Abstract
Stenotrophomonas maltophilia is a pathogen with unique resistance patterns. We assessed 70 combat casualties with S. maltophilia clinical isolates to examine its role as a nosocomial pathogen in critically-ill trauma patients. Incidence density was 0.36 S. maltophilia infections per 100 patient-days (95% CI: 0.29–0.44). Patients predominantly had blast trauma (97%) and were critically injured (injury severity score [ISS] >25; 80%). Restricting to patients with ISS >15, 50 patients with S. maltophilia infections were compared to 441 patients with infections attributed to other gram-negative bacilli. Patients with S. maltophilia infections had significantly more operating room visits prior to isolation, traumatic or early surgical amputations, longer hospitalization (median 71 vs 47 days), and higher overall mortality (10% vs 2%; p=0.01). Initial and serial (≥7 days between initial and subsequent isolation) S. maltophilia isolates had high susceptibility to trimethoprim-sulfamethoxazole and minocycline. Evaluation of newer agents awaiting CLSI breakpoints, including moxifloxacin, showed promising results.
Keywords: Stenotrophomonas maltophilia, wound infections, trauma-related infections, combat-related infections
1. INTRODUCTION
Advancements in point of injury care and medical capabilities have contributed to increased survival following previously fatal injuries for U.S. combat casualties (Murray et al., 2009). Nevertheless, infectious complications remain a leading cause of morbidity and mortality (Blyth et al., 2015) with high rates of multidrug-resistant organism (MDRO) colonization and infections providing further challenges (Campbell et al., 2017; Gilbert et al., 2016; Hospenthal et al., 2011; Tribble et al., 2011; Weintrob et al., 2018). Frequent pathogens associated with combat-related infections include coagulase-negative staphylococci, Enterococcus spp. (17–26% vancomycin-resistant), Escherichia coli (73–91% MDR), and Acinetobacter calcoaceticus-baumannii complex (86–95% MDR) (Weintrob et al., 2018). Other organisms are also increasingly being recognized as clinically-relevant pathogens within this population.
Stenotrophomonas maltophilia has emerged as a concerning nosocomial pathogen in critically ill trauma patients (Hanes et al., 2002). In particular, S. maltophilia has demonstrated a trend toward pathogenicity in compromised hosts, including trauma patients with attributable mortality rates of up to 37.5% (Falagas et al., 2009; Hanes et al., 2002; Schaumann et al., 2001; Senol, 2004). Ventilator-associated pneumonia was the most commonly identified syndrome associated with S. maltophilia, with the organism recovered from trauma patients following multiple antimicrobial exposures (Mueller et al., 2005). Neutropenia, higher injury severity score (ISS), presence of central venous catheters, use of broad-spectrum antibiotics, and increased length of hospital stay have been independently associated with infection risk (Hanes et al., 2002; Senol, 2004).
S. maltophilia has demonstrated intrinsic resistance to commonly used broad-spectrum antibiotics, including carbapenems, piperacillin-tazobactam, and newer cephalosporin/beta-lactamase combinations (Legacé-Wiens et al., 2014; Nicodemo and Garcia Paez, 2007). Trimethoprim-sulfamethoxazole is currently the first-line therapy, but mechanisms of resistance to this drug, including plasmid-mediated, have been identified (Nicodemo and Garcia Paez, 2007). In vitro activity has been reported with newer fluoroquinolones (e.g., clinafoxacin, levofloxacin, gatifloxacin, moxifloxacin, and sitafloxacin), but empiric therapy may prove difficult given regional variations in resistance rates (Nicodemo and Garcia Paez, 2007). Minocycline has shown activity against S. maltophilia, but there are concerns that frequent use in treating A. calcoaceticus-baumannii complex may have potential for inducing increased resistance in S. maltophilia (Milne and Gould, 2012).
While S. maltophilia has traditionally been considered a low virulence pathogen, its clinical significance and resistance patterns in a severely-injured trauma population have not yet been fully elucidated. Given this organism’s multiple resistance mechanisms, empiric antibiotics in critically ill patients may not provide adequate coverage. We examined the prevalence and clinical features of S. maltophilia in U.S. wounded military personnel and evaluated patterns of resistance.
2. METHODS
2.1. Study Population and Design
Clinical and microbiological data collected from June 1, 2009 through September 1, 2014 as part of the Trauma Infectious Disease Outcomes Study (TIDOS) was assessed. Patients were eligible for inclusion in TIDOS if they were active-duty personnel or Department of Defense beneficiaries, 18 years of age or older, injured during deployment and required evacuation through Landstuhl Regional Medical Center (LRMC), Germany, before ultimately being transferred to a participating military hospital in the United States (Brooke Army Medical Center [BAMC] or a hospital in the National Capital Region [NCR]), as previously described (Tribble et al., 2011). The study was approved by the Institutional Review Board of the Uniformed Services University of the Health Sciences.
As part of TIDOS, specimens collected from surveillance cultures and clinical infection work-ups were archived for future study in a microbiological repository. Patients were considered for inclusion if they had S. maltophilia isolated from a clinical infection work-up. Exclusion criteria were those patients with S. maltophilia isolates without linkage to a corresponding infection syndrome (as defined below). A comparator group was comprised of patients with infections attributed to gram-negative bacilli other than S. maltophilia. As preliminary analysis found the patients in the S. maltophilia group to have more severe injuries than the comparator group, both groups were restricted to patients with an ISS >15, indicating severe or critical injuries (Copes et al., 1988).
