Abstract
Preterm infants often have dysphagia. Because reducing lifetime cumulative exposure to radiation in the context of diagnosis and treatment is a continuing goal of all medical fields which use Xray imaging, efforts exist to reduce reliance on the gold standard diagnostic tool for dysphagia, VFSS. Alternatives, such as video of external hyolaryngeal movement using video recordings of the anterior surface of the neck, must be evaluated and validated against videofluoroscopy, a task for which non-human animal models are appropriate. In this study we tested the hypotheses that 1) swallows could be identified equally well from video of external hyolaryngeal movement and bolus movement in videofluoroscopy, and that 2) the two measures would be tightly temporally linked in both term and preterm infant pigs. We recorded 222 swallows in simultaneous and precisely synchronized high speed videofluoroscopy and high speed camera films of 4 preterm and 3 term infant pigs drinking milk from a bottle. In term pigs, the two measures consistently identified the same swallows in each image stream. However, in preterm pigs there was a high rate of false positives (~ 10% per feeding sequence) and false negatives (~27% per feeding sequence). The timing of hyolaryngeal elevation (external video) and bolus movement (videofluoroscopy) was correlated and consistent in terms pigs, but not in preterm pigs. Magnitude of hyolaryngeal elevation was less in preterm pig swallows than term pig swallows. Absence of epiglottal inversion in preterm pigs was not linked to variation in the timing of the two swallow events. Video of external hyolaryngeal movement, though a reliable swallow indicator in term infant pigs, was unreliable in preterm infant pigs. The coordination of swallowing events differs in preterm and term infant pigs. More research is needed into the distinctive biomechanics of preterm infant pigs.
Keywords: Deglutition, dysphagia, premature, biomechanics, pig
Introduction
Videofluoroscopic swallow studies (VFSS) are considered the gold standard for assessing and diagnosing dysphagia [1]. However, reducing lifetime cumulative exposure to radiation in the context of diagnosis and treatment is a continuing goal of all medical fields which rely on X-ray imaging. As the risks associated with radiation exposure are magnified in low body weight, neurologically developing preterm infants, where dysphagia is a frequent concern [2], there is particular interest in avenues that would reduce dependency on VFSS in the treatment of preterm infant dysphagia. Thus, there is considerable interest in finding reliable alternatives to videofluoroscopy that can be applied in preterm infants.
The usefulness of external measurements of gross hyolaryngeal movement recorded from videos of the anterior surface of the neck during swallowing relies, in part, on the degree to which hyolaryngeal movements correlate with bolus movements. Hyolaryngeal movement is part of the pharyngeal swallow, and is associated with cranial displacement of the airway, a critical part of the airway protection process in adult humans, children, and infant full term pigs [3–5]. Although a relationship between hyolaryngeal movement and bolus movement is established in adults, it may not be identical in term or preterm infants [5]. Some preterm infants in particular show poor coordination in oropharyngeal motor function, including sucking and swallowing [6–8]. Furthermore, work in the infant pig model has indicated that movement of internal structures during the swallows, such as the epiglottis, changes over early ontogeny even in term pigs [9]. Establishing what relationship exists between externally visible markers of the swallow, such as hyolaryngeal elevation, and bolus movement in term and preterm infants, is necessary before considering the potential usefulness of external video recordings as an alternative or adjunct to VFSS.
Because of their vulnerability as patients, it is unethical to conduct large scale videofluoroscopic studies on infants without a diagnostic need. In addition, concerns about radiation dosage effects, as well as standards for medical equipment, mean that frame rates are kept at 30 fps or lower [10,11], and studies in human pediatric and infant populations remain rare [12]. Thus, although recent quantitative studies using videofluoroscopy have yielded quantitative results into tongue and hyoid kinematics in infant swallowing [5,13], they are limited in scope by small samples sizes of both individuals and swallows, and frame rates that may not capture details of swallowing kinematics. Furthermore, degree of prematurity, which impacts the degree of discoordination of infant feeding function [14], is difficult to control in clinical samples. Animals models permit the collection of more and higher quality videofluoroscopic data than is possible in human populations [15]. Furthermore, animal models of preterm infants allow degree of prematurity to be experimentally manipulated, resulting in more powerful analyses with fewer confounding variables [16]. Infant pigs are a clinically relevant model for infant feeding, owing to similar size, anatomy, and feeding behavior [16–19]. Thus, we use a pig model of term and preterm infants to establish the degree to which externally visible movements of the feeding apparatus correlate with internal bolus movements.
The purpose of this study was to test the reliability and validity of using externally visible markers of the swallow to infer bolus movement in term and preterm pigs. Furthermore, we aimed to determine whether the temporal relationship between bolus movement and hyoid kinematics was the same in term and preterm pigs, given the documented discoordination of feeding movements in preterm versus term infants. Our specific hypotheses are that, using videofluoroscopy as the definitive determination of the occurrence and timing of a swallow:
Swallows, determined from videofluoroscopy, can be accurately identified from simultaneous video of external hyolaryngeal movement in term and preterm pigs.
