Abstract
Objective
National guidelines recommend oral vancomycin over oral metronidazole as first-line treatment for nonsevere Clostridioides difficile infection (CDI) in adults. Guidelines recommend metronidazole for children with nonsevere CDI, emphasizing that comparative effectiveness studies comparing the relative efficacy of vancomycin and metronidazole are lacking in children.
Method
We conducted an observational study of hospitalized children with nonsevere CDI treated with metronidazole versus vancomycin using an inverse probability of treatment-weighted propensity-score analysis. All of the following criteria had to be present for children with positive CDI testing for study eligibility: (1) ≥3 new-onset unformed stools within a 24-hour period; (2) 2–17 years of age; (3) hospitalization for ≥48 hours for CDI; (4) no laxative use ≤48 hours; (5) no alternate etiology for diarrhea; (6) no previous episode of CDI ≤3 months; (7) no concurrent non-CDI–targeted antibiotic therapy, and (8) no severe or fulminant CDI.
Results
One hundred ninety-two patients met eligibility criteria; 141 (73.4%) received oral metronidazole and 51 (26.6%) children received oral vancomycin. Baseline characteristics were similar between the 2 groups in the weighted cohort. Of 141 patients, 101 (71.7%) children receiving metronidazole had clinical improvement by day 5, whereas 44 of 51 (86.3%) cases resolved with vancomycin (odds ratio, 0.40; 95% confidence interval, 0.17–0.97; P = .04). The odds of CDI recurrence within 12 weeks were similar between the groups.
Conclusions
Our study suggests that children with nonsevere CDI have earlier resolution of clinical symptoms when prescribed vancomycin compared with metronidazole. Large interventional studies are necessary to evaluate the reproducibility of our findings.
Keywords: C. difficile, Clostridium difficile, CDAD, diarrhea, pediatrics
INTRODUCTION
Clostridioides difficile infections (CDI) are the leading cause of healthcare-associated infections in the United States and are associated with significant morbidity [1, 2]. Historically, both oral metronidazole and oral vancomycin were commonly used for the treatment of nonsevere CDI. As of 2018, the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) recommend vancomycin over metronidazole as the first-line treatment for nonsevere CDI in adults [3]. This is a strong recommendation based primarily on the results of 2 randomized controlled trials [4, 5], in which findings were driven mostly by severe CDI cases, as well as the findings of an observational study that focused on mild to moderate CDI cases [6]. Others have shown that the median duration of CDI symptoms is shorter with vancomycin compared to metronidazole [7, 8].
Studies evaluating the relative efficacy of metronidazole versus vancomycin for nonsevere CDI in children are lacking. Because of the scarcity of evidence addressing this question in the pediatric population, the IDSA/SHEA guidelines recommend oral metronidazole as a weak recommendation with a low quality of evidence for children with nonsevere CDI, specifically stating, “Either metronidazole or vancomycin is recommended for the treatment of children with an initial episode or first recurrence of nonsevere CDI.” [3] Though data for the management of children with severe or fulminant CDI also are limited, experts agree because of the significant risk of poor outcomes and the strength of the evidence supporting oral vancomycin in the adult population that oral vancomycin should be used as first line therapy for both children and adults with severe or fulminant CDI.
A concern that has been raised frequently with the use of oral vancomycin for CDI therapy is the association with the subsequent emergence of vancomycin-resistant enterococci (VRE) [7]; however, this has not been consistently demonstrated in the published literature. The most robust study to date, including patients diagnosed with CDI at more than 1200 Veterans Affairs hospitals and clinics, suggests the risk of isolating VRE from a clinical culture within the subsequent 3 months is similar for the 2 agents [9–15]. Our objective was to compare the clinical outcomes of hospitalized children with nonsevere CDI treated with oral metronidazole versus oral vancomycin using a multicenter, observational cohort.
METHODS
Study Setting and Design
This study included patients under 18 years of age hospitalized at The Johns Hopkins Hospital, Bayview Medical Center, or Howard County General Hospital with nonsevere CDI between January 2012 and December 2018. As there is no validated definition for nonsevere CDI in children, the adult criteria (developed by expert opinion [3]) of peripheral white blood cell count <15 000 cells/mm3 and a serum creatinine level of <1.5 mg/dL in addition to no hypotension, ileus, or megacolon, and not requiring intensive care unit care was used to define nonsevere CDI.
