Abstract
Diets rich in fat, smoking, as well as exposure to environmental pollutants and dysbiosis of gut microbiota, increase the risk of developing colorectal cancer (CRC). Much progress has been made in combating CRC. However, options for chemoprevention from environmental insult and dysbiosis of gut microbiota remains elusive. We investigated the influence of berry-derived anthocyanidins (Anthos), with and without encapsulating them in bovine milk-derived exosomes (ExoAnthos), on the chemoprevention of bacteria-driven colon tumor development. Anthos and ExoAnthos treatment of colon cancer cells showed dose-dependent decreases in cell viability. Calculated selectivity index (SI) values for Anthos and ExoAnthos suggest that both treatments selectively targeted cancer over normal colon cells. Additionally, ExoAnthos treatment yielded higher SI values than Anthos. Anthos and ExoAnthos treatment of ApcMin/+ mice inoculated with ETBF bacteria led to significant decreases in colon tumor numbers over mice receiving vehicle treatments. Western blot analysis of normal colon, colon tumor, and liver tissue lysates showed that mice inoculated with ETBF featured increased expression of phase I enzymes in normal colon tissue and decreased expression of phase II enzymes in liver tissue. Treatment with the Anthos and ExoAnthos reverted the modulation of phase I and phase II enzymes, respectively; no significant changes in phase II enzyme expression occurred in colon tumor tissue. Treatment of HCT-116 cells with the ubiquitous carcinogen, benzo[a]pyrene led to similar modulation of phase I and II enzymes, which was partially mitigated by treatment with Anthos. These results provide a promising outlook on the impact of berry Anthos for prevention and treatment of bacteria- and benzo[a]pyrene-driven colorectal cancer.
Keywords: Anthocyanidins, Exosomes, ETBF, Colorectal cancer, Benzo[a]pyrene, Phase I and Phase II enzymes
Introduction
Although much progress has been made in the diagnosis and treatment of cancer over the last thirty years, colorectal cancer (CRC) remains a looming threat on the horizon. For instance, although the incidence of CRC has been trending downward since the mid to late 1980s for individuals 55 years or older, a recent study has found a rather disconcerting uptick in the CRC incidence for individuals below 55 years old (1). According to the CDC, the third most common form of cancer in the US is CRC. Furthermore, CRC is the third leading form attributed to cancer-related deaths each year. In fact, according to the American Cancer Society, it is estimated that 140,250 individuals will be diagnosed and 50,630 will die from CRC in the US alone in 2018. Epidemiological studies suggest that diets rich in fat, smoking, and increased alcohol consumption, as well as exposure to environmental pollutants and dysbiosis of gut microbiota, increase the risk of developing CRC (2,3). Much progress has been made in combating the disease due to advancements made in the early detection of CRC. However, options for chemoprevention from environmental insult and an understanding of how such treatments could alter the dialogue between one’s microbiome and environmental toxins remain largely elusive.
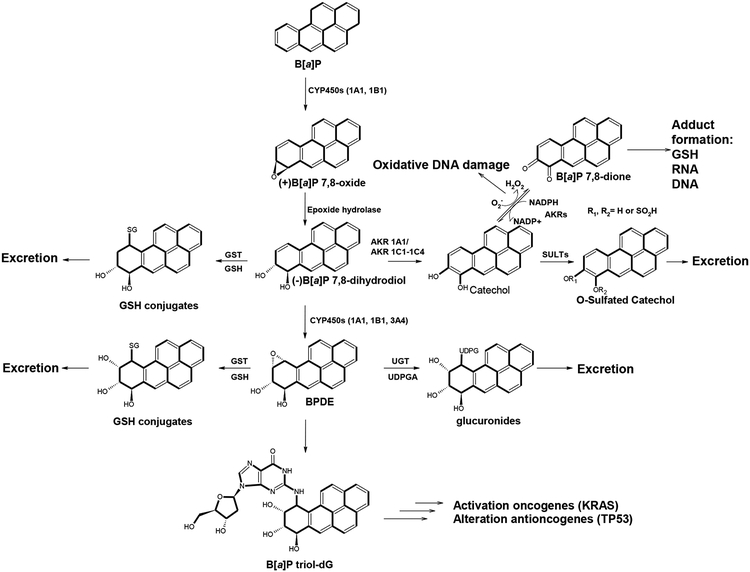
Environmental factors such as air pollution, cigarette smoke, and dietary contaminants have been mechanistically linked to an increased risk of CRC (4–6). One particular class of environmental pollutants that is especially pervasive are polycyclic aromatic hydrocarbons (PAHs). One of the most ubiquitous members of this family is benzo[a]pyrene (B[a]P) which is found in cigarette smoke, as a contaminant in many foods, car exhaust fumes, wood-burning and coal tar. In order to initiate the carcinogenic process, B[a]P undergoes bioactivation by enzymes such as the cytochrome P450 (CYP) 1A1 and/or 1B1 and microsomal epoxide hydrolase (mEH), resulting in the formation of the ultimate carcinogen benzo[a]pyrene-7,8-diol-9,10-epoxide (BPDE). Various enzymes including members of the glutathione s-transferase (GST), uridine 5’-diphospho-glucuronosyltransferase (UGT) and sulfotransferase (SULT) families are involved in detoxification of the intermediates along this bioactivation pathway (Figure 1). The metabolites of B[a]P are classified as group 1 carcinogens by the International Agency for Research on Cancer (IARC). BPDE intercalates DNA and ultimately covalently binds with guanine bases. This acts to distort the structure of DNA, disrupting the copying of DNA, which in turn causes mutations. BPDE has also been found to target p53 thereby altering the tumor suppression of cells which may ultimately lead to cancer (7). Interestingly, research that measures all of the exposures of an individual in a lifetime and how those exposures relate to health, referred to as “exposome,” has found that exposure to B[a]P leads to the enhanced susceptibility of macrophage membranes to bacterial infection and ultimately may lead to immunosuppression (8). Although one of the first carcinogens to be studied, B[a]P remains a continued threat due to its widespread presence in the environment. Given the continued relevance of B[a]P, how this toxicant and its metabolites interact with endogenous factors such as the gut microbiome is of high importance in addressing the management of several diseases including colon cancer.
Figure 1. Metabolism of benzo[a]pyrene to carcinogenic BPDE versus detoxification pathways.
In addition to traditional carcinogens such as B[a]P, recent research has begun to uncover the importance of the gut microbiome in the development of CRC. Research has shown that imbalances in intestinal microbiota lead to both an increase in inflammatory conditions as well as an increased production of carcinogenic metabolites, which may ultimately lead to neoplasia. Several bacteria have been associated with increased risk of developing CRC including, S. gallolyticus, H. pylori, virulent forms of Escherichia coli (E. coli), Fusobacterium nucleatum (F. nucleatum), Salmonella enterica (S. enterica) and enterotoxigenic B. fragilis (9). Enterotoxigenic Bacteriodes fragilis (ETBF) in particular is a highly relevant model for development of CRC due to its contribution to both familial and sporadic forms of cancer (10,11). ETBF exists asymptomatically in 12.4% of individuals overall and in 27% of individuals with diarrhea symptoms (11). Furthermore, presence of ETBF in the gut is a well-known cause of diarrheal disease globally that is accompanied by colitis in both humans and animals. The pathogenicity associated with ETBF is due to the secretion of a 20 kDa zinc-dependent metalloprotease toxin, B. fragilis toxin (BFT), which binds to colonic epithelial cells and leads to the cleavage of the tumor suppressor protein, E-cadherin, and the secretion of interleukin-8 (12). Overall, this process leads to the stimulation of proliferation and migration of human colon cancer cells (13). It should be noted that BFT has also been shown to induce pro-inflammatory cytokine secretion by further activating the NFƘB pathway (13).