Externally visible hyolaryngeal kinematics exhibit an identical and consistent temporal correlation with bolus movement established from videofluoroscopy in both term and preterm pigs.
Presence or absence of epiglottal inversion in preterm pigs does not affect the timing relationship between hyolaryngeal movements and bolus movement.
Materials and Methods
Animals and procedures
All procedures were done in accordance with a NEOMED IACUC approved animal protocol (#17-04-071). Three full term and four preterm pigs were included in this study. Full term pigs (Yorkshire strain) were purchased at age two days (Shoup Farms, Wooster, Ohio). Preterm neonatal pigs were delivered via Cesarean section from pregnant Yorkshire sows (Shoup Farms, Wooster, Ohio) at 107–109 days gestation, equivalent to 30–32 weeks in human gestation. Cesarean section ensured that the neonatal pigs were of the exact age specified in this study. For this degree of prematurity, induction of vaginal delivery is not possible. The C-section followed procedures described by this lab previously [16]. Briefly, the sow was sedated and then maintained under anesthesia using isofluorane. Under full aseptic conditions, an incision was made in the flank, the horns of the uterus located, and the piglets removed sequentially through separate incisions in the uterine wall. Umbilical vessels were clamped, and piglets were tactilely stimulated to induce spontaneous respiration. Dopram was administered if spontaneous respiration did not start soon after birth. Piglets were kept in modified human incubators until they were walking (12–24h post birth). The sow was euthanized by injection of sodium pentobarbitol after all the pigs were delivered. The neonatal pigs were fed colostrum a few hours after delivery via syringe. Infant pig formula (Solustart Pig Milk Replacement, Land o’ Lakes, Arden Mills, MN) was then administered after the colostrum was given, via syringe or gavage until animals were able to tolerate oral feeding, which occurred approximately 24 hours post birth.
Marker Placement
Term pigs underwent intra oral placement of radio opaque markers in the tongue, palate and epiglottis. Term pigs also underwent surgery under 1–3% inhalant isoflurane and full aseptic technique to place markers on the thyroid and hyoid [20,21]. Preterm pigs were found to be too fragile to undergo prolonged anesthesia and so did not receive marker implantation. As a result of this, radio opaque markers were not used in the comparison of term and preterm swallows in this study.
Videofluoroscopy Protocol and Data Recording
The study was designed as a paired experimental study with two groups of pigs (term and preterm) of the same post natal age. At seven days post-natal (roughly equivalent to 1–2 months post-natal in humans), both term and preterm groups were deemed medically stable enough to feed while being recorded simultaneously by high speed videofluoroscopy (100fps) and high speed video recording of external features (100fps). The pigs fed on milk formula (Solustart Pig Milk Replacement, Land o’ Lakes, Arden Mills, MN) mixed with a barium solution, producing a liquid with the thickness of light cream. Fluoroscopic recording used a modified C-arm fluoroscope (GE9400 C-Arm, 80 kV, 4 MA) fitted with a digital video camera (XC 1M digital video camera, Xcitex, Cambridge, MA). Simultaneous videos of external hyolaryngeal movement (100fps) were recorded (MotionXtra N3, IDT, Pasadena, CA for term pigs, XC 2M digital video cameras, Xcitex, Cambridge, MA for preterm pigs). A square wave trigger pulse was used to ensure precise frame to frame synchronization. Both sets of film images were digitally stored for subsequent frame by frame analysis. For term pigs, one sequence of swallows for each pig at seven days of age was recorded. For the preterm pigs we used the sequences of swallows recorded at seven and eight days of age, as preterm pigs fed for shorter durations on each day.
Data Extraction
The video of external hyolaryngeal movement and fluoroscopy videos were synchronized to extract the same swallows for each pig feeding sequences. Blocks of 10 to 30 swallows were identified from the fluoroscopy videos for each pig and then the corresponding duration of external video identified. The external videos and fluoroscopy videos were then blinded. All preprocessing was done by one author. The total number of swallows analyzed was 222 (Table 1).
Table 1:
Number of swallows by individual for each treatment group.
| Treatment | Number of swallows |
|---|---|
| preterm | 150 |
| Pig 1 | 39 |
| Pig 2 | 41 |
| Pig 3 | 39 |
| Pig 4 | 31 |
| term | 72 |
| Pig 5 | 11 |
| Pig 6 | 33 |
| Pig 7 | 28 |
Identification of swallows
The exact time, or frame number, of each swallow was marked in each blinded external video and fluoroscopy recording. All swallow identification was done by one author different from the author who extracted and blinded each swallow. Inter and intra rater reliability was used to assess the quality of the swallow identification method from a subset of external and X ray videos for term pigs. Inter rater reliability was assessed by calculating the percent of swallows where the discrepancy in the ID frame for two different raters was not more than one frame (1/100th of a second). For intra rater reliability, a single rater identified swallows in the same sequences with new blind names three times, with a 12 hour space between each round of scoring. Again, intra rater reliability was assessed by calculating the percent of swallows where the discrepancy in the frame of the swallows among all three trials was no more than one frame (1/100th of a second).