Demographic patient data and clinical history were obtained through the Department of Defense Trauma Registry (Eastridge et al., 2006). Infection syndromes, clinical microbiology, and antimicrobial administration were extracted from the TIDOS supplemental Infectious Disease Module (Tribble et al., 2011). Infections were identified based on a combination of clinical findings and laboratory tests, and classified in accordance with standardized definitions of the Centers for Disease Control and Prevention National Healthcare Safety Network (2019). In the absence of meeting the a priori definition, infections were included if there was an indication in the medical chart of a clinical diagnosis, as well as directed antimicrobial treatment (duration of ≥5 days for skin and soft-tissue infections (SSTIs) and ≥21 days for osteomyelitis). Infections were excluded if there was a record of an alternate diagnosis and discontinuation of antimicrobial treatment (Tribble et al., 2011).
Pre-culture antibiotics were defined as those instituted at any point during care prior to the first isolation of S. maltophilia and post-culture antibiotics as those initiated within two days following initial isolation. Broad-spectrum antibiotics were specified as any 4th generation cephalosporin, anti-pseudomonal penicillin, or carbapenem. Traumatic or early surgical amputations were defined as amputations occurring prior to admission at a military hospital in the United States. Invasive fungal wound infections (IFIs) were classified in accordance with published criteria (Weintrob et al., 2015).
2.2. Isolate Susceptibility Analysis
Confirmation of S. maltophilia speciation and antimicrobial susceptibilities were performed on all archived S. maltophilia isolates collected from clinical workups. For isolates cultured from the same patient, only unique initial and serial isolates were included in the analysis, as defined by culture site, resistance pattern, or pulsed-field gel electrophoresis (PFGE) pattern. Serial isolates were defined as isolates collected ≥7 days apart from the first isolate. Any isolate that did not meet inclusion criteria (i.e., was not associated with a clinical infectious workup, including those obtained through routine MDRO screening by groin swabs), was excluded.
S. maltophilia isolates underwent two passages on 5% sheep blood agar prior to confirmed identification using the BD Phoenix™ Automated Microbiology System (BD Diagnostics, Sparks, Maryland). BD Phoenix™ BD Emerge™ (NMIC 300) Panels were utilized for susceptibility testing. Only antibiotics with possible in vitro activity against S. maltophilia were included in the analysis. Clinical and Laboratory Standards Institute (CLSI) breakpoints were used if available for susceptibility analysis. Isolates with intermediate interpretation were considered non-susceptible. Antimicrobials without CLSI breakpoints were assessed by minimum inhibitory concentration (MIC) with breakpoints assigned for analysis based on published pharmacokinetics/pharmacodynamics data (ciprofloxacin MIC ≤2 μg/mL, moxifloxacin MIC ≤4 μg/mL, and tigecycline MIC ≤2μg/mL being considered susceptible) (Chung et al., 2012; Farrell et al., 2010; Weiss et al., 2000). The PFGE to determine the clonality of the isolates was performed according to the Centers for Disease Control and Prevention standardized PFGE protocol for Gram-negative rods (Centers for Disease Control and Prevention, 2014) with some minor modifications and using a 90% similarity cut-off value.
2.3. Statistical Analysis
Clinical, injury, and infection data of the patients in the S. maltophilia and comparator groups were analyzed. Chi-square and Fisher’s Exact Test were used to compare categorical variables. Continuous variables were analyzed by Mann-Whitney U. Statistical analysis for the microbiological data was completed using IBM SPSS Statistics 22 (IBM, NY) and SAS version 9.4 (SAS, Cary, NC) was utilized for the analysis of S. maltophilia patients and the comparator group.
3. RESULTS
3.1. Epidemiology of S. maltophilia infections
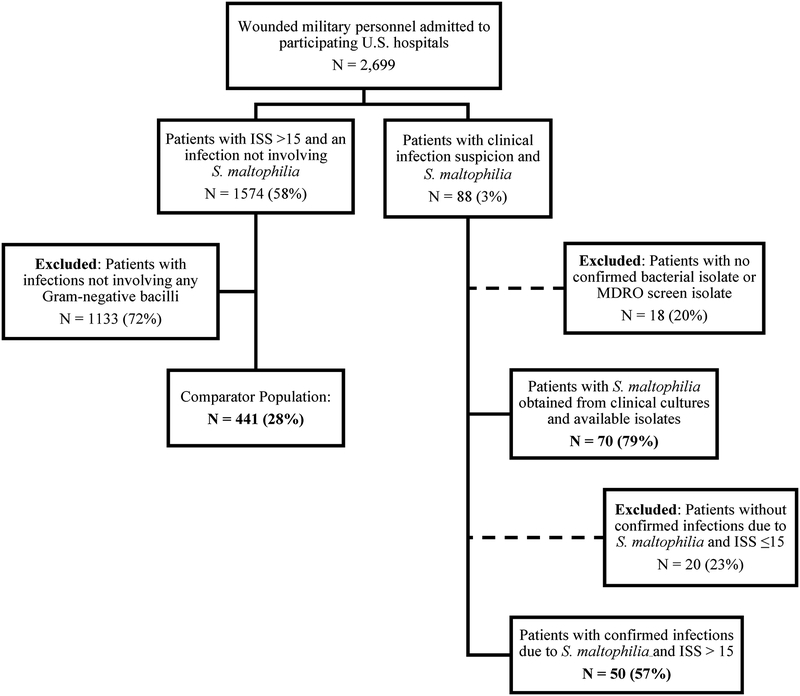
A total of 2,699 patients were transferred from LRMC to TIDOS-participating U.S. military hospitals (Figure 1). Seventy (2.6%) of these patients had 71 initial S. maltophilia isolates available in the TIDOS microbiological repository (one patient provided two initial isolates). Nine of these patients had subsequent cultures yielding 26 unique serial isolates. The patients were predominantly male (98.6%) who sustained blast-related trauma (97.1%) in Afghanistan (97.1%). Fifty-six (80%) patients had an ISS >25 (critical injury) and 57 (81.4%) patients were admitted to the intensive care unit at participating U.S. military hospitals. The time from injury to diagnosis of an infection (any pathogen) was a median of 4 days (interquartile range [IQR]: 3–6.5 days). For the patients with S. maltophilia infections, the time from injury to culture confirmation with growth of S. maltophilia was a median of 9 days (IQR: 6–15 days). Sixty-four patients (91.4%) received antibiotics prior to the S. maltophilia culture with 45 (64%) patients receiving broad-spectrum antibiotics, 25 (36%) a fluoroquinolone, 3 (4%) trimethoprim-sulfamethoxazole, and 47 (67%) a tetracycline. The length of hospitalization was a median of 65 days (IQR: 42–95 days) and 7 (10%) patients died.
