Abstract
The advent of immunotherapy using immune checkpoint inhibitors (ICIs) and targeted therapy (TT) has dramatically improved the prognosis of various cancer types. However, following ICI therapy or TT—either alone (especially ICI) or in combination with radiotherapy—imaging findings on anatomical contrast-enhanced MRI can be unpredictable and highly variable, and are often difficult to interpret regarding treatment response and outcome. This review aims at summarizing the imaging challenges related to TT and ICI monotherapy as well as combined with radiotherapy in patients with brain metastases, and to give an overview on advanced imaging techniques which potentially overcome some of these imaging challenges. Currently, major evidence suggests that imaging parameters especially derived from amino acid PET, perfusion-/diffusion-weighted MRI, or MR spectroscopy may provide valuable additional information for the differentiation of treatment-induced changes from brain metastases recurrence and the evaluation of treatment response.
Keywords: brain metastasis, FET PET, immune checkpoint inhibitors, lung cancer, melanoma, radiomics
The advent of immunotherapy using immune checkpoint inhibitors (ICIs) and targeted therapy (TT) has dramatically improved the prognosis of cancer, especially in patients with melanoma, lung cancer, or breast cancer. Although initially tested only in patients with extracranial cancer manifestations, recent trials have demonstrated that patients with brain metastases (BM) may also benefit from these agents alone or in combination with other treatment options such as radiotherapy.
Immunotherapy rests on the premise that tumors can be recognized as foreign rather than self, and that they thereby can be targeted by the activated immune system. Antibodies that block regulatory checkpoints of the immune system can facilitate an immune response that leads to inhibition of tumor growth or regression. In particular, the blockade of immune checkpoints such as the cytotoxic T-lymphocyte associated protein 4 (CTLA-4) or programmed cell death receptor 1 (PD1) axis has resulted in a significant improvement of prognosis and overall survival.1,2 Furthermore, the combination of ICIs (eg, nivolumab with ipilimumab) can generate complete or partial response of selected BM in an even greater percentage of patients, especially in melanoma.3,4 Studies on the combination of ICIs with radiotherapy in patients with BM suggest that this approach is a valuable option that may offer improved survival over ICI therapy alone.5
In addition to ICI, TT using small molecules has demonstrated activity against BM.6–8 The presence of predictive genetic alterations such as epidermal growth factor receptor (EGFR) mutation, anaplastic lymphoma kinase (ALK) or ROS1 translocation, human epidermal growth factor receptor 2 (HER2) overexpression, or BRAF V600E mutation is considered as an essential prerequisite for a response to TT.9 Similar to ICI, the combination of TT with radiotherapy also appears to be effective in patients with BM,10,11 although substantial side effects may occur following TT concurrent with radiotherapy, especially when BRAF inhibitors are used.12
Following TT or ICI therapy, either alone (especially ICI) or in combination with radiotherapy, imaging findings on anatomical contrast-enhanced MRI can be unpredictable and highly variable, and the interpretation concerning the differentiation of treatment response from tumor progression is often challenging. For example, pseudoprogression is one of the most important critical clinical and imaging challenges. It refers primarily to MRI findings that are mimicking progressive tumor, which, however, are actually due to other causes, particularly inflammation related to ICI therapy. If pseudoprogression is not correctly identified, the consequences for patients and clinicians may be substantial, such as premature discontinuation of an effective treatment with a negative impact on patient outcome. Conversely, trial results for recurrent disease may be compromised if patients with pseudoprogression are entered because this will result in overestimating the activity of the experimental intervention explored. Although the immunotherapy Response Assessment in Neuro-Oncology (iRANO) Working Group recently recommended standard MRI and clinical criteria for addressing the clinical problem of pseudoprogression following immunotherapy,13 to date the need for the acquisition of additional diagnostic information to overcome the problem of differentiating pseudoprogression from tumor progression remains of foremost importance. Furthermore, other imaging challenges (eg, the assessment of response to TT and ICI therapy) are not specifically incorporated into the iRANO criteria.
We here aim at (i) summarizing clinically relevant imaging challenges related to TT and ICI monotherapy as well as TT or ICI therapy plus radiotherapy in patients with BM, and (ii) providing an overview of advanced imaging techniques that may help to overcome these challenges.
Search Strategy, Selection Criteria, and Levels of Validation
A PubMed search of the published literature was performed with the combination of the search terms “brain metastasis/metastases,” “MRI,” “MR,” “advanced MRI,” “perfusion MRI,” “PWI,” “diffusion MRI,” “DWI,” “ADC,” “spectroscopy,” “MRS,” “PET,” “positron,” “FDG,” “amino acid,” “methionine,” “FET,” “FDOPA,” “FLT,” “radiotherapy,” “WBRT,” “radiosurgery,” “gamma knife,” “radiation-induced changes/radiation injury,” “radionecrosis,” “radiation necrosis,” “pseudoprogression,” “progression,” “delayed/mixed response,” “treatment monitoring,” “assessment of treatment response,” “hyperprogression,” “abscopal effect,” “immunotherapy,” “ipilimumab,” “nivolumab,” “pembrolizumab,” “targeted therapy,” “EGFR,” “BRAF,” “HER2,” and “ALK” before and inclusive of February 2019. Additionally, articles identified through searches of the authors’ own files were included. Only papers constituting levels 1–3 evidence according to the Oxford Centre for Evidence-based Medicine (the Oxford 2011 Levels of Evidence) were considered. In brief, a randomized controlled trial fulfills the criteria for Oxford level 1, a prospective cohort study corresponds to level 2, and a retrospective study is consistent with Oxford level 3.
Overview of Imaging Challenges Following ICI and TT in Patients with BM
Pseudoprogression
In patients undergoing immunotherapy using ICIs, intratumoral infiltrates including cytotoxic T cells (CD8+) may lead to pseudoprogressive MR imaging findings. Histopathology typically shows inflammatory cells,14 but not mitotically active tumor cells. Conversely, after ICI initiation, progressive imaging changes might represent an initial true tumor progression that ultimately becomes controlled by a delayed immune response, subsequently leading to a decrease of tumor burden. Furthermore, a transient appearance of new contrast-enhancing lesions on MRI at either local or even distant sites might occur in patients with BM receiving ICIs. These findings suggest that new contrast-enhancing lesions might represent immune responses directed against infiltrative brain tumor cells.
In extracranial solid tumors, the frequency of ICI-related pseudoprogression seems to be highest in melanoma treated with anti–cytotoxic T-lymphocyte associated protein 4 (CTLA-4) antibodies (range of 5–10% in the majority of studies)15–17 but is lower in other solid tumors, such as lung cancer treated with anti–programmed cell death protein 1 (PD1)/PD ligand 1 (PDL1) antibodies (~5%).18,19 In contrast, data on the percentage of cases with pseudoprogression in patients with BM related to ICI monotherapy or ICI combination therapy are few.14,20–22 In a recent study in patients with BM from non–small cell lung cancer (NSCLC) treated with ICIs alone (n = 1025), the rate of pseudoprogression was only 0.8%,23 suggesting that this phenomenon is scarce in BM resulting from NSCLC or even misdiagnosed.
The timing of pseudoprogressive changes in BM patients treated with ICIs has not been fully explored, but based on preliminary evidence, this phenomenon may occur early within the first weeks after initiation (range, 1.5–18 wk),14,20,21,24 but not later than 6 months.
