Abstract
Our purpose is to provide the results of a prospective study evaluating prostate-specific membrane antigen–targeted 18F-DCFPyL (2-(3-{1-carboxy-5-[(6-18F-fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid) PET/CT in patients with biochemical failure after radical prostatectomy for prostate cancer (PCa). Methods: Thirty-one patients with postprostatectomy serum prostate-specific antigen (PSA) levels of at least 0.2 ng/mL and negative conventional imaging results were enrolled in this study and imaged with 18F-DCFPyL PET/CT. A consensus central review identified foci of radiotracer uptake consistent with sites of PCa. Descriptive statistics were used. Results: Twenty-one patients (67.7%) had at least 1 finding on 18F-DCFPyL PET/CT consistent with a site of PCa. Imaging was positive in 59.1% of patients with a PSA level of less than 1.0 ng/mL and in 88.9% of patients with a PSA level of more than 1.0 ng/mL. The median SUVmax across all lesions was 11.6 (range, 1.5–57.6). Conclusion: In this prospective study using the prostate-specific membrane antigen–targeted PET agent 18F-DCFPyL, most patients with biochemical failure after radical prostatectomy had foci of suggestive uptake, even at low serum PSA levels.
Keywords: oncology, genitourinary, PET/CT, prostate-specific membrane antigen, biochemical persistence, biochemical recurrence
Recent years have witnessed the rapid adoption of PET for imaging prostate cancer (PCa) (1). Among the available PET radiotracers, those targeting prostate-specific membrane antigen (PSMA) have garnered the widest clinical interest (2). One of the most studied indications for PSMA-targeted PET imaging has been biochemical recurrence of PCa after attempted curative local therapy (3,4). Although several studies have been prospective (5,6), most reports on PSMA-targeted PET imaging of men with recurrent PCa have been retrospective and some have included patients with findings of sites of disease on conventional imaging (e.g., contrast-enhanced CT and 99mTc-methylene diphosphonate bone scanning). As such, the true added value of PSMA-targeted PET over conventional imaging for identifying sites of disease in men with recurrent PCa detected solely on the basis of an elevated prostate-specific antigen (PSA) level remains unclear. Furthermore, most reports published to date have made use of 68Ga-labeled radiotracers targeting PSMA, although there has recently been a shift toward the more widespread use of 18F-labeled agents (7).
18F offers numerous advantages over 68Ga as a radionuclide for PET imaging (8). These include a longer half-life (110 vs. 68 min) that facilitates centralized production and distribution, a lower average positron energy, and a higher positron yield. Indeed, early reports have shown improved lesion detection with 18F-DCFPyL (2-(3-{1-carboxy-5-[(6-18F-fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid) as compared with 68Ga-PSMA-11—two otherwise similar urea-based small-molecule inhibitors of PSMA (9).
Herein, we present data from a single-center prospective cohort of patients with biochemical failure after radical prostatectomy who were imaged with the 18F-labeled, PSMA-targeted agent 18F-DCFPyL.
MATERIALS AND METHODS
Patient Population
This prospective study was approved by the Johns Hopkins Medicine Institutional Review Board and was performed under a U.S. Food and Drug Administration investigational new drug application (application 121064; Clinicaltrials.gov identifier NCT02523924). Written informed consent was obtained from all patients. The inclusion criteria were an age of at least 18 y, a history of adenocarcinoma of the prostate after radical prostatectomy, a serum PSA level of at least 0.2 ng/mL at least 45 d before study enrollment, and staging evaluation with CT of the abdomen and pelvis or MRI of the pelvis, and a bone scan at least 45 d before enrollment. Exclusion criteria were a history of nonprostate malignancy (other than squamous cell or basal cell carcinoma of the skin) within the 3 y before enrollment and intention to enroll in a masked therapeutic clinical trial. Patients with definitive findings of recurrent PCa on staging conventional imaging were excluded, as were patients treated with prior systemic therapy. Descriptive statistics were used, with medians and ranges or percentages derived as appropriate.