Figure 1.
Flowchart of Study Population. ISS – injury severity score. MDRO – multidrug-resistant organisms
Initial isolates were recovered predominately from wounds (N=44; 62%), followed by respiratory (N=18; 25%), blood (N=5; 7%), urine (N=1; 1%), and other sources (N=3; 4%) with a similar pattern seen in serial isolates [wound (N=18; 69%), respiratory (N=4; 15%), blood (N=2; 8%), urine (N=1; 4%), and other (N=1; 4%)]. Thirty-seven of the 44 (84%) wound isolates were from polymicrobial wound infections. Of these, 25 had other gram-negative bacilli isolated (most frequently Pseudomonas spp. [N=11], A. calcoaceticus-baumannii complex [N=7], Enterobacter cloacae [N=6], E. coli [N=5]), 20 had gram-positive organisms (most frequently E. faecium [N=14], and coagulase-negative staphylococci [N=5]), and 7 had anaerobes (Clostridium spp. [N=3], Bacteroides [N=2], and Prevotella spp. [N=2]). Fifteen wounds also had fungi isolated, of which 7 were proven, 2 probable, and 4 possible IFIs. Of the 18 S. maltophilia respiratory isolates, 13 (72%) were associated with isolation of other gram-negatives (most frequently P. aeruginosa [N=4], E. cloacae [N=3], A. calcoaceticus-baumannii complex [N=2], and Klebsiella spp. [N=2]) and only 7 isolates had Stenotrophomonas-active agents prescribed following isolation (including 1 trimethoprim-sulfamethoxazole and a fluoroquinolone, 4 fluoroquinolones, and 2 tetracyclines).
On a patient-level, there were a median of 9 operative room (OR) visits (IQR: 5–16) following isolation of S. maltophilia in wound infections, but only a median of 3 (IQR: 1–4) OR visits were related to surgeries/procedures for the wound infected with S. maltophilia in 18 patients (14 patients with polymicrobial wound infections). In the four patients with monomicrobial S. maltophilia wound infections requiring subsequent debridements for management of the infection (1, 3, 4, and 15 debridements, respectively), all were treated with trimethoprim-sulfamethoxazole. Of the 70 patients with initial S. maltophilia infections, 59 (84%) received antimicrobials active against S. maltophilia following isolation (25 received trimethoprim/sulfamethoxazole, 29 tetracycline, and 22 fluoroquinolones).
Restricting to patients with severe or critical injury severity (ISS >15), 50 patients with an infection syndrome due to S. maltophilia were compared to 441 patients with an infection due to other gram-negative bacilli (Figure 1). There were no significant differences between the groups with regards to gender, age, region where the injury was sustained (Afghanistan or Iraq), or rates of intensive care unit (ICU) admission (Table 1). Patients in the S. maltophilia group were more likely to have had blast as the primary mechanism of injury, a higher proportion of traumatic or early surgical amputations, and more OR visits prior to isolation. Nevertheless, mechanical ventilation was employed at similar frequencies in each group with the majority of patients continuing ventilation after transfer to the U.S. for ≤ 1 week. The S. maltophilia group had later diagnoses of first infection (median of 10 vs 6 days; p=0.001), increased length of hospitalization (median of 71 vs 47 days; p<0.001), and higher proportion of mortality (10% vs 2%; p=0.01). Furthermore, S. maltophilia was identified later than other gram-negative organisms (p=0.001).
Table 1.
