Abstract
The level of evidence to provide treatment recommendations for vestibular schwannoma is low compared with other intracranial neoplasms. Therefore, the vestibular schwannoma task force of the European Association of Neuro-Oncology assessed the data available in the literature and composed a set of recommendations for health care professionals. The radiological diagnosis of vestibular schwannoma is made by magnetic resonance imaging. Histological verification of the diagnosis is not always required. Current treatment options include observation, surgical resection, fractionated radiotherapy, and radiosurgery. The choice of treatment depends on clinical presentation, tumor size, and expertise of the treating center. In small tumors, observation has to be weighed against radiosurgery, in large tumors surgical decompression is mandatory, potentially followed by fractionated radiotherapy or radiosurgery. Except for bevacizumab in neurofibromatosis type 2, there is no role for pharmacotherapy.
Keywords: diagnosis, radiotherapy, surgery, treatment, vestibular schwannoma
Key Points.
1. Observing VS is considered appropriate for incidental, asymptomatic VS. As an alternative to observation, SRS can be performed.
2. For smaller VS where preserving facial nerve and hearing function is the primary goal of treatment, SRS over microsurgery may be chosen.
3. In large VS surgery is considered as the primary treatment to reduce mass effect. The choice of surgical approach depends on tumor characteristics and surgeon’s expertise. Intraoperative neurophysiological monitoring is mandatory.
4. For large VS, tumor mass reduction followed by SRS or observation is a valid option.
5. ORisk for tumor regrowth rises with residual tumor volume.
This first guideline of the European Association of Neuro-Oncology (EANO) on the diagnosis, treatment, and follow-up of patients with vestibular schwannoma (VS) aims at guidance in an area where there is little evidence from controlled clinical trials, major variation of clinical practice across sites and countries, but urgent need for consensus and standard operating procedures. To facilitate future clinical research and to improve overall outcome for patients regarding both functional integrity of cranial nerves and long-lasting tumor control, consensus on standard operating procedures is demanded. To meet this goal, the EANO assembled a task force of experts from various European countries reflecting the multidisciplinary approach to this disease to derive recommendations for diagnostic workup and therapy.
Methods
The task force represents the clinical disciplines involved in diagnosis and treatment of patients with VS and was established upon suggestions of the EANO board. Specialists representing neurosurgery, ear nose and throat surgery, radiation oncology, pharmacotherapy, neuroradiology, neuropathology, molecular pathology, and molecular genetics were included. The topics of the guideline were distributed to groups of authors according to their clinical profession and scientific profile. The individual authors searched the Medline database from January 1990 to July 2018, the Cochrane Library from January 1990 until July 2018, as well as Embase-Ovid, Cancer Net, and Science Citation Index (all January 1990 to July 2018). Sensitive and specific keywords as well as combinations of keywords were used. All types of clinical and basic science articles in all languages represented by the members of the EANO task force were considered. The main keywords were chemotherapy, facial, function, hearing, NF2, nerve, neurofibromatosis, observation, outcome, radiosurgery, radiotherapy, schwannoma, sheath, surgery, tumor, vestibular, in various combinations.
In the consensus process with participation of all authors, the literature was evaluated and 174 papers were chosen for the final guideline. The scientific evidence was rated into classes I–IV and recommendations were labeled as levels A–C, according to guidelines of the European Federation of Neurological Societies1 (see Table 1).When sufficient evidence for recommendations was not available, the task force offered advice as a “good practice point.”
Table 1.
Evidence classes and recommendation levels
| Evidence | |
| Class I | Prospective randomized blinded trial (PRBT) or review of PRBTs |
| Class II | Prospective matched pair cohort studies |
| Class III | Any controlled trial (incl. retrospective controls) |
| Class IV | Uncontrolled studies, case series, case reports, expert opinions |
| Recommendation | |
| Level A | One class I or at least two class II studies |
| Level B | One class II or overwhelming class III evidence |
| Level C | At least two class III studies |
| “Good practice point” | Only class IV evidence |
Clinical Presentation and Epidemiology
Vestibular schwannomas, formerly termed acoustic neuromas, represent the third most common intracranial nonmalignant tumor entity after meningiomas and pituitary adenomas.1 They are the most common extra-axial posterior fossa tumors in adults, comprising over 80% of tumors in the cerebellopontine angle.2,3 In most cases the tumors present unilaterally; bilateral VS are a hallmark of neurofibromatosis type 2 (NF2) (see below).
Clinically, most patients present with unilateral sensorineural hearing loss (94%) and tinnitus (83%). The frequency of the vestibular symptoms vertigo and unsteadiness varies widely (17‒75% of patients), but they are likely underreported.4 Large tumors may cause trigeminal and facial neuropathies as well as brainstem compression and hydrocephalus.
According to the Central Brain Tumor Registry of the United States, from 2004 to 2010 the overall incidence of VS was 1.09 per 100 000/year.5 This incidence increased with age to a peak of 2.93 per 100 000/year in the 65–74 year age group without sex difference. Worldwide there is substantial geographical variation in VS incidence: a recent analysis of the Surveillance, Epidemiology, and End Results (SEER) database in the US including a total of 9782 VS patients among 822 million person-years revealed the median annual disease incidence to be lowest among black and Hispanic and highest among Caucasian populations (P < 0.001).6 The VS incidence in Denmark, Sweden, Finland, and Norway varies widely by country, with highest incidence in Denmark.7 These differences in VS incidence may be due to genetic and environmental factors as well as different diagnostic practices. Improved screening protocols for asymmetrical hearing loss, better access to advanced imaging, and improved resolution of MRI have led to an increased number of VS diagnoses and a decreased average tumor size at the time of diagnosis.8
Risk factors for VS have scarcely been investigated. A population-based case-control study of VS risk factors in the UK and Nordic countries revealed an elevated risk for VS in parous compared with nulliparous women.9 There was no relation to age at first birth or the number of children. Tumor risk was lower in current but not in ex-smokers. The biological mechanisms, if any, underlying these observations remain unclear.
Pathogenesis
Inactivation of the NF2 tumor suppressor gene is considered a major event in the tumorigenesis of conventional schwannoma. A recent whole exome sequencing study demonstrated that 77% of VS show evidence of genomic inactivation of NF2 via loss of chromosome 22q or NF2 gene mutation.10NF2 inactivation is the most highly recurrent genomic alteration in VS. Biallelic inactivation can be demonstrated by exome sequencing in 45% of cases, whereas in 41% of cases only one hit either by heterozygous chromosome 22q deletion or NF2 mutation is evident. In 14% of cases no genomic hit in NF2 can be detected by exome sequencing. However, the consistent lack of the NF2 gene product merlin in the tumor cells of VS suggests that in cases without evidence for genetic inactivation, epigenetic mechanisms of NF2 silencing or mutational events in regions not covered by exome sequencing likely exist.11 Another recent whole exome sequencing study reports concordant results regarding NF2 alterations in VS.12 However, there are discrepancies between both studies regarding alterations in non-NF2 genes. While one study found ARID1A (14%), ARID1B (18%), DDR1 (11%), TSC1 (9%), TSC2 (7%), CAST (8%), ALPK2 (8%), LZTR1 (8%), and TAB3 (3%) as additional genes recurrently altered in (vestibular) schwannomas, the other study did not find recurrent somatic mutations in these genes but in CDC27 (11%) and USP8 (7%).10 Therefore, further studies are required to clarify the role of non-NF2 gene mutations in schwannoma pathogenesis.
RNA sequencing revealed recurrent SH3PXD2A-HTRA1 fusions on chromosome 10 in about 10% of VS associated with a male predominance and partly co-occurring with genetic NF2 inactivation.10 Although the precise biochemical consequences of acquiring this fusion remain to be elucidated, activation of the MEK-ERK pathway seems to be involved.
Biallelic inactivation of PRKAR1A by deletion and/or mutation is considered a major event in the pathogenesis of melanotic schwannoma.13 In addition, melanotic schwannomas frequently show monosomies of chromosomes 1, 2, 17, and 22q as well as variable whole chromosomal gains.14,15
Neurofibromatosis 2
Vestibular schwannomas are usually solitary tumors; however, about 4–6% are associated with NF2, an autosomal dominant monogenic condition caused by pathogenic variants in the NF2 gene on chromosome 22q.16–18 NF2 has a birth incidence of about 1 in 25 000–33 000 with a diagnostic prevalence of around 1 in 60–70 000.16,19 Rarely schwannomatosis caused by pathogenic variants in the leucine zipper like transcription regulator 1 (LZTR1) gene can cause isolated VS or VS that can be misdiagnosed as NF2.20,21 NF2 can be diagnosed when the criteria in Table 2 are fulfilled or when a pathogenic mutation in the NF2 gene is found in constitutional DNA or in 2 anatomically distinct tumors.17,18 Although NF2 usually presents with bilateral VS, it may present with unilateral VS with other NF2 features in up to 15% of patients.17,18,22–25 Furthermore LZTR1 pathogenic variants can also present with apparently isolated VS at young ages, particularly <25 years, where of 106 patients with an apparently isolated VS, 9 patients (8.5%) had an NF2 and 3 patients (2.9%) had LZTR1 pathogenic variants. First affected individuals in a family and particularly those with unilateral presentation are often (30–35%) mosaic for the causative gene variant, as this occurs during early embryogenesis and was not present in the gamete.21,25 NF2 tumors are often multifocal, caused by different clonal events and multiple second hits affecting the NF2 gene in the internal auditory meatus with both roots of the vestibular nerve being affected throughout its course in the canal.26,27 This makes surgery and other interventions such as radiation treatment more difficult with higher rates of recurrence.28 Radiation should be used with caution in young NF2 patients because of the risk of malignant transformation and secondary tumor induction.28,29 Although NF2 is very variable in its course, there are strong genotype-phenotype correlations with truncating variants in exons 2–13 associated with poorest life expectancy.30 NF2 can cause schwannomas throughout the central and peripheral nervous system and patients may also develop meningiomas and spinal ependymomas. The associated morbidity affects quality of life severely and reduces life expectancy.29,30
Table 2.
Definition of neurofibromatosis type 2
| A | Bilateral vestibular schwannomas | ||
| B | Family history of NF2 | PLUS | Unilateral VS or any 2 of: meningioma, glioma,* neurofibroma, schwannoma, posterior subcapsular lenticular opacities |
| C | Unilateral VS | PLUS | Any 2 of: meningioma, glioma,* neurofibroma, schwannoma, posterior subcapsular lenticular opacities |
| D | Multiple meningioma (2 or more) | PLUS | Unilateral VS or any 2 of: meningioma, glioma,* neurofibroma, schwannoma, posterior subcapsular lenticular opacities |
Note: “any two of” refers to individual tumors or cataract, not to tumor types.
*Usually spinal cord ependymoma.
When to Consider NF2?