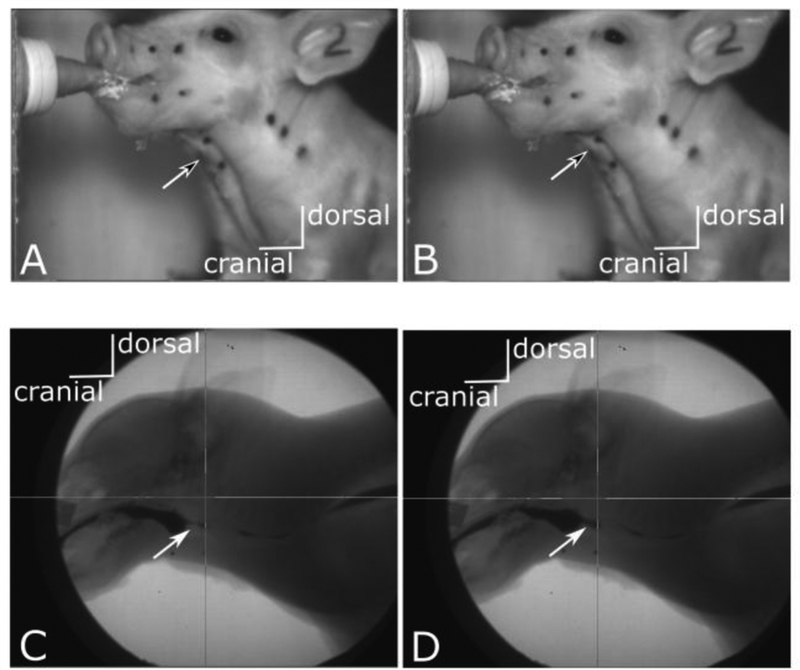
A swallow event in the x-ray was defined as the frame where the bolus of milk just began to move past the epiglottis and enter the pharynx [16] (figure 1 C,D). Each swallow was also scored on a binary scale for whether or not the epiglottis fully inverted during the swallow based on the videofluoroscopic recording.
Figure 1:
Stills from external (top row) and videofluoroscopic (bottom row) videos of the same swallow in a pig. Left hand images (A, C) are from before swallow initiation. Right hand images (B, D) are those scored as marking the swallow. Black tipped arrows in the top row point to the laryngeal prominence and show elevation and change of angle with the hyoid in the left hand image. The two stills are two frames apart. White tipped arrows in the bottom row point to increase in milk volume in the pyriform recess in D, marking initiation of posterior bolus movement. Again, the two stills are two frames apart. Refer to supplemental materials for videos from which stills were taken.
Swallows in the video of external hyolaryngeal movement were defined as the frame of onset of rapid acceleration of the hyolaryngeal region (figure 1 A, B). The videos were replayed at 25 frames a second initially to distinguish rapid elevation of the throat (swallows) from other movements of the anterior neck region due to e.g. pig movement. Within each identified swallow the exact frame of onset of hyolaryngeal movement was found by slowly scrolling through each frame and scoring the most abrupt change in hyoid elevation.
Assessment of reliability of swallow identification
After identifying the time of the swallow, both sets of film were unblinded to match light and videofluoroscopy sequences. Total number of putative swallows were counted in external video and videofluoroscopy video for each pig feeding sequence. Interswallow interval, the time between swallows, was also calculated. Where a discrepancy existed between a pair of external video and videofluoroscopy sequences, swallows were aligned manually using interswallow interval as a guide to identifying pairs of frames from the video recordings of the neck and video fluoroscopy that corresponded to the same swallows within a feeding sequence.
In addition to differences in timing of swallow, we also identified two additional types of error in identifying swallows from external videos of the anterior neck. We counted firstly, swallows correctly identified in the videofluoroscopy sequence not identified in the external video sequence, and secondly, movement in the external video sequence that were identified as swallows but did not match swallows in the videofluoroscopy sequence. The percentage of each type of error was calculated for each pig feeding sequence. The unit of analysis for the statistical assessment of reliability of swallow identification was the feeding sequence.
Regularity of timing between external hyoid movement and bolus movement
Using only swallows that were correctly identified in both external video and videofluoroscopy sequences, we calculated the time difference between the hyolaryngeal movement in the external video and the movement of the bolus in the videofluoroscopy. A Wilcoxon rank sum non-parametric test was used to determine whether the timing relationship between hyolaryngeal movement and bolus movement was the same in term and preterm pigs. A Levene’s test was used to test whether the variation in this timing differed between term and preterm groups. The unit of analysis for this test was the swallow.