Interestingly, a bidirectional dialogue has been found to exist between the gut microbiome and environmental chemicals, with bacteria metabolizing the pollutants contributing to host toxicity and the contaminants altering the composition of gut microbiota (3). This dynamic interaction between the host microbiome and environmental carcinogens is becoming ever more prevalent and relevant in the modern era. Understanding the impact of gut bacteria such as ETBF on the expression of phase I/II enzymes and identifying a chemopreventive method to combat this omnipresent insult is of great importance.
Several plant bioactives have been an invaluable source of medicines for humans. The family of plant pigments, known as the anthocyanins, have been identified with a variety of health benefits including chemopreventive and therapeutic effects due to their roles as anti-inflammatory, antioxidant agents and modulators of cytochrome P450 enzymes, CYP1A1 and CYP1B1 (14,15). Found in dark-colored vegetables, fruits, grains, and flowers, anthocyanins provide the characteristic red, purple and blue hues. Anthocyanins are, in part, converted to anthocyanidins (Anthos), the aglycone moieties, and, in fact, have higher antiproliferative and anti-inflammatory activities than the anthocyanins (16) presumably due to higher cell uptake.
The berry Anthos presents a potential chemopreventive option for individuals to avoid developing CRC. Berries were shown to reduce the oral dysplasia and carcinoma-in-situ by approximately 50% in animals previously treated with a mixture of the cigarette smoke carcinogens, B[a]P and NNK (17). Previous work from our laboratory against breast and lung cancer has shown that Anthos possess both chemopreventive and therapeutic effects due to their roles as anti-inflammatory, antioxidant agents and modulators of CYP1A1 and CYP1B1 (18). We demonstrated that intervention with the anthocyanidin, delphinidin favorably modulated the underlying mechanisms of potent PAHs (19). Furthermore, although work has been conducted to research the impact of anthocyanins on CRC (20,21), no data have been reported to study the impact of Anthos or ETBF bacteria on alterations in phase I and II enzyme expression.
With this in mind, the aim of the series of studies presented in this manuscript was to assess how treatment with a native mixture of anthocyanidins derived from bilberry (i.e., Anthos) and an exosomal formulation of Anthos (ExoAnthos) influence proliferation and modulation of expression of key phase I and II enzymes both in vitro and in a bacterially-induced in vivo model of colorectal cancer. Furthermore, the influence of gut bacterial dysbiosis induced by ETBF on phase I/II enzyme expression was also investigated.
Materials and Methods
Chemicals
B[a]P was handled carefully with all safety procedures as it is a highly carcinogenic and hazardous chemical. B[a]P (B-1760, St. Louis, MO) was purchased from Sigma-Aldrich.
Isolation of bilberry-derived Anthos
The native bilberry Anthos mixture (>85%), composed of delphinidin, cyanidin, petunidin, malvidin, and peonidin, was generously provided by 3P Biotechnologies, Inc. (Louisville, KY). The native Anthos was further enriched using C18 Sep-Pak cartridges (Waters, Milford, MA) and eluted in acidified (0.1% HCl) ethanol. The enriched extract was then dried using a Savant SC210A Speed-Vac (ThermoFisher Scientific, Waltham, MA) and stored at −20 °C. Purity, batch to batch consistency and reproducibility of the Anthos was determined by HPLC-PDA-UV. The enriched bilberry extract available commercially is highly standardized and it provided similar ratios of the individual anthcyanidins isolated from different batches of the bilberry extract. Briefly, 15 μl samples were analyzed using a Shimadzu Premier C18 reverse-phase column (250×4.6 mm i.d., 5 μm). Mobile phase A was composed of water: formic acid: acetonitrile (87:10:3) and mobile phase B was composed of water: formic acid: acetonitrile (40:10:50). The flow rate was 0.6 ml/min and the gradient condition was 0–5 min 5% B; 5–15 min 15% B; 15–20 min 25% B; 20–30 min 35% B; 30–40 min 45% B; 40–45 min 100% B; 45–50 min 5% B. Detection of Anthos was at 520 nm by PDA-UV and total Anthos concentration was calculated using a standard curve. The reference compounds were purchased from Chromadex (Irvine, CA) and Cayman Chemical Company (Ann Arbor, MI).
Isolation of milk-derived exosomes
Exosomes from cow colostrum, isolated using differential centrifugation as described by Munagala et al., (22), were generously provided by 3P Biotechnologies (Louisville, KY).
Protein determination
Protein estimation for exosomes was assessed using a bicinchoninic acid (BCA) assay (Thermo Scientific, Rockford, IL). In order to determine protein concentration, diluted exosomal preparations were compared, in triplicate, to a serially diluted bovine serum albumin (BSA) standard curve.
Preparation of ExoAnthos
Anthos were loaded onto exosomes by mixing Anthos (dissolved in 1:1 mixture of ethanol and water) with the exosomes suspension in a 1:5 (Anthos:Exosomal protein, w/w) ratio at room temperature (22 °C). Unbound Anthos and any coagulated exosomes were removed by low-speed centrifugation (10,000×g for 10 min). The exosomal formulation of Anthos was collected by ultracentrifugation (135,000 xg for 1.5 h). The pellet was then suspended in PBS and passed through a 0.22 μ syringe filter and stored at −80 °C. The percent loading was determined using solvent extraction, as described (22,23).
Analysis of Anthos loading
In order to determine the load of Anthos in the ExoAnthos formulation, the protein and Anthos concentrations were measured. Briefly, a 50-μl aliquot of ExoAnthos formulation was mixed with 950 μl of acidified ethanol (0.1% HCl) and incubated at 4 °C for 30–60 min. The precipitated proteins were separated by centrifugation (10,000 × g for 10 minutes). The Anthos contained in the supernatant was then analyzed using a SpectraMax M2 spectrometer (Molecular Devices, Sunnyvale, CA). Anthos were detected at 520 nm and total Anthos concentration was calculated using a standard curve. Anthos concentrations were confirmed via HPLC-PDA against reference compounds. The pelleted exosomal proteins were determined by the BCA method described above. The percent Anthos load was calculated by dividing the amount of Anthos by exosomal proteins × 100 (22). Individual anthocyanidins present in the Anthos mixture were loaded onto exosomes equally as confirmed using HPLC-DAD.
