Abstract
Objective:
We hypothesized that the risk of CRC in night-shift workers might be different according to insulin receptor substrates status.
Methods:
Among 77,470 eligible women having night work assessed in the Nurses’ Health Study, we documented a total of 1,397 CRC cases, of which 304 or 308 had available data on IRS1 and IRS2, respectively. We used duplication method Cox proportional hazards regression analysis for competing risks to calculate HRs and 95% CIs for each CRC subtype. We measured tumor IRS1 or IRS2 expression by immunohistochemistry.
Results:
Compared with women who never worked night-shifts, those working ≥ 15 years night-shifts had a marginal trend of increased overall risk of CRC (Ptrend = 0.06, multivariable HR = 1.20, 95% CI, 0.99 to 1.45). Longer duration of night-shift work was associated with a higher risk of IRS2-positive tumors (multivariable HR = 2.69, 95% CI 1.48 to 4.89, Ptrend = 0.001, ≥ 15 years night-shifts vs. never) but not with IRS2-negative tumors (multivariable HR = 0.90, 95% CI 0.54 to 1.51, Ptrend = 0.72, Pheterogeneity for IRS2 = 0.008). Similarly, the corresponding multivariable HRs were 1.81 for IRS1-positive tumors (95% CI 0.94 to 3.48, Ptrend = 0.06) and 1.13 for IRS1-negative tumors (95% CI 0.71 to 1.80, Ptrend = 0.56, Pheterogeneity for IRS1 = 0.02).
Conclusion:
Our molecular pathological epidemiology data suggest a potential role of IRS in mediating carcinogenesis induced by night-shift work.
Impact:
Although these findings need validation, rotating night-shift might increase CRC risk in women with abnormal insulin receptor pathway.
Keywords: Cancer prevention, circadian disruption, cohort study, colon cancer, etiologic heterogeneity, insulin receptor substrate, metabolism, molecular pathological epidemiology, rectal cancer, rotating night-shift work
INTRODUCTION
Shift work is considered as a “probable” (class 2A) carcinogen to human by the International Agency for Research on Cancer.(1,2) Accumulating evidence suggests that shift work involving circadian rhythm disruption is associated with increased risk of some types of cancers such as breast and colorectal cancer (CRC).(3,4) Schernhammer et al previously reported an increased CRC risk with longer duration of night-shift work in the Nurses’ Health study (NHS) in 2003.(4) Subsequent studies (3,5,6) including a recent meta-analysis (7) reported similar findings. However, other studies (8,9) including a most recent one from Papantoniou et al failed to replicate these findings.(9) It is conceivable that dealing with inherently heterogeneous CRC as a single entity might have diluted any risk association with shift work that might exist for a specific molecular subtype of CRC. Further exploring the underlying biological mechanisms would be helpful to better understand these inconsistent associations. Given that CRC is a highly heterogeneous disease, night-shift work might have different effects on the development of different subgroups of CRC defined by tumor molecular characteristics. Thus, molecular pathological epidemiology (MPE) approach that integrates molecular pathology into epidemiological research (10) can link a certain exposure (such as shift work) to specific pathological signatures, thereby better elucidating the possible pathogenic effect of night-shift work on CRC development.
Recent experimental studies suggested that circadian rhythm disruption may be associated with β-cell dysfunction, glucose intolerance, and improper insulin secretion.(11,12) Similarly, population studies showed that night shift worker tended to have lower insulin sensitivity, hyperinsulinemia, insulin resistance, and develop metabolic syndrome, accordingly.(13–17) Insulin resistance is an adaptive process in insulin-sensitive tissues characterized by reduced insulin receptor substrate 1 (IRS1), and increased IRS1 serine phosphorylation and attenuated downstream signaling. However, there is some evidence demonstrating that the presence of high insulin levels may not necessarily cause insulin resistance, but instead was associated with IRS1 or IRS2 expression and / or tyrosine phosphorylation, which could activate downstream the PI3K / MTOR pathway and subsequently promote mitogenesis and cell proliferation, as shown in colon cancer cells and mouse skeletal muscle cells.(18,19) In addition, human evidence reported positive association between high levels of insulin and risk of colon cancer.(20,21) Since IRS1 and IRS2 are two primary mediators of insulin-dependent mitogenesis and regulation of glucose metabolism in most cell types,(22) and abundantly expressed in CRC,(23) it appears plausible that IRS1 and IRS2 play a key role in colorectal carcinogenesis as part of the chronic metabolic disorder observed in night-shift workers.(24)
In light of this evidence, we hypothesized that longer duration of night-shift work might be associated with an increased risk of colorectal cancer overexpressing IRSs. To test our hypothesis, we prospectively investigated the association of duration of night-shift work with CRC risk according to tumor IRS1 or IRS2 expression in the Nurses’ Health Study (NHS).
In this cohort, we have previously found that women who worked rotating night-shifts for at least 15 years were at an increased risk of CRC.(4) Integrating host factors (such as night-shift work) and tumor molecular features (such as IRSs expression) may enhance our understanding of the mechanisms through which night-shift work may act on colorectal carcinogenesis.
METHODS
Study population and assessment of night-shift work duration
Participants were identified from the Nurses’ Health Study (NHS). Details for the study design and the population have been reported elsewhere.(25–27) A total of 121,700 female registered nurses aged 30 to 55 years were enrolled at baseline in 1976 in the U.S. A biennial questionnaire has been sent to all the participants since 1976 to collect updated information regarding demographics, lifestyle factors, and medical history. Returning the questionnaires was considered to imply informed consent. All procedures of the study were in accordance with the Declaration of Helsinki. The study protocol was approved by the institutional review boards at the Brigham and Women’s Hospital, Boston, Massachusetts.
As described previously,(4,28) NHS participants were asked “how many years have you worked rotating night-shifts including at least 3 nights per month, in addition to days or evenings in that month” in 1988. Information on lifetime years of rotating night-shift work was collected in 8 pre-specified categories, which are never, 1 - 2, 3 - 5, 6 - 9, 10 - 14, 15 - 19, 20 - 29, and ≥ 30 years. We excluded women with a history of any cancer other than non-melanoma skin cancer, polyposis syndrome, ulcerative colitis, or Crohn’s disease in or before 1988, or who did not report their night-shift work duration. A total of 77,470 women were included in this analysis. (Figure 1)
Figure 1.