The clinical microbiology laboratory provided the investigators with a list of all children with a positive stool nucleic acid amplification test (NAAT) for C. difficile toxin B during the study period. The microbiology laboratory routinely rejects samples for CDI testing from children (1) with formed stool, (2) who had previous CDI testing within 7 days, or (3) who received a laxative within 48 hours. Infectious diseases consultation is recommended for any child under 2 years of age for whom CDI testing is requested.
Manual chart review was conducted for all children with a positive C. difficile NAAT to identify those meeting eligibility criteria. The Epic Care Everywhere network that includes inpatient and outpatient records from a large number of healthcare facilities in the United States was reviewed for all patients to identify relevant pre- and post-discharge data. Similarly, the Chesapeake Regional Information System for Our Patients (CRISP), which includes inpatient, outpatient, and emergency department information for children in the state of Maryland and the District of Columbia, also was reviewed. This study was approved by The Johns Hopkins University School of Medicine Institutional Review Board with a waiver of informed consent.
All of the following criteria had to be present for children with positive CDI testing for inclusion in the study: (1) at least 3 new-onset unformed stools within a 24 hour period; (2) at least 2 years of age and under 18 years of age; (3) hospitalization for at least 48 hours for CDI; (4) no laxative use within the previous 48 hours; (5) no plausible alternate explanation for diarrhea (as determined by adjudication by both a gastroenterologist (J.W.) and an infectious diseases physician (P.D.T.); (6) receipt of either oral metronidazole or oral vancomycin therapy (as explained in more detail below); (7) no previous episode of CDI within the past 3 months [16] (children who had a nonsevere episode of CDI more than 3 months prior to the current episode with complete resolution of symptoms were included); (8) no concurrent non-CDI–targeted antibiotic therapy while receiving CDI treatment; and (9) not meeting criteria for severe or fulminant CDI.
Exposures and Outcomes
The primary exposure was receipt of oral metronidazole therapy. To be eligible for the oral metronidazole group, patients had to receive oral metronidazole from day 1 of treatment and for at least 5 days. Patients initially prescribed oral vancomycin who were converted to oral metronidazole within the first 48 hours of antibiotic therapy (and remained on metronidazole for the duration of therapy) also were designated to the exposed group. Unexposed patients were those prescribed oral vancomycin as initial therapy for the duration of therapy. As with the exposed group, patients transitioned from oral metronidazole to oral vancomycin within 48 hours of the initiation of CDI-targeted therapy were included in the unexposed group. Any patient receiving oral metronidazole or oral vancomycin and transitioned to the alternate treatment after 48 hours were excluded from further analysis. Furthermore, any child who received vancomycin as a tapered and pulsed regimen also were excluded.
The primary outcome was documented resolution or improvement of diarrhea (by the treating clinician) within 5 days of antibiotic treatment initiation. For patients discharged prior to day 5 with no post-discharge records available (after evaluating the medical chart, Epic Care Everywhere, and CRISP) an assumption was made that resolution or improvement of diarrhea occurred by day 5.
Recurrence of disease, defined as occurrence of another episode of CDI following resolution of the previous episode of CDI, within a 12-week period was a secondary outcome. To be considered a recurrent episode, the same clinical and laboratory criteria as the initial CDI episode was necessary. Additionally, incident VRE isolation from clinical cultures within 12 weeks after the initiation of treatment for CDI was collected for both groups.
Demographic, pre-existing medical conditions, microbiology, treatment, and outcomes data were manually collected and entered into a secure REDCap database. Surveillance case definitions included the following: community-onset (symptoms within 3 days of admission and no previous admission within the past 28 days), facility-associated community-onset healthcare (history of hospital admission in the past 28 days), and healthcare facility-onset (onset greater than 3 days after admission) [17].
Statistical Analysis
Using multivariable logistic regression, propensity scores were calculated for each patient with the dependent variable being metronidazole therapy. Covariates used for generating propensity scores included: previous episode of CDI (ie, nonsevere episode of CDI more than 3 months prior to the current episode with complete resolution of symptoms), chemotherapy within the previous 6 months, hematologic stem-cell transplantation (HSCT) within the previous 12 months, solid organ transplant recipient, ulcerative colitis, Crohn’s disease, and surveillance case definitions of CDI.