Regarding the occurrence of pseudoprogression in patients with BM related to TT monotherapy, data also remain scarce. In an NSCLC patient with ALK translocation, progressive MRI findings occurred after 12 months of alectinib treatment. Interestingly, histopathology was considered consistent with radiation necrosis, although radiotherapy had been performed 7 years before the start of alectinib.25
Assessment of Treatment Response
In patients with extracranial tumors treated with immunotherapy, Wolchok and colleagues described that basically 4 different patterns of response may occur: (i) rapid regression of baseline lesions without new lesions, (ii) durable stable disease (in some patients followed by a slow, steady decline in total tumor burden), (iii) an initial increase in tumor burden followed by (delayed) tumor regression, and (iv) the appearance of new lesions followed by a decrease in overall tumor burden.15 As stated above, the initial increase in tumor size or number of lesions in the latter 2 patterns does not always reflect actual disease progression, but may be related to pseudoprogression due to the influx of inflammatory cells. This important issue is also considered in frequently used immune-related response criteria (ie, irRC [immune-related response criteria],15 irRECIST [immune related Response Evaluation Criteria In Solid Tumors],26 and immunotherapy RECIST).27
To rule out pseudoprogression following treatment for intracranial neoplasms, the iRANO criteria stipulate that within 6 months of initiating ICI therapy, early increases in lesion size and/or the development of new lesions do not define progressive disease unless further progressive changes are confirmed upon follow-up MR imaging, provided that patients do not have clinical deterioration.13 After worsening of the first MR study after ICI therapy initiation, the iRANO criteria recommend a 3-month window for confirmation of progression.13 Besides, progressive imaging changes more than 6 months after immunotherapy initiation are more likely to reflect an actual tumor progression.13,28,29
Thus, the early assessment of treatment response to ICI therapy may be thereby complicated by pseudoprogression. Furthermore, clinical evaluation of immunotherapy is also hampered by the absence of response criteria that can comprehensively describe all patterns of antitumor activity associated with such agents. In addition to the above stated 4 response patterns, lesions may show “mixed” responses, consisting of regression in some lesions, while others remain stable, progress, or appear simultaneously.15,30 This pattern has been termed dissociated response.31
Hyperprogression
In extracranial tumors, it has been observed that a subset of patients might experience a paradoxical acceleration of tumor growth kinetics after initiation of ICI therapy using anti-PD1/PDL1 antibodies, which may lead to a considerably reduced overall survival. This phenomenon has been termed hyperprogression or hyperprogressive disease.32–34 The reported frequency for hyperprogression is in the range of 6–29% and varies considerably across different solid tumor types.32 The highest rates of hyperprogression have been observed in patients with head and neck squamous cell carcinoma (29%) and NSCLC (14%).35,36
In clinical practice, the differentiation of hyperprogression from progressive tumors with a naturally aggressive phenotype remains a major challenge. To date, most of the current immune-related response criteria aim at identifying pseudoprogression but not hyperprogression. To recognize hyperprogression, it is important to integrate pretreatment tumor kinetics (tumor growth rate) by estimating the tumor size increase 2- or 3-dimensionally over time between 2 imaging studies. Subsequently, tumor growth rates can be used to compare the growth rate before and after initiating ICI. In several studies, at least a 2-fold increase of tumor growth on-treatment versus before ICI therapy has been considered as defining hyperprogressive disease.34,35
In patients with BM, reports on hyperprogression after initiation of ICI monotherapy remain scarce, and it is therefore still not yet clear whether hyperprogression may really occur in the CNS following ICI therapy. Kaito and coworkers reported a series of NSCLC patients (n = 32) with a poor performance status or BM with severe exacerbations or manifestations of the primary disease related to nivolumab.37 The treatment was discontinued in 8 patients with BM due to severe exacerbation of neurologic symptoms (eg, headache, gait disorder, disturbance of consciousness), indicating that hyperprogression may also occur in BM. However, BM growth rates before and after initiating ICI were not provided.
Further Unsolved Imaging Challenges
Several phase II and III trials in patients with BM have suggested that response to ICIs or TT on contrast-enhanced MRI based on frequently used response criteria15,26,27,38,39 is associated with considerably prolonged survival.3,4,40 However, there is an unmet need for the prediction of treatment response, such as by the evaluation of the tumor mutational burden41 and molecular markers or non-invasively by using neuroimaging biomarkers, ideally before the initiation of TT or ICI therapy. This is also of high clinical relevance, as these agents may cause severe side effects (ie, Common Terminology Criteria for Adverse Events grades 3 and 4), especially in patients with BM.
Role of Radiotherapy in Combination with ICI or TT
Synergistic Effects of Radiotherapy Combined with ICI or TT
Besides response, the therapeutic efficacy of any radiotherapy technique is usually determined in terms of the achieved local control rate of the irradiated lesion as well as the distant intracranial failure rate. Nowadays, radiosurgery is the dominant type of primary radiotherapy for patients with a limited number of small to middle-sized BM.42 Radiosurgery has high local efficacy, but does not target microscopic lesions distant to the lesions detected by brain imaging, and therefore the rate of distant BM in the further course of disease is usually high.43–46
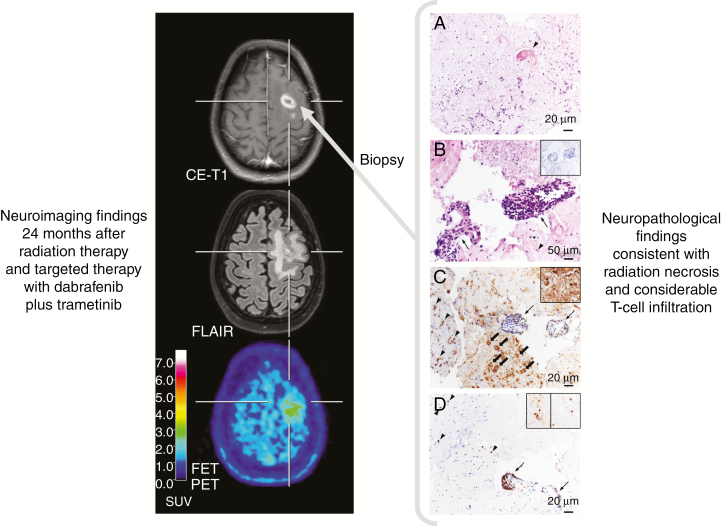
The combination of radiosurgery with immunotherapy or TT may have synergistic effects on both irradiated and non-irradiated, distant regions. Within the target volume, the release of tumor cell antigens due to post-irradiation mitotic cell death may stimulate a cytotoxic immune response directed to the remaining tumor cells,47 leading to increased local response rates. Moreover, activated immune cells may also attack microscopic tumor cell clusters distant from the irradiated region, leading to a so-called abscopal effect48 and a potential protection from the occurrence of distant BM. Figure 1 shows neuropathological findings consistent with a distinct immune response most probably related to radiation therapy combined with targeted therapy.
Fig. 1.
Radiation necrosis and chronic inflammation in a patient with brain metastases of a BRAF-mutated malignant melanoma who had been treated with whole-brain radiation therapy and concurrently with dabrafenib plus trametinib. Twenty-four months later, the contrast-enhanced MRI suggests brain metastasis recurrence (left panel), whereas the FET PET shows only an insignificant uptake, consistent with treatment-related effects. Neuropathological findings obtained following stereotactic biopsy revealed besides signs of radiation necrosis a considerable infiltration of intra- and perivascular T cells (right panel). (A) Hyaline, eosinophilic necrosis with only single leukocytes and cell detritus. A necrotic vessel wall is hyalinized and thickened (arrowhead). Hematoxylin and eosin (H&E) staining; original magnification x200. (B) Adjacent to necrosis, small fragments of vital brain parenchyma harbor activated microglial cells (arrowhead) and reactive astrocytes (asterisk). Two blood vessels are heavily infiltrated by lymphocytes (arrows). Tumor cells are absent (insert). H&E staining; original magnification x500; insert: immunohistochemistry with monoclonal mouse anti-HMB45 (DCS Diagnostics) and slight counterstaining with hemalum; original magnification, x200. (C) Adjacent to the inflamed blood vessels (arrows), foamy CD68+ macrophages are in the process of resorption of necrosis (block arrows). In the brain parenchyma, microglial cells (arrowheads) and astrocytes (insert, asterisks) are activated. Immunohistochemistry with monoclonal mouse anti-CD68 (DCS Diagnostics) and slight counterstaining with hemalum; original magnification, x200; insert: immunohistochemistry with monoclonal mouse anti–glial fibrillary acidic protein (BioGenex) and slight counterstaining with hemalum; original magnification, x500. (D) CD3+ T cells are the major population of intra- and perivascular infiltrates (arrow). Both CD4+ (left insert) and CD8+ (right insert) T cells contribute to the infiltrates. Immunohistochemistry with monoclonal rabbit anti-CD3 (DCS Diagnostics) and slight counterstaining with hemalum; original magnification, x200; inserts: immunohistochemistry with monoclonal mouse anti-CD4 (left, BioGenex) and with monoclonal rabbit anti-CD8 (right, DCS Diagnostics), slight counterstaining with hemalum; original magnification, x400.
Several predominantly retrospective studies have addressed the effects of combined therapy (ie, radiosurgery and ICI or TT) compared with radiosurgery alone. Further studies have focused on the optimal timing of systemic TT or ICI therapy relative to the time point of radiosurgery (Table 1). Studies of patients with BM secondary to melanoma comparing radiosurgery and ICI or TT with radiosurgery alone suggest that combined therapies have the potential to increase response and local control rates compared with radiosurgery alone and can prevent distant BM at least to some extent.49–53 Additionally, the synergistic effects observed in patients with melanoma BM have also been observed in patients with BM from breast cancer.54–56 However, one study of patients with BM secondary to NSCLC did not find any synergistic effects of anti-PD1 therapies in combination with radiosurgery.57
Table 1.
