Abstract
Background:
Findings on structural brain volume associated with pediatric posttraumatic stress disorder (PTSD) have been variable, and it is unclear whether any structural differences are specific to pediatric PTSD in comparison to adult PTSD or other co-occurring pediatric psychiatric conditions.
Methods:
We tested volumetric brain differences between pediatric groups with and without PTSD in a region-of-interest meta-analysis. We conducted meta-regressions to test the effects of age and sex on heterogeneous study findings. To assess specificity, we compared pediatric PTSD to adult PTSD, pediatric trauma exposure without PTSD, pediatric depression, and pediatric anxiety.
Results:
In 15 studies examined, pediatric PTSD was associated with smaller total gray matter and cerebral, temporal lobe (total, right, left), total cerebellar vermis, and hippocampal (total, right, left) volumes, compared to peers without PTSD. Pediatric PTSD, but not the comparison group, was also associated with a trend toward smaller total, right, and left amygdala volumes. In an external comparison, smaller hippocampal volume was not significantly different between adult and pediatric PTSD. Qualitative comparisons to pediatric trauma exposure without PTSD, depression, and anxiety revealed differences that may be unique to pediatric PTSD and others that may be convergent with these related clinical conditions in youth.
Conclusions:
Pediatric PTSD is associated with structural differences that parallel those associated with adult PTSD. Furthermore, pediatric PTSD appears to be distinct from other related pediatric conditions at the structural level. Future studies employing longitudinal, dimensional, and multimodal neuroimaging approaches will further elucidate the nature of neurobiological differences in pediatric PTSD.
Keywords: Posttraumatic stress, pediatric, trauma, youth, brain structure, magnetic resonance imaging
INTRODUCTION
Exposure to traumatic events during childhood is prevalent, with more than 65% of children in the United States having experienced at least one traumatic event (Criterion A) as defined by the Diagnostic Statistical Manual of Mental Disorders (DSM) (1–4). Cross-nationally, approximately 15% of children and adolescents develop PTSD following exposure to traumatic stress (5). Thus, childhood trauma exposure is prevalent and increases risk for PTSD, but there is also vast heterogeneity in outcome.
In the last two decades, structural magnetic resonance imaging (sMRI) studies have examined the association between exposure to traumatic stress (both with and without PTSD) and regional volumes in the developing brain (6–8). Volumetric differences have been identified in some structures (e.g., gray matter, cerebral, temporal lobe volumes) (9–14), whereas no differences between groups or mixed findings have emerged for other regions (e.g., hippocampus, amygdala) (9,15–17). To better characterize the heterogeneity in findings, it is important to statistically compile existing results in a single meta-analytic study (8,18).
In the present study, we aimed to collectively analyze key findings from all structural volumetric studies in pediatric PTSD using region-of-interest (ROI) meta-analyses. A comprehensive online database built for a recent meta-analysis of sMRI studies in adult PTSD (19) also included a separate database of pediatric PTSD studies. However, the pediatric findings had not been examined independently in a meta-analysis. Therefore, we updated the database of all sMRI studies using a systematic MEDLINE search in pediatric PTSD and carried out a comprehensive meta-analysis across 15 studies for 13 brain regions: total gray matter, total cerebral, total, left, and right temporal lobe, total, left, and right hippocampus, total, left and right amygdala, and total vermis volumes, and corpus callosum structure.
We predicted that meta-analytic findings for gray matter, cerebral, and temporal lobe volumes would be consistent with results from numerous previous studies suggesting that smaller volumes are associated with pediatric PTSD, relative to no PTSD (without trauma exposure) (9–14). Multiple studies have suggested no differences in hippocampal and amygdala volumes between pediatric groups with and without PTSD (9–11,15–16,20), although smaller hippocampal, but not amygdala, volumes have been strongly associated with adult PTSD (19). For hippocampal volume, we thus had competing hypotheses that our meta-analytic findings may show the same pattern of no difference in hippocampal volume between pediatric PTSD and no PTSD or that the meta-analysis may reveal a difference in hippocampal volume that had previously not been identified in individual studies. For amygdala volume, we hypothesized that there would be no difference between pediatric PTSD and no PTSD groups. Furthermore, in order to determine potential sources of variation across studies included in the final meta-analysis, we also conducted meta-regression analyses for key variables of interest. Specifically, given the nonlinear changes that take place across brain development (21–23) and based on prior literature highlighting structural differences in pediatric PTSD that are associated with factors like age and sex (6,12), we hypothesized that age and sex might relate to potential variability in effect sizes for key regions of interest, such as the hippocampus and amygdala.
In order to discern the extent to which structural differences in pediatric PTSD were specific to pediatric PTSD versus other related conditions, we conducted four comparison analyses with external datasets. First, we compared pediatric PTSD to adult PTSD (effect sizes from pediatric PTSD versus no PTSD were compared to those from adult PTSD versus no PTSD) to investigate whether structural alterations associated with PTSD differ between children and adolescents and adults. Adult PTSD is associated with significant volumetric differences in numerous regions, and the sensitivity analysis including children with PTSD revealed differential findings (e.g., smaller gray matter, cerebral, and amygdala volumes in PTSD versus no PTSD) (19). Thus, we hypothesized that children and adolescents with PTSD would display different structural patterns than adults with PTSD, potentially due to dynamic developmental changes in brain architecture in which prefrontal structures undergo more protracted development than subcortical structures during typical maturation (21–23).
Second, it remains unclear whether differences that have been observed in pediatric PTSD are associated with the disorder or with trauma exposure itself (16). Given the heterogeneity in volumetric brain differences in pediatric PTSD (18) and the fact that not all children and adolescents who experience traumatic events develop PTSD (3,24), it is important to disentangle the neurobiological effects of trauma versus those of PTSD itself. To this end, we compared pediatric PTSD to pediatric trauma exposure without a PTSD diagnosis.
Finally, depression and anxiety often co-occur with PTSD following trauma exposure (1,3,24), with up to 54% and 23% of children and adolescents with PTSD also meeting criteria for major depressive disorder and for an anxiety disorder, respectively (24). Therefore, neurobiological differences observed in pediatric PTSD (relative to a group without PTSD or trauma exposure) may overlap with those of pediatric depression and anxiety, potentially contributing to comorbidity across these conditions. It is also possible that neurobiological differences associated with pediatric PTSD are secondary to another pediatric condition and not directly related to pediatric PTSD. We addressed the specificity of structural differences in pediatric PTSD by comparing them to findings in pediatric depression and pediatric anxiety.
METHODS
Database of Imaging Studies in Pediatric PTSD
In a previously reported and publicly-available online database (19) including studies from 1992 to 2016, 17 sMRI studies in pediatric PTSD were identified but not directly analyzed. To update this database to present time and ensure completeness, we conducted a systematic MEDLINE search using the following terms: ((“Stress Disorders, Traumatic”[MeSH] OR “PTSD” OR “post traumatic stress” OR “posttraumatic stress” OR “posttraumatic stress disorder” OR “maltreatment”) AND (MRI OR “gray matter” OR “volume”) AND (pediatric OR youth OR adolescent OR child) (Figure 1, Table S1). Studies were included in the current database if they were performed in a pediatric population (i.e., included participants less than 18 years of age), included sMRI ROI analyses with volumetric data accessible as principal summary measures of group means and standard deviations, and included participants diagnosed with PTSD (see Table 1 for diagnostic criteria). If data were not available in the published paper or supplemental information, data were acquired through direct correspondence with the original study investigators. Studies were excluded from the entire database if only voxel-based morphometry analyses were conducted, the reported brain volumes did not include at least one ROI that was common to any study in the database, samples were overlapping with at least one other study included in the database for all ROIs, or the data of interest were not available (Figure 1, Table S1). No studies were excluded based on type of trauma exposure (see Table S2 for types of trauma exposures).
Figure 1.