Cells, culture conditions and treatments
The APC wild-type HCT 116 (ATCC® CCL-247™) and APC mutant HT-29 (ATCC® HTB-38D™) colon cancer cell lines and CCD-18Co (ATCC® CRL-1459™) normal colon cells were acquired from American Type Culture Collection (Manassas, VA). HCT-116 and HT-29 cells were maintained in McCoy’s 5A medium (Gibco, Grand Island, NY) supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin in a humidified atmosphere containing 5% CO2 at 37°C. Based upon dosages derived from cell viability assays, cells were pre-treated with Anthos alone (25, 50, 100, 200 μM) for 24 h and treated with a mixture of Anthos (25, 50, 100, 200 μM) and B[a]P (20 μM) for 24 h.
Measurement of cell viability
The cytotoxicity of bilberry Anthos in colon cancer cell lines was assessed by enzymatic reduction of the tetrazolium dye MTT. Briefly, 3.0 ×103 cells/well were grown in 96-well tissue culture plates and were then exposed to varying concentrations of the Anthos, ExoAnthos or vehicle control 24 h after seeding. After 72 h treatment, cells were incubated with 5 mg/ml MTT reagent for 2 h. Resulting formazan crystals were subsequently solubilized in DMSO and measured spectrophotometrically at 570 nm (Bio-rad, Philadelphia, PA). IC50 values were then determined using Calcysyn software version 2.1 (Biosoft, Cambridge, England).
Western-blot analysis
For western-blot analysis, 40 μg of protein from in vivo and in vitro tissue lysates was resolved using SDS-polyacrylamide gel electrophoresis and electro-transferred to polyvinylidene difluoride membranes by semi-dry transfer (Biorad Trans-blot SD, Hercules, CA). Blots were blocked with 4% dry powder milk or BSA for 1 h and then incubated with primary antibodies β-actin, UGT1A6, SULT1, GSTM1, GSTM2, CYP1A1, CYP1B1, PXR, Nrf2, AhR, AhRR, and ARNT1 which were all acquired from Santa Cruz Biotech (Santa Cruz, CA) at 4°C overnight and secondary antibodies (Santa Cruz Biotech, Santa Cruz, CA) conjugated to peroxidase for 1 h at room temperature. Blots were then developed with an ECL detection system. Densitometric analysis was then performed using ImageJ 1.x software (24).
In vivo CRC studies
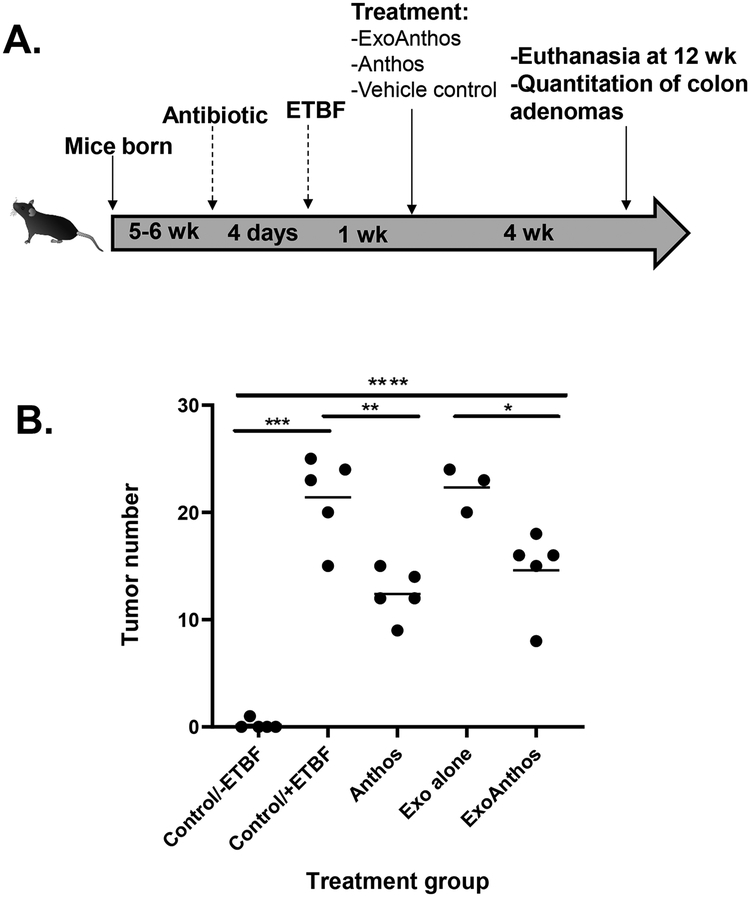
Animal experiments were performed in agreement with an approved protocol by the Institutional Animal Care and Use Committee at the University of Louisville. Breeding colonies were established in collaboration with Dr. Nejat K. Egilmez’s lab (25) at the University of Louisville using C57BL/6J Min/+ (ApcMin/+) mice that were originally procured from Jackson Laboratories (Bar Harbour, ME). Mice were genotyped for the APC mutation using PCR according to the protocol established by Jackson Laboratories. Mice were fed a standard chow diet and received water ad libitum and were maintained on a standard light/dark cycle for the duration of the study. At 5–6 weeks of age, animals were administered antibiotics (clindamycin (0.1 g/L) and streptomycin (5 g/L)). Four days later the animals were administered ETBF to promote tumorigenesis in the colon and one week following ETBF inoculation, animals began their respective treatment regimen. Male (n=2) and female (n=3) ApcMin/+ mice were orally administered (by gavage) an average of 8.6 mg/kg/day Anthos or ExoAnthos or vehicle control 3 days a week for 4 weeks. The Anthos dosage selected is roughly equivalent to 400 g of fresh blueberries per day for a 70 kg individual, based upon the level of anthocyanidins commonly found in this fruit (26). Animals were culled in the fed state at 12 weeks, colon tumors were counted and tissues were harvested.
Data analysis
Statistical analysis was performed using Graph Pad Prism statistical software version 4.03 (La Jolla, CA) and RStudio software version 1.0.153 (Boston, MA) Lattice package (27,28). One-way ANOVA was used for assessing the significance of mean differences across the various treatments for animal tumor and western data. IC50 values were determined using CalcuSyn software version 2.1 (Biosoft, Cambridge, England). Heat maps were constructed using RStudio software version 1.0.153 (Boston, MA) gplot package (28,29).
