Abstract
Background & Aims:
Endoscopic screening reduces incidence and mortality of colorectal cancer (CRC) because precursor lesions, such as conventional adenomas or serrated polyps, are removed. Individuals with polypectomies are advised to undergo colonoscopy surveillance to prevent CRC. However, guidelines for surveillance intervals after diagnosis of a precursor lesion, particularly for individuals with serrated polyps, vary widely, and lack sufficient supporting evidence. Consequently, some high-risk patients do not receive enough surveillance and lower-risk subjects receive excessive surveillance.
Methods:
We examined the association between findings from first endoscopy and CRC risk among 122,899 participants who underwent flexible sigmoidoscopy or colonoscopy in the Nurses’ Health Study 1 (1990–2012), Nurses’ Health Study 2 (1989–2013), or the Health Professionals Follow-up Study (1990–2012). Endoscopic findings were categorized as no polyp, conventional adenoma, or serrated polyp (hyperplastic polyp, traditional serrated adenoma, or sessile serrated adenoma, with or without cytological dysplasia). Conventional adenomas were classified as advanced (≥10 mm, high-grade dysplasia, or tubulovillous or villous histology) or non-advanced, and serrated polyps were assigned to categories of large (≥10 mm) or small (< 10 mm). We used Cox proportional hazards regression model to calculate the hazard ratios (HRs) of CRC incidence, after adjusting for various potential risk factors.
Results:
After a median follow-up period of 10 years, we documented 491 incident cases of CRC: 51 occurred in 6161 participants with conventional adenomas, 24 in 5918 participants with serrated polyps, and 427 in 112,107 participants with no polyp. Compared to participants with no polyp detected during initial endoscopy, the multivariable HR for incident CRC in individuals with an advanced adenoma was 4.07 (95% CI, 2.89–5.72) and the HR for CRC in individuals with a large serrated polyp was 3.35 (95% CI, 1.37–8.15). In contrast, there was no significant increase in risk of CRC in patients with non-advanced adenomas (HR, 1.21; 95% CI, 0.68–2.16, P=.52) or small serrated polyps (HR, 1.25; 95% CI, 0.76–2.08; P=.38).
Conclusions:
These findings provide support for guidelines that recommend repeat lower endoscopy within 3 years of a diagnosis of advanced adenoma and large serrated polyps. In contrast, patients with non-advanced adenoma or small serrated polyps may not require more intensive surveillance than patients without polyps.
Keywords: polypectomy, interval cancer, early detection, secondary prevention
Graphical Abstract
Lay Summary:
In an analysis of colonoscopy data and outcomes from a large group of subjects, we show that patients found to have advanced adenomas or large serrated polyps at their screening colonoscopy have a 3-fold to 4-fold increase in risk of colorectal cancer, compared to patients found to have no polyps. Patients with non-advanced adenoma or small serrated polyps do not seem to have an increase in colorectal cancer risk, compared to patients with no polyps.
Introduction
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer death in the world.1 Endoscopic screening with colonoscopy or flexible sigmoidoscopy in asymptomatic populations has been shown to reduce CRC incidence and mortality by removal of precursor lesions.2–6 Conventional adenomas (including tubular, tubulovillous and villous adenomas) and serrated polyps (SPs, including hyperplastic polyps [HPs], sessile serrated adenoma/polyps [SSA/Ps] and traditional serrated adenomas [TSAs]) represent two groups of precursors for CRC and are believed to arise from distinct etiologic pathways. Therefore, individuals diagnosed with either conventional adenomas or SPs via screening endoscopy are advised to undergo colonoscopy surveillance to prevent subsequent cancer.7–12
Several guidelines have been proposed by different professional societies for the intervals of colonoscopy surveillance, depending on the most advanced findings on baseline colonoscopy.7–9 However, these guidelines vary widely and are largely based on low or modest supporting evidence regarding CRC risk after polypectomy. For example, individuals with removal of 1–2 small (<10 mm) tubular adenoma(s) are advised to undergo repeated colonoscopy in 5 to 10 years by the United States Multi-Society Task Force [USMSTF], but in 10 years by the European Society of Gastrointestinal Endoscopy guidelines [ESGE].8, 11 However, there is limited evidence to inform guidelines to distinguish between a 5- or 10-year interval. Recently, a study in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer randomized trial examined CRC risk based on the findings on diagnostic colonoscopy prompted by a positive initial screening flexible sigmoidoscopy and found an increased CRC risk for individuals with advanced adenomas, but no risk elevation among those with 1–2 non-advanced adenoma(s), compared to those with no adenoma.13 However, it is unknown whether these findings are generalizable to individuals without positive findings from an initial flexible sigmoidoscopy.13
For SPs, the recommendations for colonoscopy surveillance are even more inconsistent, due to sparse data and the largely unknown natural history of SPs. While the USMSTF recommends a 5-year surveillance interval for SPs of <10 mm and a 3-year interval for SPs of >10 mm, ESGE recommends a 10- and 3-year surveillance interval for SPs of <10 mm and >10 mm, respectively; and the British Society of Gastroenterology [BSG] guideline recommends no surveillance for SPs of <10 mm and one surveillance colonoscopy at 3 years for SPs of >10 mm.8, 11, 12 Two recent nationwide studies in Norway and Denmark reported that individuals with large SPs or SSA/Ps have an increased risk of CRC.14, 15 However, these studies have several limitations, including small sample size14, lack of clinical data on polyp features (such as number and size)15, and lack of information on major CRC risk factors.14, 15
Therefore, to fill these knowledge gaps, we prospectively examined the risk of CRC among individuals who underwent removal of conventional adenomas and SPs at their time of the first lower gastrointestinal endoscopies in three large cohort studies, the Nurses’ Health Study (NHS), the Nurses’ Health Study 2 (NHS2), and the Health Professionals Follow-up Study (HPFS) with biennially updated information on endoscopy over 20 years of follow-up.