NF2 should be considered when an individual presents with a unilateral vestibular or other sporadic schwannoma at <30 years or meningioma at <25 years.21,24,28 Germline pathogenic variants can be identified in 1–10% of cases. NF2 should also be considered in older patients with two NF2 related tumors. Although detection rates in germline are low, mosaic NF2 can be confirmed if an identical NF2 pathogenic variant is found in both tumors.31
Diagnostic Procedures
Imaging
MRI is the method of choice for the identification of suspected VS, with contrast-enhanced T1-weighted scans considered to be the gold standard for the initial evaluation and postoperative assessment of recurrence or residual tumors.32,33 Computed tomography has a complementary role in the evaluation of VS. It provides useful preoperative information about the surgical anatomy of the skull base, especially the petrous bone.34 The MRI protocol should include standard T1- and T2-weighted sequences, diffusion-weighted imaging (DWI), and fluid-attenuated inversion recovery sequences. DWI is useful to differentiate VS from arachnoid or epidermoid cysts. At least one T2-weighted sequence is mandatory to rule out a potential brainstem pathology mimicking VS symptoms, such as multiple sclerosis or glioma. Axial submillimetric heavily T2-weighted sequence is the most important sequence in order to evaluate the vestibulocochlear nerve and its branches and depict the nerve as a linear hypointense structure surrounded by hyperintense CSF within adjacent cisterns (FIESTA [fast imaging employing steady-state acquisition], CISS [constructive interference in steady state], or DRIVE [driven equilibrium pulse]).35 There is a general agreement that MRI protocols should include axial T1-weighted sequences before and after gadolinium administration. Thin slice spin echo or turbo spin echo/fast spin echo T1-weighted sequences or submillimetric T1-weighted 3D gradient echo sequences can be used.34,36
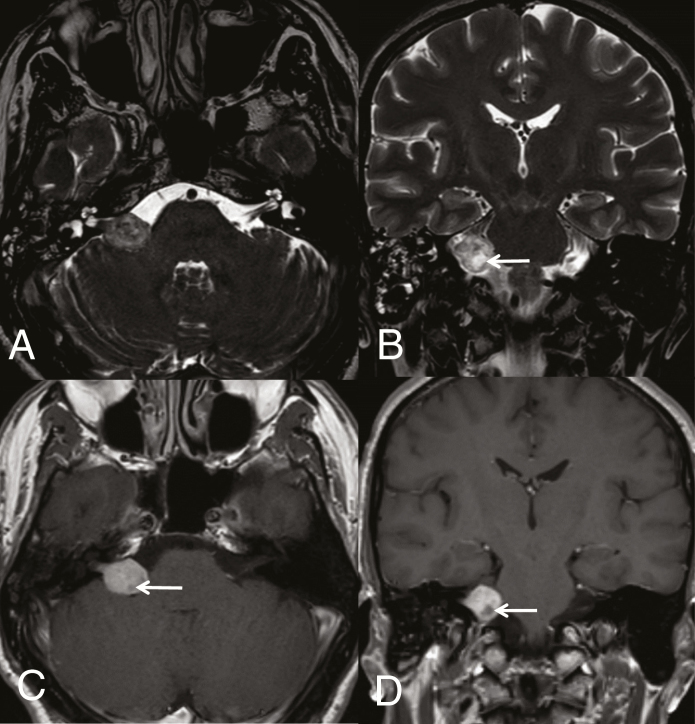
VS presents as a solid nodular mass with an intracanalicular component in the internal acoustic canal (IAC) that often results in its widening. Larger lesions can protrude into the cerebellar pontine cistern, whereas smaller lesions are often localized only inside the IAC or the labyrinth. The mass is usually isointense on T1-weighted imaging, with strong enhancement after gadolinium administration. On T2-weighted imaging, the lesion is heterogeneously hyperintense, while larger lesions may show scattered cystic degenerative changes and hemorrhagic areas (Fig. 1). Calcifications are typically absent.
Fig. 1.
Intracanalicular and cisternal VS (Koos grade III). Axial 3D heavily T2-weighted sequence (DRIVE) (A) shows a VS expanding from the internal porus acusticus into the cerebellopontine-angle cistern. Coronal T2-weighted image (B) depicts slight mass effect on middle cerebellar peduncle. Cystic degenerative changes seen on T2 are well evident on axial (C) and coronal (D) T1-weighted images after gadolinium (arrows).
Despite the current debate about omitting the post-gadolinium T1-weighted sequences,37,38 T1-weighted MRI using gadolinium-based contrast is still considered the gold standard in the diagnostic workup of VS.32,33
Histopathology
Vestibular schwannomas, formerly thought to originate from Schwann cells in the glial-Schwannian transitional zone of the vestibulocochlear nerve, do in fact arise anywhere along the eighth cranial nerve.39 In about 80% of cases they are found in the vestibular portion and in about 20% of cases in the cochlear portion.40 The diagnosis is made according to the World Health Organization (WHO) 2016 classification.41 The histological picture of conventional VS on hematoxylin/eosin-stained sections parallels that of schwannomas in other localizations and is specific enough for a morphological diagnosis in the vast majority of cases. Cellular Antoni A areas with interlacing bundles of spindle cells alternating with hypocellular, loose microcystic Antoni B areas are characteristic. Verocay bodies consisting of arrangements of palisading nuclei alternating with zones containing cell processes is another typical architecture of schwannomas. Immunohistochemically, VS are diffusely positive for S100B and SOX10. Cellular schwannoma and melanotic schwannoma are variants which may raise important differential diagnostic considerations. Cellular schwannoma is characterized by hypercellularity and predominant or exclusive presence of an Antoni A pattern without (well-formed) Verocay bodies.42 These tumors are considered benign, and therefore the distinction from malignant peripheral nerve sheath tumors (MPNSTs) is important.15,43,44 Melanotic schwannoma is recognized by the WHO as a distinct entity that rarely may also affect cranial nerves.45–47 Melanotic schwannoma is grossly pigmented and expresses melanocytic markers like HMB45 or melan-A, raising a separate differential diagnosis, including melanoma. The subvariant of psammomatous melanotic schwannoma has a 50% association with the Carney complex, an autosomal dominant clinical condition comprising myxomas, hyperpigmentation, and endocrine overactivity. In contrast to conventional or cellular schwannoma, there is a 10% risk for malignant transformation in melanotic schwannoma.48
Molecular Pathology
Currently, molecular analyses do not have a role in diagnosis, prognostication, or guiding therapy. Hotspot mutations in GNAQ/GNA11, BRAF, and pTERT are helpful for differentiating melanotic schwannoma (wild type) from melanocytoma (often GNAQ/GNA11 mutant) or cutaneous melanoma (often BRAF or pTERT mutant).14,49,50 Epigenetic analyses using genome-wide methylation profiles emerge as a superb tool for differentiating biologically distinct tumor groups. Most VS form a distinctive methylation cluster compared with schwannomas from other localizations. Methylation profiles also separate (cellular) schwannomas from histological mimics.14,15 A reference set of conventional and melanotic schwannomas is included in the recently developed DNA methylation based brain tumor classifier tool.51 Additional studies are necessary to clarify whether SH3PXD2A-HTRA1 fusion or any other molecular alteration in VS is of prognostic relevance.
Therapeutic Strategies
Observation
Observing VS with serial MRI scanning and audiological monitoring without any tumor-directed treatment is considered appropriate for incidental, asymptomatic VS (evidence class III, recommendation level C).52 Compliance of patients has to be taken into account, since noncompliance could lead to a failure of follow-up.53 The task of observational management is to monitor tumor growth and hearing function to obtain data for a potential decision for therapy. There are hardly any clinical parameters which reliably predict growth in a newly diagnosed tumor. There are studies reporting age, sex, hearing loss, imbalance, initial size, tumor location, and even sidedness as predictors of future growth, but these are mostly single studies at low evidence levels which were not reproduced.54 The proportion of growing tumors at follow-up varies considerably with reported ranges of 30–70% over different periods of time, the variation most likely being due to methodological issues.55,56 On average, approximately 50% of tumors may be expected to grow over a 5-year period.54,57,58 Series employing quantitative measurements of VS growth with long-term follow-up have shown a mean maximum diameter growth of 2.9 mm/year (maximum diameter).59 Two recent studies found that 50% of patients lost functional hearing during a 3–4 year period.60,61 A full speech discrimination score was considered a good predictor for preservation of functional hearing.61
Four nonrandomized studies compared outcomes from observation and stereotactic radiosurgery (SRS) showing better tumor control after SRS (evidence class II, recommendation level B).53,62–64 Some studies reported less hearing loss in patients with SRS, whereas in others hearing outcome and complaints were not different.53,62–64 Two studies compared either conservative management, surgery, or SRS using various quality of life questionnaires after 5–7 years of follow-up.65,66 Both reports showed that patients with conservative management only responded more favorably in the questionnaires than those who were treated up front. In addition, hearing and facial nerve outcomes were better in observed patients, the latter only in comparison with surgery. However, in nearly all observed patients the tumors had stayed stable in size, which may represent a relevant bias but also indicates the importance of thoughtful indication to treat.
Surgery
Surgical management of VS should take into account tumor size and morphology at time of diagnosis as well as the patient’s symptoms, comorbidities, and preferences.67 There are various classification systems for tumor size which support decision making.68–70 Of those, the Koos classification system is the most commonly used (see Table 3).69 In large VS (Koos grade IV), surgery is considered as the primary treatment to remove a symptomatic lesion or potentially life-threatening mass effect.69 Surgery may also be considered for smaller tumors, if cystic degeneration is present or if cure is the primary goal of treatment (evidence class IV, good practice point).71–73
Table 3.
The Koos grading system69
| Koos Grade | Tumor Description |
|---|---|
| I | Small intracanalicular tumor |
| II | Small tumor with protrusion into the cerebellopontine angle; no contact with the brainstem |
| III | Tumor occupying the cerebellopontine cistern with no brainstem displacement |
| IV | Large tumor with brainstem and cranial nerve displacement |
The choice of surgical approach depends on hearing status, tumor characteristics, patient’s preferences, and surgeon’s expertise. The experience of the surgical team is an important factor affecting the outcome, suggesting that VS should be treated in high volume centers (evidence class IV, good practice point).74–76 Surgery-related mortality is 0.5% in large series.77 The probability of hearing preservation in patients with normal hearing (Gardner–Robertson class A; see Table 4) was >50–75% immediately after surgery, as well as after 2 and 5 years, and >25–50% after 10 years.78,79 Factors influencing preservation of serviceable hearing after microsurgery are tumor size <1 cm, presence of a distal internal auditory canal CSF fluid fundal cap, as well as good preoperative hearing function.78 The risk of persisting facial palsy is between 3% and 46%.80,81 It depends on tumor size and the occurrence of an immediate paresis.80 To improve the rate of functional preservation, intraoperative monitoring is mandatory for surgery of VS and should include somatosensoric evoked potentials and monitoring of the facial nerve comprising direct electrical stimulation and free-running electromyography (evidence class III, recommendation level B). Facial motor evoked potentials are currently being evaluated.74,82 Intraoperative facial nerve monitoring leads to improved functional outcome and can be used to accurately predict favorable facial nerve function after surgery.74 Brainstem auditory evoked responses should also be used when hearing preservation is attempted (evidence class III, recommendation level B).78,83,84 In case of large lesions, electromyography of the lower cranial nerves is recommended (evidence class IV, good practice point).