Results
Anti-proliferative effects of Anthos and ExoAnthos on colon cancer cells
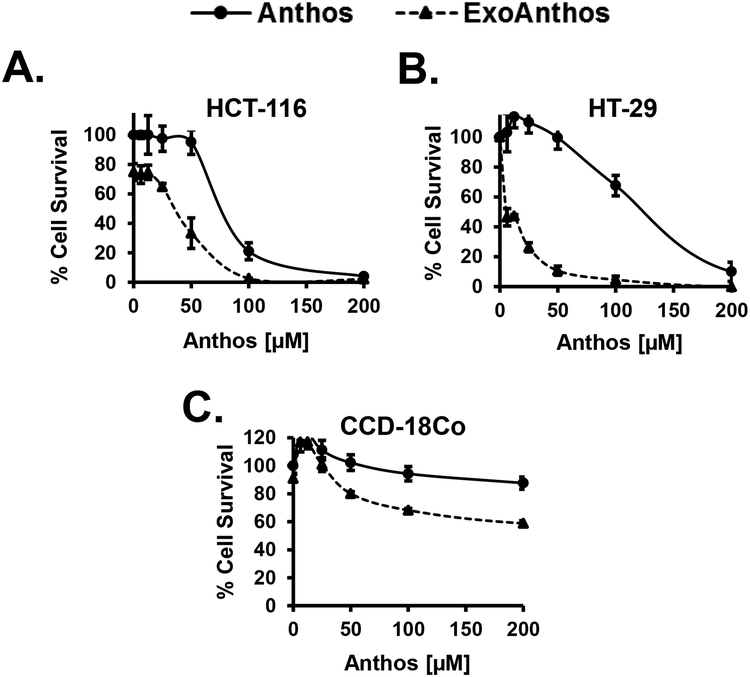
Previous work from our laboratory has shown that exosomal formulation yielded enhanced therapeutic potency and efficacy for therapeutics such as paclitaxel, celastrol, curcumin and Anthos against lung cancer due to increased stability and bioavailability of these compounds (22,30–32). Prior to carrying out our in vivo work, we first compared the relative activity of ExoAnthos and Anthos treatment on proliferation of HCT-116 and HT-29 colon cancer cell lines and CCD-18Co normal colon cells (Figure 2). Results from these studies showed a clear increase in the anti-proliferative properties of Anthos against colon cancer cells, with 4–16-fold decreases in the IC50 values of ExoAnthos as compared to the free Anthos (Figure 2). One can posit that the improved anti-proliferative effects of the ExoAnthos formulation over Anthos alone is most likely due to the increased cell uptake and stability in media of the ExoAnthos over Anthos. Part of the higher efficacy of ExoAnthos may be attributed to the intrinsic effect of the exosomes alone (22,23).
Figure 2. Antiproliferative activity of Anthos and ExoAnthos against colon normal cells and cancer cells in vitro.
Colon normal cells, CCD-18Co, and colon cancer cells, HCT-116 and HT-29 were treated with various concentrations of bilberry-derived Anthos or ExoAnthos for 72 h and the effect on cell growth inhibition was assessed using an MTT assay. Data represent average ± SEM (n=4).
In order to determine whether Anthos and ExoAnthos were selective toward colon cancer over normal colon cells in vitro, we determined the selectivity index (SI) values for both HCT 116 and HT-29 colon cancer cells compared to normal colon CCD-18Co cells. The results (Table 1) showed that not only were Anthos and ExoAnthos selective for colon cancer cells over normal colon cells but that ExoAnthos enhanced this selectivity, with the greatest increase yielded in HT-29 cells, which increased from an SI value of 9 for Anthos to 51 for ExoAnthos. Overall, these results confirm that Anthos and ExoAnthos did not show any significant toxicity for the normal CCD-18Co colon cells and that the cytotoxicity is specific for colon cancer cells.
Table 1: Selectivity Index (SI) values for Anthos and ExoAnthos treatments.
The selectivity index values were calculated by dividing the IC50 value of normal CCD-18Co colon cells by the IC50 value of HCT116 or HT-29 colon cancer cells.
| Cell line | IC50 Anthos (μM) | SI Anthos | IC50 ExoAnthos (μM) | SI ExoAnthos | Fold difference in IC50 values |
|---|---|---|---|---|---|
| CCD-18Co | 1050 | - | 407 | - | - |
| HCT116 | 75 | 14 | 20 | 20 | 4 |
| HT-29 | 124 | 9 | 8 | 51 | 16 |
Impact of Anthos treatment on tumor number in vivo
After the promising results attained in vitro, we next sought to test our ExoAnthos formulation, which makes use of an exosomal nano delivery method to determine if a greater therapeutic effect could be achieved. Results from the comparison of Anthos and ExoAnthos treatment showed that ExoAnthos lead to a similar reduction in colon tumor burden as Anthos alone, when given at the same dose (P=0.30). When compared to control, a significant reduction in colon tumors was noted in the ExoAnthos-treated animals versus exosome vehicle control (P=0.019) and Anthos treated animals versus vehicle control (P=0.0025), when given at 8.6 mg/kg/day (Figure 3). No significant difference was found between the tumor numbers in control vs. exosome alone treated animals (P=0.728).
Figure 3. Anti-tumor activities of Anthos and ExoAnthos against colon tumors.
(A) Study overview, ApcMin/+ mice inoculated with ETBF were treated via oral gavage with Anthos or ExoAnthos at 8.6 mg/kg/day or vehicle controls. (B) Data represent the distribution of animal colon tumor counts, with the average noted. Control ApcMin/+ mice without ETBF bacteria versus ApcMin/+ mice with ETBF bacteria (P=0.0003), Anthos versus control (P=0.0025), control versus exosomes alone-treated animals (P=0.728), ExoAnthos treated animals versus exosomes vehicle control (P=0.019), Anthos versus ExoAnthos (P=0.30). **** signifies P<0.0001, *** signifies P<0.001, ** signifies P<0.01, and * signifies P<0.05.
Impact of ETBF bacteria on phase I/II enzyme expression in colon/liver
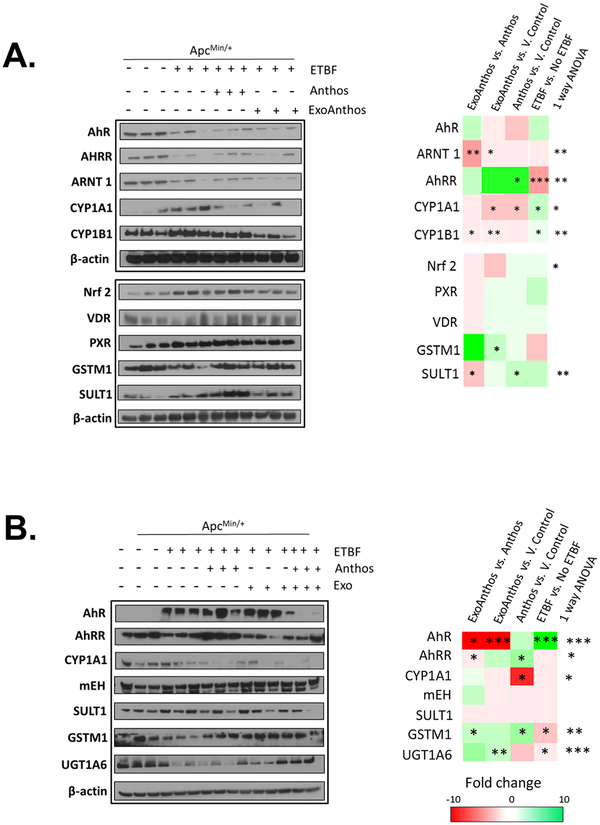
ApcMin/+ mice treated with the bacteria showed significant increases in the expression of phase I enzymes CYP1A1 and CYP1B1 in normal colon tissue (Figure 4A). Additionally, a significant decrease in the expression of the phase II enzyme GSTM1 in normal colon tissue was noted. In order to assess how ETBF bacteria influences the expression of phase I and II enzymes we assessed the expression of AhR, AhRR, ARNT1, Nrf2, VDR, and PXR in ApcMin/+ mice, with and without ETBF treatment. Results from this survey (Figure 4A) showed that mice treated with the bacteria had significant decreases in the expression of the AhR repressor, AhRR. No significant changes in the expression of PXR, Nrf2, and VDR expression were noted in colon tissue samples taken from the mice that received bacteria compared to untreated mice. Liver samples taken from bacteria-treated mice also featured decreased expression of phase II enzymes UGT1A6 and GSTM1 and increased expression of AhR when compared to ApcMin/+ mice that did not receive bacteria treatment (Figure 4B).
