as well as those of PTH, Ca, P, and iFGF23 l [14]. However, since our M1/M21-DIKO animals have Cyp27b1 expression that is insensitive to rescue, the expression levels of Cyp27b1 remained extremely low. Therefore, the low expression of Cyp27b1 and the increased Cyp24a1 expression caused the levels of 1,25(OH)2D3 present in the circulation to fall from 10 pg/mL down to below the limit of detection (LOD < 5 pg/mL) as more 1,24,25(OH)3D3 catabolic product is made from CYP24A1 enzymatic turnover of the 1,25(OH)2D3 substrate. The rescue diets and our genetic manipulations only affect gene expression and enzymatic activities in the kidney. NRTCs expression was unchanged in these animals before or after the dietary rescue, leading to an excellent model with which to study 1,25(OH)2D3 production from non-renal sources in healthy animals as well as disease models of inflammation.
5. Metabolite predictions and conclusions
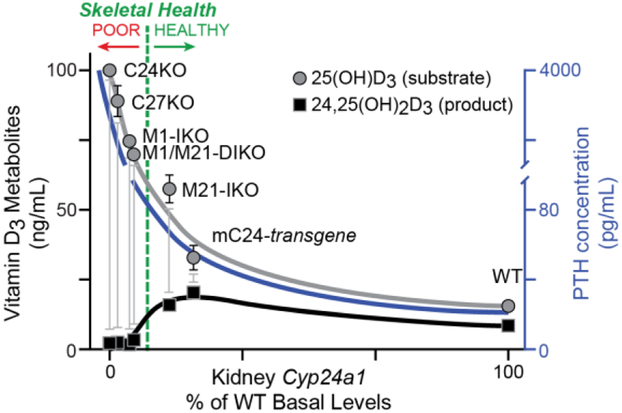
Through these studies, we have unearthed many new tantalizing details about the genomic mechanism of Cyp27b1 and Cyp24a1 regulation and the impact that these genes have on mineral metabolism. While our initial goal was to completely eliminate the impact of Cyp27b1 and 1,25(OH)2D3 production in the kidney, and one we did achieve in the M1/M21-DIKO mouse, the various enhancer deletion models also allowed us to see a wide spectrum of Cyp27b1 activity from basal suppression (M21) to a dramatic 95% suppression (M1, M1/M21), and finally a basal increase (M1-IKOD). We learned through these studies that perhaps the kidney Cyp24a1 expression and CYP24A1 enzymatic activity may prove to be more vital than the levels of Cyp27b1 expression [19]. The levels of 1,25(OH)2D3 are controlled by both enzymes, thus complicating the predictive relationship, however, the concentration of Cyp24a1 helps control the maintenance of appropriate levels of circulating 25(OH)D3. 25(OH)D3 status (blood levels) is a clinically relevant question that remains highly controversial in its ability to be predictive or beneficial to overall health [42]. This is best illustrated by the metabolite conversion schematic shown in Fig. 2 [19]. As our Cyp27b1 enhancer knockout models had an accommodation in Cyp24a1 by lowering its expression, less conversion of 25(OH)D3 to the first catabolic product 24,25(OH)2D3 produced by the CYP24A1 enzyme was observed. When we aligned the relative values for our Cyp27b1 enhancer deletion animals, along with the serum PTH status of these models, we found a tight relationship of the ratio of 25(OH)D3: 24,25(OH)2D3 and skeletal health. As the ratio of 25(OH)D3:24,25(OH)2D3 maintains at a steady 1.6 – 2.0, the animals remain healthy. As the relative expression of Cyp24a1 decreases, and the PTH levels begin to increase, there is less enzymatic turnover of 25(OH)D3 and the 25(OH)D3 levels start to climb which increases the 25(OH)D3:24,25(OH)2D3 ratio dramatically. We see an inflection point in this relationship as we approach the M21-IKO mouse, whose skeleton remains healthy despite lowered levels of both Cyp27b1 and Cyp24a1, however the ratio increases out of range quickly as the 24,25(OH)2D3 product decreases and the PTH climbs leading to skeletal deformities in the M1-IKO mice. Since the levels of 25(OH)D3 are nearly 1,000 fold greater than 1,25(OH)2D3 (15 ng/mL to 25 pg/mL) and more easily measured with a larger dynamic range, this relationship may prove predictive in clinical applications as well.