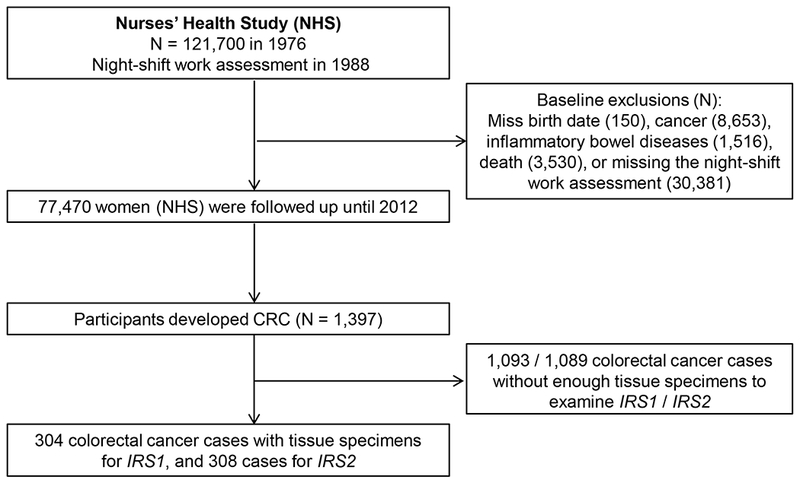
Flow chart of the study population in the Nurses’ Health Study.
Assessment of covariates
We collected information on potential CRC risk factors including height, body weight, physical activity (METS-hours/week), cigarette smoking, history of sigmoidoscopy or colonoscopy screening, family history of colorectal cancer, history of type 2 diabetes, aspirin use, and menopausal status and use of menopausal hormones at baseline and updated in biennial follow-up questionnaires. Body mass index (BMI) was calculated based on reported height and weight. In addition, we collected information on dietary factors including consumption of alcohol, vitamin D, folate, calcium, red meat, processed meat using a validated food frequency questionnaire, with updates almost every 4 years.(29,30) Furthermore, in 1986, 2000, 2002 and 2008, we asked how many hours a woman slept, on average, in a 24-hour period (5 hours or less, 6 hours, 7 hours, 8 hours, 9 hours, 10 hours, or 11 hours or more). Self-reported sleep duration correlated well with sleep duration assessed by sleep diaries in this cohort (Spearman r = 0.79; P < 0.0001).(31)
Ascertainment of incident colorectal cancer cases
Participants or next-of-kin were asked for written permission to obtain medical records and pathological reports if they reported cancer on biennial questionnaires. Study researchers blinded to exposure status further reviewed medical and pathological records to confirm all possible CRC cancer cases, and extracted the information on anatomic location, stage and histological type of the cancer. CRC cases were defined as primary tumors with International Classification of Diseases-9 (ICD-9) codes 153 and 154 and with the histological subtypes, adenocacinoma, signet-ring cell cancer, adenosquamous cancer, as well as undifferentiated cancer (excluding carcinoid, squamous cell cancer, and non-epithelial malignancies, such as sarcoma and lymphoma). We identified unreported fatal CRC and death from state vital statistics records and the National Death Index.
Immunohistochemistry for IRS1 and IRS2 expression
We collected formalin-fixed paraffin-embedded (FFPE) archival tissue specimens of colorectal carcinoma resections from hospitals and laboratories, and constructed tissue microarrays (TMA) from CRC blocks as previously described.(32) Methods for tumor IRS1 and IRS2 immunohistochemistry have been previously described.(33) TMA sections were deparaffinized, rehydrated, and heated in a pressure cooker for 30 min at 95°C in Antigen Retrieval Citra Solution, pH 6 (BioGenex Laboratories, Fremont, CA, USA). Sections were incubated with Dual Endogenous Enzyme Block (Dako, Carpinteria, CA, USA) for 30 min, followed by the treatment with 10% fetal bovine serum (Life Technologies, Carlsbad, CA, USA) in Tris-buffered saline (TBS) for 30 min. Samples were then incubated at 4℃ for 16 h with IRS1 antibody (rabbit 06-248, Millipore, Billerica, MA, USA; 1:200 dilution) or IRS2 antibody (rabbit 06-506, Millipore; 1:500). After washing thoroughly in TBS, sections were incubated with anti-rabbit IgG (Vector Laboratories, Burlingame, CA, USA) for 30 min, then treated with streptavidin-peroxidase (ABC kit, Vector Laboratories) according to the manufacturer’s instructions. Specimens were visualized using diaminobenzidine (Dako) and counterstained with hematoxylin. Sections processed with the replacement of primary antibody by TBS were used as a negative control.
Immunohistochemical assessment for IRS1 and IRS2 in all cases were interpreted by a pathologist (T.M.), and a random group of 76 cases was independently reviewed by a second pathologist (S.A.K.). Both pathologists were blinded to any information concerning the CRC cases. Concordance between the two pathologists indicated substantial agreement for both IRS1 status (four levels) with a weighted κ of 0.74 (95% CI 0.61 to 0.86) and IRS2 status (four levels) with a weighted κ of 0.77 (95% CI 0.65 to 0.89). Tumor cytoplasmic IRS1 and IRS2 expression status were scored as 1 (no or minimal staining), 2 (weak staining), 3 (moderately intense staining), and 4 (intense staining) based on the staining intensity in colorectal carcinoma cells.