Magnitude of hyolaryngeal excursion during swallows
For each swallow correctly identified in a video of external features (after cross-validation with the videofluoroscopic recordings), we used digital marker tracking software (ProAnalyst, Excitex, Cambridge, MA) to identify the most caudal and most cranial position of the thyroid eminence during the swallow. The caudal-most point of the eye was also digitized in the corresponding frames. The distance between the two thyroid eminence positions was calculated. The distance moved by the eye between the two frames of thyroid eminence movement was also calculated, and if it was more than 10% of eye length, that swallow was discarded. This procedure controlled for movement artifact as there were no markers on the pig we could use to normalize thyroid position. Thyroid eminence movement was standardized to eye length to make measurements comparable between pigs. The effect of prematurity on magnitude of thyroid eminence movement was tested using a Wilcoxon rank sum test.
Relationship between epiglottal movement and correlation between two measures of swallow
No swallows without epiglottal movement were seen in term pigs, but some were seen in the preterm animals. In preterm pigs, Wilcoxon rank sum test and Levene’s test was used to test whether a difference in median or variance existed between swallows with and without epiglottal inversion with regard to the duration between onset of hyolaryngeal movement and onset of bolus movement. The unit of analysis for this test was the swallow.
Results
Characteristics of individuals
Key biological characteristics of the two groups of pigs at time of recording are presented below (table 2). By and large, preterm pigs were worse at thermoregulating and maintaining posture than term pigs at 7 days post birth.
Table 2:
weight and sex distributions of the two groups of pigs at 7 days post birth (time of recording).
| Group | Weight at time of recording (minimum -maximum) (kg) | sex |
|---|---|---|
| Term | 1.7 – 2.5 | 2 male, 1 female |
| preterm | 0.5–1.5 | 3 male, 1 female |
Intra and inter rater reliability scores were high in the training dataset
Intra and inter rater reliability scores of between 80 and 100 % were achieved for both external and X-ray video identification of swallows in the training dataset (Table 3.)
Table 3.:
Intra and inter rater reliability scores for swallow identification in a training dataset of both external and Xray videos.
| Data source | Inter rater percent agreement +/− 1 frame | Intra rater percent It agreement +/1 frame |
|---|---|---|
| Xray video | 100% | 85% |
| External video | 81% | 90% |
Swallows were missed and falsely identified in the external video footage more often in preterm than in term infant pigs
On average in term pigs, only 1% of swallows (no more than one or two per sequence) identified in the videofluoroscopy recordings were not identified in the high speed external video recording. However, in preterm pigs, up to 10% of swallows per sequence that were identified in the videofluoroscopy recordings were missed in the external video recordings (Table 2). In preterm pigs, even after multiple rounds of cross validation, up 27% of the hyoid movements identified as swallows in the external video did not match up with any swallows seen in the videofluoroscopy recording. This was never observed in the term pigs (Table 4).
Table 4:
Average frequency and range of the two types of error in swallow identification in preterm and term pigs per feeding sequence
| Swallow identification error per sequence | Avg percent term (min-max) | Avg percent preterm (min-max) |
|---|---|---|
| Identified in fluoroscopy but not in external video | 1% (0–3%) | 3.5% (0–10%) |
| Identified in external video but not seen in fluoroscopy | 0% (0–0%) | 9.5% (0–27%) |
The time difference between hyoid movement and beginning of bolus movement is different and more variable in preterm pigs
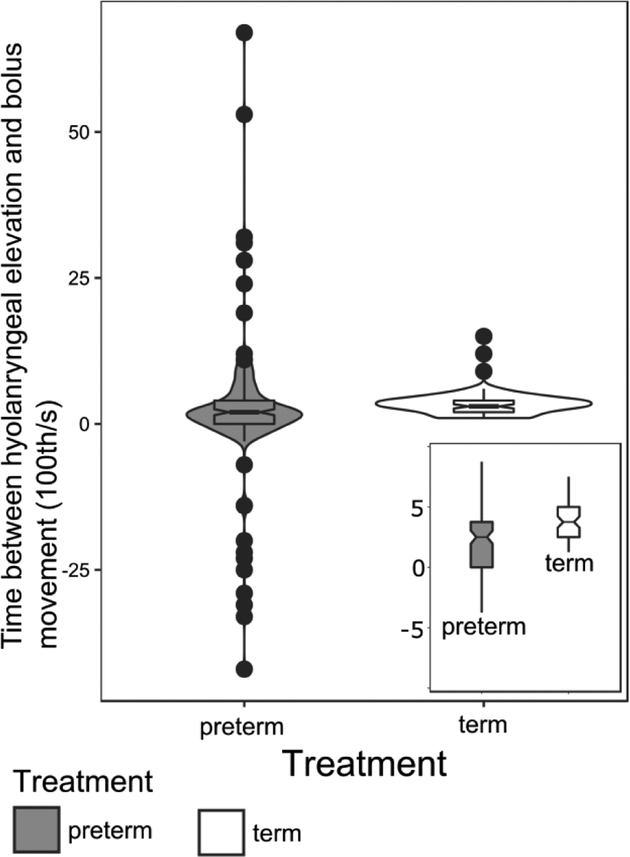
The mean delay between frame of beginning of hyoid acceleration identified in the external video and frame of beginning of bolus movement identified in the videofluoroscopy was significantly shorter in preterms than terms (Wilcox rank sum test, W=7312, P<0.001). However, the variance in that delay was much greater in preterms, indicating a weaker correlation between the two variables in preterm pigs than term pigs (Levene’s test of equal variance, p<0.001 and figure 2).