Overview of selected studies (2016–2019) evaluating the rate of radiation necrosis in BM patients treated with radiosurgery alone in comparison to BM patients treated with radiosurgery in combination with TT or ICI therapy
| First Author | Year | n | Study Design | Primary Tumor | Treatment Groups | ICI/TT Timing | Rate of RN | Comment |
|---|---|---|---|---|---|---|---|---|
| Colaco129 | 2016 | 180 | R | MM | SRS + CT SRS + ICI or TT |
ipi, erlo (< / > 6 mo of SRS) | 17% 38% / 25% |
Increased RN risk for SRS + ICI, no effect of timing |
| Patel52 | 2017 | 54 | R | MM | SRS SRS + ICI |
ipi within 4 mo of SRS | 21% 30% |
Insignificantly increased RN risk for SRS + ICI |
| Yusuf53 | 2017 | 51 | P, NR | MM | SRS SRS + ICI |
ipi, pembro within 3 mo of SRS | 12% 3% |
No increased RN risk for SRS + ICI |
| Kaidar- Person130 | 2017 | 58 | R | MM | SRS SRS + ICI |
ipi, pembro, nivo | 0% 28% |
Increased RN risk for SRS + ICI |
| Kotecha51 | 2018 | 191 | R | MM | SRS SRS + TT or ICI |
vemura, ipi within 4 wk of SRS | 6% 0% / 2% |
No increased RN risk for SRS + TT or ICI |
| Diao60 | 2018 | 91 | R | MM | SRS SRS + ICI |
ipi (< / > 4 wk of SRS) | 3% 9% / 7% |
Insignificantly increased RN risk for SRS + ICI |
| Rahman63 | 2018 | 74 | R | MM | SRS + ICI | ipi, pembro, nivo (< / > 4 wk of SRS) |
11% / 13% | Timing was not associated with an increased risk for RN |
| Nardin61 | 2018 | 25 | R | MM | SRS + ICI | pembro (< / > 4 wk of SRS) |
16% overall | Increased risk for RN, no effect of timing |
| Du Four131 | 2018 | 142 | P, NR | MM | SRT + ICI | pembro before and after SRS | 13% overall | Increased risk for RN |
| Pires da Silva132 | 2019 | 135 | R | MM | SRT + ICI | ipi concurrent/after SRS | 17% | Increased risk for RN, no effect of timing |
| Kim133 | 2017 | 1650 | R | Various | SRS SRS + TT |
various TT concurrent to SRS | 5% 9% |
Increased RN risk for SRS + TT |
| Weingarten134 | 2019 | 57 | R | Various | SRS + ICI | ipi, pembro, nivo, durva, treme before, concurrent and after SRS | 7% overall | Increased RN risk for SRS + ICI |
| Hubbeling135 | 2018 | 94 | R | NSCLC | SRS SRS + ICI |
pembro, nivo, atezo before and after SRS | 34% 31% |
Increased RN risk for SRS + ICI |
| Kim54 | 2019 | 84 | R | Breast | SRS SRS + TT |
lapa concurrent to SRS | 4% 1% |
No increased RN risk for SRS + TT |
| Parsai56 | 2019 | 126 | R | Breast | SRS SRS + TT |
lapa concurrent to SRS | 6% 1% |
No increased RN risk for SRS + TT |
atezo = atezolizumab; breast = breast cancer, CT = cytotoxic chemotherapy; durva = durvalumab; erlo = erlotinib; ipi = ipilimumab; lapa = lapatinib; MM = malignant melanoma; nivo = nivolumab; pembro = pembrolizumab; P, NR = prospective, non-randomized; R = retrospective; RN = radiation necrosis; SRS = stereotactic radiosurgery; treme = tremelimumab; vemura = vemurafenib.
Regarding the optimal timing of systemic ICI therapy or TT and radiosurgery in melanoma patients with BM eligible for both approaches, the majority of these studies suggest that a faster and more pronounced or a more durable local response rate as well as a reduced distant intracranial failure rate were associated with a time interval of less than 4 weeks between initiation of systemic therapy and radiosurgery.58–65 However, randomized trials are needed to clarify whether radiosurgery should be applied upfront or delayed at progression.
Does ICI Therapy or TT Increase the Rate of Radiation Necrosis After Radiosurgery of Brain Metastases?
After radiosurgery, approximately 30% of the lesions increase in size and change their pattern of contrast enhancement, with a peak at 12–18 months after irradiation.66 Focal radiation necrosis is the most important type of late toxicity after radiosurgery. Histologically, radiation necrosis is characterized by a central area of necrosis surrounded by regions of vascular hyalinization, vasculitis, demyelination, macrophage and T-cell infiltration, and reactive astrocytosis.67,68 As these tissue changes clearly involve immunogenic reactions, an interference with immunomodulatory therapy can be expected. In clinical routine, treatment-related changes on MRI are frequently used as surrogate markers for radiation necrosis. Usually, the diagnosis is based upon serial MR images, although the diagnostic criteria may differ between institutions.
Table 1 shows the rate of radiation necrosis in BM patients treated with radiosurgery alone in comparison to BM patients treated with radiosurgery combined with TT or ICI therapy. These selected studies (2016–2019; Table 1) suggest that an increased risk for radiation necrosis cannot be excluded when radiosurgery is applied in combination with ICI therapy, while the combination of radiosurgery with TT seems to be less prone to radiation necrosis.
Pseudoprogression and Radiosurgery in Combination with ICI
The occurrence of pseudoprogression after radiosurgery in combination with ICI therapy has so far not been well recognized. Compared with radiation necrosis, pseudoprogression may differ in terms of the time course of development (typically earlier) and the tissue reactions involved. A recent study observed that approximately 20% of the treated BM showed a transient, reversible increase in size 3–6 months after combined treatment compared with 5% after radiosurgery alone.24 Rahman et al63 reported that about 50% of melanoma patients concurrently treated with ipilimumab, pembrolizumab, or nivolumab and radiosurgery had an earlier tumor progression compared with those treated with ICI therapy with more time elapsed since radiosurgery. Despite these earlier tumor progressions, the concurrent patients had a better intracranial progression-free survival (30% vs 12% at 12 mo). The phenomenon of pseudoprogression has also been observed in melanoma BM patients treated with PD1 antagonists administered less than 6 weeks after radiosurgery.69 These findings warrant consideration during follow-up when interpreting conventional MRI.
PET and Advanced MRI as Neuroimaging Tools to Overcome Challenges of Conventional MRI
Currently, ICIs and TT are being investigated in clinical trials while already being used in clinical practice for patients with BM. While these therapies hold great promise, management of patients undergoing these treatments can be complicated due to brain imaging findings on standard MRI, such as immune-related pseudoprogression caused by ICI therapy or equivocal MRI findings related to radiation in combination with TT. Thus, ICIs and TT impose specific requirements on neuroimaging which are not met by anatomical MRI. Metabolic PET imaging and advanced MR techniques may provide helpful objective information to overcome these imaging challenges. An overview is presented in Table 2.
Table 2.
Overview of main results derived from metabolic PET imaging and advanced MR techniques to overcome imaging challenges related to radiotherapy, ICI therapy and TT in patients with BM
| PET | Advanced MRI | |||||||
|---|---|---|---|---|---|---|---|---|
| FDG | AA | AA PET Radiomics | Other Tracers | PWI | MRS | DWI | MRI Radiomics | |
| Differentiation of radiation-induced changes from BM relapse | Diagnostic performance varies considerably85‒90 | For FET, MET, and FDOPA consistently high diagnostic performance, 91‒98 SN and SP 80‒90% | FET PET textural feature analysis is of value,104,105 SN and SP 80–90% | n.a. | For rCBV, thresholds and diagnostic performance vary considerably89,95,110–113 | Available studies suggest high SP, but low SN112,114 | ADC values seem not to be helpful90,115 | Initial results suggest high SP116 |
| Identification of pseudoprogression related to ICI | n.a. | FET is potentially helpful20 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Evaluation of response to ICI or TT | n.a. | FET is of value,73,108 reduction of tracer uptake despite unchanged MRI | n.a. | FLT is of value,106 reduction of proliferative activity despite unchanged MRI | n.a. | n.a. | n.a. | n.a. |
| Evaluation of response to radiotherapy | n.a. | n.a. | n.a. | n.a. | Various PWI parameters allow the prediction of radiotherapy outcome117‒120 | n.a. | ADC values allow the prediction of radiotherapy outcome122‒125 | n.a. |
AA = radiolabeled amino acids, ie, [11C]-methyl-L-methionine (MET), 3,4-dihydroxy-6-[18F]-fluoro-L-phenylalanine (FDOPA), or O-(2-[18F]-fluoroethyl)-L-tyrosine (FET); ADC = apparent diffusion coefficient; DWI = diffusion-weighted imaging; FDG = [18F]-2-fluoro-2-deoxy-D-glucose; FLT = 3´-deoxy-3´-[18F]-fluorothymidine; MRS = MR spectroscopy; n.a. = not available; PWI = perfusion-weighted imaging; SN = sensitivity; SP = specificity.