Patients who received metronidazole treatment were weighted by the inverse of the propensity score and patients who received oral vancomycin were weighted by the inverse of 1 minus the propensity score. A new weighted pseudo-population was created to ensure improved balance of baseline characteristics between the groups. Individuals who received an “unexpected” antibiotic (ie, received oral vancomycin when the propensity score suggested the patient would be more likely to receive oral metronidazole) based on their baseline characteristics were given an increased weight and individuals who received the “expected” antibiotic were assigned a decreased weight as they already were represented adequately in the study population. Weighted t test or χ 2 tests were used to compare baseline characteristics after inverse probability of treatment weighting was applied. Stabilized weights were used to reduce the influence of extreme weights. Standardized differences—that describe the between-group differences in standard deviation units—also were used to assess balance, with values less than 10% considered appropriately balanced. In the final analysis, weighted logistic regression was used to estimate odds ratios and 95% confidence intervals (CI) for the outcome, adjusting for variables with standardized differences greater than 10%. A 2-sided P value <.05 was considered statistically significant for all tests. Statistical analysis was completed using STATA version 13.0 (StataCorp, College Station, TX).
RESULTS
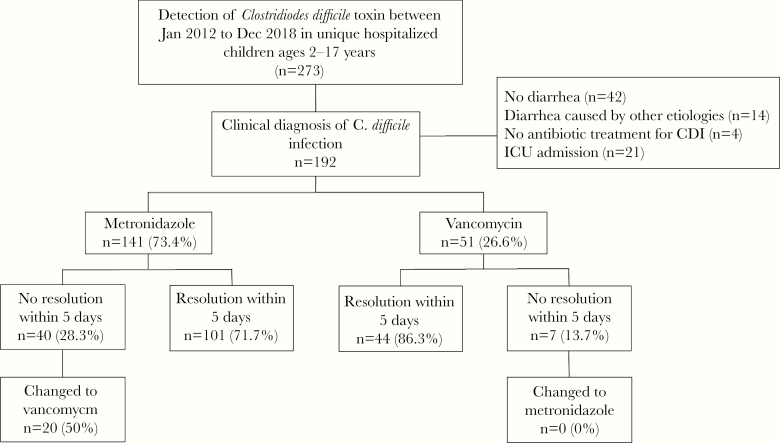
There were 273 hospitalized children with a positive C. difficile NAAT for the toxin B gene identified between January 2012 and December 2018 (Figure 1). A total of 192 patients met eligibility criteria, of which 141 (73.4%) received oral metronidazole and 51 (26.6%) children received oral vancomycin. As shown in Table 1, there was no significant difference in demographics, pre-existing medical conditions, including immunocompromised status or underlying inflammatory bowel disease (IBD) or surveillance categories between children in the metronidazole group and the vancomycin group. However, children in the vancomycin group had a higher likelihood of having a previous CDI episode within 2 years of the current episode 9.9% versus 29.4%.
Figure 1.
Eligibility criteria for observational study comparing oral metronidazole and oral vancomycin for the treatment of nonsevere Clostridioides difficle infection in hospitalized children.
Table 1.