Figure 2:
Violin plot showing distribution, and notched boxplot with median, quartiles, whiskers to 1.5 times the interquartile distance, and outliers of time between hyoid elevation and beginning of bolus movement (in 100th of a sec) in preterm and term pigs. Inset: magnified version of box and whisker plots to show difference in medians otherwise obscures by the difference in range of data between the two groups.
Thyroid eminence excursion in the swallow is less in preterm pigs than in term pigs
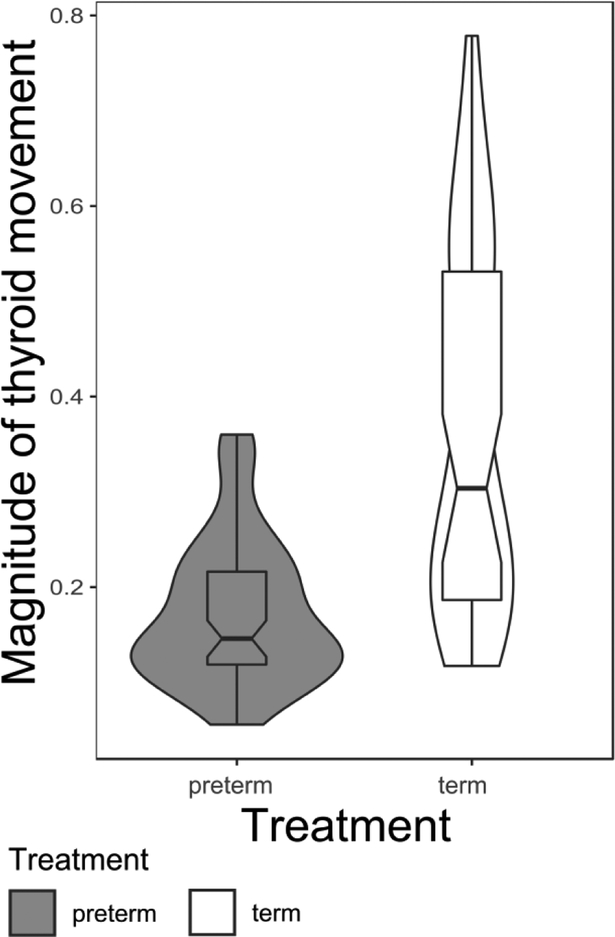
The magnitude of thyroid elevation measured from external videos was consistently less in preterm pigs than in term pigs (Wilcoxon rank sum test, W=2647, p<0.001; figure 3).
Figure 3:
Violin plot showing distribution, and notched boxplot with median, quartiles, whiskers to 1.5 times the interquartile distance of magnitude of thyroid eminence movement during swallows in preterm and term pigs.
Epiglottal inversion is less frequent in preterm pigs, but uncorrelated with the variation in time between hyoid movement and bolus movement
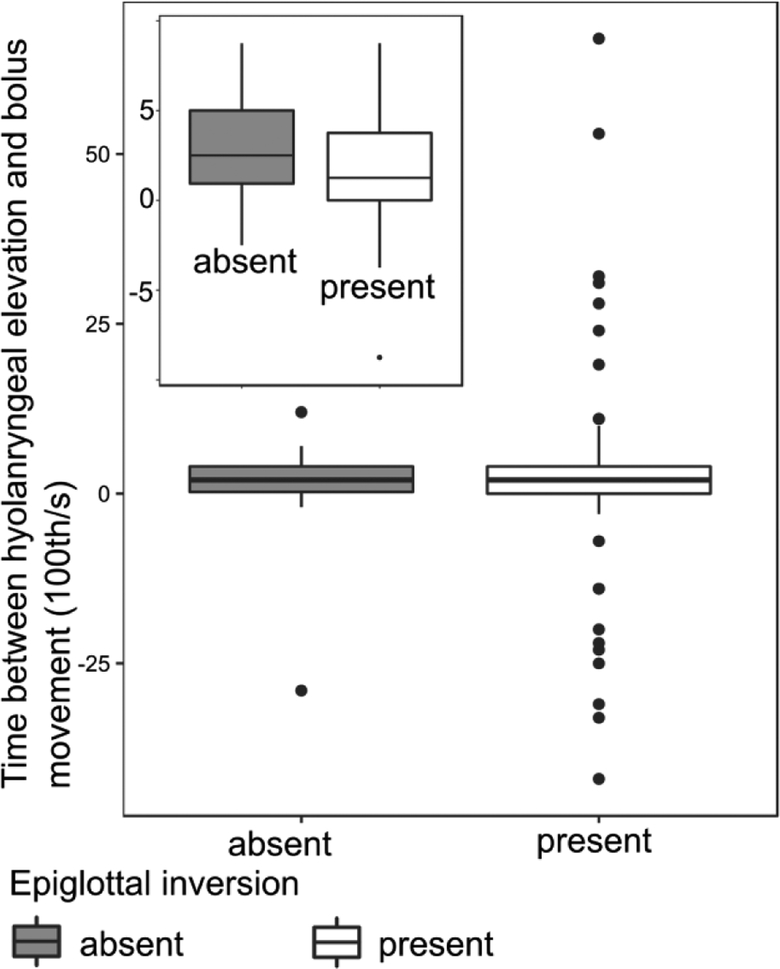
Swallows without an epiglottal inversion were found to occur in preterm pigs, though not in term pigs (figure 4). However, the status of the epiglottal inversion in a swallow does not affect either mean or variance of the delay between hyoid elevation and bolus movement in preterm pigs (Wilcox rank sum test W=1074, p=0.501, Levene’s test F=0.63, p=0.429).