Methods
Study population
The NHS included 121,700 US female nurses aged 30 to 55 at enrollment in 1976. The NHS2 included 116,430 registered US female nurses aged 25 to 42 years at the time of enrollment in 1989. The HPFS enrolled 51,529 male health professionals aged 40 to 75 at enrollment in 1986. Participants were mailed a questionnaire at baseline and every two years thereafter that inquired detailed medical and lifestyle information, including history of endoscopic examinations and diagnosis of colorectal polyps and CRC. The average follow-up rate has been greater than 90% in all three cohorts.
Because detailed histological information of polyps was not collected until 1990 for the NHS/HPFS, we used 1990 as baseline for the two cohorts and 1989 for the NHS2. We included participants who had undergone their first flexible sigmoidoscopy or colonoscopy since then. At baseline, we excluded participants who had a history of cancer (except non-melanoma skin cancer), prior colorectal polyp, inflammatory bowel disease, or previous lower gastrointestinal endoscopy. We also excluded CRC cases who occurred within 1 year after removal of polyps. A total of 43,147 participants in the NHS, 63,073 in the NHS2, and 16,679 in the HPFS were included in the current study (shown in Supplementary Figure 1). The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required.
Ascertainment of colorectal polyp and cancer
On each biennial follow-up questionnaire, participants were asked if they had undergone a flexible sigmoidoscopy or colonoscopy, and if any colorectal polyp or CRC had been diagnosed in the past two years. For participants who reported yes, we acquired permission to collect their endoscopic and pathologic reports from their providers. All records were centrally reviewed by study physicians to confirm the diagnosis, and extract relevant histopathologic data. While we did not systematically collect medical records for participants who reported having had endoscopy but no polyps, our previous validation studies indicate good accuracy of self-reported data. In random samples of participants who reported having negative endoscopy (n=114 in the NHS and 140 in the HPFS), we collected endoscopic records and observed high concordance rate with self-reported negative endoscopy (97% in the NHS and 100% in the HPFS).16–18 Participants who died from CRC and did not respond to our regular follow-up questionnaires were identified and confirmed through various sources, including next-of-kin, the National Death Index, and death certificates. We asked permission from next of kin to review the medical records.
According to the 2010 WHO classification schema, SPs include HPs, SSA/Ps and TSAs.19 Because all initial endoscopies in our study were performed before 2010 when consensus on the diagnostic criteria of specific subtypes of SPs remained lacking and recommendations were still evolving along with the pathologic diagnosis criteria20, we were unable to distinguish HPs from SSA/Ps and TSAs, and defined SP to include HPs, TSAs, and SSA/Ps. If a participant had both SPs and conventional adenomas in an endoscopy, we recorded each type of the polyps separately.
Covariate Assessment
In the baseline and biennial follow-up questionnaires, we assessed a variety of CRC risk factors, including family history of CRC, smoking, height, body weight, alcohol intake, physical activity, and regular use of aspirin. All covariable data were derived from the questionnaire in which the first endoscopy was reported. Participants were defined as having a positive family history of CRC if at least one of their parents or siblings had been diagnosed with CRC. For physical activity, weekly energy expenditure was estimated by multiplying the typical intensity expressed in metabolic equivalent of task (MET) (the ratio of metabolic rate during the activity to metabolic rate at rest) by the reported hours spent per week.21 Consistent with our prior analyses, regular aspirin use was defined as use of at least two standard tablets (325 mg) of aspirin per week.22
Statistical Analysis
For each participant, we calculated follow-up time from the age at which their first endoscopy was performed until the age at the date of death, CRC diagnosis, loss to follow-up, or end of follow-up (June 1, 2012 for the NHS, June 1, 2013 for the NHS2, or January 31, 2012 for the HPFS), whichever came first. Kaplan-Meier analysis was used to calculate the cumulative incidence of CRC. We computed hazard ratio (HR) and 95% confidence intervals (CI) of CRC in relation to the first endoscopic findings using Cox proportional hazards model. In model 1, we adjusted for age and calendar year of the first endoscopy, and cohorts. In model 2, we further adjusted for several CRC risk factors, including family history of CRC, pack-year of smoking, body mass index (BMI), physical activity, alcohol intake, and regular aspirin use, and reason for the first endoscopy (Details about covariate assessment are provided in the Supplementary Methods). In model 3, we further adjusted for number of endoscopies performed after the index endoscopy. The frequency of surveillance endoscopies in major comparison groups is shown in Supplementary Table 1.
We first evaluated the overall association of SPs and conventional adenomas with subsequent risk of CRC. To facilitate clinical translation of our findings, we then examined the associations according to polyp subgroups as categorized in the USMSTF guideline on the basis of polyp size,number, risk classification (for conventional adenomas only: advanced and non-advanced), and anatomic subsite. Advanced conventional adenomas were defined as at least one conventional adenoma of ≥10 mm in diameter or with advanced histology (tubulovillous/villous histological features or high-grade dysplasia).8, 13, 22 For subsite, polyps in the cecum, ascending colon, hepatic flexure, transverse colon, or splenic flexure were classified as proximal, polyps in the descending or sigmoid colon as distal, and those in the rectum or rectosigmoid junction as rectal.
To examine the influence of endoscopic indication on results, we performed a sensitivity analysis by restricting to participants who had the index endoscopy for the purpose of screening only. To test the robustness of our findings to the secular trend of examination methods, we performed a sensitivity analysis by restricting to participants whose first endoscopy was colonoscopy. Finally, we performed T test and Fisher’s exact test to compare the characteristics of CRC cases that developed in different polyp groups.
We used SAS 9.4 for all the analyses. All statistical tests were two-sided. A p value of <0.05 was considered statistically significant.