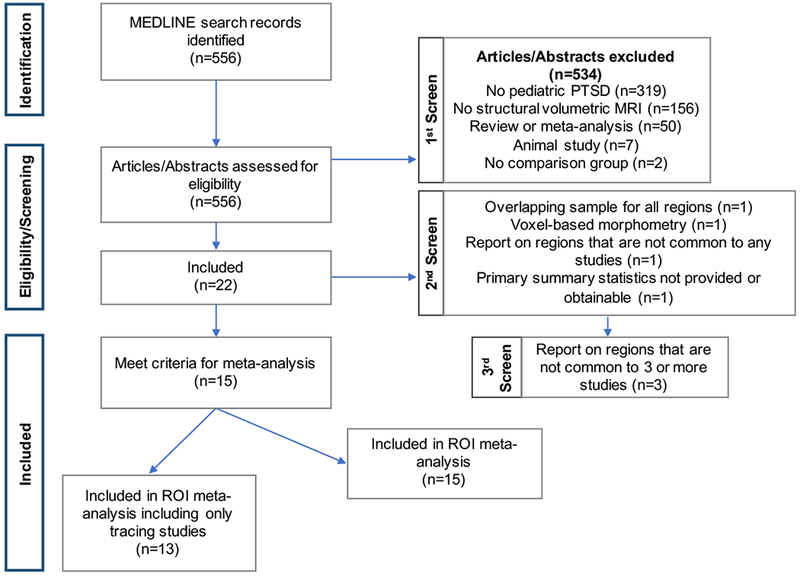
Flowchart indicating the number of studies excluded, reasons for exclusion, and total number of studies included.
Table 1.
15 MRI studies included in region-of-interest meta-analysis comparing participants with PTSD to participants without PTSD.
| Study | Participants with PTSD (N) | Comparison Participants without PTSD (N) | Comparison Participants without Trauma Exposure (N) | Comparison Participants with Trauma Exposure (N) | Diagnosti c Criteria | Average Agea | Method for ROI segmentation |
|---|---|---|---|---|---|---|---|
| Ahmed et al. (15) | 21 | 32 | - | 32 | DSM-IV | 15.3 | Automatedc |
| Carrion et al. (9)b | 24 | 24 | 24 | - | DSM-IV | 11.0 | Manual |
| Carrion et al. (68)b | 24 | 24 | 24 | - | DSM-IV | 11.0 | Manual |
| De Bellis et al. (10)b | 44 | 61 | 61 | - | DSM-III-R | 12.1 | Manual |
| De Bellis et al. (20) | 9 | 9 | 9 | - | DSM-IV | 10.5 | Manual |
| De Bellis et al. (11)b | 28 | 66 | 66 | - | DSM-IV | 11.5 | Manual |
| De Bellis et al. (70)b | 43 | 61 | 61 | - | DSM-IV | 12.1 | Manual |
| De Bellis et al. (12)b | 61 | 122 | 122 | - | DSM-IV | 11.7 | Manual |
| De Bellis et al. (71)b | 58 | 98 | 98 | - | DSM-IV | 12.0 | Manual |
| De Bellis et al. (14) | 38 | 59 | 59 | 35 | DSM-IV | 10.5 | Manual Automatedc + |
| Morey et al. (16) | 31 | 57 | 57 | 32 | DSM-IV | 10.4 | Manual |
| Mutluer et al. (74) | 23 | 21 | 21 | - | DSM-IV | 15.4 | Manual |
| Postel et al. (75) | 15 | 24 | 24 | - | DSM-IV | 16.0 | Manual |
| Weems et al. (79)b | 24 | 24 | 24 | - | DSM-IV | 11.0 | Manual |
| Weems et al. (42)b | 28 | 26 | 26 | - | DSM-IV | 13.8 | Automatedc |
Average age of all participants in sample (PTSD and comparison groups). None of the comparison participants listed above had a PTSD diagnosis at the time of the study
Despite overlapping samples present in overall database, none of the studies included in the individual ROI meta-analyses reported here contained overlapping samples
Automated method used was FreeSurfer image analysis suite.
“-” = not stated in the study.
Statistical Analysis
Pediatric PTSD Region-of-Interest Meta-Analysis
ROI meta-analytic methods using the public meta-analytic Excel pipeline (www.ptsdmri.uk) are as previously described in Bromis et al., 2018 (19). The composite comparison group for the meta-analysis primarily included participants without PTSD without trauma exposure. Study estimates were combined using a random-effects inverse weighted variance model (25,26). Effect sizes were calculated as Hedges’ g (Cohen’s d with small sample bias correction). Given that an individual meta-analysis was conducted for each brain region, we report results that survived Bonferroni correction for multiple comparisons. For the corpus callosum ROI, studies that examined either volume or area were both included. Finally, given heterogeneity in methodological approaches used to determine regions of interest in sMRI studies that can lead to differences in study findings, especially in developmental samples (28), we conducted a separate additional analysis in which we only included studies that used manual (hand tracing) methods (Table S3, Figure S1).
Pediatric PTSD Region-of-Interest Meta-Regression
To assess variation across studies due to heterogeneity, we calculated heterogeneity using the Cochran Q and I2 statistics (27). We next investigated the association between potential moderator variables (age and sex) and heterogeneity in hippocampal and amygdala effect sizes using a meta-regression analysis (29). We also conducted meta-regressions for the supplemental analysis including only studies that used tracing methods (Table S4).
Comparison to Related Clinical Groups
To assess the specificity of any volumetric differences detected in pediatric PTSD, we also compared these meta-analytical results with effect size differences in four relevant comparison groups: adult PTSD versus no PTSD, pediatric trauma exposure without PTSD versus no trauma exposure, pediatric depression versus no depression, and pediatric anxiety versus no anxiety. First, we statistically compared pediatric PTSD to adult PTSD to test whether findings in pediatric PTSD may be developmentally specific by using the study published by Bromis et al. in 2018, which included 66 studies of adult PTSD in an ROI meta-analysis (Table S5). Next, we sought to compare pediatric PTSD to pediatric trauma exposure without PTSD to dissociate the extent to which structural alterations might be associated with the disorder itself versus with trauma exposure. Finally, we also aimed to compare pediatric PTSD with pediatric depression and anxiety because these disorders also arise in children and adolescents following trauma and often co-occur with pediatric PTSD (1,3,24).
To identify studies for the three comparisons, we conducted a MEDLINE search to identify comprehensive meta-analyses for structural volumetric MRI studies using search terms “(pediatric OR child OR adolescent OR youth) AND (anxiety OR depression OR maltreatment OR stress OR trauma) AND MRI AND meta-analysis”. We did not find any published meta-analytic studies of this nature. Therefore, separately for these three related conditions, we identified an ROI study with the largest sample size that met the following inclusion criteria: the study was performed in a pediatric population, included structural volumetric MRI ROI analyses for the amygdala and hippocampus with available principal summary measures, and were related to anxiety, depression, or early-life stress and trauma (see Supplemental Material for full search terms). Studies selected for the comparison analyses and their respective demographic information are listed in Table S5. Given the lack of meta-analytic studies and the use of a single study for comparison, we did not perform a formal statistical comparison between pediatric PTSD and pediatric depression, anxiety, or exposure to trauma without PTSD. Instead we nominally compared effect sizes between pediatric PTSD and the three comparison conditions.
To limit the number of comparisons performed, we focused our analyses on the hippocampus and amygdala as these were the most commonly examined regions in the studies included in the present meta-analysis (Figure S2). For each comparison study, except adult PTSD, we calculated Hedges’ g effect sizes using mean volumes and standard deviations for the amygdala and hippocampus. For adult PTSD, we used Hedges’ g effect sizes and respective p-values directly from the meta-analytic study (19). To carry out the statistical comparison between pediatric and adult PTSD, we calculated the z-statistics and derived the p-value for the comparison. We also conducted statistical and qualitative comparisons for the supplemental analysis including only studies that used tracing methods (Tables S6–S9).
Results
Database of Imaging Studies in Pediatric PTSD
Of the 17 pediatric imaging studies included in the original database, 14 met the criteria listed above (Table S1). Our additional comprehensive MEDLINE search identified five eligible studies for our database per inclusion criteria (Figure 1; “1st Screen”). Of these five studies that were added to the database, four were included in the ROI meta-analysis and one was excluded because summary measures were not available. Thus, a total of 18 studies passed the second screen for eligibility (Figure 1).
