Abstract
Background:
The insula has a well-established role in nicotine dependence and is a node of the salience network (SN), which integrates internal and external information to guide behavior. Recent findings reveal that internal and external processing occurs in the ventral and dorsal anterior insula (vAI/dAI), respectively. Whether vAI/dAI network connectivity differentially reflects internally-generated craving and externally-triggered smoking cue reactivity was tested.
Methods:
Thirty-six male and female nicotine-dependent individuals smoked 1 hour before functional magnetic resonance imaging. Baseline craving was measured, followed by resting-state and smoking cue-reactivity scans, and another assessment of craving. Craving and cue-reactivity interactions were measured by focusing on specific nodes of the SN: the vAI/dAI and anterior cingulate cortex (ACC).
Results:
Resting-state vAI/dAI networks overlapped with the prototypical SN, yet they possess distinct patterns, linking the vAI with nodes of the internally-focused default mode network (DMN) and the dAI with nodes of the external/goal-related frontoparietal network (FPN). Internally generated baseline craving was associated with enhanced vAI connectivity while rostral ACC (rACC) reactivity to external smoking cues was associated with greater dAI connectivity. We also found that cue reactivity in the rACC was associated with a rise in subjective cue-induced craving, while baseline subjective craving did not influence brain cue-reactivity.
Conclusions:
These data show that brain reactivity to smoking cues is associated with a subsequent increase in craving. Additionally, separate insula networks play a role in an individual’s vulnerability to internally-related craving and externally-triggered cue-reactivity, which may guide the development of new neurobiologically-targeted therapies.
Keywords: Anterior Insula, Smoking, Nicotine, Cues, Craving, Connectivity
Introduction
The insula’s role in nicotine dependence is well established as it has been implicated in emotional/internal states associated with craving, withdrawal, and in the goal-directed responsivity to external nicotine cues(1-4). Such findings in nicotine dependence reflect the insula’s global involvement in emotion, cognition, and emotion-cognition integration(5,6), supporting the concept that the insula contributes to nicotine dependence by integrating information from both internal and external triggers(7). However, the insula is not homogeneous as there is anatomic specialization within this region. Specifically, the ventral anterior insula (vAI) is linked with affect, while the dorsal anterior insula (dAI) is associated with goal-related behavior(8). Distinctions between the ventral/dorsal anterior insula have recently been re-conceptualized to suggest that the vAI is part of an internally-oriented system related more with the default mode network (DMN) and limbic regions, while the dAI is part of an externally-oriented system coupled with goal-directed functional networks(9; e.g., the frontoparietal network; FPN). Translating to nicotine dependence, we hypothesize that the vAI is involved in internally-generated craving, while the dAI plays a role in reactivity to external smoking cues.
The idea that cue-reactivity and baseline (unprovoked) craving are distinct factors is suggested by the literature. For example, cue-reactive and non-reactive individuals can be identified from a population that otherwise shows similar craving at baseline(10) and greater nicotine dependence is associated with more baseline craving, but not cue-reactivity(11). Such findings provide evidence that baseline craving does not necessarily predict how smokers respond to cues and that different elements trigger each type of craving. This is not to say that under certain situations baseline craving and cue-reactivity cannot influence each other, but the propensity to evoke each type of craving appears to be driven either by internal or external triggers.
Thus far, the existing literature on AI connectivity has focused on baseline craving. During nicotine withdrawal, functional integration is enhanced between the internally focused DMN and the salience network (SN), which includes the bilateral anterior insula and the anterior cingulate cortex(12, ACC). While AI sub-regions have not been evaluated, this network-based finding supports the idea that during withdrawal, when craving is enhanced by physiologic factors, there is more integration between functional networks involving the AI and those supporting internally directed attention. The present study extended this finding to verify the more specific relationship between baseline craving and vAI connectivity, while also confirming the role of dAI connectivity in cue-reactivity. Understanding the neurobiology contributing to cue-reactivity may also help explain why certain individuals display a cue-reactive phenotype, which is not necessarily related to endogenous craving levels(2,10,13-14), rendering such individuals more likely to relapse particularly when treated with therapies that do not attenuate cue-reactivity(2,15).