Figure 2:
Schematic representation of Cyp24a1 expression levels correlated to vitamin D3 metabolites may help predict skeletal health. The percentage of Cyp24a1 gene expression levels (x-axis) plotted against 25(OH)D3 (grey circles), 24,25(OH)2D3 (black squares) concentrations (left y-axis) and serum PTH values (right y-axis) in Cyp24a1-null (C24KO), Cyp27b1-null (C27KO), M1-IKO, M1/M21-DIKO, M21-IKO, mouse Cyp24a1 transgene (mC24-transgene), and wildtype littermate control (WT) mice. Skeletal health of the animals is depicted by a green dashed line. Animals to the right of the line are “healthy” and to the left “poor” skeletal health relative to Ca and P levels as well as circulating concentrations of PTH, FGF23 and 1,25(OH)2D3. A variation of this figure first appeared in Meyer, Lee, et. al. (2019) [19].
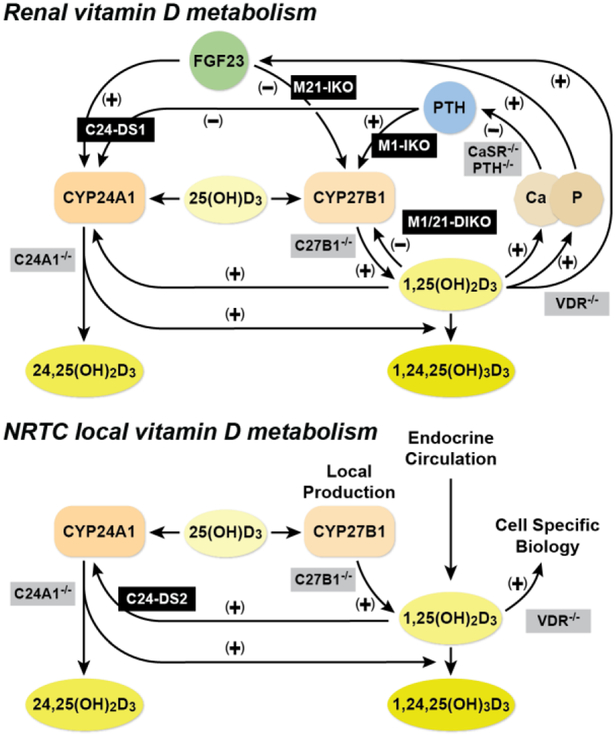
Through our investigations and our enhancer deletion models, we can now add our knowledge into the existing paradigm of vitamin D metabolism in the kidney as well as the NRTCs. As illustrated in Fig. 3, previous gene deletion models (grey) provided the knowledge necessary to describe the physiology surrounding the key components of the vitamin D metabolism. Now, we can describe and, importantly, separate the genomic elements for specific control of both Cyp27b1 and Cyp24a1 by PTH, FGF23, or 1,25(OH)2D3 in the kidney (top). The non-renal pathways of vitamin D metabolism (bottom) do not include control by endocrine PTH or FGF23, but are further complicated by inflammatory regulation as well as 25(OH)D3 availability. While all cells may contain the machinery for 1,25(OH)2D3 production, they may not contain necessary 25(OH)D3 substrate levels relevant for the enzyme. Our current and future studies are now focused on the next phase of mechanistic homeostasis and determination of non-renal control of Cyp27b1 activity utilizing the unique model system created in the M1/M21-DIKO rescued animal. By completing these studies in vivo, we will gain a complete view of the genomic control governing 1,25(OH)2D3 production and the homeostatic forces necessary for maintaining a healthy skeleton.