Comparison of Baseline Characteristics of 192 Hospitalized Children With Mild to Moderate Clostridioides difficile Infection Treated With Oral Metronidazole Versus Oral Vancomycin in the Full Cohort and Propensity-Score Weighted Cohort
| Full Cohort | Weighted Cohorta | |||||||
|---|---|---|---|---|---|---|---|---|
| Metronidazole (n = 141, 73 %) | Vancomycin (n = 51, 27 %) | P value | Standardized difference | Metronidazole (n = 142, 74 %) | Vancomycin (n = 50, 26%) | P value | Standardized difference | |
| Age, median (interquartile range) | 8 years (4–13) | 9 years (4–15) | .242 | 0.188 | 8 years (8–13) | 9 years (4–15) | .212 | 0.215 |
| Male, n (%) | 76 (53.9) | 27 (52.9) | .906 | 0.019 | 77 (54.1) | 26 (51.2) | .740 | 0.058 |
| Race/ethnicity, n (%) | ||||||||
| Black | 36 (25.5) | 11 (21.6) | .573 | -0.093 | 35 (24.6) | 11 (20.9) | .614 | -0.087 |
| White | 77 (54.6) | 35 (68.6) | .082 | 0.289 | 81 (56.9) | 34 (68.0) | .191 | 0.231 |
| Latino | 7 (5.0) | 2 (3.9) | .763 | -0.050 | 6 (4.4) | 2 (3.5) | .783 | -0.045 |
| Asian | 8 (5.7) | 0 | .082 | -0.346 | 8 (5.7) | 0 | .094 | -0.347 |
| Other | 13 (9.2) | 3 (5.9) | .460 | -0.126 | 12 (8.5) | 4 (7.5) | .855 | -0.034 |
| Previous episode of Clostridioides difficile infection within past 2 years, n (%)b | 14 (9.9) | 15 (29.4) | .001 | 0.502 | 23 (15.9) | 8 (16.1) | .967 | 0.006 |
| Pre-existing medical conditions, n (%) | ||||||||
| Chemotherapy within the past 6 months | 29 (20.6) | 6 (11.8) | .163 | -0.239 | 25 (17.9) | 8 (16.2) | .823 | -0.043 |
| Hematopoietic stem cell transplant in past 12 months | 15 (10.6) | 5 (9.8) | .867 | -0.027 | 15 (10.2) | 5 (10.5) | .955 | 0.010 |
| Solid organ transplant recipient | 8 (5.7) | 3 (5.9) | .956 | 0.009 | 8 (5.7) | 3 (5.3) | .917 | -0.018 |
| Crohn’s disease | 10 (7.1) | 5 (9.8) | .536 | 0.097 | 11 (8.0) | 4 (8.1) | .989 | 0.002 |
| Ulcerative colitis | 8 (5.7) | 6 (11.7) | .152 | 0.215 | 11 (7.8) | 4 (8.0) | .970 | 0.006 |
| Surveillance categoriesc | ||||||||
| Community-onset | 35 (24.8) | 18 (35.3) | .152 | 0.228 | 41 (28.6) | 15 (30.4) | .813 | 0.040 |
| Community-onset healthcare, facility-associated | 48 (34.0) | 15 (29.4) | .546 | -0.099 | 45 (31.9) | 15 (30.0) | .817 | -0.041 |
| Healthcare facility-onset | 58 (41.1) | 18 (35.3) | .465 | -0.120 | 56 (39.5) | 20 (39.6) | .993 | 0.001 |
aWeighted cohort numbers rounded to whole numbers.
bChildren who experienced a previous Clostridioides difficile infection episode within the past 3 months were excluded.
In the weighted propensity-score cohort, 101 of 141 (71.7%) children with nonsevere CDI receiving oral metronidazole had clinical improvement by day 5, whereas 44 of 51 (86.3%) cases resolved with oral vancomycin treatment. The odds of clinical resolution were lower in the metronidazole group than in the vancomycin group (odds ratio [OR], 0.40; 95% CI, 0.17–0.97; P = .04), after additional adjustment for variables with standard biases greater than 0.10. Among the 40 children not experiencing clinical resolution by day 5 of initial oral metronidazole, 20 (50%) of these children were changed to oral vancomycin. The median duration of CDI treatment was 10 days (interquartile range [IQR] 10–13 versus IQR 10–12) in both treatment arms (P = .98).
Limiting the evaluation to children with resolution of symptoms by day 5, recurrence of disease within 12 weeks following complete resolution of the prior CDI episode was observed in 22 of 101 (21.7%) children treated with metronidazole and 7 of 44 (15.9%) children treated with oral vancomycin. The overall odds of CDI recurrence within 12 weeks were similar between the metronidazole and vanocmycin groups (OR, 1.47; 95% CI, 0.58–3.75; P = .42). There were 6 incident episodes of VRE isolated from clinical cultures in the 192 children meeting eligibility criteria: 4 (2.8%) in the metronidazole arm and 2 (3.9%) in the vancomycin arm, P = .43.
DISCUSSION
In this multicenter, observational, propensity-score–weighted cohort of 192 children with nonsevere CDI, patients receiving vancomycin were more likely to experience clinical resolution by day 5 compared with those receiving metronidazole. The odds of recurrent CDI within 12 weeks were similar between the 2 groups. Consistent with data from adult patients [6–8, 18], our findings suggest that vancomycin may have more favorable clinical efficacy than metronidazole for children with nonsevere CDI. To our knowledge, this is the first comparative effectiveness study of metronidazole and vancomycin for children hospitalized with nonsevere CDI.