Inter-related analyses were chosen to address the central hypothesis that baseline craving and cue reactivity are dissociable factors related to the connectivity strength of the vAI and dAI, respectively. To achieve this goal, we built upon prior research to show that : 1) baseline subjective report of craving is not associated with subsequent reactivity to smoking cues, fitting with the work of others who showed no relationship between subjective baseline and cue-induced craving(10), and that 2) brain reactivity to smoking cues is associated with a rise in subjective craving following exposure to smoking cues(16-17). This second analysis also controls for baseline craving to better isolate the specific role of brain reactivity to smoking cues. We then assess the functional connectivity of the v/dAI networks and their associations with craving and brain cue-reactivity, respectively. Brain reactivity to smoking cues was calculated for nodes of the SN, which typically react to smoking cues(18). These a priori defined regions include the four AI sub-regions and dorsal and rostral anterior cingulate cortex (dACC and rACC, respectively). Of these regions, the rACC is the most consistently linked with cue-induced subjective report of craving(19-20) and stimulus-reward associations more broadly(21). Whether enhanced dAI connectivity prior to task performance is associated with the cue-induced activation of such regions may help determine how AI network connectivity enhances vulnerability for cue-triggered craving. Collectively, these analyses will aid in our understanding of factors driving the motivation to smoke and how neurobiological variance may contribute to individual vulnerabilities.
Methods
Participants
Participants included 36 nicotine-dependent individuals (13 women) who reported an interest in quitting smoking (Table 1). A Fagerström Test for Nicotine Dependence(22) (FTND) score ≥ 4 and an expired carbon monoxide concentration of ≥ 5 ppm at the time of screening were required. Serious medical illness, pregnancy (confirmed by urinalysis) drug or alcohol (except nicotine) dependence, major depressive disorder within the past 6 months, and current or lifetime history of schizophrenia, schizoaffective disorder, bipolar disorder, or psychotic disorders not otherwise specified (confirmed via Structured Clinical Interview for DSM (SCID) IV) were exclusionary criteria. Abstinence from drug/alcohol use was confirmed by urine/breath samples respectively (QuickTox11 Panel Drug Test Card, Branan Medical Corporation, Irvine California; Alco-Sensor IV, Intoximeters Inc., St. Louis, MO). All procedures were completed at McLean Hospital and were approved by the Partners Human Research Committee. Participants provided written and verbal informed consent after receiving a complete study description.
Table 1:
Demographics
| Means (standard deviation) | |
|---|---|
| Age (Years) | 29.66 (11.81) |
| Education (Years) | 14.94 (1.74) |
| FTND | 5.6 (1.61) |
| Average Cig/Day | 13.61 (5.86) |
| Pack -Year | 8.70 (7.56) |
| Age started smoking (Years) | 17.91 (3.89) |
| Years Smoked | 11.76 (7.29) |
| QSU (PRE) | 19.63 (7.86) |
| Positive Mood (Pre, PANAS) | 29.62 (6.57) |
| Negative Mood (Pre, PANAS) | 12.41 (3.55) |
Procedures
As in our prior research with an independent sample(23), participants were asked to smoke as usual prior to the study visit. To standardize all procedures relative to the last cigarette smoked, participants smoked one of their own cigarettes in the lab ~1 hour prior to scanning. To evaluate pre-cue exposure craving state, fifteen minutes prior to scanning, participants completed the Questionnaire of Smoking Urges(24; QSU). In the scanner, participants completed a 6-minute resting state scan where they were told to keep their eyes open and think of nothing in particular. Next, participants performed the smoking cue-reactivity task, which has been described elsewhere(23). Briefly, participants viewed smoking and neutral images matched for general content (e.g., hands holding a cigarette or a neutral object, faces in a neutral or smoking related context), and pressed a button when the target image of an animal was shown. Button presses confirmed participants were awake and attending to the task but were not included in subsequent analyses. After exiting the scanner, craving was measured again with the QSU and the change in craving was calculated (QSU post – QSU pre).
Functional neuroimaging
Scanning was performed on a Siemens Prisma 3T scanner (Erlagen, Germany) with a 64-channel head coil. Functional scans (resting and cue reactivity) were acquired with TR = 720 ms, TE = 30 ms, slices = 66, phase encode direction posterior to anterior, Flip angle = 66o voxel size = 2.5 × 2.5 × 2×5 mm, GRAPPA factor of 2, and a multi-band acceleration factor = 6. After all functional scans, multiecho multi-planar rapidly acquired gradient echo-structural images were acquired with the following: TR = 2530 ms, TE1= 3.3 ms, TE2 = 6.98 ms, TE3 = 8.79 ms, TE4 = 10.65 ms, flip angle 7o, resolution = 1.33 × 1 × 1 mm.
fMRI Pre-processing
Data were analyzed using tools from the Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL: www.fmrib.ox.ac.uk/fsl). Standard pre-processing was conducted on both the resting and task-based data including: motion correction with MCFLIRT, brain extraction using BET, slice time correction, spatial smoothing with a Gaussian kernel of full-width half-max of 6mm, and high pass filtering at 0.01 Hz. The only non-FSL tool used was spikefix (https://github.com/bbfrederick/spikefix), which evaluates fMRI data for motion/intensity spikes. For task-based data, these spikes are removed and single point confound regressors representing noise-related timepoints were generated. Resting-state data was denoised via independent component analysis using the FSL tool for multivariate exploratory linear decomposition into independent components (MELODIC). As in our prior studies(23,25,26), MELODIC was run for each subject and spatial/temporal information for each independent component was visually inspected to identify noise-related components, which were then regressed out of the resting-state fMRI data to generate a denoised fMRI timeseries.