From these 18 studies, a total of 46 brain regions were reported. Out of these 46 regions, we selected the 13 regions for the ROI meta-analysis that contained at least three studies (Figure S2) with means and standard deviations for both PTSD and comparison groups in order to include a sufficient number of studies in each meta-analysis (19). During this third screen, 3 out of the 18 studies only reported on regions that did not meet these criteria and were thus not included in the meta-analyses of 13 brain regions (Figure 1). Thus, a total of 15 studies were included in the present meta-analysis (Table 1). Studies were excluded from individual ROI meta-analyses if samples were overlapping with other studies for the same region. They were not, however, excluded from the entire database.
Out of the 15 studies included in the ROI meta-analysis, 12 studies compared participants with PTSD to participants without PTSD and with no exposure to trauma. Of the remaining 3 studies, 2 studies compared participants with PTSD to participants without PTSD both with and without trauma exposure (14, 16), and 1 study compared participants with PTSD to only participants without PTSD with trauma exposure (15). For the 2 studies that included two comparison groups (with and without trauma exposure) (14,16), we only included data for comparison participants without PTSD who did not have trauma exposure in order to ensure consistency with the 12 other studies and to maintain balanced sample sizes between groups with and without PTSD (Table 2), which is consistent with the approach used in the recent adult PTSD meta-analysis (19). Within the database of 15 studies, there were a total of 471 pediatric participants with a DSM diagnosis of PTSD and 676 participants without any diagnosis or trauma exposure (Table 2).
Table 2.
Demographic and clinical data from participants in the database of 15 MRI Studies included in the 13-brain region meta-analysis comparing participants with PTSD to participants without PTSD.
| Variable | Pooled Number of Participants in Database | Number of Studies Reporting Variable | Mean Value or Percentage per Study | Between-Study SD |
|---|---|---|---|---|
| N | N | Mean value | SD | |
| Number of participants with PTSD | 471 | 15 | 33 | 15 |
| Number of comparison participants without trauma exposure | 676 | 14 | 48 | 33 |
| Number of comparison participants with trauma exposure | 99 | 3 | 33 | 2.0 |
| Age (years) | ||||
| Participants with PTSD, mean | 15 | 12.3 | 2.0 | |
| Participants with PTSD, SD | 12 | 2.2 | 0.5 | |
| Comparison participants (no trauma), mean | 15 | 12.3 | 1.9 | |
| Comparison participants (no trauma), SD | 12 | 2.2 | 0.5 | |
| Comparison participants (with trauma), mean | 3 | 11.4 | 2.6 | |
| Comparison participants (with trauma), SD | 3 | 2.5 | 0.2 | |
| N | N | Mean % | SD | |
| Medication status | ||||
| Medication free | 9 | 100 | 0 | |
| Sex of participants with PTSD (female/male) | 239/232 | 15 | 53/47 | 4.4 |
| Sex of comparison participants without trauma (female/male) | 339/337 | 14 | 51/49 | 6.2 |
| Sex of comparison participants with trauma (female/male) | 51/48 | 3 | 52/48 | 2.6 |
Pediatric PTSD Region-of-Interest Meta-Analysis
Significantly smaller total gray matter, total cerebral, temporal lobe (total, right, and left), total cerebellar vermis, and hippocampal (total, right, and left) volumes were associated with pediatric PTSD compared to no PTSD and no trauma exposure. There were trend-level differences in total (p=0.052), right (p=0.060), and left (p=0.073) amygdala volumes between pediatric PTSD and no PTSD such that smaller amygdala volume was associated with pediatric PTSD (relative to no PTSD). In contrast, there were no significant differences in corpus callosum structure between children and adolescents with and without pediatric PTSD (Table 3, Figure 2). Importantly, there was a significant publication bias for total cerebral volume (p=0.04), but not for any of the other regions.
Table 3.
Meta-analysis results of comparison between pediatric participants with PTSD and all participants without PTSD.
For the meta-analytic comparison between participants with and without PTSD, significant differences are noted in boldface text. All results remained significant after Bonferroni correction for multiple comparisons of 13 brain structures. Effect sizes are reported as Hedges’ g values. Negative effect sizes indicate that the region is smaller in pediatric participants with PTSD, whereas positive effect sizes indicate that the region is larger in pediatric participants with PTSD compared to participants without PTSD.
| Sample Size |
Comparison of participants with and without PTSD |
Heterogeneity |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | Studies (n) | PTSD Group (n) | Comparison Group (n) | Effect Size | 95% CI | p | I2 (%) | p | Small-Study Bias |
| Gray Matter (total) | 3 | 123 | 205 | −0.56 | −0.83, −0.28 | <0.001 | 25.24 | 0.26 | 0.28 |
| Cerebral Volume (total) | 3 | 123 | 205 | −0.56 | −0.79, −0.33 | <0.001 | 0.00 | 0.59 | 0.04 |
| Temporal Lobe (total) | 4 | 105 | 160 | −0.6 | −0.86, −0.35 | <0.001 | 0.00 | 0.79 | 0.57 |
| Temporal Lobe (right) | 3 | 96 | 151 | −0.58 | −0.85, −0.32 | <0.001 | 0.00 | 0.60 | 0.43 |
| Temporal Lobe (left) | 3 | 96 | 151 | −0.54 | −0.80, −0.27 | <0.001 | 0.00 | 0.50 | 0.75 |
| Hippocampus (total) | 8 | 195 | 294 | −0.51 | −0.88, −0.13 | 0.007 | 72.68 | 0.00 | 0.24 |
| Hippocampus (right) | 7 | 171 | 270 | −0.51 | −0.93, −0.09 | 0.016 | 75.28 | 0.00 | 0.25 |
| Hippocampus (left) | 7 | 171 | 270 | −0.46 | −0.87, −0.04 | 0.030 | 74.69 | 0.00 | 0.34 |
| Amygdala (total) | 8 | 208 | 296 | −0.28 | −0.56, 0.00 | 0.052 | 54.31 | 0.03 | 0.48 |
| Amygdala (right) | 8 | 208 | 296 | −0.23 | −0.47, 0.01 | 0.060 | 39.69 | 0.11 | 0.50 |
| Amygdala (left) | 8 | 208 | 296 | −0.29 | −0.61, 0.03 | 0.073 | 64.29 | 0.01 | 0.46 |
| Vermis (total) | 3 | 120 | 181 | −0.46 | −0.88, −0.04 | 0.033 | 64.51 | 0.06 | 0.18 |
| Corpus Callosum (total) | 3 | 106 | 178 | −0.30 | −0.87,0.27 | 0.307 | 76.74 | 0.01 | 0.63 |
Figure 2.
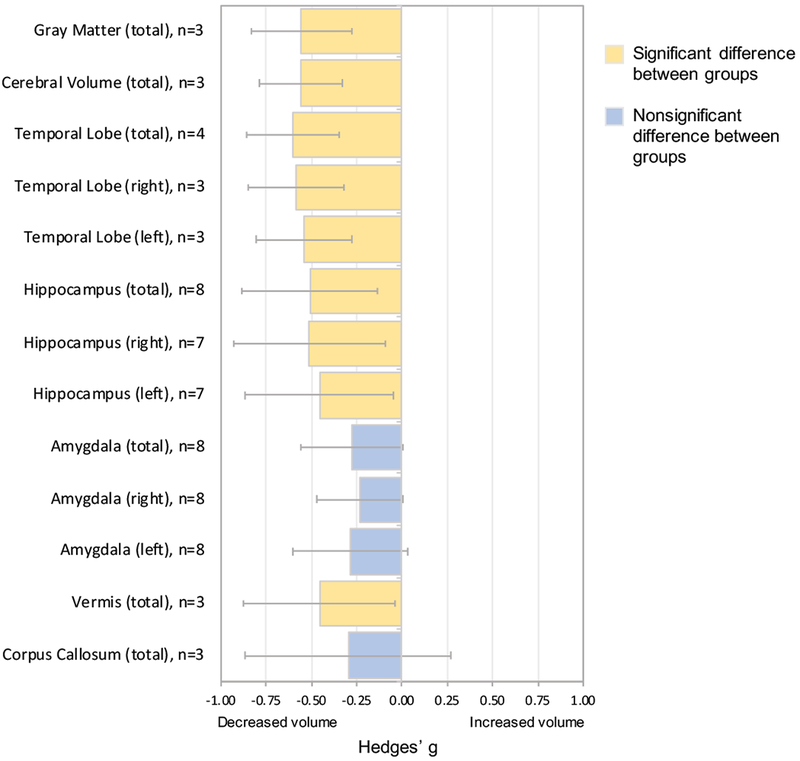
Meta-analysis comparing pediatric PTSD to no PTSD.