Table 4.
Gardner–Robertson scale for hearing function79
| Grades | Pure Tone Audiogram (dB) | Speech Discrimination (%) |
|---|---|---|
| I | 0–30 | 70–100 |
| II | 31–50 | 50–69 |
| III | 51–90 | 5–49 |
| IV | 91–max | 1–4 |
| V | Not testable | 0 |
Goal of surgery should be total or near-total resection, since residual tumor volume correlates with rate of recurrence (evidence class III, recommendation level B). A series of 116 patients with VS who were treated by gross total resection (GTR), near-total resection (NTR), or subtotal resection (STR) yielded recurrence rates of 3.8%, 9.4%, and 27.6%, respectively.85 The mean time to recurrence was 22 months, ranging from 6 to 143 months. In a recent study of 103 sporadic VS patients who underwent NTR or STR, those with STR experienced recurrences over 13 times more often than those treated with NTR.86 In a retrospective study with 111 incomplete excisions (NTR and STR), the 7 patients who showed evidence of tumor regrowth had all undergone STR.87 Several further series also showed a considerably greater risk of regrowth with increasing residual tumor volumes.88–90 For large VS, the lower risk of recurrence after GTR should be weighed against the higher risk for facial nerve dysfunction and lower rates of hearing preservation, since there seems to be a relationship between tumor volume and functional outcome.91,92
For these cases, partial resection followed by SRS (see below) has become increasingly popular.93–96 With this combined approach, the results reported so far show superior outcome regarding facial nerve function and hearing preservation when compared with total resection, with comparable tumor control rates. However, the studies are still small and retrospective (evidence class IV, good practice point).93,96 After intentional NTR or STR, a watch and scan policy is warranted as only a minority of remnants do progress; however, the risk increases with the size of the remnant.96 In cases of recurrences after radiosurgery, both reoperation and radiosurgical retreatment are possible. However, the functional risk for the facial nerve upon surgery is higher after previous irradiation, and a very meticulous, conservative dissection technique might be necessary (evidence class IV, good practice point). In VS recurring after surgery, radiosurgery may be used preferentially because the risk of damage to the facial nerve is lower than with a second operation (evidence class III, recommendation level C).97–100
The following approaches are commonly used:
The suboccipital retrosigmoid (retromastoid) approach is favored by neurosurgeons and is particularly indicated for tumors located primarily in the cerebellopontine cistern or tumors with significant mass effect. It allows removal of tumors of various sizes and offers the possibility of hearing preservation. It provides excellent visualization of the brainstem, cranial nerves, and relevant vascular structures but requires some cerebellar retraction and allows only limited access to the fundus of the IAC.101,102 The procedure can be performed with the patient in either a (semi)sitting or a horizontal positioning. Although there are some small retrospective studies reporting on superior functional outcome associated with the semi-sitting position, current data do not support favoring a particular positioning technique (class IV, good practice point).103–105
The translabyrinthine approach, usually performed by ENT surgeons, can be used to remove tumors of all sizes. A labyrinthectomy will result in complete loss of function of the inner ear and is therefore not suitable for patients seeking hearing preservation. The approach provides access to the IAC after labyrinthectomy and exposure of the facial nerve within the Fallopian canal. The approach has the advantage of excellent tumor access without the need of occipital or temporal lobe retraction.106 It offers superior visualization of the entire facial nerve from brainstem to its entry into the labyrinthine portion of the Fallopian canal.107
The middle fossa approach may be considered for patients with small tumors who would like to preserve residual hearing. Suitable access requires temporal craniotomy above the zygoma and dissection of dura up to the arcuate eminence.
This approach requires careful patient selection. Tumor extending to the fundus and extending below the transverse crest is more difficult to remove than tumors where there is a CSF cap filling the lateral end of the IAC.108,109 Endoscope assisted resection has been reported as an aid for far lateral tumor dissection.110,111 Postoperative hearing outcome improves with smaller tumors, and optimal tumor size is less than 1 cm intracranial tumor diameter.112 This approach has the potential disadvantage of increased facial nerve manipulation because of the anterosuperior course of the facial nerve through the IAC, especially for those tumors that arise from the inferior vestibular nerve.113
Altogether, there are no sufficient data supporting the superiority of any approach in terms of radical tumor resection and nerve function preservation.114 Therefore, no recommendation can be made, and the approach should be chosen upon the experience of the treating center.
Radiosurgery and Radiotherapy
Stereotactic radiosurgery defines delivery of high dose irradiation with high conformity and precision in a single fraction and is commonly used for small to medium sized VS. SRS can be performed using a cobalt-60 based GammaKnife or linear accelerator techniques like CyberKnife using doses from 11 to 14 Gy.81,115–117 The definition of a hypofractionated schedule of up to 5 fractions as “radiosurgery” remains controversial in Europe. In case of larger tumors, fractionation is mandatory. For these cases, fractionated radiotherapy or hypofractionated stereotactic radiotherapy (SRT) using up to 10 fractions is increasingly used. There are no data providing an outcome-based comparison of GammaKnife or linear accelerator techniques.52
Five prospective studies without randomization (evidence class II) have revealed that SRS is superior to microsurgery for patients with VS <3 cm in terms of preserving facial nerve and hearing function.81,116,118–120 As upper limit for radiosurgery, a mass effect on the brainstem (Koos IV) is considered, but there is no clear definition by diameter or volume alone (evidence class IV, good practice point).
Several retrospective cohort studies evaluated SRS using GammaKnife with at least 100 patients, 2 years follow-up, and objective audiometric assessment. One linear accelerator series comprising over 100 patients with 2 years follow-up did not report functional hearing outcome.115 Pioneer series of SRS included patients treated with very high dose regimens. Contemporary SRS series using GammaKnife with tumor marginal doses between 12 and 14 Gy revealed 5-year tumor control rates of 90–99%, hearing preservation rates of 41–79%, facial nerve preservation rates of 95–100%, and trigeminal preservation rates of 79–99%. Numerous authors have found that the key predictor for functional hearing preservation is the quality of hearing at time of radiosurgery.121–126 A recent review revealed a relevant decline in hearing after SRS, even in patients with normal hearing function (Gardner–Robertson grade 1).78 The probability to preserve hearing was >75–100% after 2 years, >50–75% after 5 years, and >25–50% after 10 years. After 5 and 10 years, the rates of hearing preservation were similar to patients having microsurgery.78 However, it has to be considered that the latter data are based on selected surgical cases with a special attempt to preserve hearing.
The maximum dose at the modiolus of the cochlea has been reported to be a negative predictor for functional hearing preservation with a threshold around 4 Gy.122,123,127–130 However, these series comprise small retrospective cohorts of patients. Cochlear dose is likely to be one of many variables associated with hearing preservation. The recommendation is to use SRS with a dose of 11–14 Gy at the margin and 11–12 Gy when the risk of hearing loss is a critical issue (evidence class III, recommendation level C).52
There are no randomized, prospective studies comparing SRS and SRT. Six nonrandomized studies found that functional hearing preservation is similar but the rate of facial palsy and trigeminal nerve dysfunction seems higher with SRT than SRS.131–138
There are little data about the incidence of malignant VS after radiation of spontaneous, non-NF2 VS. The spontaneous risk of malignancy was addressed in a large retrospective study using the SEER database. The incidence of MPNSTs of the eighth cranial nerve with no history of prior radiation was 0.017 per 1 million persons/year. Compared with the incidence of benign VS, 1041 VS were present for every 1 MPNST arising from the eighth cranial nerve. There is no evidence that spontaneous MPNST is a feature of NF2 as opposed to NF1, nonetheless around half of MPNSTs reported after radiation treatment are in NF2 patients.139 This baseline rate of malignancy should be considered when estimating the risk of malignant transformation following SRS for VS.140 In a retrospective single center review, Pollock et al did not find any radiation-induced tumors in 11 264 patient-years of follow-up after SRS.141 In a review by Maducdoc et al, only 8 cases with malignant transformation after surgery or SRS were found; 4 of these patients had surgery only.142
Numerous studies have reported about transient enlargement of VS occurring within 3 years after radiosurgery.143–147 This MR change observed in up to 30% of the patients is related to the therapeutic effect of SRS and is termed “pseudoprogression.” It is not a predictor of failure.143–147 We recommend clinical and radiological observation within this time frame and performing annual MRI scans (expert opinion, good practice point).
Systemic Treatment Options
Local therapies are the mainstay for the treatment of VS. There is no level I evidence for any systemic treatment, and even level II evidence for treatment with the anti–vascular endothelial growth factor antibody bevacizumab is disputable and only valid for schwannomas arising in patients with NF2. In the sequence of therapies, a safe surgical resection and radiotherapy are considered as superior. Thus, systemic medical treatment options have been generally used in locally pretreated patients, potentially limiting efficacy and more importantly interfering with the ability to assess efficacy.
Bevacizumab has been successfully used for patients with progressive VS associated with NF2.148 Patients experienced an improvement of hearing and objective (>20% reduced tumor volume) radiographic responses.149 In NF2 patients with progressive VS, a prospective, multi-institutional, uncontrolled phase II study with 14 patients using 7.5 mg/kg bevacizumab administered every 3 weeks revealed a hearing improvement in 36% of patients, and there was no patient with a hearing decline in the trial period of 12 months.150 Volumetric assessments demonstrated a partial radiographic response of volume reduction of 20% or more in 43% (6/14 patients), making bevacizumab a potential treatment option for NF2 patients (evidence class II, recommendation level B).
Other pathways have been addressed based on preclinical or immunohistochemical target expression.151 Again, patients studied had VS in the context of NF2. Neither epidermal growth factor receptor (EGFR) pathway inhibition using erlotinib nor ErbB2 (and EGFR) pathway blockade applying lapatinib has been associated with relevant radiographic responses or impact on the hearing function.152,153 The mechanistic target of rapamycin (mTOR) signaling cascade has also been proposed for the treatment of NF2-related tumors, since the mTOR pathway is considered a key driver of tumor growth in merlin (NF2)-deficient tumors. Similarly, a small (n = 10 patients), single-institution trial study of the mTOR complex 1 inhibitor everolimus was not associated with tumor shrinkage or hearing improvement.154
Supportive Care
There are almost no data about the value of supportive care in VS patients. Therefore, only expert opinion–based recommendations are possible (good practice point).
