Abstract
Background
Age is a well‐established risk factor for thromboembolic events in patients with atrial fibrillation (AF). However, the mechanism underlying the association between age and thromboembolic events in AF remains unknown.
Methods
The prognostic value of age as a risk factor for thromboembolic events was analyzed using data from the Korean National Health Insurance Service (NHIS). In a large‐scale single‐center registry, cardiac hemodynamic parameters were examined to elucidate the cause of increased risk of thromboembolic events in older patients.
Results
NHIS sample cohort data including 5896 patients with AF revealed that the risk of thromboembolic complication differed significantly according to age despite equal CHA2DS2‐VASc score. In the registry of 2801 patients, age showed significant correlations with left atrium (LA) diameter, LA volume, E/e′, pulmonary artery pressure, and LA appendage flow velocity. Older patients had a significantly higher prevalence of spontaneous echocontrast (odds ratio [OR] = 1.030; P < .001). Age (OR = 1.031; P < .001), E/e′ (OR = 1.065; P = .004), and LA appendage flow velocity (OR = .988; P = .009) were significant predictors for thromboembolic events in multivariate analyses. In data from the NHIS, CHA2DS2‐VASc score did not outperform age to predict thromboembolic events.
Conclusions
Age is a significant risk factor for thromboembolic events in patients with AF, and old age is associated with adverse cardiac hemodynamics. This study suggests that older patients with AF are at high risk of thromboembolic events regardless of CHA2DS2‐VASc score.
Keywords: age, atrial fibrillation, ischemic stroke, thromboembolic complication, transient ischemic attack
Abbreviations
- AAD
antiarrhythmic drug
- AF
atrial fibrillation
- AUC
area under curve
- BMI
body mass index
- EF
ejection fraction
- HR
hazard ratio
- LA
left atrium
- LAA
left atrial appendage
- LR
late recurrence
- LV
left ventricle
- OR
odds ratio
- RFCA
radiofrequency catheter ablation
- ROC
receiver operating characteristic
- SEC
spontaneous echocontrast
- TEE
transesophageal echocardiography
- TIA
transient ischemic attack.
- TTE
transthoracic echocardiography
1. INTRODUCTION
A substantial proportion of ischemic stroke, transient ischemic attack (TIA), and systemic embolism are caused by atrial fibrillation (AF).1, 2, 3 The thromboembolic events in AF patients are associated with significantly increased morbidity and mortality. Significant efforts are made to identify high risk patients and prevent these catastrophic complications.4, 5, 6, 7, 8, 9 The CHA2DS2‐VASc scoring system is the most common system used to stratify risk of thromboembolic events.6, 7, 10, 11 CHA2DS2‐VASc score is calculated by summing seven components: congestive heart failure, hypertension, age, diabetes, previous stroke/TIA/systemic embolism, vascular disease, and sex category.7, 12 For age, one point is given for age 65‐74 and two points are given for patients equal or older than 75 years.7 The use of anticoagulation therapy to prevent thromboembolic events in patients with AF is guided by the CHA2DS2‐VASc scoring system, and anticoagulation should be recommended to patients with CHA2DS2‐VASc score ≥ 2.10, 11 The CHA2DS2‐VASc system can identify patients at low risk for thromboembolic events.13 However, CHA2DS2‐VASc is not perfect and the C‐statistic was 0.606 according to the Euro Heart Survey on AF.7, 14 A previous study by Chao et al. showed that assigning additional points to those older than 50 years improved the C‐statistic and enabled identification of low risk patients more precisely in East Asian patients.15 A recent study also demonstrated that age is the most powerful predictor of ischemic stroke in patients with AF.16 However, the mechanism underlying age as a dominant risk factor for thromboembolic events in patients with AF is not fully understood.
We aimed to evaluate the relative importance of age as a risk factor for thromboembolic events and to elucidate the underlying pathophysiology of the association between age and thromboembolic events. We used both the Korean National Health Insurance Service (K‐NHIS) sample cohort data and the Korea University Medical Center Anam Hospital radiofrequency catheter ablation (RFCA) registry (KUMC registry).