Image Acquisition
Our standard 18F-DCFPyL PET/CT acquisition protocol was followed (10). 18F-DCFPyL was synthesized according to current good manufacturing practices as previously described (11). Patients were instructed to remain nil per os except for water and medications for at least 4 h before radiotracer injection. Intravenous injection of no more than 333 MBq (≤9 mCi) of 18F-DCFPyL was followed 60 min later by PET/CT acquisition on either a Discovery RX 64-slice system (GE Healthcare) or a Biograph mCT 128-slice system (Siemens). The PET/CT scanners were operated in 3-dimensional emission mode with CT for attenuation correction. Acquisitions were performed from the mid thigh to the skull vertex. Images were reconstructed using ordered-subset expectation maximization algorithms provided by the manufacturers.
Image Analysis
All attenuation correction CT, 18F-DCFPyL PET, and fusion PET/CT images were reviewed on a SyngoVia Workstation (Siemens Healthineers). A consensus central review was performed by 2 nuclear medicine physicians with experience in reading PSMA-targeted PET studies. Sites of uptake above the background level that were consistent with potential sites of recurrent PCa were noted as putative sites of disease. SUVmax corrected for lean body mass was recorded.
RESULTS
Thirty-one patients were accrued. The median patient age was 63 y (range, 45–74 y), and 27 patients (87.1%) were white. The patients had a broad distribution of pathologic stages at the time of radical prostatectomy (11/31 [35.5%] were pT2, 14/31 [45.2%] were pT3a, 6/31 [19.4%] were pT3b, and 8/31 [25.8%] were pN1). They were imaged a median of 30 mo (range, 4–152 mo) after radical prostatectomy. In total, 20 patients (64.5%) experienced biochemical recurrence after surgery, whereas 11 (35.5%) had a postprostatectomy persistently elevated PSA level. At the time of 18F-DCFPyL PET/CT, the patients had a median PSA level of 0.4 ng/mL (range, 0.2–28.3 ng/mL), and 21 patients (67.7%) had a PSA level below 0.6 ng/mL. Additional information is provided in Table 1.
TABLE 1.
Selected Demographics and Clinical Data for Study Cohort
| Parameter | Data |
| Age (y) | 63 (45–74) |
| White race | 27 (87.1) |
| Months since radical prostatectomy | 30 (4–152) |
| Gleason grade group | |
| 1 | 2 (6.4) |
| 2 | 6 (19.4) |
| 3 | 13 (41.9) |
| 4 | 1 (3.2) |
| 5 | 9 (29.0) |
| Pathologic stage | |
| pT2 | 11/31 (35.5) |
| pT3a | 14/31 (45.2) |
| pT3b | 6/31 (19.4) |
| N1 | 8/31 (25.8) |
| Positive surgical margin | 13/31 (41.9) |
| PSA (ng/mL) | 0.4 (0.2–28.3) |
Qualitative data are expressed as numbers followed by percentages in parentheses; continuous data are expressed as median followed by range in parentheses.
In total, 21 (67.7%) of the 31 patients had at least 1 site of 18F-DCFPyL uptake consistent with PCa (Table 2). Sites of disease included the prostate bed in 8 patients (25.8%), the pelvic lymph nodes in 14 (45.1%), nonpelvic lymph nodes in 2 (6.5%), and bone in 2 (6.5%). More than 1 site of disease was found in 5 patients (16.1%). For PSA levels between 0.2 and 1.0 ng/mL, 13 of 22 patients (59.1%) had findings compatible with sites of PCa. Each of these patients had only 1 putative site of disease. For those patients with PSA levels higher than 1.0 ng/mL, the detection efficiency improved to 8 of 9 (88.9%). Among these 9 patients, 5 (55.6%) had more than 1 suspected site of disease.
TABLE 2.