Statistical analysis
We calculated person-years for each participant from 1988 when the shift work questionnaire was returned, to the date of death, CRC diagnosis, or the end of follow-up (June 1, 2012), whichever came first. We used the duplication-method Cox proportional hazards regression for competing risks data to calculate age-adjusted and multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for each CRC subtype.(34) Multivariable hazard ratios were adjusted for age, BMI, smoking, history of colorectal cancer in a parent or sibling, history of sigmoidoscopy / colonoscopy, postmenopausal status and hormone use, physical activity, regular aspirin use, alcohol consumption, total intake of vitamin D, folate, calcium, red meat and processed meat, sleep duration, and history of type 2 diabetes. For covariates, when appropriate, we have calculated the cumulative averages by averaging all the prior intakes up to each questionnaire cycle. All the models were stratified by age (in months) and year of questionnaire return (every two years since baseline questionnaire return). To retain sufficient statistical power in the analysis, we divided the duration of rotating shift work into three categories as never, 1 – 14, and ≥ 15 years as main exposure. When appropriate, we calculated cumulative averages for covariates including consumptions of alcohol, vitamin D, folate, calcium, red meat and processed meat. For each covariate with missing data (generally 2 – 3%), we assigned a separate “missing” indicator to include those participants in the multivariable Cox models. We found no violation of the proportional hazard assumption.
To retain statistical power in subgroup analysis, tumors were classified as IRS1- or IRS2-positive (moderate / intense) and IRS1- or IRS2-negative (negative / weak) with the score ranging from 3 to 4 and 1 to 2, respectively. We examined the statistical significance of the difference in associations according to cancer subtypes using the likelihood ratio test that compared the model fit that allowed separate associations by different tumor IRS1 or IRS2 expression status with the model fit that assumed a common effect. Linear trend tests were conducted using the median of each category of night-shift work duration as a continuous variable, and the P value for trend was calculated using a Wald test. We also conducted a sensitivity analysis using inverse probability weighting method as previously described to reduce the potential bias due to the availability of tumor samples.(34,35) We used duplication-method Cox regression cause-specific hazards regression for competing risk data (34) to assess the association between night-shift work and risks of colorectal cancer subtypes by IRS1 or IRS2. In this competing risk model, the outcome of interest is the incidence of a specific subtype of CRC, but not CRC, and therefore, cases without the specific biomarker data were treated as censored as the diagnosis of colorectal cancer. The weight was set as the reciprocal of the predictive probability for each case with the corresponding IRS1 or IRS2 marker, whereas, it was set as 1 for non-cases or cases without the corresponding IRS1 or IRS2 marker in the weighted Cox regression models.
We did a secondary data analysis stratified by primary tumor location (colon vs. rectum). All analyses were performed using the SAS software (SAS Institute, Version 9.2, Cary, NC), and a two-sided P value less than 0.05 was considered statistically significant for the overall risk testing. For subtype analysis, the primary hypothesis test was the heterogeneity in the association with various colorectal cancer subtypes. To account for multiple testing for two biomarkers (IRS1 and IRS2), we adjusted for the statistical significance level to 0.025 (0.05/2).
RESULTS
Among 77,470 eligible participants reporting their night-shift work history in 1988 with 1,708,790 person-years of follow up, we documented a total of 1,397 incident colorectal cancer cases, of which 304 or 308 had available IRS1 or IRS2 expression data respectively (Figure 1). Compared with women who never worked rotating night-shifts, women with longer duration of rotating night-shift work were more likely to be a smoker, overweight, sleepless, and developing type 2 diabetes (Table 1). Additionally, demographic or clinical features were similar according to availability of tumor IRS1 or IRS2 status (Supplementary Table S1). Among the CRC cases with available tissue for IRS1 or IRS2 expression analysis, 86 (28.2%) and 102 (33.1%) had moderate or intense IRS1 and IRS2 expression, respectively.
Table 1.
Age-adjusted baseline characteristics of participants by night-shift work duration in the Nurses’ Health Study (Women, at 1988)
| Night-shift work duration |
|||
|---|---|---|---|
| Characteristic | Never | 1 - 14 years | ≥ 15 years |
| N of participants | 31,382 | 40,359 | 5,729 |
| Age, years* | 54.7 (7.2) | 54.7 (7.1) | 54.8 (7.1) |
| Race (White), % | 97.9 | 97.5 | 96.5 |
| Body mass index, kg/m2 | 25.3 (4.8) | 25.6 (4.9) | 26.9 (5.5) |
| Family history of colorectal cancer, % | 11.4 | 11.6 | 12.1 |
| History of sigmoidoscopy / endoscopy, % | 12.4 | 12.6 | 11.4 |
| Postmenopausal status, % | 72.1 | 72.5 | 76.0 |
| Postmenopausal hormone use, % | 37.5 | 38.1 | 35.5 |
| Total activity, METS - hours/week§ | 14.6 (20.8) | 16.0 (22.0) | 16.6 (24.0) |
| Regular aspirin use (2 or more tablets/week), % | 39.3 | 40.9 | 42.6 |
| Smoking status, % | |||
| Never, % | 45.6 | 43.3 | 42.2 |
| Smoking, pack years 0 −10, % | 16.8 | 17.4 | 14.4 |
| Pack years > 10, % | 36.3 | 37.9 | 41.7 |
| Total alcohol intake, g/day | 6.2 (10.6) | 6.3 (10.7) | 5.2 (10.3) |
| Total vitamin D, IU/day | 341 (252) | 343 (253) | 337 (255) |
| Total folate intake, μg/day | 403 (221) | 407 (224) | 395 (218) |
| Total energy intake, kcal/day | 1747 (519) | 1782 (525) | 1789 (556) |
| Red meat, servings/week | 2.2 (1.4) | 2.2 (1.4) | 2.2 (1.4) |
| Processed meat, servings/week | 1.0 (1.3) | 1.0 (1.3) | 1.1 (1.3) |
| Total calcium intake, mg/day | 1093 (514) | 1088 (507) | 1056 (508) |
| Sleep duration, % | |||
| Sleep < 6h, % | 3.1 | 3.8 | 8.4 |
| Sleep 6h -< 7h, % | 20.5 | 22.4 | 28.2 |
| Sleep 7h -< 8h, % | 50.2 | 49.5 | 44.0 |
| Sleep 8h -< 9h, % | 21.9 | 20.3 | 15.7 |
| Sleep ≥ 9h, % | 4.3 | 3.9 | 3.6 |
| History of type 2 diabetes, % | 4.1 | 4.3 | 6.7 |
Values were means ± standard deviation (SD) or percentages and were standardized to the age distribution of the study population.
Value was not age adjusted.
METS, metabolic equivalent task score.