Metronidazole has been traditionally prescribed for both children and adults as the first-line treatment for CDI due to its reported clinical efficacy, lower cost, and perceived reduced risk of adverse events, including subsequent VRE colonization, compared to oral vancomycin [3]. This practice was supported by initial small-scale randomized controlled trials (RCTs) in adults, which suggested that metronidazole and vancomycin were equivalent for the treatment of CDI [19, 20]. Recent RCTs studies have refuted these findings, particularly for patients with severe CDI [4, 5]. Although the measurable impact of oral vancomycin being associated with improved outcomes compared to oral metronidazole for nonsevere CDI has not been apparent across all studies that have attempted to address this question [4–6, 21], no studies have demonstrated improved clinical outcomes with the use of metronidazole. Interventional studies comparing oral metronidazole and oral vancomycin for nonsevere CDI do not exist in the pediatric population. Although some observational studies have attempted to explore this question, insufficient sample sizes have precluded its adequate investigation [22, 23].
There are several possible explanations for the apparent improved effectiveness of vancomycin compared to metronidazole for the treatment of CDI. Some studies have suggested that metronidazole minimum inhibitory concentrations against C. difficile are rising, although vancomycin resistance remains rare [24–27]. The reduced effectiveness of oral metronidazole may be compounded by the differential absorption and fecal concentrations of the 2 agents. Vancomycin is poorly absorbed, which results in high fecal concentrations after oral administration [28]. In contrast, metronidazole is very well absorbed; only 6–15% is excreted in stool [29].
An important consideration with the use of oral vancomycin is the cost of vancomycin capsules—even when generic [30]. For hospitalized patients, as a cost-containment measure, inpatient pharmacies often compound an oral solution from intravenous vancomycin [31], but this can prove challenging for outpatients or for patients at the time of hospital discharge if the duration of the outpatient treatment course is not provided by the inpatient pharmacy. Ideally, with the publication of the most recent CDI guidelines [3], both the cost and barriers associated with the inconsistent coverage of oral vancomycin by insurance coverage will be reduced. In the meantime, it is incumbent upon clinicians to weigh the costs of oral vancomycin—particularly for outpatients—into their decision making.
Approximately 15% of our cohort consisted of children with IBD, with equal distribution in both treatment groups. The small numbers of patients with IBD limited a meaningful subgroup analysis of this population. Differentiating IBD exacerbations from CDI remains challenging due to the overlapping clinical presentations of the 2 conditions. To complicate matters, patients with IBD are at an increased risk for CDI. Inflammatory bowel disease is associated with a 4.8-fold increased risk of CDI compared to the absence of IBD, with no differences observed between ulcerative colitis and Crohn’s disease [32]. Unique underlying IBD-related risk factors include the use of chronic immunosuppression, frequent healthcare exposures, and an ongoing inflammatory state, which disrupts the normal intestinal symbiotic state [33]. Ultimately, we included patients with IBD if we were in agreement with the treating gastroenterologist that more likely than not the presenting symptoms seemed consistent with CDI and warranted CDI-targeted therapy. We realize some misclassification still may have occurred; however, when taking into consideration real-world clinical decision making for patients with IBD, we believe the practical constraints in confidently assigning a diagnosis of CDI should not prohibit them from inclusion in comparative effectiveness studies for the management of CDI.
This study has several limitations. First, only 192 children were included. We deliberately imposed a strict eligibility criteria—including the need for hospitalization—even though in reality the majority of patients with nonsevere CDI are not hospitalized [13]. We did so understanding that this would compromise the sample size of our population but would increase the validity of our findings. Second, although this study involved 3 hospitals, all 3 hospitals are located within the same geographic region, have a central laboratory, and share the same local CDI diagnosis and treatment guidelines. Further studies are needed to verify the generalizability of our findings across regions where the epidemiology of C. difficile ribotypes and general clinical management practices may differ. Third, this is a retrospective observational study. In addition to institutional electronic medical records, the Epic Care Everywhere and CRISP databases were accessed to identify relevant healthcare encounters before and after discharge, but the possibility of missing data remains. In the absence of records to suggest treatment failure, we assumed clinical resolution. As a result, the proportions of clinical resolution may have been overestimated, although there is no reason to suspect that missing data would have had a greater impact on 1 treatment group compared with the other. Fourth, we used propensity score inverse probability of treatment weighting to address imbalances in measured confounders. Because patients were not randomized to treatment, the possibility of unmeasured confounding remains. Finally, this study used the IDSA/SHEA definitions to stratify disease severity [3]. These definitions were developed for adult patients and may not be able to accurately identify children with severe disease, potentially leading to some misclassification. However, available data suggest that these definitions likely “overcall” severe CDI in children [34, 35] and therefore is unlikely to have impacted our results.