Sites of Recurrent or Metastatic PCa Detected on PSMA-Targeted 18F-DCFPyL PET/CT
| Anatomic location | All patients (n = 31) | PSA 0.2–1.0 ng/mL (n = 22) | PSA > 1.0 ng/mL (n = 9) |
| Prostate bed | 8 (25.8) | 6 (27.2) | 2 (22.2) |
| Pelvic lymph node | 14 (45.1) | 6 (27.2) | 8 (88.9) |
| Nonpelvic lymph node | 2 (6.5) | 0 (0.0) | 2 (22.2) |
| Bone | 2 (6.5) | 1 (4.5) | 1 (11.1) |
| Viscera | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Any site | 21 (67.7) | 13 (59.1) | 8 (88.9) |
| >1 site | 5 (16.1) | 0 (0.0) | 5 (55.6) |
Data are expressed as numbers followed by percentages in parentheses.
The median SUVmax for all lesions was 11.6 (range, 1.5–57.6). For prostate bed lesions, the median SUVmax was 3.4 (range, 1.9–16.3), and for pelvic lymph node lesions, the median SUVmax was 11.6 (range, 1.5–55.6). The median SUVmax for nonpelvic lymph node lesions was 23.9 (range, 1.8–57.6). The 2 visualized bone lesions had an SUVmax of 17.3 and 19.9. Examples of uptake in the prostate bed and a pelvic lymph node are found in Figure 1 and Figure 2, respectively.
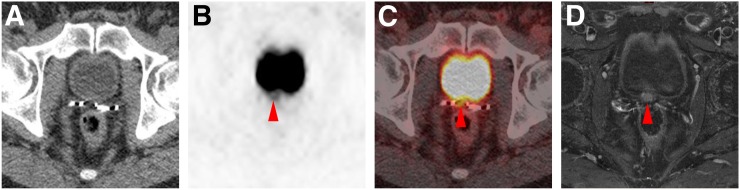
FIGURE 1.
Local recurrence detected on 18F-DCFPyL PET/CT in patient with PSA level of 0.3 ng/mL. (A–C) Axial attenuation-correction CT (A), axial 18F-DCFPyL PET (B), and axial 18F-DCFPyL PET/CT (C) demonstrate subtle uptake in prostate bed (SUVmax, 3.8; arrowheads). (D) Given the subtlety, confirmation was sought with pelvic MRI, which demonstrated corresponding nodular enhancement (arrowhead) on axial T1-weighted enhanced, fat-saturation sequence.
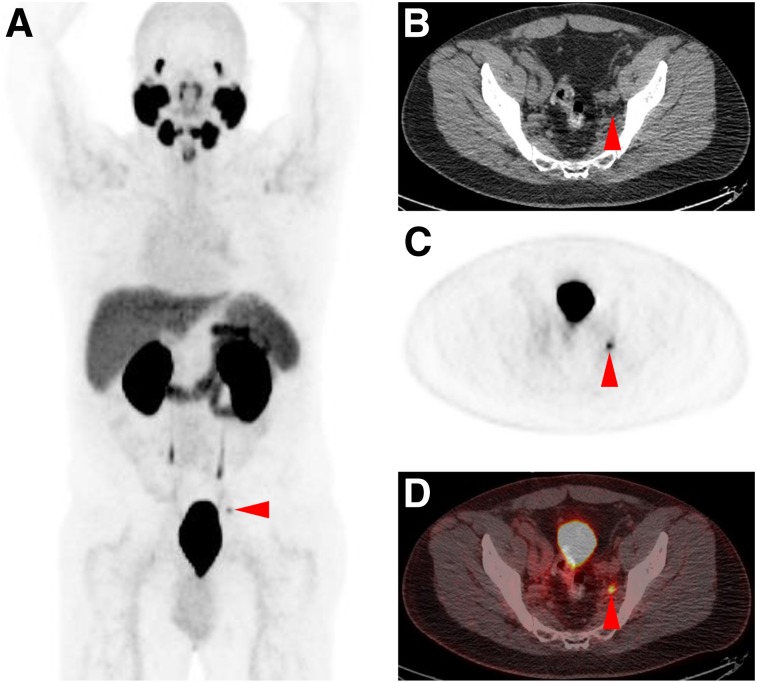
FIGURE 2.