Results
Based on the findings of the first lower endoscopies among 122,899 participants, we identified 6,161 cases with at least one conventional adenoma, 5,918 with SP (1287 [22%] of those also had conventional adenomas), and 112,107 with no polyp. As shown in Table 1, compared with participants with no polyp at the first endoscopy, those with polyps were more likely to have a family history of CRC, smoke, drink alcohol, and have a higher BMI; and were less likely to exercise and regularly use aspirin. Among participants with conventional adenomas, 1,985 (32%) had a large lesion and 1,539 (25%) had advanced histology (1,145 [19%] had tubulovillous adenomas, 228 [4%] had villous adenomas, and 166 [2%] had high-grade dysplasia), giving rise to a total of 2,453 cases (40%) with advanced adenoma. Among SP cases, 566 participants (10%) had at least one large SP and 579 (10%) had ≥3 SPs.
Table 1.
Basic characteristics of study participants at their first endoscopy in three cohort studies (NHS, NHS2 and HPFS) a
| Non-polyps | Conventional adenomas | Serrated polyps | |
|---|---|---|---|
| No. of participants | 112,107 | 6,161 | 5,918 |
| Age, year | 57±10 | 59±10 | 57±9 |
| Age<50 years, % | 20 | 14 | 19 |
| Female, % | 87 | 77 | 85 |
| Year of endoscopy, % | |||
| Before 1995 | 18 | 10 | 8 |
| 1995-2000 | 22 | 22 | 21 |
| 2000-2005 | 28 | 35 | 41 |
| After 2005 | 32 | 32 | 30 |
| Reason for first endoscopy, % | |||
| Symptom | 29 | 31 | 29 |
| Routine screening | 67 | 62 | 65 |
| Unknown | 4 | 7 | 6 |
| Number of surveillance endoscopy, % | |||
| 0 | 24 | 9 | 10 |
| 1 | 28 | 20 | 21 |
| 2 | 19 | 22 | 22 |
| >2 | 29 | 49 | 47 |
| Family history of colorectal cancer, % | 16 | 21 | 22 |
| Pack-years of smoking | 8.0±14.5 | 9.9±16.4 | 12.3±17.9 |
| Current smoking status, % | |||
| Never smokers | 56 | 51 | 45 |
| Past smokers | 38 | 40 | 42 |
| Current smokers | 6 | 9 | 13 |
| Body mass index, kg/m2 | 26.7±5.6 | 27.2±5.7 | 27.6±5.8 |
| Physical activity, MET-hours/week b | 21.9±26.9 | 20.8±25.5 | 20.3±24.0 |
| Alcohol intake, g/day | 5.7±10.0 | 6.6±11.1 | 7.1±11.9 |
| Regular aspirin use, % c | 26 | 26 | 27 |
| Number of polyps | |||
| 1-2, n (%) | 5,543 (90) | 4,957 (84) | |
| ≥3, n (%) | 599 (10) | 579 (10) | |
| Missing, n (%) | 19 (0) | 382 (6) | |
| Size of polyps | |||
| <10mm, n (%) | - | 3,902 (63) | 5,010(85) |
| ≥10mm, n (%) | - | 1,985 (32) | 566(10) |
| Missing, n (%) | 274 (5) | 342 (5) | |
| Location of polyps | |||
| Proximal colon, n (%) | - | 1,975 (32) | 1,347 (23) |
| Distal colon, n (%) | - | 2,448 (40) | 1,968 (33) |
| Rectum, n (%) | - | 763 (12) | 1,530 (26) |
| More than one region, n (%) | 964 (15) | 915(15) | |
| Missing, n (%) | 11 (0) | 158 (3) | |
| Histology | |||
| Tubular adenoma, n (%) | - | 4,622 (75) | - |
| Tubulovillous adenoma, n (%) | - | 1,145 (19) | - |
| Villous adenoma, n (%) | - | 228 (4) | - |
| Adenoma with high-grade dysplasia, n (%) | - | 166 (2) | - |
Abbreviations: NHS, the Nurses’ Health Study; NHS2, the Nurses’ Health Study 2; HPFS, the Health Professionals Follow-up Study; MET, metabolic equivalent task.
The presented data are based on baseline information for each participant at their first endoscopy. Variables including current smoking status, body mass index, physical activity, alcohol intake and regular aspirin use, are adjusted for age and sex. Mean ±SD is presented for continuous variables and number of participants (percentage) for categorical variables.
Physical activity is represented by the product sum of the MET of each specific recreational activity and hours spent on that activity per week.
A standard tablet contains 325 mg aspirin, and regular users were defined as those who used at least two standard tablets per week.
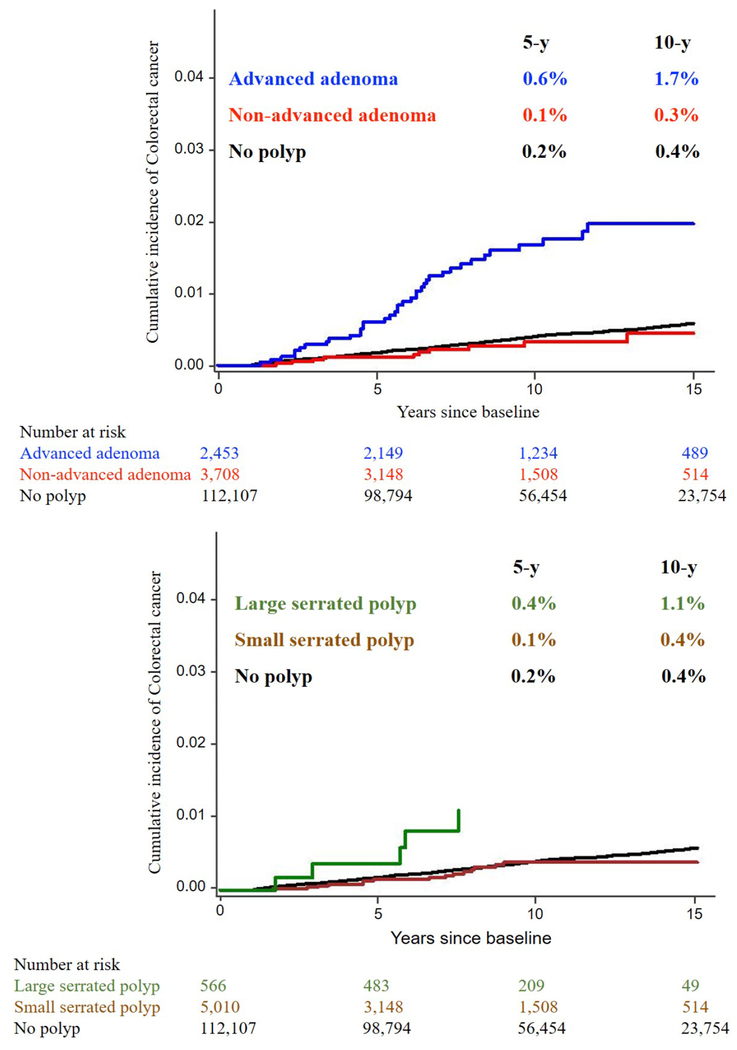
During a median of 10 years of follow-up, we documented 491 incident CRC cases. As shown in Figure 1, the 5-year and 10-year cumulative incidences of CRC were 0.2% and 0.4% for participants with no polyp, 0.1% and 0.3% for non-advanced adenomas, 0.6% and 1.7% for advanced adenomas, 0.1% and 0.4% for small SPs, 0.4% and 1.1% for large SPs, respectively. Of note, all CRC cases in the large SP group developed within 6 years after polyp diagnosis.