Positron Emission Tomography
Oncologic PET imaging using [18F]-2-fluoro-2-deoxy-D-glucose (FDG) has evolved over the last decades into the paramount clinical PET modality for cancer diagnostics.70 Increased glucose metabolism as assessed by an increased FDG uptake is commonly seen in proliferating tumor cells due to an increased expression of glucose transporters and the enzyme hexokinase, which converts FDG to a phosphorylated product. However, the physiological high FDG uptake in the normal brain parenchyma hinders the delineation of brain tumors,71 and cerebral inflammatory processes may also exhibit high FDG uptake, thereby diminishing the diagnostic performance.72
Radiolabeled amino acids are of particular interest for brain tumor imaging using PET because of their increased uptake in neoplastic tissue but low uptake in normal brain parenchyma, resulting in an improved tumor-to-brain contrast.72 A key feature of amino acid tracers is their ability to pass the intact blood–brain barrier, which allows the depiction of glioma tissue beyond contrast enhancement in MRI72 and to differentiate tumor progression from nonspecific, treatment-related changes, especially in patients with BM.73 Recently, the RANO group analyzed the clinical value of amino acid PET in the diagnostic evaluation of brain tumors. It strongly recommended the use of this imaging technique in addition to conventional MRI, especially for the delineation of brain tumor extent, treatment response assessment, evaluation of prognosis of newly diagnosed brain tumors, and the differentiation of treatment-related changes from tumor progression.71,73–76 Within the group of amino acid PET tracers, [11C]-methyl-L-methionine (MET), 3,4-dihydroxy-6-[18F]-fluoro-L-phenylalanine (FDOPA), and O-(2-[18F]-fluoroethyl)-L-tyrosine (FET) are frequently used.72,77,78 In both gliomas and BM, increased uptake of MET, FET, and FDOPA is related to L-type amino acid transporters (LATs, subtypes LAT1 and LAT2), which are overexpressed in tumor tissue.79–82 Thus, the LAT overexpression in BM makes intracranial metastases a compelling target for amino acid PET imaging.82
In patients with BM, a few PET imaging studies have used other tracers than FDG or radiolabeled amino acids. For example, the PET tracer 3′-deoxy-3′-[18F]-fluorothymidine (FLT) is an analog to the nucleoside thymidine and was developed to assess cellular proliferation by tracking the thymidine salvage pathway.83 The few data thus far available suggest that in patients with brain tumors, including BM, this tracer may be of great value.84
Importantly, in the USA only FDG is FDA approved, and all other radiotracers are typically only available as part of a clinical trial.
Differentiation of Radiation-Induced Changes from Brain Metastasis Recurrence
FDG PET has been studied to differentiate radiation-induced changes from BM relapse. Interestingly, the diagnostic performance of FDG PET varied considerably (range of sensitivity, 40–95%; range of specificity, 50–100%).85–90 Most probably, these results are related to a low number of patients and by variations in methodology.
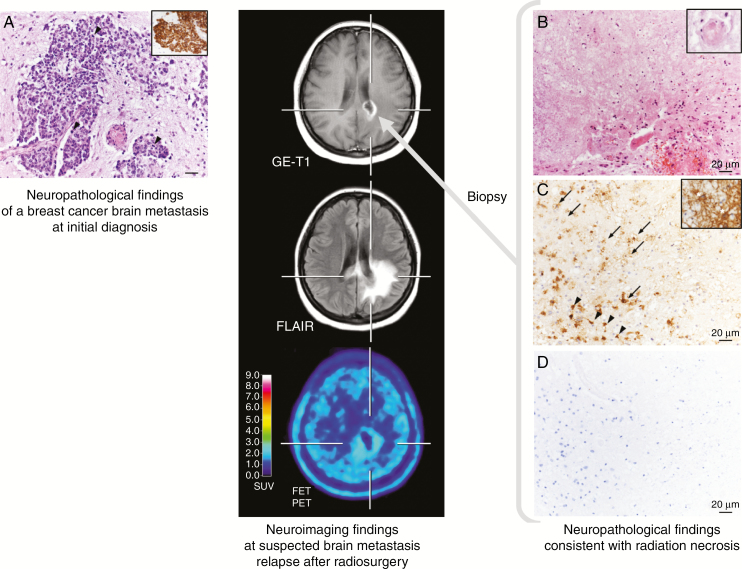
In contrast, FDOPA PET and MET PET have consistently demonstrated higher sensitivity and specificity of approximately 80% in differentiating treatment effect from BM recurrence.91–94 Another study has reported a high accuracy for differentiating radiation-induced changes from BM relapse after radiosurgery using FDOPA PET, outperforming perfusion MRI parameters 91% to 76%.95 Similarly, static and dynamic FET PET parameters showed a high diagnostic performance, with a sensitivity and specificity of 80–90% for the differentiation of radiation-induced changes from locally recurrent BM.96–98 An illustrative case is presented in Figure 2. Furthermore, the diagnostic performance of amino acid PET seems to be superior to both glucose PET and perfusion- and diffusion-weighted MR imaging.90,95
Fig. 2.
Radiation necrosis in a patient with brain metastases secondary to a breast cancer (ductal carcinoma, HER2 negative, estrogen and progesterone receptor positive) (left panel). Five months after external fractionated radiation therapy, contrast-enhanced MRI suggests BM relapse (middle panel). In contrast, FET PET shows no increased metabolic activity, indicating treatment-related changes. Neuropathological findings obtained following stereotactic biopsy were consistent with radiation necrosis (right panel). (A) Epithelial, pleomorphic tumor with increased mitotic activity (arrowheads) in the brain parenchyma expressing cytokeratin (CK) 8 (insert) at initial diagnosis. Hematoxylin and eosin (H&E) staining; original magnification x200. Insert: immunohistochemistry with monoclonal mouse anti-CK8 (BioGenex) and slight counterstaining with hemalum; original magnification, x100. (B) Hyaline, eosinophilic necrosis with only single leukocytes. A necrotic vessel wall is hyalinized and thickened (insert). Adjacent vital brain parenchyma shows reactive alterations with activated microglial cells and reactive astrocytes. H&E staining; original magnification x200; insert: H&E staining; original magnification, x500. (C) Necrosis is infiltrated by foamy macrophages (arrows). In the brain parenchyma, microglial cells (arrowheads) and astrocytes (insert, asterisks) are activated. Immunohistochemistry with monoclonal mouse anti–major histocompatibility complex class I antigen (DCS Diagnostics) and slight counterstaining with hemalum; original magnification x200; insert: immunohistochemistry with monoclonal mouse anti–glial fibrillary acidic protein (BioGenex) and slight counterstaining with hemalum; original magnification, x500. (D) Epithelial tumor cells were absent from necrosis and vital brain parenchyma. Immunohistochemistry with monoclonal mouse anti-CK8 (BioGenex) and slight counterstaining with hemalum; original magnification, x200.
Recent literature highlights the value of radiomics and artificial intelligence in the field of neuro-oncology.99–101 Radiomics enables the high-throughput extraction of quantitative imaging features from MRI as well as PET.102,103 Using FET PET, it has been demonstrated that radiomic textural feature analysis helps distinguish treatment-related changes from BM recurrence.104 For this important clinical question, radiomics analysis using the combination of textural features obtained from FET PET and contrast-enhanced MRI achieved a high diagnostic specificity (>90%).105
As stated above, pseudoprogression may occur in patients with BM treated with (mono)immunotherapy using checkpoint inhibitors such as antibodies to CTLA-4 (eg, ipilimumab), PD1 (eg, pembrolizumab, nivolumab), or PDL1 (eg, atezolizumab). A small pilot study (n = 5 patients) highlighted the potential of FET PET to identify pseudoprogression in patients with BM secondary to melanoma treated with the ICI ipilimumab.20 In that study, FET PET imaging findings were correlated with the patients’ clinical course after ICI therapy initiation. In the case of pseudoprogression, FET PET showed in contrast to progressive MRI only minimal or even no uptake and the outcome was favorable (>6 mo).
Assessment of Treatment Response
In patients (n = 5) with melanoma BM (n = 22) treated with TT or ICI therapy, a small prospective study found in a subset of patients that metabolic responders may show a proliferative reduction on FLT PET despite unchanged findings on standard MRI.106 Furthermore, FLT PET responders had a survival of more than 12 months after therapy initiation. The pilot data suggest that FLT PET also has the potential to detect a reduction of proliferative tumor activity despite apparent morphologic progression on conventional MRI (ie, pseudoprogression).
While the value of amino acid PET for the assessment of treatment response in gliomas is well established,107 studies on BM still remain scarce. Single case reports suggest that amino acid PET has the potential to add valuable information to standard MRI for the assessment of treatment response. Similar to FLT PET, a reduction of metabolic activity in BM patients secondary to melanoma or NSCLC treated with TT could be identified by FET PET, whereas findings on standard MRI remained unchanged.73,108
Advanced MRI
While conventional MRI is exceptional in providing detailed anatomical information of both the central nervous system and brain tumors, advanced MRI methods offer the ability to yield valuable information concerning tumor biology, especially at the functional, physiologic, and molecular levels. Commonly used advanced MR techniques include perfusion-weighted imaging (PWI), MR spectroscopy (MRS), and diffusion-weighted imaging (DWI). Due to a better scanner resolution, smaller lesions (~5 mm in diameter) can be better evaluated by MRI techniques than by PET (optimal lesion diameter, 10 mm or more).