2. METHODS
2.1. A nationwide sample cohort
The NHIS is the single medical insurer in the Republic of Korea managed by the government. The majority of Korean people (97.1%) are mandatory subscribers, and the database is open to medical researchers. The K‐NHIS sample cohort was created and released by NHIS in 2014 and contains 1 025 340 individuals representing the entire Korean population from the beginning of 2002, accounting for 2.2% of the entire Korean population in the K‐NHIS system. Patients included in the K‐NHIS sample cohort were followed until 2013, and the database contains demographics, diagnosis codes, use of inpatient and outpatient services, pharmacy dispensing claims, and mortality data. The diagnosis of AF required one inpatient or two outpatient records of international classification of disease, tenth revision (ICD‐10) codes in the database. Years 2002 to 2004 were used as a screening period, and patients newly diagnosed with AF beginning from January 2005 were included in the analysis. Diagnosis of hypertension, diabetes, heart failure, vascular disease, ischemic stroke, TIA, and systemic embolism were performed using data of the screening period. Patients diagnosed with ischemic stroke, TIA, and systemic embolism in the screening period were excluded from the analysis to prevent misclassification of old thromboembolic events as newly diagnosed thromboembolic events. The exact diagnosis codes for AF, hypertension, diabetes, heart failure, vascular disease, ischemic stroke, TIA, and systemic embolism are presented in Table S1. If a patient was prescribed anticoagulants for more than 6 months after the diagnosis of AF, he or she was considered to have had anticoagulation therapy. The study protocols were approved by the official review committee of the Korean government.
2.2. Registry‐based data
The KUMC registry consisted of consecutive patients with AF who underwent their first RFCA in KUMC Anam Hospital from June 1998 to December 2017. All patients who underwent RFCA in the institution were included, and there were no specific exclusion criteria. The exact profile of the registry is reported elsewhere.8, 17 The current study was approved by the institutional review board, which ensured appropriate ethical and bioethical conduct. Informed consent was waived since this was a retrospective study. The protocol was consistent with the ethical guidelines of the 2008 Helsinki Declaration.
In the KUMC registry, both transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) were performed before RFCA. Left atrial (LA) size, left ventricular (LV) ejection fraction (EF), mitral valve inflow velocity (E), and mitral annular tissue velocity (e′) were measured during TTE evaluation. For TEE evaluation, multiple views (high esophageal 0°, 45°, 60°, and 120° views) were obtained and emptying (forward), filling (backward), and average flow velocity of the LA appendage (LAA) were measured. The presence of spontaneous echocontrast (SEC) or thrombus was carefully evaluated. SEC was divided into grades of very mild (minimal echogenicity, only detectable transiently, or increasing gain setting required for the detection), mild (detectable without increasing gain setting), moderate (dense, swirling echogenic material; echogenic signal is dense in LAA compared to LA), or severe (dense, swirling echogenic material; echogenic signal is equivocal in LAA, and LA). Dense SEC was defined as a composite of moderate and severe SEC.
2.3. Study end points
The occurrence of ischemic stroke or TIA was the end point in analysis using the K‐NIHS sample cohort. The relative influences of age and other risk factors were evaluated through various approaches. In analysis with the KUMC registry, previous history of ischemic stroke, TIA, and systemic embolism was the end point. The impact of age and other clinical risk factors on thromboembolic events was evaluated. The influence of various cardiac hemodynamic parameters measured with TTE and TEE was examined, and the relationship between age and cardiac hemodynamics was also analyzed.
2.4. Statistical analysis
Continuous variables were described as mean ± SD, and were compared using a Student's t‐test. Categorical variables were presented as percentile values, and were compared with a chi‐square test or Fisher's exact test as appropriate. Pearson product‐moment correlation analysis was performed to examine correlation between two continuous variables. Thromboembolic event‐free survival was depicted by Kaplan‐Meier survival curve analysis, and the difference between groups was assessed using a log‐rank test. Cox regression analysis was performed to calculate the hazard ratio (HR) and 95% confidence interval (CI). Receiver operating characteristic (ROC) curve analysis with calculation of the area under the curve (AUC) was performed to evaluate the efficacy of CHA2DS2‐VASc score and to predict thromboembolic events. Comparison of AUCs of two ROC curves was performed using the statistical method suggested by Hanley and McNeil.18 Logistic regression analysis was performed to calculate the odds ratio (OR) with 95% CI. All significance tests were two‐tailed, and P values equal to or less than .05 were considered statistically significant. All statistical analyses were performed with SPSS version 21.0 (IBM, Armonk, NY).