Regions of Interest
All regions of interest (ROIs) were defined a priori. Four anatomically defined anterior insula (AI) sub-regions, divided into the right and left hemispheres and the dorsal and ventral components(27), were used as ROIs in both the resting state and task-based analyses. The ACC was only assessed during cue-reactivity and was divided into two ROIs based on our prior work - the dorsal ACC(23) and rostral ACC(25). All ROIs are shown in Supplemental Figure 1.
Cue Reactivity
Cue reactivity methods largely replicate our prior work(23). During each of the 5 cue-reactivity runs, 10 smoking, 10 neutral, and 2 target images were shown for 4 seconds each. Images were separated by a jittered inter-trial-interval averaging 10 seconds and ranging from 6 -14 seconds in steps of 2 seconds. Subject-level general linear models (GLMs) were implemented with task regressors representing the presentation of smoking, neutral, and target images convolved with the standard gamma heymodynamic response function. Confound regressors included motion time courses (x, y, z translation and rotation) and the motion/intensity artifacts defined by the spikefix tool described above. Given that participants completed 5 cue-reactivity runs, a second-level fixed effects GLM was implemented to combine the runs for each subject and then beta weights for the smoking vs. neutral contrast were extracted for each subject, e.g., the average value computed for each of the 6 ROIs, from the second-level regression maps.
Resting State Connectivity: AI Networks
A dual regression approach(28) was used to calculate subject specific time courses and spatial maps reflecting the functional connectivity for each of the four insula sub-regions. In the first step of dual regression, a whole brain multivariate spatial regression was conducted using spatial maps of all insula ROIs. These resulting time courses were then each normalized to unit variance and used as regressors in a second multivariate regression against each subject’s dataset to identify participant-specific spatial maps reflecting the functional connectivity for each insula sub-region in that participant. This approach allows the functional connectivity maps for each ROI to be disentangled into the set of networks for which each ROI is connected, while screening out any overlapping network connectivity with the other ROIs. These v/dAI connectivity maps were used for further statistical analyses to assess relationships with baseline craving and brain cue-reactivity.
Statistical Analyses
SPSS version 24 was used to conduct two regression analyses to confirm that pre-existing baseline craving does not influence subsequent cue-induced reactivity, while brain reactivity to smoking cues predicts the resulting change in craving. First, a multivariate regression was fit with baseline craving as the predictor and beta weights from the 6 ROIs for the smoking vs. neutral contrast as dependent variables. A second multiple linear regression was run with the 6 ROI beta weights for the smoking vs. neutral contrast as predictors and the change in QSU score as the dependent variable. Baseline craving was included in the model to control for this factor.
For resting state functional connectivity analyses, group-level analyses implemented GLMs with non-parametric permutation testing via FSL Randomise with cluster-based thresholding (Z = 3.1, number of permutations = 5000)(29) . This was done to determine relationships between 1) vAI network connectivity and baseline craving, and 2) dAI network connectivity and smoking cue-reactivity, with control of family-wise error at p<0.05. Predictors included baseline craving, and brain reactivity to smoking vs. neutral cues in the 6 ROIs noted above. The contrasts of interest were specific to the AI subregion and aimed to confirm that baseline craving corresponds with vAI connectivity while brain reactivity to smoking cues is related to dAI connectivity.
Results
Insula Resting State Networks
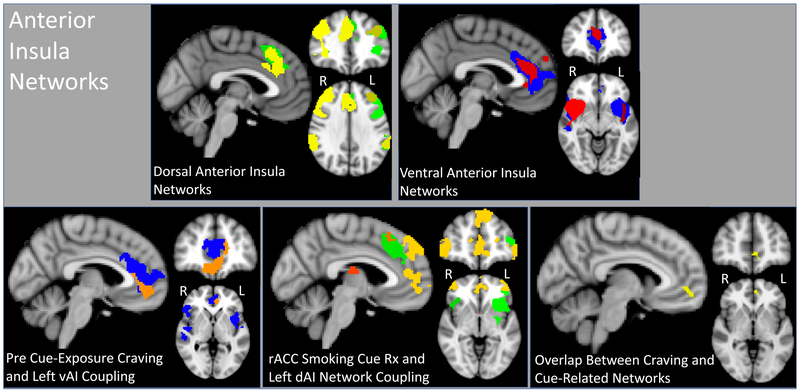
Group average maps for each of the four insula networks contained aspects of the typical SN yet showed distinct patterns (Figure 1). Ventral AI networks included more rostral portions of the ACC, with the left vAI network extending into the mPFC. Dorsal AI networks included caudal portions of the ACC extending dorsally into Brodmann (BA) area 8. Dorsal AI networks also overlapped with regions of the FPN, including the dorsolateral prefrontal cortex (DLPFC) and lateral parietal cortex. Dorsal AI networks were either right or left dominant, corresponding to the dAI ROI.