Differentiation of Radiation-Induced Changes from Brain Metastasis Recurrence
A recent meta-analysis by Chuang and colleagues109 examined the value of various imaging parameters derived from PWI and MRS for the differentiation of recurrent tumor from radiation-induced necrosis in brain tumor patients. Of 397 brain tumor patients encompassed by 13 studies, 95 patients suffered from BM, and the remaining patients had gliomas. The main finding of that meta-analysis was that MRS and MR perfusion might increase the accuracy of differentiating recurrent tumor from radiation-induced necrosis in patients with gliomas or BM. In particular, the relative cerebral blood volume (rCBV) derived from PWI as well as various MRS metabolite ratios in contrast-enhancing lesions was significantly different in BM recurrence compared with radiation injury.
Regarding the diagnostic performance of PWI, the available studies revealed a considerable variability of sensitivity and specificity (range of sensitivity, 56–100%; range of specificity, 68–100%) and rCBV thresholds (range, 1.52–2.14).89,95,110–113 Although PWI separates radiation-induced changes from BM recurrence with a relatively good accuracy in individual studies, significant variabilities in optimal reported thresholds and methodology indicate that further studies and standardization are warranted.
For MRS, the specificity for the detection of BM recurrence seems to be high (100% across all studies), whereas the sensitivity is relatively low (range, 33–50%).112,114 Of note, MRS studies evaluating this clinical question remain comparatively rare and may be limited by a small lesion size (ie, <2 cm3).
Apparent diffusion coefficients (ADCs) obtained from DWI seem to be inferior to amino acid PET using MET for distinguishing radiation-induced injury from BM recurrence (area under the curve obtained from receiver operating characteristic curve analyses, 0.60 vs 0.81).90 Furthermore, in contrast to the rCBV, ADC values seem not to be of value for the detection of treatment-related changes after stereotactic radiotherapy of BM.115
A radiomics prediction model based on contrast-enhanced T1 and fluid attenuated inversion recovery (FLAIR) images has been used for distinguishing actual tumor progression from radionecrosis after stereotactic radiosurgery for BM patients.116 After cross-validation of the model, radiomics analysis revealed a sensitivity and specificity of 65% and 87%, respectively (area under the curve, 0.81).
Evaluation of Response to Radiotherapy
For the evaluation of treatment response in patients with BM, a variety of parameters obtained from dynamic susceptibility contrast (DSC), dynamic contrast-enhanced (DCE), or arterial spin labeling (ASL) perfusion MRI have been evaluated, including predominantly the rCBV, the relative cerebral blood flow (rCBF), and Ktrans (which reflects the efflux rate of gadolinium contrast from blood plasma into the tissue).
Taunk and coworkers evaluated pre- and post-treatment stereotactic radiosurgery effects in 41 NSCLC patients with 53 BM using DCE PWI.117 Already within the first 12 weeks after radiosurgery, the PWI parameter Ktrans could be used to predict long-term response (median follow-up, 11 mo) in this group of patients to stereotactic radiosurgery. Similar findings regarding the parameter Ktrans have been observed in previous PWI studies.118,119
In 25 patients with 28 BM treated with radiosurgery, rCBF alterations after 6 weeks as assessed using DSC or ASL allowed the prediction of the treatment effect (median follow-up, 6 mo).120 Similarly, Essig et al found that a decrease of the rCBV at the 6-week follow-up helped to predict the treatment outcome with a sensitivity of more than 90%. In contrast, the pre-therapeutic rCBV was unable to help predict treatment outcome.121
In patients with BM, predominantly ADC values obtained from DWI have been used to evaluate treatment response, especially the response to radiosurgery. A few studies have suggested that in patients with treatment-responsive BM, the ADC values increased during follow-up after radiosurgery.122–124 Conversely, Jakubovic and colleagues evaluated 42 patients with 70 BM and observed—in contrast to the aforementioned studies—that especially lower ADC values already at one week and one month identified responders to radiosurgery.125 Regarding the prediction of tumor response, Lee found that initial (pretreatment) ADC values of 107 patients with 144 BM were able to predict response to radiosurgery with a sensitivity and specificity of 86% and 73%, respectively.126
Additionally, more sophisticated imaging postprocessing techniques of DWI, such as the calculation of the diffusion abnormality probability function or functional diffusion maps, seem to provide a reliable prediction of BM response to radiotherapy.127,128
Summary and Outlook
Advanced MRI and PET techniques have the great potential to noninvasively investigate the molecular, cellular, and structural components of the tumor and its microenvironment. In the light of recent treatment options for patients with BM, such as ICI and TT, and their potential side effects as well as ensuing imaging challenges, it is of paramount interest to both visualize and quantify metabolic and (patho)physiological changes, especially inflammation, before and during treatment.
Currently, significant evidence suggests that imaging parameters—especially derived from amino acid PET, PWI, DWI, or MRS—may provide valuable additional information for the differentiation of treatment-induced changes from BM recurrence and the evaluation of treatment response. The PET/RANO group has recently published various recommendations about which imaging modality should be preferred73: Amino acid PET may be more useful than advanced MRI, whereas FDG PET appears to be inferior. However, at present, direct comparisons of advanced MRI versus PET are limited. When using PET for this indication, amino acid tracers should be preferred because present studies consistently show high diagnostic accuracy. Nevertheless, only few data are currently available for evaluation of ICI/TT-treated BM patients using these advanced imaging techniques.
It is tempting to speculate that a multimodal approach combining parameters derived from each of these advanced imaging techniques may improve diagnostic performance. To further improve diagnostic accuracy and to assess the resulting clinical impact, multicenter studies are warranted that also standardize imaging protocols as well as postprocessing procedures.
Funding
This work was supported by the Wilhelm-Sander Stiftung, München, Germany.
Conflict of interest statement. Related to the present work, the authors disclosed no potential conflicts of interest.
Authorship statement. Study design: N.G. Data acquisition: N.G., G.C., J-M.W. Writing of manuscript drafts: N.G., M.K. Preparation of neuropathological images: A.B., M.D. Revising manuscript, approving final content of manuscript: all.
References
- 1. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kluger HM, Chiang V, Mahajan A, et al. Long-term survival of patients with melanoma with active brain metastases treated with pembrolizumab on a phase II trial. J Clin Oncol. 2019;37(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379(8):722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19(5):672–681. [DOI] [PubMed] [Google Scholar]
- 5. Stokes WA, Binder DC, Jones BL, et al. Impact of immunotherapy among patients with melanoma brain metastases managed with radiotherapy. J Neuroimmunol. 2017;313:118–122. [DOI] [PubMed] [Google Scholar]
- 6. Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087–1095. [DOI] [PubMed] [Google Scholar]
- 7. Wu YL, Ahn MJ, Garassino MC, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3). J Clin Oncol. 2018;36(26):2702–2709. [DOI] [PubMed] [Google Scholar]
- 8. Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14(1):64–71. [DOI] [PubMed] [Google Scholar]
- 9. Berghoff AS, Preusser M. New developments in brain metastases. Ther Adv Neurol Disord. 2018;11:1756286418785502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gaudy-Marqueste C, Dussouil AS, Carron R, et al. Survival of melanoma patients treated with targeted therapy and immunotherapy after systematic upfront control of brain metastases by radiosurgery. Eur J Cancer. 2017;84:44–54. [DOI] [PubMed] [Google Scholar]
- 11. Trino E, Mantovani C, Badellino S, Ricardi U, Filippi AR. Radiosurgery/stereotactic radiotherapy in combination with immunotherapy and targeted agents for melanoma brain metastases. Expert Rev Anticancer Ther. 2017;17(4):347–356. [DOI] [PubMed] [Google Scholar]
- 12. Kroeze SG, Fritz C, Hoyer M, et al. Toxicity of concurrent stereotactic radiotherapy and targeted therapy or immunotherapy: a systematic review. Cancer Treat Rev. 2017;53:25–37. [DOI] [PubMed] [Google Scholar]