Characteristics, No. (%), of Severely Injured Trauma Patients by Infectiona
| Characteristic | Patients with S. maltophilia Infection (N = 50) |
Patients with Other Gram-negative Infection (N = 441) |
P-value |
|---|---|---|---|
| Age, median years (IQR) | 24 (22–27) | 24 (21–28) | 0.95 |
| Male | 50 (100) | 435 (98.6) | 1.00 |
| Afghanistan theater | 49 (98.0) | 410 (93.0) | 0.50 |
| U.S. Admission Facility | 0.52 | ||
| National Capital Regionb | 37 (74.0) | 344 (78.0) | |
| Brooke Army Medical Center | 13 (26.0) | 97 (22.0) | |
| Composite ISS | 0.26 | ||
| 16 – 25 (severe) | 6 (12.0) | 81 (18.4) | |
| > 25 (critical) | 44 (88.0) | 360 (81.6) | |
| Injury Mechanism | <0.01 | ||
| Blast | 50 (100) | 358 (81.2) | |
| Other (e.g., gunshot, motor vehicle crash) | 0 | 79 (17.9) | |
| Pre-culture Antibioticsc | |||
| None | 2 (4.0) | 15 (3.4) | 0.69 |
| Broad-spectrumd | 38 (76.0) | 131 (29.7) | <0.01 |
| Fluoroquinolone | 19 (38.0) | 129 (29.3) | NAe |
| Fluoroquinolones without broad-spectrum antibiotics | 7 (14.0) | 78 (17.7) | 0.51 |
| Trimethoprim-sulfamethoxazole | 3 (6.0) | 2 (0.5) | NAe |
| Tetracycline | 43 (86.0) | 302 (68.5) | NAe |
| Post-culture Antibioticsc | |||
| None | 4 (8.0) | 11 (2.5) | 0.06 |
| Broad-spectrumd | 28 (56.0) | 331 (75.1) | <0.01 |
| Fluoroquinolone | 16 (32.0) | 184 (41.7) | NAe |
| Fluoroquinolones without broad-spectrum antibiotics | 7 (14.0) | 51 (11.6) | 0.61 |
| Trimethoprim-sulfamethoxazole | 24 (48.0) | 17 (3.9) | NAe |
| Tetracycline | 19 (38.0) | 228 (51.7) | NAe |
| Hospitalization, median days (IQR) | 71 (46–117) | 47 (34–66) | <0.01 |
| Time to Culture, median days post-injury (IQR) | 10 (6–17) | 7 (4–13) | <0.01 |
| OR Visits Pre-culturec, median (IQR) | 3 (1–6) | 2 (1–4) | 0.02 |
| Injury to 1st infection diagnosisf, median days post-injury (IQR) | 9.5 (6, 17) | 6 (3,13) | <0.01 |
| ICU Admission | 0.34 | ||
| None | 3 (6.0) | 40 (9.1) | |
| LRMC only | 4 (8.0) | 61 (13.8) | |
| U.S. hospital with/without LRMC | 43 (86.0) | 336 (76.2) | |
| Traumatic/Early Surgical Amputationg | 39 (78.0) | 240 (54.4) | <0.01 |
| Inpatient Mechanical Ventilation | 0.11 | ||
| None | 7 (14.0) | 104 (23.5) | |
| LRMC Only | 7 (14.0) | 103 (23.3) | |
| LRMC plus U.S. hospital ≤ 1 week | 33 (66.0) | 219 (49.6) | |
| LRMC plus U.S. hospital ≥ 2 weeks | 1 (2.0) | 6 (1.3) | |
| Died | 5 (10.0) | 10 (2.3) | 0.01 |
ICU – intensive care unit; ISS – injury severity score; IQR – interquartile range; LRMC – Landstuhl Regional Medical Center; OR – operating room
Patients are restricted to those with an injury severity score >15. Comparator group includes patients with infections attributed to gram-negative bacillus other than Stenotrophomonas maltophilia. In the S. maltophilia infection group, 2 patients did not have documentation of mechanical ventilation status and in the comparator group, the operation theater, mechanism of injury, ICU status, and mechanical ventilation status was not known for 8, 4, 4, and 9 patients respectively. As such, these numbers were excluded from the analysis.
Includes National Naval Medical Center and Walter Reed Army Medical Center, which merged to become Walter Reed National Military Medical Center in September 2011
Pre-culture is the period prior to the date of collection of the culture that grew S. maltophilia. Post-culture is defined as at least one day after the date of collection of the culture that grew S. maltophilia.
4th generation cephalosporin, anti-pseudomonal penicillin, or carbapenem
Use of antibiotics was not mutually exclusive for the patients, a p-value could not be calculated.
Initial infection post-injury regardless of infecting pathogen.
Amputations occurred prior to admission at U.S. hospitals
On the infection-level, 95 infection syndromes were identified in the S. maltophilia group (50 patients) and 866 infections in the comparator group (441 patients) with SSTIs comprising the majority of infections in both groups (Table 2). Incidence density for S. maltophilia in this group was 0.36 infections per 100 patient days (95% CI: 0.29–0.44). P. aeruginosa (10.1%) was the most common gram-negative bacterium recovered in the comparison group followed by Escherichia coli (9.3%), A. calcoaceticus-baumannii complex (8.2%), E. cloacae (5.5%), Serratia marcescens (2.6%), and Klebsiella pneumoniae (2.4%).
Table 2.