Figure 4:
Box plot with median, quartiles, whiskers to 1.5 times the interquartile distance, and outliers for time between hyolaryngeal elevation and beginning of bolus movement relative to presence or absence of epiglottal inversion in preterm pigs.
Discussion
Video of External hyolaryngeal motion is unreliable for identifying swallows in preterm pigs
The accuracy and precision of hyolaryngeal movement measured from external videos for indicating beginning of bolus movement differs between preterm and term infant pigs, refuting our initial hypothesis. In term pigs, false positives (wrongly identifying hyoid movements as swallows) and false negatives (failing to identify a swallow from a hyoid movement) are rare. In preterm pigs, both are more frequent. The poor ability to identify preterm swallows from external video recordings was despite using high speed, high resolution cameras and professional lighting in a highly controlled animal feeding environment. This failure to correctly identify swallow events from hyolaryngeal movement in preterm pigs would have significant impacts on the measurement of many parameters of interest in the study of infant pig oropharyngeal function. Important parameters of feeding, such as swallow rate, interswallow interval, swallow regularity, and suck-swallow coordination, rely on the ability to accurately and precisely measure the time of the swallow event. External hyoid movements would be unreliable to measure any of these in preterm pigs, based on our findings. Crucially, because the reliability of external hyolaryngeal movements for identifying swallows was higher in term pigs, only a direct assessment of this metric in preterm infant pigs specifically, as conducted in this study, revealed the inaccuracy.
The differences in reliability between the two groups of infant pigs may reflect measurement error owing to different sizes of the animals making swallow associated hyoid movements more difficult to see in preterm pigs. The reduced hyolaryngeal movement seen in external videos of preterm versus term pigs suggests that smaller magnitude of motion may be part of what makes swallow detection less reliable in the preterms. However, this explanation does not account for the hyoid movements identified as swallows that did not correspond to swallows identified in videofluoroscopy. The existence of these false positives points instead to less stereotyped hyoid movement in the preterms accounting for the discrepancy. Specifically, the immature musculoskeletal system of preterm pigs, partially responsible for their impaired oropharyngeal function [6,14], may also impair their ability to maintain the hyoid in a stable position during different oropharyngeal activities [22]. Further study into the variability of hyoid movement in different oral behaviors, for example sucking and swallowing, of term and preterm pigs would improve our understanding of specific biomechanical effects of musculoskeletal immaturity on preterm infant pig feeding.
Preterm pigs show discoordination of hyoid elevation and bolus movement relative to term pigs
Beyond the problems in identifying swallows from external hyolaryngeal movements in preterm pigs discussed above, the biomechanical relationship between hyolaryngeal elevation and bolus movement differs between preterm and term infant pigs. In term pigs the delay between the two events (bolus movement and onset of hyolaryngeal elevation) is highly consistent even between individuals. In preterm pigs however, that duration differs in mean value, but more crucially it is far more variable. Thus, there are many swallows in preterm pigs where hyolaryngeal movement occurs before or after bolus movement by an order of magnitude more than the mean duration length. Preterm pigs exhibit less consistent timing between bolus movement and hyoid movement than term pigs.
Although hyoid movement is critical in ensuring airway protection during the swallow, initiation of bolus movement is primarily controlled by the tongue and the soft palate [13]. Thus, a potential explanation for the pattern seen here is that immature development of preterm infant pig musculoskeletal and neuromuscular systems leads to poor coordination of movements of the tongue and the soft palate with hyolaryngeal elevation in swallowing. Poor coordination of different oropharyngeal structures is characteristic of preterm infants [7,14]. Whether such poor coordination derives from poor muscle function or neurological immaturity of central nervous system circuits requires further investigation.
The clear difference between term and preterm pigs in the coordination of hyolaryngeal elevation with bolus movement has implications for bolus dynamics. If bolus movement is initiated out of sequence with other swallowing events, this may potentially affect effectiveness of airway protection, as airway protection mechanisms such as laryngeal vestibule closure are tightly linked to hyolaryngeal elevation [4]. Differences in bolus size were observed between our term and preterm pigs. We are currently in the process of collecting data on levels of aspiration in term and preterm pigs, along with more detailed kinematic data, to test this hypothesis. The changes in the relationship between hyolaryngeal movement and timing of bolus passage in preterm pigs may affect aspects of bolus dynamics and bolus control. Such aspects of bolus movement have been shown to be associated with airway protection deficits [23]. Further investigation of bolus dynamics and airway protection in preterm pigs is needed to determine what impact these modified kinematic relationships have on preterm infant pig swallowing performance.