Figure 4. In vivo changes in phase I and II enzyme expression and related xenobiotic- sensing nuclear receptors in normal colon and liver tissue following treatment with ETBF bacteria alone, bilberry-derived Anthos or ExoAnthos.
Changes in the expression of key phase I and II enzymes along with key nuclear receptors including CYP1A1, CYP1B1, GSTM1, SULT1, AhR, AhRR, ARNT1, Nrf2, PXR and VDR in normal colon (A) and liver (B) tissue (A) taken from ETBF-inoculated ApcMin/+ mice after treatment with bilberry-derived Anthos, ExoAnthos or vehicle control as assessed using western blot analysis compared to with ApcMin/+ mice that received no bacteria; β-actin served as loading control. Representative heat maps depict fold changes in expression between each group and the corresponding statistical significance. *** signifies P<0.001, ** signifies P<0.01, and * signifies P<0.05.
Impact of Anthos treatment on phase I/II enzyme expression in colon and liver tissues
There have been no prior literature reports to assess whether Anthos modulate phase I and II enzyme expression in the colon or liver tissue in animals treated with ETBF bacteria. Results from our survey of the impact of Anthos and ExoAnthos on key phase I and II enzymes involved in the metabolism of the environmental carcinogen B[a]P as well as other carcinogens, demonstrated significant modulation of the phase I enzymes CYP1A1 and CYP1B1 and phase II enzymes GSTM1 and SULT1 by Anthos and ExoAnthos in normal colon tissue (Figure 4A). Our survey of nuclear transcription factors and associated proteins including AhR, AhRR, ARNT1, Nrf2, VDR, and PXR found that Anthos led to increases in the expression of the AhR repressor AhRR when compared to ETBF alone treated animals. Interestingly, ExoAnthos treatment did not affect the expression of AhRR; however, it led to decreased expression of the aryl hydrocarbon receptor nuclear translocator, ARNT1. No significant changes were noted in the expression of PXR, Nrf2, or VDR in colon samples from Anthos- or ExoAnthos-treated animals. Results from the enzyme expression analysis of liver tissues taken from the same animals noted similar decreases in the expression of CYP1A1 in Anthos-treated mice and increases in the phase II enzymes GSTM1 and UGT1A6 (Figure 4B). No significant changes in expression of mEH or SULT1 were noted in liver tissue. A significant decrease in AhR was noted in ExoAnthos-treated mice and a significant increase in AhRR expression was noted with the Anthos treatment as compared to ETBF ApcMin/+ control mice.
Impact of Anthos treatment on phase I/II enzyme expression in tumor tissue
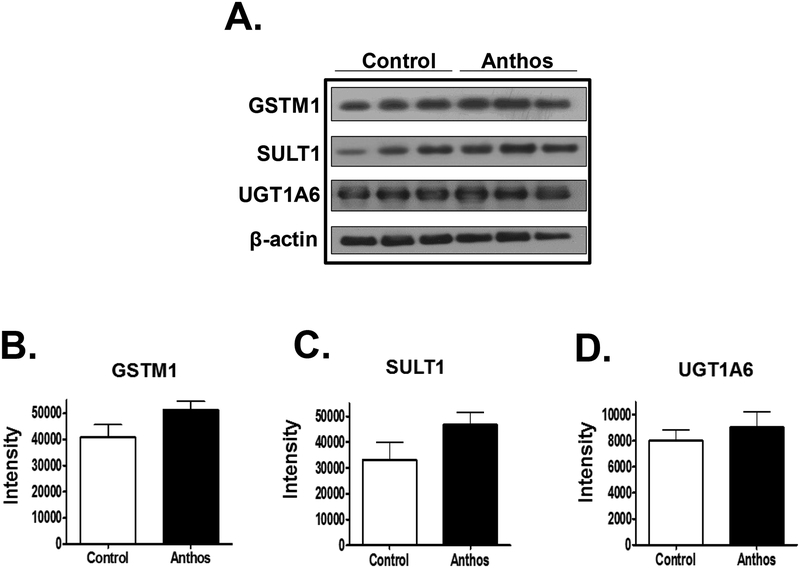
Alterations of phase I and II enzymes have been implicated in the development of chemo-resistance in cancer (33,34). Since modulation of phase I and II enzymes was demonstrated in normal colon and liver tissues, we sought to determine whether additional chemo-resistance could arise for individuals undergoing chemotherapy if they were taking Anthos or whether Anthos could help alleviate offsite toxicity associated with chemotherapeutic drugs if phase II enzymes would be favorably modulated in normal vs. tumor tissue. In order to do this, we assessed the expression of select phase II enzymes in the colon tumor tissue taken from Anthos- and ETBF alone-treated mice showed that Anthos treatment did not result in any significant changes in the expression of UGT1A6 (P>0.5), GSTM1 (P>0.1), or SULT1 (P>0.1) (Figure 5).
Figure 5. Impact of in vivo Anthos treatment on phase II enzyme expression in colon tumor tissue:
changes in the expression of key phase II enzymes including GSTM1 (P>0.1), SULT1 (P>0.1) and UGT1A6 (P>0.5) taken from ETBF-inoculated ApcMin/+ mice after treatment with bilberry-derived Anthos or vehicle control as assessed using western blot analysis compared to β-actin loading control.
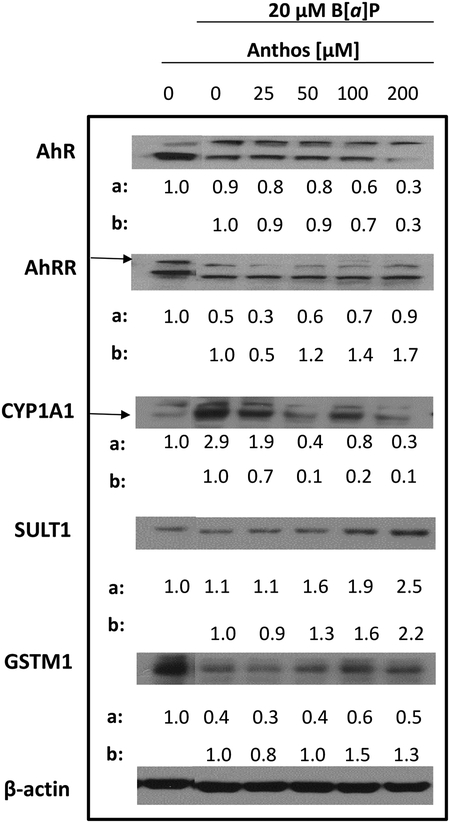
Impact of Anthos treatment on alterations induced by B[a]P treatment in vitro
Alterations in the expression of phase I enzymes had been previously reported by our laboratory in an ACI rat model for breast cancer (18). Given this background, we next sought to determine if Anthos and ExoAnthos treatment would alter this shift in phase I/II enzyme expression induced by B[a]P treatment. Results showed that cells treated overnight with B[a]P (20 μM) featured increased expression of CYP1A1 accompanied by decreased expression of GSTM1 (Figure 6). Furthermore, treatment with Anthos led to decreases in the expression of CYP1A1 while increasing the phase II enzymes GSTM1 and SULT1. Anthos treatment, at the highest dose tested, led to approximately 3-fold reductions in the expression of a key transcription factor AhR, which is involved in the expression of CYP1A1, in HCT-116 colon cancer cells. Furthermore, Anthos treatment simultaneously led to a nearly 2-fold increase in the expression of the AhR repressor, AhRR. Anthos treatment also led to 2- to 10-fold increase in the expression of a key nuclear receptor, PXR.