Figure. 1.
The 5-year and 10-year cumulative incidences of CRC based on different findings at the initial endoscopy. As shown, the 5-year and 10-year cumulative incidences of CRC were 0.2% and 0.4% for participants with no polyp, 0.1% and 0.3% for non-advanced adenomas, 0.6% and 1.7% for advanced adenomas, 0.1% and 0.4% for small SPs, 0.4% and 1.1% for large SPs, respectively.
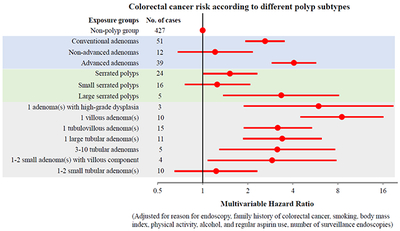
Table 2 shows the association between various subtypes of conventional adenomas, and CRC risk. Compared with participants without any polyp, those with conventional adenomas were more likely to develop CRC, with a multivariable HR of 2.61 (95%, 1.93-3.52). This positive association appeared to be driven by advanced adenomas (HR, 4.07; 95% CI, 2.89-5.72; P<0.001), whereas no association was found for non-advanced adenoma (HR, 1.21; 95% CI, 68-2.16; P=0.52). Individuals who had at least one adenoma with advanced histology and of any size were at higher risk of developing CRC, with the multivariable HR increasing from 3.17 (95% CI, 1.88-5.36, P<0.001) for tubulovillous adenoma to 8.51 (95% CI, 4.50-16.1, P<0.001) for villous adenoma and 5.95 (95% CI, 1.88-18.8, P=0.002) for high-grade dysplasia. We also observed an increased risk of CRC among individuals with 1 to 2 small conventional adenoma(s) with villous component (HR, 2.91; 95% CI, 1.08-7.82). For tubular adenomas, both the large size and multiplicity were associated with increased CRC risk, with an HR of 3.40 (95% CI, 1.86-6.24, P<0.001) for individuals with at least one ≥10 mm tubular adenoma and 3.15 (95% CI, 1.29-7.67; P=0.01) for those with 3-10 tubular adenomas of any size. In contrast, no risk elevation was found for 1-2 small tubular adenoma(s) (HR, 1.23; 95% CI, 0.65-2.31; P=0.52). No statistically significant heterogeneity was detected by the sublocation of adenomas (Pinteraction=0.91; Supplementary Table 2).
Table 2.
Conventional adenoma diagnosed at the first endoscopy and subsequent risk of CRC in the three cohorts (NHS [1990-2012], NHS2 [1989-2013] and HPFS [1990-2012]) a
| No. of participants | No. of CRC cases | Model 1 b | P | Model 2 c | P | Model 3 d | P | |
|---|---|---|---|---|---|---|---|---|
| Non-polyp group | 112,107 | 427 | 1(ref) | 1(ref) | 1(ref) | |||
| Conventional adenomas | 6,161 | 51 | 1.88 (1.40-2.52) | <0.001 | 1.72 (1.28-2.32) | <0.001 | 2.61 (1.93-3.52) | <0.001 |
| Non-advanced conventional adenomas | 3,708 | 12 | 0.86 (0.48-1.53) | 0.6 | 0.80 (0.45-1.43) | 0.46 | 1.21 (0.68-2.16) | 0.52 |
| Advanced conventional adenomas | 2,453 | 39 | 2.96 (2.13-4.13) | <0.001 | 2.67 (1.91-3.73) | <0.001 | 4.07 (2.89-5.72) | <0.001 |
| 1-2 small (<10 mm) tubular adenomas | 3,259 | 10 | 0.86 (0.46-1.61) | 0.64 | 0.81 (0.43-1.52) | 0.51 | 1.23 (0.65-2.31) | 0.52 |
| 1-2 small (<10 mm) adenoma with villous component | 351 | 4 | 2.21 (0.83-5.92) | 0.11 | 1.97 (0.74-5.29) | 0.18 | 2.91 (1.08-7.82) | 0.03 |
| 3-10 tubular adenomas | 369 | 5 | 2.65 (1.09-6.43) | 0.03 | 2.36 (0.97-5.74) | 0.06 | 3.15 (1.29-7.67) | 0.01 |
| one or more tubular adenomas ≥10 mm | 914 | 11 | 2.54 (1.39-4.64) | 0.002 | 2.25 (1.23-4.12) | 0.008 | 3.40 (1.86-6.24) | <0.001 |
| one or more tubulovillous adenomas, any size | 1,145 | 15 | 2.35 (1.40-3.95) | 0.001 | 2.13 (1.27-3.58) | 0.004 | 3.17 (1.88-5.36) | <0.001 |
| one or more villous adenomas, any size | 228 | 10 | 5.88 (3.13-11.1) | <0.001 | 5.16 (2.74-9.72) | <0.001 | 8.51 (4.50-16.1) | <0.001 |
| one or more adenomas with high-grade dysplasia, any size | 166 | 3 | 4.31 (1.38-13.5) | 0.01 | 3.89 (1.24-12.2) | 0.02 | 5.95 (1.88-18.8) | 0.002 |
Abbreviations: CRC, colorectal cancer; NHS, the Nurses’ Health Study; NHS2, the Nurses’ Health Study 2; HPFS, the Health Professionals Follow-up Study.
