Abstract
Naturally occurring smallpox has been eradicated but research stocks of variola virus (VARV), the causative agent of smallpox, still exist in secure laboratories. Clandestine stores of the virus or resurrection of VARV via synthetic biology are possible and have led to concerns that VARV could be used as a biological weapon. The US government has prepared for such an event by stockpiling smallpox vaccines and TPOXX®, SIGA Technologies’ smallpox antiviral drug. While vaccination is effective as a pre-exposure prophylaxis, protection is limited when administered following exposure. Safety concerns preclude general use of the vaccine unless there is a smallpox outbreak. TPOXX is approved by the FDA for use after confirmed diagnosis of smallpox disease. Tecovirimat, the active pharmaceutical ingredient in TPOXX, targets a highly conserved orthopoxviral protein, inhibiting long-range dissemination of virus. Although indications for use of the vaccine and TPOXX do not overlap, concomitant use is possible, especially if the TPOXX indication is expanded to include post-exposure prophylaxis. It is therefore important to understand how vaccine and TPOXX may interact. In studies presented here, monkeys were vaccinated with the ACAM2000TM live attenuated smallpox vaccine and concomitantly treated with tecovirimat or placebo. Immune responses to the vaccine and protective efficacy versus a lethal monkeypox virus (MPXV) challenge were evaluated. In two studies, primary and anamnestic humoral immune responses were similar regardless of tecovirimat treatment while the third study showed reduction in vaccine elicited humoral immunity. Following lethal MPXV challenge, all (12 of 12) vaccinated/placebo treated animals survived, and 12 of 13 vaccinated/tecovirimat treated animals survived. Clinical signs of disease were elevated in tecovirimat treated animals compared to placebo treated animals. This suggests that TPOXX may affect the immunogenicity of ACAM2000 if administered concomitantly. These studies may inform on how vaccine and TPOXX are used during a smallpox outbreak.
Keywords: Smallpox, TPOXX®, ACAM2000, Biodefense, Tecovirimat, ST-246®, Monkeypox virus, Vaccinia, Variola, Antiviral, Animal Rule
1. Introduction
Smallpox was one of the most devastating diseases of human history [1] and is estimated to have killed 300 million people in the 20th century alone [2], [3]. Naturally occurring smallpox has not been documented since 1977 [4] and the last known cases were the result of a laboratory accident in 1978 [5]. Smallpox is caused by variola virus (VARV). Nearly all susceptible individuals will contract disease if exposed and, historically, 30% or more of unvaccinated individuals succumb to disease [6]. Although smallpox was officially declared eradicated worldwide and immunization was discontinued in 1980 [7], potential for use of VARV for biowarfare or bioterrorism has renewed interest in smallpox antiviral drugs [6]. Monkeypox in humans, which resembles smallpox, is a less virulent emerging zoonosis caused by monkeypox virus (MPXV) [8], [9]. The deliberate release of either pathogen in an attack is considered possible by the Centers for Disease Control and Prevention (CDC), and VARV is considered a material threat to national security by the Department of Homeland Security [10].
TPOXX oral capsule formulation is the only drug currently approved by the FDA for smallpox treatment. TPOXX is the proprietary name of tecovirimat, a small molecule antiviral drug with activity against multiple orthopoxvirus species [11], [12]. Tecovirimat inhibits the VARV VP37 protein, and its homologs in other orthopoxviruses [13], that is required for formation and release of enveloped virions, which are associated with increased virulence of orthopoxviruses [14], [15].
In pre-clinical studies tecovirimat was highly protective against lethal challenge with vaccinia virus (VACV), ectromelia virus, and cowpox virus in mice [16], MPXV [17] and VARV [18] in nonhuman primates (NHP), and rabbitpox virus in rabbits [19]. Tecovirimat provided protection from disease when treatment was initiated up to 72 h post-infection in small animals [13], [16], [19], five days post-infection in NHPs challenged intravenously [17], and up to eight days following aerosol challenge in NHPs [20]. In all cases, 100% survival was observed when tecovirimat administration was initiated prior to the appearance of disease signs.
The vaccines used to eradicate smallpox were live vaccines based on isolates of VACV, which is closely related to VARV [21], [22]. The smallpox vaccine ACAM2000 TM (Emergent Biosolutions) is a plaque-purified product derived from Dryvax® (Wyeth) and manufactured using modern cell culture technology [22], [23]. Severe adverse events have been observed with ACAM2000 similar to the parental vaccine [24]. Following eradication of smallpox, vaccination of the general public was discontinued due to safety concerns [25], resulting in an growing numbers of naive individuals vulnerable to an accidental or intentional release of smallpox, monkeypox, or engineered orthopoxvirus variants.
In the event of a smallpox outbreak individuals with known or suspected exposure to VARV will be vaccinated with a second generation vaccine, such as ACAM2000 [26]. Patients with confirmed smallpox diagnosis will be treated with TPOXX. Post-exposure prophylaxis using smallpox vaccine is estimated to be 80–93% effective in preventing disease if administered within three days of exposure [27], and rapidly loses efficacy becoming ineffective after the appearance of clinical signs of smallpox [28], [29]. Therefore, it is likely that those with known or suspected exposure to VARV who have received the vaccine may also receive prophylactic TPOXX treatment, making it essential to understand how TPOXX interacts with the vaccine. Considering that tecovirimat interferes with orthopoxvirus maturation and release from infected cells, co-administration of TPOXX with live vaccines may alleviate adverse events but may also affect vaccine efficacy.
Human testing with either VARV or MPXV would be unethical, therefore studies of tecovirimat effects on ACAM2000 efficacy must be performed in “well-characterized” animal models accepted by the Food and Drug Administration as adequate for the evaluation of smallpox antivirals [30]. The natural route of transmission for human smallpox is by aerosol, and it would be preferable to utilize an animal model in which viral challenge is by the more natural aerosol route. Unfortunately, the aerosol challenge model in NHPs does not mimic human smallpox to the extent that it could be used to evaluate smallpox antivirals [20]. VARV IV challenge in NHPs is a poor model of human smallpox also, while NHPs challenged with MPXV by the IV route closely mimic the lesional stage of human smallpox disease and provide more consistent outcomes than the other models [31].
The models in which tecovirimat has been tested are designed to mimic lethal exposure of naive individuals to VARV and subsequent therapeutic treatment with TPOXX. In this report, these models are used to evaluate the effect of tecovirimat treatment on vaccine following ACAM2000 vaccination in NHPs with respect to vaccine “take”, potential vaccine adverse events, and on vaccine-induced protection from subsequent lethal MPXV challenge.
2. Materials and methods
2.1. Study locations
The studies described here are designated Study 1, 2, and 3. Study designs are shown in Table 1 . Study 1 (original study #11051.12, Part #2, Study Director: Peter Silvera) and Study 3 (original study #SR11-011F, Study Director: Jon Prigge) were conducted at Southern Research Institute, Frederick, MD. Study 2 (original study #1218-100004544, Study Director: Catherine Bigger) was conducted at Battelle Biomedical Research Center, West Jefferson, OH.
Table 1.
Study Design Information.
| Study ID | Study Group Assignment | Vaccination Day | MPXV Challenge Day | MPXV Target Challenge Dose (PFU, IV) |
|---|---|---|---|---|
| Study 1 | Controls | 0 | 45 | 5.0E + 07 |
| ACAM2000 + Placebo | ||||
| ACAM2000 + Tecovirimat | ||||
| Study 2 | ACAM2000 + Placebo | 0 | 30 | 5.0E + 07 |
| ACAM2000 + Tecovirimat | ||||
| Study 3 | ACAM2000 + Placebo | 0 | 32 | 5.0E + 07 |
| ACAM2000 + Tecovirimat | ||||
2.2. Biosafety
All laboratory and animal work with ACAM2000 and tecovirimat was performed in Biosafety Level-2 facilities or higher. Prior to work with MPXV, animals were transferred to Biosafety Level-3 (BSL-3), where they were housed for the remainder of each study. All laboratory work with MPXV was performed in a BSL-3 laboratory.