Consistent with our previous report,(4) we observed a trend of increased overall risk of CRC with at least 15 years of night-shift work (Ptrend = 0.06). Additionally, this positive association appeared to persist when we restricted our analyses to women with available IRS1 or IRS2 expression data (Table 2). We also examined the association between night-shift work duration and CRC risk by primary tumor sites. We found a similar significant trend of increasing risk of rectal cancer (15+years vs. never: multivariable HR=1.54; 95%CI, 1.03 to 2.29) as in Papantoniou et al (same comparison, multivariable HR=1.60; 95%CI, 1.09 to 2.34).
Table 2.
Night-shift work duration and colorectal cancer risk according to tumor IRS1 and IRS2 expression status in the Nurses’ Health Study
| Night-shift work duration |
|||||
|---|---|---|---|---|---|
| Never | 1 - 14 years | ≥ 15 years | Ptrend* | Pheterogeneity¶ | |
| Total colorectal cancer in the full cohorts | |||||
| No. cases (N = 1,397) | 536 | 718 | 143 | ||
| Age-adjusted HR (95% CI) | 1 (ref) | 1.01 (0.90 to 1.13) | 1.28 (1.06 to 1.54) | 0.008 | |
| Multivariable HR (95% CI)§ | 1 (ref) | 1.01 (0.90 to 1.13) | 1.20 (0.99 to 1.45) | 0.06 | |
| Total colorectal cancer among women with IRS1 data | |||||
| No. cases (N = 304) | 122 | 146 | 36 | ||
| Age-adjusted HR (95% CI) | 1 (ref) | 0.91 (0.71 to 1.16) | 1.42 (0.97 to 2.06) | 0.05 | |
| Multivariable HR (95% CI)§ | 1 (ref) | 0.90 (0.70 to 1.14) | 1.31 (0.89 to 1.91) | 0.13 | |
| IRS1 | |||||
| Negative / weak | |||||
| No. cases (N = 218) | 90 | 105 | 23 | ||
| Age-adjusted HR (95% CI) | 1 (ref) | 0.88 (0.66 to 1.17) | 1.23 (0.77 to 1.94) | 0.36 | 0.02 |
| Multivariable HR (95% CI)§ | 1 (ref) | 0.87 (0.65 to 1.15) | 1.13 (0.71 to 1.80) | 0.56 | 0.02 |
| Moderate / intense | |||||
| No. cases (N = 86) | 32 | 41 | 13 | ||
| Age-adjusted HR (95% CI) | 1 (ref) | 0.98 (0.62 to 1.56) | 1.96 (1.02 to 3.77) | 0.03 | |
| Multivariable HR (95% CI)§ | 1 (ref) | 0.97 (0.61 to 1.54) | 1.81 (0.94 to 3.48) | 0.06 | |
| Total colorectal cancer among women with IRS2 data | |||||
| No. cases (N = 308) | 119 | 153 | 36 | ||
| Age-adjusted HR (95% CI) | 1 (ref) | 0.98 (0.77 to 1.25) | 1.46 (1.00 to 2.13) | 0.04 | |
| Multivariable HR (95% CI)§ | 1 (ref) | 0.97 (0.76 to 1.23) | 1.35 (0.92 to 1.97) | 0.11 | |
| IRS2 | |||||
| Negative / weak | |||||
| No. cases (N = 206) | 90 | 98 | 18 | ||
| Age-adjusted HR (95% CI) | 1 (ref) | 0.83 (0.62 to 1.11) | 0.98 (0.59 to 1.63) | 0.95 | 0.008 |
| Multivariable HR (95% CI)§ | 1 (ref) | 0.83 (0.62 to 1.10) | 0.90 (0.54 to 1.51) | 0.72 | 0.008 |
| Moderate / intense | |||||
| No. cases (N = 102) | 29 | 55 | 18 | ||
| Age-adjusted HR (95% CI) | 1 (ref) | 1.46 (0.93 to 2.29) | 2.92 (1.61 to 5.30) | 0.0004 | |
| Multivariable HR (95% CI)§ | 1 (ref) | 1.42 (0.90 to 2.22) | 2.69 (1.48 to 4.89) | 0.001 | |
CI, confidence interval; HR, hazard ratio.
Duplication-method Cox proportional cause-specific hazards regression for competing risks data was used to compute HRs and 95% CIs.
All analyses were stratified by age (in month) and year of questionnaire return.
Linear trend test using the median years of each category.
The likelihood ratio test was used to test for the heterogeneity of the associations between night-shift work duration (median) and colorectal cancer risk according to the expression of IRS1 and IRS2 (ordinal).
Multivariable hazard ratios were adjusted for age (in month), adult BMI (< 25, 25 -< 27.5, 27.5 -< 30, or ≥ 30 kg/m2), smoking (0, 1-10, or > 10 pack-years), history of colorectal cancer in a parent or sibling (yes or no), history of sigmoidoscopy/colonoscopy (yes or no), postmenopausal status and hormone use (premenopause, postmenopause and never use hormone, postmenopause and current use hormone, postmenopause and past use hormone), physical activity (< 3, 3 -< 27, ≥ 27 METS - hours/week), regular aspirin use (yes or no), alcohol consumption (0 -< 5, 5 -< 15, or ≥ 15 g/day), total intake of vitamin D, folate, calcium, red meat and processed meat (all in tertiles), sleep duration (< 6h, 6 -< 7h, 7 -< 8h, 8 -< 9h, or ≥ 9h), and history of type 2 diabetes (yes or no).
We then tested our primary hypothesis that the association between duration of rotating night-shift work and CRC risk might differ according to IRS1 or IRS2 expression. We found that the positive association of longer duration of rotating night-shift work appeared to differ by tumoral IRS1 or IRS2 status. Compared with women who never worked rotating night-shifts, women with at least 15 years of rotating night-shift work had a trend of an increased risk for IRS1-positive tumors (multivariable HR = 1.81, 95%CI 0.94 to 3.48, Ptrend = 0.06), but not for IRS1-negative tumors (multivariable HR = 1.13, 95%CI 0.71 to 1.80, Ptrend = 0.56, Pheterogeneity for IRS1 subtypes = 0.02). Likewise, a stronger association was observed for the IRS2-positive tumors (multivariable HR = 2.69, 95% CI 1.48 to 4.89, Ptrend = 0.001) but not for the IRS2-negative tumors (multivariable HR = 0.90, 95% CI 0.54 to 1.51, Ptrend = 0.72, Pheterogeneity for IRS2 subtypes = 0.008) (Table 2).