Figure 3:
Vitamin D metabolism in the kidney and non-renal tissues. Schematic diagram for the regulation of vitamin D metabolism and serum calcium and phosphate homeostasis in the kidney (top) and non-renal target cells (NRTCs, bottom). Our genetic models (black) and previously existing models (grey) are overlaid on or near the pathways they disrupt. This figure first appeared in Meyer, Lee, et. al. (2019) [19].
Highlights.
Tissue-specific enhancers mediate expression of Cyp27b1 and Cyp24a1 in the kidney
PTH and FGF23 fine tune Cyp27b1 and Cyp24a1 expression in vitamin D metabolism
Deleting both Cyp27b1 enhancers results in an endocrine-deficient pseudo-null mouse
Its skeleton can be rescued, providing a unique model for investigating non-renal 1,25(OH)2D3
Acknowledgements:
We thank members of the Pike Laboratory and Kathy Krentz in the University of Wisconsin Biotechnology Center - Genome Editing and Animal Models Core for generating the CRISPR/Cas9 enhancer deleted mice. We also thank Drs. Martin Kaufmann and Glenville Jones at Queen’s University for exhaustive vitamin D metabolite measurements that have proven crucial to our understanding of these animals and mechanisms.
Funding: This work was supported by the Department of Biochemistry, University of Wisconsin – Madison, University of Wisconsin Carbone Cancer Center Support Grant P30, and by the NIH-NIDDK award number R01-DK117475 to J.W.P.
Abbreviations:
- FGF23
fibroblast growth factor 23
- PTH
parathyroid hormone
- PTG
parathyroid gland
- TPTG
thyroparathyroid gland
- NRTC
non-renal target cell
- CT
calcitonin
- Ca
calcium
- P
phosphate
- CREB
phosphorylated cAMP response element-binding protein
- M1-IKO
Mettl1 intronic knockout
- M21-IKO
Mettl21b intronic knockout
- M1/M21-DIKO
Mettl1 and Mettl21b double intronic knockout
- Cyp24-DS1
Cyp24a1 downstream deletion 1
- Cyp24-DS2
Cyp24a1 downstream deletion 2
- BMD
bone mineral density
Footnotes
Conflict of interest: The authors declare that they have no conflicts of interest with the contents of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].DeLuca HF, Overview of general physiologic features and functions of vitamin D., Am J Clin Nutr 80(6 Suppl) (2004) 1689S–1696S. [DOI] [PubMed] [Google Scholar]
- [2].Jones G, Prosser DE, Kaufmann M, Cytochrome P450-mediated metabolism of vitamin D, J Lipid Res 55(1) (2014) 13–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Garabedian M, Holick MF, Deluca HF, Boyle IT, Control of 25-hydroxycholecalciferol metabolism by parathyroid glands, Proc Natl Acad Sci U S A 69(7) (1972) 1673–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].DeLuca HF, Parathyroid hormone as a trophic hormone for 1,25-dihydroxyvitamin D3, the metabolically active form of vitamin D, N Engl J Med 287(5) (1972) 250–251. [DOI] [PubMed] [Google Scholar]
- [5].Tanaka Y, DeLuca H, Rat renal 25-hydroxyvitamin D3 1- and 24-hydroxylases: their in vivo regulation., Am J Physiol 246(2 Pt 1) (1984) E168–173. [DOI] [PubMed] [Google Scholar]
- [6].Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T, Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism., J Clin Invest 113(4) (2004) 561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shimada T, Urakawa I, Yamazaki Y, Hasegawa H, Hino R, Yoneya T, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T, FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa., Biochem Biophys Res Commun 314(2) (2004) 409–414. [DOI] [PubMed] [Google Scholar]
- [8].Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS, Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease., J Steroid Biochem Mol Biol 103(3-5) (2007) 316–321. [DOI] [PubMed] [Google Scholar]