2.3. Animals
Each study protocol was approved by the respective Institutional Animal Care and Use Committee. Treatment of animals adhered to U.S. Government “Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Education”, the Guide for the Care and Use of Laboratory Animals [32], the Animal Welfare Act, and other applicable public laws and regulations. Prior to placement on study all macaques were determined to be free of pre-existing antibodies against poxviruses.
Study 1: Sixteen cynomolgus macaques, (8 females and 8 males between 2 and 8 years of age) were acquired from NIH Animal Center (Poolesville, MD). Animal weights on Day 0 post-challenge ranged from 2.55 to 8.08 kg, with a median weight of 4.54 kg. Tests for pathogenic viral and parasitic infections performed during quarantine were negative.
Study 2: Six cynomolgus macaques (3 females and 3 males) weighing ≥2 kg were acquired from Covance Research Products (Princeton, NJ). Age was not reported for this study. Tests for pathogenic viral and parasitic infections performed during quarantine were negative.
Study 3: Six Indian rhesus macaques (3 males and 3 females), aged between 2.26 and 4.75 years were obtained from Primate Products, Inc. (Immokalee, FL). Macaque weights ranged from 3.50 to 7.72 kg at study start. Tests for pathogenic viral and parasitic infections performed during quarantine were negative.
All animals were confirmed to be in good health prior to placement on their respective studies and were randomized into study groups based on weight and sex using SAS or comparable software tool.
2.4. ACAM2000 administration
ACAM2000 and diluent were provided by the CDC (Atlanta, GA). Lot #VV04-003-A (ACAM2000) and Lot # DV01C01 (Diluent) were used in studies 1 and 3. The Study 2 report did not include this information. The vaccine was stored at 2–8 °C, reconstituted with diluent, and held at 2–8 °C until used. Each vial of diluent contained 0.6 mL of 50% (v/v) Glycerin USP, 0.25% (v/v) phenol USP in water for injection USP. The concentration of VACV in the reconstituted suspension was 1.0–5.0 × 108 PFU/mL (2.5–12.5 × 105 PFU/dose). During the vaccination procedure reconstituted vaccine was held at room temperature. Monkeys in Studies 1–3 were anesthetized with Telazol (1–6 mg/kg) prior to administration of either diluent or ACAM2000 by percutaneous scarification. In any specific study, all NHPs were vaccinated on the same date. The skin between the shoulder blades was shaved, cleaned with alcohol, and allowed to dry completely prior to scarification. ACAM2000 or diluent were administered in a volume of 0.0025 mL to the intrascapular region using 15 punctures with bifurcated needles.
2.5. Vaccination site reaction
Following vaccination, the vaccination site was checked for development of the characteristic lesion. Vaccine lesion area was determined by cross-sectional measurement and reported as cm2.
2.6. MPXV and intravenous challenge
Prior to challenge, all animals were anesthetized with Ketamine HCL, 10–30 mg/kg IM. Monkeys were challenged with a dose between 1.65x107 and 5.4x107 PFU of MPXV (Zaire 79 strain V79-I-005, BEI Resources, Manassas VA) via the saphenous vein. Virus stock was thawed in a 37 °C water bath, sonicated, then diluted to 5x107 PFU/mL for challenge in Dulbecco's Modified Eagle's Media (DMEM) supplemented with 4 mM L-glutamine and 2% Fetal Bovine Serum (FBS) in Studies 1 and 3, and a HEPES buffer in Study 2.
2.7. Tecovirimat and placebo administration
Tecovirimat was formulated as a 2 mg/mL liquid suspension in vehicle (1% (w/v) hydroxypropyl methylcellulose with 0.5% (w/v) Tween® 80 in sterile water for injection). The vehicle without tecovirimat was used as the placebo. For dosing, animals were anesthetized with Telazol (1–6 mg/kg). Animals were administered either 10 mg/kg tecovirimat or an equal volume/weight dose of placebo by oral gavage, followed by 5 ± 0.5 mL/kg of a 30% suspension of hydrated homogenized monkey biscuits. All animals on all studies included here received placebo or tecovirimat for 14 consecutive days (interval 24 ± 2 h) starting on the day of vaccination, following the vaccination procedure (including sham vaccinated animals).
2.8. Clinical observations and sample collection
Monkeys were observed twice daily throughout the quarantine and study periods for signs of moribundity and mortality. Detailed observations were performed at least once daily. Animals on Study 1 and 3 were provided supplemental hydration (subcutaneous Lactated Ringers) if necessary throughout the study period. Following MPXV challenge, total body lesions were counted on the first day lesions were observed and then at regular intervals, generally every third day from Day 3 post-challenge until animals succumbed to disease or fully resolved their lesions. Lesion counts were performed on animals euthanized at an unscheduled interval or found dead. When lesions were too numerous to count (TNTC), the count was estimated.
2.9. DNA isolation and quantitative Real-time PCR
Blood samples were collected in potassium EDTA tubes from each monkey at regular intervals throughout each study (Table 2, Table 3 ) and at time of unscheduled euthanasia. DNA was extracted from fresh or frozen blood samples using the QIAGEN QIAamp DNA mini kit (Valencia, CA) (Study 1 and 3) or the bioMérieux NUCLISENS easyMAG kit (Durham, NC) (Study 2). Viral loads were measured using a real-time quantitative PCR method for detection of MPXV genomes in peripheral blood adapted from Kulesh, et al [33].
Table 2.
Blood collection relative to vaccination on Day 0.
| Study Day: | −3 | −2 | 0 | 7 | 14 | 28 | 30 | 42 | 45 |
|---|---|---|---|---|---|---|---|---|---|
| PRNT | |||||||||
| Study 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Study 2 | ✓ | ✓ | ✓ | ✓ | |||||
| Study 3 | ✓ | ✓ | ✓ | ✓ | |||||
| ELISA | |||||||||
| Study 3 | ✓ | ✓ | ✓ | ✓ | |||||
Table 3.
Post-challenge blood collection.
| Study Day: | 0* | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 28 | 30 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRNT | ||||||||||||
| Study 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Study 2 | ✓ | ✓ | ||||||||||
| Study 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Viral Load (qPCR) | ||||||||||||
| Study 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Study 2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Study 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
Monkeypox virus (Zaire strain V79-I-005; NR-2324); I.V., 1 mL, 5x107 PFU/ml
*Day of MPXV challenge.
2.10. Plaque reduction neutralizing titer (PRNT) and ELISA assays
For measurement of humoral immune responses, blood samples were collected at regular intervals in serum separator tubes during the vaccination and challenge phases (Table 2, Table 3) and at the time of scheduled or unscheduled euthanasia, if possible.
Samples from Study 3 were analyzed using a VACV-specific ELISA to measure antibody responses during the vaccination and challenge phases. Positive and negative control sera were included on each plate.
A PRNT assay was used to evaluate the virus neutralizing antibody response during the vaccination and challenge phases for all three studies. Naive serum and immune serum from infected animals served as a negative and positive control, respectively. Using point-to-point linear regression analysis, neutralization endpoint titers were calculated based on the reciprocal dilution of the test serum that produced 50% plaque reduction (PRNT50) compared to the virus control.