3. RESULTS
3.1. Patients
Among 1 025 340 patients in the K‐NHIS sample cohort, 5896 patients were diagnosed with AF from January 2005 to December 2012. Baseline demographic data including individual components of the CHA2DS2‐VASc score were obtained during the screening period from January 2002 to December 2004. Baseline characteristics of the study population are summarized in Table S2. Male patients comprised 54.7% of the study population, and mean CHA2DS2‐VASc score was 2.99 ± 1.96. The KUMC registry included a total of 2801 patients with AF who underwent RFCA for the first time. Mean age and CHA2DS2‐VASc score was 55.58 ± 10.97 and 1.25 ± 1.26, respectively. Baseline patient demographics are described in Table S2. TTE, TEE, and cardiac MRI was performed in 2742 (97.89%), 2580 (92.10%), and 932 (33.27%) patients, respectively.
3.2. K‐NHIS sample cohort
Cumulative incidence of thromboembolic events differed significantly according to age and CHA2DS2‐VASc score (Figure 1A,B). Age had a significant impact on thromboembolic events among patients with equal CHA2DS2‐VASc scores. In patients with CHA2DS2‐VASc score 0, patients <45 years old had significantly lower risk of thromboembolic events compared to patients 45‐55 or 55‐65 years old (Figure 2A). Patients with CHA2DS2‐VASc scores of 1, 2, and ≥ 3 also showed significantly different risks of thromboembolic events according to age group (Figure 2B‐D). Patients under 45 years had a low risk of thromboembolic events irrespective of the CHA2DS2‐VASc score (Figure 2A‐D).
Figure 1.

Risk of thromboembolic events stratified by age and CHA2DS2‐VASc score. Risk of thromboembolic events increased gradually as age, A, and CHA2DS2‐VASc score, B, increased. CI, confidence interval; HR, hazard ratio
Figure 2.

Impact of age within equal CHA2DS2‐VASc score. Risk of thromboembolic events was significantly influenced by age in patients with CHA2DS2‐VASc score 0, A, 1, B, 2, C, and ≥ 3, D. CI, confidence interval; HR, hazard ratio
In patients with CHA2DS2‐VASc score 1, those with 1 point due to age (65 ≤ age < 75) had significantly higher cumulative incidence of thromboembolic events compared to those with CHA2DS2‐VASc score of 1 for reasons other than age criteria (HR = 1.635; 95% CI = 1.02‐2.61; P = .038; Figure 3A). Patients with two points in CHA2DS2‐VASc score for age older than 75 years had a significantly higher risk of thromboembolic events compared to patients with two points for reasons other than age (HR = 2.713; 95% CI = 1.54‐4.78; P < .001; Figure 3B). In subgroup analysis, the difference in the risk of thromboembolic events between the two aforementioned groups was significant in patients who did not take anticoagulants (HR = 3.403; 95% CI = 1.83‐6.33; P < .001) but was not significant in patients who took anticoagulants (HR = 1.121; 95% CI = 0.26‐4.82; P = .878). Patients with CHA2DS2‐VASc score of 0 and aged between 50 and 65 years showed significantly higher risk of thromboembolic events compared to patients with CHA2DS2‐VASc score of 1 or 2 but less than 50 years old (HR = 2.297; 95% CI = 1.43‐3.70; P < .001; Figure 3C). Patients between 50 and 65 years had significantly higher risk of thromboembolic events compared to those under 50 years (HR = 2.508, 95% CI = 1.88‐3.34; P < .001) in multivariate analysis including individual components of CHA2DS2‐VASc score. On ROC curve analysis, CHA2DS2‐VASc score and age showed similar efficacy to predict future thromboembolic events (AUC = 0.639 vs 0.634; P = .154; Figure 4).