In order to reduce possible bias due to the availability of tumor specimens after diagnosis of CRC, we conducted a sensitivity analysis using the inverse probability weighting (IPW) method as previously described. We observed similar differential associations by both IRS1 (Pheterogeneity = 0.001) and IRS2 status (Pheterogeneity = 0.001) (Supplementary Table S2). In addition, the similar pattern was observed regardless of tumor locations in either colon or rectum, although the heterogeneity test did not reach statistical significance (Supplementary Table S3).
DISCUSSION
As the third most commonly diagnosed cancer both in women and men in US and worldwide,(36,37) CRC comprises a group of heterogeneous diseases in which each tumor arises and behaves in a unique fashion due to its distinctive genetic and epigenetic background. The potential pro-tumorigenic effects of night-shift work on CRC may thus differ by specific tumor molecular subtypes. In this large U.S. prospective cohort of nurses, we found that working a rotating night-shift for at least 15 years was associated with higher risk of IRS2-positive and had a trend of higher risk of IRS1-positive CRCs, but not negative tumors, compared with women who never worked rotating night-shifts.
Consistent with previous studies including Schernhammer’s in 2003,(3–5) we observed positive associations between rotating night-shift work and CRC risk. However, Papantoniou et al published an updated analysis of Schernhammer et al of night shift work and CRC in NHS, and newly adding data from the NHS2 cohort,(9) which we did not include in the current paper due to lack of tumor marker data in NHS2. Our findings regarding to overall association of night-shift work duration (i.e., 15+ years night shift work vs. never) and CRC risk in the NHS cohort (multivariable HR=1.20, 95%CI: 0.99 to 1.45) are consistent with these results (multivariable HR=1.15, 95%CI: 0.95 to 1.39). There were also some differences in the inclusion criteria and covariate adjustment between these two studies. Specifically, Papantoniou included 130 additional CRC cases (N=1,527) compared to ours (N=1,397), as these 130 CRC cases were histologic subtypes of malignancies (carcinoid, leiomyosarcoma, and squamous cell cancer), which may have different pathogenesis and were therefore not suitable for MPE analysis. Lastly, we adjusted for additional covariates in our paper that were not included in Papantoniou’s paper, all of which likely contributed to the slight difference in magnitude of the aforementioned associations.
Working at night and rotating shifts could lead to a series of unfavorable alterations of the sleep cycle and cell cycle,(38) lipid and carbohydrate metabolism, and insulin resistance.(39,40) Because these alterations play a role in regulation of cell proliferation, the observed positive association is biological plausible.(1,41) The insulin resistance system involving the insulin receptor (INSR) and IGF1R pathways is the primary system responsible for many manifestations of metabolic disorders. Insulin is also considered as a growth factor for tumor formation by stimulating proliferation, inhibiting apoptosis or activating the INSR and IGF1R pathway.(42) Recent population studies showed that circulation IGF1 and IGF2, and some of genetic variants in the INSR or IGF1R pathway (such as single-nucleotide polymorphisms in IGF1, IGFBP3, INSR and IRS), were associated with CRC risk. (43–48) Therefore, the influence of night-shift working on colorectal cancer might partially act through the INSR and IGF1R pathway. The positive associations of CRC risk and night-shift work observed for IRS2-positive tumors, and the marginally significant association for IRS1-positive tumors support this possibility.
IRS proteins are a family of cytoplasmic proteins composed of six members (IRS1 to 6) that regulate numerous processes such as growth, metabolism, survival and proliferation.(49) IRS1 and IRS2 were identified as the first two dominant members of the IRS family, which act as the mediators of the INSR and IGF1R pathway and play a central role in maintaining diverse cellular functions, such as metabolism and proliferation.(24,50) In normal metabolic regulation, these proteins contribute to the insulin-regulated glucose homeostasis through promoting glucose uptake and utilization, and regulating the biosynthesis of macromolecules that are required for cell growth and proliferation.(50) When human circadian rhythms are disrupted, such as in night-shift workers, glucose homeostasis is dysregulated, leading to hyperinsulinemia and insulin insensitivity, as well as potentially insulin resistance. In vitro, high levels of insulin may stimulate IRS1 tyrosine phosphorylation, which is associated with activation of PI3K / AKT and MAPK pathway, and mitogenesis in mouse skeletal muscle cells.(19) Similarly, chronic insulin exposure may be associated with IRS1 and IRS2 expression, AKT activation and chemo-resistance in some colon cancer cells.(18) Phosphorylation of IRS1 tyrosine sites could activate downstream pathways including PI3K / AKT, MAPK, and PAK1, which increase proliferation and cell survival in cancer cell.(51,52) Many studies have focused on the increased expression level or activity of IRSs in different human cancers including colorectal cancer, and correlated these with poor prognosis, potentially defining them as oncogenic proteins.(23,53) In light of this evidence, night-shift workers may experience different degrees of metabolic disorders such as insulin oscillations or hyperinsulinemia, which can stimulate IRSs and its downstream signaling. This disruption can eventually result in tumor occurrence as the duration of exposure (i.e., night-shift work) increases. Hence, it is plausible that the higher risk of longer duration of shift work appeared in IRSs-positive CRC but not IRSs-negative tumors.
We also observed slightly stronger positive associations with IRS2-positive than IRS1-positive tumors, suggesting a possible different role of IRS1 and IRS2 in tumorigenesis. To date, most such research has focused on breast cancer. Using the PyV-MT mouse model of mammary tumor progression, it was reported that tumor onset and growth were equivalent in the absence of either IRS1 or IRS2.(54,55) However, the absence of IRS2 was associated with the regression of mammary tumor metastasis but IRS1 cannot compensate for this loss.(55) And in irs1−/− tumors, IRS2 activation was enhanced and associated with a higher frequency of metastasis.(54) Moreover, IRS1 was expressed predominantly in estrogen receptor positive, well-differentiated breast cancer cell lines, whereas IRS2 was expressed in estrogen receptor negative, poorly-differentiated metastatic breast cancer cells.(56,57) Taken together, these studies suggest that IRS2 might play a different role than IRS1 in tumor initiation, aggressiveness, and progression. Further functional studies in colon cancer pre-clinical models are warranted to further clarify these potential biological mechanisms.