3. Results
3.1. Effect of tecovirimat on vaccination site lesion
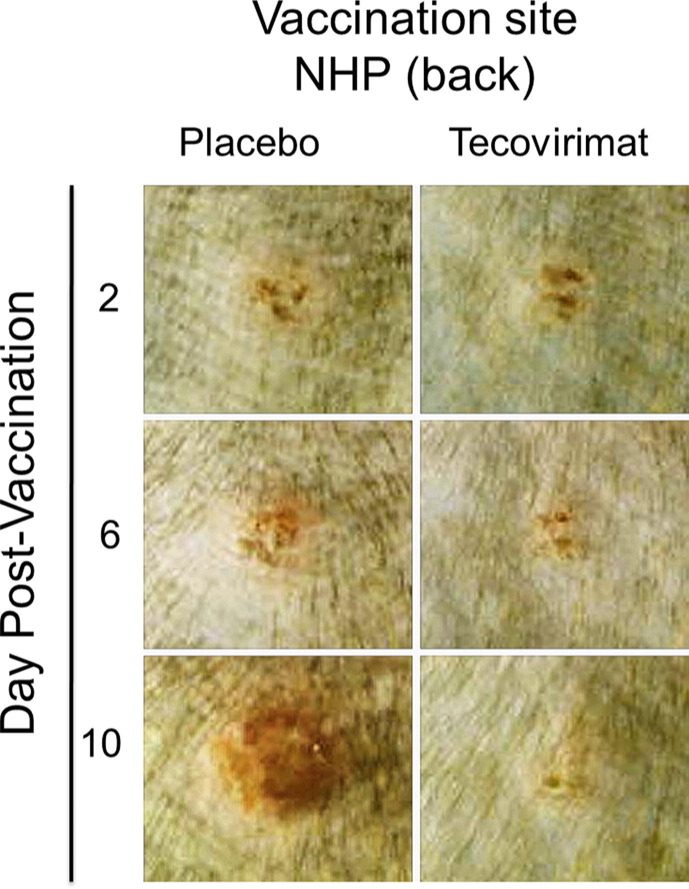
The appearance of a characteristic pustule at the vaccination site within seven days of vaccination with live VACV-based vaccine is the classic marker of successful vaccination and is accepted as evidence of acquired immunity against smallpox [34]. Lesion progression following ACAM2000 vaccination in individual NHPs, with or without concurrent tecovirimat treatment shown in Fig. 1 is representative of lesion progression observed in all animals, dependent on their treatment. Two days following vaccination the sites appear similar between the two representative animals. Infection is not evident in either animal. At later times the presence of active infection and the development of the characteristic lesion is clear in the placebo-treated animal, while the reaction at the vaccination site in the tecovirimat-treated animal is milder and appears nearly resolved by 10 days following vaccination.
Fig. 1.
Effect of tecovirimat on vaccination site lesion formation and progression in cynomolgus macaques. NHPs were inoculated by epidermal scarification with ACAM2000. NHPs were concurrently treated with placebo or tecovirimat (10 mg/kg, once daily) for 14 days. Vaccination site was photographed on multiple days following vaccination. Representative images at the specified intervals are presented. The effect of tecovirimat was to reduce severity and duration of vaccination site lesions.
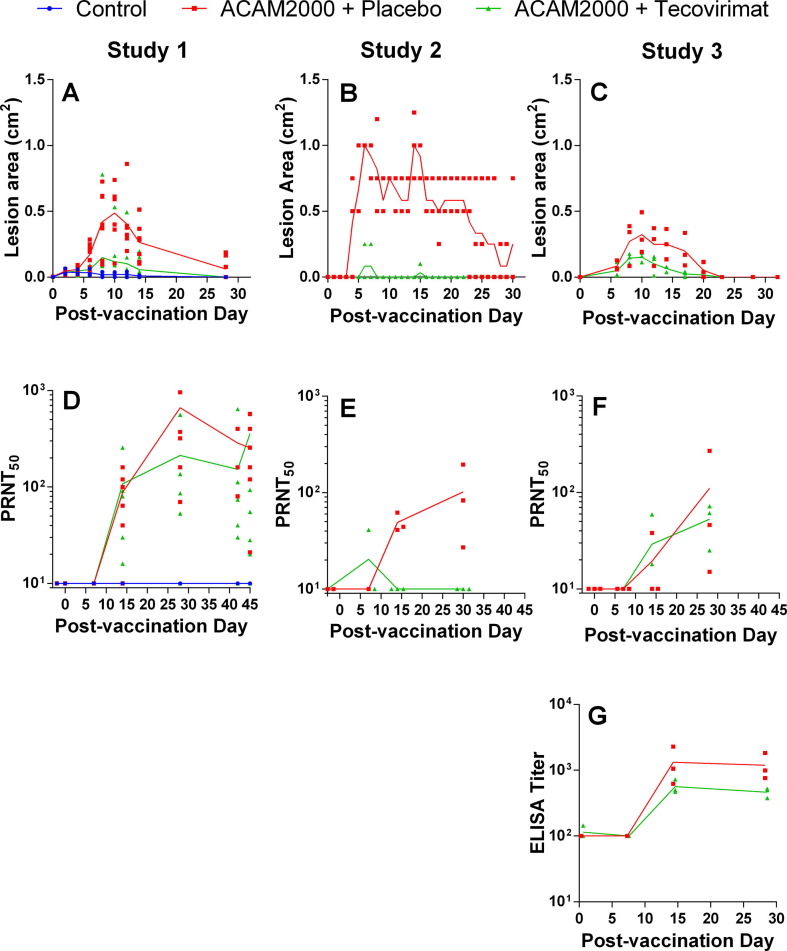
Shown in Fig. 2 (A–C) is the effect of tecovirimat treatment on the group mean lesion area following ACAM2000 vaccination. The control animals (Diluent + placebo; panel A), showed a slight reaction at the inoculation site due to the trauma of the procedure. Following vaccination, all groups receiving placebo treatment (ACAM2000 + placebo) displayed lesions at the vaccination site that increased in size over time and peaked in size between days five (Study 2) and 10 (Studies 1 and 3). Group mean lesion area in Study 2 also showed a transient spike over days 14–15.
Fig. 2.
Effect of tecovirimat treatment on response to ACAM2000 vaccination in cynomolgus (Studies 1 and 2) and rhesus (Study 3) macaques. NHPs were inoculated by epidermal scarification with ACAM2000 or vaccine diluent and concurrently treated with placebo or tecovirimat (10 mg/kg, once daily) for 14 days. Vaccination site lesion area (A-C) and VACV PRNT50 (D-E) were monitored on all studies and anti-VACV ELISA titers were determined on study 3 (G). PRNT50 data were collected up to the day of MPXV challenge in all studies and the time between vaccination and MPXV challenge varied between 28 and 45 days depending on the study. 45 days are shown on the x-axis for all studies regardless of time between vaccination and MPXV challenge to allow presentation of all pre-challenge data for Study 1. Study Group sizes were as follows: Study 1 Control (n = 3), ACAM2000 + Placebo (n = 7) and ACAM2000 + tecovirimat (n = 7); Study 2 ACAM2000 + Placebo (n = 3) and ACAM2000 + tecovirimat (n = 3); Study 3 ACAM2000 + Placebo (n = 3) and ACAM2000 + tecovirimat (n = 3). Individual animal data are plotted on all figures. Connecting lines are plotted through group mean values for lesion area and group geometric mean values for PRNT50 and ELISA measurements. The unusual appearance of individual animal data in panel B is due to reporting of vaccination site lesion area in increments of 0.25 cm2.
In groups treated with tecovirimat (ACAM2000 + tecovirimat), vaccination lesion formation was attenuated and lesions resolved more quickly than in placebo-treated animals. Of the 13 ACAM2000 + tecovirimat animals, seven exhibited a reaction at the vaccination site indicative of a positive vaccine take. However, the vaccine take rate in tecovirimat treated animals was variable among the three studies: 3/7 in Study 1, 1/3 in Study 2, and 3/3 in Study 3 exhibited a reaction at the vaccination site. In all studies, vaccination lesions were mostly resolved by 32 days following vaccination regardless of tecovirimat treatment status.
3.2. Post–vaccination PRNT50 and anti-VACV ELISA titer
Measurement of PRNT50 (Fig. 2, D-F) shows peak geometric mean neutralizing antibody titers between 57 and 424 for ACAM2000 + placebo groups, and 20 to 107 for ACAM2000 + tecovirimat groups. Peak individual animal PRNT50 values are presented in Table 5. Maximum individual PRNT50 values were observed 28–42 days following vaccination in ACAM2000 + placebo animals and 7–45 days in ACAM2000 + tecovirimat animals. All unvaccinated control animals, and two tecovirimat-treated animals in Study 2 showed no detectable neutralizing antibody response.