Advanced conventional adenomas were defined as at least one conventional adenoma of ≥10 mm in diameter or with advanced histology (tubulovillous/villous histological features or high grade or severe dysplasia), otherwise were defined as non-advanced conventional adenomas.
Model 1 was adjusted by age at first endoscopy, study cohort (NHS, NHS2, HPFS), and year of first endoscopy (continuous).
Model 2 was further adjusted for reason for the first endoscopy (routine screening or symptom), family history of colorectal cancer (yes or no), pack-year of smoking (continuous), body mass index (continuous), physical activity (<7.5, 7.5-14.9, 15-29.9, and ≥30 MET-hours/week), alcohol intake (no drinking, <3.5, 3.5-6.9, and ≥7.0 g/day for women; no drinking, <7.0, 7.0-13.9, or ≥14.0 g/day for men), and regular aspirin use (yes or no), based on Model 1.
Model 3 was additionally adjusted for number of surveillance endoscopies (0, 1, 2, >2), based on model 2.
Table 3 shows the association between SPs with various subtypes, and CRC risk. SPs overall were suggestively associated with higher CRC risk (HR, 1.52, 95% CI, 1.00-2.31, P=0.05). This positive association seemed driven by large SPs, with an HR of 3.35 (95% CI, 1.37-8.15; P=0.008). No association was observed for small SPs (HR, 1.25; 95% CI, 0.76-2.08; P=0.38). We did not observe any association according to the multiplicity and sublocation of SPs.
Table 3.
Serrated polyps diagnosed at the first endoscopy and subsequent risk of CRC in the three cohorts (NHS [1990-2012], NHS2 [1989-2013] and HPFS [1990-2012]) a
| No. of participants | No. of CRC cases | Model 1 b | P | Model 2 c | P | Model 3 d | P | |
|---|---|---|---|---|---|---|---|---|
| Non-polyp group | 112,107 | 427 | 1(ref) | 1(ref) | 1(ref) | |||
| Serrated polyps | 5,918 | 24 | 1.15 (0.76-1.74) | 0.5 | 1.05 (0.69-1.59) | 0.82 | 1.52 (1.00-2.31) | 0.05 |
| Size of serrated polyps | ||||||||
| <10 mm serrated polyps | 5,010 | 16 | 0.93 (0.56-1.53) | 0.77 | 0.85 (0.51-1.41) | 0.53 | 1.25 (0.76-2.08) | 0.38 |
| ≥10 mm serrated polyps | 566 | 5 | 2.82 (1.16-6.82) | 0.02 | 2.55 (1.05-6.18) | 0.04 | 3.35 (1.37-8.15) | 0.008 |
| Number of serrated polyps | ||||||||
| 1-2 | 4,957 | 19 | 1.07 (0.68-1.70) | 0.76 | 0.98 (0.62-1.56) | 0.93 | 1.41 (0.89-2.25) | 0.15 |
| ≥3 | 579 | 3 | 1.92 (0.61-6.01) | 0.26 | 1.70 (0.54-5.35) | 0.36 | 2.57 (0.82-8.09) | 0.11 |
| Location of serrated polyps | ||||||||
| Proximal colon | 1,347 | 4 | 1.11 (0.42-2.99) | 0.83 | 1.05 (0.39-2.81) | 0.93 | 1.73 (0.64-4.66) | 0.28 |
| Distal colon | 1,968 | 6 | 0.81 (0.36-1.81) | 0.61 | 0.74 (0.33-1.66) | 0.46 | 1.03 (0.46-2.32) | 0.94 |
| Rectum | 1,530 | 7 | 1.25 (0.59-2.64) | 0.56 | 1.12 (0.53-2.37) | 0.77 | 1.54 (0.73-3.27) | 0.26 |
Abbreviations: CRC, colorectal cancer; NHS, the Nurses’ Health Study; NHS2, the Nurses’ Health Study 2; HPFS, the Health Professionals Follow-up Study.
Adjusted by age at first endoscopy, study cohort (NHS, NHS2, HPFS), and year of first endoscopy (continuous).
Model 1 was adjusted by age at first endoscopy, study cohort (NHS, NHS2, HPFS), and year of first endoscopy (continuous).
Model 2 was further adjusted for reason for the first endoscopy (routine screening or symptom), family history of colorectal cancer (yes or no), pack-year of smoking (continuous), body mass index (continuous), physical activity (<7.5, 7.5-14.9, 15-29.9, and ≥30 MET-hours/week), alcohol intake (no drinking, <3.5, 3.5-6.9, and ≥7.0 g/day for women; no drinking, <7.0, 7.0-13.9, or ≥14.0 g/day for men), and regular aspirin use (yes or no), based on Model 1.
Model 3 was additionally adjusted for number of surveillance endoscopies (0, 1, 2, >2), based on model 2.
Compared to individuals with no polyps, those with synchronous SPs and conventional adenomas were at higher risk of CRC (HR, 2.69; 95% CI, 1.46-4.93; P=0.002; Supplementary Table 3). In the sensitivity analysis, we restricted to participants who had colonoscopies only for their first examinations and obtained similar results (Supplementary Table 4). We also restricted to participants who had the index endoscopy for screening only. The association with CRC risk was similar for conventional adenomas and did not achieve statistical significance for large SPs (Supplementary Table 5).