Our study has several strengths, including the prospective design with a large sample size, long-term follow-up with high follow-up rate, and validated CRC outcomes. The repeated assessments of a variety of dietary and lifestyle risk factors allowed better confounding control. Furthermore, the availability of tumor IRS1 and IRS2 data in these cohorts enabled us to identify tumor subtypes that are more susceptible to night-shift work, which provide potential mechanistic insights.
Our study has some potential limitations. First, information on lifetime shift work exposure was self-reported, and only inquire once with no further updates beyond 1988. We are unable to evaluate the impact of changes or different intensities or patterns of night-shift work. However, it is likely that these self-reported data among these nurses were reliable because other self-reported measures by these nurses have been reasonably accurate.(4) Second, not all colorectal cancer cases in the NHS cohort have tumor specimen data from which we can assess their IRS status. However, patients with or without IRS data were highly comparable. Additionally, to address possible bias due to the availability of tumor specimens, we used IPW in sensitivity analyses and results remained essentially unchanged. Nonetheless, the number of cases with IRS data in our study was limited and chance can therefore not be ruled out. Finally, due to sparse data in certain tumor subtype analyses especially in the long-term shift worker group, we could not have enough power to analyze these association by stratify or adjust for other potential confounding molecular features. So, these results should be interpreted cautiously.
In conclusion, our prospective cohort study showed that working at least 15 years of rotating night-shift was associated with higher risk of CRC, particularly for IRS2-positive tumors, and with a trend for higher risk of IRS1-positive CRCs with increasing duration of night shift work. Our findings suggest a role of IRSs, especially for IRS2, in mediating pro-tumorigenic effects of night-shift work on CRC. Future studies with more available tumor specimens and functional experiments are needed to confirm these findings and better clarify the underlying mechanisms.
Supplementary Material
Acknowledgement
We would like to thank the participants and staff of the Nurses’ Health Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data. This work was supported by U.S. National Institutes of Health (NIH) grants (P01 CA87969, UM1 CA186107 to M.J. Stampfer; P50 CA127003 to C.S.F.; R01 CA137178, K24 DK098311 to A.T.C.; R01 CA151993, R35 CA197735 to S.O.; R01 OH009803 to E.S.S., K07 CA190673 to R.N.; and R03 CA176717, K07 CA188126 to X.Z.); Nodal Award (to S.O.) from the Dana-Farber Harvard Cancer Center; and by grants from The Project P Fund for Colorectal Cancer Research, The Friends of the Dana-Farber Cancer Institute, Bennett Family Fund, and the Entertainment Industry Foundation through National Colorectal Cancer Research Alliance. Y.S. is supported by the grants from National Natural Science Foundation of China No.81402016, Beijing Municipal Natural Science Foundation No.7152140 and Beijing Nova Program XXJH2015B098. L.L. is supported by a grant from National Natural Science Foundation of China No. 81302491, a scholarship grant from Chinese Scholarship Council and a fellowship grant from Huazhong University of Science and Technology. K.K. is supported by a grant from Overseas Research Fellowship from Japanese Society for the Promotion of Science (JP2017-775). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Role of the sponsors: The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Abbreviations:
- CI
confidence interval
- CRC
colorectal cancer
- FFPE
formalin-fixed paraffin-embedded
- FFQ
food frequency questionnaire
- HR
hazard ratio
- IGF1R
insulin like growth factor 1 receptor
- INSR
insulin receptor
- IRS
insulin receptor substrate
- METS
metabolic equivalent task score
- MPE
molecular pathological epidemiology
- NHS
Nurses’ Health Study
- TBS
Tris-buffered saline
- TMA
tissue microarrays
Footnotes
Use of standardized official symbols: We use HUGO (Human Genome Organisation)-approved official symbols (or root symbols) for genes and gene products, including AKT, IGF1R, INSR, IRS1, IRS2, and PIK3CA; all of which are described at www.genenames.org. The official symbols are italicized to differentiate from non-italicized colloquial names that are used along with the official symbols. This format enables readers to familiarize themselves with the official symbols for genes and gene products together with common colloquial names.
Conflict of interest statement: None declared.