All limitations notwithstanding, our study suggests that children hospitalized with nonsevere CDI have earlier resolution of clinical findings when prescribed oral vancomycin compared with oral metronidazole. Larger interventional studies are necessary to evaluate the reproducibility of our findings.
Acknowledgments
Financial support. The work was supported by funding from the National Institutes of Health (K23-AI127935 [P.D.T.], T32-AI052071 [R.G.S.], and K23-AI123525 [L.K.K.]).
Potential conflicts of interest. L.K.K. is a scientific advisor for Synthetic Biologics. All other authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sammons JS, Localio R, Xiao R, et al. Clostridium difficile infection is associated with increased risk of death and prolonged hospitalization in children. Clin Infect Dis 2013; 57:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:e1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–7. [DOI] [PubMed] [Google Scholar]
- 5. Johnson S, Louie TJ, Gerding DN, et al. ; Polymer Alternative for CDI Treatment (PACT) investigators Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis 2014; 59:345–54. [DOI] [PubMed] [Google Scholar]
- 6. Siegfried J, Dubrovskaya Y, Flagiello T, et al. Initial therapy for mild to moderate Clostridium difficile infection. Infect Dis Clin Pract 2016; 24:210–6. [Google Scholar]
- 7. Wilcox MH, Howe R. Diarrhoea caused by Clostridium difficile: response time for treatment with metronidazole and vancomycin. J Antimicrob Chemother 1995; 36:673–9. [DOI] [PubMed] [Google Scholar]
- 8. Al-Nassir WN, Sethi AK, Nerandzic MM, et al. Comparison of clinical and microbiological response to treatment of Clostridium difficile-associated disease with metronidazole and vancomycin. Clin Infect Dis 2008; 47:56–62. [DOI] [PubMed] [Google Scholar]
- 9. Karanfil LV, Murphy M, Josephson A, et al. A cluster of vancomycin-resistant Enterococcus faecium in an intensive care unit. Infect Control Hosp Epidemiol 1992; 13:195–200. [DOI] [PubMed] [Google Scholar]
- 10. Boyle JF, Soumakis SA, Rendo A, et al. Epidemiologic analysis and genotypic characterization of a nosocomial outbreak of vancomycin-resistant enterococci. J Clin Microbiol 1993; 31:1280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carmeli Y, Eliopoulos GM, Samore MH. Antecedent treatment with different antibiotic agents as a risk factor for vancomycin-resistant Enterococcus. Emerg Infect Dis 2002; 8:802–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Donskey CJ, Chowdhry TK, Hecker MT, et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med 2000; 343:1925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stevens VW, Khader K, Echevarria K, et al. Use of oral vancomycin for Clostridioides difficile infection (CDI) and the risk of vancomycin-resistant Enterococci (VRE). Clin Infect Dis 2019; ciz871. doi:10.1093/cid/ciz871. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14. Al-Nassir WN, Sethi AK, Li Y, Pultz MJ, Riggs MM, Donskey CJ. Both oral metronidazole and oral vancomycin promote persistent overgrowth of vancomycin-resistant enterococci during treatment of Clostridium difficile-associated disease. Antimicrob Agents Chemother 2008; 52:2403–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McKinnell JA, Kunz DF, Moser SA, et al. Patient-level analysis of incident vancomycin-resistant enterococci colonization and antibiotic days of therapy. Epidemiol Infect 2016; 144:1748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kociolek LK, Patel SJ, Shulman ST, Gerding DN. Molecular epidemiology of Clostridium difficile infections in children: a retrospective cohort study. Infect Control Hosp Epidemiol 2015; 36:445–51. [DOI] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention. MDRO and CDI Module. https://www.cdc.gov/nhsn/PDFs/pscManual/12pscMDRO_CDADcurrent.pdf. Accessed September 19, 2019.