Table 5.
Peak individual animal measurements of study endpoints following lethal MPXV challenge.
| Study Group Assignment | Study ID | Animal ID | Peak PRNT50 Pre-challengea | Peak PRNT50 Post-challengea | Peak Lesion Count | Peak Viral Load copies/mlb |
|---|---|---|---|---|---|---|
| Controls | Study 1c | 4463 | <10 | 520 (d11) | >3250 (d6) | 6.70E + 07 (d11) |
| 4465 | <10 | 88 (d11) | >3250 (d6) | 3.34E + 07 (d11) | ||
| 4466 | <10 | 400 (d6) | >3250 (d6) | 2.02E + 08 (d8) | ||
| Mean | <10 | 263 | >3250 | 7.67E + 07 | ||
| ACAM2000 + Placebo | Study 1c | 4458 | 1408 (d28) | >10240 (d12) | 3 (d6) | <5.00E + 03 (d0) |
| 4460 | 160 (d28) | >10240 (d12) | 0 | <5.00E + 03 (d0) | ||
| 4464 | 400 (d42) | >10240 (d6) | 31 (d9) | <5.00E + 03 (d0) | ||
| 4467 | 80 (d42) | >10240 (d6) | 11 (d9) | <5.00E + 03 (d0) | ||
| 4470 | 1360 (d28) | >10240 (d6) | 7 (d6) | <5.00E + 03 (d0) | ||
| 4473 | 400 (d42) | >10240 (d6) | 3 (d6) | <5.00E + 03 (d0) | ||
| Mean | 397 | >10240 | 9.2 | <5.00E + 03 | ||
| Study 2d | A12660 | 83 (d30) | 8925 (d30) | 0 | <1.00E + 02 (d3) | |
| A12662 | 41 (d14) | 1794 (d30) | 0 | 5.24E + 02 (d3) | ||
| A12712 | 195 (d30) | 195 (d0) | 0 | <1.00E + 02 (d3) | ||
| Mean | 87 | 1462 | 0 | 1.74E + 04 | ||
| Study 3e | 4900 | 46 (d28) | 5579 (d12) | 2 (d3) | 1.26E + 05 (d18) | |
| 4908 | 270 (d28) | >10240 (d6) | 3 (d9) | 9.74E + 03 (d15) | ||
| 4916 | 15 (d28) | >10240 (d6) | 6 (d6) | 6.97E + 03 (d15) | ||
| Mean | 57 | 8363 | 3.7 | 2.05E + 04 | ||
| ACAM2000 + Tecovirimat | Study 1c | 4459 | 1280 (d45) | >10240 (d6) | 1 (d6) | 6.04E + 03 (d15) |
| 4461 | 93 (d45) | >10240 (d24) | 10 (d9) | 2.54E + 04 (d9) | ||
| 4469 | 16 (d28) | 5120 (d28) | TNTC (d6) | 7.18E + 05 (d6) | ||
| 4472 | 136 (d28) | >10240 (d6) | 5 (d9) | <5.00E + 03 (d0) | ||
| 4474 | 256 (d14) | >10240 (d6) | 312 (d6) | 5.42E + 04 (d3) | ||
| 4476 | 120 (d14) | >10240 (d6) | TNTC (d6) | 6.26E + 04 (d6) | ||
| 4477 | 1024 (d45) | >10240 (d6) | 0 | <5.00E + 03 (d0) | ||
| Mean | 188 | 9223 | 975.42 | 1.89E + 04 | ||
| Study 2 | A12673 | 41 (d7) | 662 (d12) | 2800 (d9) | 2.03E + 08 (d9) | |
| A12720 | N/A | 5146 (d30) | 495 (d9) | 3.26E + 06 (d6) | ||
| A12721 | N/A | 10,779 (d30) | 230 (d9) | 9.04E + 04 (d6) | ||
| Mean | N/A | 3324 | 1175.00 | 3.91E + 06 | ||
| Study 3e | 4907 | 61 (d28) | >10240 (d12) | 25 (d9) | 1.04E + 04 (d15) | |
| 4910 | 72(d28) | >10240 (d6) | 6 (d6) | <5.00E + 03 (d3) | ||
| 4912 | 25 (d28) | >10240 (d6) | 203 (d9) | 1.20E + 04 (d9) | ||
| Mean | 48 | >10240 | 78.00 | 8.55E + 03 | ||
Parentheses indicate the day when the peak value was initially observed, subsequent to ACAM2000 vaccination or MPXV challenge. Mean values incorporating any ULOQ values represent a lower bound for the group mean.
In Study 1 PRNT50 was measured for VACV, in Studies 2 and 3 MPXV was used to determine PRNT50. Mean values presented are geometric means. If no samples measured over 50% neutralization, or logit analysis failed to converge to a meaningful result, value reported is N/A.
Mean values presented for viral load are geometric means.
Peak PRNT of 10,240 is the ULOQ. Peak lesion count of 3250 is the ULOQ reported when lesions are too numerous to count. LLOQ for viral genome count is 5000. This value is reported for cases where no genomes were detected by PCR.
LLOQ for viral genome count is 100.
Peak PRNT of 10,240 is the ULOQ. LLOQ for viral genome count is 5000.
Anti-VACV ELISA results in Fig. 2G suggest slight reduction in anti-VACV titer in the ACAM2000 + tecovirimat group although the difference is not statistically significant (P = 0.19).
3.3. Survival
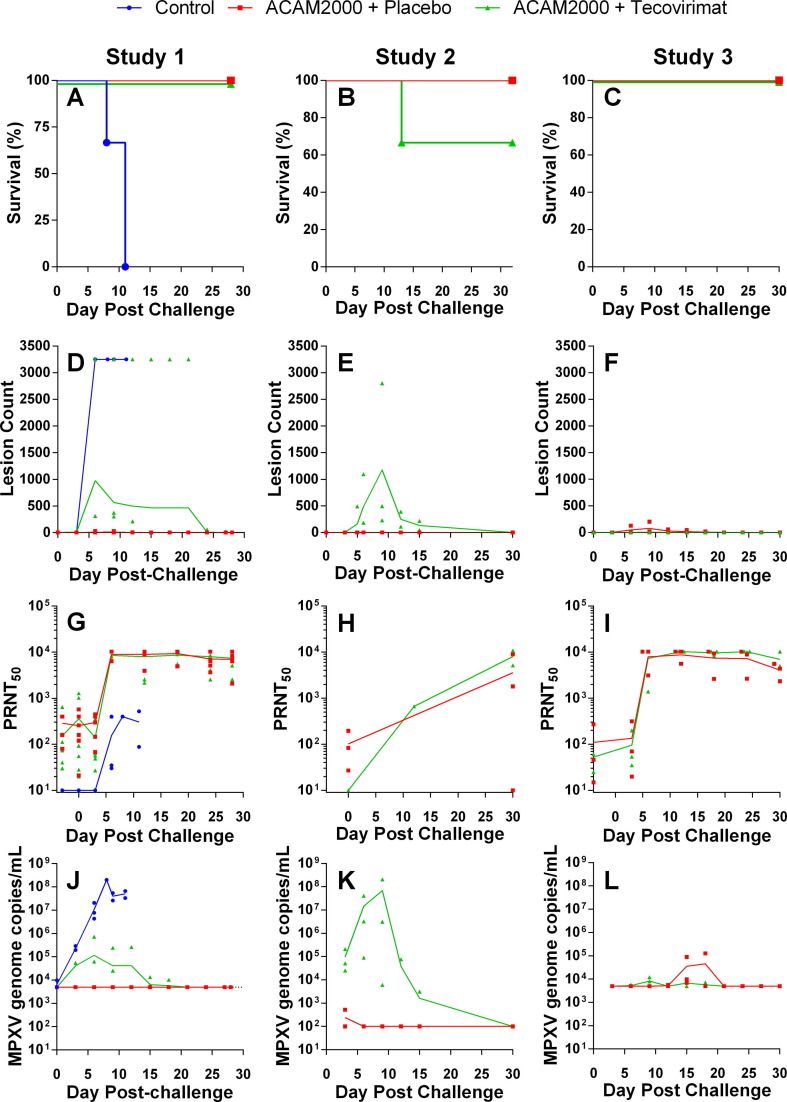
All groups were lethally challenged with MPXV between 30 and 45 days following vaccination. Kaplan-Meier survival plots are presented in Fig. 3 (Panels A–C) and survival data in Table 4 . All three unvaccinated control animals (Study 1) succumbed to disease by 11 days following challenge. All 12 ACAM2000 + placebo animals survived to study conclusion, and 12 of 13 ACAM2000 + tecovirimat animals (92.3%) survived to study conclusion. A single animal in Study 2 (A12673) required euthanasia 12 days following challenge.