REFERENCES
- 1.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of shift-work, painting, and fire-fighting. The Lancet Oncology 2007;8(12):1065–6 doi 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- 2.group IMV. Carcinogenicity of night shift work. The Lancet Oncology 2019. doi 10.1016/S1470-2045(19)30455-3. [DOI] [PubMed] [Google Scholar]
- 3.Papantoniou K, Castano-Vinyals G, Espinosa A, Turner MC, Alonso-Aguado MH, Martin V, et al. Shift work and colorectal cancer risk in the MCC-Spain case-control study. Scandinavian journal of work, environment & health 2017;43(3):250–9 doi 10.5271/sjweh.3626. [DOI] [PubMed] [Google Scholar]
- 4.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, et al. Night-shift work and risk of colorectal cancer in the nurses’ health study. Journal of the National Cancer Institute 2003;95(11):825–8. [DOI] [PubMed] [Google Scholar]
- 5.Tsai RJ, Luckhaupt SE, Sweeney MH, Calvert GM. Shift work and cancer screening: do females who work alternative shifts undergo recommended cancer screening? Am J Ind Med 2014;57(3):265–75 doi 10.1002/ajim.22285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parent ME, El-Zein M, Rousseau MC, Pintos J, Siemiatycki J. Night work and the risk of cancer among men. American journal of epidemiology 2012;176(9):751–9 doi 10.1093/aje/kws318. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Ji A, Zhu Y, Liang Z, Wu J, Li S, et al. A meta-analysis including dose-response relationship between night shift work and the risk of colorectal cancer. Oncotarget 2015;6(28):25046–60 doi 10.18632/oncotarget.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartzbaum J, Ahlbom A, Feychting M. Cohort study of cancer risk among male and female shift workers. Scandinavian journal of work, environment & health 2007;33(5):336–43. [DOI] [PubMed] [Google Scholar]
- 9.Papantoniou K, Devore EE, Massa J, Strohmaier S, Vetter C, Yang L, et al. Rotating night shift work and colorectal cancer risk in the nurses’ health studies. International journal of cancer 2018;143(11):2709–17 doi 10.1002/ijc.31655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogino S, Nowak JA, Hamada T, Milner DA Jr., Nishihara R. Insights into Pathogenic Interactions Among Environment, Host, and Tumor at the Crossroads of Molecular Pathology and Epidemiology. Annu Rev Pathol 2019;14:83–103 doi 10.1146/annurev-pathmechdis-012418-012818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang K, Sun Y, Lin P, Song J, Zhao R, Li W, et al. Liraglutide Activates AMPK Signaling and Partially Restores Normal Circadian Rhythm and Insulin Secretion in Pancreatic Islets in Diabetic Mice. Biological & pharmaceutical bulletin 2015;38(8):1142–9 doi 10.1248/bpb.b15-00024. [DOI] [PubMed] [Google Scholar]
- 12.Saini C, Petrenko V, Pulimeno P, Giovannoni L, Berney T, Hebrok M, et al. A functional circadian clock is required for proper insulin secretion by human pancreatic islet cells. Diabetes, obesity & metabolism 2016;18(4):355–65 doi 10.1111/dom.12616. [DOI] [PubMed] [Google Scholar]
- 13.Ulhoa MA, Marqueze EC, Burgos LG, Moreno CR. Shift work and endocrine disorders. Int J Endocrinol 2015;2015:826249 doi 10.1155/2015/826249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sookoian S, Gemma C, Fernandez Gianotti T, Burgueno A, Alvarez A, Gonzalez CD, et al. Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. J Intern Med 2007;261(3):285–92 doi 10.1111/j.1365-2796.2007.01766.x. [DOI] [PubMed] [Google Scholar]
- 15.Esquirol Y, Bongard V, Ferrieres J, Verdier H, Perret B. Shiftwork and higher pancreatic secretion: early detection of an intermediate state of insulin resistance? Chronobiology international 2012;29(9):1258–66 doi 10.3109/07420528.2012.719959. [DOI] [PubMed] [Google Scholar]
- 16.Korsiak J, Tranmer J, Day A, Aronson KJ. Sleep duration as a mediator between an alternating day and night shift work schedule and metabolic syndrome among female hospital employees. Occupational and environmental medicine 2018;75(2):132–8 doi 10.1136/oemed-2017-104371. [DOI] [PubMed] [Google Scholar]
- 17.Hulsegge G, Boer JM, van der Beek AJ, Verschuren WM, Sluijs I, Vermeulen R, et al. Shift workers have a similar diet quality but higher energy intake than day workers. Scandinavian journal of work, environment & health 2016;42(6):459–68 doi 10.5271/sjweh.3593. [DOI] [PubMed] [Google Scholar]
- 18.Baricevic I, Roberts DL, Renehan AG. Chronic insulin exposure does not cause insulin resistance but is associated with chemo-resistance in colon cancer cells. Horm Metab Res 2014;46(2):85–93 doi 10.1055/s-0033-1354414. [DOI] [PubMed] [Google Scholar]
- 19.Conejo R, Lorenzo M. Insulin signaling leading to proliferation, survival, and membrane ruffling in C2C12 myoblasts. J Cell Physiol 2001;187(1):96–108 doi . [DOI] [PubMed] [Google Scholar]
- 20.Giovannucci E Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr 2001;131(11 Suppl):3109S–20S doi 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 21.Lu CC, Chu PY, Hsia SM, Wu CH, Tung YT, Yen GC. Insulin induction instigates cell proliferation and metastasis in human colorectal cancer cells. Int J Oncol 2017;50(2):736–44 doi 10.3892/ijo.2017.3844. [DOI] [PubMed] [Google Scholar]
- 22.White MF. IRS proteins and the common path to diabetes. American journal of physiology Endocrinology and metabolism 2002;283(3):E413–22 doi 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]
- 23.Mardilovich K, Pankratz SL, Shaw LM. Expression and function of the insulin receptor substrate proteins in cancer. Cell communication and signaling : CCS 2009;7:14 doi 10.1186/1478-811X-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw LM. The insulin receptor substrate (IRS) proteins: at the intersection of metabolism and cancer. Cell cycle 2011;10(11):1750–6 doi 10.4161/cc.10.11.15824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belanger CF, Hennekens CH, Rosner B, Speizer FE. The nurses’ health study. The American journal of nursing 1978;78(6):1039–40. [PubMed] [Google Scholar]
- 26.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Annals of internal medicine 1995;122(5):327–34. [DOI] [PubMed] [Google Scholar]
- 27.Colditz GA, Hankinson SE. The Nurses’ Health Study: lifestyle and health among women. Nature reviews Cancer 2005;5(5):388–96 doi 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 28.Wegrzyn LR, Tamimi RM, Rosner BA, Brown SB, Stevens RG, Eliassen AH, et al. Rotating Night-Shift Work and the Risk of Breast Cancer in the Nurses’ Health Studies. American journal of epidemiology 2017;186(5):532–40 doi 10.1093/aje/kwx140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. American journal of epidemiology 1992;135(10):1114–26; discussion 27-36. [DOI] [PubMed] [Google Scholar]