Pelvic lymph node recurrence detected on 18F-DCFPyL PET/CT in patient with PSA level of 0.5 ng/mL. Maximum-intensity projection (A), axial attenuation-correction CT (B), axial 18F-DCFPyL PET (C), and axial 18F-DCFPyL PET/CT (D) demonstrate positive uptake in small left pelvic lymph node (SUVmax, 4.3; arrowheads).
DISCUSSION
This study provides a prospective evaluation of the 18F-labeled PSMA-targeted radiotracer 18F-DCFPyL in men with biochemical failure after radical prostatectomy. Despite negative results on conventional imaging, no prior treatment with systemic therapy, and relatively low PSA levels, 18F-DCFPyL PET/CT yielded a high detection rate for putative sites of disease. Although these findings should be confirmed in larger multicenter trials, the findings in this study nonetheless suggest a future role for PSMA-targeted PET imaging in supplanting evaluation with conventional imaging in patients with biochemical failure. Further, as the number of men worldwide with access to PSMA-targeted PET imaging continues to increase, the intrinsic advantages of 18F as a radionuclide will become more apparent, and studies that build on previous findings with 68Ga-labeled PSMA-targeted PET agents will be of increasing importance.
Given the overall low serum PSA levels of most patients, the preponderance of lesions in the pelvis is not surprising. Only 4 of 31 patients (12.9%) were found to have occult M1a or M1b disease. When taking into account differences in inclusion criteria, the overall detection rate of 18F-DCFPyL in this trial is in line with other recent studies with 18F-labeled agents, including a larger cohort of patients imaged with 18F-DCFPyL (12) and the compound 18F-PSMA-1007 (7).
Although the current study addresses some weaknesses of the available literature on PSMA-targeted PET imaging, it nonetheless has limitations. First, given that the findings of suspected disease in these patients did not have definitive conventional imaging correlates, confirmatory biopsy was not practical to perform and histopathologic confirmation is not available. This is somewhat ameliorated by the high specificity of PSMA-targeted PET in other clinical contexts (13), although the known pitfalls of PSMA-targeted PET interpretation leave open the possibility of false-positive uptake (14). Second, given that this was an externally funded, prospective trial, we were limited in the number of patients who could be accrued. A multicenter trial with this radiotracer is soon expected (i.e., the CONDOR trial; ClinicalTrials.gov NCT03739684). Lastly, the imaging central review was performed such that the readers were asked to make a definitive decision on the presence or absence of findings of PCa. A more nuanced approach allowing for gradations of confidence in findings (15) may more closely mirror a real-world clinical setting.
CONCLUSION
In this prospective study using the PSMA-targeted PET agent 18F-DCFPyL, significant added value for the detection of lesions compatible with sites of PCa was found over conventional imaging. This was true even at low serum PSA levels and adds to the evidence that PSMA-targeted PET is a promising modality for imaging patients with biochemical failure.
DISCLOSURE
This work was funded by Progenics Pharmaceuticals, Inc.; the Prostate Cancer Foundation; National Institutes of Health grants CA134675, CA183031, CA184228, and EB024495; and philanthropy raised by the James Buchanan Brady Urological Institute and Department of Urology. Martin G. Pomper is a coinventor on a patent covering 18F-DCFPyL and is entitled to a portion of any licensing fees and royalties generated by this technology. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict-of-interest policies. He has also received research funding from Progenics Pharmaceuticals, the licensee of 18F-DCFPyL. Michael A. Gorin has served as a consultant to, and has received research funding from, Progenics Pharmaceuticals. Kenneth J. Pienta and Steven P. Rowe have also received research funding from Progenics Pharmaceuticals. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: How frequently can PSMA-targeted PET with 18F-DCFPyL detect sites of suspected recurrent PCa in men with negative conventional imaging results?