Fig. 3.
Protective efficacy of ACAM2000 given in combination with tecovirimat in cynomolgus (Studies 1 and 2) and rhesus (Study 3) macaques. NHPs were inoculated by epidermal scarification with ACAM2000 or vaccine diluent and concurrently treated with placebo or tecovirimat (10 mg/kg, once daily) for 14 days. NHPs were challenged with a lethal dose of MPXV, Zaire 79 via the intravenous route 45 (Study 1), 30 (Study 2) or 32 (Study 3) days following vaccination with ACAM200 or vaccine diluent. Following MPXV challenge, NHPs were monitored for survival (A–C), group mean total body lesion counts (D–F), PRNT50 (G–I), and MPXV genome copies in blood (J–L). LLOQ for MPXV genome copies/mL in Studies 1 and 3 (Panels J and K respectively) was 5 × 103. Study 2 (Panel K) LLOQ was 1 × 102. Study Group sizes were as follows: Study 1 Unvaccinated + placebo Control (n = 3), ACAM2000 + Placebo (n = 6) and ACAM2000 + tecovirimat (n = 7); Study 2 ACAM2000 + Placebo (n = 3) and ACAM2000 + tecovirimat (n = 3); Study 3 ACAM2000 + Placebo (n = 3) and ACAM2000 + tecovirimat (n = 3). Study Group sizes were as described in Fig. 2, with the exception that the Study 1 ACAM2000 + Placebo group size is reduced by one from the pre-challenge group size due to censoring of one NHP prior to MPXV challenge due to health issues unrelated to study procedures. Individual animal data are plotted on all figures. Connecting lines are plotted through group mean values for lesion counts and group geometric mean values for PRNT50 and MPXV genome measurements.
Table 4.
Survival following lethal MPXV challenge.
| Study ID | Study Group Assignment | MPXV Target Challenge Dose (PFU, IV) | Survival |
|---|---|---|---|
| Study 1 | Controls | 5.0E + 07 | 0/3 |
| ACAM2000 + Placebo | 5.0E + 07 | 6/6 | |
| ACAM2000 + Tecovirimat | 5.0E + 07 | 7/7 | |
| Study 2 | ACAM2000 + Placebo | 5.0E + 07 | 3/3 |
| ACAM2000 + Tecovirimat | 5.0E + 07 | 2/3 | |
| Study 3 | ACAM2000 + Placebo | 5.0E + 07 | 3/3 |
| ACAM2000 + Tecovirimat | 5.0E + 07 | 3/3 | |
3.4. Lesion counts
Group mean total lesion counts following challenge are shown in Fig. 3 (Panels D-F). Individual animal lesion counts varied from TNTC (counted as 3250 for plotting purposes) to zero. Peak individual animal lesion counts are presented in Table 5 . Lesions in the unvaccinated control animals (Study 1) were TNTC six days after challenge and persisted until death. In Study 2 no lesions were observed at any time following challenge in ACAM2000 + placebo animals, and in Studies 1 and 3 individual peak counts ranged from 0 to 31. In all ACAM2000 + tecovirimat groups, lesions were observed following MPXV challenge. In Study 1, the number of lesions observed in the ACAM2000 + tecovirimat group was significantly elevated (t-test; P < 0.05) compared to the ACAM2000 + placebo group. Mean lesion counts in the Study 2 ACAM2000 + tecovirimat group were elevated compared to the ACAM2000 + placebo group, but the observed difference was not significant. In Study 3, group mean lesion counts between the two groups differed only slightly.
In the ACAM2000 + tecovirimat group in Study 1 the peak group mean lesion count and extended time to resolution were biased by elevated lesion counts (TNTC) in a single animal. In the ACAM2000 + tecovirimat group in Study 2, the peak mean lesion count nine days after challenge is biased by a single animal with elevated lesion counts that was declared moribund and euthanatized 12 days after challenge.
3.5. Post-MPXV challenge PRNT
PRNT50 values observed following lethal MPXV challenge are shown in Fig. 3 (G–I) and peak individual animal PRNT50 is presented in Table 5. Control animals from Study 1 showed an antibody response five days following challenge that was lower than vaccinated animals and was insufficient to provide protection from the challenge. In vaccinated animals from studies 1 and 3, PRNT50 values increased sharply five days following challenge and remained elevated until study conclusion. Most Study 1 and 3 animals, regardless of tecovirimat treatment status, showed a peak PRNT50 value in excess of the upper limit of quantitation (ULOQ; 10240). In contrast, Study 2 results were all within quantifiable limits. All but a single animal showed increased PRNT50 following MPXV challenge, and one of the ACAM2000 + tecovirimat animals that showed no measurable PRNT50 following vaccination showed the highest PRNT50 following MPXV challenge, which was notably higher than the maximum value observed in the ACAM2000 + placebo group.
3.6. MPXV load in blood
Group mean blood viral loads are presented in Fig. 3 (J-L) and individual animal peak viral loads are provided in Table 5. Unvaccinated control animals (Study 1) showed a steep increase in viremia following challenge, as high as 2x108 genome copies/mL, which remained elevated until animals succumbed to disease. Vaccinated animals in studies 1 and 3, regardless of tecovirimat treatment status, showed peak viral loads one thousandfold or more lower than observed in the control animals, which fell below the lower limit of quantitation (LLOQ) by the study conclusion. In Study 1 the ACAM2000 + placebo animals did not show measurable viremia at any time following challenge. The results from Study 2 are noteworthy in that while the viral loads in ACAM2000 + placebo animals are similar to the other two studies, the observed viral loads in the ACAM2000 + tecovirimat group peak higher than observed in the other studies by more than a hundredfold. Regardless of tecovirimat treatment status, all surviving animals cleared detectable MPXV genomes from the blood by study conclusion.
4. Discussion
Since routine vaccination of the general public was discontinued in 1980 [7], orthopoxvirus immunity has declined in the population, raising concerns about susceptibility to emerging zoonotic orthopoxviruses, such as MPXV, and vulnerability to VARV as a bioweapon [6], [35], [36]. In response to these concerns, the United States maintains over 300 million doses of ACAM2000 [37], [38] as well as 2 million treatment courses of TPOXX in the Strategic National Stockpile to be used in the event of a smallpox incident.
A single confirmed case of smallpox would be considered a public health emergency, thus compelling the CDC to establish a response plan to be implemented should this occur. According to the plan, the response to a smallpox outbreak would include isolation of confirmed cases of smallpox and treatment with TPOXX. First and second degree contacts of those infected would be vaccinated, or if the outbreak were large enough, mass community vaccination would be implemented, including individuals with contraindications [39], [40]. In addition to vaccination, it is possible that TPOXX would be administered to those with known or suspected exposure to VARV prior to diagnosis of smallpox, concurrent with or following inoculation with ACAM2000, or to mitigate vaccine adverse events [41], [42]. This would constitute “off-label” use of TPOXX, and raises concern that TPOXX could interfere with complete acquisition of protective immunity if simultaneously administered with the vaccine. As a follow-up to this work, animal studies are planned to further investigate the impact of TPOXX intervention on vaccine efficacy as well as the timing of TPOXX intervention relative to administration of ACAM2000 vaccination and challenge with MPXV.