Table 4 shows the basic characteristics of CRC diagnosed in different polyp groups. Participants who had large SPs at the first endoscopy tended to develop CRC at a younger age (median, 59 years; interquartile, 52-77 years) than those who did not have a polyp (median, 72 years; interquartile, 65-78 years) or had advanced adenoma (median, 73 years; interquartile, 62-79 years). CRC cases in the non-polyp group were more likely to have family history of CRC (28%) than those in the polyp group (ranging from 14% to 20%). CRC patients in the conventional adenoma group drank more alcohol (7.8 g/day) than those in the non-polyp (1.5 g/day) or SP group (3.4 g/day). The distribution of subsite and stage of CRC did not differ between cases with no polyp, conventional adenoma, and SP. However, given the limited number of cases, these results should be interpreted with caution.
Table 4.
Characteristic of CRC cases according to participants’ first endoscopic findings in the three cohorts (NHS [1990-2012], NHS2 [1989-2013] and HPFS [1990-2012]) a
| Non-polyp |
Overall polyps |
High-risk polyps |
|||
|---|---|---|---|---|---|
| (n=427) | Conventional adenomas (n=51) | Serrated polyps (n=24) | Advanced conventional adenomas (n=39) | Large serrated polyps (≥10 mm) (n=5) | |
| Age at diagnosis, year | 72 (65-78) | 74 (62-80) | 73 (61-78) | 73 (62-79) | 59 (52-77) |
| P | 0.69 | 0.79 | 0.99 | 0.09 | |
| Interval between the first endoscopy and diagnosis of CRC, year | 6 (3-10) | 6 (4-8) | 6 (4-8) | 6 (4-8) | 6 (3-6) |
| P | 0.95 | 0.26 | 0.55 | 0.30 | |
| Family history of CRC, n (%) | |||||
| No | 309 (72) | 43 (84) | 20 (83) | 32 (82) | 4 (80) |
| Yes | 118 (28) | 8 (16) | 4 (17) | 7 (18) | 1 (20) |
| P | 0.09 | 0.34 | 0.26 | 1.00 | |
| Pack-years of smoking | 2.0 (0-23.0) | 7.0 (0-28.0) | 10.5 (0.5-25.5) | 8.5 (0-33.0) | 8.0 (0-17.0) |
| P | 0.59 | 0.34 | 0.32 | 0.65 | |
| Body mass index, kg/m2 | 25.8 (23.1-28.8) | 26.1 (23.4-28.3) | 25.0 (23.1-27.7) | 26.1 (23.4-28.1) | 30.4 (22.7-45.8) |
| P | 0.67 | 0.89 | 0.81 | 0.29 | |
| Alcohol intake, g/day | 1.5 (0-6.9) | 7.8 (0.4-18.2) | 3.4 (0-15.0) | 8.4 (2.2-18.2) | 3.7 (1.1-6.0) |
| P | 0.009 | 0.16 | 0.007 | 0.81 | |
| Colorectal cancer subsite, n (%) | |||||
| Proximal colon | 238 (56) | 28 (55) | 16 (67) | 19 (49) | 3 (60) |
| Distal colon | 77 (18) | 11 (22) | 3 (13) | 10 (26) | 0 |
| Rectum | 67 (16) | 4 (8) | 3 (13) | 3 (8) | 2 (40) |
| Unidentified or multi-region | 45 (11) | 8 (16) | 2 (8) | 7 (18) | 0 |
| P | 0.35 | 0.74 | 0.26 | ||
| Colorectal cancer stage, n (%) | |||||
| Stage I | 96 (22) | 9 (18) | 5 (21) | 7 (18) | 1 (20) |
| Stage II | 94 (22) | 13 (25) | 5 (21) | 10 (26) | 1 (20) |
| Stage III or IV | 148 (35) | 13 (25) | 8 (33) | 9 (23) | 2 (40) |
| Unknown | 89 (21) | 16 (31) | 6 (25) | 13 (33) | 1 (20) |
| P | 0.26 | 0.97 | 0.22 | 1.00 | |
Abbreviations: CRC, colorectal cancer; SP, serrated polyp; NHS, the Nurses’ Health Study; HPFS, the Health Professionals Follow-up Study; OR, odd ratio; CI, confidence interval.
Median (interquartiles) is presented for continuous variables and number of participants (percentage) for categorical variables. Advanced conventional adenomas were defined as at least one conventional adenoma of ≥10 mm in diameter or with advanced histology (tubulovillous/villous histological features or high grade or severe dysplasia), otherwise were defined as non-advanced conventional adenomas.
Discussion
Within three large prospective US cohorts with a median follow-up of 10 years since the first endoscopy, we observed that individuals with advanced adenoma or large SP were more likely to develop CRC compared to participants with no polyp. Through detailed analysis according to adenoma features, we found that large size, multiplicity and villous histology all predicted a higher CRC risk. In contrast, individuals with non-advanced adenoma or small SP did not have an increased risk of CRC. Our findings provide further evidence for improving the existing guidelines for colonoscopy surveillance after polypectomy.
Current guidelines uniformly recommend that individuals with advanced conventional adenomas undergo routine surveillance by repeating colonoscopy within 3 years, based on the data that risk for CRC and recurrent advanced neoplastic findings on subsequent colonoscopy was higher among these individuals compared to the general population.23–25 Consistent with these data and extending the findings of the recent PLCO study that included individuals with positive flexible sigmoidoscopy results only,13 we found a strong positive association between detection of advanced adenomas at the first endoscopy and subsequent risk of CRC. These data provide further support for periodic, ongoing surveillance colonoscopy in patients diagnosed with an advanced adenoma.