Care should focus on clinical symptoms and treatment complications. Trigeminal and facial neuropathies, as well as brainstem compression or hydrocephalus, could be symptoms of large VS as well as treatment complications. Patients with facial nerve palsy can have various types of ocular complications like lagophthalmus, which can lead to exposure keratopathy, corneal breakdown, ulcers, and even perforation. Patient management should be based on the severity of the ocular findings and ranges from symptomatic treatment like moistening eye drops to surgical reanimation of the facial nerve via hypoglossal facial nerve anastomosis and symptomatic plastic measure for the eye or face.155–158 Patients with facial nerve palsy who present at earlier stages can benefit from conservative treatment. Therapy with corticosteroids is frequently administered in this case; however, evidence for this treatment is lacking. Decreased hearing can be supported by various hearing aid. We refer the reader to specific reviews about multidisciplinary treatment and rehabilitation of facial palsy and lower cranial nerve deficits as possible complications of surgical therapy.159–161
Quality of Life
Tumor size seems to be a predictor for quality of life (QoL) in VS patients.65,162,163 Several retrospective studies addressed the question of which treatment modality provides the best QoL but led to inconsistent results. Observation, resection, radiosurgery, and radiotherapy were compared using different study designs.65,116,162,164–169 Although there are significant discrepancies between these studies, it might be concluded that QoL in patients with VS cannot be predicted based on management strategy alone. As expected, poor QoL is more likely in patients with large, symptomatic tumors that were resected.65,162,163
Monitoring and Follow-Up
Follow-up after treatment as well as with conservative management requires a program of MRI scanning, audiometry, and outpatient consultation. There are plenty of retrospective data describing tumor growth in different situations over time (see above); however, prospective data focusing on control intervals are rare. We support annual MRI follow-up for 5 years in patients with untreated tumors; thereafter, the follow-up intervals can be prolonged.33 There are also recommendations for a size-dependent follow-up with a recommendation of 6-month intervals in large tumors.169 In a prospective collection of 196 patients, no onset of growth was found in intracanalicular tumors which were stable for 5 years after diagnosis.57 In another large prospectively followed cohort of extra- and intracanalicular tumors, 7.2 % exhibited growth after a stable period of 5 years following diagnosis.170 Thus, even if the likelihood of growth declines with time, imaging albeit at larger intervals is recommended for untreated tumors.
Based on these data, we recommend annual follow-up intervals with microbeam radiation therapy and audiometry in patients with conservatively treated, radiated, and incompletely resected VS for 5 years. In case of stable tumor size, the intervals can be doubled thereafter (good practice point). In patients with GTR, MRI controls postoperatively and after 2, 5, and 10 years are sufficient (expert opinion, good practice point).
Specific Recommendations
VS in General
Many tumors are managed in single departments or institutions offering one particular treatment. Since there are several therapeutic options possible, especially in medium sized tumors, we recommend discussion of patients with VS in multidisciplinary tumor boards. Besides treatment decisions, follow-up including pseudoprogression can be evaluated by specialists from all disciplines involved (expert opinion, good practice point).
If surgery is indicated, surgical treatment at a high-volume center is recommended, since surgical experience affects outcome (evidence class IV, good practice point).
Sporadic Unilateral VS
Small asymptomatic tumor (Koos grades I–II)
Management of sporadic, non-NF2 related unilateral VS should depend on symptoms and signs and the size of the tumor. In small, asymptomatic tumor with regular cranial nerve function, observation is the management of choice. The data available provide evidence level III and recommendation level C.
As an alternative to observation, SRS can be performed to stop tumor growth and preserve long-term nerve function. However, there still is a small risk of deterioration of nerve function or QoL. There is evidence level II and recommendation level B for SRS in asymptomatic patients.
If long-term preservation of nerve function is the primary aim of management, even surgery can be chosen; however, the risk of any functional deterioration is considerable, ranging up to 50% (evidence class III). Therefore, we recommend not to perform surgery in these patients (recommendation level C).
Small tumor with impaired hearing (Koos grades I–II)
If patients with small tumors become symptomatic with vestibular and/or auditory symptoms, therapy should be discussed to avoid further deterioration. In these cases, SRS offers a better rate of hearing preservation and a lower risk for facial paresis than surgery (recommendation level C).
Small tumor with complete hearing loss (Koos grades I–II)
In these patients, the aim of therapy can be cure or tumor control while preserving facial nerve function. All options can be justified in these cases. Observation is usually the first option, since there is no function endangered for a long period of time (evidence class III, recommendation level C). SRS or surgery carries a low risk of facial nerve damage and may provide long-term control or cure, respectively. Besides facial nerve function, SRS carries a lower risk profile than surgery, making SRS the first option if tumor control is regarded sufficient by the patient (evidence class II, recommendation level B).
Medium sized tumors (Koos grades III–IV, <3 cm).
—Most patients with medium sized tumors present with vestibular or cochlear symptoms. Facial paresis is rare and might even be a hint for a facial schwannoma. Due to the symptomatic burden and considerable tumor volume, therapy should be performed. Surgery or radiosurgery can be recommended at a very similar level (recommendation level C). The risk profile of SRS is lower than that of surgery, however, surgery can offer complete removal of the tumor. Altogether, this situation should be meticulously discussed with the patients and all options explained. Also subtotal resection to preserve function might be an option, if subsequent SRS of a growing tumor rest can be provided (good practice point).
Large tumor with brainstem compression (Koos grade IV, >3 cm)
These patients typically suffer from eighth cranial nerve symptoms for a long time. A considerable number of these patients present with additional symptoms like facial nerve paresis and gait ataxia. Primary goal of therapy is decompression of the brainstem and stretched cranial nerves, which makes surgery the only option. Since there are no prospective studies for this clinical situation, recommendation level is good practice point. Surgery in large tumors is accompanied by a considerable risk for loss or deterioration of cranial nerve function. For this reason, tumor mass reduction by incomplete resection, followed by SRS or observation, is a valid option (evidence class IV, good practice point).
NF2 with Uni- or Bilateral VS
Patients with NF2 suffer from bilateral VS or unilateral VS and other intracranial and/or spinal tumors (see Table 1). The high incidence of newly developing tumors, the fast tumor growth and early tumor regrowth, as well as the lack of a cure make patient management challenging.171 Generally, observation and follow-up should use shorter intervals compared with spontaneous VS, particularly in younger patients. Follow-up intervals of 6–12 months are recommended (evidence level IV, good practice point). In most patients, several consecutive therapies have to be performed to preserve cranial nerve and brainstem function. Bilateral VS may lead to intense brainstem compression, making surgical decompression mandatory. If incomplete resection is performed to preserve seventh and/or eighth cranial nerve function, residual tumors may be treated by SRS; in small tumors, observation may be appropriate. In inevitable recurrent situations, therapy depends on the clinical condition of the patient, cranial nerve symptoms, tumor size, and therapies performed earlier. Re-surgery and re-SRS commonly need to be performed depending on the respective risk profile (good practice point). Prospective data show positive effects of bevacizumab on hearing and tumor growth (evidence class II), making bevacizumab a good treatment option (recommendation level B).172–174 Due to impaired hearing function, auditory rehabilitation is of utmost importance for these patients. There are no prospective data on general management, surgery, or SRS, especially for NF2 patients, allowing only recommendation as good practice points.
Key recommendations are summarized in Table 5.
Table 5.
Key recommendations
| Clinical Situation | Recommendation | Evidence Class | Recomm. Level |
|---|---|---|---|
| Spontaneous VS, small asymptomatic | Observation | III | C |
| OR | |||
| SRS | II | B | |
| Spontaneous VS, small, complete hearing loss | Observation | III | C |
| OR | |||
| SRS | II | B | |
| superior to | |||
| Surgery | III | C | |
| Spontaneous VS, large with brainstem compression | Surgery | IV | Good practice point (GPP) |
| inferior to | |||
| Combination surgery + SRS | IV | GPP | |
| NF 2 | Surgery | IV | GPP |
| and/or | |||
| SRS | IV | GPP | |
| and/or | |||
| Bevacizumab | II | B | |
| and/or | |||
| SRT | IV | GPP | |
| dependent on situation |
Research Outlook
This guideline presents the recommendations derived from the current state of the literature. To generate progress in the area of VS management, we recommend to focus on the following issues:
The value of surgery versus SRS is difficult to evaluate in a prospective manner. Randomization is conceivable only in medium and small sized tumors and we suspect that patients will be reluctant to undergo randomization. An improved multidisciplinary communication in tumor boards might help to solve this problem.
Since considerable late effects on hearing have been encountered, more information about the long time effects of SRS is needed by extending clinical and radiological follow-up in VS patients more than 10 years.
According to other putative benign intracranial tumors like meningiomas, molecular profiling of VS should be intended on a broad scientific basis to (i) learn more about the reasons for clinically different growth behavior within a distinct WHO grade I tumor entity and (ii) define targets for a tailored pharmacotherapy.
Funding
None.
Conflict of interest statement.
None.
Authorship statement.
Each author contributed to the text according to his/her specialty and each author reviewed and approved the final version of the manuscript.