Preterm infant pig swallowing is different to term infant pig swallowing
Epiglottal inversion is known to mature in term pigs over the first week following birth [9]. As expected, given their immature state of development, preterm pigs have many swallows without epiglottal inversion at a post birth age where epiglottal inversion occurs consistently in term pigs. However, presence of epiglottal inversion is not correlated with close timing of hyolaryngeal movement and bolus movement. Thus, the presence of epiglottal inversion, a process associated with mature swallowing in term pigs, is not correlated with more term-like kinematic patterns in the relationship between hyoid elevation and bolus movement. Thus, even when preterm pig swallows resemble term pig swallows in some respects, epiglottal inversion, they do not in others. This novel pairing pairing between epiglottal movement and hyolaryngeal kinematics suggests preterm pig swallowing is not simply like immature term pig swallowing, but rather is kinematically distinct. Similarly, preterm infants humans show multiple forms of disordered swallowing, some that can be likened to prolonging the swallowing maturation seen in term infants, and some that appear specific to the neuromuscular immaturity of preterm infants [6,13,14]. Finding similar patterns in two species suggests preterm infant swallowing may be physiologically and kinematically distinct from term infant swallowing, requiring development and validation of preterm specific metrics. More research is needed to understand the specific biomechanical and neurological characteristics of preterm infant swallowing.
Relevance to clinical practice and concerns
Although results from animal studies cannot be directly applied to human research, the results of this study have a number of implications for the design of studies, and the development of clinical protocols, in human infants. A key result of this study is that approaches developed and validated in term infants may not be applicable in preterm infants owing to either differences in sensitivity (because of smaller size) or differences in preterm infant function. Thus, metrics for assessing preterm swallowing function should be tested and validated in preterm infants. Secondly, difference between term and preterm infants in terms of swallowing function are significant even in the absence of other co-morbidities. Work in human preterm infants is showing a range of ways in which preterm infant swallowing differs from term infant swallowing [8,13,24]. Comparisons of term and preterm swallowing function, as in this paper, can help identify key loci of difference or dysfunction in preterm swallowing. In turn, this understanding of the physiological characteristics of preterm swallowing as distinct from term swallowing may help inform the development of therapies targeted specifically at preterm needs [25].
Limitations of study
This study had several limitations owing largely to the difficulty of working with vulnerable preterm infant pigs. Preterm infant pigs have difficulty standing and feeding independently, and thermoregulate poorly, which limited our ability to get data from them in our fluoroscopy suite. Although our sample of swallows was large, our sample of individuals was small. Interindividual effects are known to be significant in other studies of infant pig swallowing [20,26] although they seem to have a limited impact in this study. The vulnerability of preterm infant pigs means that radiopaque markers could not be surgically attached to the hyoid. As a result, automated marker tracking could not be used so our measurement of hyolaryngeal elevation was done manually. Future work will explore alternatives, such as reflective surface markers, to improve reliability of identification of hyolaryngeal elevation.
Supplementary Material
Acknowledgments
Grant support: NIH R01 HD088561 to Rebecca German
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Bibliography
- 1.Logemann JA. Role of the Modified Barium Swallow in Management of Patients with Dysphagia. Otolaryngol Neck Surg. 1997;116:335–8. [DOI] [PubMed] [Google Scholar]
- 2.Lefton-Greif MA, Arvedson JC. Pediatric Feeding and Swallowing Disorders: State of Health, Population Trends, and Application of the International Classification of Functioning, Disability, and Health. Semin Speech Lang. 2007;28:161–5. [DOI] [PubMed] [Google Scholar]
- 3.Molfenter SM, Steele CM. Physiological Variability in the Deglutition Literature: Hyoid and Laryngeal Kinematics. Dysphagia. 2011;26:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gross A, Ohlemacher J, German R, Gould F. LVC Timing in Infant Pig Swallowing and the Effect of Safe Swallowing. Dysphagia. 2018;33:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riley A, Miles A, Steele CM. An Exploratory Study of Hyoid Visibility, Position, and Swallowing-Related Displacement in a Pediatric Population. Dysphagia. 2019;34:248–56. [DOI] [PubMed] [Google Scholar]
- 6.Gewolb IH, Vice FL, Schweitzer-Kenney EL, Taciak VL, Bosma JF. Developmental patterns of rhythmic suck and swallow in preterm infants. Dev Med Child Neurol. 2001;43:22–7. [DOI] [PubMed] [Google Scholar]