For each of the 13 regions in the meta-analysis, Hedges’ g values are reported with 95% confidence intervals. Positive Hedges’ g values indicate increased structural volume in pediatric participants diagnosed with PTSD. Negative Hedges’ g values indicate smaller structural volumes in participants with pediatric PTSD. The number of studies included in the meta-analysis (n) that report on the relevant region is listed for each region. Error bars represent width of 95% confidence interval.
Meta-Regression Analyses
There was significant heterogeneity across studies for total, right, and left hippocampal as well as total and left amygdala meta-analyses (Table 3). Meta-regression results revealed that age was a significant moderator of heterogeneity for total, right and left hippocampal regions, accounting for more than 30% of the heterogeneity across effect sizes from studies included in the regional meta-analysis (Table 4). Specifically, older age was associated with larger negative effect sizes for total hippocampal volume (relative to studies with younger participants) (Figure S3). Age was not a significant moderator for total or left amygdala regions. Sex was a significant moderator of heterogeneity for all hippocampal and amygdala regions, accounting for more than 50% of heterogeneity across studies’ effect sizes (Table 4). Specifically, a higher percentage of female participants was associated with larger negative effect sizes (relative to studies with equal number of male and female participants) (Figure S5).
Table 4.
Meta-regression results for age and sex moderators for primary database.
I2: meta-analysis heterogeneity, R2: amount of heterogeneity accounted for by moderator.
| Moderator: Age |
Moderator: Sex |
|||||||
|---|---|---|---|---|---|---|---|---|
| Region | I2 % | I2 % (p-value) | R2 % | Test of moderator (QM) | Test of moderator (p-value) | R2 % | Test of moderator (QM) | Test of moderator (p-value) |
| Hippocampus (total) | 72.68 | 0.00 | 34.86 | 5.23 | 0.022 | 84.79 | 13.49 | 0.0002 |
| Hippocampus (right) | 75.28 | 0.00 | 41.92 | 5.63 | 0.018 | 79.59 | 11.32 | 0.0008 |
| Hippocampus (left) | 74.69 | 0.00 | 33.37 | 4.73 | 0.030 | 100.00 | 19.60 | 0.0001 |
| Amygdala (total) | 54.31 | 0.03 | 0.00 | 0.69 | 0.407 | 63.70 | 5.82 | 0.016 |
| Amygdala (right) | 39.69 | 0.11 | 12.77 | 1.46 | 0.226 | 77.61 | 4.51 | 0.034 |
| Amygdala (left) | 64.29 | 0.01 | 0.00 | 0.30 | 0.581 | 51.57 | 5.99 | 0.015 |
Comparison to Related Clinical Groups
To assess the specificity of the meta-analytic findings, we compared volumetric differences in the hippocampus and amygdala in pediatric PTSD to differences in key clinical groups.
Pediatric PTSD versus adult PTSD.
To statistically compare meta-analytic results between pediatric PTSD and adult PTSD, we used the recently published meta-analysis in adult PTSD (19) (Table S5). There were no significant differences between effect sizes in pediatric versus adult PTSD for any hippocampal or amygdala regions (Figure 3, Table S10).
Figure 3.
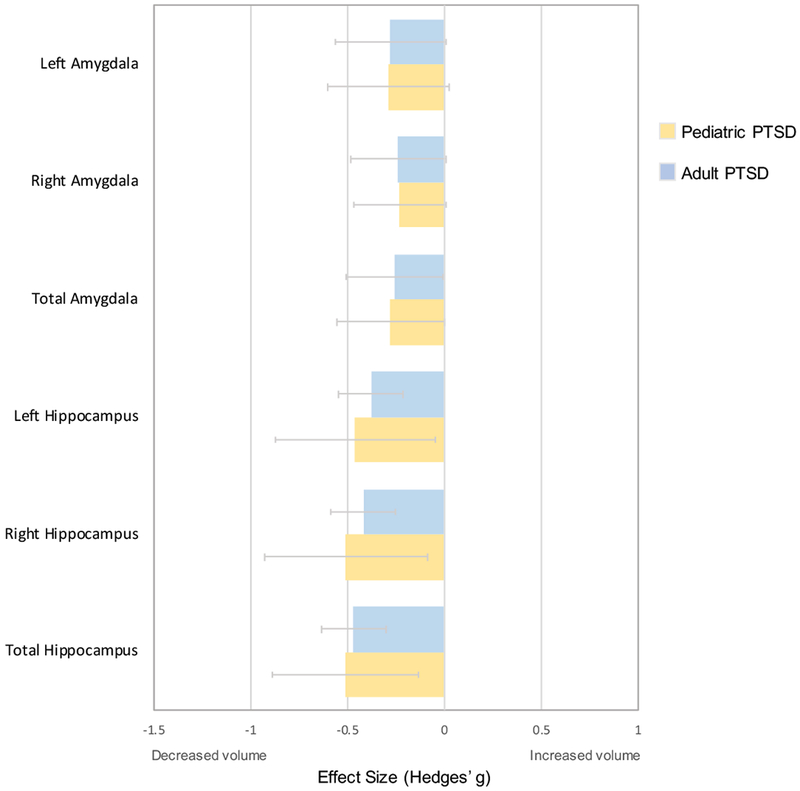
Comparison analysis of hippocampal and amygdala volumes between pediatric PTSD and adult PTSD.
The comparison adult PTSD study is a meta-analysis of 89 adult structural MRI studies (Bromis et al., 2018). Positive Hedges’ g values indicate increased structural volume in pediatric participants with the condition indicated in the legend. Negative Hedges’ g values indicate smaller structural volumes in participants with the condition indicated in the legend. Error bars represent width of 95% confidence interval.
Pediatric PTSD versus Pediatric Exposure to Trauma without PTSD Diagnosis.
Given that a meta-analysis of pediatric exposure to trauma without PTSD was not available, we selected the study with that largest sample size obtained from our MEDLINE search that included hippocampal and amygdala volumetric findings to conduct an indicative qualitative comparison, as using a single study for comparison precluded the use of formal statistical analyses (16) (See Table S5 for demographic characteristics of comparison studies). Our qualitative comparison revealed a difference between pediatric PTSD and pediatric exposure to trauma without PTSD. Specifically, whereas there was a medium negative effect size and small negative effective size for total hippocampal (ES=−0.51) and amygdala volume (ES=−0.28) in pediatric PTSD, respectively, there was a small positive effect size for total hippocampal (ES=0.24) and small to medium positive effect sizes for total (ES=0.42) and left (ES=0.46) amygdala volumes, respectively, in pediatric trauma exposure without PTSD (Figure 4, Table S11).
Figure 4.
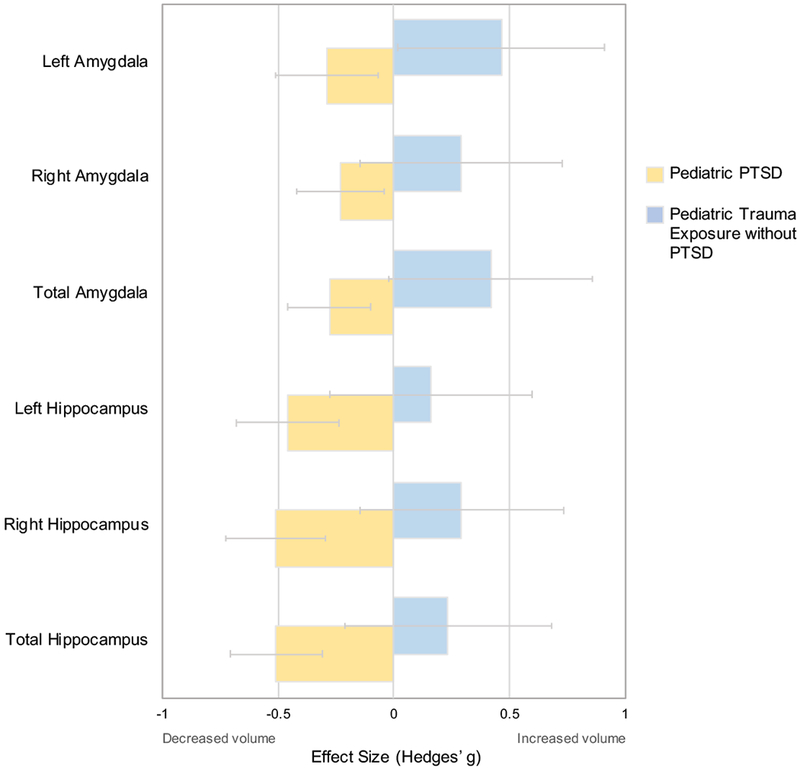
Comparison analysis of hippocampal and amygdala volumes between pediatric PTSD and pediatric exposure to trauma without PTSD.