Figure 1: Anterior Insula networks and their relationship with baseline craving and cue reactivity.
Top two panels represent the group-level dorsal anterior insula (dAI: MNI coordinates X = 0, Y = 42, Z = −6) and ventral anterior insula (vAI: x = 0, y = 35, z = 32) networks. Yellow overlay represents the right dAI, green dAI, blue and red overly represents left vAI and right vAI, respectively. Bottom three panels show: 1) the association between the left vAI (blue) and baseline craving (associated rise in coupling shown in orange: X = −4, Y = 46, Z = 0), 2) the relationship between the left dAI (green) and rACC reactivity to smoking vs. neutral cues (associated rise in coupling shown in yellow/orange: X = −4, Y = 54, z = −2) and 3) the overlap between the baseline craving and rACC cue-reactivity networks (yellow: X = −4, Y = 50, Z = −2). Network maps were thresholded using a cluster-forming threshold of z = 3.1, and a family-wise error corrected threshold of p < 0.05.
Insula Network Connectivity Associated with Craving and Cue-Reactivity
Only baseline craving and rACC reactivity to smoking vs. neutral cues were associated with insula network resting-state connectivity, but in distinct AI networks in line with the stated a priori hypotheses. Baseline craving was associated with increased resting-state connectivity between the left vAI and three additional regions including midline rACC/mPFC, right and left superior parietal lobule and adjacent angular gyrus (Figure 1, Table 2). Rostral ACC reactivity to smoking cues was related to increased connectivity between the left dAI and the bilateral thalamus, bilateral frontal pole, dorsal/rostral anterior cingulate cortex, superior frontal gyrus, and middle frontal gyrus. Enhanced connectivity also was noted between the left dAI and the left lateralized superior parietal cortex/angular gyrus and occipital cortex (Figure 1, Table 3).
Table 2:
Left vAI Network Coupling Associated with pre Cue-exposure Craving
| Brain Region | Brodmann Area |
Number of voxels |
X (COG) |
Y (COG) |
Z (COG) |
p- value |
|---|---|---|---|---|---|---|
| Rostral Anterior Cingulate Cortex, Medial Prefrontal Cortex | 10, 24, 32 | 483 | 45.9 | 85.8 | 33 | 0.02 |
| Right Superior Parietal Lobule, Angular Gyrus | 7, 40 | 435 | 24.4 | 35.7 | 61.8 | 0.023 |
| Left Superior Parietal Lobule, Angular Gyrus | 7,40 | 400 | 66.8 | 32.3 | 60.2 | 0.026 |
Table 3:
Left dAI Network Coupling Associated with Rostral Anterior Cingulate Cortex Reactivity to Smoking vs. Neutral Cues
| Brain Region | Brodmann Area |
Number of voxels |
X (COG) |
Y (COG) |
Z (COG) |
p- value |
|---|---|---|---|---|---|---|
| Bilateral Frontal Pole, Anterior Cingulate Cortex, Superior Frontal Gyrus | 8, 9, 10, 32 | 3061 | 44 | 89.6 | 42.4 | 0.008 |
| Bilateral Superior Frontal Gyrus | BA 6 | 474 | 40.9 | 76.1 | 62.3 | 0.026 |
| Right Middle Frontal Gyrus | BA 8, 6 | 415 | 28.3 | 67.9 | 62.3 | 0.031 |
| Left Lateral Occipital Cortex | BA 19 | 395 | 60.9 | 19.9 | 46.6 | 0.032 |
| Bilateral Thalamus | 352 | 43 | 56.4 | 43.1 | 0.033 | |
| Left middle frontal gyrus | BA 8, 6 | 237 | 60 | 77.4 | 61.1 | 0.048 |
| Left Angular Gyrus | BA 39 | 231 | 73.2 | 34 | 48.9 | 0.049 |
These resultant connectivity maps were multiplied to identify the area of spatial overlap between these AI networks as impacted by baseline craving and rACC reactivity to cues. A single region in the medial prefrontal cortex (BA 10) was noted (voxels = 44, Center of gravity: X =47.5, Y = 88.4, Z = 33.2, Figure 1).