Infectious Syndromes among Subjects with Stenotrophomonas maltophilia Infections and Other Gram-negative Bacilli Infections (Comparator Group)
| Infection Syndrome, No. (%) |
S. maltophilia Infections N=95 |
Other Gram-negative bacilli Infections N=866 |
P-value |
|---|---|---|---|
| Skin and soft-tissue infection | 56 (59.0) | 394 (45.5) | 0.07 |
| Bloodstream infection | 9 (9.5) | 101 (11.7) | 0.37 |
| Osteomyelitis | 6 (6.3) | 68 (7.9) | 0.85 |
| Pneumoniaa | 11 (11.6) | 143 (16.5) | 0.15 |
| Sepsis | 9 (9.5) | 48 (5.5) | 0.19 |
| Urinary tract infection | 1 (1.1) | 73 (8.4) | NC |
| Central nervous system infection | 0 | 10 (1.2) | NC |
| Otherb | 3 (3.2) | 29 (3.3) | NC |
Includes ventilator and non-ventilator associated pneumonia
Includes sinusitis, empyema without pneumonia, lung abscess without pneumonia, tracheobronchitis, intra-abdominal infection
3.2. Isolate Susceptibility Analysis
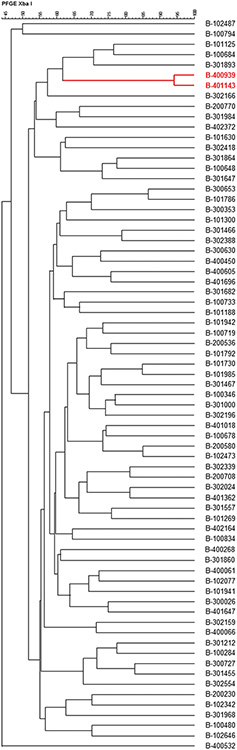
A total of 226 S. maltophilia isolates were recovered, of which 71 (31.4%) met inclusion criteria for unique, initial isolates and 26 (11.5%) for serial isolates. The PFGE analysis revealed distinct patterns in all except two initial isolates from BAMC (Figure 2). Antimicrobial susceptibilities of initial and serial isolates are shown in Table 3. There were trends towards initial isolate resistance (defined as resistance to any Stenotrophomonas-active agent) in patients with an ISS>25 (p=0.056). Among the initial isolates, 21 (47%) of 45 wound, 4 (80%) of 5 blood, 7 (38%) of 18 respiratory, and 1 (33%) of 3 other isolates (p=0.437) had Stenotrophomonas-active agents prescribed following isolation. Thirty-six (82%) wound isolates had resistance on initial isolation compared to 9 (50%) respiratory, 3 (60%) blood, 1 (100%) urine, and 1 (33%) other isolate (p=0.060). Treatment status with Stenotrophomonas-active agents following initial isolation was not associated with serial isolation (p=1.0).
Figure 2.
Dendrogram for 71 unique initial Stenotrophomonas maltophilia isolates. Two isolates, highlighted in red, showed the same pulsed-field gel electrophoresis (PFGE) type. All other initial isolates had unique PFGE types.
Table 3.
Antimicrobial Susceptibilities of Initial and Serial Stenotrophomonas maltophilia Isolates, No. (%)
| Ceftazidime | Levofloxacin | Minocycline | TIM | SXT | Ciprofloxacina | Moxifloxacina | Tigecyclinea | |
|---|---|---|---|---|---|---|---|---|
| Initial Isolates (N=71) | 30 (42.3) | 59 (83.1) | 71 (100) | 48 (67.6) | 70 (98.6) | 10 (28.2) | 69 (97.2) | 62 (87.3) |
| Serial Isolates (N=26) | 1 (3.8) | 8 (30.8) | 26 (100) | 8 (30.8) | 21 (80.8) | 2 (7.7) | 23 (88.5) | 6 (23.1) |
SXT – trimethoprim-sulfamethoxazole; TIM – ticarcillin-clavulanic acid
Susceptibility defined for ciprofloxacin, moxifloxacin, and tigecycline by MIC ≤ 2 μg/ml, ≤ 4 μg/ml, and ≤ 2 μg/ml respectively based on values used in published literature
Initial isolates obtained from patients with ISS >25 had a higher proportion of resistance to ceftazidime (38 [64%] of 60 isolates vs 2 [20%] of 10 isolates; p=0.018). Ceftazidime resistance was also encountered more frequently in levofloxacin-resistant isolates (12 [100%] of 12 isolates vs 29 [49%] of 59 levofloxacin-susceptible isolates; p=0.001). In addition, there was a trend toward increased tigecycline resistance with prior receipt of agents with Stenotrophomonas-activity (4 [31%] of 13 isolates vs 5 [9%] of 58 isolates; p=0.052). Overall, there was a more frequent recovery of serial isolates when the initial isolates demonstrated resistance (9 [18%] of 50 isolates vs 0 of 21 isolates; p=0.050) and also with initial isolation at BAMC (6 [43%] of 14 isolates vs 2 [8%] of 26 isolates from LRMC and 1 [3%] of 31 isolates from NCR; p=0.001).
The comparison of the susceptibility data between the 71 initial isolates and 26 serial isolates (collected from 9 patients; 4 with ≥1 serial isolate) revealed an increased percentage of resistance in the serial isolates, with exception of minocycline (Table 3). In particular, the proportion of susceptible isolates decreased from 42% to 4% for ceftazidime, 82% to 31% for levofloxacin, and 87% to 23% for tigecycline. Thirteen of the 26 serial isolates were from the same patient [from wound, respiratory, blood (on two separate occasions), and other specimens], who was treated with varying combinations of carbapenems, aminoglycosides, trimethoprim-sulfamethoxazole, and fluoroquinolones with increasing MICs to moxifloxacin, ciprofloxacin, and tigecycline, but preserved susceptibilities to minocycline and trimethoprim-sulfamethoxazole.
Restricting to the nine patients with serial isolates, final serial isolates were analyzed against the respective initial isolates, with no significant change in susceptibility patterns. There were minor decreases in susceptibility for trimethoprim-sulfamethoxazole (100% to 78%; p=0.47) without intervening trimethoprim-sulfamethoxazole exposure. Moxifloxacin susceptibility also decreased (89% to 67%; p=0.58) with one of the patients who developed new resistance having had 20 days of levofloxacin in the intervening period (another patient with 14 days of fluoroquinolone exposure had moxifloxacin MIC increase from 0 to 4, but remained within extrapolated susceptibility range). The greatest changes in susceptibility were for levofloxacin and tigecycline, which both decreased from 67% in initial to 11% in the final serial isolate (p=0.05). Of the patients with initial levofloxacin-susceptible isolates, the only patient with continued levofloxacin susceptibility received 8 days of trimethoprim-sulfamethoxazole between cultures. Three patients with newly levofloxacin-resistant isolates had >7 days of interceding fluoroquinolone exposure. Ciprofloxacin susceptibility decreased from 11% to 0 in final isolates. Of the six patients with increased tigecycline MICs (five had new resistance), three had ≥20 days cumulative exposure to tetracyclines (2 doxycycline, 1 minocycline); however, all retained minocycline susceptibility.