For patients with 1 to 2 small adenoma(s) with villous histology, the European Union and British guidelines recommend a repeat endoscopy in 10 years, while the ESGE and USMSTF guidelines recommend a repeat colonoscopy in 3 years.7–9, 11 No study has yet specifically examined CRC risk among these patients. In the current study, we found that participants with 1-2 small tubulovillous or villous adenomas had a higher risk of CRC than those with no polyp. The risk elevation was similar to those with one or more tubulovillous adenoma of any size, or with one or more tubular adenomas >10 mm (HR=1.97, 2.13, and 2.25, respectively), suggesting that villous histology, multiplicity, and large size all predicted a higher CRC risk. These findings support the ESGE and USMSTF guidelines8, 11 for more intensive surveillance among these patients.
For patients with 1 to 2 small tubular adenoma(s), the current USMSTF guidelines recommend repeated colonoscopy between 5 and 10 years, while others recommend patients return for routine screening within 10 years.7–9, 11 Consistent with the UK Flexible Sigmoidoscopy Trial and the PLCO study, we found that participants with 1 to 2 small tubular adenomas had no increased risk of developing CRC compared to polyp-free individuals.6, 13 However, our current study, along with others, was unable to directly test whether a 5- or 10-year interval would be sufficient to minimize future CRC risk. This question is currently being investigated in the ongoing randomized clinical trials, including the European Polyp Surveillance trial (EPoS), in which participants with low-risk adenomas (1-2 tubular adenomas of size <10mm, low-grade dysplasia) are randomized to undergo a surveillance colonoscopy at an interval of 5 or 10 years26
Increasing evidence indicates that SPs represent a distinct group of CRC precursors. Unlike conventional adenomas that develop through sequential mutations in oncogenes and tumor suppressor genes, the serrated pathway is characterized by hypermethylation of CpG islands in gene promoters, BRAF mutation, and microsatellite instability.27 However, because SPs were generally not appreciated in clinical practice until 2003–2005, their natural history remains largely unknown. Limited evidence suggests that size is an important predictor for the malignant potential of SP and that patients with SPs of >10 mm are more likely to have synchronous and metachronous advanced neoplasia than those with no polyp or with small SPs. 10,28,29 Based on these data, several guidelines8–12 have proposed that patients with large SPs should undergo a repeat colonoscopy within 3 years. Two recent longitudinal studies have assessed future CRC risk following removal of SPs. A study in the Norwegian Colorectal Cancer Prevention trial reported that detection of large SPs at the first endoscopy was an independent risk factor for subsequent CRC and the association (HR, 4.2; 95% CI, 1.3-13.3) was even stronger than that for advanced adenomas (HR, 3.3; 95% CI, 2.1-5.2).14 Another registry-based case-control study in Denmark found that patients with SSA/Ps were at higher risk of CRC than those with no polyp, with an odd ratio (OR) of 3.07 (95% CI, 2.30-4.10).15 In line with these findings, we found that, compared with polyp-free individuals, those with large SPs had a higher risk of CRC, suggesting that large SPs may warrant intensive surveillance colonoscopy similar to that for advanced adenomas.
Our study has several strengths, including the long-term follow-up, ascertainment of colorectal polyps and cancers with detailed histopathological information, and repeated collection of detailed lifestyle data that allow for adjustment for confounding by CRC risk factors. Several limitations of our study need to be noted. First, because of the evolving nature and lack of consensus regarding the diagnostic criteria of specific subsets of SPs, we were unable to distinguish HPs from SSA/Ps and TSAs. However, as indicated by prior data and as proposed by an expert panel, SPs larger than 10 mm is a good indicator for SSA/P.10 Moreover, our findings for the positive association of large SPs with CRC are consistent with those of previous studies of SSA/P that had limited sample size, although our effect estimates for small SPs may have been heavily weighted by diminutive HPs that have little malignant potential. Second, data on surveillance colonoscopy were self-reported and subject to measurement error. Third, data on quality of endoscopy was unavailable and thus there may be residual confounding by the performance of the index endoscopies. Finally, some of the analyses were based on a small number of events and need to be interpreted with caution.
In conclusion, our findings provide support for current guidelines which recommend repeat lower endoscopy within 3 years of a diagnosis of advanced adenomas and large SPs. In contrast, non-advanced adenomas or small SPs may not require more intensive surveillance compared to individuals without polyps.
Supplementary Material
What you need to know:
BACKGROUND AND CONTEXT:
Individuals with polypectomies during screening endoscopies are advised to undergo colonoscopy surveillance to prevent colorectal cancer (CRC). However, guidelines for surveillance intervals after diagnosis of a precursor lesion vary. We performed a prospective study to examine the association between findings from first endoscopy and CRC risk among 122,899 participants who underwent flexible sigmoidoscopy or colonoscopy.
NEW FINDINGS:
Compared to participants with no polyp detected during initial endoscopy, individuals with an advanced adenoma had a more than 4-fold increase in risk for CRC and individuals with a large serrated polyp had a 3.35-fold increase in risk. In contrast, there was no significant increase in risk of CRC in patients with non-advanced adenomas or small serrated polyps.
LIMITATIONS:
This was a retrospective analysis of data from 3 large cohort studies.
IMPACT:
These findings provide support for guidelines that recommend repeat lower endoscopy within 3 years of a diagnosis of advanced adenoma and large serrated polyps. In contrast, patients with non-advanced adenoma or small serrated polyps may not require more intensive surveillance than patients without polyps.