- 18. Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis 2005; 40:1586–90. [DOI] [PubMed] [Google Scholar]
- 19. Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet 1983; 2:1043–6. [DOI] [PubMed] [Google Scholar]
- 20. Wenisch C, Parschalk B, Hasenhündl M, et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis 1996; 22:813–8. [DOI] [PubMed] [Google Scholar]
- 21. Stevens VW, Nelson RE, Schwab-Daugherty EM, et al. Comparative effectiveness of vancomycin and metronidazole for the prevention of recurrence and death in patients with Clostridium difficile infection. JAMA Intern Med 2017; 177:546–53. [DOI] [PubMed] [Google Scholar]
- 22. Khanna S, Baddour LM, Huskins WC, et al. The epidemiology of Clostridium difficile infection in children: a population-based study. Clin Infect Dis 2013; 56:1401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim J, Shaklee JF, Smathers S, et al. Risk factors and outcomes associated with severe Clostridium difficile infection in children. Pediatr Infect Dis J 2012; 31:134–8. [DOI] [PubMed] [Google Scholar]
- 24. Adler A, Miller-Roll T, Bradenstein R, et al. A national survey of the molecular epidemiology of Clostridium difficile in Israel: the dissemination of the ribotype 027 strain with reduced susceptibility to vancomycin and metronidazole. Diagn Microbiol Infect Dis 2015; 83:21–4. [DOI] [PubMed] [Google Scholar]
- 25. Snydman DR, McDermott LA, Jacobus NV, et al. U.S.-based national sentinel surveillance study for the epidemiology of Clostridium difficile-associated diarrheal isolates and their susceptibility to fidaxomicin. Antimicrob Agents Chemother 2015; 59:6437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Freeman J, Vernon J, Morris K, et al. ; Pan-European Longitudinal Surveillance of Antibiotic Resistance among Prevalent Clostridium difficile Ribotypes’ Study Group Pan-European longitudinal surveillance of antibiotic resistance among prevalent Clostridium difficile ribotypes. Clin Microbiol Infect 2015; 21:248.e9–248.e16. [DOI] [PubMed] [Google Scholar]
- 27. Jin D, Luo Y, Huang C, et al. Molecular epidemiology of Clostridium difficile infection in hospitalized patients in Eastern China. J Clin Microbiol 2017; 55:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Keighley MR, Burdon DW, Arabi Y, et al. Randomised controlled trial of vancomycin for pseudomembranous colitis and postoperative diarrhoea. Br Med J 1978; 2:1667–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bolton RP, Culshaw MA. Faecal metronidazole concentrations during oral and intravenous therapy for antibiotic associated colitis due to Clostridium difficile. Gut 1986; 27:1169–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bunnell KL, Danziger LH, Johnson S. Economic barriers in the treatment of Clostridium difficile infection with oral vancomycin. Open Forum Infect Dis 2017; 4:ofx078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bass SN, Lam SW, Bauer SR, Neuner EA. Comparison of oral vancomycin capsule and solution for treatment of initial episode of severe Clostridium difficile infection. J Pharm Pract 2015; 28:183–8. [DOI] [PubMed] [Google Scholar]
- 32. Singh H, Nugent Z, Yu BN, et al. Higher incidence of Clostridium difficile infection among individuals with inflammatory bowel disease. Gastroenterology 2017; 153:430–438.e2. [DOI] [PubMed] [Google Scholar]
- 33. Ananthakrishnan AN. Detecting and treating Clostridium difficile infections in patients with inflammatory bowel disease. Gastroenterol Clin North Am 2012; 41:339–53. [DOI] [PubMed] [Google Scholar]
- 34. Kociolek LK, Patel SJ, Shulman ST, Gerding DN. Concomitant medical conditions and therapies preclude accurate classification of children with severe or severe complicated Clostridium difficile infection. J Pediatric Infect Dis Soc 2015; 4:e139–42. [DOI] [PubMed] [Google Scholar]
- 35. Tschudin-Sutter S, Tamma PD, Milstone AM, Perl TM. The prediction of complicated Clostridium difficile infections in children. Infect Control Hosp Epidemiol 2014; 35:901–3. [DOI] [PubMed] [Google Scholar]