4. DISCUSSION
To our knowledge, this is the first study that has examined S. maltophilia infections in a previously healthy, young, trauma population. Patients with S. maltophilia infections were more severely injured than those acquiring non-S. maltophilia gram-negative infections. S. maltophilia was most frequently isolated from wound and respiratory sources, and in >70% of these cases in the setting of other organisms.
Similar to prior studies identifying S. maltophilia infections at higher frequencies in immunocompromised and ICU patients (Brooke, 2012; Falagas et al., 2009; Garcia Paez and Costa, 2008), we found infections tend to predominate in patients with higher injury severity. Although previously healthy, our study population was likely transiently immunosuppressed from the severity of the trauma (i.e., 80% of patients had an ISS > 25 indicating critical injury, 94% admitted to intensive care, and 10% crude mortality). When the 50 patients with S. maltophilia infections were assessed against the comparator group with similarly high ISS severity, S. maltophilia was recovered more frequently in patients that had a traumatic amputation or required a surgical amputation prior to reaching definitive care in a U.S. facility. Notably, the majority of S. maltophilia isolates were cultured in the setting of polymicrobial infections, which makes interpretation of its attributable morbidity difficult to assess, which is a similar challenge in examining these complex extremity wound infections. Especially notable is the fact that almost a third of S. maltophilia wound isolates were associated with IFIs. Interestingly, despite frequent co-isolation of gram-positive organisms in wounds (45%), S. aureus was not isolated in any wounds in which S. maltophilia was cultured. Additionally, even S. maltophilia isolates from respiratory sources were frequently associated with other gram-negative organisms, of which some are also thought to be less virulent. Overall, 84% of patients with isolation of S. maltophilia had antimicrobials targeting this organism prescribed following isolation, including fluoroquinolones and tetracyclines, which were frequently prescribed for other indications (e.g., coverage of other pathogens or continuation of malaria chemoprophylaxis). Despite this, serial isolation was a rare event, potentially due to surgical management. Being ubiquitous in the environment (Brooke, 2012; Denton and Kerr, 1998), this organism has been linked to nosocomial transmission via water sources, disinfectant solutions, and other healthcare-related modes of transmission (Khardori et al., 1990; Mukhopadhyay et al., 2003; Sakhnini et al., 2002), raising the suspicion that infections occur primarily through a common contaminated source. Nevertheless, our analysis showed no evidence for clonal spread, suggesting that trauma patients are acquiring unique healthcare-associated infections selected through antimicrobial pressure, again emphasizing the role for antimicrobial stewardship in these critically ill patients.
When examining the susceptibility of S. maltophilia isolates, susceptibility to trimethoprim-sulfamethoxazole was documented in 99% of initial isolates with levofloxacin susceptibility in only 83%, confirming the role of trimethoprim-sulfamethoxazole as a first-line therapy for S. maltophilia in the absence of contraindications to the drug (Farrell et al., 2010). Susceptibility to minocycline was high (100%) and persisted in serial isolates, demonstrating a similar potential for minocycline to be employed as an alternative therapy, as described in other studies (Hand et al., 2016; Neela et al., 2012). Furthermore, MICs to moxifloxacin appeared favorable in 97% of initial isolates when using previously described cutoffs (Weiss et al., 2000), suggesting that it may be a more effective agent than levofloxacin and warrants further clinical evaluation. Although serial isolates are less common, these isolates were noted to harbor more resistance to S. maltophilia-targeted antibiotics versus the unique initial isolates with 81% retaining susceptibility to trimethoprim-sulfamethoxazole, but only 31% being susceptible to levofloxacin. Previous studies have described various resistance mechanisms employed by S. maltophilia and that increased resistance to trimethoprim-sulfamethoxazole may be associated with acquisition of the sul1 gene and class 1 integron (Chang et al., 2007; Song et al., 2010; Toleman et al., 2007). Moreover, the presence of smeDEF RNA increases resistance to multiple antimicrobial agents through action of an efflux pump (Alonso and Martinez, 2000). As the nine patients with serial isolates in our study had severe injuries, predisposing them to longer hospital stays and duration of antibiotic use, it may suggest activation or new acquisition of these resistance genes over the course of their care, which warrants further investigation. Minocycline susceptibility was preserved against all isolates and moxifloxacin MICs were favorable in 89% of the serial isolates, indicating that these may be useful alternative agents. Despite favorable MICs for moxifloxacin, recent data suggests poor bactericidal activity of fluoroquinolones against S. maltophilia in time-kill studies (Grillon et al., 2016), and perhaps limiting in vivo effectiveness.
This study includes limitations inherent to its retrospective nature. Despite starting with 226 isolates from 2,699 eligible patients, only 71 isolates met criteria as a unique initial isolate and only nine patients had unique serial isolates, limiting the statistical power to identify predisposing factors for infection. Taking the study limitations into consideration, our analysis reveals that S. maltophilia is an uncommon pathogen in trauma patients. When isolated, it is most frequent in later, polymicrobial wound and respiratory infections. We again demonstrate the utility of trimethoprim-sulfamethoxazole and suggest minocycline and moxifloxacin as potential alternatives, though further clinical data is needed.