Figure 3.

Relative risk of age compared with other risk factors. Increase in CHA2DS2‐VASc score due to age criteria was associated with greater risk compared to other risk factors in patients with CHA2DS2‐VASc score 1, A, or 2, B. Age had a significant impact on thromboembolic events even across different CHA2DS2‐VASc scores, C. CI, confidence interval; HR, hazard ratio
Figure 4.
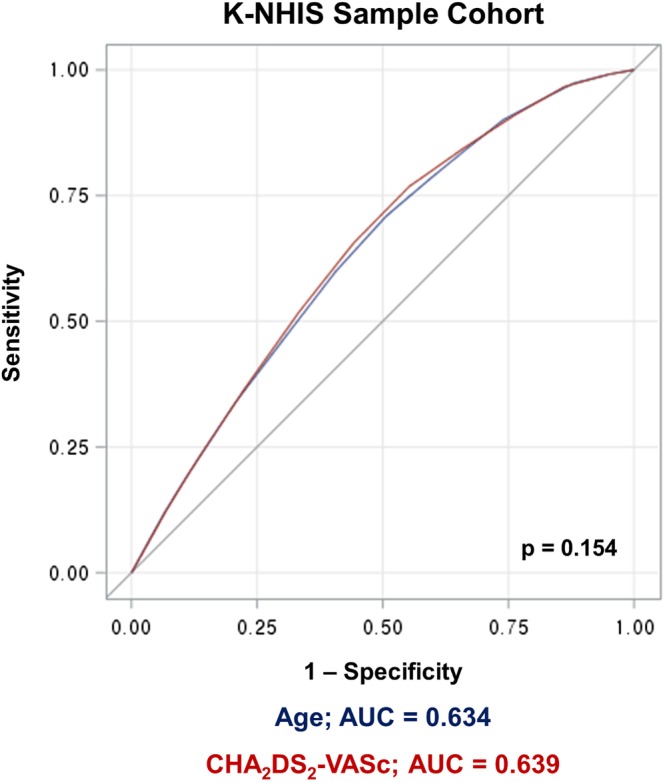
ROC curve analysis: age vs CHA2DS2‐VASc score. CHA2DS2‐VASc score and age showed similar efficacy to predict thromboembolic events in K‐NHIS sample cohort data. AUC, area under curve; CI, confidence interval; ROC, receiver operating characteristic
3.3. KUMC registry
In KUMC registry, age (OR = 1.031; 95% CI = 1.014‐1.048; P < .001), E/e′ (OR = 1.065; 95% CI = 1.021‐1.110; P = .004), and LAA flow velocity (OR = 0.988; 95% CI = 0.980‐0.997; P = .009) were independent risk factors associated with thromboembolic events in multivariate analysis (Table 1). In correlation analysis, old age was associated with significantly increased E/e′ (0.362; P < .001) and decreased LAA flow velocity (r = −0.159; P < .001) (Table 2). Older patients had a significantly higher prevalence of spontaneous echocontrast (OR = 1.030 for every one year increase in age; P < .001).
Table 1.
Clinical and echocardiographic risk factors for thromboembolic events
| Univariate analysis | Multivariate analysisa | ||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age | 1.041 | 1.027‐1.054 | .000 | 1.031 | 1.014–1.048 | .000 | |
| LA diameter | 1.033 | 1.010‐1.055 | .004 | 0.992 | 0.963‐1.022 | .587 | |
| LV EF | 0.975 | 0.956‐0.994 | .012 | 0.984 | 0.956‐1.013 | .278 | |
| PAP | 1.006 | 0.979‐1.034 | .683 | ||||
| E/e′ | 1.095 | 1.058‐1.133 | .000 | 1.065 | 1.021–1.110 | .004 | |
| LAA flow velocity | 0.987 | 0.980‐0.994 | .000 | 0.988 | 0.980–0.997 | .009 | |
| SEC | 1.487 | 1.085‐2.039 | .014 | 0.966 | 0.648‐1.439 | .863 | |
| CHA2DS2‐VASc | 1.226 | 1.095‐1.373 | .000 | ||||
Abbreviations: CI, confidence interval; E/e′: E over e′; HR, hazard ratio; LA, left atrium; LAA, LA appendage; LV EF, left ventricular ejection fraction; PAP, pulmonary artery pressure; SEC, spontaneous echocontrast.