- 13. Okada H, Weller M, Huang R, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16(15):e534–e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cohen JV, Alomari AK, Vortmeyer AO, et al. Melanoma brain metastasis pseudoprogression after pembrolizumab treatment. Cancer Immunol Res. 2016;4(3):179–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. [DOI] [PubMed] [Google Scholar]
- 16. Nishino M, Giobbie-Hurder A, Manos MP, et al. Immune-related tumor response dynamics in melanoma patients treated with pembrolizumab: identifying markers for clinical outcome and treatment decisions. Clin Cancer Res. 2017;23(16):4671–4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hodi FS, Hwu WJ, Kefford R, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34(13):1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kebir S, Rauschenbach L, Galldiks N, et al. Dynamic O-(2-[18F]fluoroethyl)-L-tyrosine PET imaging for the detection of checkpoint inhibitor-related pseudoprogression in melanoma brain metastases. Neuro Oncol. 2016;18(10):1462–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vander Jagt TA, Davis LE, Thakur MD, Franz C, Pollock JM. Pseudoprogression of CNS metastatic disease of alveolar soft part sarcoma during anti-PDL1 treatment. Radiol Case Rep. 2018;13(4):882–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Melian M, Lorente D, Aparici F, Juan O. Lung brain metastasis pseudoprogression after nivolumab and ipilimumab combination treatment. Thorac Cancer. 2018;9(12):1770–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hendriks LEL, Henon C, Auclin E, et al. Outcome of patients with non-small cell lung cancer and brain metastases treated with checkpoint inhibitors. J Thorac Oncol. 2019;14(7):1244–1254. [DOI] [PubMed] [Google Scholar]
- 24. Trommer-Nestler M, Marnitz S, Kocher M, et al. Robotic stereotactic radiosurgery in melanoma patients with brain metastases under simultaneous anti-PD-1 treatment. Int J Mol Sci. 2018;19(9). pii: E2653. doi:10.3390/ijms19092653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ou SH, Weitz M, Jalas JR, et al. Alectinib induced CNS radiation necrosis in an ALK+NSCLC patient with a remote (7 years) history of brain radiation. Lung Cancer. 2016;96:15–18. [DOI] [PubMed] [Google Scholar]
- 26. Tazdait M, Mezquita L, Lahmar J, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer. 2018;88:38–47. [DOI] [PubMed] [Google Scholar]
- 27. Seymour L, Bogaerts J, Perrone A, et al. ; RECIST working group iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Okada H, Kalinski P, Ueda R, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29(3):330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sampson JH, Heimberger AB, Archer GE, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(31):4722–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kruit WH, van Ojik HH, Brichard VG, et al. Phase 1/2 study of subcutaneous and intradermal immunization with a recombinant MAGE-3 protein in patients with detectable metastatic melanoma. Int J Cancer. 2005;117(4):596–604. [DOI] [PubMed] [Google Scholar]
- 31. Borcoman E, Kanjanapan Y, Champiat S, et al. Novel patterns of response under immunotherapy. Ann Oncol. 2019;30(3): 385–396. [DOI] [PubMed] [Google Scholar]
- 32. Champiat S, Ferrara R, Massard C, et al. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol. 2018;15(12):748–762. [DOI] [PubMed] [Google Scholar]
- 33. Sharon E. Can an immune checkpoint inhibitor (sometimes) make things worse? Clin Cancer Res. 2017;23(8):1879–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23(8):1920–1928. [DOI] [PubMed] [Google Scholar]
- 35. Saâda-Bouzid E, Defaucheux C, Karabajakian A, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2017;28(7):1605–1611. [DOI] [PubMed] [Google Scholar]
- 36. Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4(11):1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kanai O, Fujita K, Okamura M, Nakatani K, Mio T. Severe exacerbation or manifestation of primary disease related to nivolumab in non-small-cell lung cancer patients with poor performance status or brain metastases. Ann Oncol. 2016;27(7):1354–1356. [DOI] [PubMed] [Google Scholar]
- 38. Lin NU, Lee EQ, Aoyama H, et al. ; Response Assessment in Neuro-Oncology (RANO) group Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16(6):e270–e278. [DOI] [PubMed] [Google Scholar]
- 39. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 40. Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18(7):863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ready N, Hellmann MD, Awad MM, et al. First-line nivolumab plus ipilimumab in advanced non-small-cell lung cancer (CheckMate 568): outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J Clin Oncol. 2019;37(12):992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kocher M, Wittig A, Piroth MD, et al. Stereotactic radiosurgery for treatment of brain metastases. A report of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlenther Onkol. 2014;190(6):521–532. [DOI] [PubMed] [Google Scholar]
- 43. Aoyama H, Tago M, Shirato H; Japanese Radiation Oncology Study Group 99-1 (JROSG 99-1) Investigators Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: secondary analysis of the JROSG 99-1 randomized clinical trial. JAMA Oncol. 2015;1(4):457–464. [DOI] [PubMed] [Google Scholar]
- 44. Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamamoto M, Serizawa T, Higuchi Y, et al. A Multi-institutional prospective observational study of stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901 Study Update): irradiation-related complications and long-term maintenance of mini-mental state examination scores. Int J Radiat Oncol Biol Phys. 2017;99(1):31–40. [DOI] [PubMed] [Google Scholar]
- 47. Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell. 2017;31(3):326–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Walle T, Martinez Monge R, Cerwenka A, Ajona D, Melero I, Lecanda F. Radiation effects on antitumor immune responses: current perspectives and challenges. Ther Adv Med Oncol. 2018;10:1758834017742575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Acharya S, Mahmood M, Mullen D, et al. Distant intracranial failure in melanoma brain metastases treated with stereotactic radiosurgery in the era of immunotherapy and targeted agents. Adv Radiat Oncol. 2017;2(4):572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ahmed KA, Abuodeh YA, Echevarria MI, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiosurgery and anti-PD-1 therapy, anti-CTLA-4 therapy, BRAF/MEK inhibitors, BRAF inhibitor, or conventional chemotherapy. Ann Oncol. 2016;27(12):2288–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kotecha R, Miller JA, Venur VA, et al. Melanoma brain metastasis: the impact of stereotactic radiosurgery, BRAF mutational status, and targeted and/or immune-based therapies on treatment outcome. J Neurosurg. 2018;129(1):50–59. [DOI] [PubMed] [Google Scholar]
- 52. Patel KR, Shoukat S, Oliver DE, et al. Ipilimumab and stereotactic radiosurgery versus stereotactic radiosurgery alone for newly diagnosed melanoma brain metastases. Am J Clin Oncol. 2017;40(5):444–450. [DOI] [PubMed] [Google Scholar]
- 53. Yusuf MB, Amsbaugh MJ, Burton E, Chesney J, Woo S. Peri-SRS administration of immune checkpoint therapy for melanoma metastatic to the brain: investigating efficacy and the effects of relative treatment timing on lesion response. World Neurosurg. 2017;100:632–640.e4. [DOI] [PubMed] [Google Scholar]
- 54. Kim JM, Miller JA, Kotecha R, et al. Stereotactic radiosurgery with concurrent HER2-directed therapy is associated with improved objective response for breast cancer brain metastasis. Neuro Oncol. 2019;21(5):659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Miller JA, Kotecha R, Ahluwalia MS, et al. Overall survival and the response to radiotherapy among molecular subtypes of breast cancer brain metastases treated with targeted therapies. Cancer. 2017;123(12):2283–2293. [DOI] [PubMed] [Google Scholar]
- 56. Parsai S, Miller JA, Juloori A, et al. Stereotactic radiosurgery with concurrent lapatinib is associated with improved local control for HER2-positive breast cancer brain metastases. J Neurosurg. 2019:1–9. [DOI] [PubMed] [Google Scholar]
- 57. Singh C, Qian JM, Yu JB, Chiang VL. Local tumor response and survival outcomes after combined stereotactic radiosurgery and immunotherapy in non-small cell lung cancer with brain metastases. J Neurosurg. 2019:1–6. [DOI] [PubMed] [Google Scholar]
- 58. An Y, Jiang W, Kim BYS, et al. Stereotactic radiosurgery of early melanoma brain metastases after initiation of anti-CTLA-4 treatment is associated with improved intracranial control. Radiother Oncol. 2017;125(1):80–88. [DOI] [PubMed] [Google Scholar]
- 59. Cohen-Inbar O, Shih HH, Xu Z, Schlesinger D, Sheehan JP. The effect of timing of stereotactic radiosurgery treatment of melanoma brain metastases treated with ipilimumab. J Neurosurg. 2017;127(5):1007–1014. [DOI] [PubMed] [Google Scholar]