Figure 6. Changes in expression of key phase I and II enzymes and related nuclear receptors following treatment with B[a]P versus B[a]P and Anthos.
Colon cancer cell line HCT-116 was pre-treated with various concentrations of Anthos for 24 h followed by co-treatment with B[a]P and Anthos for 24 h and the effect on the expression of AhR, AhRR, CYP1A1, SULT1, and GSTM1 was assessed using western blot analysis and compared to β-actin loading control. Densitometry values listed are the ratio of each dose to the vehicle control (a) and B[a]P control (b), corrected for β-actin loading control. Treatment and controls were run on the same gels.
Discussion
Given the recent uptick in cases of CRC diagnosed in younger individuals (1), CRC appears to be making a comeback and thus warrants research into identifying potential chemopreventive methods to combat this disease. Although much research has been conducted on the role of carcinogens such as B[a]P on the development of colorectal cancer, little has been achieved in the successful prevention of this disease in populations who are exposed to such environmental carcinogens on a daily basis. It should be noted that many studies have been conducted on a variety of plant-derived chemopreventive agents but few agents have successfully been translated. For instance, anthocyanins have been attributed to a variety of health-promoting benefits including chemopreventive and therapeutic effects due to their roles as anti-inflammatory and antioxidant agents (14,15). However, no studies investigating the impact of Anthos on the balance of phase I/II enzymes in the colon have been reported. Furthermore, the impact of bacteria such as ETBF on this balance in the colon and liver has also yet to be elucidated. Given the lack of understanding in these areas, we sought to uncover the impact of ETBF bacteria, Anthos and B[a]P on this crucial enzymatic balance.
Several recent studies have confirmed the link between dysbiosis of the gut microbiome and colorectal cancer. Several bacteria, in particular, have been associated with increased risk of developing CRC including S. gallolyticus, H. pylori, virulent forms of Escherichia coli (E. coli), Fusobacterium nucleatum (F. nucleatum), Salmonella enterica (S. enterica) and enterotoxigenic B. fragilis (9). Although much research has been conducted on the role of bacteria on inflammation and carcinogenesis (10,35,36), no reports have been published regarding the role of ETBF on the expression of phase I and II enzymes. Results from our assessment of the impact that ETBF has on the expression of phase I and II enzymes in ApcMin/+ mice showed that treatment with the bacteria led to significant increases in the expression of phase I enzymes CYP1A1 and CYP1B1 in normal colon tissue. Furthermore, significant increases in the expression of AhR and significant decreases in the expression of phase II enzymes GSTM1 and UGT1A6 were noted in liver tissue samples taken from ETBF mice when compared to mice that did not receive the bacteria. The results gathered in this study provide an additional link between how “bad” bacteria such as ETBF can ultimately contribute to the development of cancer beyond the initially elucidated inflammatory and gut barrier breakdown pathways (13,35).
After elucidating how ETBF bacteria increases the expression of phase I enzymes while decreasing the expression of the phase II enzymes in normal colon and liver tissue, we next determined if Anthos or ExoAnthos treatment could alter this enzyme imbalance. Previous work from our laboratory has shown that Anthos treatment led to decreased expression and activity of CYP1A1 and CYP1A2 in an estrogen-driven ACI rat model for breast cancer (18). Results from this series of studies showed that Anthos treatment significantly decreased the expression of phase I enzymes CYP1A1 and CYP1B1, while increasing the expression of the phase II enzymes GSTM1 and SULT1. Our survey of AhR, ARNT1 and AhRR expression suggest that the modulation of phase I and II enzymes could be attributable to the altered expression of AhRR and ARNT1 induced by Anthos treatment. It should be noted that AhRR is a key protein in the AhR signaling cascade that acts as a repressor of AhR-dependent gene expression. Structural work shows that AhRR acts by competitively repressing AhR binding to ARNT and target DNA (37). Furthermore, AhRR levels have been shown to decline in a variety of disease states ranging from rheumatoid arthritis (38) to lung cancer (39). Interestingly, DNA methylation at AhRR has also been shown to be a marker for smoking and was correlated with future smoking morbidity and mortality (40). As noted above, ARNT1 is also a key component of the AhR signaling cascade and functions by binding to the ligand-bound form of AhR and aiding in the movement of the AhR complex to the nucleus. ARNT has also been shown to be upregulated under hypoxic conditions by a HIF-1α dependent mechanism in Hep3B cells (41).
Phase II enzymes such as UGT1A6, SULT1, and GSTM1 play an important role in the breakdown of chemotherapeutic drugs such as irinotecan and cisplatin (42,43). Therefore, increased expression of phase II enzymes in target tumor tissue would not be desirable. Importantly results from this study showed that no significant changes in the expression of phase II enzymes UGT1A6, SULT1 or GSTM1 occurred in colon tumor tissue taken from animals treated with Anthos. Therefore, potential negative effects due to increased breakdown of chemotherapeutic drugs that may result from increased phase II enzyme expression, as evolves in many tumors, should not be a cause for concern with Anthos treatment. With this in mind, the selective increase in phase II enzyme expression in normal tissue over tumor tissue may actually be an advantage in decreasing off-target toxicity to healthy tissue for drugs such as irinotecan (42). However, additional studies would be needed to confirm this hypothesis.
Exposure to environmental pollutants is now considered to be one of the reasons behind the increasing rates of individuals with disorders ranging from obesity and type 2 diabetes to cancer (4,6). Up to 90% of CRC cases are of sporadic origin and it is estimated that diet contributes to 80% of known cases of CRC. The role of chemicals that contaminate food and ultimately contribute to the development of CRC has been of great interest (6). The PAH, B[a]P is of special relevance due to its presence in a variety of common sources of exposure ranging from charcoal-cooked food to cigarette smoke as well as several environmental sources and importantly, its epidemiological correlation with increased risk of CRC (44,45). Similar to the alterations in phase I and II metabolism found to exist in vivo following ETBF inoculation, we found that cells treated with B[a]P (20 μM) featured increased expression of the phase I enzyme CYP1A1 with decreased expression of the phase II enzyme GSTM1 as well as the AhR repressor, AhRR. These results suggest that dysbiosis of the gut microbiome and exposure to the environmental carcinogen B[a]P both lead to dysfunction of the balance between phase I and II enzymes in colon tissue. Furthermore, treatment with Anthos effectively shifted this balance in expression levels of AhRR, AhR, PXR, CYP1A1, and SULT1 to greater favor a state of detoxification in B[a]P treated cells. It should be noted that the beneficial effects attributed to the consumption of Anthos in this study may vary from person to person depending upon the presence of genetic polymorphisms of these enzymes. However, since the expression of several enzymes were shown to be modulated by these compounds, individuals may still benefit overall by way of alternative enzymes.