The model included age, LA diameter, LV EF, E/e′, LAA flow velocity, SEC, sex, congestive heart failure, hypertension, diabetes mellitus, and vascular disease.
Table 2.
Correlation among age and cardiac hemodynamic parameters
| All patients (N = 2801) | Paroxysmal AF (n = 1656) | Non‐paroxysmal AF (n = 1145) | ||||
|---|---|---|---|---|---|---|
| r | P value | r | P value | r | P value | |
| LA diameter | 0.230 | < .001 | 0.243 | < .001 | 0.211 | < .001 |
| LA volume | 0.254 | < .001 | 0.334 | < .001 | 0.202 | < .001 |
| LV EF | 0.020 | .296 | 0.042 | .095 | 0.029 | .332 |
| PAP | 0.223 | < .001 | 0.255 | < .001 | 0.175 | < .001 |
| E/e′ | 0.362 | < .001 | 0.371 | < .001 | 0.352 | < .001 |
| LAA flow velocity | −0.159 | < .001 | −0.175 | < .001 | −0.151 | < .001 |
Abbreviations: E/e′, E over e′; LA, left atrium; LAA, LA appendage; LV EF, left ventricular ejection fraction; PAP, pulmonary artery pressure.
4. DISCUSSION
The major findings of our study are summarized as follows: (a) risk of thromboembolic events in patients with AF differs significantly according to age; (b) increased CHA2DS2‐VASc score due to age criteria is associated with higher risk of thromboembolic events; (c) intermediate age group (50‐65) has an increased risk of thromboembolic events although they do not receive age points in the CHA2DS2‐VASc scoring system; (d) old age is associated with adverse cardiac hemodynamics; (e) old age, increased E/e′, and decreased LAA flow velocity are independent risk factors for thromboembolic events in patients with AF. The current study utilized not only insurance data but also registry data to analyze echocardiographic parameters and clinical factors.
4.1. Age and thromboembolic events
Age is a critical risk factor for thromboembolic events in patients with AF.6, 15, 16, 19 In accordance with previous studies, our study showed that age is the most important risk factor for thromboembolic events in AF patients with low to intermediate CHA2DS2‐VASc score (0‐2).6, 20, 21, 22 In addition, our study also demonstrated that age is a critical risk factor in patients with AF and high CHA2DS2‐VASc score (≥ 3).
Anticoagulation is recommended in patients with CHA2DS2‐VASc score ≥ 2. However, previous report by Chao et al. suggested that not all risk factors in CHA2DS2‐VASc score carry an equal risk and age criteria (65 to 74 years) was associated with the highest stroke risk.23 Intermediate age (50 to 64 years) was also associated with increased risk of ischemic stroke.21, 22 Our study also found that patients who had a CHA2DS2‐VASc score of 1 for being older than 65 years showed a greater risk of thromboembolic events than patients with CHA2DS2‐VASc score of 1 due to other risk factors (Figure 2A). Previous study by Freiberg et al. suggested that routine anticoagulation therapy is not justified in patients with CHA2DS2‐VASc score of 1.24 However, our study indicate that the risk of thromboembolic event differ significantly depending on age in patients with CHA2DS2‐VASc score of 1 and certain subgroup of patients might benefit from anticoagulation therapy (Figure 2B). Furthermore, we also revealed that age had a significant influence on thromboembolic events beyond CHA2DS2‐VASc scores. Patients with CHA2DS2‐VASc score of 0 but between 50 and 65 years old were at significantly higher risk of thromboembolic events compared to patients with CHA2DS2‐VASc score of 1 or 2 but under 50 years.