- 30.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. Journal of the American Dietetic Association 1993;93(7):790–6. [DOI] [PubMed] [Google Scholar]
- 31.Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, et al. A prospective study of sleep duration and mortality risk in women. Sleep 2004;27(3):440–4. [DOI] [PubMed] [Google Scholar]
- 32.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. The New England journal of medicine 2007;356(21):2131–42 doi 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 33.Hanyuda A, Kim SA, Martinez-Fernandez A, Qian ZR, Yamauchi M, Nishihara R, et al. Survival Benefit of Exercise Differs by Tumor IRS1 Expression Status in Colorectal Cancer. Ann Surg Oncol 2016;23(3):908–17 doi 10.1245/s10434-015-4967-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang M, Spiegelman D, Kuchiba A, Lochhead P, Kim S, Chan AT, et al. Statistical methods for studying disease subtype heterogeneity. Statistics in medicine 2016;35(5):782–800 doi 10.1002/sim.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu L, Nevo D, Nishihara R, Cao Y, Song M, Twombly TS, et al. Utility of inverse probability weighting in molecular pathological epidemiology. European journal of epidemiology 2017. doi 10.1007/s10654-017-0346-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians 2018;68(1):7–30 doi 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 37.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a cancer journal for clinicians 2015;65(2):87–108 doi 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 38.Greene MW. Circadian rhythms and tumor growth. Cancer letters 2012;318(2):115–23 doi 10.1016/j.canlet.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Al-Naimi S, Hampton SM, Richard P, Tzung C, Morgan LM. Postprandial metabolic profiles following meals and snacks eaten during simulated night and day shift work. Chronobiology international 2004;21(6):937–47. [DOI] [PubMed] [Google Scholar]
- 40.Morgan L, Hampton S, Gibbs M, Arendt J. Circadian aspects of postprandial metabolism. Chronobiology international 2003;20(5):795–808. [DOI] [PubMed] [Google Scholar]
- 41.Fritschi L, Glass DC, Heyworth JS, Aronson K, Girschik J, Boyle T, et al. Hypotheses for mechanisms linking shiftwork and cancer. Medical hypotheses 2011;77(3):430–6 doi 10.1016/j.mehy.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Gupta K, Krishnaswamy G, Karnad A, Peiris AN. Insulin: a novel factor in carcinogenesis. The American journal of the medical sciences 2002;323(3):140–5. [DOI] [PubMed] [Google Scholar]
- 43.Chi F, Wu R, Zeng YC, Xing R, Liu Y. Circulation insulin-like growth factor peptides and colorectal cancer risk: an updated systematic review and meta-analysis. Mol Biol Rep 2013;40(5):3583–90 doi 10.1007/s11033-012-2432-z. [DOI] [PubMed] [Google Scholar]
- 44.de Kort S, Simons C, van den Brandt PA, Janssen-Heijnen MLG, Sanduleanu S, Masclee AAM, et al. Diabetes mellitus, genetic variants in the insulin-like growth factor pathway and colorectal cancer risk. International journal of cancer 2019. doi 10.1002/ijc.32365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung SY, Zhang ZF. The effects of genetic variants related to insulin metabolism pathways and the interactions with lifestyles on colorectal cancer risk. Menopause 2019;26(7):771–80 doi 10.1097/GME.0000000000001301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pechlivanis S, Pardini B, Bermejo JL, Wagner K, Naccarati A, Vodickova L, et al. Insulin pathway related genes and risk of colorectal cancer: INSR promoter polymorphism shows a protective effect. Endocr Relat Cancer 2007;14(3):733–40 doi 10.1677/ERC-07-0107. [DOI] [PubMed] [Google Scholar]
- 47.Pechlivanis S, Wagner K, Chang-Claude J, Hoffmeister M, Brenner H, Forsti A. Polymorphisms in the insulin like growth factor 1 and IGF binding protein 3 genes and risk of colorectal cancer. Cancer Detect Prev 2007;31(5):408–16 doi 10.1016/j.cdp.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 48.Simons CC, Schouten LJ, Godschalk RW, van Engeland M, van den Brandt PA, van Schooten FJ, et al. Genetic Variants in the Insulin-like Growth Factor Pathway and Colorectal Cancer Risk in the Netherlands Cohort Study. Sci Rep 2015;5:14126 doi 10.1038/srep14126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Machado-Neto JA, Fenerich BA, Rodrigues Alves APN, Fernandes JC, Scopim-Ribeiro R, Coelho-Silva JL, et al. Insulin Substrate Receptor (IRS) proteins in normal and malignant hematopoiesis. Clinics (Sao Paulo) 2018;73(suppl 1):e566s doi 10.6061/clinics/2018/e566s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong X, Park S, Lin X, Copps K, Yi X, White MF. Irs1 and Irs2 signaling is essential for hepatic glucose homeostasis and systemic growth. The Journal of clinical investigation 2006;116(1):101–14 doi 10.1172/JCI25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang XF, Chen JZ. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes Rev 2009;10(6):610–6 doi 10.1111/j.1467-789X.2009.00607.x. [DOI] [PubMed] [Google Scholar]
- 52.Ding XZ, Fehsenfeld DM, Murphy LO, Permert J, Adrian TE. Physiological concentrations of insulin augment pancreatic cancer cell proliferation and glucose utilization by activating MAP kinase, PI3 kinase and enhancing GLUT-1 expression. Pancreas 2000;21(3):310–20. [DOI] [PubMed] [Google Scholar]
- 53.Dearth RK, Cui X, Kim HJ, Kuiatse I, Lawrence NA, Zhang X, et al. Mammary tumorigenesis and metastasis caused by overexpression of insulin receptor substrate 1 (IRS-1) or IRS-2. Molecular and cellular biology 2006;26(24):9302–14 doi 10.1128/MCB.00260-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma Z, Gibson SL, Byrne MA, Zhang J, White MF, Shaw LM. Suppression of insulin receptor substrate 1 (IRS-1) promotes mammary tumor metastasis. Molecular and cellular biology 2006;26(24):9338–51 doi 10.1128/MCB.01032-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagle JA, Ma Z, Byrne MA, White MF, Shaw LM. Involvement of insulin receptor substrate 2 in mammary tumor metastasis. Molecular and cellular biology 2004;24(22):9726–35 doi 10.1128/MCB.24.22.9726-9735.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shaw LM. Identification of insulin receptor substrate 1 (IRS-1) and IRS-2 as signaling intermediates in the alpha6beta4 integrin-dependent activation of phosphoinositide 3-OH kinase and promotion of invasion. Molecular and cellular biology 2001;21(15):5082–93 doi 10.1128/MCB.21.15.5082-5093.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jackson JG, White MF, Yee D. Insulin receptor substrate-1 is the predominant signaling molecule activated by insulin-like growth factor-I, insulin, and interleukin-4 in estrogen receptor-positive human breast cancer cells. The Journal of biological chemistry 1998;273(16):9994–10003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