In our previous studies of pre-exposure vaccination with Dryvax [43] or ACAM2000 [44] in combination with tecovirimat in mice, vaccination site lesion severity and resolution time were reduced in tecovirimat-treated animals vs untreated animals, and mild impacts on measures of immune responses were observed. Mice were fully protected against subsequent lethal challenge with VACV Western Reserve suggesting no impact of tecovirimat on acquisition of protective immunity. In earlier studies of post-exposure ACAM2000 vaccination with concurrent initiation of tecovirimat treatment in cynomolgus macaques three days following lethal IV MPXV challenge, all tecovirimat-treated animals survived regardless of whether or not ACAM2000 was administered. All MPXV-infected animals treated with tecovirimat survived a lethal MPXV re-challenge without any external signs of disease strongly suggesting that tecovirimat did not interfere with the development of a fully protective immune response to MPXV infection with or without concurrent ACAM2000 vaccination [45].
4.1. Pre-MPXV challenge
Our findings demonstrate that concurrent tecovirimat treatment during the first 14 days following vaccination appears to reduce vaccination site lesion size and time to resolution. All ACAM2000 + placebo animals developed a characteristic take following vaccination although variation in lesion area at different study sites was noted, possibly due to variation between study sites in the determination of individual lesion size or in vaccine administration. Approximately half of the ACAM2000 + tecovirimat animals formed a primary lesion that was less severe and resolved more quickly than ACAM2000 + placebo animals, while the remaining ACAM2000 + tecovirimat animals developed small lesions likely due to the multiple puncture vaccine administration and minimal viral replication.
In Studies 1 and 3, all vaccinated animals, regardless of tecovirimat treatment status, had measurable PRNT50 titers following vaccination. In ACAM2000 + tecovirimat animals, the group mean PRNT50 titers appeared lower than for ACAM2000 + placebo animals but higher than neutralizing titers said to be protective against smallpox in humans [46], [47]. In Study 2, ACAM2000 + placebo animals showed PRNT50 titers similar to observations in the other studies, but in the tecovirimat-treated group only a single animal had a measurable titer following vaccination and prior to MPXV challenge. This animal did not develop a vaccination lesion and was the only animal in the ACAM2000 + tecovirimat group to succumb to lethal MPXV challenge. Anti-VACV ELISA titers were measured in Study 3 and showed a small difference between tecovirimat-treated and placebo-treated animals following vaccination.
These results suggest that tecovirimat administered in combination with ACAM2000 attenuates reactogenicity and in some animals completely inhibits the characteristic, visible vaccine “take”. The reason is unclear but possibilities include individual animal variability in response to vaccination or tecovirimat treatment, or variability in the vaccine administration technique, which we consider most likely. This procedure leaves room for human error as it requires delivery of a 2.5 µl droplet to the dermis via a bifurcated needle and 15 punctures to ensure delivery. In placebo-treated animals, suboptimal vaccination (i.e., lower than standard vaccine dose administered) is “self-correcting” as the virus may replicate and spread locally prior to immune control. Tecovirimat treatment does not prevent viral replication but efficiently inhibits dissemination. In a suboptimal vaccination, this may result in self-limiting infections and resolution prior to elicitation of robust immunity. This may explain the low PRNT results seen in some animals, the only death observed in the ACAM2000 + tecovirimat group in Study 2, and the generally more severe disease seen in most ACAM2000 + tecovirimat animals in all three studies.
In contrast, when NHPs are challenged with a lethal dose of MPXV by the IV route and receive tecovirimat treatment, the immune response is always robust (unpublished data), and in studies with a rechallenge, was protective. In comparing the neutralizing antibody response in vaccinated animals treated with tecovirimat, with MPXV-challenged animals treated with the same dose regimen, the development of immune responses occur slightly earlier, and the magnitude of the response is dramatically higher for MPXV-challenged animals. This is likely due to the high MPXV challenge dose and the route of challenge (IV vs. intradermal for vaccine).
Similarly, tecovirimat does not seem to affect immune responses to the non-replicating Modified Vaccinia Ankara based smallpox vaccine, IMVAMUNE®/JYNNEOS™ (unpublished data). In one study, immune responses and protective efficacy were similar between animals vaccinated and treated with tecovirimat or a placebo. This is likely due to the high dose of virus administered with the vaccine and since the vaccine is non-replicative in mammalian cells, the mechanism targeted by tecovirimat is non-functional.
4.2. Post-MPXV challenge
Following lethal MPXV challenge, unvaccinated control animals succumbed to monkeypox by 11 days after challenge. Of 25 vaccinated animals, one succumbed to monkeypox. This animal, an ACAM2000 + tecovirimat animal from Study 2, showed no apparent vaccination site lesion, and was the only animal in that group with a measurable PRNT50 titer prior to challenge. The other two animals in this group did not show a measurable PRNT50 prior to MPXV challenge and one of them did not show a vaccination site lesion, yet they both survived. Of the 10 ACAM2000 + tecovirimat treated animals from Studies 1 and 3, four failed to show a characteristic vaccine take and yet were protected from lethal MPXV challenge. These results suggest that administration of tecovirimat concurrently with ACAM2000 has minor impact on survival of a subsequent lethal IV MPXV challenge. However, there may be some impact on ACAM2000-elicited protection from morbidity.
Numerous lesions (TNTC) were observed in all unvaccinated control animals by five days following challenge and persisted until animals succumbed to disease. Lesion development in ACAM2000 + placebo animals was irregular, lesion counts were generally low or absent with a peak count of 31 in one animal. In ACAM2000 + tecovirimat animals, there was substantial variability in lesion counts. Most (12 of 13) of these animals showed at least one lesion following challenge, and two animals exhibited lesions TNTC. Lesion counts in ACAM2000 + tecovirimat animals on any specific study was similar between animals with the exception of a few outliers in Studies 1 and 2 that were as much as 10-fold higher than the rest of their groups. Note that in Study 2 the ACAM2000 + tecovirimat animal that did not survive exhibited a peak lesion count over 5-fold higher than the other two animals in the group.
Following lethal MPXV challenge, unvaccinated control animals mounted a weak primary immune response, indicated by measurable PRNT50 starting six days following challenge. This response was insufficient and all control animals eventually succumbed to disease. Vaccinated animals, regardless of tecovirimat treatment status (particularly in studies 1 and 3), showed strong anamnestic responses to MPXV challenge, with group mean PRNT50 values for tecovirimat-treated and untreated groups essentially indistinguishable.
In Study 2, PRNT50 was only evaluated prior to challenge, and at the study conclusion or animal removal due to death or moribund euthanasia. Increases in PRNT50 by the end of the study were smaller than observed in Studies 1 and 3. The final neutralizing titers in surviving ACAM2000 + tecovirimat animals was 10-fold higher than the ACAM2000 + placebo animals, and similar to the final neutralizing titers observed in the other two studies.
The blood viral load results show a nearly complete clearance of virus from the blood of ACAM2000 + placebo animals as soon as 3 days following MPXV challenge. The elevated MPXV genome copies observed on challenge day in Study 2 result from sample collection shortly following challenge for verification of MPXV delivery. In the other studies, challenge day samples were collected prior to challenge. All three ACAM2000 + placebo animals on Study 3 showed MPXV genome counts above the LLOQ between 12 and 18 days after challenge for unknown reasons. These genome counts were orders of magnitude lower than observations in the unvaccinated control animals in Study 1.
In ACAM2000 + tecovirimat animals a discrepancy is noted between viral loads observed in Studies 1 and 3, and in Study 2. Results from the animal that succumbed to challenge look much like the results of the control animals. MPXV genome counts for this animal continued to increase up to nine days after challenge and peaked at 2.03 × 108 copies/mL. The surviving animals showed higher MPXV genome counts than in the other two studies and cleared virus from their blood by the end of the study. In Studies 1 and 3 very low MPXV genome counts were observed shortly after challenge in most of the animals and were detectable in some animals up to 18 days following challenge. Peak counts were several orders of magnitude lower than observed in control animals and were not indicative of a productive infection. Since these assays measure DNA and not necessarily infectious virus, it cannot be determined if virus detected in the blood was potentially infectious virus, neutralized virus particles, or genome fragments.