We revealed that young patients (< 45) were at low risk of thromboembolic events irrespective of CHA2DS2‐VASc score and that the risk and benefit profile should be reviewed before giving anticoagulants to these patients. Therefore, anticoagulation therapy based solely on CHA2DS2‐VASc score might miss a subgroup of patients who require anticoagulation therapy despite low CHA2DS2‐VASc score and can also result in overtreatment of patients who might not need anticoagulation therapy despite high CHA2DS2‐VASc score. Patients with a CHA2DS2‐VASc score of 2 due to age ≥ 75 years had a significantly higher risk of thromboembolic events than patients with a CHA2DS2‐VASc score of 2 due to factors other than age criteria. Anticoagulation is strongly recommended in patients older than 75 years.
4.2. Age and cardiac hemodynamics
The KUMC registry included both TTE and TEE data for a substantial proportion of patients. Analysis of this registry revealed that older patients had significantly worse cardiac hemodynamics, including LA diameter, LV EF, pulmonary artery pressure, E/e′, LAA flow velocity, and SEC. Cardiac hemodynamic parameters were associated with increased risk of thromboembolic events (Table 1). However, the association between age and thromboembolic events was not fully explained by cardiac hemodynamics, since age was still an independent risk factor after adjusting echocardiographic and clinical parameters. In addition to age, E/e′ and LAA flow velocity were also independently associated with the risk of thromboembolic events. Our data suggest that worse cardiac hemodynamics in older patients partially explains the association between age and increased risk of thromboembolic events in patients with AF. However, additional underlying mechanisms remain unclear and must be elucidated in future studies.
5. LIMITATIONS
This study used the K‐NHIS sample cohort and KUMC registry data to perform retrospective analysis; therefore, is not free from the intrinsic limitations of such data. The K‐NHIS sample cohort data is based on an administrative database and is potentially susceptible to errors from inaccurate coding. Differentiation between paroxysmal and non‐paroxysmal AF was not possible in the K‐NHIS sample cohort data. Evaluation of cardiac imaging studies was also not possible. However, KUMC registry data included echocardiographic data. Furthermore, TEE was performed in a substantial number of patients, and important cardiac hemodynamic factors were evaluated. The K‐NHIS sample cohort data and KUMC registry do not represent the exact same population since the KUMC registry consists of patients with AF undergoing RFCA.
6. CONCLUSIONS
Age is a robust risk factor for thromboembolic events in patients with AF and older patients showed significantly worse cardiac hemodynamics. Despite equal CHA2DS2‐VASc score, the risk of thromboembolic events can be significantly different depending on age.
CONFLICT OF INTEREST
The authors declare no potential conflict of interests.
Supporting information
Supplementary Table S1 Diagnosis codes.
Supplementary Table S2. Baseline characteristics of K‐NHIS sample cohort and KUMC registry.
Kim YG, Choi J‐I, Boo KY, et al. Impact of age on thromboembolic events in patients with non‐valvular atrial fibrillation. Clin Cardiol. 2020;43:78–85. 10.1002/clc.23293
Funding information National Health Insurance Service
REFERENCES
- 1. Lip GY, Tse HF, Lane DA. Atrial fibrillation. Lancet. 2012;379:648‐661. [DOI] [PubMed] [Google Scholar]
- 2. Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet. 2009;373:155‐166. [DOI] [PubMed] [Google Scholar]
- 3. Savelieva I, Camm J. Update on atrial fibrillation: part ii. Clin Cardiol. 2008;31:102‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Steger C, Pratter A, Martinek‐Bregel M, et al. Stroke patients with atrial fibrillation have a worse prognosis than patients without: data from the Austrian stroke registry. Eur Heart J. 2004;25:1734‐1740. [DOI] [PubMed] [Google Scholar]
- 5. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA. 2001;285:2864‐2870. [DOI] [PubMed] [Google Scholar]