The comparison study is one in which pediatric exposure to trauma without PTSD (n=32) was compared no trauma exposure in an age-matched group (n=57) (Morey et al., 2016). Positive Hedges’ g values indicate increased structural volume in pediatric participants with the condition indicated in the legend. Negative Hedges’ g values indicate smaller structural volumes in participants with the condition indicated in the legend. Error bars represent width of 95% confidence interval.
Pediatric PTSD versus Pediatric Depression.
Because a meta-analysis of structural volumetric studies in pediatric depression was not available, we selected the largest study obtained from our MEDLINE search that included hippocampal and amygdala volumetric findings in pediatric depression (30) (Table S5). Our qualitative comparison showed that while pediatric PTSD was associated with medium and small negative effect sizes for total hippocampal (ES=−0.51) and amygdala (ES=−0.28) volumes, respectively, pediatric depression was only associated with small positive effect sizes for both total hippocampal (ES=0.20) and amygdala (ES=0.20) volumes (Figure 5, Table S12).
Figure 5.
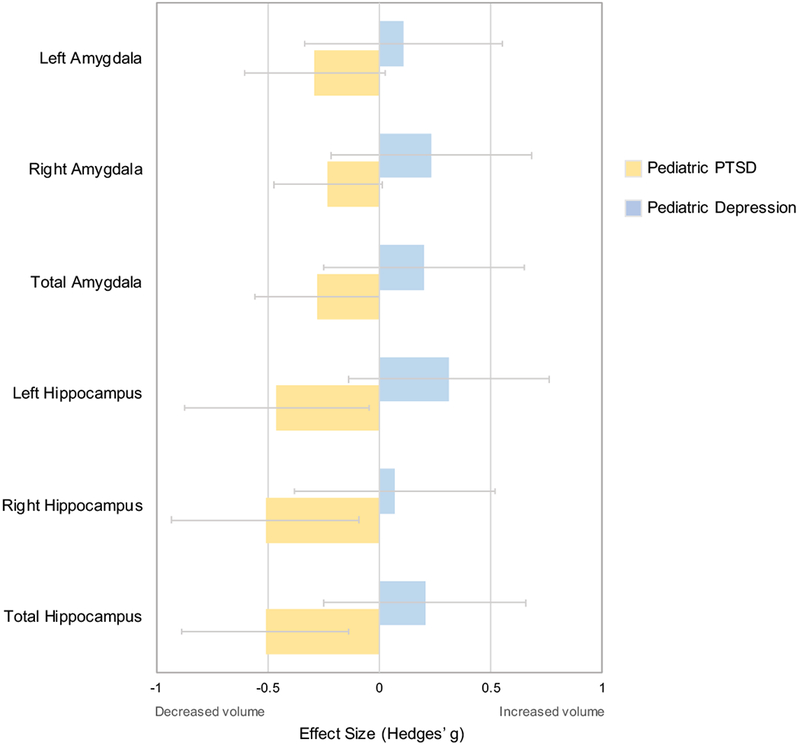
Comparison analysis of hippocampal and amygdala volumes between pediatric PTSD and pediatric depression.
The comparison study is one in which pediatric depression (n=30) was compared to a pediatric group without depression (n=56) at the second timepoint in a longitudinal study (Whittle et al., 2016). Positive Hedges’ g values indicate increased structural volume in pediatric participants with the condition indicated in the legend. Negative Hedges’ g values indicate smaller structural volumes in participants with the condition indicated in the legend. Error bars represent width of 95% confidence interval.
Pediatric PTSD versus Pediatric Anxiety.
A meta-analysis of structural volumetric studies in pediatric anxiety was not available; thus, we selected the largest study obtained from our MEDLINE search that included hippocampal and amygdala volumetric findings in pediatric anxiety (31) (Table S5). Our qualitative comparison revealed that both pediatric PTSD (ES=−0.51) and pediatric anxiety (ES=−0.38) were associated with negative effect sizes for total hippocampal volume (Figure S7, Table S13). Pediatric anxiety (ES=−0.38) and PTSD (ES=−0.51) were both also associated with smaller right hippocampal volume. Finally, whereas pediatric PTSD was associated with small negative effect size for total amygdala volume (ES=−0.28), smaller total amygdala volume was not associated with pediatric anxiety (ES=−0.17) (Figure S7, Table S13).
DISCUSSION
Our meta-analysis of 15 ROI volumetric sMRI studies showed significantly smaller total gray matter, total cerebral, temporal lobe (total, right, and left), total vermis, and hippocampal (total, right, and left) volumes in pediatric PTSD when compared to no PTSD. Additionally, a trend toward smaller amygdala volume (total, right, and left) was associated with pediatric PTSD relative to no PTSD. However, we found no significant differences in corpus callosum structure between pediatric PTSD and no PTSD. Importantly, there was a notable qualitative difference between pediatric PTSD and pediatric exposure to trauma without PTSD such that the former was associated with smaller amygdala volume (trend-level), while the latter with larger total amygdala volume.
The finding of smaller total hippocampal volume in pediatric PTSD in the present meta-analysis highlights volumetric differences in a region commonly involved in emotional memory and learning, both of which are disrupted in PTSD (32,33). This finding is particularly important as prior meta-analyses of neuroimaging studies in pediatric PTSD have not shown significantly smaller hippocampal volume to be associated with pediatric PTSD (8, 34). The inconsistency in meta-analytic findings may be attributed to the specific studies included in each regional meta-analysis. For example, the meta-analysis conducted by Woon and Hedges in 2008 (34) included only four studies, whereas Milani et al. (8) included four studies (N=343), one (17) of which was excluded from our database due to an overlapping sample. Our meta-analysis of total hippocampal volume included a total of eight studies (N=489) with non-overlapping samples. Thus, the examination of different studies likely accounts for the disparate findings regarding hippocampal volume in pediatric PTSD across these meta-analyses.
Whether smaller hippocampal volume is a risk factor for PTSD versus a consequence of trauma or PTSD remains an open question. Evidence from monozygotic twins discordant for trauma exposure suggests that smaller hippocampal volume may predispose some individuals to developing PTSD following trauma exposure (35). However, another study shows that hippocampal volume is not associated with increased risk for PTSD following trauma exposure (36). In addition, neurobiological differences associated with trauma and PTSD might reflect pre-existing vulnerabilities rather than consequences of trauma exposure (37). The current meta-analysis suggests developmental differences that warrant future investigations on the temporal relationship between structural changes, trauma exposure, and disorder onset.
With regard to the amygdala, which plays a central role in fear learning and threat reactivity (38), our meta-analytic findings show trend-level volumetric difference between children and adolescents with and without PTSD. Although there was a trend-level decrease in amygdala volumes in pediatric PTSD, the supplemental meta-analysis that only included studies using tracing methods revealed that pediatric PTSD is associated with significantly smaller amygdala volumes. Previous reviews of this literature (6, 18) have proposed that a lack of significant findings regarding amygdala volume in pediatric PTSD (9–11,16,20) may stem from developmental changes in limbic structures (39–41) that have not been taken into consideration. That is, the association between amygdala volume and PTSD may vary depending on age, such that an effect could be obscured in studies that do not examine age-related changes (18,42). Although we find that age is a significant moderator of variability for hippocampal but not amygdala regions in our primary analysis, age does emerge as a significant moderator for amygdala regions when only tracing studies are included in the meta-analysis. This finding may be explained by the fact that there is greater variability in ROI delineation using automated approaches for smaller subcortical regions (28), thus developmental effects may have been less likely to be detected in our analysis that included studies using automated methods. Taken together, these findings indicate the need to employ a developmental approach to understand the relationship between PTSD and brain structure, in addition to traditional reliance on comparisons between age-matched clinical and control groups. Although we focus here on the amygdala and hippocampus, the current meta-analysis also shows differences in the temporal lobe and vermis in pediatric PTSD. Little is known about structural changes in these regions following trauma exposure, and, thus, interpretation of these findings will depend on future research.