Acknowledgements:
We are indebted to the Infectious Disease Clinical Research Program Trauma Infectious Disease Outcomes Study team of clinical coordinators, microbiology technicians, data managers, clinical site managers, and administrative support personnel for their tireless hours to ensure the success of this project.
Funding: This work (IDCRP-024) was conducted by the Infectious Disease Clinical Research Program, a DoD program executed through the Uniformed Services University of the Health Sciences, Department of Preventive Medicine and Biostatistics through a cooperative agreement with The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (HJF). This project has been funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Inter-Agency Agreement Y1-AI-5072, Military Infectious Disease Research Program (HU0001-15-2-0045), and the Department of the Navy under the Wounded, Ill, and Injured Program (HU0001-10-1-0014).
Footnotes
Publisher's Disclaimer: Disclaimer: The views expressed herein are those of the authors and do not reflect the official policy or position of Uniformed Services University of the Health Sciences, Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., National Institutes of Health or the Department of Health and Human Services, Brooke Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Departments of the Air Force, Navy, or the Army, the Department of Defense, or the U.S. Government.
REFERENCES
- Alonso A and Martinez J. Cloning and characterizations of SmeDEF, a novel multidrug efflux pump from Stenotrophomonas maltophilia. Antimicrob Agents Chemother 2000;44:3079–86. doi: 10.1128/aac.44.11.3079-3086.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyth D, Yun H, Tribble D, Murray C. Lessons of war: combat-related injury infections during the Vietnam War and Operation Iraqi and Enduring Freedom. J Trauma Acute Care Surg 2015;79:S227–35. doi: 10.1097/TA.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke J. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev 2012;25:2–41. Doi: 10.1128/CMR.00019-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell W, Li P, Whitman T, Blyth D, Schnaubelt E, Mende K, Tribble D. Multi-drug-resistant gram-negative infections in deployment-related trauma patients. Surg Infect 2017;18:357–67. doi: 10.1089/sur.2017.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Modified Pulse-Net Procedure for Pulsed-Field Gel Electrophoresis of Select Gram Negative Bacilli. Document No. CEM.TE.C.0002. https://www.cdc.gov/hai/pdfs/labsettings/Modified-PulsedNet-procedure-GNB.pdf. (accessed 8 August 2019).
- Centers for Disease Control and Prevention; National Healthcare Safety Network. CDC/NHSN Surveillance Definitions for Specific Types of Infection. http://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf. (accessed 30 July 2019).
- Chang L, Lin H, Chang C, Lu P. Increased incidence of class 1 integrons in trimethoprim/sulfamethoxazole-resistant clinical isolates of Stenotrophomonas maltophilia. J Antimicrob Chemother 2007;59:1038–9. doi: 10.1093/jac/dkm034 [DOI] [PubMed] [Google Scholar]
- Chung H, Hong S, Lee Y, Yong D, Jeong S, Lee K, Chong Y. Antimicrobial susceptibility of Stenotrophomonas maltophilia isolates from a Korean tertiary care hospital. Yonsei Med J 2012;53:439–41. doi: 10.3349/ymj.2012.53.2.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copes W, Champion H, Sacco W, Lawnick M, Keast S, Bain L. The injury severity score revisited. 1988. J Trauma 28:69–77. [DOI] [PubMed] [Google Scholar]
- Denton M and Kerr K. Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clin Microbiol Rev 1998;11:57–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastridge B, Jenkins D, Flaherty S, Schiller H, Holcomb J. Trauma system development in a theater of war: Experiences from Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma 2006;61:1366–72. [DOI] [PubMed] [Google Scholar]
- Falagas M, Kastoris A, Vouloumanou E, Rafailidis P, Kapaskelis A, Dimopoulos G. Attributable mortality of Stenotrophomonas maltophilia infections: a systematic review of the literature. Future Microbiol 2009;4:1103–9. doi: 10.2217/fmb.09.84. [DOI] [PubMed] [Google Scholar]
- Farrell D, Sader H, Jones R. Antimicrobial susceptibilities of a worldwide collection of Stenotrophomonas maltophilia isolates tested against tigecycline and agents commonly used for S. maltophilia infections. J Antimicrob Agents Chemother 2010;54:2735–7. Doi: 10.1128/AAC.01774-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Paez J and Costa S. Risk factors associated with mortality of infections caused by Stenotrophomonas maltophilia: a systematic review. J Hosp Infect 2008;70:101–8. doi: 10.1016/j.jhin.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Gilbert L, Li P, Murray C, Yun H, Aggarwal D, Weintrob A, Tribble D. Multidrug-resistant gram-negative bacilli colonization risk factors among trauma patients. Diagn Microbiol Infect Dis 2016;84:358–60. doi: 10.1016/j.diagmicrobio.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon A, Schramm F, Kleinberg M, Jehl F. Comparative activity of ciprofloxacin, levofloxacin and moxifloxacin against Klebsiella pneumoniae, Pseudomonas aeruginosa and Stenotrophomonas maltophilia assessed by minimum inhibitory concentrations and time-kill studies. PLoS One 2016;11: e0156690. doi: 10.1371/journal.pone.0156690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand E, Kim T, Duhon B. Monotherapy with minocycline or trimethoprim/sulfamethoxazole for treatment of Stenotrophomonas maltophilia infections. J Antimicrob Chemother 2016;71:1071–5. doi: 10.1093/jac/dkv456. [DOI] [PubMed] [Google Scholar]