Although our initial hypothesis was that ExoAnthos would enhance therapeutic efficacy over Anthos, the findings presented in this manuscript showed no significant difference in tumor numbers between the Anthos and ExoAnthos treated animals. Ultimately, both Anthos and ExoAnthos yielded similarly significant decreases in tumor numbers compared with vehicle treatments, respectively. This lack of enhanced therapeutic efficacy could perhaps be due to higher absorption of ExoAnthos prior to reaching the gut and enhanced delivery to distant sites (31). Further studies would be needed to further elucidate this notion.
Overarching results from this series of studies stress the importance of integrating the gut microbiome into the study of carcinogen metabolism and carcinogenesis. With the ever omnipresent threat and buildup of carcinogens within the industrialized environment and the resulting inevitable daily exposure of the colon to these compounds, a more integrated approach to the prevention of cancer is needed. Future studies assessing potential synergy that may arise when dysbiosis of the gut microbiome is combined with exposure to environmental carcinogens such as B[a]P could lead to a better understanding and a potential explanation for the current upward tick in colorectal cancer cases.
Acknowledgments:
This work was supported by Agnes Brown Duggan Endowment, Helmsley Trust Fund, US National Institutes of Health [grant numbers AI092133 and CA100656], and the National Institute of Environmental Sciences [grant number USPHS T32-ES011564]. A Mudd was supported by the IPIBS fellowship and the NIEHS training grant. The authors thank Dr. Farrukh Aqil and Dr. Manicka Vadhanam for useful discussion during the course of the work. We also thank Dr. Wendy Spencer of 3P Biotechnologies, Inc. (Louisville, KY) for generously providing highly enriched berry Anthos as well as colostrum-derived exosomes used in our studies.
Abbreviations
- B[a]P
Benzo[a]pyrene
- CRC
colorectal cancer
- PAHs
polycyclic aromatic hydrocarbons, Anthos, native anthocyanidins mixture from billberry
- CYP
cytochrome P450
- mEH
microsomal epoxide hydrolase
- BPDE
benzo[a]pyrene-7,8-diol-9,10-epoxide
- GST
glutathione s-transferase
- UGT
uridine 5’-diphospho-glucuronosyltransferase
- SULT
sulfotransferase
- AhR
aryl hydrocarbon receptor
- AhRR
aryl hydrocarbon receptor repressor
- PXR
pregnane X receptor
- UDPGT
Glucuronosyltransferase
- APC
adenomatous polyposis coli
- COX-2
cyclo-oxygenase-2
- Cy
cyanidin
- Dp
delphinidin
- Pt
petunidin
- Mv
malvidin
- Pe
peonidin
- HPLC
high performance liquid chromatography
- PDA
photodiode array detector
- UV
ultraviolet
- ETBF
enterotoxigenic Bacteriodes fragilis
- BFT
B. fragilis toxin
Footnotes
Conflict of interest disclosure: The authors declare no potential conflicts of interest.
References:
- 1.Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS, et al. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. Journal of the National Cancer Institute 2017;109(8) doi 10.1093/jnci/djw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shivappa N, Zucchetto A, Montella M, Serraino D, Steck SE, La Vecchia C, et al. Inflammatory potential of diet and risk of colorectal cancer: a case-control study from Italy. The British journal of nutrition 2015;114(1):152–8 doi 10.1017/s0007114515001828. [DOI] [PubMed] [Google Scholar]
- 3.Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? 2017;3:17001 doi 10.1038/npjbiofilms.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beamish LA, Osornio-Vargas AR, Wine E. Air pollution: An environmental factor contributing to intestinal disease. Journal of Crohn’s & colitis 2011;5(4):279–86 doi 10.1016/j.crohns.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Wei P-L, Lin S-Y, Chang Y-J. Cigarette Smoking and Colorectal Cancer: From Epidemiology to Bench. Journal of Experimental & Clinical Medicine 2011;3(6):257–61 doi 10.1016/j.jecm.2011.10.002. [DOI] [Google Scholar]
- 6.Diggs DL, Huderson AC, Harris KL, Myers JN, Banks LD, Rekhadevi PV, et al. Polycyclic aromatic hydrocarbons and digestive tract cancers: a perspective. Journal of environmental science and health Part C, Environmental carcinogenesis & ecotoxicology reviews 2011;29(4):324–57 doi 10.1080/10590501.2011.629974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene 2002;21(48):7435–51 doi 10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]
- 8.Clark RS, Pellom ST, Booker B, Ramesh A, Zhang T, Shanker A, et al. Validation of research trajectory 1 of an Exposome framework: Exposure to benzo(a)pyrene confers enhanced susceptibility to bacterial infection. Environmental research 2016;146:173–84 doi 10.1016/j.envres.2015.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun J, Kato I. Gut microbiota, inflammation and colorectal cancer. Genes & diseases 2016;3(2):130–43 doi 10.1016/j.gendis.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dejea CM, Fathi P, Craig JM, Boleij A, Taddese R, Geis AL, et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science (New York, NY) 2018;359(6375):592–7 doi 10.1126/science.aah3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang G, Svenungsson B, Karnell A, Weintraub A. Prevalence of enterotoxigenic Bacteroides fragilis in adult patients with diarrhea and healthy controls. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 1999;29(3):590–4. [DOI] [PubMed] [Google Scholar]
- 12.Rhee K-J, Wu S, Wu X, Huso DL, Karim B, Franco AA, et al. Induction of Persistent Colitis by a Human Commensal, Enterotoxigenic Bacteroides fragilis, in Wild-Type C57BL/6 Mice. Infection and immunity 2009;77(4):1708–18 doi 10.1128/IAI.00814-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu S, Powell J, Mathioudakis N, Kane S, Fernandez E, Sears CL. Bacteroides fragilis enterotoxin induces intestinal epithelial cell secretion of interleukin-8 through mitogen-activated protein kinases and a tyrosine kinase-regulated nuclear factor-kappaB pathway. Infection and immunity 2004;72(10):5832–9 doi 10.1128/iai.72.10.5832-5839.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Wang L, Wu Z, Yao L, Wu Y, Huang L, et al. Anthocyanin-rich fractions from red raspberries attenuate inflammation in both RAW264.7 macrophages and a mouse model of colitis. Scientific reports 2014;4:6234 doi 10.1038/srep06234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He J, Giusti MM. Anthocyanins: natural colorants with health-promoting properties. Annual review of food science and technology 2010;1:163–87 doi 10.1146/annurev.food.080708.100754. [DOI] [PubMed] [Google Scholar]