In addition to performing an analysis of regional brain structure in pediatric PTSD, we aimed to assess the degree to which these findings were specific to pediatric PTSD. First, we compared pediatric PTSD with adult PTSD. The hippocampus and amygdala undergo dynamic changes with neurodevelopment (21–23;43–44), suggesting they may be differentially influenced by trauma or involved in PTSD-related symptoms depending on developmental stage. Our analysis revealed that both adult and pediatric PTSD are associated with significant smaller hippocampal volumes (relative to no PTSD) and that there is no significant difference between adult and pediatric PTSD for this finding. This pattern could be due to the fact that although adults may have experienced more traumatic stress (e.g., greater cumulative exposure to trauma over more years of life) than children and adolescents, the hippocampus has been shown to be especially vulnerable earlier in life (45–47). There were also no significant differences in amygdala volumes between pediatric and adult PTSD.
Second, we compared ROI volumes in pediatric PTSD to trauma exposure without PTSD. Given that most studies in pediatric PTSD to date, and thus also the current ROI meta-analysis, have focused on pediatric groups with PTSD compared pediatric groups without any exposure to trauma, much remains unknown about the extent to which volumetric differences observed in PTSD relate to the disorder versus trauma exposure. A qualitative comparison between the present meta-analysis to a study of pediatric exposure to maltreatment without PTSD (16) revealed a notable difference between effect sizes in pediatric PTSD versus pediatric exposure to trauma for hippocampal and amygdala volumes. Specifically, regional volumes were smaller in pediatric PTSD versus no PTSD (the present meta-analysis) but larger in trauma versus no trauma exposure (16). Notably, within the individual study used in the comparison analysis (16), left amygdala volume was significantly larger in trauma exposure relative to no trauma exposure. These results suggest that exposure to trauma itself may be associated with amygdala differences with a general trend toward larger volume. In contrast, we observed a trend toward smaller amygdala volume in pediatric PTSD. Taken together, amygdala and hippocampal volumetric differences in pediatric PTSD are unlikely to be explained solely by exposure to trauma. Although the present study compares pediatric trauma exposure with and without PTSD in a qualitative manner, a meta-analysis of studies including groups with trauma exposure but without PTSD was not conducted due to the limited number of studies with a trauma exposure without PTSD group.
Finally, we aimed to dissociate volumetric effects in pediatric PTSD versus psychiatric disorders that commonly co-occur following trauma (3,24). We found that while pediatric PTSD was associated with smaller and trend toward smaller total hippocampal and amygdala volumes, respectively, pediatric depression was not associated with any notable volumetric differences (relative to individuals without depression). Notably, for the qualitative comparison with pediatric anxiety, we observed that both pediatric PTSD and pediatric anxiety were associated with smaller in total hippocampal volumes. Only pediatric PTSD was associated with smaller total amygdala volume (trend-level). Investigation of co-presenting pediatric PTSD with depression and with anxiety in the same sample, and inclusion of psychiatric comparison groups with depression or anxiety only, will further clarify the specificity of structural differences in pediatric PTSD. This approach will provide a foundation for future investigations that aim to enhance risk identification using neuroimaging correlates or to elucidate mechanisms that can inform clinical practice.
Three considerations are important to contextualize our findings and their interpretation within the broader literatures on PTSD and structural imaging. First, the use of categorical DSM-defined PTSD diagnoses and the definition of criterion A trauma exposure remain debated in the field (48–53), and differences in trauma exposure or clinical presentation across individuals included in different studies may be due in part to this substantial heterogeneity in classification (54). Although clinicians and researchers use the PTSD diagnosis to maintain consistency in clinical and scientific practices, this categorical approach may obscure meaningful complexity in traumatic experiences or symptom presentations. Although we were able to investigate the association between age and sex as moderators of hippocampal and amygdala findings, we were underpowered to investigate the role of a number of other key variables such as age at PTSD onset, age of first traumatic event, and duration of trauma exposures and PTSD. It is possible, however, that age of PTSD diagnosis may relate to key neurobiological changes associated with pediatric PTSD (6). Thus, it is important that future studies investigate the complex contributions of these elements to neurobiological changes following trauma exposure and PTSD. Finally, recent work has suggested the use of a network approach to identify related symptom subgroups that may share ontological origins (50,52,53), which could allow for more direct mapping to neurobiological correlates.
Second, the functional and mechanistic relevance of identifying volumetric differences using structural neuroimaging in humans remains unclear. Cross-species studies suggest potential mechanisms underlying hippocampal and amygdala volumetric differences. For example, chronic stress in rodents can lead to smaller hippocampal dendritic spine density, dendritic remodeling, and neurogenesis (55–59). Evidence also shows that stress activates inflammatory and anti-inflammatory responses (60) and that early-life stress in mice is associated with increased density and morphological differences in hippocampal microglia (61,62). Finally, in primates, direct cortisol administration to the hippocampus results in dendritic atrophy and shrinkage of the soma (63). In the amygdala, by contrast, chronic stress in rodents is associated with increased dendritic remodeling, growth, and spine density (64). These lines of evidence from animal studies illustrate mechanisms by which structural volumetric changes can occur.
While the etiology underlying volumetric differences in human neuroimaging is unknown, a recent study aimed to probe this very question (65). The authors showed that increased axonal myelination in the human visual cortex underlies the developmental decrease in cortical thickness that had previously been interpreted as cortical thinning (65). These processes can be regionally dependent, however; thus, it is unclear whether increased myelination underlying decreased cortical thickness generalizes beyond human visual cortex to other cortical or subcortical structures. Therefore, investigating volumetric differences in humans does not currently provide conclusive evidence of the mechanisms underlying neurodevelopmental differences following trauma. Future investigations utilizing multimodal structural and functional approaches within the same samples and longitudinal design will provide further insight into observed structural differences.
A final consideration surrounding the current findings is the nature of structural differences with regard to behavior and adaptation following stress. Though volumetric differences following trauma exposure are often characterized as pathologic, they may also reflect ways in which the brain has adapted to meet the needs of an adverse environment (6, 66). For instance, some evidence suggests that neurobiological differences in children and adolescents exposed to early adversity are ontogenetic adaptations that confer some functional benefit, at least in the short term (67), though long-term consequences remain to be explored. To better inform whether specific neurobiological differences are adaptive, research investigating structural brain differences in PTSD will benefit from examining behavioral correlates and mechanistic processes, as well as longitudinal designs to evaluate potential adaptations in the context of development and changing environmental circumstances.