- [9].Bikle DD, Patzek S, Wang Y, Physiologic and pathophysiologic roles of extra renal CYP27b1: Case report and review, Bone Rep 8 (2018) 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Adams JS, Rafison B, Witzel S, Reyes RE, Shieh A, Chun R, Zavala K, Hewison M, Liu PT, Regulation of the extrarenal CYP27B1-hydroxylase, J Steroid Biochem Mol Biol 144 Pt A (2014) 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Adams JS, Singer FR, Gacad MA, Sharma OP, Hayes MJ, Vouros P, Holick MF, Isolation and structural identification of 1,25-dihydroxyvitamin D3 produced by cultured alveolar macrophages in sarcoidosis, J Clin Endocrinol Metab 60(5) (1985) 960–966. [DOI] [PubMed] [Google Scholar]
- [12].Barbour GL, Coburn JW, Slatopolsky E, Norman AW, Horst RL, Hypercalcemia in an anephric patient with sarcoidosis: evidence for extrarenal generation of 1,25-dihydroxyvitamin D, N Engl J Med 305(8) (1981) 440–443. [DOI] [PubMed] [Google Scholar]
- [13].Hewison M, Zehnder D, Bland R, Stewart P, 1alpha-Hydroxylase and the action of vitamin D., J Mol Endocrinol 25(2) (2000) 141–148. [DOI] [PubMed] [Google Scholar]
- [14].Meyer MB, Benkusky NA, Kaufmann M, Lee SM, Redfield RR, Jones G, Pike JW, Targeted genomic deletions identify diverse enhancer functions and generate a kidney-specific, endocrine-deficient Cyp27b1 pseudo-null mouse, J Biol Chem 294(24) (2019) 9518–9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Meyer MB, Benkusky NA, Kaufmann M, Lee SM, Onal M, Jones G, Pike JW, A kidney-specific genetic control module in mice governs endocrine regulation of the cytochrome P450 gene, J Biol Chem 292(42) (2017) 17541–17558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen KS, DeLuca HF, Cloning of the human 1 alpha,25-dihydroxyvitamin D-3 24-hydroxylase gene promoter and identification of two vitamin D-responsive elements, Biochim Biophys Acta 1263(1) (1995) 1–9. [DOI] [PubMed] [Google Scholar]
- [17].Zierold C, Darwish H, DeLuca H, Two vitamin D response elements function in the rat 1,25-dihydroxyvitamin D 24-hydroxylase promoter., J Biol Chem 270(4) (1995) 1675–1678. [DOI] [PubMed] [Google Scholar]
- [18].Meyer MB, Goetsch PD, Pike JW, A downstream intergenic cluster of regulatory enhancers contributes to the induction of CYP24A1 expression by 1alpha,25-dihydroxyvitamin D3, J Biol Chem 285(20) (2010) 15599–15610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Meyer MB, Lee SM, Carlson AH, Benkusky NA, Kaufmann M, Jones G, Pike JW, A chromatin-based mechanism controls differential regulation of the cytochrome P450 gene Cyp24a1 in renal and non-renal tissues, J Biol Chem (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Turner BM, Defining an epigenetic code, Nat Cell Biol 9(1) (2007) 2–6. [DOI] [PubMed] [Google Scholar]
- [21].Janzen WP, Wigle TJ, Jin J, Frye SV, Epigenetics: Tools and Technologies., Drug Discov Today Technol 7(1) (2010) e59–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ramsey S, Knijnenburg T, Kennedy K, Zak D, Gilchrist M, Gold E, Johnson C, Lampano A, Litvak V, Navarro G, Stolyar T, Aderem A, Shmulevich I, Genome-wide histone acetylation data improve prediction of mammalian transcription factor binding sites., Bioinformatics 26(17) (2010) 2071–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ, Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position, Nat Methods 10(12) (2013) 1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Buenrostro JD, Wu B, Chang HY, Greenleaf WJ, ATAC-seq: A Method for Assaying Chromatin Accessibility Genome-Wide, Curr Protoc Mol Biol 109 (2015) 21.29.21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].de Wit E, de Laat W, A decade of 3C technologies: insights into