Prediction Analysis
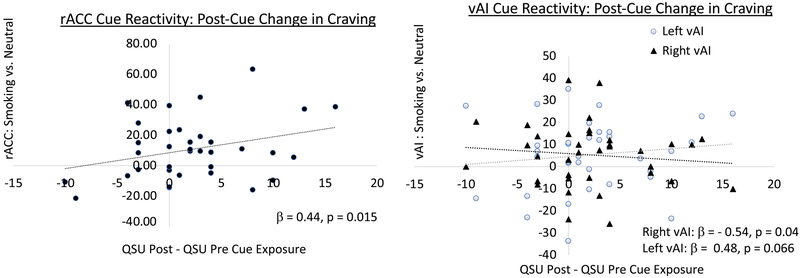
While baseline craving was not associated with brain activation to smoking cues, brain reactivity to smoking cues was associated with the change in subjective craving following cue presentation. The model showing brain reactivity to smoking cues predicts the rise in cue-induced craving was significant (F (7, 28) = 2.82, p = 0.024, R =0.64, adjusted R2 = 0.27). Figure 2 shows the results of the predictors associated with cue-induced craving including a positive relationship with smoking-cue reactivity in the rACC (unstandardized β = 0.13, standardized β = 0.44, t = 2.59, p = 0.015) and a negative relationship with cue-reactivity in the right vAI (unstandardized β = − 0.21, standardized β = − 0.54, t = −2.15, p = 0.040). There was a trend positive association between cue-induced subjective craving and left vAI reactivity to smoking cues (unstandardized β = 0.17, standardized β = 0.48, t = 1.92, p = 0.066). No other individual predictors were significant.
Figure 2: Association between cue-induced change in craving and brain reactivity to smoking cues.
All 6 ROIs were included in a single model that was significant (F (7, 28) = 2.82, p = 0.024, R =0.64, adjusted R2 = 0.27). While rACC reactivity to smoking cues significantly predicted the subsequent rise in craving (left plot, black circles, standardized β = 0.44, p = 0.015), the right vAI showed the opposite effect (right plot, black triangles, standardized β = −0.54, p = 0.04) and there was a trend effect in the left vAI, which was similar to the rACC findings (right plot gray circles, standardized β = 0.48, p = 0.066).
Discussion
The present findings deepen our understanding of the neurobiological mechanisms maintaining tobacco use by demonstrating that distinct, yet overlapping anterior insula resting-state networks were linked with 1) baseline craving and 2) subsequent brain reactivity to smoking cues. We also show that baseline craving and cue-reactivity are distinct at least during relative satiety, as baseline craving did not impact cue-reactivity, yet brain activation to smoking cues was associated with a subsequent rise in craving. These findings fit with the stated hypothesis, confirm the work of others(10), and support the concept that the propensity to respond to internal and external craving triggers can be differentiated.
In defining group-level ventral/dorsal AI resting-state networks, both included aspects of the canonical SN, yet had unique patterns. Specifically, vAI networks included the vmPFC, a primary node of the internally focused DMN, while dAI networks coupled with regions of the externally oriented FPN such as the DLPFC and lateral parietal cortex. These patterns reflect the vAI/dAI network definitions observed by Wang and colleagues(8) and support the differentiation of function along the ventral/dorsal AI axis. Associations with baseline craving and cue reactivity align with this internal/external distinction. Baseline craving, conceptualized as an internally driven process, was associated with enhanced resting-state connectivity of the left vAI, while brain reactivity to external smoking cues was linked to greater resting-state connectivity of the left dAI.
Greater baseline craving was associated with more vAI resting-state connectivity with regions of the DMN (rACC, adjacent mPFC, and angular gyrus). This finding supports and extends prior research demonstrating that craving during abstinence is related to alterations in SN connectivity; specifically, stronger integration with the DMN and weaker connectivity with the FPN(12). While Lerman and colleagues focused on the SN as a unit(12), the present study offers empirical evidence that the vAI portion of the SN exhibits greater craving-related connectivity with DMN nodes. The vAI has been posited to act as an affective component of the insula(7), suggesting that greater baseline craving may be due to stronger integration between regions involved in affect and internal focus. Unlike a past study(12), the present study was not designed to evaluate abstinence, but focused on the relationship between baseline craving and resting-state vAI-DMN connectivity following recent smoking. We propose that this enhanced connectivity may render individuals more likely to experience craving-related internal states even prior to protracted abstinence.
The idea that some individuals are more likely to attend to internal states is supported by the literature on major depressive disorder (MDD). Those with MDD have greater connectivity between the DMN and regions including the insula(30) and are more distracted by internal thoughts due to this stronger engagement of the DMN(31,32). Collectively, it is likely that within any population there is variability in the propensity to attend to internal states, which may be related to the strength of functional integration between regions of the DMN and areas such as the vAI. Nicotine withdrawal appears to enhance this process(12) and the overall proneness to internal focus. Thus, both the pharmacological impact of nicotine and individual variability impinge on the same circuit to influence internally generated craving.