References
- 1. Ostrom QT, Gittleman H, Liao P, et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro-Oncol. 2017;19(suppl_5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Babu R, Sharma R, Bagley JH, Hatef J, Friedman AH, Adamson C. Vestibular schwannomas in the modern era: epidemiology, treatment trends, and disparities in management. J Neurosurg. 2013;119(1):121–130. [DOI] [PubMed] [Google Scholar]
- 3. Jeyakumar A, Seth R, Brickman TM, Dutcher P. The prevalence and clinical course of patients with ‘incidental’ acoustic neuromas. Acta Otolaryngol. 2007;127(10):1051–1057. [DOI] [PubMed] [Google Scholar]
- 4. Andersen JF, Nilsen KS, Vassbotn FS, et al. . Predictors of vertigo in patients with untreated vestibular schwannoma. Otol Neurotol. 2015;36(4):647–652. [DOI] [PubMed] [Google Scholar]
- 5. Kshettry VR, Hsieh JK, Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Incidence of vestibular schwannomas in the United States. J Neurooncol. 2015;124(2):223–228. [DOI] [PubMed] [Google Scholar]
- 6. Carlson ML, Marston AP, Glasgow AE, et al. . Racial differences in vestibular schwannoma. Laryngoscope. 2016;126(9):2128–2133. [DOI] [PubMed] [Google Scholar]
- 7. Larjavaara S, Feychting M, Sankila R, et al. . Incidence trends of vestibular schwannomas in Denmark, Finland, Norway and Sweden in 1987–2007. Br J Cancer. 2011;105(7):1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stangerup SE, Caye-Thomasen P. Epidemiology and natural history of vestibular schwannomas. Otolaryngol Clin North Am. 2012;45(2):257–268, vii. [DOI] [PubMed] [Google Scholar]
- 9. Schoemaker MJ, Swerdlow AJ, Auvinen A, et al. . Medical history, cigarette smoking and risk of acoustic neuroma: an international case-control study. Int J Cancer. 2007;120(1):103–110. [DOI] [PubMed] [Google Scholar]
- 10. Agnihotri S, Jalali S, Wilson MR, et al. . The genomic landscape of schwannoma. Nat Genet. 2016;48(11):1339–1348. [DOI] [PubMed] [Google Scholar]
- 11. Sainz J, Huynh DP, Figueroa K, Ragge NK, Baser ME, Pulst SM. Mutations of the neurofibromatosis type 2 gene and lack of the gene product in vestibular schwannomas. Hum Mol Genet. 1994;3(6):885–891. [DOI] [PubMed] [Google Scholar]
- 12. Håvik AL, Bruland O, Myrseth E, et al. . Genetic landscape of sporadic vestibular schwannoma. J Neurosurg. 2018;128(3):911–922. [DOI] [PubMed] [Google Scholar]
- 13. Wang L, Zehir A, Sadowska J, et al. . Consistent copy number changes and recurrent PRKAR1A mutations distinguish Melanotic Schwannomas from Melanomas: SNP-array and next generation sequencing analysis. Genes Chromosomes Cancer. 2015;54(8):463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koelsche C, Hovestadt V, Jones DT, et al. . Melanotic tumors of the nervous system are characterized by distinct mutational, chromosomal and epigenomic profiles. Brain Pathol. 2015;25(2):202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Röhrich M, Koelsche C, Schrimpf D, et al. . Methylation-based classification of benign and malignant peripheral nerve sheath tumors. Acta Neuropathol. 2016;131(6):877–887. [DOI] [PubMed] [Google Scholar]
- 16. Evans DG, Moran A, King A, Saeed S, Gurusinghe N, Ramsden R. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. Otol Neurotol. 2005;26(1):93–97. [DOI] [PubMed] [Google Scholar]
- 17. Evans DG, Huson SM, Donnai D, et al. . A clinical study of type 2 neurofibromatosis. Q J Med. 1992;84(304):603–618. [PubMed] [Google Scholar]
- 18. Evans DG. Neurofibromatosis type 2. Handb Clin Neurol. 2015;132:87–96. [DOI] [PubMed] [Google Scholar]
- 19. Evans DG, Howard E, Giblin C, et al. . Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A. 2010;152A(2):327–332. [DOI] [PubMed] [Google Scholar]
- 20. Smith MJ, Bowers NL, Bulman M, et al. . Revisiting neurofibromatosis type 2 diagnostic criteria to exclude LZTR1-related schwannomatosis. Neurology. 2017;88(1):87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pathmanaban ON, Sadler KV, Kamaly-Asl ID, et al. . Association of genetic predisposition with solitary schwannoma or meningioma in children and young adults. JAMA Neurol. 2017;74(9):1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Evans DG, Lye R, Neary W, et al. . Probability of bilateral disease in people presenting with a unilateral vestibular schwannoma. J Neurol Neurosurg Psychiatry. 1999;66(6):764–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Evans DG, Ramsden RT, Shenton A, et al. . What are the implications in individuals with unilateral vestibular schwannoma and other neurogenic tumors? J Neurosurg. 2008;108(1):92–96. [DOI] [PubMed] [Google Scholar]
- 24. Evans DG, Ramsden RT, Gokhale C, Bowers N, Huson SM, Wallace A. Should NF2 mutation screening be undertaken in patients with an apparently isolated vestibular schwannoma? Clin Genet. 2007;71(4):354–358. [DOI] [PubMed] [Google Scholar]
- 25. Evans DG, Ramsden RT, Shenton A, et al. . Mosaicism in neurofibromatosis type 2: an update of risk based on uni/bilaterality of vestibular schwannoma at presentation and sensitive mutation analysis including multiple ligation-dependent probe amplification. J Med Genet. 2007;44(7):424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dewan R, Pemov A, Kim HJ, et al. . Evidence of polyclonality in neurofibromatosis type 2-associated multilobulated vestibular schwannomas. Neuro Oncol. 2015;17(4):566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stivaros SM, Stemmer-Rachamimov AO, Alston R, et al. . Multiple synchronous sites of origin of vestibular schwannomas in neurofibromatosis Type 2. J Med Genet. 2015;52(8):557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Evans DGR, Salvador H, Chang VY, et al. . Cancer and central nervous system tumor surveillance in pediatric neurofibromatosis 2 and related disorders. Clin Cancer Res. 2017;23(12):e54–e61. [DOI] [PubMed] [Google Scholar]
- 29. Evans DG, Baser ME, O’Reilly B, et al. . Management of the patient and family with neurofibromatosis 2: a consensus conference statement. Br J Neurosurg. 2005;19(1):5–12. [DOI] [PubMed] [Google Scholar]
- 30. Hexter A, Jones A, Joe H, et al. ; English Specialist NF2 Research Group Clinical and molecular predictors of mortality in neurofibromatosis 2: a UK national analysis of 1192 patients. J Med Genet. 2015;52(10):699–705. [DOI] [PubMed] [Google Scholar]
- 31. Evans DG, Raymond FL, Barwell JG, Halliday D. Genetic testing and screening of individuals at risk of NF2. Clin Genet. 2012;82(5):416–424. [DOI] [PubMed] [Google Scholar]
- 32. Hentschel M, Scholte M, Steens S, Kunst H, Rovers M. The diagnostic accuracy of non-imaging screening protocols for vestibular schwannoma in patients with asymmetrical hearing loss and/or unilateral audiovestibular dysfunction: a diagnostic review and meta-analysis. Clin Otolaryngol. 2017;42(4):815–823. [DOI] [PubMed] [Google Scholar]
- 33. Dunn IF, Bi WL, Mukundan S, et al. . Congress of neurological surgeons systematic review and evidence-based guidelines on the role of imaging in the diagnosis and management of patients with vestibular schwannomas. Neurosurgery. 2018;82(2):E32–E34. [DOI] [PubMed] [Google Scholar]
- 34. De Foer B, Kenis C, Van Melkebeke D, et al. . Pathology of the vestibulocochlear nerve. Eur J Radiol. 2010;74(2):349–358. [DOI] [PubMed] [Google Scholar]
- 35. Stuckey SL, Harris AJ, Mannolini SM. Detection of acoustic schwannoma: use of constructive interference in the steady state three-dimensional MR. AJNR Am J Neuroradiol. 1996;17(7):1219–1225. [PMC free article] [PubMed] [Google Scholar]
- 36. Borges A, Casselman J. Imaging the cranial nerves: Part I: methodology, infectious and inflammatory, traumatic and congenital lesions. Eur Radiol. 2007;17(8):2112–2125. [DOI] [PubMed] [Google Scholar]
- 37. Curtin HD. Rule out eighth nerve tumor: contrast-enhanced T1-weighted or high-resolution T2-weighted MR? AJNR Am J Neuroradiol. 1997;18(10):1834–1838. [PMC free article] [PubMed] [Google Scholar]
- 38. Schmalbrock P, Chakeres DW, Monroe JW, Saraswat A, Miles BA, Welling DB. Assessment of internal auditory canal tumors: a comparison of contrast-enhanced T1-weighted and steady-state T2-weighted gradient-echo MR imaging. AJNR Am J Neuroradiol. 1999;20(7):1207–1213. [PMC free article] [PubMed] [Google Scholar]
- 39. Xenellis JE, Linthicum FH Jr. On the myth of the glial/schwann junction (Obersteiner-Redlich zone): origin of vestibular nerve schwannomas. Otol Neurotol. 2003;24(1):1. [DOI] [PubMed] [Google Scholar]
- 40. Roosli C, Linthicum FH Jr, Cureoglu S, Merchant SN. What is the site of origin of cochleovestibular schwannomas? Audiol Neurootol. 2012;17(2):121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Louis DN, Perry A, Reifenberger G, et al. . The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 42. Woodruff JM, Godwin TA, Erlandson RA, Susin M, Martini N. Cellular schwannoma: a variety of schwannoma sometimes mistaken for a malignant tumor. Am J Surg Pathol. 1981;5(8):733–744. [PubMed] [Google Scholar]
- 43. White W, Shiu MH, Rosenblum MK, Erlandson RA, Woodruff JM. Cellular schwannoma. A clinicopathologic study of 57 patients and 58 tumors. Cancer. 1990;66(6):1266–1275. [DOI] [PubMed] [Google Scholar]
- 44. Pekmezci M, Reuss DE, Hirbe AC, et al. . Morphologic and immunohistochemical features of malignant peripheral nerve sheath tumors and cellular schwannomas. Mod Pathol. 2015;28(2):187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miller RT, Sarikaya H, Sos A. Melanotic schwannoma of the acoustic nerve. Arch Pathol Lab Med. 1986;110(2):153–154. [PubMed] [Google Scholar]
- 46. Saint-Blancard P, Goasguen O, Kossowski M, Dulou R. [A rare primary tumor of the cerebellopontine angle: melanotic schwannoma, a pigmented tumor with unpredictable prognosis]. Rev Med Interne. 2008;29(7):587–590. [DOI] [PubMed] [Google Scholar]