- 6. Olesen JB, Lip GY, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263‐272. [DOI] [PubMed] [Google Scholar]
- 8. Kim YG, Shim J, Oh SK, Lee KN, Choi JI, Kim YH. Electrical isolation of the left atrial appendage increases the risk of ischemic stroke and transient ischemic attack regardless of postisolation flow velocity. Heart Rhythm. 2018;15:1746‐1753. [DOI] [PubMed] [Google Scholar]
- 9. Kim YG, Choi JI, Kim MN, et al. Non‐vitamin k antagonist oral anticoagulants versus warfarin for the prevention of spontaneous echo‐contrast and thrombus in patients with atrial fibrillation or flutter undergoing cardioversion: a trans‐esophageal echocardiography study. PLoS ONE. 2018;13:e0191648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American college of cardiology/American heart association task force on practice guidelines and the heart rhythm society. Circulation. 2014;130:2071‐2104. [DOI] [PubMed] [Google Scholar]
- 11. Kirchhof P, Benussi S, Kotecha D, et al. 2016 esc guidelines for the management of atrial fibrillation developed in collaboration with eacts. Eur Heart J. 2016;37:2893‐2962. [DOI] [PubMed] [Google Scholar]
- 12. Camm AJ, Savelieva I. Female gender as a risk factor for stroke associated with atrial fibrillation. Eur Heart J. 2017;38:1480‐1484. [DOI] [PubMed] [Google Scholar]
- 13. Potpara TS, Polovina MM, Licina MM, Marinkovic JM, Prostran MS, Lip GYH. Reliable identification of "truly low" thromboembolic risk in patients initially diagnosed with "lone" atrial fibrillation: the Belgrade atrial fibrillation study. Circ Arrhythm Electrophysiol. 2012;5:319‐326. [DOI] [PubMed] [Google Scholar]
- 14. Sun Y, Hu D, Li K, Zhou Z. Predictors of stroke risk in native chinese with nonrheumatic atrial fibrillation: retrospective investigation of hospitalized patients. Clin Cardiol. 2009;32:76‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chao TF, Lip GY, Liu CJ, et al. Validation of a modified cha2ds2‐vasc score for stroke risk stratification in Asian patients with atrial fibrillation: a nationwide cohort study. Stroke. 2016;47:2462‐2469. [DOI] [PubMed] [Google Scholar]
- 16. Kim TH, Yang PS, Yu HT, et al. Age threshold for ischemic stroke risk in atrial fibrillation. Stroke. 2018;49:1872‐1879. [DOI] [PubMed] [Google Scholar]
- 17. Kim YG, Choi JI, Boo KY, et al. Clinical and echocardiographic risk factors predict late recurrence after radiofrequency catheter ablation of atrial fibrillation. Sci Rep. 2019;9:6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839‐843. [DOI] [PubMed] [Google Scholar]
- 19. Camm AJ, Coleman CI, Larsen TB, Nielsen PB, Tamayo CS. Understanding the value of real‐world evidence: focus on stroke prevention in atrial fibrillation with rivaroxaban. Thromb Haemost. 2018;118:S45‐S60. [DOI] [PubMed] [Google Scholar]
- 20. Okumura K, Hori M, Tanahashi N, John Camm A. Special considerations for therapeutic choice of non‐vitamin k antagonist oral anticoagulants for japanese patients with nonvalvular atrial fibrillation. Clin Cardiol. 2017;40:126‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chao TF, Wang KL, Liu CJ, et al. Age threshold for increased stroke risk among patients with atrial fibrillation: a nationwide cohort study from Taiwan. J Am Coll Cardiol. 2015;66:1339‐1347. [DOI] [PubMed] [Google Scholar]
- 22. Chao TF, Lip GYH, Lin YJ, et al. Age threshold for the use of non‐vitamin k antagonist oral anticoagulants for stroke prevention in patients with atrial fibrillation: insights into the optimal assessment of age and incident comorbidities. Eur Heart J. 2019;40:1504‐1514. [DOI] [PubMed] [Google Scholar]
- 23. Chao TF, Liu CJ, Wang KL, et al. Should atrial fibrillation patients with 1 additional risk factor of the cha2ds2‐vasc score (beyond sex) receive oral anticoagulation? J Am Coll Cardiol. 2015;65:635‐642. [DOI] [PubMed] [Google Scholar]
- 24. Friberg L, Skeppholm M, Terent A. Benefit of anticoagulation unlikely in patients with atrial fibrillation and a cha2ds2‐vasc score of 1. J Am Coll Cardiol. 2015;65:225‐232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1 Diagnosis codes.
Supplementary Table S2. Baseline characteristics of K‐NHIS sample cohort and KUMC registry.