These results indicate that the immune response to ACAM2000 vaccination is protective against lethal IV MPXV challenge even when tecovirimat is administered concurrently with vaccination. Even in cases where tecovirimat prevented a characteristic vaccination take, animals were protected from mortality following a lethal MPXV challenge, and morbidity associated with infection, such as lesion development, was reduced.
5. Conclusions
TPOXX is FDA-approved only as therapy for clinically evident smallpox, which is usually diagnosed 12 to 17 days post-exposure. Therefore, there is a “window of vulnerability” in the progression of smallpox for which no treatment options are approved by FDA: between the 1–5 day window, where post-exposure vaccination appears to be effective, and the 12–17 day window, after which clinical diagnosis is possible and TPOXX treatment is indicated. Although TPOXX is fully effective in terms of survival when initiated after disease is clinically evident in animal models, pilot studies have demonstrated that earlier TPOXX intervention provides additional benefit in terms of reduced morbidity and shedding of virus from the infected host [17], [18], [48]. Hence, treatment indications for vaccine and TPOXX administration may overlap in a smallpox outbreak. Therefore, to inform on the best use of vaccine and TPOXX in an outbreak, the impact of TPOXX on vaccine efficacy was evaluated.
The protective efficacy of the vaccine measured by survival of lethal MPXV challenge is largely unaffected by concurrent TPOXX treatment, but humoral response to the vaccine may be adversely affected. The diminished humoral responses may have contributed to increased morbidity in ACAM2000 + tecovirimat treated animals lethally challenged with MPXV, since lesion counts and viral load in the blood fell between observations in the ACAM2000 + placebo and unvaccinated control groups. This suggests that in a pre-exposure prophylaxis scenario, with a near zero risk of exposure to VARV, TPOXX likely should not be administered concurrently with smallpox vaccine as it may slightly reduce vaccine protective efficacy. If exposure to VARV is suspected, even if prior to clinical signs of disease, TPOXX is likely the best intervention option [49], as post-exposure vaccination efficacy wanes quickly with progress of infection, while TPOXX remains effective well after clinical disease is evident. Additional animal studies are necessary to explore the priority of TPOXX relative to vaccination in various disease scenarios to determine the optimal use of each agent based on assessed risk of smallpox exposure.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [Andrew Russo, Doug Grosenbach, and Dennis Hruby are employees of SIGA Technologies, Inc. and hold stock or equity interests in the company. Aklile Berhanu is a former employee of SIGA Technologies. All other authors have no conflicts to declare.].
Acknowledgments
Acknowledgements
We gratefully acknowledge Helen F. Schiltz, PhD, and Judith Hewitt, PhD, for technical and administrative support.
The following reagent was obtained through the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: Monkeypox Virus, Zaire 79 (V79-I-005), NR-2324.
Funding
These studies were supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health [Contract No. N01-AI-30063]; and SBIR/STTR Phase 2 Grant [Grant Number: 5R44AI075747]. MPXV stock supply of Monkeypox Virus, Zaire 79 (V79-I-005), NR-2324 by BEI Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
All authors attest they meet the ICMJE criteria for authorship.
Footnotes
Previous Presentations. Material in this paper has been presented at the XIX International Poxvirus, Asfarvirus and Iridovirus Conference, 2012, Salamanca Spain.
Impact of ST-246 on Smallpox Vaccination in Immunodeficient Non-Human Primates. Doug Grosenbach, Aklile Berhanu, Jon Prigge, Peter Silvera, Catherine Bigger, and Dennis Hruby.
References
- 1.Henderson D.A. Proceedings of the Royal Society of London Series B Biological Sciences. 1977. Smallpox Eradication; pp. 199–283. [DOI] [PubMed] [Google Scholar]
- 2.Melamed S., Israely T., Paran N. Challenges and Achievements in Prevention and Treatment of Smallpox. Vaccines (Basel) 2018;6 doi: 10.3390/vaccines6010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geddes A.M. The history of smallpox. Clin Dermatol. 2006;24:152–157. doi: 10.1016/j.clindermatol.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Deria A., Jezek Z., Markvart K., Carrasco P., Weisfeld J. The world's last endemic case of smallpox: surveillance and containment measures. Bull World Health Organ. 1980;58:279–283. [PMC free article] [PubMed] [Google Scholar]
- 5.Barclay W.R. The conquest of smallpox. JAMA. 1978;240:1991–1992. doi: 10.1001/jama.1978.03290180065033. [DOI] [PubMed] [Google Scholar]
- 6.Henderson D.A., Inglesby T.V., Bartlett J.G., Ascher M.S., Eitzen E., Jahrling P.B., et al. Working Group on Civilian Biodefense. JAMA; 1999. Smallpox as a biological weapon: medical and public health management; pp. 2127–2137. [DOI] [PubMed] [Google Scholar]
- 7.Breman J.G., Arita I. The confirmation and maintenance of smallpox eradication. N Engl J Med. 1980;303:1263–1273. doi: 10.1056/NEJM198011273032204. [DOI] [PubMed] [Google Scholar]
- 8.Yinka-Ogunleye A., Aruna O., Ogoina D., Aworabhi N., Eteng W., Badaru S., et al. Reemergence of Human Monkeypox in Nigeria, 2017. Emerg Infect Dis. 2018;24:1149–1151. doi: 10.3201/eid2406.180017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sklenovska N., Van Ranst M. Emergence of Monkeypox as the Most Important Orthopoxvirus Infection in Humans. Front Public Health. 2018;6:241. doi: 10.3389/fpubh.2018.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.BARDA. Smallpox Antiviral for the Strategic National Stockpile. 2009. p. Request for Proposal.