- 47. Torres-Mora J, Dry S, Li X, Binder S, Amin M, Folpe AL. Malignant melanotic schwannian tumor: a clinicopathologic, immunohistochemical, and gene expression profiling study of 40 cases, with a proposal for the reclassification of “melanotic schwannoma”. Am J Surg Pathol. 2014;38(1):94–105. [DOI] [PubMed] [Google Scholar]
- 48. Carney JA. Psammomatous melanotic schwannoma. A distinctive, heritable tumor with special associations, including cardiac myxoma and the Cushing syndrome. Am J Surg Pathol. 1990;14(3):206–222. [PubMed] [Google Scholar]
- 49. Küsters-Vandevelde HV, van E ngen-van G runsven IA, Küsters B, et al. . Improved discrimination of melanotic schwannoma from melanocytic lesions by combined morphological and GNAQ mutational analysis. Acta Neuropathol. 2010;120(6):755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Küsters-Vandevelde HV, Klaasen A, Küsters B, et al. . Activating mutations of the GNAQ gene: a frequent event in primary melanocytic neoplasms of the central nervous system. Acta Neuropathol. 2010;119(3):317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Capper D, Jones DTW, Sill M, et al. . DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Germano IM, Sheehan J, Parish J, et al. . Congress of neurological surgeons systematic review and evidence-based guidelines on the role of radiosurgery and radiation therapy in the management of patients with vestibular schwannomas. Neurosurgery. 2018;82(2):E49–E51. [DOI] [PubMed] [Google Scholar]
- 53. Hillman TA, Chen DA, Quigley M, Arriaga MA. Acoustic tumor observation and failure to follow-up. Otolaryngol Head Neck Surg. 2010;142(3):400–404. [DOI] [PubMed] [Google Scholar]
- 54. Paldor I, Chen AS, Kaye AH. Growth rate of vestibular schwannoma. J Clin Neurosci. 2016;32:1–8. [DOI] [PubMed] [Google Scholar]
- 55. Varughese JK, Wentzel-Larsen T, Vassbotn F, Moen G, Lund-Johansen M. Analysis of vestibular schwannoma size in multiple dimensions: a comparative cohort study of different measurement techniques. Clin Otolaryngol. 2010;35(2):97–103. [DOI] [PubMed] [Google Scholar]
- 56. Varughese JK, Breivik CN, Wentzel-Larsen T, Lund-Johansen M. Growth of untreated vestibular schwannoma: a prospective study. J Neurosurg. 2012;116(4):706–712. [DOI] [PubMed] [Google Scholar]
- 57. Caye-Thomasen P, Hansen S, Dethloff T, Stangerup SE, Thomsen J. Sublocalization and volumetric growth pattern of intracanalicular vestibular schwannomas. Laryngoscope. 2006;116(7):1131–1135. [DOI] [PubMed] [Google Scholar]
- 58. Hunter JB, Francis DO, O’Connell BP, et al. . Single institutional experience with observing 564 vestibular schwannomas: factors associated with tumor growth. Otol Neurotol. 2016;37(10):1630–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sughrue ME, Yang I, Aranda D, et al. . The natural history of untreated sporadic vestibular schwannomas: a comprehensive review of hearing outcomes. J Neurosurg. 2010;112(1):163–167. [DOI] [PubMed] [Google Scholar]
- 60. Tveiten OV, Carlson ML, Goplen F, Vassbotn F, Link MJ, Lund-Johansen M. Long-term auditory symptoms in patients with sporadic vestibular schwannoma: an international cross-sectional study. Neurosurgery. 2015;77(2):218–227; discussion 227. [DOI] [PubMed] [Google Scholar]
- 61. Stangerup SE, Tos M, Thomsen J, Caye-Thomasen P. Hearing outcomes of vestibular schwannoma patients managed with ‘wait and scan’: predictive value of hearing level at diagnosis. J Laryngol Otol. 2010;124(5):490–494. [DOI] [PubMed] [Google Scholar]
- 62. Regis J, Carron R, Park MC, et al. . Wait-and-see strategy compared with proactive Gamma Knife surgery in patients with intracanalicular vestibular schwannomas: clinical article. J Neurosurg 2013;119(Suppl):105–111. [DOI] [PubMed] [Google Scholar]
- 63. Breivik CN, Nilsen RM, Myrseth E, et al. . Conservative management or Gamma Knife radiosurgery for vestibular schwannoma: tumor growth, symptoms, and quality of life. Neurosurgery. 2013;73(1):48–56; discussion 56–57. [DOI] [PubMed] [Google Scholar]
- 64. Yomo S, Arkha Y, Delsanti C, Roche PH, Thomassin JM, Regis J. Repeat Gamma Knife surgery for regrowth of vestibular schwannomas. Neurosurgery. 2009;64(1):48–54; discussion 54–55. [DOI] [PubMed] [Google Scholar]
- 65. Carlson ML, Tveiten OV, Driscoll CL, et al. . Long-term quality of life in patients with vestibular schwannoma: an international multicenter cross-sectional study comparing microsurgery, stereotactic radiosurgery, observation, and nontumor controls. J Neurosurg 2015;122(4):833–842. [DOI] [PubMed] [Google Scholar]
- 66. Robinett ZN, Walz PC, Miles-Markley B, Moberly AC, Welling DB. Comparison of long-term quality-of-life outcomes in vestibular schwannoma patients. Otolaryngol Head Neck Surg. 2014;150(6):1024–1032. [DOI] [PubMed] [Google Scholar]
- 67. Rutherford SA, King AT. Vestibular schwannoma management: what is the ‘best’ option? Br J Neurosurg. 2005;19(4):309–316. [DOI] [PubMed] [Google Scholar]
- 68. Matthies C, Samii M. Management of 1000 vestibular schwannomas (acoustic neuromas): clinical presentation. Neurosurgery. 1997;40(1):1–9; discussion 9–10. [DOI] [PubMed] [Google Scholar]
- 69. Koos WT, Day JD, Matula C, Levy DI. Neurotopographic considerations in the microsurgical treatment of small acoustic neurinomas. J Neurosurg 1998;88(3):506–512. [DOI] [PubMed] [Google Scholar]
- 70. Hitselberger WE, House WF. Classification of acoustic neuromas. Arch Otolaryngol (Chicago, Ill.: 1960). 1966;84(3):245–246. [PubMed] [Google Scholar]
- 71. Link MJ, Driscoll CL, Foote RL, Pollock BE. Radiation therapy and radiosurgery for vestibular schwannomas: indications, techniques, and results. Otolaryngol Clin North Am 2012;45(2):353–366, viii-ix. [DOI] [PubMed] [Google Scholar]
- 72. Charabi S, Mantoni M, Tos M, Thomsen J. Cystic vestibular schwannomas: neuroimaging and growth rate. J Laryngol Otol. 1994;108(5):375–379. [DOI] [PubMed] [Google Scholar]
- 73. Nutik SL, Babb MJ. Determinants of tumor size and growth in vestibular schwannomas. J Neurosurg 2001;94(6):922–926. [DOI] [PubMed] [Google Scholar]
- 74. Vivas EX, Carlson ML, Neff BA, et al. . Congress of neurological surgeons systematic review and evidence-based guidelines on intraoperative cranial nerve monitoring in vestibular schwannoma surgery. Neurosurgery. 2018;82(2):E44–E46. [DOI] [PubMed] [Google Scholar]
- 75. Barker FG 2nd, Carter BS, Ojemann RG, Jyung RW, Poe DS, McKenna MJ. Surgical excision of acoustic neuroma: patient outcome and provider caseload. Laryngoscope. 2003;113(8):1332–1343. [DOI] [PubMed] [Google Scholar]
- 76. Mangham CA., Jr Retrosigmoid versus middle fossa surgery for small vestibular schwannomas. Laryngoscope. 2004;114(8):1455–1461. [DOI] [PubMed] [Google Scholar]
- 77. McClelland S 3rd, Kim E, Murphy JD, Jaboin JJ. Operative mortality rates of acoustic neuroma surgery: a national cancer database analysis. Otol Neurotol. 2017;38(5):751–753. [DOI] [PubMed] [Google Scholar]
- 78. Carlson ML, Vivas EX, McCracken DJ, et al. . Congress of neurological surgeons systematic review and evidence-based guidelines on hearing preservation outcomes in patients with sporadic vestibular schwannomas. Neurosurgery. 2018;82(2):E35–E39. [DOI] [PubMed] [Google Scholar]
- 79. Gardner G, Robertson JH. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1988;97(1):55–66. [DOI] [PubMed] [Google Scholar]
- 80. Morton RP, Ackerman PD, Pisansky MT, et al. . Prognostic factors for the incidence and recovery of delayed facial nerve palsy after vestibular schwannoma resection. J Neurosurg 2011;114(2):375–380. [DOI] [PubMed] [Google Scholar]
- 81. Myrseth E, Moller P, Pedersen PH, Lund-Johansen M. Vestibular schwannoma: surgery or Gamma Knife radiosurgery? A prospective, nonrandomized study. Neurosurgery. 2009;64(4):654–661; discussion 661–663. [DOI] [PubMed] [Google Scholar]
- 82. Acioly MA, Liebsch M, de Aguiar PH, Tatagiba M. Facial nerve monitoring during cerebellopontine angle and skull base tumor surgery: a systematic review from description to current success on function prediction. World Neurosurg. 2013;80(6):e271–e300. [DOI] [PubMed] [Google Scholar]
- 83. Piccirillo E, Hiraumi H, Hamada M, Russo A, De Stefano A, Sanna M. Intraoperative cochlear nerve monitoring in vestibular schwannoma surgery—does it really affect hearing outcome? Audiology & Neuro-Otol. 2008;13(1):58–64. [DOI] [PubMed] [Google Scholar]
- 84. Tonn JC, Schlake HP, Goldbrunner R, Milewski C, Helms J, Roosen K. Acoustic neuroma surgery as an interdisciplinary approach: a neurosurgical series of 508 patients. J Neurol Neurosurg Psychiatry. 2000;69(2):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Seol HJ, Kim CH, Park CK, et al. . Optimal extent of resection in vestibular schwannoma surgery: relationship to recurrence and facial nerve preservation. Neurol Med Chir. 2006;46(4):176–180; discussion 180–181. [DOI] [PubMed] [Google Scholar]
- 86. Jacob JT, Carlson ML, Driscoll CL, Link MJ. Volumetric analysis of tumor control following subtotal and near-total resection of vestibular schwannoma. Laryngoscope. 2016;126(8):1877–1882. [DOI] [PubMed] [Google Scholar]
- 87. Chen Z, Prasad SC, Di L ella F, et al. . The behavior of residual tumors and facial nerve outcomes after incomplete excision of vestibular schwannomas. J Neurosurg. 2014;120(6):1278–1287. [DOI] [PubMed] [Google Scholar]
- 88. Bloch DC, Oghalai JS, Jackler RK, Osofsky M, Pitts LH. The fate of the tumor remnant after less-than-complete acoustic neuroma resection. Otolaryngol Head Neck Surg. 2004;130(1):104–112. [DOI] [PubMed] [Google Scholar]
- 89. Park CK, Jung HW, Kim JE, Son YJ, Paek SH, Kim DG. Therapeutic strategy for large vestibular schwannomas. J Neurooncol. 2006;77(2):167–171. [DOI] [PubMed] [Google Scholar]
- 90. van de Langenberg R, Hanssens PE, van Overbeeke JJ, et al. . Management of large vestibular schwannoma. Part I. Planned subtotal resection followed by Gamma Knife surgery: radiological and clinical aspects. J Neurosurg 2011;115(5):875–884. [DOI] [PubMed] [Google Scholar]