The a priori hypothesis was further supported by the finding that enhanced dAI resting-state connectivity was associated with more brain reactivity to smoking cues in the subsequent cue-reactivity task. Specifically, greater resting-state connectivity between the dAI and regions such as the dorsomedial thalamus, sensory integration areas, and dorsomedial and ventral lateral portions of the PFC preceded enhanced rACC cue-reactivity. The dAI has been implicated in goal-directed behavior(7), as have these other functionally connected regions. For instance, the mPFC and lateral PFC form functional connections(33) allowing the integration of cognitive resources to impact the evaluation and/or behavioral response to rewards(34-36). Greater integration between these brain regions at rest prior to cue-exposure may render individuals more prone to engage with external information to guide behavioral and affective responding. As the present study did not measure behavior, the connection between activation and action needs to be confirmed. However, others have shown that higher ACC activity in a region falling between our dACC and rACC seeds precedes better performance on reward-related tasks(34), supporting the idea that the link between enhanced dAI connectivity at rest and subsequent ACC reactivity to smoking cues may have a behavioral correlate. As discussed below, results from the present study demonstrate an affective relationship given that rACC reactivity to smoking cues was associated with both greater dAI connectivity and the rise in cue-induced subjective craving.
A direct, causal influence needs verification, but the interpretation of data from the present study suggests that pre-existing dAI resting-state connectivity may represent a larger sensitivity to external processing, resulting in more cue-induced craving mediated by the rACC. The specific involvement of the rACC is consistent with this region’s role in contingency learning and goal-directed behavior(21,37). A meta-analysis also defined the rACC as specifically involved in craving when urge intensity is high(20), which complements the study by Hanlon (16) and others who demonstrated that ventral prefrontal brain regions including the rACC are involved in promoting craving while more dorsal prefrontal regions including the dACC can play a role in resisting craving. While we have conceptually split the AI into internally and externally focused networks, this was not meant to imply that these processes cannot interact. In the case of the current findings, greater dAI resting state connectivity represents enhanced sensitivity to external smoking cues that evoke the rise in rACC-mediated subjective craving, which is arguably the internal response to such images. The specific involvement of the rACC is likely due to the current study's focus on subjective craving, while other outcomes such as smoking behavior may exhibit stronger relationships with additional brain regions.
The rostral mPFC (BA 10) was the point of overlap between the vAI/baseline craving and dAI/cue-reactive resting-state networks described above. Assigning a single role to mPFC BA 10 is difficult given that this region has been linked with the internal “mentalizing” aspects associated with the DMN(38), the coordination of multiple cognitive tasks(39), and the representation of reward choice(35) that occurs prior to the initiation of the related behavioral response(40). A meta-analysis concluded that a rostral-caudal gradient of function exists where more rostral regions of the mPFC are involved in multi-task integration, while caudal regions are more consistently active during internal thought(41). Despite evidence for functional separation along the rostral-caudal axis, this same report indicated that there was substantial spatial overlap between the portions of the mPFC associated with these roles. Thus, it is difficult to assign a specific function to the area of the mPFC noted in the present study. However, our data suggest that this portion of medial BA 10 contributes to the cognitive aspects relevant to both internal/craving and external/smoking cue reactivity, potentially related to this region’s role in manipulating multiple task-related elements and reward choice selection.
In addition to the rACC, vAI reactivity to smoking cues also was associated with a subsequent change in craving. Right vAI smoking-cue reactivity predicted less cue-induced craving while there was a trend for left vAI reactivity showing the opposite effect. This hemispheric specificity fits with Craig’s proposal that “the right and left anterior insulae may be organized asymmetrically in an opponent fashion” (pp. 72)( 42). More precisely, the homeostatic model Craig sets forth suggests that the left insula is associated with positive, while the right is associated with negative, feelings(43), which may also explain the left lateralized connectivity findings discussed above. The opposing roles of the right and left insula in the present study fit with this model yet needs further confirmation because the specific influence of the left vAI was just below significance and both regions exhibited a weaker effect than the rACC.
There are limitations to this study requiring comment. First, the sample size limits the ability to fully test the impact of sex differences. However, within the current sample there was no effect of sex. No differences were noted between men and women in any of the craving or brain cue-reactivity measures assessed. Including sex in the model did not change the influence of baseline craving on cue-reactivity nor the influence of brain reactivity to smoking cues on the subsequent change in craving. A future, larger study should be conducted to determine if this null effect extends beyond the current sample as well as to verify the current findings. Second, despite the relationships revealed by the analyses, a direct causal influence of AI connectivity on craving and cue-reactivity needs to be tested and related to treatment outcome. Third, no data were collected from controls making it necessary for future work to explore whether there are group differences between smokers and non-smokers in addition to the relationships currently identified. Finally, while all significant values below 0.05 are provided for the imaging regression data, some findings are clearly stronger than others suggesting that the weaker values be taken with more caution.