Acknowledgements:
We would like to thank the participants and staff of the Nurses’ Health Study, the Nurses’ Health Study 2 and the Health Professionals Follow-up Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Funding:
This work was supported by the American Cancer Society Mentored Research Scholar Grant (MRSG-17-220-01 - NEC to M.S.); by the U.S. National Institutes of Health (NIH) grants [P01 CA87969 to M.J. Stampfer; UM1 CA186107 to M.J. Stampfer; P01 CA55075, to W.C. Willett; UM1 CA167552 to W.C. Willett; P50 CA127003 to C.S. Fuchs; K24 DK098311, R01 CA137178, R01 CA202704, R01 CA176726, to A.T.C.; R01 CA151993, R35 CA197735 to S.O.; CA202704, R01 CA176726, to A.T.C.; R01 CA151993, R35 CA197735 to S.O.; R03 CA197879 to KW; R21 CA230873 to K.W. and S.O.; R21 CA230873 to K.W. and S.O.; K99 CA215314 and R00 CA215314 to M.S.]; by National Key R&D Program of China [2017YFC1308800 to X.H.; 2017YFC0908300 to D.H.] and by grants from the American Institute for Cancer Research (K.W.), the Project P Fund for Colorectal Cancer Research, The Friends of the Dana-Farber Cancer Institute, Bennett Family Fund, and the Entertainment Industry Foundation through National Colorectal Cancer Research Alliance. Dr. Chan is a Stuart and Suzanne Steele MGH Research Scholar. The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Abbreviations:
- BMI
body mass index
- BSG
British Society of Gastroenterology
- CI
confidence interval
- CRC
colorectal cancer
- ESGE
European Society of Gastrointestinal Endoscopy guidelines
- HR
hazard ratio
- HP
hyperplastic polyp
- HPFS
the Health Professionals Follow-up Study
- MET
metabolic equivalent of task
- NHS
the Nurses’ Health Study
- NHS2
the Nurses’ Health Study 2
- SSA/P
sessile serrated adenomas/polyp
- SP
serrated polyp
- TSA
traditional serrated adenoma
- USMSTF
United States Multi-Society Task Force
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: Andrew T. Chan previously served as a consultant for Bayer Healthcare and Pfizer Inc. for work unrelated to the topic of this manuscript. This study was not funded by Bayer Healthcare or Pfizer Inc. No other conflict of interest exists.
Reference
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012;366:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 2013;369:1095–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holme O, Loberg M, Kalager M, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA 2014;312:606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkin W, Wooldrage K, Parkin DM, et al. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK Flexible Sigmoidoscopy Screening randomised controlled trial. Lancet 2017;389:1299–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010;375:1624–33. [DOI] [PubMed] [Google Scholar]
- 7.Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010;59:666–89. [DOI] [PubMed] [Google Scholar]
- 8.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143:844–857. [DOI] [PubMed] [Google Scholar]
- 9.Atkin WS, Valori R, Kuipers EJ, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition--Colonoscopic surveillance following adenoma removal. Endoscopy 2012;44 Suppl 3:SE151–63. [DOI] [PubMed] [Google Scholar]
- 10.Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012;107:1315–29; quiz 1314, 1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassan C, Quintero E, Dumonceau JM, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2013;45:842–51. [DOI] [PubMed] [Google Scholar]
- 12.East JE, Atkin WS, Bateman AC, et al. British Society of Gastroenterology position statement on serrated polyps in the colon and rectum. Gut 2017;66:1181–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Click B, Pinsky PF, Hickey T, et al. Association of Colonoscopy Adenoma Findings With Long-term Colorectal Cancer Incidence. JAMA 2018;319:2021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holme O, Bretthauer M, Eide TJ, et al. Long-term risk of colorectal cancer in individuals with serrated polyps. Gut 2015;64:929–36. [DOI] [PubMed] [Google Scholar]
- 15.Erichsen R, Baron JA, Hamilton-Dutoit SJ, et al. Increased Risk of Colorectal Cancer Development Among Patients With Serrated Polyps. Gastroenterology 2016;150:895–902 e5. [DOI] [PubMed] [Google Scholar]
- 16.Giovannucci E, Ascherio A, Rimm EB, et al. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med 1995;122:327–34. [DOI] [PubMed] [Google Scholar]
- 17.Wark PA, Wu K, van ‘t Veer P, et al. Family history of colorectal cancer: a determinant of advanced adenoma stage or adenoma multiplicity? Int J Cancer 2009;125:413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kavanagh AM, Giovannucci EL, Fuchs CS, et al. Screening endoscopy and risk of colorectal cancer in United States men. Cancer Causes Control 1998;9:455–62. [DOI] [PubMed] [Google Scholar]
- 19.Snover DC, Ahnen DJ, Burt RW, et al. Serrated polyps of the colon and rectum and serrated polyposis In: Bosman FT, Carneiro F, Hruban RH, et al. , eds. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon: IARC Press, 2010. [Google Scholar]
- 20.Gill P, Wang LM, Bailey A, et al. Reporting trends of right-sided hyperplastic and sessile serrated polyps in a large teaching hospital over a 4-year period (2009–2012). J Clin Pathol 2013;66:655–8. [DOI] [PubMed] [Google Scholar]
- 21.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 1993;25:71–80. [DOI] [PubMed] [Google Scholar]
- 22.He X, Wu K, Ogino S, et al. Association Between Risk Factors for Colorectal Cancer and Risk of Serrated Polyps and Conventional Adenomas. Gastroenterology 2018;155:355–373 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lieberman DA, Weiss DG, Harford WV, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology 2007;133:1077–85. [DOI] [PubMed] [Google Scholar]
- 24.Martinez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology 2009;136:832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med 1992;326:658–62. [DOI] [PubMed] [Google Scholar]
- 26.Jover R, Bretthauer M, Dekker E, et al. Rationale and design of the European Polyp Surveillance (EPoS) trials. Endoscopy 2016;48:571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.JE IJ, Vermeulen L, Meijer GA, et al. Serrated neoplasia-role in colorectal carcinogenesis and clinical implications. Nat Rev Gastroenterol Hepatol 2015;12:401–9. [DOI] [PubMed] [Google Scholar]
- 28.Schreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology 2010;139:1497–502. [DOI] [PubMed] [Google Scholar]
- 29.Hiraoka S, Kato J, Fujiki S, et al. The presence of large serrated polyps increases risk for colorectal cancer. Gastroenterology 2010;139:1503–10, 1510 e1–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.