- 60. Diao K, Bian SX, Routman DM, et al. Stereotactic radiosurgery and ipilimumab for patients with melanoma brain metastases: clinical outcomes and toxicity. J Neurooncol. 2018;139(2):421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nardin C, Mateus C, Texier M, et al. Tolerance and outcomes of stereotactic radiosurgery combined with anti-programmed cell death-1 (pembrolizumab) for melanoma brain metastases. Melanoma Res. 2018;28(2):111–119. [DOI] [PubMed] [Google Scholar]
- 62. Qian JM, Yu JB, Kluger HM, Chiang VL. Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer. 2016;122(19):3051–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rahman R, Cortes A, Niemierko A, et al. The impact of timing of immunotherapy with cranial irradiation in melanoma patients with brain metastases: intracranial progression, survival and toxicity. J Neurooncol. 2018;138(2):299–306. [DOI] [PubMed] [Google Scholar]
- 64. Skrepnik T, Sundararajan S, Cui H, Stea B. Improved time to disease progression in the brain in patients with melanoma brain metastases treated with concurrent delivery of radiosurgery and ipilimumab. Oncoimmunology. 2017;6(3):e1283461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mastorakos P, Xu Z, Yu J, et al. BRAF V600 mutation and BRAF kinase inhibitors in conjunction with stereotactic radiosurgery for intracranial melanoma metastases: a multicenter retrospective study. Neurosurgery. 2019;84(4):868–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Patel TR, McHugh BJ, Bi WL, Minja FJ, Knisely JP, Chiang VL. A comprehensive review of MR imaging changes following radiosurgery to 500 brain metastases. AJNR Am J Neuroradiol. 2011;32(10):1885–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Szeifert GT, Atteberry DS, Kondziolka D, Levivier M, Lunsford LD. Cerebral metastases pathology after radiosurgery: a multicenter study. Cancer. 2006;106(12):2672–2681. [DOI] [PubMed] [Google Scholar]
- 68. Szeifert GT, Kondziolka D, Levivier M, Lunsford LD. Histopathology of brain metastases after radiosurgery. Prog Neurol Surg. 2012;25:30–38. [DOI] [PubMed] [Google Scholar]
- 69. Nordmann N, Hubbard M, Nordmann T, Sperduto PW, Clark HB, Hunt MA. Effect of gamma knife radiosurgery and programmed cell death 1 receptor antagonists on metastatic melanoma. Cureus. 2017;9(12):e1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Herholz K, Langen KJ, Schiepers C, Mountz JM. Brain tumors. Semin Nucl Med. 2012;42(6):356–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Albert NL, Weller M, Suchorska B, et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18(9):1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Langen KJ, Galldiks N, Hattingen E, Shah NJ. Advances in neuro-oncology imaging. Nat Rev Neurol. 2017;13(5):279–289. [DOI] [PubMed] [Google Scholar]
- 73. Galldiks N, Langen KJ, Albert NL, et al. PET imaging in patients with brain metastasis-report of the RANO/PET group. Neuro Oncol. 2019;21(5):585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Langen KJ, Watts C. Neuro-oncology: amino acid PET for brain tumours—ready for the clinic? Nat Rev Neurol. 2016;12(7):375–376. [DOI] [PubMed] [Google Scholar]
- 75. Law I, Albert NL, Arbizu J, et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2019;46(3):540–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Galldiks N, Albert NL, Sommerauer M, et al. PET imaging in patients with meningioma-report of the RANO/PET Group. Neuro Oncol. 2017;19(12):1576–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Galldiks N, Langen KJ, Pope WB. From the clinician’s point of view—what is the status quo of positron emission tomography in patients with brain tumors? Neuro Oncol. 2015;17(11):1434–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Langen KJ, Stoffels G, Filss C, et al. Imaging of amino acid transport in brain tumours: positron emission tomography with O-(2-[18F]fluoroethyl)-L-tyrosine (FET). Methods. 2017;130:124–134. [DOI] [PubMed] [Google Scholar]
- 79. Okubo S, Zhen HN, Kawai N, Nishiyama Y, Haba R, Tamiya T. Correlation of L-methyl-11C-methionine (MET) uptake with L-type amino acid transporter 1 in human gliomas. J Neurooncol. 2010;99(2):217–225. [DOI] [PubMed] [Google Scholar]
- 80. Wiriyasermkul P, Nagamori S, Tominaga H, et al. Transport of 3-fluoro-L-α-methyl-tyrosine by tumor-upregulated L-type amino acid transporter 1: a cause of the tumor uptake in PET. J Nucl Med. 2012;53(8):1253–1261. [DOI] [PubMed] [Google Scholar]
- 81. Youland RS, Kitange GJ, Peterson TE, et al. The role of LAT1 in (18)F-DOPA uptake in malignant gliomas. J Neurooncol. 2013;111(1):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Papin-Michault C, Bonnetaud C, Dufour M, et al. Study of LAT1 expression in brain metastases: towards a better understanding of the results of positron emission tomography using amino acid tracers. PLoS One. 2016;11(6):e0157139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Shields AF, Grierson JR, Dohmen BM, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998;4(11):1334–1336. [DOI] [PubMed] [Google Scholar]
- 84. Jacobs AH, Thomas A, Kracht LW, et al. 18F-fluoro-L-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. J Nucl Med. 2005;46(12):1948–1958. [PubMed] [Google Scholar]
- 85. Chao ST, Suh JH, Raja S, Lee SY, Barnett G. The sensitivity and specificity of FDG PET in distinguishing recurrent brain tumor from radionecrosis in patients treated with stereotactic radiosurgery. Int J Cancer. 2001;96(3):191–197. [DOI] [PubMed] [Google Scholar]
- 86. Belohlavek O, Simonova G, Kantorova I, Novotny J Jr, Liscak R. Brain metastases after stereotactic radiosurgery using the Leksell gamma knife: can FDG PET help to differentiate radionecrosis from tumour progression? Eur J Nucl Med Mol Imag. 2003;30(1):96–100. [DOI] [PubMed] [Google Scholar]
- 87. Chernov M, Hayashi M, Izawa M, et al. Differentiation of the radiation-induced necrosis and tumor recurrence after gamma knife radiosurgery for brain metastases: importance of multi-voxel proton MRS. Minim Invasive Neurosurg. 2005;48(4):228–234. [DOI] [PubMed] [Google Scholar]
- 88. Lai G, Mahadevan A, Hackney D, et al. Diagnostic accuracy of PET, SPECT, and arterial spin-labeling in differentiating tumor recurrence from necrosis in cerebral metastasis after stereotactic radiosurgery. AJNR Am J Neuroradiol. 2015;36(12):2250–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hatzoglou V, Yang TJ, Omuro A, et al. A prospective trial of dynamic contrast-enhanced MRI perfusion and fluorine-18 FDG PET-CT in differentiating brain tumor progression from radiation injury after cranial irradiation. Neuro Oncol. 2016;18(6):873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tomura N, Kokubun M, Saginoya T, Mizuno Y, Kikuchi Y. Differentiation between treatment-induced necrosis and recurrent tumors in patients with metastatic brain tumors: comparison among 11C-methionine-PET, FDG-PET, MR permeability imaging, and MRI-ADC-preliminary results. AJNR Am J Neuroradiol. 2017;38(8):1520–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Terakawa Y, Tsuyuguchi N, Iwai Y, et al. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med. 2008;49(5):694–699. [DOI] [PubMed] [Google Scholar]
- 92. Tsuyuguchi N, Sunada I, Iwai Y, et al. Methionine positron emission tomography of recurrent metastatic brain tumor and radiation necrosis after stereotactic radiosurgery: is a differential diagnosis possible? J Neurosurg. 2003;98(5):1056–1064. [DOI] [PubMed] [Google Scholar]
- 93. Minamimoto R, Saginoya T, Kondo C, et al. Differentiation of brain tumor recurrence from post-radiotherapy necrosis with 11C-methionine PET: visual assessment versus quantitative assessment. PLoS One. 2015;10(7):e0132515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lizarraga KJ, Allen-Auerbach M, Czernin J, et al. (18)F-FDOPA PET for differentiating recurrent or progressive brain metastatic tumors from late or delayed radiation injury after radiation treatment. J Nucl Med. 2014;55(1):30–36. [DOI] [PubMed] [Google Scholar]
- 95. Cicone F, Minniti G, Romano A, et al. Accuracy of F-DOPA PET and perfusion-MRI for differentiating radionecrotic from progressive brain metastases after radiosurgery. Eur J Nucl Med Mol Imaging. 2015;42(1):103–111. [DOI] [PubMed] [Google Scholar]
- 96. Galldiks N, Stoffels G, Filss CP, et al. Role of O-(2-(18)F-fluoroethyl)-L-tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J Nucl Med. 2012;53(9):1367–1374. [DOI] [PubMed] [Google Scholar]
- 97. Ceccon G, Lohmann P, Stoffels G, et al. Dynamic O-(2-18F-fluoroethyl)-L-tyrosine positron emission tomography differentiates brain metastasis recurrence from radiation injury after radiotherapy. Neuro Oncol. 2017;19(2):281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Romagna A, Unterrainer M, Schmid-Tannwald C, et al. Suspected recurrence of brain metastases after focused high dose radiotherapy: can [18F]FET- PET overcome diagnostic uncertainties? Radiat Oncol. 2016;11(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kickingereder P, Götz M, Muschelli J, et al. Large-scale radiomic profiling of recurrent glioblastoma identifies an imaging predictor for stratifying anti-angiogenic treatment response. Clin Cancer Res. 2016;22(23):5765–5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rudie JD, Rauschecker AM, Bryan RN, Davatzikos C, Mohan S. Emerging applications of artificial intelligence in neuro-oncology. Radiology. 2019;290(3):607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lohmann P, Kocher M, Steger J, Galldiks N. Radiomics derived from amino-acid PET and conventional MRI in patients with high-grade gliomas. Q J Nucl Med Mol Imaging. 2018;62(3):272–280. [DOI] [PubMed] [Google Scholar]