PERTINENT FINDINGS: In this prospective study, 67.7% of patients had at least 1 site of 18F-DCFPyL uptake consistent with PCa. Even with low PSA values (<1.0 ng/mL), 59.1% of patients had findings compatible with sites of PCa.
IMPLICATIONS FOR PATIENT CARE: PSMA-targeted PET with 18F-DCFPyL is sensitive for detecting suspected sites of recurrent PCa and may obviate the need for conventional imaging.
REFERENCES
- 1.Li R, Ravizinni GC, Gorin MA, et al. The use of PET/CT in prostate cancer. Prostate Cancer Prostatic Dis. 2018;21:4–21. [DOI] [PubMed] [Google Scholar]
- 2.Rowe SP, Gorin MA, Allaf ME, et al. PET imaging of prostate-specific membrane antigen in prostate cancer: current state of the art and future challenges. Prostate Cancer Prostatic Dis. 2016;19:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afshar-Oromieh A, Zechmann CM, Malcher A, et al. Comparison of PET imaging with a 68Ga-labelled PSMA ligand and 18F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–674. [DOI] [PubMed] [Google Scholar]
- 5.Morigi JJ, Stricker PD, van Leeuwen PJ, et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56:1185–1190. [DOI] [PubMed] [Google Scholar]
- 6.Ceci F, Castellucci P, Graziani T, et al. 68Ga-PSMA-11 PET/CT in recurrence prostate cancer: efficacy in different clinical stages of PSA failure after radical therapy. Eur J Nucl Med Mol Imaging. 2019;46:31–39. [DOI] [PubMed] [Google Scholar]
- 7.Giesel FL, Knorr K, Spohn F, et al. Detection efficacy of [18F]PSMA-1007 PET/CT in 251 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2019;60:362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez-Crespo A. Comparison of gallium-68 and fluorine-18 imaging characteristics in positron emission tomography. Appl Radiat Isot. 2013;76:55–62. [DOI] [PubMed] [Google Scholar]
- 9.Dietlein M, Kobe C, Kuhnert G, et al. Comparison of [18F]DCFPyL and [68Ga]Ga-PSMA-HBED-CC for PSMA-PET imaging in patients with relapsed prostate cancer. Mol Imaging Biol. 2015;17:575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rowe SP, Gorin MA, Hammers HJ, et al. Imaging of metastatic clear cell renal cell carcinoma with PSMA-targeted 18F-DCFPyL PET/CT. Ann Nucl Med. 2015;29:877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Pullambhatla M, Foss CA, et al. 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid, [18F]DCFPyL, a PSMA-based PET imaging agent for prostate cancer. Clin Cancer Res. 2011;17:7645–7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rousseau E, Wilson D, Lacroix-Poisson F, et al. A prospective study on 18F-DCFPyL PSMA PET/CT imaging in biochemical recurrence of prostate cancer. J Nucl Med. April 12, 2019. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorin MA, Rowe SP, Patel HD, et al. Prostate specific membrane antigen targeted 18F-DCFPyL positron emission tomography/computerized tomography for the preoperative staging of high risk prostate cancer: results of a prospective, phase II, single center study. J Urol. 2018;199:126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheikhbahaei S, Afshar-Oromieh A, Eiber M, et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur J Nucl Med Mol Imaging. 2017;44:2117–2136. [DOI] [PubMed] [Google Scholar]
- 15.Rowe SP, Pienta KJ, Pomper MG, Gorin MA. Proposal for a structured reporting system for prostate-specific membrane antigen-targeted PET Imaging: PSMA-RADS version 1.0. J Nucl Med. 2018;59:479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]