- 91. Gurgel RK, Dogru S, Amdur RL, Monfared A. Facial nerve outcomes after surgery for large vestibular schwannomas: do surgical approach and extent of resection matter? Neurosurg Focus. 2012;33(3):E16. [DOI] [PubMed] [Google Scholar]
- 92. Samii M, Gerganov VM, Samii A. Functional outcome after complete surgical removal of giant vestibular schwannomas. J Neurosurg 2010;112(4):860–867. [DOI] [PubMed] [Google Scholar]
- 93. Iwai Y, Ishibashi K, Watanabe Y, Uemura G, Yamanaka K. Functional preservation after planned partial resection followed by Gamma Knife radiosurgery for large vestibular schwannomas. World Neurosurg. 2015;84(2):292–300. [DOI] [PubMed] [Google Scholar]
- 94. Anaizi AN, Gantwerker EA, Pensak ML, Theodosopoulos PV. Facial nerve preservation surgery for Koos grade 3 and 4 vestibular schwannomas. Neurosurgery. 2014;75(6):671–675; discussion 676–677; quiz 677. [DOI] [PubMed] [Google Scholar]
- 95. Brokinkel B, Sauerland C, Holling M, et al. . Gamma Knife radiosurgery following subtotal resection of vestibular schwannoma. J Clin Neurosci 2014;21(12):2077–2082. [DOI] [PubMed] [Google Scholar]
- 96. Daniel RT, Tuleasca C, George M, et al. . Preserving normal facial nerve function and improving hearing outcome in large vestibular schwannomas with a combined approach: planned subtotal resection followed by gamma knife radiosurgery. Acta Neurochir. 2017;159(7):1197–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Roche PH, Ribeiro T, Khalil M, Soumare O, Thomassin JM, Pellet W. Recurrence of vestibular schwannomas after surgery. Prog Neurol Surg. 2008;21:89–92. [DOI] [PubMed] [Google Scholar]
- 98. Husseini ST, Piccirillo E, Taibah A, Almutair T, Sequino G, Sanna M. Salvage surgery of vestibular schwannoma after failed radiotherapy: the Gruppo Otologico experience and review of the literature. Am J Otolaryngol. 2013;34(2):107–114. [DOI] [PubMed] [Google Scholar]
- 99. Wise SC, Carlson ML, Tveiten OV, et al. . Surgical salvage of recurrent vestibular schwannoma following prior stereotactic radiosurgery. Laryngoscope. 2016;126(11):2580–2586. [DOI] [PubMed] [Google Scholar]
- 100. Slattery WH., 3rd Microsurgery after radiosurgery or radiotherapy for vestibular schwannomas. Otolaryngol Clin North Am 2009;42(4):707–715. [DOI] [PubMed] [Google Scholar]
- 101. Gharabaghi A, Samii A, Koerbel A, Rosahl SK, Tatagiba M, Samii M. Preservation of function in vestibular schwannoma surgery. Neurosurgery. 2007;60(2 Suppl 1):ONS124–127; discussion ONS127–128. [DOI] [PubMed] [Google Scholar]
- 102. Rabelo de Freitas M, Russo A, Sequino G, Piccirillo E, Sanna M. Analysis of hearing preservation and facial nerve function for patients undergoing vestibular schwannoma surgery: the middle cranial fossa approach versus the retrosigmoid approach—personal experience and literature review. Audiol & Neuro-otol. 2012;17(2):71–81. [DOI] [PubMed] [Google Scholar]
- 103. Rath GP, Bithal PK, Chaturvedi A, Dash HH. Complications related to positioning in posterior fossa craniectomy. J Clin Neurosci 2007;14(6):520–525. [DOI] [PubMed] [Google Scholar]
- 104. Roessler K, Krawagna M, Bischoff B, et al. . Improved postoperative facial nerve and hearing function in retrosigmoid vestibular schwannoma surgery significantly associated with semisitting position. World Neurosurg. 2016;87:290–297. [DOI] [PubMed] [Google Scholar]
- 105. Spektor S, Fraifeld S, Margolin E, Saseedharan S, Eimerl D, Umansky F. Comparison of outcomes following complex posterior fossa surgery performed in the sitting versus lateral position. J Clin Neurosci 2015;22(4):705–712. [DOI] [PubMed] [Google Scholar]
- 106. Moffat DA, Quaranta N, Chang P. Management of the high jugular bulb in translabyrinthine surgery. Laryngoscope. 2003;113(3):580–582. [DOI] [PubMed] [Google Scholar]
- 107. Ahmad RA, Sivalingam S, Topsakal V, Russo A, Taibah A, Sanna M. Rate of recurrent vestibular schwannoma after total removal via different surgical approaches. Ann Otol Rhinol Laryngol. 2012;121(3):156–161. [DOI] [PubMed] [Google Scholar]
- 108. Driscoll CL, Jackler RK, Pitts LH, Banthia V. Is the entire fundus of the internal auditory canal visible during the middle fossa approach for acoustic neuroma? American Journal of Otology. 2000;21(3):382–388. [DOI] [PubMed] [Google Scholar]
- 109. Goddard JC, Schwartz MS, Friedman RA. Fundal fluid as a predictor of hearing preservation in the middle cranial fossa approach for vestibular schwannoma. Otol Neurotol. 2010;31(7):1128–1134. [DOI] [PubMed] [Google Scholar]
- 110. Master AN, Roberts DS, Wilkinson EP, Slattery WH, Lekovic GP. Endoscope-assisted middle fossa craniotomy for resection of inferior vestibular nerve schwannoma extending lateral to transverse crest. Neurosurgical focus. 2018;44(3):E7. [DOI] [PubMed] [Google Scholar]
- 111. Wackym PA, King WA, Poe DS, et al. . Adjunctive use of endoscopy during acoustic neuroma surgery. Laryngoscope. 1999;109(8):1193–1201. [DOI] [PubMed] [Google Scholar]
- 112. Jacob A, Robinson LL Jr, Bortman JS, Yu L, Dodson EE, Welling DB. Nerve of origin, tumor size, hearing preservation, and facial nerve outcomes in 359 vestibular schwannoma resections at a tertiary care academic center. Laryngoscope. 2007;117(12):2087–2092. [DOI] [PubMed] [Google Scholar]
- 113. DeMonte F, Gidley PW. Hearing preservation surgery for vestibular schwannoma: experience with the middle fossa approach. Neurosurg Focus. 2012;33(3):E10. [DOI] [PubMed] [Google Scholar]
- 114. Hadjipanayis CG, Carlson ML, Link MJ, et al. . Congress of neurological surgeons systematic review and evidence-based guidelines on surgical resection for the treatment of patients with vestibular schwannomas. Neurosurgery. 2018;82():E40–E43. [DOI] [PubMed] [Google Scholar]
- 115. Friedman WA, Bradshaw P, Myers A, Bova FJ. Linear accelerator radiosurgery for vestibular schwannomas. J Neurosurg. 2006;105(5):657–661. [DOI] [PubMed] [Google Scholar]
- 116. Regis J, Pellet W, Delsanti C, et al. . Functional outcome after gamma knife surgery or microsurgery for vestibular schwannomas. J Neurosurg 2002;97(5):1091–1100. [DOI] [PubMed] [Google Scholar]
- 117. Wu CC, Guo WY, Chung WY, et al. . Magnetic resonance imaging characteristics and the prediction of outcome of vestibular schwannomas following Gamma Knife radiosurgery. J Neurosurg 2017;127(6):1384–1391. [DOI] [PubMed] [Google Scholar]
- 118. Karpinos M, Teh BS, Zeck O, et al. . Treatment of acoustic neuroma: stereotactic radiosurgery vs. microsurgery. Int J Radiat Oncol Biol Phys. 2002;54(5):1410–1421. [DOI] [PubMed] [Google Scholar]
- 119. Pollock BE, Lunsford LD, Kondziolka D, et al. . Outcome analysis of acoustic neuroma management: a comparison of microsurgery and stereotactic radiosurgery. Neurosurgery. 1995;36(1):215–224; discussion 224–229. [DOI] [PubMed] [Google Scholar]
- 120. Pollock BE, Driscoll CL, Foote RL, et al. . Patient outcomes after vestibular schwannoma management: a prospective comparison of microsurgical resection and stereotactic radiosurgery. Neurosurgery. 2006;59(1):77–85; discussion 77–85. [DOI] [PubMed] [Google Scholar]
- 121. Akpinar B, Mousavi SH, McDowell MM, et al. . Early radiosurgery improves hearing preservation in vestibular schwannoma patients with normal hearing at the time of diagnosis. Int J Radiat Oncol Biol Phys. 2016;95(2):729–734. [DOI] [PubMed] [Google Scholar]
- 122. Tamura M, Carron R, Yomo S, et al. . Hearing preservation after gamma knife radiosurgery for vestibular schwannomas presenting with high-level hearing. Neurosurgery. 2009;64(2):289–296; discussion 296. [DOI] [PubMed] [Google Scholar]
- 123. Jacob JT, Carlson ML, Schiefer TK, Pollock BE, Driscoll CL, Link MJ. Significance of cochlear dose in the radiosurgical treatment of vestibular schwannoma: controversies and unanswered questions. Neurosurgery. 2014;74(5):466–474; discussion 474. [DOI] [PubMed] [Google Scholar]
- 124. Carlson ML, Jacob JT, Pollock BE, et al. . Long-term hearing outcomes following stereotactic radiosurgery for vestibular schwannoma: patterns of hearing loss and variables influencing audiometric decline. J Neurosurg 2013;118(3):579–587. [DOI] [PubMed] [Google Scholar]
- 125. Lobato-Polo J, Kondziolka D, Zorro O, Kano H, Flickinger JC, Lunsford LD. Gamma knife radiosurgery in younger patients with vestibular schwannomas. Neurosurgery. 2009;65(2):294–300; discussion 300–301. [DOI] [PubMed] [Google Scholar]
- 126. Mousavi SH, Niranjan A, Akpinar B, et al. . Hearing subclassification may predict long-term auditory outcomes after radiosurgery for vestibular schwannoma patients with good hearing. J Neurosurg 2016;125(4):845–852. [DOI] [PubMed] [Google Scholar]
- 127. Linskey ME, Johnstone PA, O’Leary M, Goetsch S. Radiation exposure of normal temporal bone structures during stereotactically guided gamma knife surgery for vestibular schwannomas. J Neurosurg 2013;119(Suppl):800–806. [DOI] [PubMed] [Google Scholar]
- 128. Linskey ME. Hearing preservation in vestibular schwannoma stereotactic radiosurgery: what really matters? J Neurosurg 2013;119(Suppl):129–136. [DOI] [PubMed] [Google Scholar]
- 129. Wackym PA, Runge-Samuelson CL, Nash JJ, et al. . Gamma knife surgery of vestibular schwannomas: volumetric dosimetry correlations to hearing loss suggest stria vascularis devascularization as the mechanism of early hearing loss. Otol Neurotol. 2010;31(9):1480–1487. [DOI] [PubMed] [Google Scholar]
- 130. Kano H, Kondziolka D, Khan A, Flickinger JC, Lunsford LD. Predictors of hearing preservation after stereotactic radiosurgery for acoustic neuroma: clinical article. J Neurosurg 2013;119(Suppl):863–873. [DOI] [PubMed] [Google Scholar]
- 131. Anderson BM, Khuntia D, Bentzen SM, et al. . Single institution experience treating 104 vestibular schwannomas with fractionated stereotactic radiation therapy or stereotactic radiosurgery. J Neurooncol. 2014;116(1):187–193. [DOI] [PubMed] [Google Scholar]