- 102. Peeken JC, Nüsslin F, Combs SE. “Radio-oncomics”: The potential of radiomics in radiation oncology. Strahlenther Onkol. 2017;193(10):767–779. [DOI] [PubMed] [Google Scholar]
- 103. Galldiks N, Langen KJ. Amino acid PET in neuro-oncology: applications in the clinic. Expert Rev Anticancer Ther. 2017;17(5):395–397. [DOI] [PubMed] [Google Scholar]
- 104. Lohmann P, Stoffels G, Ceccon G, et al. Radiation injury vs. recurrent brain metastasis: combining textural feature radiomics analysis and standard parameters may increase 18F-FET PET accuracy without dynamic scans. Eur Radiol. 2017;27(7):2916–2927. [DOI] [PubMed] [Google Scholar]
- 105. Lohmann P, Kocher M, Ceccon G, et al. Combined FET PET/MRI radiomics differentiates radiation injury from recurrent brain metastasis. Neuroimage Clin. 2018;20:537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Nguyen NC, Yee MK, Tuchayi AM, Kirkwood JM, Tawbi H, Mountz JM. Targeted therapy and immunotherapy response assessment with F-18 fluorothymidine positron-emission tomography/magnetic resonance imaging in melanoma brain metastasis: a pilot study. Front Oncol. 2018;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Langen KJ, Galldiks N. Update on amino acid PET of brain tumours. Curr Opin Neurol. 2018;31(4):354–361. [DOI] [PubMed] [Google Scholar]
- 108. Abdulla DSY, Scheffler M, Brandes V, et al. Monitoring treatment response to erlotinib in EGFR-mutated non-small-cell lung cancer brain metastases using serial O-(2-[18F]fluoroethyl)-L-tyrosine PET. Clin Lung Cancer. 2019;20(2):e148–e151. [DOI] [PubMed] [Google Scholar]
- 109. Chuang MT, Liu YS, Tsai YS, Chen YC, Wang CK. Differentiating radiation-induced necrosis from recurrent brain tumor using MR perfusion and spectroscopy: a meta-analysis. PLoS One. 2016;11(1):e0141438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Mitsuya K, Nakasu Y, Horiguchi S, et al. Perfusion weighted magnetic resonance imaging to distinguish the recurrence of metastatic brain tumors from radiation necrosis after stereotactic radiosurgery. J Neurooncol. 2010;99(1):81–88. [DOI] [PubMed] [Google Scholar]
- 111. Barajas RF, Chang JS, Sneed PK, Segal MR, McDermott MW, Cha S. Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol. 2009;30(2):367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Huang J, Wang AM, Shetty A, et al. Differentiation between intra-axial metastatic tumor progression and radiation injury following fractionated radiation therapy or stereotactic radiosurgery using MR spectroscopy, perfusion MR imaging or volume progression modeling. Magn Reson Imaging. 2011;29(7):993–1001. [DOI] [PubMed] [Google Scholar]
- 113. Hoefnagels FW, Lagerwaard FJ, Sanchez E, et al. Radiological progression of cerebral metastases after radiosurgery: assessment of perfusion MRI for differentiating between necrosis and recurrence. J Neurol. 2009;256(6):878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chernov MF, Hayashi M, Izawa M, et al. Multivoxel proton MRS for differentiation of radiation-induced necrosis and tumor recurrence after gamma knife radiosurgery for brain metastases. Brain Tumor Pathol. 2006;23(1):19–27. [DOI] [PubMed] [Google Scholar]
- 115. Knitter JR, Erly WK, Stea BD, et al. Interval change in diffusion and perfusion MRI parameters for the assessment of pseudoprogression in cerebral metastases treated with stereotactic radiation. AJR Am J Roentgenol. 2018;211(1):168–175. [DOI] [PubMed] [Google Scholar]
- 116. Peng L, Parekh V, Huang P, et al. Distinguishing true progression from radionecrosis after stereotactic radiation therapy for brain metastases with machine learning and radiomics. Int J Radiat Oncol Biol Phys. 2018;102(4):1236–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Taunk NK, Oh JH, Shukla-Dave A, et al. Early posttreatment assessment of MRI perfusion biomarkers can predict long-term response of lung cancer brain metastases to stereotactic radiosurgery. Neuro Oncol. 2018;20(4):567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kuchcinski G, Le Rhun E, Cortot AB, et al. Dynamic contrast-enhanced MR imaging pharmacokinetic parameters as predictors of treatment response of brain metastases in patients with lung cancer. Eur Radiol. 2017;27(9):3733–3743. [DOI] [PubMed] [Google Scholar]
- 119. Jakubovic R, Sahgal A, Soliman H, et al. Magnetic resonance imaging-based tumour perfusion parameters are biomarkers predicting response after radiation to brain metastases. Clin Oncol (R Coll Radiol). 2014;26(11):704–712. [DOI] [PubMed] [Google Scholar]
- 120. Weber MA, Thilmann C, Lichy MP, et al. Assessment of irradiated brain metastases by means of arterial spin-labeling and dynamic susceptibility-weighted contrast-enhanced perfusion MRI: initial results. Invest Radiol. 2004;39(5):277–287. [DOI] [PubMed] [Google Scholar]
- 121. Essig M, Waschkies M, Wenz F, Debus J, Hentrich HR, Knopp MV. Assessment of brain metastases with dynamic susceptibility-weighted contrast-enhanced MR imaging: initial results. Radiology. 2003;228(1):193–199. [DOI] [PubMed] [Google Scholar]
- 122. Chen Z, Zu J, Li L, Lu X, Ni J, Xu J. Assessment of stereotactic radiosurgery treatment response for brain metastases using MRI based diffusion index. Eur J Radiol Open. 2017;4:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Huang CF, Chiou SY, Wu MF, Tu HT, Liu WS, Chuang JC. Apparent diffusion coefficients for evaluation of the response of brain tumors treated by Gamma Knife surgery. J Neurosurg. 2010;113(Suppl):97–104. [PubMed] [Google Scholar]
- 124. Huang CF, Chou HH, Tu HT, Yang MS, Lee JK, Lin LY. Diffusion magnetic resonance imaging as an evaluation of the response of brain metastases treated by stereotactic radiosurgery. Surg Neurol. 2008;69(1):62–68;discussion 68. [DOI] [PubMed] [Google Scholar]
- 125. Jakubovic R, Zhou S, Heyn C, et al. The predictive capacity of apparent diffusion coefficient (ADC) in response assessment of brain metastases following radiation. Clin Exp Metastasis. 2016;33(3):277–284. [DOI] [PubMed] [Google Scholar]
- 126. Lee CC, Wintermark M, Xu Z, Yen CP, Schlesinger D, Sheehan JP. Application of diffusion-weighted magnetic resonance imaging to predict the intracranial metastatic tumor response to gamma knife radiosurgery. J Neurooncol. 2014;118(2):351–361. [DOI] [PubMed] [Google Scholar]
- 127. Ruiz-Espana S, Jimenez-Moya A, Arana E, Moratal D. Functional diffusion map: a biomarker of brain metastases response to treatment based on magnetic resonance image analysis. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:4282–4285. [DOI] [PubMed] [Google Scholar]
- 128. Farjam R, Tsien CI, Feng FY, et al. Investigation of the diffusion abnormality index as a new imaging biomarker for early assessment of brain tumor response to radiation therapy. Neuro Oncol. 2014;16(1):131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Colaco RJ, Martin P, Kluger HM, Yu JB, Chiang VL. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J Neurosurg. 2016;125(1):17–23. [DOI] [PubMed] [Google Scholar]
- 130. Kaidar-Person O, Zagar TM, Deal A, et al. The incidence of radiation necrosis following stereotactic radiotherapy for melanoma brain metastases: the potential impact of immunotherapy. Anticancer Drugs. 2017;28(6):669–675. [DOI] [PubMed] [Google Scholar]
- 131. Du Four S, Janssen Y, Michotte A, et al. Focal radiation necrosis of the brain in patients with melanoma brain metastases treated with pembrolizumab. Cancer Med. 2018;7(10):4870–4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Pires da Silva I, Glitza IC, Haydu LE, et al. Incidence, features and management of radionecrosis in melanoma patients treated with cerebral radiotherapy and anti-PD-1 antibodies. Pigment Cell Melanoma Res. 2019;32(4):553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kim JM, Miller JA, Kotecha R, et al. The risk of radiation necrosis following stereotactic radiosurgery with concurrent systemic therapies. J Neurooncol. 2017;133(2):357–368. [DOI] [PubMed] [Google Scholar]
- 134. Weingarten N, Kruser TJ, Bloch O. Symptomatic radiation necrosis in brain metastasis patients treated with stereotactic radiosurgery and immunotherapy. Clin Neurol Neurosurg. 2019;179:14–18. [DOI] [PubMed] [Google Scholar]
- 135. Hubbeling HG, Schapira EF, Horick NK, et al. Safety of combined PD-1 pathway inhibition and intracranial radiation therapy in non-small cell lung cancer. J Thorac Oncol. 2018;13(4):550–558. [DOI] [PubMed] [Google Scholar]