The present meta-analysis leverages prior research to delineate differences in brain structure in pediatric PTSD and investigates the extent to which structural differences are specific to pediatric PTSD relative to trauma exposure, commonly comorbid pediatric affective disorders, and PTSD in adults. Furthermore, it highlights the relative paucity of research investigating regional differences in pediatric PTSD, as we only identified 22 studies conducted since 1992 that met criteria for the present study. There remains an important need in the field for future studies to better elucidate neurobiological changes following exposure to trauma, including multimodal investigations of structural and functional connectivity to examine circuit-based differences in pediatric PTSD. Future work will benefit from considering the complex and co-occurring psychiatric presentations following trauma that are the reality in clinical settings, dimensional approaches to better capture heterogeneous symptoms, and developmental designs to elucidate age-dependent effects of trauma and PTSD. Taken together, the current study identifies key structural brain differences in pediatric PTSD and provides concrete directions for future research that will further elucidate the nature of brain development following trauma exposure and in children and adolescents with PTSD.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health (NIH) Director’s Early Independence Award (DP5OD021370) to DGG, Brain and Behavior Research Foundation NARSAD Young Investigator Award to DGG, Jacobs Foundation Early Career Research Fellowship to DGG, and NIH Medical Scientist Training Program Training Grant (T32) to SK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Copeland WE, Keeler G, Angold A, Costello EJ (2007): Traumatic Events and Posttraumatic Stress in Childhood. Arch Gen Psychiatry. 64: 577–584. [DOI] [PubMed] [Google Scholar]
- 2.Copeland WE, Shanahan L, Hinesley J, Chan RF, Aberg KA, Fairbank JA, et al. (2018): Association of Childhood Trauma Exposure With Adult Psychiatric Disorders and Functional Outcomes. JAMA Network Open. 1: e184493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLaughlin KA, Koenen KC, Hill ED, Petukhova M, Sampson NA, Zaslavsky AM, Kessler RC (2013): Trauma exposure and posttraumatic stress disorder in a national sample of adolescents. J Am Acad Child Adolesc Psychiatry. 52: 815–830. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Publishing. [Google Scholar]
- 5.Alisic E, Zalta AK, van Wesel F, Larsen SE, Hafstad GS, Hassanpour K, Smid GE (2014): Rates of post-traumatic stress disorder in trauma-exposed children and adolescents: meta-analysis. Br J Psychiatry. 204: 335–340. [DOI] [PubMed] [Google Scholar]
- 6.Weems CF, Russell JD, Neill EL, McCurdy BH (2018): Annual Research Review: Pediatric posttraumatic stress disorder from a neurodevelopmental network perspective. J Child Psychol Psychiatry. 60: 395–408. [DOI] [PubMed] [Google Scholar]
- 7.Herringa RJ (2017): Trauma, PTSD, and the Developing Brain. Current Psychiatry Reports.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milani ACC, Hoffmann EV, Fossaluza V, Jackowski AP, Mello MF (2017): Does pediatric post-traumatic stress disorder alter the brain? Systematic review and meta-analysis of structural and functional magnetic resonance imaging studies: Review of MRI findings in pediatric PTSD. Psychiatry Clin Neurosci. 71: 154–169. [DOI] [PubMed] [Google Scholar]
- 9.Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, Reiss AL (2001): Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry. 50: 943–951. [DOI] [PubMed] [Google Scholar]
- 10.De Bellis MDD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, et al. (1999): Developmental Traumatology Part II: Brain Development. Biol Psychiatry. 14. [DOI] [PubMed] [Google Scholar]
- 11.De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, Moritz G (2002): Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry. 52: 1066–1078. [DOI] [PubMed] [Google Scholar]
- 12.De Bellis MD, Keshavan MS (2003): Sex differences in brain maturation in maltreatment-related pediatric posttraumatic stress disorder. Neurosci Biobehav Rev. 27: 103–117. [DOI] [PubMed] [Google Scholar]
- 14.De Bellis MD, Hooper SR, Chen SD, Provenzale JM, Boyd BD, Glessner CE, et al. (2015): Posterior structural brain volumes differ in maltreated youth with and without chronic posttraumatic stress disorder. Dev Psychopatholology. 27: 1555–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed F, Spottiswoode BS, Carey PD, Stein DJ, Seedat S (2012): Relationship between Neurocognition and Regional Brain Volumes in Traumatized Adolescents with and without Posttraumatic Stress Disorder. Neuropsychobiology. 66: 174–184. [DOI] [PubMed] [Google Scholar]
- 16.Morey RA, Haswell CC, Hooper SR, De Bellis MD (2016): Amygdala, Hippocampus and Ventral Medial Prefrontal Cortex Volumes Differ in Maltreated Youth with and without Chronic Posttraumatic Stress Disorder. Neuropsychopharmacology. 41: 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tupler LA, De Bellis MD (2006): Segmented Hippocampal Volume in Children and Adolescents with Posttraumatic Stress Disorder. Biol Psychiatry. 59: 523–529. [DOI] [PubMed] [Google Scholar]
- 18.Weems CF (2017): Severe stress and the development of the amygdala in youth: A theory and its statistical implications. Developmental Review. 46: 44–53. [Google Scholar]
- 19.Bromis K, Calem M, Reinders AATS, Williams SCR, Kempton MJ (2018): Meta-Analysis of 89 Structural MRI Studies in Posttraumatic Stress Disorder and Comparison With Major Depressive Disorder. Am JPsychiatry. 175: 989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G (2001): A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry. 50: 305–309. [DOI] [PubMed] [Google Scholar]
- 21.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. (2004): Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 101: 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. (1999): Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 2: 861–863. [DOI] [PubMed] [Google Scholar]
- 23.Lenroot RK, Giedd JN (2006): Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience & Biobehavioral Reviews. 30: 718–729. [DOI] [PubMed] [Google Scholar]
- 24.Lewis SJ, Arseneault L, Caspi A, Fisher HL, Matthews T, Moffitt TE, et al. (2019): The epidemiology of trauma and post-traumatic stress disorder in a representative cohort of young people in England and Wales. The Lancet Psychiatry. 6: 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N (1986): Meta-analysis in clinical trials. Control Clin Trials. 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 26.Han B, Eskin E (2011): Random-effects model aimed at discovering associations in meta-analysis of genome-wide association studies. Am J Hum Genet. 88: 586–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JPT, Thompson SG (2002): Quantifying heterogeneity in a meta-analysis. StatMed. 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 28.Schoemaker D, Buss C, Head K, Sandman CA, Davis EP, Chakravarty MM, et al. (2016): Hippocampus and amygdala volumes from magnetic resonance images in children: Assessing accuracy of FreeSurfer and FSL against manual segmentation. Neuroimage. 129: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson SG, Higgins JPT (2002): How should meta-regression analyses be undertaken and interpreted? Statistics in Medicine. 21: 1559–1573. [DOI] [PubMed] [Google Scholar]
- 30.Whittle S, Lichter R, Dennison M, Vijayakumar N, Schwartz O, Byrne ML, et al. (2014): Structural Brain Development and Depression Onset During Adolescence: A Prospective Longitudinal Study. Am JPsychiatry. 171: 564–571. [DOI] [PubMed] [Google Scholar]
- 31.Gold AL, Steuber ER, White LK, Pacheco J, Sachs JF, Pagliaccio D, et al. (2017): Cortical Thickness and Subcortical Gray Matter Volume in Pediatric Anxiety Disorders. Neuropsychopharmacology. 42: 2423–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jovanovic T, Kazama A, Bachevalier J, Davis M (2012): Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology. 62: 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. (2009): Neurobiological Basis of Failure to Recall Extinction Memory in Posttraumatic Stress Disorder. Biol Psychiatry. 66: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woon FL, Hedges DW (2008): Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus. 18: 729–736. [DOI] [PubMed] [Google Scholar]
- 35.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK (2002): Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 5: 1242–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonne O, Brandes D, Gilboa A, Gomori JM, Shenton ME, Pitman RK, Shalev AY (2001): Longitudinal MRI Study of Hippocampal Volume in Trauma Survivors With PTSD. Am J Psychiatry. 158: 1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danese A, Moffitt TE, Arseneault L, Bleiberg BA, Dinardo PB, Gandelman SB, et al. (2017): The Origins of Cognitive Deficits in Victimized Children: Implications for Neuroscientists and Clinicians. Am J Psychiatry. 174: 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phelps EA, LeDoux JE (2005): Contributions of the Amygdala to Emotion Processing: From Animal Models to Human Behavior. Neuron. 48: 175–187. [DOI] [PubMed] [Google Scholar]