- 16.Aqil F, Jeyabalan J, Kausar H, Munagala R, Singh IP, Gupta R. Lung cancer inhibitory activity of dietary berries and berry polyphenolics. Journal of Berry Research 2016;6(2):105–14 doi 10.3233/JBR-160120. [DOI] [Google Scholar]
- 17.Casto BC, Kresty LA, Kraly CL, Pearl DK, Knobloch TJ, Schut HA, et al. Chemoprevention of oral cancer by black raspberries. Anticancer research 2002;22(6c):4005–15. [PubMed] [Google Scholar]
- 18.Aiyer HS, Gupta RC. Berries and ellagic acid prevent estrogen-induced mammary tumorigenesis by modulating enzymes of estrogen metabolism. Cancer prevention research (Philadelphia, Pa) 2010;3(6):727–37 doi 10.1158/1940-6207.capr-09-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell GK, Gupta RC, Vadhanam MV. Effect of phytochemical intervention on dibenzo[a,l]pyrene-induced DNA adduct formation. Mutat Res 2015;774:25–32 doi 10.1016/j.mrfmmm.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomasset S, Berry DP, Cai H, West K, Marczylo TH, Marsden D, et al. Pilot study of oral anthocyanins for colorectal cancer chemoprevention. Cancer prevention research (Philadelphia, Pa) 2009;2(7):625–33 doi 10.1158/1940-6207.capr-08-0201. [DOI] [PubMed] [Google Scholar]
- 21.Lala G, Malik M, Zhao C, He J, Kwon Y, Giusti MM, et al. Anthocyanin-rich extracts inhibit multiple biomarkers of colon cancer in rats. Nutrition and cancer 2006;54(1):84–93 doi 10.1207/s15327914nc5401_10. [DOI] [PubMed] [Google Scholar]
- 22.Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett 2016;371(1):48–61 doi 10.1016/j.canlet.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munagala R, Aqil F, Jeyabalan J, Agrawal AK, Mudd AM, Kyakulaga AH, et al. Exosomal formulation of anthocyanidins against multiple cancer types. Cancer Lett 2017;393:94–102 doi 10.1016/j.canlet.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Meth 2012;9(7):671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu T, De Jesus M, Gallagher HC, Burris TP, Egilmez NK. Oral IL-10 suppresses colon carcinogenesis via elimination of pathogenicCD4+ T-cells and induction of antitumor CD8+ T-cell activity. OncoImmunology 2017:e1319027 doi 10.1080/2162402X.2017.1319027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corona G, Tang F, Vauzour D, Rodriguez-Mateos A, Spencer J. Assessment of the anthocyanidin content of common fruits and development of a test diet rich in a range of anthocyanins. Journal of Berry Research 2011;1:45–52 doi 10.3233/JBR-2011-022. [DOI] [Google Scholar]
- 27.Warnes G, Bolker B, Lumley T. gplots: Various R programming tools for plotting data. R package version 2.6.0. [Google Scholar]
- 28.Team R. RStudio: Integrated Development for R. Boston, MA: RStudio, Inc.; 2016. [Google Scholar]
- 29.Sarkar D. Lattice: multivariate data visualization with R. Springer Science & Business Media; 2008. [Google Scholar]
- 30.Aqil F, Kausar H, Agrawal AK, Jeyabalan J, Kyakulaga AH, Munagala R, et al. Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Experimental and molecular pathology 2016;101(1):12–21 doi 10.1016/j.yexmp.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Aqil F, Jeyabalan J, Agrawal AK, Kyakulaga AH, Munagala R, Parker L, et al. Exosomal delivery of berry anthocyanidins for the management of ovarian cancer. Food & function 2017;8(11):4100–7 doi 10.1039/c7fo00882a. [DOI] [PubMed] [Google Scholar]
- 32.Agrawal AK, Aqil F, Jeyabalan J, Spencer WA, Beck J, Gachuki BW, et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine: nanotechnology, biology, and medicine 2017. doi 10.1016/j.nano.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, et al. Drug Resistance in Cancer: An Overview. Cancers 2014;6(3):1769–92 doi 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Advanced Pharmaceutical Bulletin 2017;7(3):339–48 doi 10.15171/apb.2017.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung L, Thiele Orberg E, Geis AL, Chan JL, Fu K, DeStefano Shields CE, et al. Bacteroides fragilis Toxin Coordinates a Pro-carcinogenic Inflammatory Cascade via Targeting of Colonic Epithelial Cells. Cell host & microbe 2018;23(2):203–14.e5 doi 10.1016/j.chom.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med 2009;15(9):1016–22 doi 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakurai S, Shimizu T, Ohto U. The crystal structure of the AhRR-ARNT heterodimer reveals the structural basis of the repression of AhR-mediated transcription. The Journal of biological chemistry 2017;292(43):17609–16 doi 10.1074/jbc.M117.812974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng L, Qian L, Wang GS, Li XM, Li XP. Genetic association of aromatic hydrocarbon receptor and its repressor gene polymorphisms with risk of rheumatoid arthritis in Han Chinese populations. Medicine 2017;96(15):e6392 doi 10.1097/md.0000000000006392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Elgizouli M, Schottker B, Holleczek B, Nieters A, Brenner H. Smoking-associated DNA methylation markers predict lung cancer incidence. Clinical epigenetics 2016;8:127 doi 10.1186/s13148-016-0292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bojesen SE, Timpson N, Relton C, Davey Smith G, Nordestgaard BG. AHRR (cg05575921) hypomethylation marks smoking behaviour, morbidity and mortality. Thorax 2017;72(7):646–53 doi 10.1136/thoraxjnl-2016-208789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandl M, Lieberum MK, Depping R. A HIF-1alpha-driven feed-forward loop augments HIF signalling in Hep3B cells by upregulation of ARNT. Cell death & disease 2016;7(6):e2284 doi 10.1038/cddis.2016.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen S, Yueh MF, Bigo C, Barbier O, Wang K, Karin M, et al. Intestinal glucuronidation protects against chemotherapy-induced toxicity by irinotecan (CPT-11). Proceedings of the National Academy of Sciences of the United States of America 2013;110(47):19143–8 doi 10.1073/pnas.1319123110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rose MC, Kostyanovskaya E, Huang RS. Pharmacogenomics of Cisplatin Sensitivity in Non-small Cell Lung Cancer. Genomics, Proteomics & Bioinformatics 2014;12(5):198–209 doi 10.1016/j.gpb.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kazerouni N, Sinha R, Hsu C-H, Greenberg A, Rothman N. Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food and Chemical Toxicology 2001;39(5):423–36 doi 10.1016/S0278-6915(00)00158-7. [DOI] [PubMed] [Google Scholar]
- 45.Sinha R, Kulldorff M, Gunter MJ, Strickland P, Rothman N. Dietary benzo[a]pyrene intake and risk of colorectal adenoma. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2005;14(8):2030–4 doi 10.1158/1055-9965.epi-04-0854. [DOI] [PubMed] [Google Scholar]