The results of the present study document a brain-based distinction between baseline craving and cue-reactivity. Specifically, it is shown that the AI can be subdivided along the ventral/dorsal axis into two functional networks linked with an internal and external focus respectively. This distinction is further supported by the finding that stronger connectivity of the internal-vAI network relates to internally generated baseline craving while smoking cue-reactivity is associated with greater connectivity of the external-dAI network. This conceptual framework of networks being involved in internal/external processes theoretically maps on well to other areas of neuroscience research(8,30-32), suggesting a common theme across neuropsychiatric disorders. The discovery of these relationships advances the basic neuroscientific understanding of nicotine dependence, which may guide the development of more targeted therapies that address both the internal and external factors contributing to continued nicotine use. For instance, cognitive behavioral therapy (CBT) in asthmatic patients reduces connectivity between the left vAI and the rACC(44). It is intriguing to posit that such strategies, which modulate functional connectivity in targeted regions, would be useful in nicotine dependence; further research is needed to determine if this would indeed be a valid approach to develop neuroscience-guided therapeutics.
Supplementary Material
Acknowledgments:
This research study was funded by the National Institute of Drug Abuse (ACJ: R01DA039135, K02DA042987).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Naqvi NH, Bechara A. (2010): The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. 214: 435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janes AC, Pizzagalli DA, Richardt S, deB Frederick B, Chuzi S, Pachas G, et al. (2010): Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. 67: 722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forget B, Pushparaj A, Le Foll B. (2010): Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. 68: 265–271. [DOI] [PubMed] [Google Scholar]
- 4.Pushparaj A, Hamani C, Yu W, Shin DS, Kang B, Nobrega JN, et al. (2012): Electrical Stimulation of the Insular Region Attenuates Nicotine-Taking and Nicotine-Seeking Behaviors. doi: 10.1038/npp.2012.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu X, Liu X, Van Dam NT, Hof PR, Fan J. (2013): Cognition-emotion integration in the anterior insular cortex. 23: 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. (2013): Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. 23: 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wager TD, Feldman-Barrett L (2004): From affect to control: Functional specialization of the insula in motivation and regulation. PsychExtra published online.
- 8.Wang Y, Zhu L, Zou G, Cui Q, Liao W, Duan X, et al. (2018): Frequency dependent hub role of the dorsal and ventral right anterior insula. 165: 112–117. [DOI] [PubMed] [Google Scholar]
- 9.Sridharan D, Levitin DJ, Menon V. (2008): A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. 105: 12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiffman S, Shadel WG, Niaura R, Khayrallah MA, Jorenby DE, Ryan CF, Ferguson CL (2003): Efficacy of acute administration of nicotine gum in relief of cue-provoked cigarette craving. Psychopharmacology. 166: 343–350. [DOI] [PubMed] [Google Scholar]
- 11.Dunbar MS, Shiffman S, Kirchner TR, Tindle HA, Scholl SM (2014): Nicotine dependence, “background” and cue-induced craving and smoking in the laboratory. Drug and Alcohol Dependence. 142: 197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, Stein EA (2014): Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry. 71: 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flagel SB, Watson SJ, Akil H, research TRBB, 2008. (n.d.): Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Elsevier. doi: 10.1016/j.bbr.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahler SV, de Wit H (2010): Cue-reactors: individual differences in cue-induced craving after food or smoking abstinence. PLoS ONE. 5: e15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janes AC, Gilman JM, Radoman M, Pachas G, Fava M, Evins AE (2017): Revisiting the role of the insula and smoking cue-reactivity in relapse: A replication and extension of neuroimaging findings. Drug and Alcohol Dependence. 179: 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanlon CA, Hartwell KJ, Canterberry M, Li X, Owens M, LeMatty T, et al. (2013): Reduction of cue-induced craving through realtime neurofeedback in nicotine users: The role of region of interest selection and multiple visits. Psychiatry Res. 213: 79–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumais KM, Franklin TR, Jagannathan K, Hager N, Gawrysiak M, Betts J, et al. (2017): Multi-site exploration of sex differences in brain reactivity to smoking cues: Consensus across sites and methodologies. Drug and Alcohol Dependence. 178: 469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, et al. (2012): Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. NeuroImage. 60: 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, et al. (2002): Brain metabolic changes during cigarette craving. 59: 1162–1172. [DOI] [PubMed] [Google Scholar]