- 39.Albaugh MD, Nguyen T-V, Ducharme S, Collins DL, Botteron KN, D’Alberto N, et al. (2017): Age-related volumetric change of limbic structures and subclinical anxious/depressed symptomatology in typically developing children and adolescents. Biol Psychol. 124: 133–140. [DOI] [PubMed] [Google Scholar]
- 40.Uematsu A, Matsui M, Tanaka C, Takahashi T, Noguchi K, Suzuki M, Nishijo H (2012): Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS ONE. 7: e46970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goddings A-L, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore S-J (2014): The influence of puberty on subcortical brain development. Neuroimage. 88: 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weems CF, Klabunde M, Russell JD, Reiss AL, Carrion VG (2015): Post-traumatic stress and age variation in amygdala volumes among youth exposed to trauma. Social Cognitive and Affective Neuroscience. 10: 1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gee DG, Bath KG, Johnson CM, Meyer HC, Murty VP, van den Bos W, Hartley CA (2018): Neurocognitive Development of Motivated Behavior: Dynamic Changes across Childhood and Adolescence. J Neurosci. 38: 9433–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casey BJ, Heller AS, Gee DG, Cohen AO (2019): Development of the emotional brain. Neurosci Lett. 693: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH (2008): Preliminary Evidence for Sensitive Periods in the Effect of Childhood Sexual Abuse on Regional Brain Development. J Neuropsychiatry Clin Neurosci. 20: 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luby JL, Belden A, Harms MP, Tillman R, Barch DM (2016): Preschool is a sensitive period for the influence of maternal support on the trajectory of hippocampal development. Proc Natl Acad Sci. 113: 5742–5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Humphreys KL, King LS, Sacchet MD, Camacho MC, Colich NL, Ordaz SJ, et al. (2018): Evidence for a sensitive period in the effects of early life stress on hippocampal volume. Dev Sci. e12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.American Psychiatric Association (1980): Diagnostic and Statistical Manual of Mental Disorders, 3rd ed. Washington, DC: American Psychiatric Publishing. [Google Scholar]
- 49.Karter JM, Kamens SR (2019): Toward Conceptual Competence in Psychiatric Diagnosis: An Ecological Model for Critiques of the DSM In: Steingard S, editor. Critical Psychiatry: Controversies and Clinical Implications. Cham: Springer International Publishing, pp 17–69. [Google Scholar]
- 50.McNally RJ (2003): Progress and controversy in the study of posttraumatic stress disorder. Annu Rev Psychol. 54: 229–252. [DOI] [PubMed] [Google Scholar]
- 51.Summerfield D (2001): The invention of post-traumatic stress disorder and the social usefulness of a psychiatric category. BMJ. 322: 95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McNally RJ, Robinaugh DJ, Wu GWY, Wang L, Deserno MK, Borsboom D (2015): Mental Disorders as Causal Systems: A Network Approach to Posttraumatic Stress Disorder. Clinical Psychological Science. 3: 836–849. [Google Scholar]
- 53.Russell JD, Neill EL, Carrion VG, Weems CF (2017): The Network Structure of Posttraumatic Stress Symptoms in Children and Adolescents Exposed to Disasters. J Am Acad Child Adolesc Psychiatry. 56: 669–677. e5. [DOI] [PubMed] [Google Scholar]
- 54.Galatzer-Levy IR, Bryant RA (2013): 636,120 Ways to Have Posttraumatic Stress Disorder. Perspectives on Psychological Science. 8: 651–662. [DOI] [PubMed] [Google Scholar]
- 55.Isgor C, Kabbaj M, Akil H, Watson SJ (2004): Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 14: 636–648. [DOI] [PubMed] [Google Scholar]
- 56.McEwen BS (2007): Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 87: 873–904. [DOI] [PubMed] [Google Scholar]
- 57.Kim JJ, Yoon KS (1998): Stress: metaplastic effects in the hippocampus. Trends Neurosci. 21: 505–509. [DOI] [PubMed] [Google Scholar]
- 58.Sapolsky RM (1996): Stress, Glucocorticoids, and Damage to the Nervous System: The Current State of Confusion. Stress. 1: 1–19. [DOI] [PubMed] [Google Scholar]
- 59.Sunanda, Rao MS, Raju TR (1995): Effect of chronic restraint stress on dendritic spines and excrescences of hippocampal CA3 pyramidal neurons--a quantitative study. Brain Res. 694: 312–317. [DOI] [PubMed] [Google Scholar]
- 60.Danese A, Baldwin JR (2017): Hidden Wounds? Inflammatory Links Between Childhood Trauma and Psychopathology. Annual Review of Psychology. 68: 517–544. [DOI] [PubMed] [Google Scholar]
- 61.García-Bueno B, Caso JR, Leza JC (2008): Stress as a neuroinflammatory condition in brain: damaging and protective mechanisms. Neurosci Biobehav Rev. 32: 1136–1151. [DOI] [PubMed] [Google Scholar]
- 62.Delpech J-C, Wei L, Hao J, Yu X, Madore C, Butovsky O, Kaffman A (2016): Early life stress perturbs the maturation of microglia in the developing hippocampus. Brain Behav Immun . 57: 79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sapolsky RM, Uno H, Rebert CS, Finch CE (1990): Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 10: 2897–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roozendaal B, McEwen BS, Chattarji S (2009): Stress, memory and the amygdala. Nat Rev Neurosci. 10: 423–433. [DOI] [PubMed] [Google Scholar]
- 65.Natu VS, Gomez J, Barnett M, Jeska B, Kirilina E, Jaeger C, et al. (2018): Apparent thinning of visual cortex during childhood is associated with myelination, not pruning. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kentner AC, Cryan JF, Brummelte S (2019): Resilience priming: Translational models for understanding resiliency and adaptation to early life adversity. Dev Psychobiol. 61: 350–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, et al. (2013): Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci. 110: 15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carrion VG, Weems CF, Watson C, Eliez S, Menon V, Reiss AL (2009): Converging evidence for abnormalities of the prefrontal cortex and evaluation of midsagittal structures in pediatric posttraumatic stress disorder: An MRI study. Psychiatry Research: Neuroimaging. 172: 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carrion VG, Weems CF, Richert K, Hoffman BC, Reiss AL (2010): Decreased Prefrontal Cortical Volume Associated with Increased Bedtime Cortisol in Traumatized Youth. Biol Psychiatry. 68: 491–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Bellis MD, Keshavan MS, Frustaci K, Shifflett H, Iyengar S, Beers SR, Hall J (2002): Superior temporal gyrus volumes in maltreated children and adolescents with ptsd. Biological Psychiatry. 51: 544–552. [DOI] [PubMed] [Google Scholar]
- 71.De Bellis MD, Kuchibhatla M (2006): Cerebellar Volumes in Pediatric Maltreatment-Related Posttraumatic Stress Disorder. Biol Psychiatry. 60: 697–703. [DOI] [PubMed] [Google Scholar]
- 72.Keding TJ, Herringa RJ (2015): Abnormal structure of fear circuitry in pediatric post-traumatic stress disorder. Neuropsychopharmacology. 40: 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Klabunde M, Weems CF, Raman M, Carrion VG (2017): The moderating effects of sex on insula subdivision structure in youth with posttraumatic stress symptoms. Depression and Anxiety. 34: 51–58. [DOI] [PubMed] [Google Scholar]
- 74.Mutluer T, Şar V, Kose-Demiray Ç, Arslan H, Tamer S, Inal S, Kaçar AŞ (2018): Lateralization of Neurobiological Response in Adolescents with Post-Traumatic Stress Disorder Related to Severe Childhood Sexual Abuse: the Tri-Modal Reaction (T-MR) Model of Protection. J Trauma Dissociation. 19: 108–125. [DOI] [PubMed] [Google Scholar]
- 75.Postel C, Viard A, André C, Guénolé F, Flores R de, Baleyte J-M, et al. (2019): Hippocampal subfields alterations in adolescents with post-traumatic stress disorder. Human Brain Mapping. 40: 1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Richert KA, Carrion VG, Karchemskiy A, Reiss AL (2006): Regional differences of the prefrontal cortex in pediatric PTSD: an MRI study. Depress Anxiety. 23: 17–25. [DOI] [PubMed] [Google Scholar]
- 77.Rinne-Albers MA, Pannekoek JN, van Hoof M-J, van Lang ND, Lamers-Winkelman F, Rombouts SA, et al. (2017): Anterior cingulate cortex grey matter volume abnormalities in adolescents with PTSD after childhood sexual abuse. Eur Neuropsychopharmacol. 27: 1163–71. [DOI] [PubMed] [Google Scholar]
- 78.Thomas LA, De Bellis MD (2004): Pituitary volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry. 55: 752–758. [DOI] [PubMed] [Google Scholar]
- 79.Weems CF, Scott BG, Russell JD, Reiss AL, Carrion VG (2013): Developmental Variation in Amygdala Volumes Among Children With Posttraumatic Stress. Developmental Neuropsychology. 38: 481–495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