- 7.McGrattan KE, Sivalingam M, Hasenstab KA, Wei L, Jadcherla SR. The physiologic coupling of sucking and swallowing coordination provides a unique process for neonatal survival. Acta Paediatr. 2016;n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldfield EC, Smith V, Buonomo C, Perez J, Larson K. Preterm Infant Swallowing of Thin and Nectar-Thick Liquids: Changes in Lingual–Palatal Coordination and Relation to Bolus Transit. Dysphagia. 2013;28:234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crompton AW, German RZ, Thexton AJ. Development of the movement of the epiglottis in infant and juvenile pigs. Zoology. 2008;111:339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CASPLO. Practice standards and guidelines for dysphagia interventions by speech language pathologists. Toronto, Ontario: CASPLO; 2018. [Google Scholar]
- 11.Rappazzo CA, Turk CL. The Videofluoroscopic Swallow Study: Introduction, Limitations, and Challenges In: Ongkasuwan J, Chiou EH, editors. Pediatr Dysphagia Chall Controv [Internet]. Cham: Springer International Publishing; 2018. [cited 2019 Apr 4]. p. 67–86. Available from: 10.1007/978-3-319-97025-7_5 [DOI] [Google Scholar]
- 12.Henderson M, Miles A, Holgate V, Peryman S, Allen J. Application and Verification of Quantitative Objective Videofluoroscopic Swallowing Measures in a Pediatric Population with Dysphagia. J Pediatr. 2016;178:200–205.e1. [DOI] [PubMed] [Google Scholar]
- 13.Goldfield EC, Buonomo C, Fletcher K, Perez J, Margetts S, Hansen A, et al. Premature infant swallowing: Patterns of tongue-soft palate coordination based upon videofluoroscopy. Infant Behav Dev. 2010;33:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gewolb IH, Vice FL. Maturational changes in the rhythms, patterning, and coordination of respiration and swallow during feeding in preterm and term infants. Dev Med Child Neurol. 2006;48:589–94. [DOI] [PubMed] [Google Scholar]
- 15.German RZ, Crompton AW, Gould FDH, Thexton AJ. Animal Models for Dysphagia Studies: What Have We Learnt So Far. Dysphagia [Internet]. 2017. [cited 2017 Feb 2]; Available from: http://link.springer.com/10.1007/s00455-016-9778-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballester A, Gould F, Bond L, Stricklen B, Ohlemacher J, Gross A, et al. Maturation of the Coordination Between Respiration and Deglutition with and Without Recurrent Laryngeal Nerve Lesion in an Animal Model. Dysphagia. 2018;1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.German RZ, Crompton AW, McCluskey C, Thexton AJ. Coordination between respiration and deglutition in a preterm infant mammal, Sus scrofa. Arch Oral Biol. 1996;41:619–22. [DOI] [PubMed] [Google Scholar]
- 18.Sangild PT, Thymann T, Schmidt M, Stoll B, Burrin DG, Buddington RK. Invited Review: The preterm pig as a model in pediatric gastroenterology. J Anim Sci. 2013;91:4713–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gould FDH, Lammers AR, Ohlemacher J, Ballester A, Fraley L, Gross A, et al. The Physiologic Impact of Unilateral Recurrent Laryngeal Nerve (RLN) Lesion on Infant Oropharyngeal and Esophageal Performance. Dysphagia. 2015;30:714–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gould FDH, Ohlemacher J, Lammers AR, Gross A, Ballester A, Fraley L, et al. Central nervous system integration of sensorimotor signals in oral and pharyngeal structures: oropharyngeal kinematics response to recurrent laryngeal nerve lesion. J Appl Physiol. 2016;120:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holman SD, Campbell-Malone R, Ding P, Gierbolini-Norat EM, Lukasik SL, Waranch DR, et al. Swallowing kinematics and airway protection after palatal local anesthesia in infant pigs: Swallowing After Palatal Anesthesia. The Laryngoscope. 2014;124:436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.German RZ, Campbell-Malone R, Crompton AW, Ding P, Holman S, Konow N, et al. The Concept of Hyoid Posture. Dysphagia. 2011;26:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gould FDH, Yglesias B, Ohlemacher J, German RZ. Pre-pharyngeal Swallow Effects of Recurrent Laryngeal Nerve Lesion on Bolus Shape and Airway Protection in an Infant Pig Model. Dysphagia [Internet]. 2016. [cited 2017 Jan 16]; Available from: http://link.springer.com/10.1007/s00455-016-9762-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amaizu N, Shulman RJ, Schanler RJ, Lau C. Maturation of oral feeding skills in preterm infants. Acta Paediatr. 2008;97:61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barlow SM, Lee J, Wang J, Oder A, Hall S, Knox K, et al. Frequency-modulated orocutaneous stimulation promotes non-nutritive suck development in preterm infants with respiratory distress syndrome or chronic lung disease. J Perinatol. 2014;34:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeLozier KR, Gould FDH, Ohlemacher J, Thexton AJ, German RZ. The impact of recurrent laryngeal nerve lesion on oropharyngeal muscle activity and sensorimotor integration in an infant pig model. J Appl Physiol [Internet]. 2018. [cited 2018 Jun 14]; Available from: https://www.physiology.org/doi/abs/10.1152/japplphysiol.00963.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.