- 11.Grosenbach D.W., Jordan R., Hruby D.E. Development of the small-molecule antiviral ST-246 as a smallpox therapeutic. Future Virol. 2011;6:653–671. doi: 10.2217/fvl.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan R., Leeds J.M., Tyavanagimatt S., Hruby D.E. Development of ST-246(R) for treatment of poxvirus infections. Viruses. 2010;2:2409–2435. doi: 10.3390/v2112409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang G., Pevear D.C., Davies M.H., Collett M.S., Bailey T., Rippen S., et al. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus Challenge. J Virol. 2005;79:13139–13149. doi: 10.1128/JVI.79.20.13139-13149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Payne L.G. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J Gen Virol. 1980;50:89–100. doi: 10.1099/0022-1317-50-1-89. [DOI] [PubMed] [Google Scholar]
- 15.Smith G.L., Vanderplasschen A., Law M. The formation and function of extracellular enveloped vaccinia virus. J Gen Virol. 2002;83:2915–2931. doi: 10.1099/0022-1317-83-12-2915. [DOI] [PubMed] [Google Scholar]
- 16.Quenelle D.C., Buller R.M., Parker S., Keith K.A., Hruby D.E., Jordan R., et al. Efficacy of delayed treatment with ST-246 given orally against systemic orthopoxvirus infections in mice. Antimicrob Agents Chemother. 2007;51:689–695. doi: 10.1128/AAC.00879-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan R., Goff A., Frimm A., Corrado M.L., Hensley L.E., Byrd C.M., et al. ST-246 antiviral efficacy in a nonhuman primate monkeypox model: determination of the minimal effective dose and human dose justification. Antimicrob Agents Chemother. 2009;53:1817–1822. doi: 10.1128/AAC.01596-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mucker E.M., Goff A.J., Shamblin J.D., Grosenbach D.W., Damon I.K., Mehal J.M., et al. Efficacy of tecovirimat (ST-246) in nonhuman primates infected with variola virus (Smallpox) Antimicrob Agents Chemother. 2013;57:6246–6253. doi: 10.1128/AAC.00977-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nalca A., Hatkin J.M., Garza N.L., Nichols D.K., Norris S.W., Hruby D.E., et al. Evaluation of orally delivered ST-246 as postexposure prophylactic and antiviral therapeutic in an aerosolized rabbitpox rabbit model. Antiviral Res. 2008;79:121–127. doi: 10.1016/j.antiviral.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Russo A.T., Grosenbach D.W., Brasel T.L., Baker R.O., Cawthon A.G., Reynolds E., et al. Effects of treatment delay on efficacy of tecovirimat following lethal aerosol monkeypox virus challenge in cynomolgus macaques. J Infect Dis. 2018;218:1490–1499. doi: 10.1093/infdis/jiy326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metzger W., Mordmueller B.G. Vaccines for preventing smallpox. Cochrane Database of Syst Rev. 2007 doi: 10.1002/14651858.CD004913.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fenner F.D., Henderson I., Arita Z., Jezek I. Smallpox and its eradication. 1988. Ladnyi Smallpox vaccine and vaccination in the intensified smallpox eradication programme; pp. 539–592. [Google Scholar]
- 23.Nalca A., Zumbrun E.E. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des Develop Therapy. 2010;4:71–79. doi: 10.2147/dddt.s3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kretzschmar M., Wallinga J., Teunis P., Xing S., Mikolajczyk R. Frequency of adverse events after vaccination with different vaccinia strains. PLoS Med. 2006;3:e272. doi: 10.1371/journal.pmed.0030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Overton E.T., Stapleton J., Frank I., Hassler S., Goepfert P.A., Barker D., et al. Safety and Immunogenicity of modified vaccinia ankara-bavarian nordic smallpox vaccine in vaccinia-naive and experienced human immunodeficiency virus-infected individuals: an open-label, controlled clinical phase II trial. open forum. Infect Dis. 2015;2:ofv040. doi: 10.1093/ofid/ofv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen B.W., Damon I.K., Pertowski C.A., Meaney-Delman D., Guarnizo J.T., Beigi R.H., et al. Clinical guidance for smallpox vaccine use in a postevent vaccination program. MMWR Recomm Rep. 2015;64:1–26. [PubMed] [Google Scholar]
- 27.Massoudi M.S., Barker L., Schwartz B. Effectiveness of postexposure vaccination for the prevention of smallpox: results of a delphi analysis. J Infect Dis. 2003;188:973–976. doi: 10.1086/378357. [DOI] [PubMed] [Google Scholar]
- 28.Fenner F., Henderson D., Arita I., Jezek Z., Ladnyi I. Geneva, Switzerlans: World Health Organization. 1988. The clinical features of smallpox. smallpox and its eradication; pp. 1–68. [Google Scholar]
- 29.Keckler M.S., Reynolds M.G., Damon I.K., Karem K.L. The effects of post-exposure smallpox vaccination on clinical disease presentation: addressing the data gaps between historical epidemiology and modern surrogate model data. Vaccine. 2013;31:5192–5201. doi: 10.1016/j.vaccine.2013.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.USFDA. Product Development Under the Animal Rule, Guidance for Industry. Rockville, MD: United States Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research/Center for Biologics Evaluation and Research; 2015.
- 31.Cann J.A., Jahrling P.B., Hensley L.E., Wahl-Jensen V. Comparative pathology of smallpox and monkeypox in man and macaques. J Comp Pathol. 2013;148:6–21. doi: 10.1016/j.jcpa.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Council NR. Guide for the care and use of laboratory animals. Eighth ed: National Academies Press; 2011. [PubMed]
- 33.Kulesh D.A., Baker R.O., Loveless B.M., Norwood D., Zwiers S.H., Mucker E., et al. Smallpox and pan-orthopox virus detection by real-time 3'-minor groove binder TaqMan assays on the roche LightCycler and the Cepheid smart Cycler platforms. J Clin Microbiol. 2004;42:601–609. doi: 10.1128/JCM.42.2.601-609.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fenner F., Henderson D., Arita I., Jezek Z., Ladnyi I. Smallpox and its eradication. 1988. Developments in vaccination and control between 1900 and 1966; pp. 302–307. [Google Scholar]
- 35.Nalca A., Rimoin A.W., Bavari S., Whitehouse C.A. Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clin Infect Dis. 2005;41:1765–1771. doi: 10.1086/498155. [DOI] [PubMed] [Google Scholar]
- 36.Duraffour S., Mertens B., Meyer H., van den Oord J.J., Mitera T., Matthys P., et al. Emergence of cowpox: study of the virulence of clinical strains and evaluation of antivirals. PLoS One. 2013;8:e55808. doi: 10.1371/journal.pone.0055808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Organization WH. WHO Advisory committee on variola virus research: report of the nineteenth meeting, 1-2 November 2017, Geneva, Switzerland. World Health Organization; 2018.
- 38.Ratto-Kim S, Yoon I-K, Paris RM, Excler J-L, Kim JH, O'Connell RJ. The US Military Commitment to Vaccine Development: A Century of Successes and Challenges. Frontiers in immunology. 2018;9:1397. [DOI] [PMC free article] [PubMed]
- 39.Artenstein A.W., Grabenstein J.D. Smallpox vaccines for biodefense: need and feasibility. Expert review of vaccines. 2008;7:1225–1237. doi: 10.1586/14760584.7.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control A, GA. Smallpox: Bioterrorism Response Planning. Available at: https://www.cdc.gov/smallpox/bioterrorism-response-planning/index.html.
- 41.Greenberg M. Complications of vaccination against smallpox. Am J Dis Child. 1948;76:492–502. doi: 10.1001/archpedi.1948.02030030505002. [DOI] [PubMed] [Google Scholar]
- 42.Bray M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 2003;58:101–114. doi: 10.1016/s0166-3542(03)00008-1. [DOI] [PubMed] [Google Scholar]
- 43.Grosenbach D.W., Jordan R., King D.S., Berhanu A., Warren T.K., Kirkwood-Watts D.L., et al. Immune responses to the smallpox vaccine given in combination with ST-246, a small-molecule inhibitor of poxvirus dissemination. Vaccine. 2008;26:933–946. doi: 10.1016/j.vaccine.2007.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berhanu A., King D.S., Mosier S., Jordan R., Jones K.F., Hruby D.E., et al. Impact of ST-246(R) on ACAM2000 smallpox vaccine reactogenicity, immunogenicity, and protective efficacy in immunodeficient mice. Vaccine. 2010;29:289–303. doi: 10.1016/j.vaccine.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berhanu A., Prigge J.T., Silvera P.M., Honeychurch K.M., Hruby D.E., Grosenbach D.W. Treatment with the smallpox antiviral tecovirimat (ST-246) alone or in combination with ACAM2000 vaccination is effective as a postsymptomatic therapy for monkeypox virus infection. Antimicrob Agents Chemother. 2015;59:4296–4300. doi: 10.1128/AAC.00208-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mack T.M., Noble J., Jr, Thomas D.B. A prospective study of serum antibody and protection against smallpox. American J Trop Med Hyg. 1972;21:214–218. doi: 10.4269/ajtmh.1972.21.214. [DOI] [PubMed] [Google Scholar]
- 47.Sarkar J.K., Mitra A.C., Mukherjee M.K. The minimum protective level of antibodies in smallpox. Bull World Health Organ. 1975;52:307–311. [PMC free article] [PubMed] [Google Scholar]
- 48.Huggins J., Goff A., Hensley L., Mucker E., Shamblin J., Wlazlowski C., et al. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob Agents Chemother. 2009;53:2620–2625. doi: 10.1128/AAC.00021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finin P., Kosaraju A., Rose E., Rubin H. The role of vaccination, antiorthopoxvirus drug, and social cooperativity in a mathematical model of smallpox control. Biosecur Bioterror. 2013;11:59–72. doi: 10.1089/bsp.2012.0037. [DOI] [PubMed] [Google Scholar]