- Hanes S, Demirkan K, Tolley E, Boucher B, Croce M, Wood G, Fabian T. Risk factors for late-onset nosocomial pneumonia caused by Stenotrophomonas maltophilia in critically ill trauma patients. Clin Infect Dis 2002;35:228–35. doi: 10.1086/341022. [DOI] [PubMed] [Google Scholar]
- Hospenthal D, Crouch H, English J, Leach F, Pool J, Conger N, et al. Multidrug-resistant bacterial colonization of combat-injured personnel at admission to medical centers after evacuation from Afghanistan and Iraq. J Trauma 2011;71:S52–7. doi: 10.1097/TA.0b013e31822118fb. [DOI] [PubMed] [Google Scholar]
- Khardori N, Elting L, Wong E, Schable B, Bodey G. Nosocomial infections due to Xanthomonas maltophilia (Pseudomonas maltophilia) in patients with cancer. Rev Infect Dis 1990;12:997–1003. [DOI] [PubMed] [Google Scholar]
- Legacé-Wiens P, Walkty A, Karlowsky J. Ceftazidime-avibactam: an evidence-based review of its pharmacology and potential use in the treatment of Gram-negative bacterial infections. Core Evidence 2014;9:13–25. doi: 10.2147/CE.S40698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne K and Gould I. Combination antimicrobial susceptibility testing of multidrug-resistant Stenotrophomonas maltophilia from cystic fibrosis patients. Antimicrob Agents Chemother 2012;56:4071–7. doi: 10.1128/AAC.00072-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller E, Hanes S, Croce M, Wood G, Boucher B, Fabian T. Effect from multiple episodes of inadequate empiric antibiotic therapy for ventilator-associated pneumonia on morbidity and mortality among critically ill trauma patients. J Trauma 2005;58:94–101. doi: 10.1097/01.ta.0000141890.29032.9a [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay C, Bhargava A, Ayyagari A. Novel nosocomial infections by Stenotrophomonas maltophilia: first reported case from Lucknow, North India. J Clin Microbiol 2003;41:3989–90. doi: 10.1128/jcm.41.8.3989-3990.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C, Wilkins K, Molter N, Yun H, Dubick M, Spott M, et al. Infections in combat casualties during Operations Iraqi and Enduring Freedom. J Trauma 2009;66:S138–44. doi: 10.1097/TA.0b013e31819d894c. [DOI] [PubMed] [Google Scholar]
- Neela V, Rankouhi S, van Belkum A, Goering R, Awang R. Stenotrophomonas maltophilia in Malaysia: molecular epidemiology and trimethoprim-sulfamethoxazole resistance. Int J Infect Dis 2012;16:e603–7. doi: 10.1016/j.ijid.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Nicodemo A and Garcia Paez J. Antimicrobial therapy for Stenotrophomonas maltophilia infections. Eur J Clin Microbiol Infect Dis 2007;26:229–37. doi: 10.1007/s10096-007-0279-3 [DOI] [PubMed] [Google Scholar]
- Sakhnini E, Weissmann A, Oren I. Fulminant Stenotrophomonas maltophilia soft tissue infection in immunocompromised patients: an outbreak transmitted via tap water. Am J Med Sci 2002;323:269–72. doi: 10.1097/00000441-200205000-00008 [DOI] [PubMed] [Google Scholar]
- Schaumann R, Stein K, Eckhardt C, Ackermann G, Rodloff A. Infections caused by Stenotrophomonas maltophilia – a prospective study. Infection 2001;29:205–8. [DOI] [PubMed] [Google Scholar]
- Senol E. Stenotrophomonas maltophilia: the significance and role as a nosocomial pathogen. J Hosp Infect 2004;57:1–7. doi: 10.1016/j.jhin.2004.01.033. [DOI] [PubMed] [Google Scholar]
- Song J, Sung J, Kwon K, Park J, Cho J, Shin S, et al. Analysis of acquired resistance genes in Stenotrophomonas maltophilia. Korean J Lab Med 2010;30:295–300. doi: 10.3343/kjlm.2010.30.3.295. [DOI] [PubMed] [Google Scholar]
- Toleman M, Bennett P, Bennett D, Jones R, Walsh T. Global emergence of trimethoprim/sulfamethoxazole resistance in Stenotrophomonas maltophilia mediated by acquisition of sul genes. Emerg Infect Dis 2007;13:559–65. doi: 10.3201/eid1304.061378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribble D, Conger N, Fraser S, Gleeson T, Wilkins K, Antonille T, et al. Infection-associated clinical outcomes in hospitalized medical evacuees following traumatic injury-Trauma Infectious Disease Outcome Study (TIDOS). J Trauma 2011;71:S33–42. doi: 10.1097/TA.0b013e318221162e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintrob AC, Weisbrod AB, Dunne JR, Rodriguez CJ, Malone D, Lloyd BA, et al. Combat trauma-associated invasive fungal wound infections: epidemiology and clinical classification. Epidemiol Infect 2015;143:214–24. doi: 10.1017/S095026881400051X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintrob AC, Murray CK, Xu J, Krauss M, Bradley W, Warkentien TE, et al. Early infections complicating the care of combat casualties from Iraq and Afghanistan. Surg Infect 2018;19:286–97. doi: 10.1089/sur.2017.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss K, Restieri C, De Carolis E, Laverdière M, Guay H. Comparative activity of new quinolones against 326 clinical isolates of Stenotrophomonas maltophilia. J Antimicrob Chemother 2000;45:363–5. doi: 10.1093/jac/45.3.363. [DOI] [PubMed] [Google Scholar]