nuclear organization., Genes Dev 26(1) (2012) 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sashital DG, Wiedenheft B, Doudna JA, Mechanism of foreign DNA selection in a bacterial adaptive immune system, Mol Cell 46(5) (2012) 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E, A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity, Science 337(6096) (2012) 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R, One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering, Cell 153(4) (2013) 910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Meyer MB, Benkusky NA, Pike JW, Selective Distal Enhancer Control of the Mmp13 Gene Identified through Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR) Genomic Deletions, J Biol Chem 290(17) (2015) 11093–11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].E.P. Consortium, The ENCODE (ENCyclopedia Of DNA Elements) Project, Science 306(5696) (2004) 636–640. [DOI] [PubMed] [Google Scholar]
- [31].Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P, CRISPR provides acquired resistance against viruses in prokaryotes, Science 315(5819) (2007) 1709–1712. [DOI] [PubMed] [Google Scholar]
- [32].Horvath P, Barrangou R, CRISPR/Cas, the immune system of bacteria and archaea, Science 327(5962) (2010) 167–170. [DOI] [PubMed] [Google Scholar]
- [33].Burgess DJ, Technology: a CRISPR genome-editing tool, Nat Rev Genet 14(2) (2013) 80. [DOI] [PubMed] [Google Scholar]
- [34].Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F, Multiplex genome engineering using CRISPR/Cas systems, Science 339(6121) (2013) 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH, Doudna JA, Lim WA, Weissman JS, Qi LS, CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes, Cell 154(2) (2013) 442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA, Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression, Cell 152(5) (2013) 1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Perez-Pinera P, Kocak DD, Vockley CM, Adler AF, Kabadi AM, Polstein LR, Thakore PI, Glass KA, Ousterout DG, Leong KW, Guilak F, Crawford GE, Reddy TE, Gersbach CA, RNA-guided gene activation by CRISPR-Cas9-based transcription factors, Nat Methods 10(10) (2013) 973–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yeo NC, Chavez A, Lance-Byrne A, Chan Y, Menn D, Milanova D, Kuo CC, Guo X, Sharma S, Tung A, Cecchi RJ, Tuttle M, Pradhan S, Lim ET, Davidsohn N, Ebrahimkhani MR, Collins JJ, Lewis NE, Kiani S, Church GM, An enhanced CRISPR repressor for targeted mammalian gene regulation, Nat Methods 15(8) (2018) 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dardenne O, Prud'homme J, Arabian A, Glorieux F, St-Arnaud R, Targeted inactivation of the 25-hydroxyvitamin D(3)-1(alpha)-hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D-deficiency rickets., Endocrinology 142(7) (2001) 3135–3141. [DOI] [PubMed] [Google Scholar]
- [40].Shigematsu T, Horiuchi N, Ogura Y, Miyahara T, Suda T, Human parathyroid hormone inhibits renal 24-hydroxylase activity of 25-hydroxyvitamin D3 by a mechanism involving adenosine 3',5'-monophosphate in rats, Endocrinology 118(4) (1986) 1583–1589. [DOI] [PubMed] [Google Scholar]
- [41].Dardenne O, Prud'homme J, Hacking SA, Glorieux FH, St-Arnaud R, Correction of the abnormal mineral ion homeostasis with a high-calcium, high-phosphorus, high-lactose diet rescues the PDDR phenotype of mice deficient for the 25-hydroxyvitamin D-1alpha-hydroxylase (CYP27B1), Bone 32(4) (2003) 332–340. [DOI] [PubMed] [Google Scholar]
- [42].Bouillon R, Bischoff-Ferrari H, Willett W, Vitamin D and health: perspectives from mice and man, J Bone Miner Res 23(7) (2008) 974–979. [DOI] [PubMed] [Google Scholar]