- 20.Wilson SJ, Sayette MA (2014): Neuroimaging craving: urge intensity matters. Addiction. doi: 10.1111/add.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson SAW, Horst NK, Pears A, Robbins TW, Roberts AC (2016): Role of the Perigenual Anterior Cingulate and Orbitofrontal Cortex in Contingency Learning in the Marmoset. Cerebral Cortex. 26: 3273–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fagerström KO (1978): Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive behaviors. 3: 235–241. [DOI] [PubMed] [Google Scholar]
- 23.Janes AC, Farmer S, Peechatka AL, Frederick B de B, Lukas SE (2015): Insula-Dorsal Anterior Cingulate Cortex Coupling is Associated with Enhanced Brain Reactivity to Smoking Cues. Neuropsychopharmacology. doi: 10.1038/npp.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox LS, Tiffany ST, Christen AG. (2001): Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. 3: 7–16. [DOI] [PubMed] [Google Scholar]
- 25.Janes AC, Zegel M, Ohashi K, Betts J, Molokotos E, Olson D, et al. (2018): Nicotine normalizes cortico-striatal connectivity in non-smoking individuals with major depressive disorder. Neuropsychopharmacology. 33: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCarthy JM, Dumais KM, Zegel M, Pizzagalli DA, Olson DP, Moran LV, Janes AC (2018): Sex differences in tobacco smokers: Executive control network and frontostriatal connectivity. Drug and Alcohol Dependence. 195: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faillenot I, Heckemann RA, Frot M, Hammers A (2017): Macroanatomy and 3D probabilistic atlas of the human insula. Neuroimage. 150: 88–98. [DOI] [PubMed] [Google Scholar]
- 28.Nickerson L, Smith SM, Öngür D, Beckmann CF. Using Dual Regression to Investigate Network Shape and Amplitude in Functional Connectivity Analyses. Front Neurosci. 2017; 11: 115. doi: 10.3389/fnins.2017.00115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014): Permutation inference for the general linear model. Neuroimage. 92: 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knyazev GG, Savostyanov AN, Bocharov AV, Brak IV, Osipov EA, Filimonova EA, et al. (2018): Task-positive and task-negative networks in major depressive disorder: A combined fMRI and EEG study. Journal of Affective Disorders. 235: 211–219. [DOI] [PubMed] [Google Scholar]
- 31.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA (2015): Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry. 72: 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaiser RH, Andrews-Hanna JR, Spielberg JM, Warren SL, Sutton BP, Miller GA, et al. (2015): Distracted and down: neural mechanisms of affective interference in subclinical depression. Social Cognitive and Affective Neuroscience. 10: 654–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blumenfeld RS, Nomura EM, Gratton C, D'Esposito M (2013): Lateral prefrontal cortex is organized into parallel dorsal and ventral streams along the rostro-caudal axis. Cerebral Cortex. 23: 2457–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cubillo A, Makwana AB, Hare TA (2019): Differential modulation of cognitive control networks by monetary reward and punishment. Social Cognitive and Affective Neuroscience. 14: 305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grabenhorst F, Rolls ET (2011): Value, pleasure and choice in the ventral prefrontal cortex. Trends in Cognitive Sciences. 15: 56–67. [DOI] [PubMed] [Google Scholar]
- 36.Kouneiher F, Charron S, Koechlin E (2009): Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci. 12: 939–945. [DOI] [PubMed] [Google Scholar]
- 37.Bossert JM, Stern AL, Theberge FRM, Cifani C, Koya E, Hope BT, Shaham Y (2011): Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci. 14: 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, et al. (2004): Reflecting upon Feelings: An fMRI Study of Neural Systems Supporting the Attribution of Emotion to Self and Other. Journal of cognitive neuroscience. 16: 1746–1772. [DOI] [PubMed] [Google Scholar]
- 39.1. Ramnani N, Owen AM (2004): Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 5: 184–194. [DOI] [PubMed] [Google Scholar]
- 40.Wunderlich K, Rangel A, O'Doherty JP (2010): Economic choices can be made using only stimulus values. Proceedings of the National Academy of Sciences. 107: 15005–15010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, Burgess PW (2006): Functional Specialization within Rostral Prefrontal Cortex (Area 10): A Meta-analysis. http://dxdoiorgezp-prod1hulharvardedu/101162/jocn2006186932 18: 932–948. [DOI] [PubMed] [Google Scholar]
- 42.Craig ADB (2011): Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci. 1225: 72–82. [DOI] [PubMed] [Google Scholar]
- 43.Yan C, Su L, Wang Y, Xu T, Yin D-Z, Fan M-X, et al. (2016): Multivariate Neural Representations of Value during Reward Anticipation and Consummation in the Human Orbitofrontal Cortex. Sci Rep. 6: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Yang Y, Bian R, Yin Y, Hou Z, Yue Y, et al. (2017): Group Cognitive Behavior Therapy Reversed Insula Subregions Functional Connectivity in Asthmatic Patients. Front Aging Neurosci. 9: 2117–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.