- 132. Andrews DW, Suarez O, Goldman HW, et al. . Stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of acoustic schwannomas: comparative observations of 125 patients treated at one institution. Int J Radiat Oncol Biol Phys. 2001;50(5):1265–1278. [DOI] [PubMed] [Google Scholar]
- 133. Collen C, Ampe B, Gevaert T, et al. . Single fraction versus fractionated linac-based stereotactic radiotherapy for vestibular schwannoma: a single-institution experience. Int J Radiat Oncol Biol Phys. 2011;81(4):e503–509. [DOI] [PubMed] [Google Scholar]
- 134. Combs SE, Engelhard C, Kopp C, et al. . Long-term outcome after highly advanced single-dose or fractionated radiotherapy in patients with vestibular schwannomas—pooled results from 3 large German centers. Radiother Oncol. 2015;114(3):378–383. [DOI] [PubMed] [Google Scholar]
- 135. Kopp C, Fauser C, Müller A, et al. . Stereotactic fractionated radiotherapy and LINAC radiosurgery in the treatment of vestibular schwannoma—report about both stereotactic methods from a single institution. Int J Radiat Oncol Biol Phys. 2011;80(5):1485–1491. [DOI] [PubMed] [Google Scholar]
- 136. Meijer OW, Vandertop WP, Baayen JC, Slotman BJ. Single-fraction vs. fractionated linac-based stereotactic radiosurgery for vestibular schwannoma: a single-institution study. Int J Radiat Oncol Biol Phys. 2003;56(5):1390–1396. [DOI] [PubMed] [Google Scholar]
- 137. Kessel KA, Fischer H, Vogel MM, et al. . Fractionated vs. single-fraction stereotactic radiotherapy in patients with vestibular schwannoma: hearing preservation and patients’ self-reported outcome based on an established questionnaire. Strahlenther Onkol. 2017;193(3):192–199. [DOI] [PubMed] [Google Scholar]
- 138. Persson O, Bartek J Jr, Shalom NB, Wangerid T, Jakola AS, Forander P. Stereotactic radiosurgery vs. fractionated radiotherapy for tumor control in vestibular schwannoma patients: a systematic review. Acta Neurochir. 2017;159(6):1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. King AT, Rutherford SA, Hammerbeck-Ward C, et al. . Malignant peripheral nerve sheath tumors are not a feature of neurofibromatosis type 2 in the unirradiated patient. Neurosurgery. 2018;83(1):38–42. [DOI] [PubMed] [Google Scholar]
- 140. Carlson ML, Jacob JT, Habermann EB, Glasgow AE, Raghunathan A, Link MJ. Malignant peripheral nerve sheath tumors of the eighth cranial nerve arising without prior irradiation. J Neurosurg 2016;125(5):1120–1129. [DOI] [PubMed] [Google Scholar]
- 141. Pollock BE, Link MJ, Stafford SL, Parney IF, Garces YI, Foote RL. The risk of radiation-induced tumors or malignant transformation after single-fraction intracranial radiosurgery: results based on a 25-year experience. Int J Radiat Oncol Biol Phys. 2017;97(5):919–923. [DOI] [PubMed] [Google Scholar]
- 142. Maducdoc MM, Ghavami Y, Linskey ME, Djalilian HR. Evaluation of reported malignant transformation of vestibular schwannoma: de novo and after stereotactic radiosurgery or surgery. Otol Neurotol. 2015;36(8):1301–1308. [DOI] [PubMed] [Google Scholar]
- 143. Delsanti C, Roche PH, Thomassin JM, Regis J. Morphological changes of vestibular schwannomas after radiosurgical treatment: pitfalls and diagnosis of failure. Prog Neurol Surg. 2008;21:93–97. [DOI] [PubMed] [Google Scholar]
- 144. Delsanti C, Tamura M, Galanaud D, Regis J. [Changing radiological results, pitfalls and criteria of failure]. Neuro-Chirurgie. 2004;50(2–3 Pt 2):312–319. [PubMed] [Google Scholar]
- 145. Nagano O, Higuchi Y, Serizawa T, et al. . Transient expansion of vestibular schwannoma following stereotactic radiosurgery. J Neurosurg 2008;109(5):811–816. [DOI] [PubMed] [Google Scholar]
- 146. Meijer OW, Weijmans EJ, Knol DL, et al. . Tumor-volume changes after radiosurgery for vestibular schwannoma: implications for follow-up MR imaging protocol. AJNR Am J Neuroradiol. 2008;29(5):906–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Hayhurst C, Zadeh G. Tumor pseudoprogression following radiosurgery for vestibular schwannoma. Neuro Oncol. 2012;14(1):87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Plotkin SR, Stemmer-Rachamimov AO, Barker FG 2nd, et al. . Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med. 2009;361(4):358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Plotkin SR, Merker VL, Halpin C, et al. . Bevacizumab for progressive vestibular schwannoma in neurofibromatosis type 2: a retrospective review of 31 patients. Otol Neurotol. 2012;33(6):1046–1052. [DOI] [PubMed] [Google Scholar]
- 150. Blakeley JO, Ye X, Duda DG, et al. . Efficacy and biomarker study of bevacizumab for hearing loss resulting from neurofibromatosis type 2-associated vestibular schwannomas. J Clin Oncol 2016;34(14):1669–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Van Gompel JJ, Agazzi S, Carlson ML, et al. . Congress of neurological surgeons systematic review and evidence-based guidelines on emerging therapies for the treatment of patients with vestibular schwannomas. Neurosurgery. 2018;82(2):E52–E54. [DOI] [PubMed] [Google Scholar]
- 152. Plotkin SR, Halpin C, McKenna MJ, Loeffler JS, Batchelor TT, Barker FG 2nd. Erlotinib for progressive vestibular schwannoma in neurofibromatosis 2 patients. Otol Neurotol. 2010;31(7):1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Karajannis MA, Legault G, Hagiwara M, et al. . Phase II trial of lapatinib in adult and pediatric patients with neurofibromatosis type 2 and progressive vestibular schwannomas. Neuro Oncol. 2012;14(9):1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Karajannis MA, Legault G, Hagiwara M, et al. . Phase II study of everolimus in children and adults with neurofibromatosis type 2 and progressive vestibular schwannomas. Neuro Oncol. 2014;16(2):292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Samii M, Alimohamadi M, Khouzani RK, Rashid MR, Gerganov V. Comparison of direct side-to-end and end-to-end hypoglossal-facial anastomosis for facial nerve repair. World Neurosurg. 2015;84(2):368–375. [DOI] [PubMed] [Google Scholar]
- 156. Slattery WH 3rd, Cassis AM, Wilkinson EP, Santos F, Berliner K. Side-to-end hypoglossal to facial anastomosis with transposition of the intratemporal facial nerve. Otol Neurotol. 2014;35(3):509–513. [DOI] [PubMed] [Google Scholar]
- 157. Seiff SR. Surgical management of seventh nerve paralysis and floppy eyelid syndrome. Curr Opin Ophthalmol. 1999;10(4):242–246. [DOI] [PubMed] [Google Scholar]
- 158. Zwick OM, Seiff SR. Supportive care of facial nerve palsy with temporary external eyelid weights. Optometry. 2006;77(7):340–342. [DOI] [PubMed] [Google Scholar]
- 159. Butler DP, Grobbelaar AO. Facial palsy: what can the multidisciplinary team do? J Multidiscip Healthc. 2017;10:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Orestes MI, Chhetri DK. Superior laryngeal nerve injury: effects, clinical findings, prognosis, and management options. Curr Opin Otolaryngol Head Neck Surg. 2014;22(6):439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Eibling DE, Boyd EM. Rehabilitation of lower cranial nerve deficits. Otolaryngol Clin North Am 1997;30(5):865–875. [PubMed] [Google Scholar]
- 162. Di Maio S, Akagami R. Prospective comparison of quality of life before and after observation, radiation, or surgery for vestibular schwannomas. J Neurosurg 2009;111(4):855–862. [DOI] [PubMed] [Google Scholar]
- 163. Foley RW, Maweni RM, Jaafar H, McConn Walsh R, Javadpour M, Rawluk D. The impact of primary treatment strategy on the quality of life in patients with vestibular schwannoma. World Neurosurg. 2017;102:111–116. [DOI] [PubMed] [Google Scholar]
- 164. Sandooram D, Grunfeld EA, McKinney C, Gleeson MJ. Quality of life following microsurgery, radiosurgery and conservative management for unilateral vestibular schwannoma. Clin Otolaryngol Allied Sci. 2004;29(6):621–627. [DOI] [PubMed] [Google Scholar]
- 165. McLaughlin EJ, Bigelow DC, Lee JY, Ruckenstein MJ. Quality of life in acoustic neuroma patients. Otol Neurotol. 2015;36(4):653–656. [DOI] [PubMed] [Google Scholar]
- 166. Myrseth E, Moller P, Pedersen PH, Vassbotn FS, Wentzel-Larsen T, Lund-Johansen M. Vestibular schwannomas: clinical results and quality of life after microsurgery or gamma knife radiosurgery. Neurosurgery. 2005;56(5):927–935; discussion 927–935. [DOI] [PubMed] [Google Scholar]
- 167. Thakkar JP, Dolecek TA, Horbinski C, et al. . Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev. 2014;23(10):1985–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Wangerid T, Bartek J Jr, Svensson M, Forander P. Long-term quality of life and tumour control following gamma knife radiosurgery for vestibular schwannoma. Acta Neurochir. 2014;156(2):389–396. [DOI] [PubMed] [Google Scholar]
- 169. Kirchmann M, Karnov K, Hansen S, Dethloff T, Stangerup SE, Caye-Thomasen P. Ten-year follow-up on tumor growth and hearing in patients observed with an intracanalicular vestibular schwannoma. Neurosurgery. 2017;80(1):49–56. [DOI] [PubMed] [Google Scholar]
- 170. Moffat DA, Kasbekar A, Axon PR, Lloyd SK. Growth characteristics of vestibular schwannomas. Otol Neurotol. 2012;33(6):1053–1058. [DOI] [PubMed] [Google Scholar]
- 171. Lawson McLean AC, Rosahl SK. Growth dynamics of intracranial tumors in patients with neurofibromatosis type 2. World Neurosurg. 2017;98:152–161. [DOI] [PubMed] [Google Scholar]
- 172. Morris KA, Golding JF, Axon PR, et al. . Bevacizumab in neurofibromatosis type 2 (NF2) related vestibular schwannomas: a nationally coordinated approach to delivery and prospective evaluation. Neuro-Oncol Pract. 2016;3(4):281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Morris KA, Golding JF, Blesing C, et al. . Toxicity profile of bevacizumab in the UK Neurofibromatosis type 2 cohort. J Neurooncol. 2017;131(1):117–124. [DOI] [PubMed] [Google Scholar]
- 174. Li KL, Djoukhadar I, Zhu X, et al. . Vascular biomarkers derived from dynamic contrast-enhanced MRI predict response of vestibular schwannoma to antiangiogenic therapy in type 2 neurofibromatosis. Neuro Oncol. 2016;18(2):275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]